Antenatal low-dose aspirin does not alter neurodevelopmental outcomes of offspring at age 3 years.
Abstract
OBJECTIVE:
Because low-dose aspirin is now commonly prescribed in pregnancy, we sought to assess the association between early antenatal exposure and child neurodevelopment.
METHODS:
We performed a noninferiority, masked, neurodevelopmental follow-up study of children between age 33 and 39 months whose mothers had been randomized to daily low-dose aspirin (81 mg) or placebo between 6 0/7 and 13 6/7 weeks of gestation through 37 weeks. Neurodevelopment was assessed with the Bayley-III (Bayley Scales of Infant and Toddler Development, 3rd Edition) and the ASQ-3 (Ages and Stages Questionnaire, 3rd Edition). The primary outcome was the Bayley-III cognitive composite score with a difference within 4 points demonstrating noninferiority.
RESULTS:
A total of 640 children (329 in the low-dose aspirin group, 311 in the placebo group) were evaluated between September 2021 and June 2022. The Bayley-III cognitive composite score was noninferior between the two groups (−1, adjusted mean −0.8, 95% CI, −2.2 to 0.60). Significant differences were not seen in the language composite score (difference 0.7, 95% CI, −0.8 to 2.1) or the motor composite score (difference −0.6, 95% CI, −2.5 to 1.2). The proportion of children who had any component of the Bayley-III score lower than 70 did not differ between the two groups. Similarly, the communication, gross motor, fine motor, problem-solving, and personal–social components of the ASQ-3 did not differ between groups. Maternal characteristics, delivery outcomes, breastfeeding rates, breastfeeding duration, and home environment as measured by the Family Care Indicators were similar.
CONCLUSION:
Antenatal low-dose aspirin exposure was not associated with altered neurodevelopmental outcomes at age 3 years.
CLINICAL TRIAL REGISTRATION:
The ASPIRIN (Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas) trial, conducted in six low- to middle-income countries, randomized 11,976 pregnant women to low-dose aspirin (81 mg daily) or an identical placebo beginning between 6 0/7 and 13 6/7 weeks of gestation and given daily until 37 weeks or delivery. Those randomized to low-dose aspirin had lower rates of preterm birth before 37 weeks of gestation, preterm birth before 34 weeks, and preterm hypertensive disorders of pregnancy before 34 weeks and lower perinatal mortality.1 These results, coupled with recent guideline changes,2–4 have potentially broadened the number of pregnant women who receive low-dose aspirin during pregnancy,5 particularly in low- to middle-income countries.
Although the immediate effects of low-dose aspirin taken during pregnancy to both the mother and the newborn have been well documented,2,6 data on the long-term neurodevelopmental implications of antenatal low-dose aspirin are limited by both the small number of participants evaluated and the quality of studies performed. These studies have been conducted with questionnaires of the child's primary care clinician at age 12 months and parental perceptions at age 18 months7 rather than standardized assessments that have been validated to be predictive of longitudinal outcomes, or they consisted of small numbers of children using instruments that are not viewed as the gold standard.8,9 Although suboptimal in their approach, these studies have generally suggested that no differences exist in neurocognition in children who were antenatally exposed to low-dose aspirin, but the data are limited.
If pregnant women, health care clinicians, and professional societies are to more broadly endorse and accept low-dose aspirin in pregnancy, understanding the long-term health implications for offspring, including neurodevelopment, is necessary. Therefore, the objective of this study was to assess the association of daily low-dose aspirin started between 6 0/7 and 13 6/7 weeks of gestation and taken until 37 weeks or delivery with neurodevelopment of children at age 33–39 months. This was a follow-up study of offspring of mothers randomized in the ASPIRIN trial using valid and standardized screening and assessment tools.
METHODS
This was a multinational (two sites in India and one each in the Democratic Republic of Congo, Guatemala, Pakistan, and Zambia) masked follow-up study of children whose mothers had participated in the ASPIRIN study. The results of the primary ASPIRIN trial1 and its methods have been published previously.10 Before initiation of the ASPIRIN trial, the protocol and consent forms were reviewed and approved by the relevant ethics review boards of each institution and by the data-coordinating center (RTI International). The trial was registered before initiation (NCT04888377) and was performed in accordance with the International Conference on Harmonization's Guideline for Good Clinical Practice.
Women who consented to being recontacted as part of the ASPIRIN trial who had children who were currently aged 33–39 months were identified. A convenience sample of available children meeting both the age and inclusion criteria were serially approached until the required sample size was obtained. Because the primary trial was randomized equally between aspirin and placebo, children were not recruited according to treatment allocation; rather, recruitment was monitored in a masked fashion by the data-coordinating center to ensure that comparable numbers of children exposed to low-dose aspirin and placebo were enrolled. Children were included if they did not have a major congenital anomaly or other medical condition (eg, blindness or deafness) that would preclude accurate assessment of neurodevelopment. Those with acute medical conditions that could affect accurate neurodevelopmental assessment were eligible for the study once their acute condition had resolved, assuming they remained in the appropriate age range.
After parental or guardian individual consent was obtained, children were evaluated with three components of the Bayley-III (Bayley Scales of Infant and Toddler Development, 3rd Edition)—cognition, motor (fine and gross), and language (receptive and expressive)—by a trained and credentialed examiner. With the role of the child's environment (eg, zippers are not broadly known in many low- to middle-income countries) recognized, appropriate substitutions of objects for the Bayley-III were permitted once they had been cleared by the trial oversight committee and were applied across all sites. We similarly note that the Bayley-III has been validated across a large number of low- to middle-income countries.11–14 Assessors were required to demonstrate training in the Bayley-III and to have three examinations reviewed and deemed acceptable by the Bayley-III training and oversight committee (M.A.L., A.B.C.). After this initial demonstration of competency, every 10th examination was recorded and reviewed to ensure that appropriate assessments occurred. Assessors were masked to whether the mother had received low-dose aspirin or placebo during her pregnancy. Because of logistical concerns or performance issues, the examiners were permitted to perform the examination over two visits. The assessors were permitted to note that they believed an examination was invalid and asked to provide the reason. In those cases, the assessors’ reasons for defining the examination as invalid were reviewed by the Bayley training and oversight committee in a masked fashion. If the reasons for excluding the assessment were deemed valid, these examinations were excluded from the analysis.
In addition, the ASQ-3 (Ages and Stages Questionnaire, 3rd Edition) was administered to the child's parent or guardian who served as the primary caregiver for the child. Because of low literacy rates, an intentional decision was made to universally administer the ASQ-3 orally by trained assessors to the child's mother or guardian. Other instruments administered by trained staff to assess covariates that may affect neurodevelopment included the Infant and Young Child Feeding Index,15 Family Care Indicators,16 and a standardized breastfeeding history. Height was assessed with a stadiometer; weight was measured with a calibrated electronic scale by staff who were trained in standardized anthropometric measurements (Appendix 1, available online at http://links.lww.com/AOG/D568). Stunting was defined as height-for-age z score less than −2 based on sex assigned at birth appropriate WHO growth curves.17
The primary outcome was the cognitive composite score of the Bayley-III. Secondary outcomes included the motor and language composite scores of the Bayley-III and the entirety of the ASQ-3. In addition to the composite score, we evaluated the individual components of the Bayley-III (cognitive, motor, and language) for the proportion of children with scores lower than 70. As a post hoc analysis of the various components of the Bayley-III and ASQ-3, the rates of being 1 SD below the mean were compared between children who were exposed to low-dose aspirin and those who were not.
The primary hypothesis was that children antenatally exposed to low-dose aspirin would have a Bayley-III cognitive score 4 or more points lower than nonexposed children. With the assumption of a noninferiority margin of 4 points in the Bayley-III cognitive composite score as clinically significant and a true effect of low-dose aspirin of not more than a 1-point decrease, a total sample size of 620 children (310 per arm) was determined to provide 80% power for a one-sided test for noninferiority with a type 1 error of 5%. Assuming that the conditions of noninferiority were met, the prespecified secondary analysis was that children antenatally exposed to low-dose aspirin would have a mean Bayley-III cognitive score greater on average than those who were not exposed. To test this secondary hypothesis, the sample size also provided greater than 90% power at a two-sided type 1 error of 5% to detect a difference of 4 points between low-dose aspirin–exposed and nonexposed children on the basis of a two-sided test for the secondary hypothesis. The primary hypothesis of noninferiority was tested by calculating the mean difference between treatment arms and its associated 90% CI for the primary outcome, the Bayley-III cognitive composite score. If the lower bound of the 90% CI was greater than −4, the treatment was assumed to be noninferior to placebo. If noninferiority were to be assumed, a one-sided t test would test the benefit of treatment compared with placebo. All analyses were modeled controlling for site as the original randomization stratification factor. For each outcome, the adjusted mean difference between treatment groups and the associated 95% CI based on an analysis of covariance model is presented. Analyses of secondary outcomes are exploratory in nature; therefore, P values and CIs are provided for descriptive purposes only, and no adjustment for multiple comparisons was made. Analyses were performed with SAS 9.4 and R.
The ethics review committees of all six participating centers (Kinshasha School of Public Health [FWA 0003581], University of Zambia [FWA00000338], Institute for Nutrition in Central America and Panama [FWA 00000742], KLE Academy of Higher Education and Research [FWA00024127], Aga Khan University [FWA0001177], and Lata Medical Research Foundation [FWA00012971]) reviewed and approved the protocol. The data-coordinating center included three authors (J.M., T.N., E.M.M.) who had access to the complete data and oversaw the statistical analysis. The Bayley-IIII training and oversight committee (M.A.L., A.B.C.), who are experienced in implementing this measure within clinical trials, conducted the Bayley-III training and oversaw the Bayley-III assessment process. All authors attest to the fidelity of data collection, analysis, and presentation of findings in this articles and these being consistent with study protocol. Before the initiation of the study, the trial was registered at ClinicalTrials.gov (NCT04888377).
RESULTS
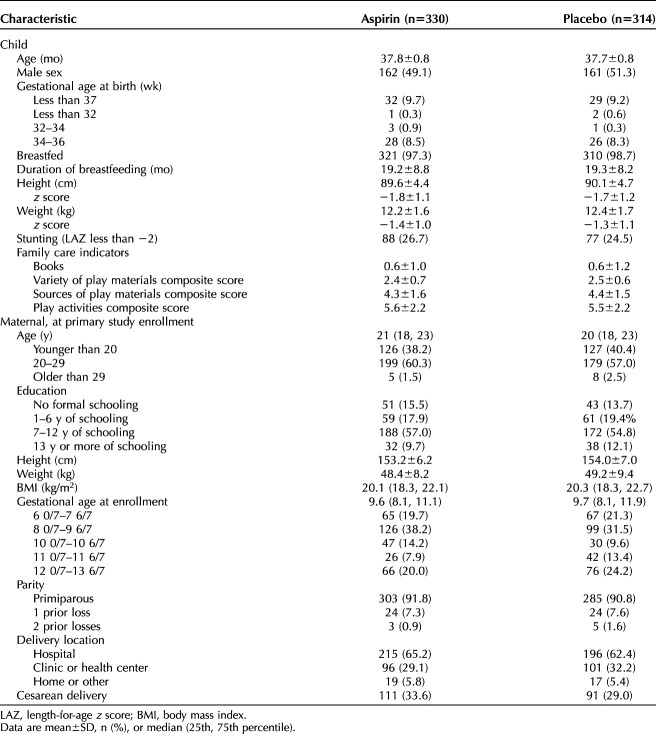
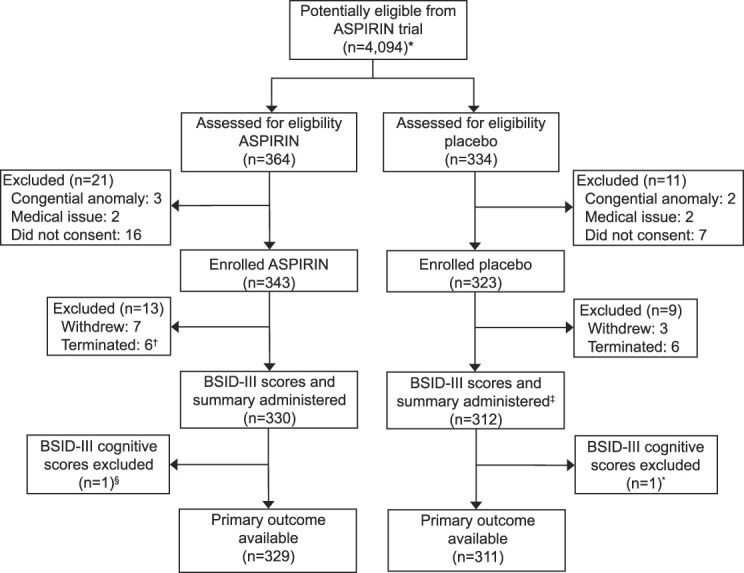
A total of 698 children were screened; of those, 666 were deemed eligible, and 642 were evaluated between September 2021 and June 2022. Two additional children (one in each group) were noted by the trained assessors to have a potentially invalid Bayley-III assessment, and the Bayley training and oversight committee agreed. We included 329 children in the low-dose aspirin group and 311 in the placebo group (Fig. 1). Maternal, antenatal, birth, and postnatal characteristics did not differ between the two groups (Table 1). Specifically, factors that are established to influence neurodevelopment, including preterm birth, maternal education, male sex, growth stunting, breastfeeding, and duration of breastfeeding, were noted to be similar.
Fig. 1. Trial profile. *Potentially eligible individuals were identified by reviewing data from ASPIRIN (Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas) main trial participants who would have children who had reached age 39 months by the end of the ASPIRIN follow-up study. †All participants who were terminated from the study were terminated because of aging out. ‡Two participants who completed the study did not complete the BSID-III (Bayley Scales of Infant and Toddler Development, 3rd Edition) assessment. §Children were excluded from analysis after review by a BSID expert on the basis of additional information provided by the BSID assessors at each site.
Hoffman. Antenatal Aspirin and Neurodevelopment at 3 Years. Obstet Gynecol 2024.
Table 1.
Child and Maternal Characteristics
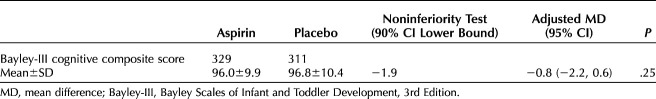
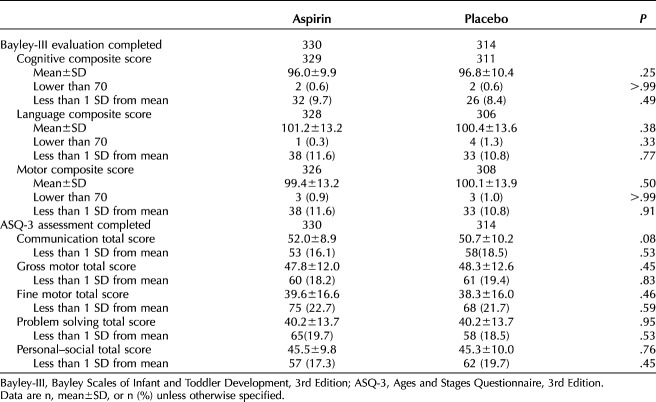
The difference in the Bayley-III cognitive composite score was noninferior between the two groups (−1.9, adjusted mean −0.8, 95% CI, −2.2 to 0.60). Specifically, the mean(±SD) cognitive composite score of the Bayley-III was similar between the two groups (low-dose aspirin 96.0±9.9 vs placebo 96.8±10.4, P=.25) (Table 2). No differences were seen in the language composite score (low-dose aspirin 101.2±13.2 vs placebo 100.4±13.6, P=.38) or the motor composite score (low-dose aspirin 99.4±13.2 vs placebo 100.1±13.9, P=.50) (Table 3). The rate of children scoring less than 1 SD below the mean and lower than a raw score of 70 points in all components of the Bayley-III did not differ between children who were antenatally exposed to low-dose aspirin and those exposed to placebo.
Table 2.
Primary Assessment of Neurodevelopment at Age 33–39 Months
Table 3.
Secondary Assessments of Neurodevelopment at Age 33–39 Months
The mean(±SD) results of the ASQ-3 similarly did not differ between children whose mothers took low-dose aspirin and those who did not (low-dose aspirin 52.0±8.9 vs placebo 50.7±10.2, P=.08). In addition, the rate of children scoring more than 1 SD below the mean of a domain of the ASQ-3 did not differ between the two groups. Although differences were noted in maternal age, mode of delivery, and other characteristics between regions (Appendix 1, http://links.lww.com/AOG/D568), no significant differences were noted between those who were exposed to low-dose aspirin and those exposed to placebo in terms of any of the measured neurodevelopmental parameters.
Post hoc subgroup sensitivity analyses of the primary outcome among women who had preterm children (before 37 weeks of gestation), among women who had anemia (hemoglobin less than 11.0 g/dL), and in the three principal regions of the study were undertaken. No differences among these subpopulations were noted (Fig. 2).
Fig. 2. Mean difference in BSID-III (Bayley Scales of Infant and Toddler Development, 3rd Edition) composite score by group sensitivity analyses. GA, gestational age.
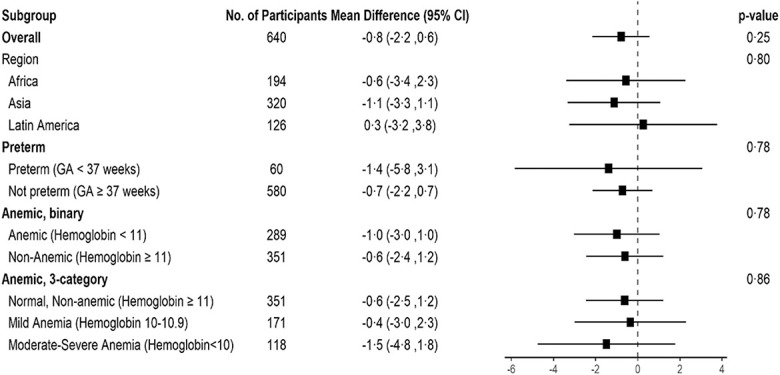
Hoffman. Antenatal Aspirin and Neurodevelopment at 3 Years. Obstet Gynecol 2024.
DISCUSSION
In this masked neurodevelopment follow-up study of children whose mothers were randomized to either 81 mg aspirin daily or an identical placebo beginning at 6 0/7 to 13 6/7 weeks of gestation and continued until delivery or 37 weeks, we found that early antenatal exposure to low-dose aspirin was not inferior to placebo exposure in offspring neurodevelopmental outcomes as measured by the cognitive component of the Bayley-III. Similarly, no differences in the remaining components of the Bayley-III or the ASQ-3 were detected. The incidence of children with low scores of developmental concern, defined as lower than 70 on the Bayley-III and less than 1 SD on both the Bayley-III and ASQ-3, did not differ between groups. We note that these tests are broadly accepted and have been validated in low- to middle-income countries.12–14 No differences in child height, weight, or stunting were noted between the two groups. In total, these findings demonstrate that low-dose aspirin is not associated with changes in neurodevelopment or postnatal growth within the parameters and age groups in which it was tested. With newer guidelines2–4 that have progressively widened the groups of eligible women and emphasized earlier initiation of low-dose aspirin during pregnancy, this study may offer reassurance to mothers, health care clinicians, professional societies, and ministries of health that neurodevelopmental delays are unlikely to be the result of antenatal aspirin exposure even when begun as early as 6 weeks of gestation.
Our findings are consistent with the follow-up investigation of CLASP (Collaborative Low-Dose Aspirin Study in Pregnancy), which assessed children whose mothers were randomized to low-dose aspirin or placebo using a standardized questionnaire of their primary care clinician at age 12 months (n=4,168) and parental questionnaire at age 18 months (n=4,365),7 which also found no differences in neurocognition in those children antenatally exposed to low-dose aspirin and those exposed to placebo. A follow-up evaluation of children using a mailed questionnaire at age 18 months among Italian mothers who participated in a randomized trial of low-dose aspirin or placebo was similarly unable to demonstrate a difference in either fetal growth or neurodevelopment. Although the findings of these two studies are reassuring, assessments at these early ages and the instruments selected to predict longitudinal outcomes are not consistent with currently accepted measures of child development that have been better validated and shown to be predictive of longitudinal outcomes. Other research has suggested potential neurodevelopmental benefits of antenatal low-dose aspirin. Marret et al8 examined neurodevelopment among children born before 33 weeks of gestation who were exposed to low-dose aspirin antenatally and noted a trend of reduction in low processing scores, behavioral difficulties, and hyperactivity. These outcomes were not assessed in our study.
Strengths of the study include that this was a masked follow-up trial of a randomized placebo-controlled trial. Because both enrollers and evaluators were masked to whether the child was randomized to antenatal low-dose aspirin exposure, selection bias of selective enrollment was mitigated.
In addition to the limitations of the parent study, several limitations of this investigation should be noted. We note that we lacked the power to detect differences less than 4 points in the Bayley-III examination that may be meaningful on a population basis. Similarly, the number of children born prematurely was small (n=61); therefore, our ability to explicitly test this hypothesis in this group of children was limited. We do, however, note that both preeclampsia18 and preterm birth19 are associated with worse neurodevelopment and that low-dose aspirin has consistently been shown to lower the risks of preeclampsia and preterm birth.
In conclusion, we were not able to demonstrate any difference in neurodevelopmental outcomes in children between age 33 and 39 months after antenatal low-dose aspirin exposure. Given the significant benefits demonstrated in the ASPIRIN trial,1 these results should offer parents, clinicians, and policy makers additional reassurance of the lack of harm when low-dose aspirin is prescribed early in pregnancy.
Authors' Data Sharing Statement
Will individual participant data be available (including data dictionaries)? No.
What data in particular will be shared? Not available.
What other documents will be available? Not available.
When will data be available (start and end dates)? Not applicable.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Not applicable.
Footnotes
This study was funded by the Thrasher Research Fund, which had no direct role in the study design, implementation, data collection, or analysis.
Financial Disclosure Nancy F. Krebs disclosed a financial relationship with Nurture, Inc, The Danone Company, Pediatric and Maternal Nutrition, and Happy Family Advisory Board. Patricia Hibberd disclosed that money was paid to her institution from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Fogarty International Center, the Foundation for the National Institutes of Health, and the Bill and Melinda Gates Foundation. Michelle A. Lobo disclosed receiving a consulting fee from the Thrasher Foundation, which supported this work. The other authors did not report any potential conflicts of interest.
Presented at the Society for Maternal-Fetal Medicine’s 43rd Annual Pregnancy Meeting, February 6–11, 2023, San Francisco, California.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D569.
REFERENCES
- 1.Hoffman MK, Goudar SS, Kodkany BS, Metgud M, Somannavar M, Okitawutshu J, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial [published erratum appears in Lancet 2020;395:e53]. Lancet 2020;395:285–93. doi: 10.1016/S0140-6736(19)32973-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson JT, Vesco KK, Senger CA, Thomas RG, Redmond N. Aspirin use to prevent preeclampsia and related morbidity and mortality: aspirin use to prevent preeclampsia and related morbidity and mortality. Accessed March 17, 2022. https://ncbi.nlm.nih.gov/books/NBK574449/ [DOI] [PubMed]
- 3.National Institute for Health Care Excellence. Hypertension in pregnancy: diagnosis and management. Accessed May 31, 2022. https://www.nice.org.uk/guidance/ng133/chapter/Recommendations#antiplatelet-agents [PubMed]
- 4.World Health Organization. WHO recommendations on antiplatelet agents for the prevention of pre-eclampsia. WHO; 2021. [PubMed] [Google Scholar]
- 5.Wheeler SM, Myers SO, Swamy GK, Myers ER. Estimated prevalence of risk factors for preeclampsia among individuals giving birth in the US in 2019. JAMA Netw Open 2022;5:e2142343. doi: 10.1001/JAMANETWORKOPEN.2021.42343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Short VL, Hoffman M, Metgud M, Kavi A, Goudar SS, Okitawutshu J, et al. Safety of daily low-dose aspirin use during pregnancy in low-income and middle-income countries. AJOG Glob Rep 2021;1:100003. doi: 10.1016/J.XAGR.2021.100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low dose aspirin in pregnancy and early childhood development: follow up of the Collaborative Low Dose Aspirin Study in Pregnancy: CLASP Collaborative Group. Br J Obstet Gynaecol 1995;102:861–8. doi: 10.1111/j.1471-0528.1995.tb10872.x [DOI] [PubMed] [Google Scholar]
- 8.Marret S, Marchand L, Kaminski M, Larroque B, Arnaud C, Truffert P, et al. Prenatal low-dose aspirin and neurobehavioral outcomes of children born very preterm. Pediatrics 2010;125:e29–34. doi: 10.1542/peds.2009-0994 [DOI] [PubMed] [Google Scholar]
- 9.Parazzini F, Bortolus R, Chatenoud L, Restelli S, Benedetto C. Follow-up of children in the Italian Study of Aspirin in Pregnancy. Lancet 1994;343:1235. doi: 10.1016/S0140-6736(94)92452-X [DOI] [PubMed] [Google Scholar]
- 10.Hoffman MK, Goudar SS, Kodkany BS, Goco N, Koso-Thomas M, Miodovnik M, et al. A description of the methods of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) study. BMC Pregnancy Childbirth 2017;17:135. doi: 10.1186/s12884-017-1312-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranjitkar S, Kvestad I, Strand TA, Ulak M, Shrestha M, Chandyo RK, et al. Acceptability and reliability of the Bayley Scales of Infant and Toddler Development-III among children in Bhaktapur, Nepal. Front Psychol 2018;9:370987. doi: 10.3389/fpsyg.2018.01265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitchik HO, Tofail F, Akter F, Shoab AKM, Sultana J, Huda TMN, et al. Concurrent validity of the Ages and Stages Questionnaire inventory and the Bayley Scales of Infant and Toddler Development in rural Bangladesh. BMC Pediatr 2023;23:93. doi: 10.1186/s12887-022-03800-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kvestad I, Hysing M, Ranjitkar S, Shrestha M, Ulak M, Chandyo RK, et al. The stability of the Bayley Scales in Early Childhood and its relationship with future intellectual abilities in a low to middle income country. Early Hum Dev 2022;170:105610. doi: 10.1016/J.EARLHUMDEV.2022.105610 [DOI] [PubMed] [Google Scholar]
- 14.Rasheed MA, Kvestad I, Shaheen F, Memon U, Strand TA. The predictive validity of Bayley Scales of Infant and Toddler Development-III at 2 years for later general abilities: findings from a rural, disadvantaged cohort in Pakistan. PLoS Glob Public Health 2023;3:e0001485. doi: 10.1371/JOURNAL.PGPH.0001485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Indicators for assessing infant and young child feeding practices. Part 2: measurement. Accessed June 18, 2023. https://apps.who.int/iris/bitstream/handle/10665/44306/9789241599290_eng.pdf?sequence=1 [Google Scholar]
- 16.Kariger P Frongillo EA Engle P Rebello Britto PM Sywulka SM, et al. Indicators of family care for development for use in multicountry surveys. J Health Popul Nutr 2012;30:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Weight-for-age. Accessed October 9, 2022. https://who.int/tools/child-growth-standards/standards/weight-for-age
- 18.Warshafsky C, Pudwell J, Walker M, Wen SW, Smith GN; Preeclampsia New Emerging Team. Prospective assessment of neurodevelopment in children following a pregnancy complicated by severe pre-eclampsia. BMJ Open 2016;6:e010884. doi: 10.1136/bmjopen-2015-010884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrini JR, Dias T, McCormick MC, Massolo ML, Green NS, Escobar GJ. Increased risk of adverse neurological development for late preterm infants. J Pediatr 2009;154:169–76. doi: 10.1016/j.jpeds.2008.08.020 [DOI] [PubMed] [Google Scholar]