Abstract
Elevated levels of TGFβ2 in the aqueous humor is associated with the pathological changes in the trabecular meshwork (TM). These changes lead to ocular hypertension (OHT), the most important risk factor for the development and progression of primary open angle glaucoma (POAG), a leading cause of blindness worldwide.
Therefore, TGFβ2 is frequently used to develop OHT models including in perfusion cultured eyes and in mouse eyes. Adenovirus-mediated overexpression of human mutant TGFβ2 has demonstrated great success in increasing intraocular pressure (IOP) in mouse eyes. However, adenoviruses have limited capacity for a foreign gene, induce transient expression, and may cause ocular inflammation. Here, we explored the potential of using lentiviral vectors carrying the mutant human TGFβ2C226S/C228S (ΔhTGFβ2C226S/C228S) gene expression cassette for the induction of OHT in C57BL/6J mice. Lentiviral vectors using CMV or EF1α promoter to drive the expression of ΔhTGFβ2C226S/C228S were injected into one of the mouse eyes and the fellow eye was injected with the same vector but expressing GFP/mCherry as controls. Both intravitreal and intracameral injection routes were tested in male and female mice. We did not observe significant IOP changes using either promoter or injection route at the dose of 8×105 PFU/eye. Immunostaining showed normal anterior chamber angle structures and a slight increase in TGFβ2 expression in the TM of the eyes receiving intracameral viral injection but not in those receiving intravitreal viral injection. At the dose of 2×106 PFU/eye, intracameral injection of the lentiviral vector with the CMV-ΔhTGFβ2C226S/C228S cassette induced significant IOP elevation and increased the expression of TGFβ2 and fibronectin isoform EDA in the TM. Our data suggest that lentiviral doses are important for establishing the TGFβ2-induced OHT model in the C57BL/6J strain.
Keywords: Intraocular pressure, Lentivirus, Ocular hypertension, Trabecular meshwork, TGFβ2, Mouse model
Elevated intraocular pressure (IOP) is the primary modifiable risk factor in the development and progression of primary open angle glaucoma (POAG) (Kwon et al., 2009). Elevated IOP in POAG eyes is due to impaired aqueous humor outflow through the trabecular meshwork (TM) (Agarwal et al., 2009). TGFβ2 causes pathologic changes in the TM (Pang et al., 2015). Specifically, TGFβ2 alters TM cell proliferation and migration (Wordinger et al., 1998), impairs TM cell phagocytosis (Cao et al., 2003), promotes the formation of cross-linked actin networks (Montecchi-Palmer et al., 2017), and increases extracellular matrix deposition (Fleenor et al., 2006b). Clinically, elevated TGFβ2 has been reported in the aqueous humor and TM tissues from POAG patients (Tovar-Vidales et al., 2011; Tripathi et al., 1994). Experimentally, TGFβ2 elevates IOP in perfusion cultured human donor eyes and in vivo mouse eyes. (Fleenor et al., 2006a; Gottanka et al., 2004; Shepard et al., 2010).
Mouse models are valuable tools for studying ocular diseases, and viral vectors have been widely used to overexpress genes of interest in mouse eyes (Campa et al., 2017; Kampik et al., 2012). In glaucoma research, TGFβ2-induced OHT models mimic POAG conditions and therefore have been used in many studies. Shephard et al. first reported using the serum type 5 adenovirus (Ad5) to transduce the mouse TM and overexpress the mutant constitutively active human TGFβ2 (Shepard et al., 2010). The authors showed that Ad5-mediated TGFβ2 expression induced OHT and decreased aqueous humor outflow facility (Shepard et al., 2010). The Ad5 adenovirus is a suitable viral vector for TM transduction due to its tropism and high transduction efficiency (Borras, 2012). However, Ad5-mediated gene expression is transient (Shepard et al., 2010). Also, due to the high immunogenicity of adenoviruses, inflammation is frequently observed when they are used at high titers (Kalesnykas et al., 2017; Lee et al., 2017).
To address these issues, we explored the potential of using a lentiviral vector to express TGFβ2 for the development of a mouse ocular hypertension model. Lentiviral vectors are retroviruses that are able to integrate their genome into the host cell, achieving long-term gene expression (Balaggan and Ali, 2012). In contrast to adenoviral vectors, lentiviral vectors are less immunogenic since most of the modern lentiviral vectors have been engineered to be replication-deficient with potential pro-inflammatory components removed (Balaggan and Ali, 2012; Borras, 2012). Several studies have already shown successful lentiviral-mediated transgene expression in retinal and corneal tissues (Basche et al., 2018; Miyazaki et al., 2011). However, no reported studies have used lentiviral vectors to overexpress TGFβ2 in the mouse TM. In the following study, we developed such a vector and evaluated its ability to induce TGFβ2 expression and OHT in vivo.
We constructed the lentiviral vectors for the expression of mutant human TGFβ2C226S/C228S (ΔhTGFβ2C226S/C228S) by subcloning the ΔhTGFβ2C226S/C228S coding sequence from the pac.Ad5-CMV-ΔhTGFβ2C226S/C228S shuttle vector (Shepard et al., 2010) (A kind gift from Alcon Research Ltd, Fort Worth, TX) into the pLVX-EF1α-mCherry-N1 (Catalog #631986) and pLVX-CMV-AcGFP1-N1 (Catalog #632154) (Clontech Laboratories Inc., Mountain View, CA) lentiviral vectors. A Kozak sequence was introduced upstream of the coding sequence, and a stop codon was introduced at the end of the coding sequence so that no fusion protein would be expressed. The coding sequence was amplified using the following primers (Sigma-Aldrich, Saint Louis, MO): Forward: 5’-CTCAAGCTTCGAATTGCCACCATGCACTACTGTGTGCTGAGC-3’ Reverse: 5’-CATGACCGGTGGATCTTAGCTGCATTTGCAAGACTTTAC-3’ The In-Fusion HD Cloning kit (Takara Bio USA Inc., Mountain View, CA) was used to insert the coding sequence after the backbone vectors were digested at the EcoRI (5’) and BamHI (3’) (Promega, Madison, WI) restriction sites following manufacturer’s instructions. Sanger sequencing was used to confirm the constructs (Genewiz, South Plainfield, NJ).
The proviruses were transfected into Lenti-X-293T cells (Takara Bio USA) with the 4th generation lentiviral packaging system (Lenti-X HTX Packaging System; Takara Bio USA) to produce lentiviruses. The pLVX-EF1α-mCherry-N1 (which overexpressed mCherry) and pLVX-CMV-AcGFP1-N1 (which overexpressed AcGFP) lentiviruses were produced similarly and were used as controls.
Conditioned medium containing lentiviruses were collected at 48 hours and 72 hours post transfection, pooled, filtered using 0.45μM filters to remove debris, and ultracentrifuged (40,000 g for 4 hours at 4°C). The concentrated lentiviruses were aliquoted into small quantities and stored at −80°C. Some lentiviruses were used for viral titer assay using the Lenti-X™ qRT-PCR Titration Kit (Catalog#: 631235; Takara Bio USA). We obtained viral titers ranging from 1 × 108 – 1 × 109 PFU/ml.
After lentiviral vector preparation, we determined if these vectors were able to induce OHT similar to adenoviral vectors. Wildtype male and female C57BL/6 mice at the age of 6–8 months (Jackson Laboratory, Bar Harbor, ME) were housed at the Indiana University School of Medicine Laboratory Animal Resource Center. All experiments followed protocol approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. The mice were maintained on a regular (not reversed) 12hr light /12hr dark cycle. Baseline IOP was measured after acclimation of the mice for about 2 weeks. The mice were placed in the anesthesia chamber (SomnoSuite, Kent Scientific; Torrington, CT), and IOP was measured using a secured Tonolab tonometer (Icare, 01510 Vantaa, Finland) within 3–5 minutes after the mice were placed in the induction chamber.
After baseline IOP establishment, the mice were anesthetized by intraperitoneal injection with a ketamine hydrochloride/xylazine mixture (90 mg/kg and 5 mg/kg, respectively) and buprenorphine (15mg/kg). Prior to injection, topical anesthesia with 0.5% proparacaine was applied. To conduct a comprehensive investigation of the effect of different expression promoters and viral injection methods, 19 mice (originally planned for 10 male and 10 female mice but one died during acclimation) were divided into 4 study groups:
Group 1: five mice (male and female) intracamerally injected with pLVX-CMV-ΔhTGFβ2C226S/C228S in one eye and pLVX-CMV-AcGFP in the fellow eye as a control. (Figure 1A).
Figure 1. The effect of the dose of lentiviral TGFβ2 expression vectors on IOP in C57BL/6J mouse eyes.
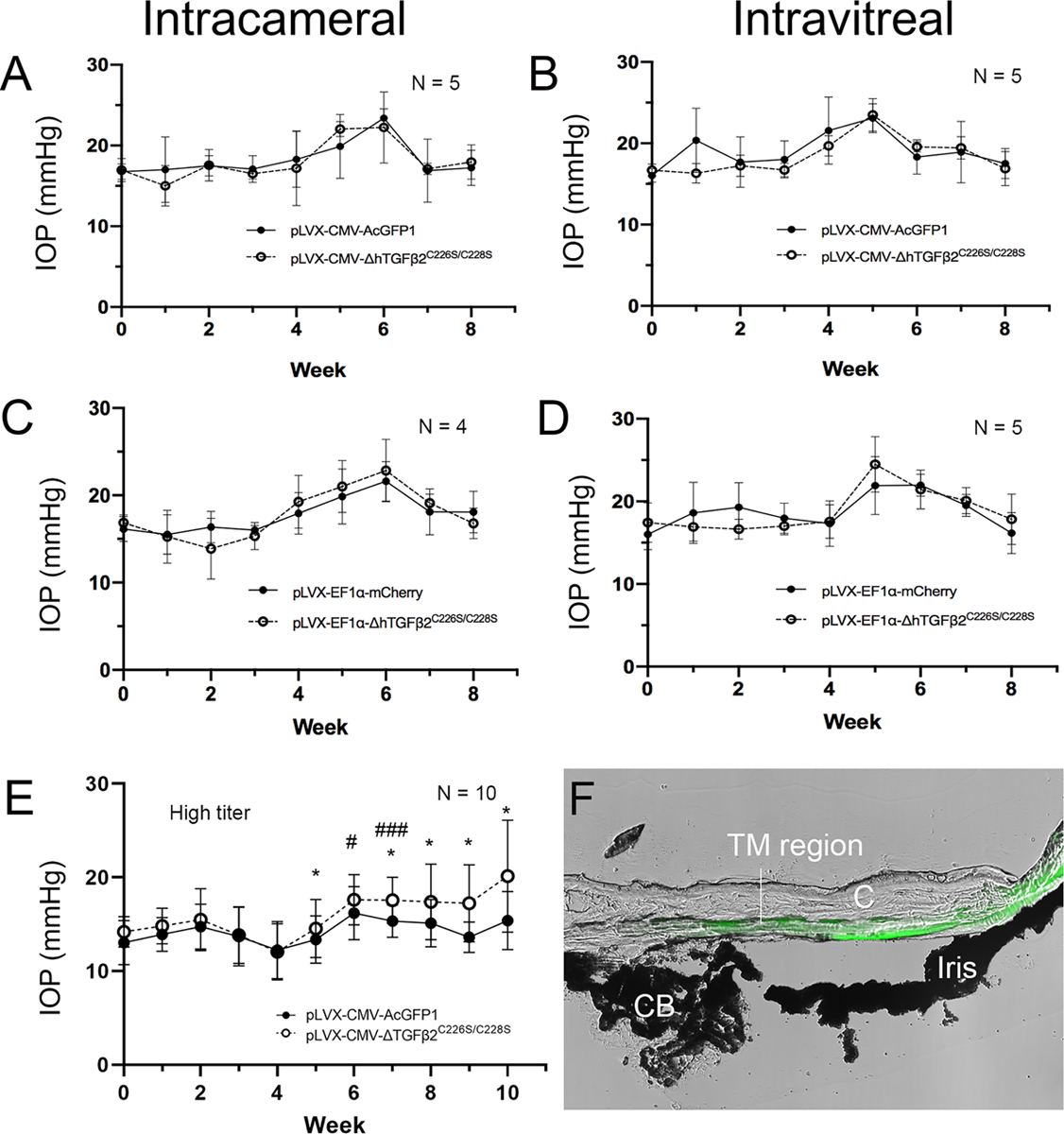
C57BL/6J mice (6–8 months) were injected with the indicated lentiviral vectors either intravitreally (A, C, E) or intracamerally (B and D) after baseline IOP establishment (Week 0). Injections were done in paired eyes: one received TGFβ2 expression lentivirus and the fellow eye with a control lentivirus (A–D: ~8 × 105 viral particles/eye; E: ~2 × 106 viral particles/eye). Each group contained a mix of male and female mice. Means and standard deviations are shown. Paired t-test did not show any significant differences in IOP between the two eyes (p>0.05) in A-D. (E) *: p<0.05 for paired t-tests between TGFβ2 and GFP eyes; #: p<0.05, ###: p<0.001 for one-way ANOVA comparing IOP after viral injection to baseline IOP. (F) A mouse eye injected with pLVX-CMV-AcGFP1 was cryosectioned (without fixation) and imaged using Nikon Advanced Modulation for morphology and the epifluorescent module for GFP (Green). The two images were merged to showed GFP localization. C: cornea. CD: ciliary body. TM: trabecular meshwork.
Group 2: five mice (male and female) intravitreally injected with pLVX-CMV-ΔhTGFβ2C226S/C228S in one eye and pLVX-CMV-AcGFP in the fellow eye as a control (Figure 1B).
Group 3: four mice (male and female) intracamerally injected with pLVX-EF1α-ΔhTGFβ2C226S/C228S in one eye and pLVX-EF1α-mCherry in the fellow eye as a control (Figure 1C).
Group 4: five mice (male and female) intravitreally injected with pLVX-EF1α-ΔhTGFβ2C226S/C228S in one eye and pLVX-EF1α-mCherry in the fellow eye as a control (Figure 1D).
To make conditions consistent, a 2μl viral suspension containing ~8×105 viral particles was injected into each eye. After injection, topical antibiotic (neomycin-polymyxin B sulfates-bacitracin zinc ophthalmic ointment) was applied. IOP was measured weekly as described previously.
Over the 8-week period, we did not observe significant differences in IOP between TGFβ2 expression virus injected eyes compared to control eyes in any of the treatment groups (Figure 1A–D). Interestingly, there was a transient IOP elevation between weeks 4–6 in both control and experimental eyes, which returned to baseline by week 7.
At the end of the study, we euthanized and enucleated the mouse eyes and fixed them with 4% paraformaldehyde in PBS at 4°C overnight. Fixed mouse eyes were embedded in paraffin, sectioned, and deparaffined for immunostaining. Tissue sections were processed in the 2100 antigen retriever (Electron Microscopy Sciences, Hatfield, PA) prior to immunostaining. These sections were blocked with Superblock (Thermo Fisher Scientific) and immunostained using the M.O.M. immunodetection kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s protocols. TGFβ2 was probed using the mouse anti-TGFβ2 primary antibody (1:100, catalog#: ab36495; Abcam, Cambridge, MA), the biotinylated horse anti-mouse IgG (included in the M.O.M. kit), and Texas Red-Avidin D (catalog#: A-20062; Vector Laboratories). The sections were then mounted with Prolong Gold mounting medium with DAPI (Thermo Fisher Scientific). Images were captured using the Nikon Eclipse Ti2 microscope (Nikon, Melville, NY). The eyes were studied and representative images of TGFβ2 are shown in Figure 2.
Figure 2. The expression of TGFβ2 in lentiviral injected mouse eyes.
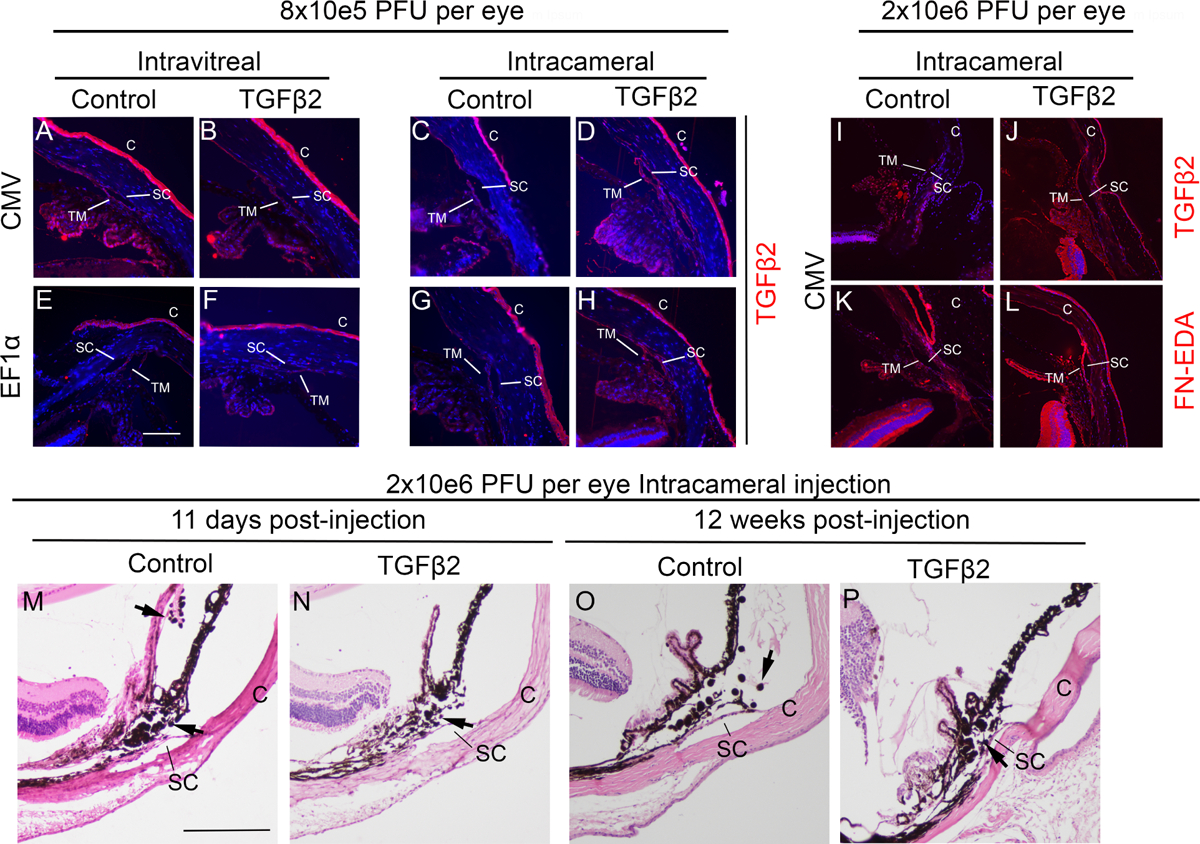
Some mouse eyes were enucleated, fixed, embedded in paraffin, and immunostained for TGFβ2 and/or fibronectin isoform EDA (FN-EDA) (shown in red) and with DAPI (blue). A-H: the eyes that received low viral doses. I-L: the eyes that received high viral doses. Representative images are shown. Blue: DAPI, Red: TGFβ2. Control: eyes injected with either pLVX-CMV-AcGFP or pLVX-EF1α-mCherry lentiviruses. TGFβ2: eyes injected with either pLVX-CMV-ΔhTGFβ2C226S/C228S or pLVX-EF1α-ΔhTGFβ2C226S/C228S lentiviruses. C: cornea, SC: Schlemm’s canal, TM: trabecular meshwork. The white scale bar: 100μm. Some mouse eyes receiving intracameral injection of 2 × 106 pLVX-CMV-AcGFP (M and O) or pLVX-CMV-ΔhTGFβ2C226S/C228S (N and P) were stained with hematoxylin and eosin to determine intraocular inflammation at early (M and N) and late (N and P) time points. SC: Schlemm’s canal, C: cornea, black arrows: inflammatory cells. The black scale bar: 200μm.
We did not observe obvious changes in anterior chamber tissue morphology including the angle, TM, or Schlemm’s canal among all groups. Also, no obvious difference in inflammation was observed at early or late time points after viral injection (Figure 2M–P), and the level of inflammation, based on our experience, was lower than that caused by adenoviruses. We observed increased TGFβ2 expression in the eyes intracamerally injected with the TGFβ2 expression lentivirus with the CMV and EF1α promoter (Figure 2A vs. 2B and 2E vs. 2F). In contrast, within the intravitreally injected eyes, there was no difference in TGFβ2 expression in treated and control eyes for either lentivirus with the CMV or EF1α promoter (Figure 2C vs. 2D and 2G vs. 2H).
Recently, Patil et al. reported that 2×106 lenti-CMV-ΔTGFβ2C226S;C228S commercially prepared lentiviral particles intravitreally into the eyes of 4-month-old C57BL/6J and Balbc/J mice (ARVO abstract, June 2021, Vol.62, 493). The authors reported an IOP increase of 3.3 mmHg among all the mice (sample size unclear) starting from 3 weeks post-injection. There was 6.19 mmHg IOP increase in some OHT mice (defined as “responders”), while the others did not show IOP increase. Therefore, we used the same number of viral particles to for our study. Since the authors already studied the intravitreal injection route, we determined if intracameral injection would also induce OHT. We only tested our lentivirus with the CMV promoter, not the EF1α promoter, to be consistent.
We used 10 mice (5 male and 5 female) at the age of 5 months and injected 2×106 pLVX-CMV-ΔhTGFβ2C226S/C228S in one eye and 2×106 pLVX-CMV-AcGFP in the fellow eye as a control (Figure 1E). IOP was measured using the same method in a masked manner as described previously. We found that a higher dose of viruses increased IOP in pLVX-CMV-ΔhTGFβ2C226S/C228S injected eyes at weeks 6 and 8 compared to their baseline IOP (Figure 1E, # signs). If the IOP in pLVX-CMV-ΔhTGFβ2C226S/C228S injected eyes was compared to their fellow control eyes, there was a significant IOP elevation from week 5 (Figure 1E, * signs). Also, four out of the 10 eyes did not show meaningful IOP elevation (<3mmHg) compared to their own baseline IOP.
To ensure the virus was expressed in the TM region, we harvested fresh eyes, embedded in Tissue-Tek® O.C.T. Compound (Sakura, Torrance, CA) without fixation before cryosectioning. The cryosection was directly imaged using Nikon Advanced Modulation for tissue morphology and epifluorescent module for GFP (Ti2, Nikon Instruments, Melville, NY). We found that GFP was expressed in the TM region (Figure 1F).
To determine the expression of TGFβ2 and fibronectin EDA isoform (FN-EDA), we collected mouse eyes 11 days post viral injection (2×106 pLVX-CMV-ΔhTGFβ2C226S/C228S or 2×106 pLVX-CMV-GFP; intracameral injection), fixed them with 4% paraformaldehyde, embedded them in paraffin, sectioned, subjected to antigen retrieval, and stained with anti-TGFβ2 antibody (described previously) or anti-FN-EDA (1:100, catalog#: ab6328, Abcam) and the M.O.M kit (described previously). We found that both TGFβ2 and FN-EDA were elevated in pLVX-CMV-ΔhTGFβ2C226S/C228S injected eyes, especially in the TM tissue, compared to pLVX-CMV-GFP injected eyes (Figure 2I–L).
Overall, in this study we did not observe the induction of OHT in C57BL/6J mice using the low dose of lentiviral vectors with either the CMV or EF1α promoter via intravitreal or intracameral injection. At the high dose, intracameral injection of the lentiviral vector with the CMV promoter induced some levels of OHT.
Several important considerations include:
a. Transduction efficiency, the number of lentiviral particles, and promoter selection
We observed increased TGFβ2 expression in the TM of intracamerally injected mouse eyes but not in intravitreally injected eyes at a low dose. However, the elevation of TGFβ2 induced by the low dose of lentiviruses did not seem to be sufficient for the development of OHT. At the high dose (2.5 times), 6/10 mice developed OHT higher than 3mmHg. However, the IOP elevation showed a large variation among individual mice.
Also, the selection of the promoter to drive the expression of TGFβ2 needs optimization. The CMV promoter is the most frequently used promoter in recombinant adenoviruses for TGFβ2 expression with success (Shepard et al., 2010). We tested the EF1α promoter but did not observe any IOP elevation at low viral titers. However, further studies are needed to determine if it induces OHT at high viral titers.
b. Injection route
Our data suggested that an intracameral injection route is suitable for lentiviral transduction at the high dose. Another study showed that intravitreal injection is also feasible (ARVO abstract, June 2021, Vol.62, 493). At the low dose, intracameral injection, compared to intravitreal injection, seemed to induce more TGFβ2 expression.
c. Timing
Lentiviral vectors usually take a longer time to express the transgene because they must first integrate their genome into host cells. In our previous studies using primary and transformed TM cell cultures, we observed transgene expression within a few days after lentiviral transduction (data not shown). In this study, we monitored the mice for over 2 months, providing sufficient time for TGFβ2 expression. In the high dose group, we did not observe OHT until week 5 or 6, which is slow compared to using adenoviruses (could be as early as week 1) (Shepard et al., 2010).
e. Mouse strain preference
Mouse strains may affect lentiviral transduction efficiency and/or TGFβ2 effects. We used C57BL/6J mice because it has been reported that this strain responds to adenovirus-mediated TGFβ2 expression in contrast to the C3H/HeJ strain which carries a spontaneous mutation in the Toll receptor 4 gene (Hernandez et al., 2017). In C57BL/6J mice, OHT was induced in 6/10 mice using a high viral dose. It is possible that other mouse strains are more sensitive or resistant to lentiviral transduction.
In summary, it is possible to use the lentivirus-mediated TGFβ2 expression system to induce OHT in C57BL/6J mice as an alternative to the adenovirus-mediated delivery system. High titers of lentiviruses are needed to ensure sufficient numbers of viral particles are delivered into mouse eyes. Also, the IOP response to lentiviral vectors is relatively slow and mild. The mouse eyes showed little to no inflammatory responses to lentiviral vectors compared to adenoviral vectors.
Highlights.
Only high doses of lentiviral TGFβ2 expression vectors induced ocular hypertension in some mouse eyes.
Increased TGFβ2 expression was observed in intracamerally injected mouse eyes.
Lentiviral transduction did not induce severe inflammation in mouse eyes.
Acknowledgements
This study was supported by National Eye Institute R01EY026962 (WM), R01EY031700 (WM), T35EY031282 (TWC and DKW), BrightFocus Foundation G2017151 (WM), Indiana University School of Medicine Showalter Scholarship (WM), and the Indiana Clinical and Translational Sciences Institute funded, in part by Award Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (WM), and a Challenge Grant from Research to Prevent Blindness (department of Ophthalmology, Indiana University School of Medicine). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- IOP
intraocular pressure
- OHT
ocular hypertension
- POAG
primary open angle glaucoma
- TM
trabecular meshwork
- FN-EDA
fibronectin isoform EDA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal R, Gupta SK, Agarwal P, Saxena R, Agrawal SS, 2009. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol 57, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaggan KS, Ali RR, 2012. Ocular gene delivery using lentiviral vectors. Gene Ther 19, 145–153. [DOI] [PubMed] [Google Scholar]
- Basche M, Kampik D, Kawasaki S, Branch MJ, Robinson M, Larkin DF, Smith AJ, Ali RR, 2018. Sustained and Widespread Gene Delivery to the Corneal Epithelium via In Situ Transduction of Limbal Epithelial Stem Cells, Using Lentiviral and Adeno-Associated Viral Vectors. Hum Gene Ther 29, 1140–1152. [DOI] [PubMed] [Google Scholar]
- Borras T, 2012. Advances in glaucoma treatment and management: gene therapy. Invest Ophthalmol Vis Sci 53, 2506–2510. [DOI] [PubMed] [Google Scholar]
- Campa C, Gallenga CE, Bolletta E, Perri P, 2017. The Role of Gene Therapy in the Treatment of Retinal Diseases: A Review. Curr Gene Ther 17, 194–213. [DOI] [PubMed] [Google Scholar]
- Cao Y, Wei H, Pfaffl MW, Da B, 2003. [Effect of transforming growth factor beta 2 on phagocytosis in cultured bovine trabecular meshwork cells]. Ophthalmologe 100, 535–538. [DOI] [PubMed] [Google Scholar]
- Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang I-H, Clark AF, 2006a. TGFβ2-Induced Changes in Human Trabecular Meshwork: Implications for Intraocular Pressure. Investigative ophthalmology & visual science 47, 226–234. [DOI] [PubMed] [Google Scholar]
- Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF, 2006b. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci 47, 226–234. [DOI] [PubMed] [Google Scholar]
- Gottanka J, Chan D, Eichhorn M, Lutjen-Drecoll E, Ethier CR, 2004. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci 45, 153–158. [DOI] [PubMed] [Google Scholar]
- Hernandez H, Medina-Ortiz WE, Luan T, Clark AF, McDowell CM, 2017. Crosstalk Between Transforming Growth Factor Beta-2 and Toll-Like Receptor 4 in the Trabecular Meshwork. Invest Ophthalmol Vis Sci 58, 1811–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnykas G, Kokki E, Alasaarela L, Lesch HP, Tuulos T, Kinnunen K, Uusitalo H, Airenne K, Yla-Herttuala S, 2017. Comparative Study of Adeno-associated Virus, Adenovirus, Bacu lovirus and Lentivirus Vectors for Gene Therapy of the Eyes. Curr Gene Ther 17, 235–247. [DOI] [PubMed] [Google Scholar]
- Kampik D, Ali RR, Larkin DF, 2012. Experimental gene transfer to the corneal endothelium. Exp Eye Res 95, 54–59. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Fingert JH, Kuehn MH, Alward WL, 2009. Primary open-angle glaucoma. N Engl J Med 360, 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, Zhao C, Zheng Z, Shu Y, Wu X, Lei J, Li Y, Zhang W, Yang C, Wu K, Wu Y, Ho S, Athiviraham A, Lee MJ, Wolf JM, Reid RR, He TC, 2017. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis 4, 43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Ikeda Y, Yonemitsu Y, Goto Y, Murakami Y, Yoshida N, Tabata T, Hasegawa M, Tobimatsu S, Sueishi K, Ishibashi T, 2011. Pigment epithelium-derived factor gene therapy targeting retinal ganglion cell injuries: neuroprotection against loss of function in two animal models. Hum Gene Ther 22, 559–565. [DOI] [PubMed] [Google Scholar]
- Montecchi-Palmer M, Bermudez JY, Webber HC, Patel GC, Clark AF, Mao W, 2017. TGFβ2 Induces the Formation of Cross-Linked Actin Networks (CLANs) in Human Trabecular Meshwork Cells Through the Smad and Non-Smad Dependent Pathways. Investigative ophthalmology & visual science 58, 1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang IH, Millar JC, Clark AF, 2015. Elevation of intraocular pressure in rodents using viral vectors targeting the trabecular meshwork. Exp Eye Res 141, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF, 2010. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci 51, 2067–2076. [DOI] [PubMed] [Google Scholar]
- Tovar-Vidales T, Clark AF, Wordinger RJ, 2011. Transforming growth factor-beta2 utilizes the canonical Smad-signaling pathway to regulate tissue transglutaminase expression in human trabecular meshwork cells. Exp Eye Res 93, 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WF, Tripathi BJ, 1994. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res 59, 723–727. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Clark AF, Agarwal R, Lambert W, McNatt L, Wilson SE, Qu Z, Fung BK, 1998. Cultured human trabecular meshwork cells express functional growth factor receptors. Invest Ophthalmol Vis Sci 39, 1575–1589. [PubMed] [Google Scholar]


