Abstract
Antibody–drug conjugates (ADCs) comprise 3 components of wildly differing sizes: antibody (150 000 Da), linker (typically <500 Da) and payload (typically <500 Da). While the drug-linker makes up only a small percent of the ADC it has a disproportionately massive impact on all aspects of the ADC. Replacing maleimide with bromoacetamide (BrAc) affords stable attachment of the linker to the antibody cysteine, supports total flexibility for linker design and affords a more homogenous ADC. Optimisation of the protease cleavable dipeptide reduces aggregation, facilitates moderation of the physicochemical properties of the ADC and enables long-term stability to facilitate subcutaneous self-administration. Payloads are designed specifically to afford the optimal ADC. Structural information and SAR guide design to improve both potency and selectivity to the small molecule target improving the therapeutic index of resulting ADCs. Minimising the solvent exposed hydrophobic surface area improves the drug-like properties of the ADC, the realisation that the attachment heteroatom can be more than just the site for linker attachment as it can also drive potency and selectivity of the payload and the adoption of a prodrug strategy at project initiation are key areas that medicinal chemistry drives. For an optimal ADC the symbiotic relationship of the three structurally disparate components requires they all function in unison and medicinal chemistry has a huge role to ensure this happens.
For an optimal ADC the symbiotic relationship of the three structurally disparate components requires they all function in unison and medicinal chemistry has a huge role to enable this.
Introduction
With more than a dozen approved antibody drug conjugates (ADC) this therapeutic class is now firmly established and a success story for drug discovery.1,2 Brentuximab vedotin3 for relapsed HL and relapsed sALCL4 represents a first generation ADC that established many critical design features for a successful ADC (Fig. 1). Brentuximab vedotin is composed of the three distinct and equally important components of antibody, linker, and payload. Targeting of the tumor is provided by the anti-CD30 chimeric monoclonal antibody which delivers the monomethyl auristatin E (MMAE) payload, a potent microtubulin inhibitor. Joining the antibody and payload is the linker which comprises maleimide (M), caproic acid (C), valine–citrulline (Val–Cit) and para-amino benzylic alcohol (PABC) and is represented as MC–Val–Cit-PABC. Maleimide facilitates attachment to cysteines from reduced inter chain Cys–Cys bonds and provides some level of drug to antibody (DAR) homogeneity due to the limited number of available Cys–Cys inter chain bonds. Payload release is a 2-step process. First, after degradation of the ADC in the lysozome the Val–Cit is cleaved by proteases which critically converts the nitrogen on the aryl ring from an amide to the more basic aniline. Increased electron density on the aniline nitrogen can then facilitate self-immolation of the PABC group releasing the MMAE payload. In addition to MMAE, carbon dioxide is also released providing a driving force for the immolation process.
Fig. 1. Design template for an antibody drug conjugate.
As all the approved ADCs are in the oncology field5 their success has energised other therapeutic areas to pursue ADCs6 with most activity seen in immunology perhaps because of the existence of the glucocorticoid receptor modulator (GRM) class of compounds. While being a first line treatment for many immune diseases their dosing is unfortunately limited by unwanted side effects observed especially with chronic dosing,7 making them an ideal candidate as ADC payload.
Linker attachment to payload oxygen
Dexamethasone, a marketed GRM, was the payload on the first immunology antibody drug conjugates (iADC). Unfortunately, despite being highly functionalised, dexamethasone does not possess a suitable nitrogen like MMAE for linker attachment. As a result, the hydroxyl group at C21 of dexamethasone was selected as the linker attachment point for the first iADC approaches. Using established medicinal chemistry prodrug approaches for cortisol-based steroids like dexamethasone, hemisuccinate on the C21 hydroxyl8 was used to enable conjugation to the steroid (Fig. 2a). After activation, the remaining free carboxylic acid of the hemisuccinate was conjugated to surface exposed lysines on a human anti-E-selection antibody affording an ADC with a heterogenous DAR with release of the dexamethasone payload by hydrolysis of the ester bond at C21.9 For conjugation to cysteine the clinically validated linker from brentuximab vedotin was deployed again with dexamethasone as the payload (Fig. 2b) and studied extensively on an anti-CD163 antibody.10–13 By attaching the para-amino benzylic alcohol to the C21 hydroxyl on dexamethasone introduces a major structural change in comparison to brentuximab vedotin. By utilizing to the secondary amine on MMAE the PABC forms a carbamate with the payload whereas for the attachment to the C21 hydroxyl on dexamethasone this forms a carbonate. As a result, dexamethasone release can occur by both cleavage of the Val–Cit dipeptide with subsequent self-immolation of the PABC and also by hydrolysis of the carbonate. From an ADC design perspective, it is noteworthy that both esters and carbonates are less stable than carbamates in circulation14 and will result in premature non-targeted release of payload.
Fig. 2. a) Dexamethasone hemisuccinate for lysine conjugation; b) Maleimide propanoic acid Val–Cit para-amino benzylic carbonate dexamethasone for Cys conjugation.
With a key design criteria for an iADC with a GRM payload being that the ADC must be stable while in circulation to minimise systemic free payload, a more stable attachment to the C21 hydroxyl than either ester or carbonate was required. Desiring to use the clinically validated carbamate, a double self-immolative release mechanism was designed. Here an amino benzylamine (ABA) group was used to link between the PABC group and payload. The aniline nitrogen of ABA was attached to the C21 hydroxyl via a carbamate with the primary aliphatic amine of ABA then acting like the nitrogen of an ADC payload and attached via a carbamate to the PABC group. Payload release is a 3-step process with a) protease cleavage of the dipeptide, b) self-immolation of the PABC group, c) self-immolation of the ABA group to afford the payload and the cyclised ABA, 1-methyl-3,4-dihydroquinazolin-2(1H)-one (Scheme 1).15
Scheme 1. Three step payload release: a) dipeptide cleavage in lysozome; b) first self-immolation (PABC); c) second self-immolation (ABA).
Drug-linkers with the PABC–ABA dual self-immolative group were conjugated to an anti-CD19 antibody and profiled for both potency and stability. Using a K562 CD19 GRE assay efficient release of payload was shown while in both PBS buffer and plasma there was no change observed by mass spectrometry over 7 days. However, while the dual self-immolative group had resulted in stable ADCs that released the payload in target expressing cells, it also caused high levels of aggregation with even DAR2 ADCs having aggregation in the 20–40% range. Identifying the hydrophobicity of the ABA group as a key factor in the high levels of aggregation, more hydrophilic alternatives were investigated. Replacement of phenyl with pyridine and the introduction of solubilizing groups like PEG and morpholine (Fig. 3) had the desired effect of lowering the levels of aggregation for ADCs on an anti-CD19 antibody though they were still considered too high at around 10%.15 As a result our focus moved to designing a GRM for the specific purpose of an ADC payload.
Fig. 3. Pyridine based second self-immolative groups.
ADC registration to enable SAR
Before embarking on the medicinal chemistry quest for a GRM designed specifically as an ADC payload it was recognised that this would require the IT infrastructure to conduct detailed structure–activity relationship (SAR) studies. A key requirement was that a different moniker be generated for each corporate ID that would immediately identify what component of the ADC it represented. For small molecules “A-” was selected, for antibodies “PR-” was selected and finally for antibody drug conjugates “DC-” was selected. It was also recognised that ADCs generated using a haloacetamide drug-linker must be assigned the same DC# regardless of whether it was the iodo, bromo or chloroacetamide. This complication was resolved by the introduction of a virtual compound called the “X-combo”. This virtual compound has an X representing the antibody and the chemical structure of the linker-drug after conjugation to the antibody (Scheme 2). An in-house software tool was subsequently developed to register the X-combo, with an A# being assigned to this virtual compound.16
Scheme 2. X-combo is a virtual compound that enables the registration system to determine if an ADC is unique. X represents the antibody to which the linker-drug is conjugated.
As part of the registration process additional data about the drug-linker is captured (Table 1). For example, released payload, linker, linker type and payload mechanism of action (MOA) were captured to facilitate filtering and searching of biological and property data.
Association of ADC components enables SAR visualization.
| ADC with MMAE | ADC with MMAF | |
|---|---|---|
| Drug-linker |

|

|
| Linker | MC–Val–Cit-PABC | MC |
| Released payload |

|

|
| Linker type | Cleavable | Non-cleavable |
| Payload MOA | Auristatin | Auristatin |
GRM design incorporating nitrogen for linker attachment
To help guide the design of a GRM specifically as an ADC payload there is over 70 years of medicinal chemistry SAR and crystallographic information.17Fig. 4 shows the key structural motifs on a glucocorticoid that either make critical interactions with the glucocorticoid receptor or moderate potency and/or selectivity. Despite their high density of functional groups glucocorticoids do not possess a nitrogen for linker attachment and so one would need to be incorporated in the design of the ADC specific GRM payload.
Fig. 4. Key structural features of a GRM for binding and functional activity.
Multiple literature GRMs were profiled for their in vitro activity and synthetic opportunity to functionalise. This analysis identified the 16α, 17α-acetal series,18 represented by des-ciclesonide19,20 (Fig. 5), as an excellent starting point for our medicinal chemistry effort. The isobutyryl ester on the C21 hydroxyl of ciclesonide is hydrolysed by endogenous esterases in the lung converting the parent compound to the active metabolite, desisobutyrylciclesonide, shortened to des-ciclesonide,21,22 thereby achieving targeted activation.23
Fig. 5. Ciclesonide, des-ciclesonide, piperidine analogue and amino cyclohexyl analogue.
Des-ciclesonide (the active metabolite of ciclesonide) was modelled in the public crystal structure of dexamethasone bound in GR24 (Fig. 6, PDB 4UDC) and maintained the key interactions with GR (C3 carbonyl, C11 hydroxyl, C20 carbonyl) and in addition the acetal oxygen on C17 overlayed well with the hydroxyl on C17 of dexamethasone. Subsequent inspection of the acetal–cyclohexyl region of des-ciclesonide suggested that the 4 position provided a promising trajectory towards solvent accessible space for the introduction of an amine for linker attachment.
Fig. 6. a) Crystal structure PDB 4UDC of dexamethasone 1 (turquoise) bound in GR with trajectory to solvent highlighted; b) model of des-ciclesonide 3 (purple) modelled in crystal structure 4UDC showing the cyclohexyl as a suitable region for introduction of an amine for linker attachment.
Unfortunately, introduction of an amine to the cyclohexyl of des-ciclesonide was not tolerated. In a GR binding assay, des-ciclesonide had an IC50 = 0.002 μM. In stark contrast, piperdine analogue P3 had a much reduced IC50 = 0.911 μM. Amino cyclohexyl analogue P4, despite being fluorinated on both C6 and C9, a structural modification known to drive potency, still had a reduced IC50 = 0.063 μM, this being at least 10-fold less potent than des-ciclesonide. In addition, payload P4 was one of four isomers generated during the reductive amination step with the stereochemistry for payload P4 being assigned arbitrarily. As such, confirmation of the stereochemistry for the four isomers and the subsequent development of a stereoselective route to the preferred isomer further deprioritised this series.25
Having established both endo- and exo-cyclic introduction of nitrogen to the cyclohexyl ring was not tolerated, analogues positioning the nitrogen further from the acetal were investigated. The Advanced Chemistry Technologies group at AbbVie had previously demonstrated the robust use of reductive amination of aryl aldehydes to generate focused libraries for an S1P5 agonist project26 which it was recognised would allow the rapid exploration of introducing a diverse set of diamines, exemplified with boc-piperazine in Scheme 3. First desonide was reacted with benzenedicarboxaldehyde 6 to afford the benzaldehyde 7, the core for the focused library. Reductive amination of 7 with a mono boc-protected diamine like boc-piperazine, afforded boc-protected diamine 8 which after treatment with TFA afforded the desired piperazine analogue P9.
Scheme 3. Synthetic route for focused libraries with mono protected diamines.
Identification of optimal dipeptide linker
Piperazine analogue P9 was again less potent than des-ciclesonide in the GR binding assay but only 4-fold less potent in the GRE cellular assay (Table 2). With the introduction of two basic nitrogens to payload P9 it was postulated that this reduction in cellular activity may be due to reduced permeability. To investigate this hypothesis payload P9 was submitted to a PAMPA assay, which showed it had extremely low predicted membrane permeability. Having established that low membrane permeability was impacting the cellular potency the decision was taken to assess payload P9 on an ADC. From an ADC payload perspective, the low permeability and subsequent cell retention may be a beneficial attribute considering the data observed with membrane permeable MMAE and membrane impermeable MMAF in the oncology ADC field.27
GR binding, GR reporter assay and PAMPA for des-ciclesonide, P9 and P10.
| ID | Structure | GR binding IC50/μM | K562 GRE EC50/μM | PAMPA/10−6 cm s−1 |
|---|---|---|---|---|
| 1 |

|
0.002 | 0.022 | — |
| P9 |

|
0.053 ± 0.039 (4) | 0.084 ± 0.02 (3) | 0.23 |
| P10 |

|
0.034 | 0.033 ± 0.01 (2) | 0.22 |
Identification of dipeptides for the efficient enzymatic release of payloads in the lysosome by chemists at BMS28 was further developed by Seattle Genetics.29 This approach was adopted and drug-linker DL14 was synthesised with payload P9 and protease cleavable linker MC–Val–Cit-PABC (Scheme 4). Drug-linker DL14 was subsequently conjugated to a mouse α-TNF antibody and both the DAR and aggregation measured. ADC17 with the MC–Val–Cit-PABC linker (Table 3) had an average DAR of 4.0 and aggregation of 3.8%. Aggregation of biologics has been associated with undesirable drug-like properties and immunogenicity.30–32 Identification of an alternative diamino acid that afforded ADCs with lower aggregation was therefore seen as critical. At the time Val–Ala was the only other dipeptide reported in the ADC literature33 and DL15 was synthesised incorporating this dipeptide. ADC18 was prepared with DL15 and unfortunately even higher aggregation of 8.1% was observed and this being with a lower DAR of 3.3. Valine is one of the most hydrophobic amino acids so replacement of this with a less hydrophobic amino acid was seen as key to securing ADCs with good drug-like properties. DL16 with the dipeptide Ala–Ala, replacing Val with Ala, was therefore synthesised. Conjugation of DL16 to the mouse α-TNF antibody afforded ADC19 which had similar aggregation to ADC17 with matching DAR. However, when comparing the hydrophobic interaction chromatography (HIC) retention times of the DAR4 peaks, ADC19 was the fastest eluting ADC of the three suggesting this was the ADC with the most hydrophilic character. Guided by this data and the more straightforward synthesis compared to Val–Cit, dipeptide Ala–Ala was selected as the dipeptide for all future ADCs.25 Extensive analyses of 30 dipeptides was also conducted that involved correlating various measured (aggregation, HIC retention time, isoelectric point) and calculated properties (cLog P, isoelectric point).34
Scheme 4. Synthetic route for drug-linkers DL14 (Val–Cit), DL15 (Val–Ala) and DL16 (Ala–Ala) with payload P9. Conditions: i) activate, NHS ester; ii) citrulline; iii) activate, PNP carbonate; iv) PABOH; v) PNP; vi) Payload P9; vii) De-Boc; viii) MC-NHS ester.
Data for mouse α-TNF ADCs ADC17, ADC18 and ADC19 comparing the impact of the dipeptide on ADC properties.

| ||||||
|---|---|---|---|---|---|---|
| ADC | DL | Dipeptide | Average DAR/n | Agg/% | HIC Ret time/min | |
| DAR2 | DAR4 | |||||
| ADC17 | DL14 | Val–Cit | 4.0 | 3.8 | 4.83 | 6.00 |
| ADC18 | DL15 | Val–Ala | 3.3 | 8.1 | 4.83 | 6.27 |
| ADC19 | DL16 | Ala–Ala | 3.9 | 3.9 | 4.77 | 5.89 |
Succinimide ring hydrolysis for stable attachment
Maleimide is an excellent group for conjugation of a drug-linker to the antibody. Unfortunately, after conjugation the resulting succinimide ring is susceptible to the reverse Michael reaction which results in loss of the drug-linker.35 Shortening the carbon chain between the succinimide ring and the dipeptide was shown to facilitate succinimide ring hydrolysis under mild alkaline conditions.36 Encouraged by this, analogues of DL16 with either one or two methylenes between the maleimide ring and the dipeptide were investigated. Drug-linkers with two methylenes were called MP (P = propionic acid) and drug-linkers with one methylene called M-Gly. Drug-linkers DL20 and DL21 (Fig. 7) were synthesised using the same conditions as DL14 except for the NHS ester for the final step.
Fig. 7. Drug-linkers with two (MP) and one (M-Gly) methylene between the maleimide and dipeptide.
Drug-linkers DL20 and DL21 were conjugated to the mouse α-TNF antibody. Following conjugation, to facilitate succinimide ring hydrolysis, ADC23 with the MP drug-linker was incubated at pH of 9 for 3 days and ADC24 with the M-Gly drug-linker was incubated at pH of 8 for 2 days37 with the milder and faster hydrolysis conditions for the M-Gly drug-linker a result of the greater electron withdrawing effect of the dipeptide relative to the MP drug-linker due to the shorter carbon chain. For the X-combos representing ADCs like ADC23 and ADC24 (Table 4) in which the succinimide ring has been hydrolysed open after conjugation, the succinimide ring hydrolysed structures were registered. As a result, during SAR analysis it would be immediately apparent that these ADCs had a stable attachment between the succinimide and the cysteine of the antibody.
Mouse α-TNF ADCs with stable attachment by succinimide ring hydrolysis.

| ||||||
|---|---|---|---|---|---|---|
| ADC | DL | Linker name | Average DAR | Agg/% | HIC Ret time/min | |
| DAR2 | DAR4 | |||||
| ADC23 | DL20 | MP-Ala–Ala | 3.9 | 3.7 | 4.58 | 5.59 |
| ADC24 | DL21 | M-Gly–Ala–Ala | 3.9 | 1.7 | 4.58 | 5.57 |
| ADC25 | DL22 | M-Gly–Ala–Ala | 3.9 | 3.2 | 4.77 | 5.80 |
The rate of succinimide ring hydrolysis for ADC23 was monitored by mass spec and showed that hydrolysis was slightly faster for the cysteine on the light chain compared to the cysteine on the heavy chain which is likely due to the light chain cysteine being more solvent exposed (Table 5).
Succinimide ring hydrolysis for ADC23 on light chain (LC) and heavy chain (HC).
| Day | 0 | 1 | 2 | 3 |
| LC % hydrolysis | 0 | 67 | 92 | 100 |
| HC % hydrolysis | 0 | 63 | 86 | 100 |
In addition to facilitating succinimide ring hydrolysis, the shorter spacers for MP and M-Gly also resulted in ADCs with improved drug-like properties compared to those with the MC linker. For an average DAR of 3.9 the aggregation for ADC23 and ADC24 was 3.7 and 1.7% respectively, lower than the aggregation observed for ADC19 with MC spacer. The HIC retention times of the DAR4 peaks of both ADC23 (5.59 min) and ADC24 (5.57 min) were also faster than the HIC retention time of the DAR4 peak for ADC19 (5.89 min) suggesting these ADCs had increased hydrophilic character.
To assess the in vitro potency of the ADCs, a GRE Reporter assay in K562 cells expressing mTNF was developed which enabled both a comparison of the potency from TNF targeted ADC delivery and the potency from non-specific uptake of the ADC. Unfortunately, the results were very disappointing with neither ADC with payload P9 showing any potency in either cell line (Table 6). As a result, DL22 with the more potent payload P10 (see Table 2) was synthesised and conjugated to the mouse α-TNF antibody providing very insightful data on the impact the payload has on the properties for the ADC. Payload P10, with fluorines on both C6 and C9 is more hydrophobic than desfluoro payload P9 and this directly translates to both the aggregation and retention time by HIC for resulting ADCs. With the same average DAR, ADC25 with payload P10 had increased aggregation (3.2%) compared to ADC24 which had the less hydrophobic payload P9. Payload hydrophobicity impact was also apparent in the HIC retention time with ADC25 having a longer retention time that ADC24 with both ADCs having the same DAR (Table 4). Disappointingly, despite the use of the more potent payload ADC25 still had low potency in the cell assay.25
In vitro potency for mouse α-TNF ADCs.
| ADC | DL | Payload | K562 mTNF GRE | K562 WT GRE |
|---|---|---|---|---|
| EC50 μg mL−1 | ||||
| ADC23 | DL20 | P9 | >50 (2) | >50 (2) |
| ADC24 | DL21 | P9 | >50 (2) | >50 (2) |
| ADC25 | DL22 | P10 | 8.1 ± 4.7 (2) | >50 (2) |
GRM design incorporating aniline for linker attachment
When conducting SAR of an ADC multiple factors complicate interpretation of data, for example, the piperazine in payloads P9 and P10 is likely protonated in the acidic environment of the lysosome which could negatively impact their lysosomal release. Aniline not only provides a suitable nitrogen for linker attachment, but it is also not susceptible to protonation in the lysozome thereby affording an optimal connection site for direct attachment of a dipeptide linker without the need for the self-immolative group. Removal of the para-amino benzylic carbamate self-immolative group would provide multiple benefits: a) improved properties of the ADC through reduction of both hydrophobicity and aggregation by removal of the hydrophobic phenyl ring, b) minimising the payload release process by removing the self-immolative step, c) providing more stable attachment of the payload to the linker via an amide bond rather than a carbamate d) no longer releasing an equivalent of para-amino benzylic alcohol per equivalent of payload.
For the medicinal chemistry campaign to identify an optimised GRM payload that incorporated an aniline for linker attachment, multiple additional points of diversity were included a) hydrogen and or fluorine at C6 and/or C9 (R1 and R2), b) stereochemistry of the acetal, c) various spacers between the two aryl rings (X), d) regiochemistry of the aniline, e) electronic and steric impact on the aniline (R3), f) both acetal isomers were synthesised and were readily separable by chromatography enabling this additional point of diversity. To make optimal use of key synthetic intermediates a convergent synthesis shown in Scheme 5 was developed employing key boc-protected aldehyde intermediates incorporating multiple points of diversity. Subsequent reaction with des-, mono- and difluorinated tetraols (R1, R2 = H: 16α-prednisolone; R1 = F, R2 = H: triamcinolone; R1, R2 = F: fluocinolone) and chromatographic separation of (R)- and (S)-acetal isomers furnished six analogues from each aldehyde intermediate.38
Scheme 5. Synthetic route for GRM payloads to enable multiple points of diversity.
Extensive screening of the resulting compounds including GR binding, GR cellular assay, selectivity screening in the nuclear hormone receptor family (NHR), HT-ADME and both mouse and rat PK identified payload P26 (Tables 7 and 8) with H at both C6 and C9, a methylene between the two aryl rings, a meta-aniline and (R)-acetal (Fig. 8). Interestingly, potency was greatly impacted by the acetal stereochemistry with (R)-isomer P26 (EC50 0.003 μM) being 15× more potent than the (S)-isomer P27 (EC50 0.043 μM) (Table 9).
In vitro profiling for payload P26.
| GR binding IC50/μM | 0.003 ± 0.001 (3) |
| GRE reporter EC50/μM | 0.003 ± 0.003 (5) |
| AR binding IC50/μM | >30 |
| ER binding IC50/μM | >30 (2) |
| MR cell EC50/μM | >30 (2) |
| PR binding IC50/μM | 0.120 ± 0.072 (5) |
| CYP1A2 IC50/mM | >40 (3) |
| CYP2C9 IC50/mM | 18.4 ± 4.1 (6) |
| CYP2D6 IC50/mM | >40 (3) |
| CYP3A4 IC50/mM | <0.020 (3) |
| Protein binding/% unbound | 0.01 ± 0.02 (2) |
| PAMPA/10 × 10−6 cm s−1 | 0.20 |
| Solubility/μM | 1.33 |
Rodent PK for payload P26.
| CD1 mouse | Sprague–Dawley rat | |||
|---|---|---|---|---|
| IV 3 mg kg−1 | PO 10 mg kg−1 | IV 0.5 mg kg−1 | PO 3 mg kg−1 | |
| t 1/2/h | 3.82 | 3.56 | 1.20 | 2.86 |
| AUC/μg h mL−1 | 3.58 | 0.22 | ||
| CLp/L h−1 kg−1 | 0.85 | 2.27 | ||
| V ss/L kg−1 | 2.91 | 2.55 | ||
| T max/h | 1.42 | 0.25 | ||
| C max/μg mL−1 | 0.04 | 0.08 | ||
| AUC 0-inf/μg h−1 mL−1 | 0.21 | 0.22 | ||
| F/% | 1.0 | 16.8 | ||
Fig. 8. Structures of (R)-acetal isomer P26 and (S)-acetal isomer P27.
GR binding and reporter assay data for payloads P26 and P27.
| Payload | Acetal isomer | GR binding IC50/μM | GRE reporter EC50/μM |
|---|---|---|---|
| P26 | (R) | 0.003 ± 0.001 (3) | 0.003 ± 0.003 (5) |
| P27 | (S) | 0.005 | 0.043 |
The crystal structure of payload P26 and the GR ligand binding domain complex with a co-activator peptide was solved (Fig. 9, PDB 8VKZ) confirming the (R)-stereochemistry of the acetal and also showed payload P26 formed the expected interactions: Gln570 and Arg611 with C3 carbonyl, Asn564 with both C11 hydroxyl and C21 hydroxyl, Thr739 with C20 carbonyl and C21 hydroxyl. In addition, the crystal structure also showed an interaction between Glu631 and aniline.39
Fig. 9. a) Binding interactions of payload P26 in GR, b) structural pose of payload P26 (orange) in the GR binding pocket with key residues highlighted (PDB 8VKZ).
MP-Ala–Ala (MP = MP = maleimide–propionamide) was selected as the linker for attachment to payload P26 and synthesis was achieved in three steps: i) coupling of Fmoc-NH–Ala–Ala–CO2H to the payload, b) removal of the Fmoc-group; iii) reaction with the activated NHS ester of carboxylic acid of MP. However, during the deprotection of compound 28 an unexpected by-product with a molecular weight 14 Da lower than the target compound was identified in addition to desired product 29. The unwanted byproduct was identified as compound 30 in which the hydroxy ketone had been oxidised to the carboxylic acid (Scheme 6).38
Scheme 6. Synthetic route with Fmoc-protected Ala–Ala.
Swapping the protection group strategy from base labile Fmoc to acid labile Boc provided an efficient route to the desired drug-linker DL33 in high purity. Commercially available Boc-NH–Ala–Ala–CO2H 31 was coupled to payload P26 to afford Boc-protected 32 which was treated with TFA to secure the desired alcohol 29 without formation of the unwanted carboxylic acid 30. Use of Boc-protection resulted in a further synthetic optimisation. Trace epimerization of the amino acids side chains was observed during removal of Fmoc while no epimerization was observed during removal of the Boc-protecting group.36 Reaction of 29 with N-succinimidyl 3-maleimidopropionate furnished drug-linker DL33 (Scheme 7). DL34 was secured using the same synthetic route starting with payload P27.
Scheme 7. Synthetic route with Boc-protected Ala–Ala. Reagents and conditions: i) HATU, 2,6-lutidine, THF; ii) TFA, DCM; iii) DIPEA, DMF.
DL33 and DL34 were both conjugated to a mouse anti-TNF antibody affording ADC35 (payload P26) and ADC36 (payload P27) respectively. ADC35 was 15× more potent than ADC36 (Table 10) which aligned perfectly with the potency of the free payloads.
Mouse α-TNF ADCs with heterogeneous DAR with DL33 and DL34.
| ADC | DL | Payload | DAR | Agg/% | RT/min | K562 mTNF GRE | K562 WT GRE | |
|---|---|---|---|---|---|---|---|---|
| DAR2 | DAR4 | EC50/μg mL−1 | ||||||
| ADC35 | DL33 | P26 | 3.9 | 0.5 | 3.31 | 4.28 | 0.450 | 3.4 |
| ADC36 | DL34 | P27 | 3.8 | 4.0 | 3.77 | 4.51 | 7.03 | >50 |
In addition to potency, the acetal stereochemistry was shown to impact the properties of the ADC, most notably hydrophobicity and aggregation. ADC35 with the (R)-acetal isomer payload P26 only had 0.5% aggregation and a HIC retention time of 3.1 minutes for the DAR2 peak. In stark contrast, ADC36 with the (S)-acetal isomer payload P27 had 4.0% aggregation and a longer HIC retention time for the DAR2 peak of 3.8 minutes. Having observed the V-shape of (R)-acetal P26 in the crystal structure it was rationalised that the lower aggregation of ADCs with this payload resulted from the smaller solvent exposed surface area of (R)-acetal P26 compared to (S)-acetal P27, which would be linear in shape.39
Fig. 10 shows the solvent exposed surfaces as a wire mesh for energy minimised conformations of P26 and P27 and confirms the hypothesis of ADC aggregation being impacted by the exposed hydrophobic surface area of a payload. As P26 and P27 are isomers, it was not surprising that the polar solvent accessible surface area, the area capable of enabling aqueous solubility, for P26 and P27 were similar (216.5 and 224.3 Å respectively). However, the solvent exposed surface areas differed significantly. (R)-Acetal P26 had a solvent accessible surface area of 718.9 Å, almost 100 Å less than (S)-acetal P27 (814.7 Å). A similar trend was observed for the solvent accessible volume with P26 (646.4 Å) substantially lower than P27 (736.1 Å). Considering this data, it is clear that while both compounds have similar solubility driving polar areas, P26 has less hydrophobic surface area to solvate than P27 (Table 11).39
Fig. 10. Solvent exposed surface area shown as a wire mesh coloured by atom for energy minimised conformations of a) (R)-acetal compound P26, b) (S)-acetal compound P27.
Solvent accessible surface area for (R)-acetal P26 and (S)-acetal P27.
| ID | Polar solvent accessible surface area/Å | Solvent accessible surface area/Å | Solvent accessible volume/Å |
|---|---|---|---|
| P26 | 216.5 | 718.9 | 646.4 |
| P27 | 224.3 | 814.7 | 736.1 |
This significant finding identified a key criteria for ADC payload design. For optimal drug-like properties of the ADC the exposed hydrophilic surface of a payload should be maximised, and probably more importantly, the exposed hydrophobic surface of a payload should be minimised.
Identification of the H-bond interaction between the aniline and Glu631 is an important design guide for ADC payloads which is that the introduced nitrogen should be considered as far more than just an attachment site for the linker. Just as during the optimisation of a small molecule drug, SAR and structural information should be used to identify additional interactions that can be made between the linker attachment heteroatom and the payload target to drive both the potency and selectivity of the payload.
To understand the acetal isomer impact on both potency and ADC properties, energy minimised conformations of both P26 and P27 were generated in Chem3D. Fig. 10 clearly shows the V-shape of (R)-acetal P26 and the linear shape of (S)-acetal P27 which will greatly impact how the compounds interact with GR thereby providing a rationale for their potency disparity. Similarly, the observation that ADC35 (with (R)-acetal P26) has lower aggregation than ADC36 (with (S)-acetal P27) at a similar DAR can be explained by the different shapes of the two payloads. While the V-shape of (R)-acetal P26 tucks the biaryl region back under the molecule minimising the area of exposed hydrophobicity, the linear shape of (R)-acetal P27 exposes a larger hydrophobic area. By considering the detrimental impact payload P27 had on the properties of ADC36 it established a key parameter for ADC payload design, that is to maximise the exposed hydrophilic surface and probably more importantly to minimise the exposed hydrophobic surface of payload.39
DL33 was conjugated to a mouse anti-TNF antibody and the resulting ADCs DAR purified by HIC to afford both the DAR2 and DAR4 ADCs prior to succinimide ring hydrolysis. Our studies had shown that it is optimal to perform the DAR purification before succinimide ring hydrolysis as this results in improved peak resolution on the HIC column facilitating higher recovery and DAR fraction purity. In addition, to affording access to consistent and homogeneous DAR, purification by HIC also removes any excess drug-linker and results in high quality material with minimal variability. Subsequent succinimide ring hydrolysis gave DAR2 ADC37 and DAR4 ADC38.
Hydrolysis of the succinimide ring stabilises attachment of the drug-linker to the antibody which is no longer susceptible to deconjugation via the reverse-Michael reaction, while direct attachment of the dipeptide linker to the aniline of the payload via an amide bond affords a stable attachment to the payload. The combination of these two approaches resulted in a highly stable ADC which was demonstrated in cynomolgus monkeys with no change in DAR over two weeks of exposure.40
Data showed that both DAR2 ADC37 and DAR4 ADC38 had low levels of aggregation and were potent in the TNF expressing cell assay with good differentiation over the wild type cells (Table 12). All of this data supported the approach of direct attachment of the dipeptide to aniline with the para-amino benzylic alcohol self-immolative group.
Mouse α-TNF ADCs with drug-linker DL33.

| |||||
|---|---|---|---|---|---|
| ADC | DAR | RT/min | Agg/% | K562 mTNF GRE | K562 WT GRE |
| EC50/μg mL−1 | |||||
| ADC37 | 2 | 4.39 | 2.2 | 0.0906 ± 0.0507 (4) | 43.7 ± 3.0 (2) |
| ADC38 | 4 | 5.29 | 2.5 | 0.0803 ± 0.0965 (9) | 5.8 ± 16.0 (9) |
Prior to testing in vivo both DAR purified ADCs were screened in both the TNF expressing and wild type K562 GRE Reporter cell assay to confirm their activity (Table 13). For assessment in vivo on the impact of an inflammatory response the acute contact hypersensitivity (CHS) model provides excellent data on not only efficacy (ear swelling) but also on important biomarkers (corticosterone and P1NP). ADC38 was dosed at both 10 mg kg−1 or 3 mg kg−1 once prior to FITC sensitization in order to evaluate the impact of the ADCs on ear swelling. To assess the impact on corticosterone and P1NP, mice were challenged with adrenocorticotropic hormone (ACTH) 72 hours following dosing of ADC38 and plasma collected 30 minutes later to assess corticosterone and P1NP levels. ADC38 was shown to inhibit ear swelling while having a reduced impact on both corticosterone and P1NP at the doses tested.38
Impact of ADC38 on ear swelling, plasma P1NP, and plasma ACTH induced corticosterone in FITC induced contact hypersensitivity model.
| Ear swelling (% inhibition ± SD) | Corticosterone (% inhibition ± SD) | P1NP (% inhibition ± SD) | |||
|---|---|---|---|---|---|
| 10 mg kg−1 | 3 mg kg−1 | 10 mg kg−1 | 3 mg kg−1 | 10 mg kg−1 | 3 mg kg−1 |
| 88 ± 10.9 | 55 ± 20.5 | 15 ± 31.2 | 6 ± 26.8 | 19 ± 19.9 | −16 ± 32.6 |
Both ADC37 and ADC38 were progressed into a mouse collagen-induced arthritis (mCIA) model to evaluate their impact on inflammation in a chronic inflammatory setting. The mCIA model recapitulates numerous pathological features and mechanisms operant in human rheumatoid arthritis such as infiltration of inflammatory cells into the joint which leads to pannus formation and bone loss. These processes are partially mitigated with TNF inhibition while they can be fully blocked with GRM treatment41,42 making it an appropriate animal model for profiling anti-mTNF GRM ADCs.
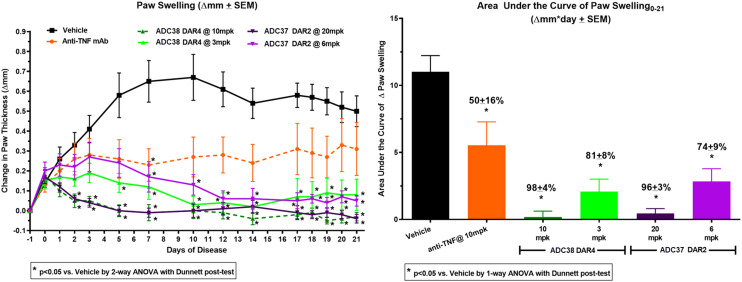
To compare the effect of DAR, ADC37 and ADC38 were tested at doses that normalised the quantity of payload per dose. DAR2 ADC37 was dosed at both 20 mg kg−1 and 6 mg kg−1 while DAR4 ADC38 was dosed at both 10 mg kg−1 and 3 mg kg−1 to dose match the quantity of payload P26. Both ADCs demonstrated inhibition of collagen-induced paw swelling at both the 20/10 mg kg−1 and 6/3 mg kg−1 doses tested (Table 14) and were significantly more efficacious than the parent mouse anti-TNF antibody.36
Impact of ADC37 and ADC38 on paw swelling in mCIA model.
| ADC | DAR | Dose | Paw swelling AUC (% inhibition ± SD) |
|---|---|---|---|
| Mouse anti-TNF mAb | — | 10 mg kg−1 | 50 ± 16 |
| ADC37 | 2 | 20 mg kg−1 | 96 ± 3 |
| 6 mg kg−1 | 74 ± 9 | ||
| ADC38 | 4 | 10 mg kg−1 | 98 ± 4 |
| 3 mg kg−1 | 81 ± 8 |
Reduction in paw swelling after a single dose in the mCIA model was maintained for >30 days for both ADCs (Fig. 11). The dose matched ADCs were shown to inhibit paw swelling to similar levels, 20 mg kg−1 DAR2 ADC37 96% compared to 10 mg kg−1 DAR4 ADC38 98% and 6 mg kg−1 DAR2 ADC37 74% compared to 3 mg kg−1 DAR2 ADC38 81% demonstrating the additional efficacy over anti-TNF antibody alone was from GRM payload P26.
Fig. 11. Mouse CIA data for DAR2 ADC37 at 6 and 20 mg kg−1, DAR4 ADC38 at 3 and 10 mg kg−1 and anti-TNF mAb at 10 mg kg−1.
The human anti-TNF version of ADC38 was designated ABBV-3373 (Fig. 12) and advanced to clinical development43 where it demonstrated clinical efficacy and the potential to provide improved outcomes for patients with rheumatoid arthritis.44,45
Fig. 12. Structure of ABBV-3373 (ADC39) human anti-TNF antibody (blue), MP-Ala–Ala linker with hydrolysed succinimide ring (black) and GRM payload P26 (red).
All of the approved oncology ADCs (oADCs) are reconstituted from the lyophilised dry powder and dosed by intravenous (IV) injection.46,47 For immunology drugs48 subcutaneous delivery of biotherapeutics49 has become the preferred dosing method and autoinjection delivery systems to facilitate self-administration by the patient have been developed for the highly successful anti-TNF monoclonal antibodies like adalimumab.50 Consequently, to enable both subcutaneous dosing51,52 and formulation at high concentrations of up to 100 mg mL−1,53 iADCs would need to have properties similar to the parent antibody.
Optimisation of drug-linker to enable long term storage of antibody-drug conjugate for subcutaneous dosing
AbbVie employs a property screening funnel54 to assess the physiochemical properties of ADCs (e.g., viscosity, conformational stability, isoelectric point, hydrophobicity and short-term solution stability). An accelerated stress test is used to ensure therapeutics have the requisite stability for development of ADCs. For the test, ADCs are incubated at 4 °C, 25 °C and 40 °C in minimal buffer (15 mM histidine) at 100 mg mL−1 for three weeks while monitoring the aggregation level to identify those that demonstrate <5.0% aggregate increase after three weeks at 40 °C. Seeking to identify ADCS that would be amenable to formulation and long-term storage at 4 °C and hence compatible with subcutaneous dosing,55 only a minimal buffer is used to provide a severe test. While adalimumab was well behaved in minimal buffer at all three temperatures, ADC39 showed an increase in aggregate at all three temperatures with a 21% increase in aggregation observed after 3 weeks at 40 °C (Table 15).
Comparison of adalimumab and ABBV-3373 in the accelerated stress test.
| Biologic | Aggregate increase after 3 weeks/% | HIC retention time/min | ||
|---|---|---|---|---|
| 4 °C | 25 °C | 40 °C | ||
| Adalimumab | 0.1 | 0.1 | 0.5 | 8.34 |
| ADC39 | 0.5 | 4.2 | 21.0 | 11.64 |
Comparing the retention time of adalimumab and ADC39 (Table 15) clearly shows the detrimental impact to hydrophobicity from the conjugation of drug-linkers to an antibody. To mitigate this increased hydrophobicity of ADC39 two strategies were employed, incorporation of a more hydrophilic linker and the introduction of a phosphate prodrug to the payload. During the drug-linker optimisation an additional change was also made to facilitate easier succinimide ring hydrolysis after conjugation. The two-methylene spacer between the maleimide and first amino acid of the peptide of DL33 (MP) was shortened to a single methylene, M-Gly. As M-Gly is more electron withdrawing than MP it facilitates succinimide ring hydrolysis after conjugation at pH 8 in 2 days. From a manufacturing perspective the shorter production would be beneficial, while the lower pH would reduce the time the antibody was exposed to stressful conditions thereby minimising the opportunity for post translational modification. These three structural modifications afforded drug-linker DL40 with a Gly–Glu dipeptide linker (Fig. 13).56
Fig. 13. DL40 with M-Gly–Glu linker and phosphate prodrug on payload P26.
DL40 was conjugated to a human anti-TNF antibody and DAR purified (Table 16). By HIC retention time it was readily apparent that the Gly–Glu dipeptide linker and phosphate resulted in a dramatic impact on the properties of the ADC (Fig. 14). While ADC39 had a HIC retention (11.64 min) that was 40% slower than the parent antibody (8.34 min), the HIC retention time of DAR4 ADC42 was only 10% slower (9.10 min) clearly indicating a large reduction in hydrophobicity.56
Drug-linkers with M-Gly and phosphate prodrug on payload.
| Human anti-TNF ADC | DL | Linker | ADC structure | cLog P |
|---|---|---|---|---|
| ADC39 | DL33 | MP-Ala–Ala |

|
2.80 |
| ADC42 | DL40 | M-Gly–Glu–PO4 |

|
0.50 |
| ADC43 | DL41 | BrAc-Gly–Glu–PO4 |

|
0.41 |
Fig. 14. Hydrophobicity ranking by HIC for ADC39, ADC42 and ADC43.
ADC42 was then subjected to the accelerated stress and shown to have much lower aggregation propensity than ADC39 (Fig. 15). Interestingly, it was observed that ADC42 gelled during storage at both 4 and 25 °C. It was hypothesised that this phenomenon resulted from the extensive negative charge on DL40 (four CO2H groups on the Glu, four CO2H groups on the hydrolysed succinimide rings and four phosphates on the payload) that caused a large decrease in the isoelectric point (Table 17) which induced strong and unfavourable molecular interactions.56
Fig. 15. Solution stability by SEC after 3 weeks at a) 40 °C; b) 25 °C; c) 4 °C. Note: data trend of ADC42 at 4 °C and 25 °C is compounded by the gelling phenomenon and doesn't reflect the actual increase in aggregates.
Physicochemical properties of parent mAb and its ADCs.
| ID | DAR | Retention time/min | pI | Absolute viscosity/cP |
|---|---|---|---|---|
| Adalimumab | — | 8.34 | 8.7 | 2.9 ± 0.0 |
| ADC39 | 4 | 11.64 | 8.3 | 2.6 ± 0.0 |
| ADC42 | 4 | 9.10 | 7.3 | 26.5 ± 3.3 |
| ADC43 | 4 | 8.99 | 7.5 | 4.9 ± 0.0 |
Succinimide ring open/closed equilibrium
Hydrolysis of the succinimide ring is critical to ensure stable attachment of the drug-linker to the antibody and remove the possibility of drug-linker loss via the reverse-Michael reaction on the ring closed succinimide. During the accelerated stress test, peptide mapping by MS showed the ring-closed succinimide in addition to the desired ring-opened succinimide (Fig. 16). In the follow-up long-term stability study of ADC39 in 15 mM Histidine at pH 5.2 it was found that the hydrolysed succinimide ring was closing to reform the succinimide ring over time at all the tested temperatures (Fig. 17).57
Fig. 16. Two forms of DL33 on ADC39, a) ring open succinimide (m/z 1332.5715 [M + H]+), b) ring closed succinimide (m/z 1314.5636 [M + H]+).
Fig. 17. Quantification of re-closed succinimide form in long-term stability study of ADC39 based on peptide mapping MS data.
After incubation at pH 9 for 3 days, analysis of ADC39 showed that the succinimide ring was ∼95% open and ∼5% closed with full hydrolysis of the succinimide ring not observed. When DL33 was conjugated to adalimumab without subsequent incubation at basic pH resulting ADC44 had ∼90% ring closed succinimide and ∼10% ring open. This indicated that even without incubation at basic pH, succinimide ring hydrolysis still occurred at a low level. These observations suggested the succinimide ring could be subject to an inevitable equilibrium of ring open and ring closed forms (Scheme 8) in formulation buffer resulting in loss of drug-linker from the ADC. Studies on ADCs with both MP and M-Gly showed the succinimide ring hydrolysis equilibrium was a universal issue.55 Comprehensive studies on how the substitution to the linker proximal to the maleimide impacts the succinimide ring equilibrium after conjugation have been conducted.58
Scheme 8. Equilibrium of hydrolysed succinimide ring open (ADC39) and succinimide ring closed (ADC44) ADCs.
Conjugation benefits of bromoacetamide (BrAc)
In order to remove any possibility of succinimide ring closure and subsequent loss of drug-linker, the approach was taken to replace maleimide with bromo acetamide (BrAc). ADC42 had demonstrated a low propensity for aggregate formation during the accelerated stress test and so the maleimide in DL40 was replaced with BrAc affording drug-linker DL41 (Fig. 18).55 Conjugation of DL41 to a huma anti-TNF antibody afforded ADC4359 which displayed multiple advantages over ADC42.
Fig. 18. DL41 with BrAc for conjugation.
Compared to ADC42 the BrAc conjugated ADC43 had a similar HIC retention time and isoelectric, but much lower viscosity (Table 17). The lower viscosity can be rationalised by the removal of the four CO2H groups that are formed by hydrolysis of the succinimide ring after conjugation. When profiled in the accelerated stress test ADC43 showed a low propensity for aggregation even at 40 °C (Fig. 15) and unlike ADC42 no gelling was observed; again, rationalised by the removal of the four CO2H groups formed by hydrolysis of the succinimide ring after conjugation.57
BrAc affords total flexibility from a linker design perspective. During succinimide ring hydrolysis, a carboxylic acid is introduced in close proximity to the cysteine of the antibody (Scheme 9); for visualization purposes this can be likened to introducing the side chain of aspartic acid to the linker close to the antibody. This additional negatively charged group is always present for any maleimide based drug-linker in which the succinimide ring is hydrolysed open. When using BrAc for conjugation, linker design can introduce the negative charge if and where it is desired at any point of the linker and so aid ADC property moderation, e.g., isoelectric point, hydrophilic surface.
Scheme 9. Succinimide ring hydrolysis generates carboxylic acid proximal to the antibody.
Finally, a much overlooked feature of maleimide-based conjugations is that they result in the formation of a chiral centre and hence isomers. Subsequently, each succinimide can be hydrolysed to one of two isomers meaning that for each maleimide six isomers can be created for every drug-linker. This presents the opportunity for isomerism (e.g., stereo- and regio-) independently at each conjugation site, with each maleimide forming 6 isomers (Scheme 10). For a DAR4 ADC, this equates to up to 24 (4 × 6) isomers, resulting in a very heterogenous ADC. In complete contrast, drug-linkers employing BrAc for conjugation form just a single isomer no matter what the DAR is, thereby resulting in a more homogenous ADC.55 Long term storage of bromoacetamide containing drug linkers is achieved by storing at −80 °C as the dry powder. Storage of solutions in DMSO is not recommended as this will result in displacement of the bromine by hydroxyl.57
Scheme 10. Maleimide conjugation and subsequent succinimide ring hydrolysis affords multiple possible isomers.
Insights to the improved ADC properties afforded by DL41 (drug-linker from ADC43) are clearly apparent in the energy minimised conformation of DL41 that was generated in Chem3D 20.1 using the MM2 force field method. As shown in Fig. 19 the bromoacetamide on the left would conjugate to the cysteine on the antibody while the V-shape of the (R)-acetal payload P26 ideally positions the hydroxyl at C21 with a trajectory pointing away from the antibody towards solvent, making it the perfect position for the introduction of the phosphate to increase the hydrophilic surface of the ADC.
Fig. 19. Energy minimised view of DL41 with BrAc showing positioning of phosphate away from the antibody.
DL41 was conjugated to a mouse anti-TNF and DAR purified by AIEX to afford DAR4 ADC45 (Table 18). In the in vitro GR cell assay, ADC45 (TNF expressing K562 EC50 = 0.073 μg mL−1) was shown to be equivalent to ADC38 (TNF expressing K562 EC50 = 0.080 μg mL−1) thereby confirming that replacement of maleimide by BrAc did not impact potency. Both ADCs were also screened in the wildtype (WT) cell line that does not express TNF with neither showing any activity.57
Mouse anti-TNF ADCs with DL1 and DL10.
| Mouse anti-TNF ADC | DL | K562 GRE/EC50 μg mL−1 | |
|---|---|---|---|
| mTNF | WT | ||
| ADC38 | DL33 | 0.080 ± 0.096 (9) | 5.81 ± 16.0 (9) |
| ADC45 | DL41 | 0.073 ± 0.010 (5) | 41.0 ± 15.5 (3) |
A mouse contact hypersensitivity (CHS) model was used to compare the impact of the mouse anti-TNF GRM ADCs ADC38 and ADC45 on acute inflammation. In this model sensitization and challenge of mice with FITC leads to a significant increase in ear thickness after 24 hours. ADC38 and ADC45 were both dosed i.p. at 0.3, 1, 3, 10, 30 and 100 mg kg−1 and shown to have the same efficacy in the CHS model (Fig. 20).55
Fig. 20. ADC38 and ADC45 in CHS model showing % inhibition of ear thickness at 0.3, 1, 3, 10, 30 and 100 mg kg−1.
The collagen-induced arthritis (CIA) mouse model was used to profile ADC38 and ADC45 in a chronic model. DBA/1 mice were immunised with type II collagen, followed by a boost with zymosan 21 days later with animals being enrolled and dosed once therapeutically, at the first clinical signs of disease. Treatment groups compared a single i.p. dose of 10 mg kg−1 of both ADC38, ADC45 and the parent anti-TNF antibody to mice treated with vehicle, dosed p.o., s.i.d. from the day of enrolment to day 21 (Fig. 21). In complete agreement with the CHS study, the collagen induced arthritis study showed that ADC38 and ADC45 were indistinguishable. While the single 3 mg kg−1 dose of both ADC38 and ADC45 resulted in a significant reduction in disease score for the duration of the study, the single 10 mg kg−1 dose of both ADC38 and ADC45 resulted in complete remission for over 21 days.55
Fig. 21. Early therapeutic mouse CIA for ADC38, ADC45 and anti-TNF antibody.
Additional comparison of the equivalence of ADC38 to ADC45 are shown in Fig. 22 with dose–response (Fig. 22a) and exposure–response (Fig. 22b) curves for ADC38 (blue lines) and ADC45 (green lines) at doses of 1, 3 and 10 mg kg−1 in the CIA study with ADC38 and ADC45 having similar EC50s, 1.34 mg kg−1 and 929 μg h mL−1 and 1.12 mg kg−1 and 1282 μg h mL−1, respectively.
Fig. 22. Mouse CIA for ADC38 and ADC45 a) dose–response (% inhibition of Paw AUC 0-21); b) exposure-response (% inhibition of Paw AUC 0-21).
ADC43 (with DL41) was subsequently designated as ABBV-154, the first ADC to be designed for subcutaneous dosing and advanced to Phase 2b clinical trials for rheumatoid arthritis,60 Crohn's disease61 and polymyalgia rheumatica.62
Conclusion
The impact of medicinal chemistry on the design and optimisation of small molecule orally bioavailable drugs is clearly recognised. However, historically medicinal chemistry has been somewhat overlooked in the field of antibody-drug conjugates (ADCs), a rather curious position when you consider the possible impact to the design and optimisation of ADCs is not only greater but also as, if not more critical than for a small molecule drug.
Many of the design criteria that have been established for small molecule drugs are directly applicable to the design of payloads for ADCs. To fully appreciate this, it must first be recognised that ADCs are not lifeboats for small molecules that have an insufficient therapeutic index. Unfortunately, ADCs are not as targeted as was initially hoped or expected. Indeed, most of the payload from an ADC becomes systemically distributed. Once this has been accepted, adoption of critical design concepts from small molecule drugs like maximising selectivity for the desired target become all too apparent. Possibly the most important small molecule design criteria to implement is the avoidance of hydrophobic payloads. Extensive analysis has shown that controlling cLog P and PSA within certain guidelines is optimal for small molecule design. As ADCs have the ability to enable highly hydrophobic payloads their use has been extensive. However, while the ADC may well enable delivery of the hydrophobic payload, once released from the ADC, at either the desired target or systemically in a non-targeted manner, the hydrophobic payload will distribute broadly and is likely to result in unwanted toxicity that is observed with many hydrophobic compounds.
For optimal payload design three key approaches have been identified. The first is that the heteroatom that is used for attachment of the linker to the payload, typically nitrogen, should be considered as more than just an attachment point. Structural information and SAR should be used to guide the design for the heteroatom incorporation in order to identify additional interactions that can be made between the linker attachment heteroatom and the payload target to drive potency at the desired target and increase selectivity over unwanted targets. Use of a prodrug is an approach that is often taken when a small molecule is found to have limited aqueous solubility. For an ADC, application of a prodrug strategy should not be an afterthought, it should be implemented at the earliest stages of payload design. Key for a successful payload prodrug is to identify the optimal location of the payload that will provide a trajectory for the prodrug away from the antibody and towards solvent after the drug-linker has been conjugated to the antibody. Lastly, attention must be paid to maximising the solvent exposed hydrophilic surface area of the payload and more importantly, minimising the solvent exposed hydrophobic surface area of the payload. Failure to do this will have a detrimental impact on the ADC properties, like aggregation.
Of paramount importance is to conduct SAR of the payload on the fully assembled ADC. This ensures that the payload undergoes intracellular delivery, is released in the lysozome to assess both stability to this harsh acidic milieu and efflux of the payload to the cytoplasm. Adopting this SAR approach also provides valuable information on the preferred DAR and optimal potency of the payload.
Early descriptions of the role of the linker component, “it just has to be stable in circulation releasing the payload in the target cell”, have been shown to be overly simplistic, and the major role the linker plays in a successful ADC are now acknowledged. For conjugation to cysteine, MC–Val–Cit-PABC became the workhorse linker. Dissecting this linker into conjugation motif (MC), protease cleavable dipeptide (Val–Cit) and self-immolative group (PABC) enables discussion on the extensive studies and optimisation that have been conducted.
Loss of drug-linkers in vivo that used MC for conjugation catalysed multiple strategies to facilitate hydrolysis of the succinimide ring resulting from conjugation to cysteine to prevent the reverse Michael reaction, and hence afford stable attachment of the drug-linker to the antibody. Maleimide was replaced by bromo actinide (BrAc) following the discovery that the hydrolysed succinimide ring was in equilibrium with the in vivo unstable ring closed succinimide. In addition to reducing the chance of drug-linker loss from the antibody, BrAc presented multiple other benefits to the use of maleimide for conjugation. Removal of the succinimide hydrolysis step that is required for maleimide conjugations shortened production by 4 days and also meant that the antibody was not exposed to stressful basic pH conditions for an extended period of time which may result in post translational modification. From a linker design perspective, unlike the hydrolysed succinimide, BrAc does not introduce a carboxylic proximal to the antibody cysteine greatly improving flexibility in linker design to moderate properties of the ADC like isoelectric point and hydrophilic surface. Finally, a much overlooked consequence of maleimide conjugation is the formation of a chiral centre when the cysteine thiol bonds to the succinimide ring which, in combination with hydrolysis of the succinimide ring means that for each maleimide six isomers can be created for every drug-linker. For a DAR4 ADC, this equates to up to 24 (4 × 6) isomers (stereo- and regio-) thus significantly increasing the heterogeneity and complexity of the resulting ADC. BrAc affords just a single isomer no matter how high the DAR resulting in a more homogeneous ADC.
Consideration of the protease cleavable dipeptide has been extensively explored. Perhaps the most important observation is that Val is the second most hydrophobic natural amino acid (Phe is the most hydrophobic). While many combinations of dipeptide have been shown to be effective as linkers for ADCs, Ala–Ala, which is synthetically straightforward, enables well behaved ADCs and does not greatly impact the properties of the parent antibody. Should moderation of the antibody properties be desired for the resulting ADC, negatively charged Ala–Glu, and positively charged Ala–Lys, are ideal options to either lower or raise the isoelectric point respectively.
When para-amino benzylic alcohol attaches to an amine on the payload it forms a carbamate. Unfortunately, carbamate is not completely stable in circulation. As a result, this not only lowers the targeted delivery of the payload, but it also results in systemic distribution of the payload. Introduction of the PABC aryl ring to the drug-linker negatively impacts the properties of the ADC by increasing the levels of aggregation. Finally, for every equivalent of released payload, one equivalent of para-amino benzylic alcohol, a small molecular weight aniline, is also released which may lead to toxicity. Taking all three of the above negative attributes of PABC together, it is better to design the payload to enable direct attachment of the protease cleavable dipeptide to either a primary amine or aniline thereby removing the need for PABC. Such an approach affords a more stable ADC, improves the drug-like properties, and does not release equivalent of para-amino benzylic alcohol per equivalent of payload.
ADCs are comprised of 3 components of wildly differing sizes: antibody (150 000 Da), linker (typically <500 Da)and payload (typically <500 Da). For a DAR4 ADC this means the while the drug-linker makes up only around 2% of the ADC, it has a disproportionately massive impact on the physicochemical properties for the ADC. While ADCs consist of three structurally disparate components, their symbiotic relationship requires that all three of them function optimally for a successful ADC. For ADC design, the goal should always be to optimise the drug-like properties of the ADC.
Associated content
Experimental procedures
For synthesis of P9, P10, DL14, DL15, DL16, DL20, DL21, DL22, ADC17, ADC18, ADC19, ADC23, ADC24 and ADC25 see ref. 25.
For synthesis of P26, P27, 28, 29, 30 31, DL33, DL34, ADC35, ADC36, ADC37, ADC38 and ADC39 see ref. 38.
For synthesis of DL40 and ADC42 see ref. 56.
For synthesis of DL41, ADC43 and ADC45 see ref. 57.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- ADC
Antibody-drug conjugate
- AIEX
Anion exchange chromatography
- Boc
tert-Butyloxy carbonyl protecting group
- BrAc
Bromo acetamide
- CIA
Collagen-induced arthritis
- CHS
Contact hypersensitivity model
- cLog P
Calculated log P
- DAR
Drug to antibody ration
- DMSO
Dimethyl sulfoxide
- FITC
Fluorescein isothiocyanate
- Fmoc
Fluorenyl methoxy carbonyl protecting group
- GRM
Glucocorticoid receptor modulator
- HIC
Hydrophobic interaction chromatography
- iADC
Immunology antibody drug conjugate
- IP
Intraperitoneal
- IV
Intravenous
- MMAE
Monomethyl auristatin E
- NHS
N-Hydroxysuccinimide
- oADC
Oncology antibody drug conjugate
- P1NP
Procollagen 1 intact N-terminal propeptide
- PABC
Para-amino benzylic alcohol
- PO
Oral dose
- PSA
Polar surface area
- sid
Semel in die (once per day)
- TFA
Trifluoro acetic acid
- TNF
Tumour necrosis factor
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
ADH is an employee of AbbVie. Dedicated to my coach, mentor, amazing supervisor and above all, great friend, Gerald “Gerry” Tometzki.
References
- Fu Z. Li S. Han S. Shi C. Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduction Targeted Ther. 2022;7:93. doi: 10.1038/s41392-022-00947-7. doi: 10.1038/s41392-022-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J. T. W. Harris P. W. R. Brimble M. A. Kavianinia I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules. 2021;26:5847. doi: 10.3390/molecules26195847. doi: 10.3390/molecules26195847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senter P. D. Sievers E. L. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 2012;30:631–637. doi: 10.1038/nbt.2289. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- Scott L. J. Brentuximab Vedotin: A review in CD30-positive Hodgkin lymphoma. Drugs. 2017;77:435–445. doi: 10.1007/s40265-017-0705-5. doi: 10.1007/s40265-017-0705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khongorzul P. Ling C. J. Khan F. U. Ihsan A. U. Zhang J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020;18:3–19. doi: 10.1158/1541-7786.MCR-19-0582. doi: 10.1158/1541-7786.MCR-19-0582. [DOI] [PubMed] [Google Scholar]
- Hobson A. D. Antibody Drug Conjugates Beyond Cytotoxic Payloads. Prog. Med. Chem. 2023;62:1–59. doi: 10.1016/bs.pmch.2023.10.001. [DOI] [PubMed] [Google Scholar]
- Rossi G. A. Cerasoli F. Cazzola M. Safety of inhaled corticosteroids: room for improvement. Pulm. Pharmacol. Ther. 2007;20:23–35. doi: 10.1016/j.pupt.2005.10.008. doi: 10.1016/j.pupt.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Yano H. Hirayama F. Arima H. Uekama K. Hydrolysis behavior of prednisolone 21-hemisuccinate/beta-cyclodextrin amide conjugate: involvement of intramolecular catalysis of amide group in drug release. Chem. Pharm. Bull. 2000;48:1125–1128. doi: 10.1248/cpb.48.1125. doi: 10.1248/cpb.48.1125. [DOI] [PubMed] [Google Scholar]
- Everts M. Kok R. J. Asgeirsdottir S. A. Melgert B. N. Moolenaar T. J. Koning G. A. van Luyn M. J. Meijer D. K. Molema G. Selective intracellular delivery of dexamethasone into activated endothelial cells using an E-selectin-directed immunoconjugate. J. Immunol. 2002;168:883–889. doi: 10.4049/jimmunol.168.2.883. doi: 10.4049/jimmunol.168.2.883. [DOI] [PubMed] [Google Scholar]
- Graversen J. H. Svendsen P. Dagnaes-Hansen F. Dal J. Anton G. Etzerodt A. Petersen M. D. Christensen P. A. Moller H. J. Moestrup S. K. Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone. Mol. Ther. 2012;20:1550–1558. doi: 10.1038/mt.2012.103. doi: 10.1038/mt.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller L. N. Knudsen A. R. Andersen K. J. Nyengaard J. R. Hamilton-Dutoit S. Moller E. M. O. Svendsen P. Moller H. J. Moestrup S. K. Graversen J. H. Mortensen F. V. Anti-CD163-dexamethasone protects against apoptosis after ischemia/reperfusion injuries in the rat liver. Ann. med. surg. 2015;4:331–337. doi: 10.1016/j.amsu.2015.09.001. doi: 10.1016/j.amsu.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen P. Graversen J. H. Etzerodt A. Hager H. Roge R. Gronbaek H. Christensen E. I. Moller H. J. Vilstrup H. Moestrup S. K. Antibody-Directed Glucocorticoid Targeting to CD163 in M2-type Macrophages Attenuates Fructose-Induced Liver Inflammatory Changes. Mol. Ther. Methods Clin. Dev. 2016;4:50–61. doi: 10.1016/j.omtm.2016.11.004. doi: 10.1016/j.omtm.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen K. L. Moller H. J. Graversen J. H. Magnusson N. E. Moestrup S. K. Vilstrup H. Gronbaek H. Anti-CD163-dexamethasone conjugate inhibits the acute phase response to lipopolysaccharide in rats. World J. Hepatol. 2016;8:726–730. doi: 10.4254/wjh.v8.i17.726. doi: 10.4254/wjh.v8.i17.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K. Brindisi M. Organic carbamates in drug design and medicinal chemistry. J. Med. Chem. 2015;58:2895–2940. doi: 10.1021/jm501371s. doi: 10.1021/jm501371s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin C. C. Hobson A. D. McPherson M. Dunstan T. A. Vargo T. R. Hayes M. E. Fettis M. M. Bischoff A. Wang L. Wang L. Hernandez Jr A. Jia Y. Oh J. Z. Tian Y. Self-Immolative Carbamate Linkers for CD19-Budesonide Antibody-Drug Conjugates. Bioconjugate Chem. 2023;34:1835–1850. doi: 10.1021/acs.bioconjchem.3c00354. doi: 10.1021/acs.bioconjchem.3c00354. [DOI] [PubMed] [Google Scholar]
- Hobson A. Packer J. Butler C. Bornemeier D. Registration of antibody drug conjugates. ADC Review. Antibody-drug Conjugates. 2017;5 doi: 10.14229/jadc.2017.14.08.002. [DOI] [Google Scholar]
- Hobson A., The Medicinal Chemistry of Glucocorticoid Receptor Modulators, SpringerBriefs in Molecular Science, Springer, Cham, 2023, ISBN 978–3–031-28732-9, 10.1007/978-3-031-28732-9 [DOI] [Google Scholar]
- Brattsand R. Thalen A. Roempke K. Kaellstroem L. Gruvstad E. Influence of 16α, 17α-acetal substitution and steroid nucleus fluorination on the topical to systemic activity ratio of glucocorticoids. J. Steroid Biochem. 1982;16:779–786. doi: 10.1016/0022-4731(82)90035-8. doi: 10.1016/0022-4731(82)90035-8. [DOI] [PubMed] [Google Scholar]
- Christie P. Ciclesonide: a novel inhaled corticosteroid for asthma. Drugs Today. 2004;40:569–576. doi: 10.1358/dot.2004.40.7.850475. doi: 10.1358/dot.2004.40.7.850475. [DOI] [PubMed] [Google Scholar]
- Reynolds N. A. Scott L. J. Ciclesonide. Drugs. 2004;64:511–519. doi: 10.2165/00003495-200464050-00005. doi: 10.2165/00003495-200464050-00005. [DOI] [PubMed] [Google Scholar]
- Guo Z. Gu Z. Howell S. R. Chen K. Rohatagi S. Cai L. Wu J. Stuhler J. Ciclesonide Disposition and Metabolism Pharmacokinetics, Metabolism, and Excretion in the Mouse, Rat, Rabbit, and Dog. Am. J. Ther. 2006;13:490–501. doi: 10.1097/01.mjt.0000209688.52571.81. doi: 10.1097/01.mjt.0000209688.52571.81. [DOI] [PubMed] [Google Scholar]
- Mutch E. Nave R. McCracken N. Zech K. Williams F. M. The role of esterases in the metabolism of ciclesonide todesisobutyryl-ciclesonide in human tissue. Biochem. Pharmacol. 2007;73:1657–1664. doi: 10.1016/j.bcp.2007.01.031. doi: 10.1016/j.bcp.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Hansel T. T. Barnes P. J. Ciclesonide An on-site-activated steroid in New Drugs for Asthma, Allergy and COPD. Prog. Respir. Res. 2001;31:91–93. doi: 10.1159/000062132. [DOI] [Google Scholar]
- Edman K. Hosseini A. Bjursell M. K. Aagaard A. Wissler L. Gunnarsson A. Kaminski T. Köhler C. Bäckström S. Jensen T. J. Cavallin A. Karlsson U. Nilsson E. Lecina D. Takahashi R. Grebner C. Geschwindner S. Lepistö M. Hogner A. C. Guallar V. Ligand Binding Mechanism in Steroid Receptors: From Conserved Plasticity to Differential Evolutionary Constraints. Structure. 2015;23:2280–2290. doi: 10.1016/j.str.2015.09.012. doi: 10.1016/j.str.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Hobson A. D. McPherson M. J. Waegell W. Goess C. A. Stoffel R. H. Li X. Zhou J. Wang Z. Yu Y. Hernandez Jr A. Bryant S. H. Mathieu S. L. Bischoff A. K. Fitzgibbons J. Pawlikowska M. Puthenveetil S. Santora L. C. Wang L. Wang L. Marvin C. C. Hayes M. E. Shrestha A. Sarris K. A. Li B. Design and Development of Glucocorticoid Receptor Modulators as Immunology Antibody-Drug Conjugate Payloads. J. Med. Chem. 2022;65:4500–4533. doi: 10.1021/acs.jmedchem.1c02099. doi: 10.1021/acs.jmedchem.1c02099. [DOI] [PubMed] [Google Scholar]
- Hobson A. D. Harris C. M. van der Kam E. L. Turner S. C. Abibi A. Aguirre A. L. Bousquet P. Kebede T. Konopacki D. B. Gintant G. Kim Y. Larson K. Maull J. W. Moore N. S. Shi D. Shrestha A. Tang X. Zhang P. Sarris K. K. Discovery of A-971432.; an orally bioavailable selective sphingosine-1-phosphate receptor 5 (S1P5) agonist for the potential treatment of neurodegenerative disorders. J. Med. Chem. 2015;58:9154–9170. doi: 10.1021/acs.jmedchem.5b00928. doi: 10.1021/acs.jmedchem.5b00928. [DOI] [PubMed] [Google Scholar]
- Li F. Emmerton K. K. Jonas M. Zhang X. Miyamoto J. B. Setter J. R. Nicholas N. D. Okeley N. M. Lyon R. P. Benjamin D. R. Law C. L. Intracellular released payload influences potency and bystander-killing effects of antibody-drug conjugates in preclinical models. Cancer Res. 2016;76:2710–2719. doi: 10.1158/0008-5472.can-15-1795. doi: 10.1158/0008-5472.can-15-1795. [DOI] [PubMed] [Google Scholar]
- de Groot F. M. H. van Berkom L. W. A. Scheeren H. W. Synthesis and biological evaluation of 2′-carbamate-linked and 2′-carbonate-linked prodrugs of paclitaxel: selective activation by the tumor-associated protease plasmin. J. Med. Chem. 2000;43:3093–3102. doi: 10.1021/jm0009078. doi: 10.1021/jm0009078. [DOI] [PubMed] [Google Scholar]
- Doronina S. O. Toki B. E. Torgov M. Y. Mendelsohn B. A. Cerveny C. G. Chace D. F. DeBlanc R. L. Gearing R. P. Bovee T. D. Siegall C. B. Francisco J. A. Wahl A. F. Meyer D. L. Senter P. D. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- Roberts C. J. Protein aggregation and its impact on product quality. Curr. Opin. Biotechnol. 2014;30:211–217. doi: 10.1016/j.copbio.2014.08.001. doi: 10.1016/j.copbio.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa E. M. Panchal J. P. Moorthy B. S. Blum J. S. Joubert M. K. Narhi L. O. Topp E. M. Immunogenicity of therapeutic protein aggregates. J. Pharm. Sci. 2016;105:417–433. doi: 10.1016/j.xphs.2015.11.002. doi: 10.1016/j.xphs.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Ratanji K. D. Derrick J. P. Dearman R. J. Kimber I. Immunogenicity of therapeutic proteins: influence of aggregation. J. Immunotoxicol. 2014;11:99–109. doi: 10.3109/1547691x.2013.821564. doi: 10.3109/1547691x.2013.821564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey S. C. Nguyen M. T. Andreyka J. B. Meyer D. L. Doronina S. O. Senter P. D. Dipeptide-based highly potent doxorubicin antibody conjugates. Bioorg. Med. Chem. Lett. 2006;16:358–362. doi: 10.1016/j.bmcl.2005.09.081. doi: 10.1016/j.bmcl.2005.09.081. [DOI] [PubMed] [Google Scholar]
- Wang L. Hobson A. D. Fitzgibbons J. Hernandez Jr A. Jia Y. Xu Z. Wang Z. Yu Y. Li X. Impact of Dipeptide on ADC Physicochemical Properties and Efficacy Identifies Ala-Ala as the Optimal Dipeptide. RSC Med. Chem. 2024;15:355–365. doi: 10.1039/D3MD00473B. doi: 10.1039/D3MD00473B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. Q. Xu K. Liu L. Raab H. Bhakta S. Kenrick M. Parsons-Reponte K. L. Tien J. Yu S. F. Mai E. Li D. Tibbitts J. Baudys J. Saad O. M. Scales S. J. McDonald P. J. Hass P. E. Eigenbrot C. Nguyen T. Solis W. A. Fuji R. N. Flagella K. M. Patel D. Spencer S. D. Khawli L. A. Ebens A. Wong W. L. Vandlen R. Kaur S. Sliwkowski M. X. Scheller R. H. Polakis P. Junutula J. R. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat. Biotechnol. 2012;30:184–189. doi: 10.1038/nbt.2108. doi: 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- Lyon R. P. Setter J. R. Bovee T. D. Doronina S. O. Hunter J. H. Anderson M. E. Balasubramanian C. L. Duniho S. M. Leiske C. I. Li F. Senter P. D. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nat. Biotechnol. 2014;32:1059–1062. doi: 10.1038/nbt.2968. doi: 10.1038/nbt.2968. [DOI] [PubMed] [Google Scholar]
- McPherson M. J., Hobson A. D., Hayes M. E., Marvin C. C., Schmidt D., Waegell W., Goess C., Oh J. Z., Hernandez A. and Randolph J. T., Preparation of glucocorticoid receptor agonist and immunoconjugates thereof, US Pat., 10668167, 2020
- Hobson A. D. McPherson M. J. Hayes M. E. Goess C. Li X. Zhou J. Wang Z. Yu Y. Yang J. Sun L. Zhang Q. Qu P. Yang S. Hernandez Jr A. Bryant S. H. Mathieu S. L. Bischoff A. K. Fitzgibbons J. Santora L. C. Wang L. Wang L. Fettis M. M. Li X. Marvin C. C. Wang Z. Patel M. V. Schmidt D. L. Li T. Randolph J. T. Henry R. F. Graff C. Tian Y. Aguirre A. L. Shrestha A. Discovery of ABBV-3373, an Anti-TNF Glucocorticoid Receptor Modulator Immunology Antibody Drug Conjugate. J. Med. Chem. 2022;65:15893–15934. doi: 10.1021/acs.jmedchem.2c01579. doi: 10.1021/acs.jmedchem.2c01579. [DOI] [PubMed] [Google Scholar]
- Hobson A. D. Zhu H. Qiu W. Judge R. Longenecker K. Minimising the payload solvent exposed hydrophobic surface area optimises the antibody-drug conjugate properties. RSC Med. Chem. 2024 doi: 10.1039/D3MD00540B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson M. J. Hobson A. D. Hernandez Jr A. Marvin C. C. Waegell W. Goess C. Oh J. Z. Shi D. Hayes M. E. Wang L. Wang L. Schmidt D. Wang Z. Pitney V. McCarthy K. Jia Y. Wang C. Kang B. N. Bryant S. Mathieu S. Ruzek M. Parmentier J. D'Cunha R. R. Pang Y. Phillips L. Brown N. J. Xu J. Graff C. Tian Y. Longenecker K. L. Qiu W. Zhu H. Liu W. Zheng P. Bi Y. Stoffel R. Discovery of a Novel anti-TNF Glucocorticoid Receptor Modulator Antibody Drug Conjugate for the Treatment of Immune-Mediated Inflammatory Diseases. Sci. Transl. Med. 2024 doi: 10.1126/scitranslmed.add8936. [DOI] [PubMed] [Google Scholar]; , just accepted
- Joosten L. A. Helsen M. M. van de Loo F. A. van den Berg W. B. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.23363. doi: 10.1002/art.23363. [DOI] [PubMed] [Google Scholar]
- Kang I. Lee W. W. Lee Y. Modulation of collagen-induced arthritis by IL-4 and dexamethasone: the synergistic effect of IL-4 and dexamethasone on the resolution of CIA. Immunopharmacology. 2000;49:317–324. doi: 10.1016/s0162-3109(00)00248-4. doi: 10.1016/s0162-3109(00)00248-4. [DOI] [PubMed] [Google Scholar]
- A Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of ABBV-3373 in Participants With Moderate to Severe Rheumatoid Arthritis (RA). https://www.ClinicalTrials.gov Identifier: NCT03823391
- Buttgereit F. Aelion J. Rojkovich B. Zubrzycka-Sienkiewicz A. Radstake T. Chen S. Arikan D. Kupper H. Amital H. OP0115 Efficacy and safety of ABBV-3373, a novel anti-TNF glucocorticoid receptor modulator antibody drug conjugate, in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy: a phase 2a proof of concept study. Ann. Rheum. Dis. 2021;80:64. doi: 10.1136/annrheumdis-2021-eular.221. doi: 10.1136/annrheumdis-2021-eular.221. [DOI] [PubMed] [Google Scholar]
- Buttgereit F. Aelion J. Rojkovich B. Zubrzycka-Sienkiewicz A. Chen S. Yang Y. Arikan D. D'Cunha R. Pang Y. Kupper H. Radstake T. Amital H. Efficacy and Safety of ABBV-3373, a Novel Anti-Tumor Necrosis Factor Glucocorticoid Receptor Modulator Antibody Drug Conjugate, in Adults with Moderate to Severe Rheumatoid Arthritis Despite Methotrexate Therapy: a Randomised, Double-Blind, Active-Controlled Proof-of-Concept Phase 2a Trial. Arthritis Rheumatol. 2023;75:879–889. doi: 10.1002/art.42415. doi: 10.1002/art.42415. [DOI] [PubMed] [Google Scholar]
- Deslandes A. Comparative clinical pharmacokinetics of antibody-drug conjugates in first-in-human Phase 1 studies. mAbs. 2014;6:859–870. doi: 10.4161/mabs.28965. doi: 10.4161/mabs.28965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklenburg L. A Brief Introduction to Antibody-Drug Conjugates for Toxicologic Pathologists. Toxicol. Pathol. 2018;46:746–752. doi: 10.1177/0192623318803059. doi: 10.1177/0192623318803059. [DOI] [PubMed] [Google Scholar]
- Viola M. Sequeira J. Seiça R. Veiga F. Serra J. Santos A. C. Ribeiro A. J. Subcutaneous delivery of monoclonal antibodies: How do we get there? J. Controlled Release. 2018;286:301–314. doi: 10.1016/j.jconrel.2018.08.001. doi: 10.1016/j.jconrel.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Bittner B. Richter W. Schmidt J. Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs. 2018;32:425–440. doi: 10.1007/s40259-018-0295-0. doi: 10.1007/s40259-018-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivitz A. Segurado O. G. HUMIRA pen: a novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody adalimumab. Expert Rev. Med. Devices. 2007;4:109–116. doi: 10.1586/17434440.4.2.109. doi: 10.1586/17434440.4.2.109. [DOI] [PubMed] [Google Scholar]
- Jain T. Sun T. Durand S. Hall A. Houston N. R. Nett J. H. Sharkey B. Bobrowicz B. Caffry I. Yu Y. Cao Y. Lynaugh H. Brown M. Baruah H. Gray L. T. Krauland E. M. Xu Y. Vásquez M. Wittrup K. D. Biophysical properties of the clinical-stage antibody landscape. Proc. Natl. Acad. Sci. U. S. A. 2017;114:944–949. doi: 10.1073/pnas.1616408114. doi: 10.1073/pnas.1616408114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski E. K. Wu L. Gupta P. Tessier P. M. Discovery-stage identification of drug-like antibodies using emerging experimental and computational methods. mAbs. 2021;13:e1895540. doi: 10.1080/19420862.2021.1895540. doi: 10.1080/19420862.2021.1895540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollar E. Balazs B. Tari T. Siro I. Development challenges of high concentration monoclonal antibody formulations. Drug Discovery Today: Technol. 2020;37:31–40. doi: 10.1016/j.ddtec.2020.08.005. doi: 10.1016/j.ddtec.2020.08.005. [DOI] [PubMed] [Google Scholar]
- Le Basle Y. Chennell P. Tokhadze N. Astier A. Sautou V. Physicochemical Stability of Monoclonal Antibodies: A Review. J. Pharm. Sci. 2020;109:169–190. doi: 10.1016/j.xphs.2019.08.009. doi: 10.1016/j.xphs.2019.08.009. [DOI] [PubMed] [Google Scholar]
- Turner M. R. Balu-Iyer S. V. Challenges and Opportunities for the Subcutaneous Delivery of Therapeutic Proteins. J. Pharm. Sci. 2018;107:1247–1260. doi: 10.1016/j.xphs.2018.01.007. doi: 10.1016/j.xphs.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson A. D. Xu J. Marvin C. C. McPherson M. J. Hollmann M. Gattner M. Dzeyk K. Fettis M. M. Bischoff A. K. Wang L. Fitzgibbons J. Wang L. Salomon P. Hernandez Jr A. Jia Y. Sarvaiya H. Goess C. A. Mathieu S. L. Santora L. C. Optimisation of Drug-Linker to Enable Long-term Storage of Antibody-Drug Conjugate for Subcutaneous Dosing. J. Med. Chem. 2023;66:9161–9173. doi: 10.1021/acs.jmedchem.3c00794. doi: 10.1021/acs.jmedchem.3c00794. [DOI] [PubMed] [Google Scholar]
- Hobson A. D. Xu J. Welch D. Marvin C. C. McPherson M. J. Gates B. Liao X. Hollmann M. Gattner M. J. Dzeyk K. Sarvaiya H. Shenoy V. M. Fettis M. M. Bischoff A. K. Wang L. Santora L. C. Wang L. Fitzgibbons J. Salomon P. Hernandez Jr A. Jia Y. Goess C. A. Mathieu S. L. Bryant S. H. Larsen M. E. Cui B. Tian Y. Discovery of ABBV-154, an anti-TNF Glucocorticoid Receptor Modulator Immunology Antibody Drug Conjugate (iADC) J. Med. Chem. 2023;66:12544–12558. doi: 10.1021/acs.jmedchem.3c01174. doi: 10.1021/acs.jmedchem.3c01174. [DOI] [PubMed] [Google Scholar]
- Wang L. Hobson A. D. Salomon P. Fitzgibbons J. Xu J. McCarthy S. Wu S. Jia Y. Hernandez Jr A. Li X. Xu Z. Wang Z. Yu Y. Li J. Tao L. Linker Substitution Influences Succinimide Ring Hydrolysis Equilibrium Impacting Stability of Attachment to the Antibody-Drug Conjugate. RSC Med. Chem. 2024;15:612–622. doi: 10.1039/D3MD00569K. doi: 10.1039/D3MD00569K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson A. D., McPherson M. J., Waegell W., Goess C., Hernandez Jr A., Wang L., Wang L., Marvin C. C. and Santora L. C., Glucocorticoid receptor agonist and immunoconjugates thereof, US Pat., 10772970, 2020
- Study to Evaluate Adverse Events and Change in Disease Activity in Participants Between 18 to 75 Years of Age Treated With Subcutaneous (SC) Injections of ABBV-154 for Moderately to Severely Active Rheumatoid Arthritis (RA). https://clinicaltrials.gov/ct2/show/NCT04888585
- A Study to Evaluate Adverse Events and Change in Disease Activity in Participants Between 18 to 75 Years of Age Treated With Intravenous (IV) Infusion and Subcutaneous (SC) Injections of ABBV-154 for Moderately to Severely Active Crohn's Disease. https://clinicaltrials.gov/ct2/show/NCT05068284
- A Study to Evaluate the Change in Disease State and Adverse Events in Adult Participants With Polymyalgia Rheumatica (PMR) Dependent on Glucocorticoid Treatment, Receiving Subcutaneous Injections of ABBV-154. https://clinicaltrials.gov/ct2/show/NCT04972968