Abstract
This 25‐parameter, 22‐color full spectrum flow cytometry panel was designed and optimized for the comprehensive enumeration and functional characterization of innate lymphoid cell (ILC) subsets in mouse tissues. The panel presented here allows the discrimination of ILC progenitors (ILCP), ILC1, ILC2, NCR+ ILC3, NCR− ILC3, CCR6+ lymphoid tissue‐inducer (LTi)‐like ILC3 and mature natural killer (NK) cell populations. Further characterization of ILC and NK cell functional profiles in response to stimulation is provided by the inclusion of subset‐specific cytokine markers, and proliferation markers. Development and optimization of this panel was performed on freshly isolated cells from adult BALB/c lungs and small intestine lamina propria, and ex vivo stimulation with phorbol 12‐myrisate 13‐acetate, ionomycin, and pro‐ILC activating cytokines.
Keywords: full spectrum flow cytometry, high‐dimensional flow cytometry, innate lymphoid cells, lung, small intestine lamina propria
1. PURPOSE AND APPROPRIATE SAMPLE TYPES
This 25‐parameter, 22‐color full spectrum flow cytometry panel was designed and optimized for the comprehensive enumeration and functional characterization of innate lymphoid cell (ILC) subsets in mouse tissues (Table 1). The panel presented here allows the discrimination of ILC progenitors (ILCP), ILC1, ILC2, NCR+ ILC3, NCR− ILC3, CCR6+ lymphoid tissue‐inducer (LTi)‐like ILC3 and mature natural killer (NK) cell populations. Further characterization of ILC and NK cell functional profiles in response to stimulation is provided by the inclusion of subset‐specific cytokine markers, and proliferation markers. Development and optimization of this panel was performed on freshly isolated cells from adult BALB/c lungs and small intestine lamina propria, and ex vivo stimulation with phorbol 12‐myrisate 13‐acetate, ionomycin, and pro‐ILC activating cytokines.
TABLE 1.
Summary table
| Purpose | Deep immunophenotyping and functional assessment of ILC subsets |
| Species | Mouse |
| Cell types | Innate lymphoid cells |
| Cross‐reference | N/A |
Abbreviation: ILC, innate lymphoid cell.
2. BACKGROUND
ILCs are a unique subset of innate effector cells enriched at mucosal surfaces, with diverse roles in host defense, tissue remodeling and repair, inflammation, and metabolic homeostasis [1]. Despite lacking rearranged antigen receptors, ILCs display remarkable homology with conventional T helper (Th) type 1 (Th1), Th2, and Th17 cells in regards to phenotype and function, and thus are similarly classified into ILC1, ILC2, and ILC3 subsets [2]. Moreover, bona fide ILC subsets have now been expanded to include both cytotoxic NK and lymphoid tissue‐inducer (LTi) cells, which are phenotypically and functionally similar to ILC1 and ILC3 respectively, yet exhibit distinct developmental trajectories [3]. However, it is increasingly recognized that ILC subsets are not fixed, and that these cells can exhibit significant plasticity depending on the local inflammatory milieu [4, 5, 6, 7, 8]. As such, ILCs demonstrate intra‐subset phenotypic heterogeneity depending on their specific microenvironment [9, 10], and deep immunophenotyping therefore requires a comprehensive array of phenotypic and functional markers to accurately capture this biological variation.
Although crucial in multiple biological settings, ILCs constitute a relatively minor population within both mouse and human lymphoid and non‐lymphoid tissues and blood, comprising 1%–5% of CD45+ leukocytes [5, 11, 12, 13, 14, 15]. Due to this inherent scarcity, acquiring as much information as possible on a single‐cell level is of upmost importance for accurate ILC discrimination. High resolution ILC characterization is further convoluted in tissues such as the lung and small intestine, where the intrinsically autofluorescent nature of the samples results in a heightened background noise‐to‐signal ratio. With this in mind, cellular characterization via full spectrum flow cytometry provides a technological advancement given its high parameter capabilities [16] combined with the capacity to extract cellular autofluorescence profiles, thus improving discrimination of rare populations compared to conventional flow cytometry [17]. As such, the full spectrum panel described herein enables the detailed enumeration and functional assessment of all ILC subsets identified within mouse tissues, with a specific focus on the characterization of lung and small intestine lamina propria (siLP) populations given the divergent repertoire of resident ILC subsets localized to these tissue compartments.
ILCs arise from common lymphoid progenitors within the bone marrow (BM), which through a series of intermediate commitment stages give rise to ILC progenitors (ILCP). While initially believed to be restricted within the BM, ILCPs are now recognized to exist within circulation and as tissue‐resident populations at distal sites in both neonates and adults, including the lungs, skin, and secondary lymphoid tissues [18, 19, 20, 21]. During early development, expression of the transcription factor promyelocytic leukemia zinc finger (PLZF; encoded by Zbtb16) dictates the bifurcation of innate lymphoid progenitors, whereby PLZF+ ILCPs are restricted to the generation of ILC1, ILC2, and ILC3, but not LTi or NK cells [22, 23]. In addition to PLZF, ILCPs display high‐level expression of Il18r1, and thus a requirement for IL‐18 signaling through IL‐18Rα for proliferation and differentiation [19]. Importantly, both ILCPs and mature ILC subset are dependent on canonical IL‐7 signaling for development and function, with expression of the receptor (IL‐7Rα) and consumption of IL‐7 dramatically greater in ILCs than their T cell counterparts [24]. Continual differentiation of IL‐7Rα+PLZF+IL‐18Rα+ ILCP into distinct ILC subsets is under the control of a series of lineage‐defining master transcription factors, with all terminal subsets lacking expression of extracellular markers routinely used to identify both lymphoid and myeloid cells, subsequently defining ILCs as lineage negative (Lin−) cells (refer to Table 2 for all appropriate lineage markers).
TABLE 2.
Reagents used for OMIP
| Specificity | Fluorochrome | Clone | Purpose |
|---|---|---|---|
| CD45 | BUV395 | 30‐F11 | Pan leukocytes |
| IL‐7Rα | PE‐Cy5 | SB/199 | Pan ILC |
| PLZF | PE | MAGS21F7 | ILCP |
| IL‐18Rα | PerCP‐eFluor 710 | P3TUNYA | ILCP and ILC1 |
| T‐bet | BV421 | 4B10 | ILC1 and ILC3 subsets |
| NKp46 | PE/Dazzle 594 | 29A1.4 | ILC1, ILC3 subsets and NK cells |
| CD49a | BUV496 | Ha31/8 | ILC1 subsets and trNK cells |
| TRAIL | PE‐Cy7 | N2B2 | ILC1 subsets |
| IFN‐γ | BUV737 | XMG1.2 | ILC1 and NK cells |
| GATA‐3 | BB700 | L50‐823 | ILC2 |
| KLRG1 | BV480 | 2F1 | ILC2 |
| ST2 | R718 | U29‐93 | ILC2 |
| IL‐5 | APC | TRFK5 | ILC2 |
| IL‐13 | eFluor 450 | eBio13A | ILC2 |
| RORγt | BV786 | Q31‐378 | ILC3 |
| CCR6 | BV711 | 140706 | ILC3 subsets |
| IL‐17A | BV650 | TC11‐18H10.1 | ILC3 |
| IL‐22 | Alexa Fluor 647 | Poly5164 | ILC3 |
| CD49b | eFluor 506 | DX5 | NK cells |
| Ki‐67 | Alexa Fluor 532 | SolA15 | Proliferating cells |
| B220 | FITC | RA3‐6B2 | B cell subsets (Lin dump) |
| CD3 | FITC | 145‐2C11 | T cell subsets (Lin dump) |
| CD4 | FITC | GK1.5 | T cell subsets (Lin dump) |
| CD5 | FITC | 53‐7.3 | T cell and B cell subsets (Lin dump) |
| CD11b | FITC | M1/70 | Myeloid/NK/Granulocytes (Lin dump) |
| CD11c | FITC | N418 | Myeloid cells/Granulocytes (Lin dump) |
| CD19 | FITC | 1D3/CD19 | B cell subsets (Lin dump) |
| F4/80 | FITC | BM8 | Macrophages (Lin dump) |
| FcεR1α | FITC | MAR‐1 | Mast cells and Basophils (Lin dump) |
| Gr‐1 | FITC | RB6‐85C | Myeloid cells/Granulocytes (Lin dump) |
| TCRβ | FITC | H57‐597 | αβ T‐cells (Lin dump) |
| TCRγδ | FITC | UC7‐13D5 | γδ T‐cells (Lin dump) |
| Ter119 | FITC | TER‐119 | Red blood cells (Lin dump) |
| Dead cells | LIVE/DEAD™ Blue | ‐ | Viable cells |
ILC1s are characterized by their constitutive expression of T‐bet (encoded by Tbx21), which is central for their production of interferon (IFN)‐γ and ensuing response to intracellular pathogens following IL‐12 and IL‐18 stimulation [18, 25]. Owing to their similarities with cytotoxic NK cells, ILC1s express NKp46, however tissue‐specific distinctions can be made between these two subsets via the preferential expression of CD49a and/or TRAIL by ILC1, in conjunction with the absence of CD11b and CD49b [3, 18, 25].
ILC2s represent the most abundant subset of ILCs in mouse lungs [5] and are dependent on the expression of GATA‐3 for maintenance and survival [26, 27]. Analogous to their Th2 counterparts, ILC2s play a key role in controlling helminth infection [26, 28, 29], while perpetuating allergen‐induced allergic inflammation [30, 31, 32, 33] via the production of IL‐5 and IL‐13 in response to alarmins IL‐33, IL‐25, and thymic stromal lymphopoietin (TSLP). Given their extensive roles in both immunity and inflammation, ILC2s are known to display dynamic expression of extracellular markers associated with pro‐inflammatory functions, exemplified via variations in IL‐33 receptor (ST2) and KLRG1 expression depending on the inflammatory signal initiating the primary response [13, 34, 35]. Moreover, the inflammation‐dependent plasticity of ILC2s has been demonstrated in both mouse lungs and human peripheral blood, whereby stimulation with IL‐1β, IL‐12, and IL‐18 promotes ILC2s to adopt an ILC1‐like transcriptional and functional profile associated with the expression of T‐bet (Tbx21) and production of IFN‐γ [5, 6, 34, 36]. Similarly, human ILC2s, in response to IL‐1β and IL‐23 stimulation, transdifferentiate into a subset exhibiting ILC3‐like characteristics, evidenced by upregulation of the ILC3 signature transcription factor RORγt and production of IL‐17 [8, 37].
ILC3s are a heterogeneous subset and contribute broad roles in combating against extracellular microbes, including fungi and bacteria, via the production of IL‐17A and IL‐22 following IL‐1β and IL‐23‐mediated activation [38, 39, 40]. Representing the dominant ILC subset within the steady state siLP [41], ILC3s are strictly reliant upon RORγt (encoded by Rorc) expression for development and function [39, 42, 43]. However, mouse ILC3s can be further classified into three subtypes based on the differential expression of NKp46 and CCR6; namely NKp46+CCR6− (NCR+), NKp46−CCR6− (NCR−), and NKp46−CCR6+ (CCR6+ LTi‐like) ILC3s [42, 44, 45]. Moreover, NCR+ ILC3s share transcriptional expression of T‐bet with ILC1s, crucial for their expression of NKp46 and endowing them with the ability to produce IFN‐γ [46, 47]. Furthermore, stimulation of mouse tissue‐specific NCR+ ILC3s with IL‐12 promotes downregulation of RORγt and concomitant upregulation of T‐bet, further promoting a phenotype associated with IFN‐γ+ ILC1s [4] and demonstrating their plasticity in response to environmental cues.
Given this microenvironmental‐driven impact on ILC heterogeneity, the developmental phase of this full spectrum flow cytometry panel involved the prioritization of definitive ILCP, ILC1, ILC2, and ILC3 transcription factors, as reflected in the gating strategy (Figure 1). An added benefit of full spectrum flow cytometry is the ability to remove red blood cell contamination by plotting side‐scatter (SSC) captured by the 405 nm violet laser against SSC‐B from the 488 nm blue laser [48] (Figure 1A), thus ensuring purity of downstream terminal cell populations. Viable leukocytes (LIVE/DEAD Blue− CD45+; Figure 1A) were selected and total ILCs were defined by their expression of IL‐7Rα+ and lack of lineage markers used to routinely define T cells, B cells, myeloid cells, and red blood cells (CD3, CD4, CD5, CD11b, CD11c, CD19, B220, F4/80, FcεR1, Gr1, TCRβ, TCRγδ, Ter119; Figure 1B). ILCPs within the lung were characterized by their ubiquitous expression of PLZF, in combination with IL‐18Rα+ (Figure 1C). Mature ILC subsets within the lung (Figure 1D) and siLP (Figure 1E) were stratified as T‐bet+RORγt−ST2−CD49b−IL‐18Rα+NKp46+CD49a+/−TRAIL+/− ILC1s, GATA‐3+T‐bet−RORγt−KLRG1+/−ST2+/− ILC2s and either RORγt+T‐bet+/−NKp46+CCR6− (NCR+), RORγt+T‐bet+/−NKp46−CCR6− (NCR−) or RORγt+T‐bet+/−NKp46−CCR6+ (CCR6+ LTi‐like) ILC3s. Regarding ILC2s, it is important to note that ST2 expression on ex vivo stimulated siLP ILC2s is largely absent (Figure 1E) [9, 49]. Moreover, while lung ILCs lack CD4 expression [50], intestinal CCR6+ LTi‐like ILC3s can be sub‐classified as CD4+ and CD4− [51]. As such, the inclusion of CD4 within the lineage cocktail may influence the characterization of ILC3 subsets on a tissue‐specific basis. ILC cytokine production and proliferation within the lung (Figure 1F) and siLP (Figure 1G) was assessed in response to subset‐specific activation signals via ex vivo stimulation with phorbol 12‐myrisate 13‐acetate (PMA), Ionomycin and Brefeldin A, and either IL‐12 and IL‐18 (pro‐ILC1), IL‐33 (pro‐ILC2) or IL‐1β and IL‐23 (pro‐ILC3). Stimulation with ILC subset‐specific cytokines clearly demonstrates a heightened propensity for IL‐13 and IL‐22 production by siLP ILC2s and ILC3 subsets respectively, whereas lung ILC1s significantly upregulate IFN‐γ compared to their siLP counterparts. Although the focus of this panel was to accurately define ILCPs and ILC1‐3 subsets, inclusion of CD11b within the lineage cocktail enables the enumeration of conventional mature CD11b+NKp46+T‐bet+ NK cells (Figure 1H) [52], with additional characterization of lung tissue‐resident (trNK) and circulating (cNK) populations on the basis of CD49a and CD49b expression [53] (Figure 1H). Markers including KLRG1 [54] and RORγt [55], and cytokines IFN‐γ, IL‐5, IL‐13 [56] and IL‐22 [57] are all reportedly expressed by NK cell subsets based on maturation, tissue localization and disease state, further highlighting the diversity of this OMIP.
FIGURE 1.
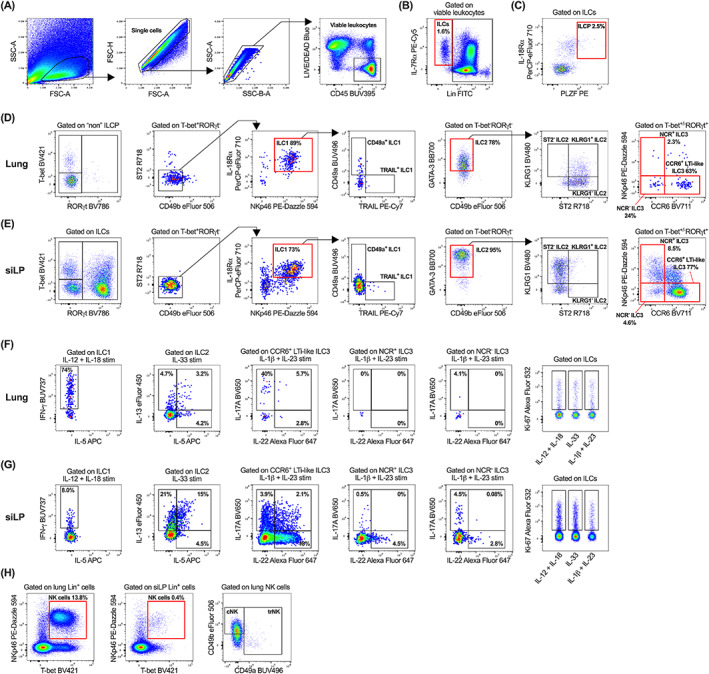
Gating strategy to characterize innate lymphoid cell (ILC) populations and their subset‐specific cytokine and proliferative profiles. After exclusion of cellular debris, doublets, red blood cell contamination and non‐viable CD45+/− cells, viable CD45+ leukocytes (A) were gated on IL‐7Rα and lineage (CD3, CD4, CD5, CD11b, CD11c, CD19, B220, F4/80, FcεR1, Gr1, TCRβ, TCRγδ, Ter119; Lin) negative cells to identify ILCs (B). ILCPs were then identified as IL‐18Rα+PLZF+ cells (C). Characterization of ILC1, ILC2, NCR+, NCR− and CCR6+ LTi‐like ILC3 subsets in the lung (D) and siLP (E). All samples in panel (A–E) were ex vivo stimulated for 5 h with PMA/Ionomycin/BFA. Single cell suspensions were ex vivo stimulated for 5 h with PMA/Ionomycin/BFA and either IL‐12 and IL‐18 (pro‐ILC1), IL‐33 (pro‐ILC2), or IL‐1β and IL‐23 (pro‐ILC3) and ILC subsets were assessed for production of subset‐specific cytokines (IFN‐γ, IL‐5, IL‐13, IL‐17A, and IL‐22) and cellular proliferation (Ki‐67) within the lung (F) and siLP (G). (H) Identification of NK cells within the lung and siLP, with downstream characterization of lung tissue‐resident NK (trNK) and circulating NK (cNK) cells. Plots in panel (A–C) are representative of individual lung samples. For panels (B–H), viable leukocytes from three technical lung or siLP replicates were concatenated into single FCS files prior to gating. All fluorochrome‐conjugated antibodies were titrated (Figure S1) during panel optimization and manual gating was determined using fluorescence minus one controls (Figure S2) where necessary [Color figure can be viewed at wileyonlinelibrary.com]
In summary, we present here the first full spectrum flow cytometry panel (Table 2) to comprehensively profile the phenotypic and functional state of ILCs in multiple mouse tissues. Moreover, by maintaining availability on the 355 nm ultraviolet laser, this panel can be expanded to incorporate additional markers of interest with little impact on the overall Complexity™ Index (a value of how distinct a collection of spectral signatures are from one other when unmixed simultaneously), thereby providing a valuable resource within the rapidly expanding field of ILC biology.
AUTHOR CONTRIBUTIONS
Kyle T Mincham: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (equal). Robert J Snelgrove: Conceptualization (supporting); funding acquisition (lead); project administration (lead); resources (lead); supervision (lead); writing – original draft (supporting); writing – review and editing (equal).
CONFLICT OF INTEREST
The author declare that no conflicts of interest exist.
3.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/cyto.a.24702.
ETHICS STATEMENT
All mouse experiments were performed in accordance with the recommendations in the Guide for the Use of Laboratory Animals of Imperial College London, with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. All animal procedures and care conformed strictly to the UK Home Office Guidelines under the Animals (Scientific Procedures) Act 1986, and the protocols were approved by the Home Office of Great Britain.
Supporting information
APPENDIX S1. Supporting Information.
MIFlowCyt Mi Flow checklist.
ACKNOWLEDGMENTS
The authors would like to thank Dr James Harker and the Imperial College London Flow Cytometry Facility South Kensington for their valuable advice on Cytek® Aurora operation during the initial design of this panel. In addition, the authors would like to thank Christopher McRandle and Dr Adam Davison from Cytek® Biosciences for their advice on data quality control using SpectroFlo® software. The authors acknowledge financial support from Imperial College London through an Imperial College Research Fellowship grant awarded to Kyle T. Mincham. Robert J. Snelgrove is a Wellcome Trust Senior Research Fellow in Basic Biomedical Sciences (209458/Z/17/Z). Parts of this work were also funded through a Rosetrees Trust/The Stoneygate Trust Project Grant (PGS21/10072).
Mincham KT, Snelgrove RJ. OMIP‐086: Full spectrum flow cytometry for high‐dimensional immunophenotyping of mouse innate lymphoid cells. Cytometry. 2023;103(2):110–116. 10.1002/cyto.a.24702
Similarities to other OMIPS: There are no published OMIPs to date characterizing ILC subsets in multiple mouse tissues.
Funding information Imperial College Research Fellowship; Rosetrees Trust/The Stoneygate Trust, Grant/Award Number: PGS21/10072; Wellcome Trust, Grant/Award Number: 209458/Z/17/Z
REFERENCES
- 1. Klose CSN, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–74. [DOI] [PubMed] [Google Scholar]
- 2. Spits H, Artis D, Colonna M, Diefenbach A, di Santo JP, Eberl G, et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–9. [DOI] [PubMed] [Google Scholar]
- 3. Vivier E, Artis D, Colonna M, Diefenbach A, di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174(5):1054–66. [DOI] [PubMed] [Google Scholar]
- 4. Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor‐expressing RORγt+ innate lymphocytes. Immunity. 2010;33(5):736–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silver JS, Kearley J, Copenhaver AM, Sanden C, Mori M, Yu L, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17(6):626–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bal SM, Bernink JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K, et al. IL‐1β, IL‐4 and IL‐12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17(6):636–45. [DOI] [PubMed] [Google Scholar]
- 7. Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed‐Nielsen M, et al. Interleukin‐12 and ‐23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity. 2015;43(1):146–60. [DOI] [PubMed] [Google Scholar]
- 8. Bernink JH, Ohne Y, Teunissen MBM, Wang J, Wu J, Krabbendam L, et al. c‐Kit‐positive ILC2s exhibit an ILC3‐like signature that may contribute to IL‐17‐mediated pathologies. Nat Immunol. 2019;20(8):992–1003. [DOI] [PubMed] [Google Scholar]
- 9. Meininger I, Carrasco A, Rao A, Soini T, Kokkinou E, Mjösberg J. Tissue‐specific features of innate lymphoid cells. Trends Immunol. 2020;41(10):902–17. [DOI] [PubMed] [Google Scholar]
- 10. Loering S, Cameron GJ, Bhatt NP, Belz GT, Foster PS, Hansbro PM, et al. Differences in pulmonary group 2 innate lymphoid cells are dependent on mouse age, sex and strain. Immunol Cell Biol. 2021;99(5):542–51. [DOI] [PubMed] [Google Scholar]
- 11. Mackley EC, Houston S, Marriott CL, Halford EE, Lucas B, Cerovic V, et al. CCR7‐dependent trafficking of RORγ+ ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun. 2015;6(1):5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gronke K, Kofoed‐Nielsen M, Diefenbach A. Isolation and flow cytometry analysis of innate lymphoid cells from the intestinal lamina propria. Methods Mol Biol. 2017;1559:255. [DOI] [PubMed] [Google Scholar]
- 13. Entwistle LJ, Gregory LG, Oliver RA, Branchett WJ, Puttur F, Lloyd CM. Pulmonary group 2 innate lymphoid cell phenotype is context specific: determining the effect of strain, location, and stimuli. Front Immunol. 2020;10:3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vély F, Barlogis V, Vallentin B, Neven B, Piperoglou C, Ebbo M, et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol. 2016;17(11):1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bennstein SB, Weinhold S, Manser AR, Scherenschlich N, Noll A, Raba K, et al. Umbilical cord blood‐derived ILC1‐like cells constitute a novel precursor for mature KIR+NKG2A‐NK cells. Elife. 2020;9:e55232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park LM, Lannigan J, Jaimes MC. OMIP‐069: forty‐color full spectrum flow cytometry panel for deep immunophenotyping of major cell subsets in human peripheral blood. Cytometry A. 2020;97(10):1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Niewold P, Ashhurst TM, Smith AL, King NJC. Evaluating spectral cytometry for immune profiling in viral disease. Cytometry A. 2020;97(11):1165–79. [DOI] [PubMed] [Google Scholar]
- 18. Klose CSN, Flach M, Möhle L, Rogell L, Hoyler T, Ebert K, et al. Differentiation of type 1 ILCs from a common progenitor to all helper‐like innate lymphoid cell lineages. Cell. 2014;157(2):340–56. [DOI] [PubMed] [Google Scholar]
- 19. Ghaedi M, Shen ZY, Orangi M, Martinez‐Gonzalez I, Wei L, Lu X, et al. Single‐cell analysis of RORα tracer mouse lung reveals ILC progenitors and effector ILC2 subsets. J Exp Med. 2020;217(3):jem.20182293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeis P, Lian M, Fan X, Herman JS, Hernandez DC, Gentek R, et al. In situ maturation and tissue adaptation of type 2 innate lymphoid cell progenitors. Immunity. 2020;53(4):775–792.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim AI, Li Y, Lopez‐Lastra S, Stadhouders R, Paul F, Casrouge A, et al. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell. 2017;168(6):1086–1100.e10. [DOI] [PubMed] [Google Scholar]
- 22. Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508(7496):397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishizuka IE, Chea S, Gudjonson H, Constantinides MG, Dinner AR, Bendelac A, et al. Single‐cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue‐inducer cell lineage. Nat Immunol. 2016;17(3):269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin CE, Spasova DS, Frimpong‐Boateng K, Kim HO, Lee M, Kim KS, et al. Interleukin‐7 availability is maintained by a hematopoietic cytokine sink comprising innate lymphoid cells and T cells. Immunity. 2017;47(1):171–182.e4. [DOI] [PubMed] [Google Scholar]
- 25. Weizman OE, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C, et al. ILC1 confer early host protection at initial sites of viral infection. Cell. 2017;171(4):795–808.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoyler T, Klose CS, Souabni A, Turqueti‐Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA‐3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37(4):634–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mjösberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37(4):649–59. [DOI] [PubMed] [Google Scholar]
- 28. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of TH2 cytokines by adipose tissue‐associated c‐Kit+Sca‐1+ lymphoid cells. Nature. 2010;463(7280):540–4. [DOI] [PubMed] [Google Scholar]
- 29. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, et al. Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature. 2010;464(7293):1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel DF, Peiró T, Bruno N, Vuononvirta J, Akthar S, Puttur F, et al. Neutrophils restrain allergic airway inflammation by limiting ILC2 function and monocyte–dendritic cell antigen presentation. Sci Immunol. 2019;4(41):eaax7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halim TYF, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell‐type cytokines in protease allergen‐induced airway inflammation. Immunity. 2012;36(3):451–63. [DOI] [PubMed] [Google Scholar]
- 32. Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134(3):671–678.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puttur F, Denney L, Gregory LG, Vuononvirta J, Oliver R, Entwistle LJ, et al. Pulmonary environmental cues drive group 2 innate lymphoid cell dynamics in mice and humans. Sci Immunol. 2019;4(36):eaav7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang Y, Guo L, Qiu J, Chen X, Hu‐Li J, Siebenlist U, et al. IL‐25‐responsive, lineage‐negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16(2):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li BWS, Stadhouders R, de Bruijn MJW, Lukkes M, Beerens DMJM, Brem MD, et al. Group 2 innate lymphoid cells exhibit a dynamic phenotype in allergic airway inflammation. Front Immunol. 2017;8:1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohne Y, Silver JS, Thompson‐Snipes LA, Collet MA, Blanck JP, Cantarel BL, et al. IL‐1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol. 2016;17(6):646–55. [DOI] [PubMed] [Google Scholar]
- 37. Ohne Y. OMIP‐066: identification of novel subpopulations of human group 2 innate lymphoid cells in peripheral blood. Cytometry A. 2020;97(10):1028–31. [DOI] [PubMed] [Google Scholar]
- 38. Gladiator A, Wangler N, Trautwein‐Weidner K, LeibundGut‐Landmann S. Cutting edge: IL‐17‐secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190(2):521–5. [DOI] [PubMed] [Google Scholar]
- 39. Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin‐23‐dependent innate intestinal pathology. Nature. 2010;464(7293):1371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Maele L, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E, et al. Activation of type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J Infect Dis. 2014;210(3):493–503. [DOI] [PubMed] [Google Scholar]
- 41. Dutton EE, Withers DR. Identification of murine innate lymphoid cell subsets in barrier tissues and their draining lymph nodes using flow cytometry. Methods Mol Biol. 2020;2121:23. [DOI] [PubMed] [Google Scholar]
- 42. Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22‐producing NKp46+ cells. Nat Immunol. 2009;10(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Satoh‐Takayama N, Vosshenrich CAJ, Lesjean‐Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29(6):958–70. [DOI] [PubMed] [Google Scholar]
- 44. Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10(1):75–82. [DOI] [PubMed] [Google Scholar]
- 45. Valle‐Noguera A, Gómez‐Sánchez MJ, Girard‐Madoux MJH, Cruz‐Adalia A. Optimized protocol for characterization of mouse gut innate lymphoid cells. Front Immunol. 2020;11:563414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klose CSN, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d'Hargues Y, et al. A T‐bet gradient controls the fate and function of CCR6–RORγt+ innate lymphoid cells. Nature. 2013;494(7436):261–5. [DOI] [PubMed] [Google Scholar]
- 47. Rankin LC, Groom JR, Chopin M, Herold MJ, Walker JA, Mielke LA, et al. The transcription factor T‐bet is essential for the development of NKp46+ innate lymphocytes via the notch pathway. Nat Immunol. 2013;14(4):389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sahir F, Mateo JM, Steinhoff M, Siveen KS. Development of a 43 color panel for the characterization of conventional and unconventional T‐cell subsets, B cells, NK cells, monocytes, dendritic cells, and innate lymphoid cells using spectral flow cytometry. Cytometry A. 2020;1–7. 10.1002/cyto.a.24288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ricardo‐Gonzalez RR, van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang HE, et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol. 2018;19(10):1093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung‐tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12(11):1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Melo‐Gonzalez F, Hepworth MR. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology. 2017;150(3):265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim S, Iizuka K, Kang HSP, Dokun A, French AR, Greco S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3(6):523–8. [DOI] [PubMed] [Google Scholar]
- 53. Sojka DK, Plougastel‐Douglas B, Yang L, Pak‐Wittel MA, Artyomov MN, Ivanova Y, et al. Tissue‐resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife. 2014;3:e01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli‐Esposti MA, et al. NK cell maturation and peripheral homeostasis is associated with KLRG1 up‐regulation. J Immunol. 2007;178(8):4764–70. [DOI] [PubMed] [Google Scholar]
- 55. Sanos SL et al. Isolation of NK cells and NK‐like cells from the intestinal lamina propria. Methods Mol Biol. 2010;612:505. [DOI] [PubMed] [Google Scholar]
- 56. Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128(2):151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL‐22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2013;6(1):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. Supporting Information.
MIFlowCyt Mi Flow checklist.


