Abstract
Background.
Does the genetic aptitude for educational attainment (GAEA) moderate the genetic risk for alcohol use disorder (AUD) and drug use disorder (DUD)?
Methods.
In the native Swedish population, born 1960–1980 and followed through 2017 (n = 1 862 435), the family genetic risk score (FGRS) for AUD and DUD and GAEA were calculated from, respectively, the educational attainment and risk for AUD and DUD, of 1st through 5th degree relatives from Swedish national registers. Analyses utilized Aalen’s linear hazards models.
Results.
Risk for AUD was robustly predicted by the main effects of FGRSAUD [b = 6.32 (95% CI 6.21–6.43), z = 64.9, p < 0.001) and GAEA [b = −2.90 (2.83–2.97), z = 44.1, p < 0.001] and their interaction [b = −1.93 (1.83–2.03), z = 32.9, p < 0.001]. Results were similar for the prediction of DUD by the main effects of FGRSDUD [b = 4.65 (CI 4.56–4.74), z = 59.4, p < 0.001] and GAEA [−2.08 (2.03–2.13), z = 46.4, p < 0.001] and their interaction [b = −1.58 (1.50–1.66)), z = 30.2, p < 0.001]. The magnitude of the interactions between GAEA and FGRSAUD and FGRSDUD in the prediction of, respectively, AUD and DUD was attenuated only slightly by the addition of educational attainment to the model.
Conclusions and relevance.
The genetic propensity to high educational attainment robustly moderates the genetic risk for both AUD and DUD such that the impact of the genetic liability to AUD and DUD on the risk of illness is substantially attenuated in those with high v. low GAEA. This effect is not appreciably mediated by the actual level of educational attainment. These naturalistic findings could form the basis of prevention efforts in high-risk youth.
Keywords: Alcohol use disorder, drug use disorder, educational attainment, genetics
Using both twin and molecular genetic strategies, we have learned a great deal in recent years about the relationship between the genetic liabilities to major psychiatric and substance use disorders (Consortium, 2013; Cross-Disorder Group of the Psychiatric Genomics Consortium, 2019; Kendler et al., 2011). We have also begun to clarify the associations between genetic liabilities to our key disorders and genetic propensity for educational attainment (Bulik-Sullivan et al., 2015; Lee et al., 2018; Rosoff, Kaminsky, McIntosh, Smith, & Lohoff, 2020). Considering the genetic aptitude for educational attainment (GAEA) and the genetic risks for alcohol use disorder (AUD) and drug use disorder (DUD), heritability has been shown to be substantial for all three phenotypes from twin models (Baker, Treloar, Reynolds, Heath, & Martin, 1996; Branigan, McCallum, & Freese, 2013; Kendler et al., 2015a; Tambs, Sundet, Magnus, & Berg, 1989; Tsuang et al., 1996; Verhulst, Neale, & Kendler, 2015). High educational attainment, socio-economic status (SES), and intelligence are consistently associated with lower levels of alcohol consumption and/or problematic drinking in Sweden (Sjölund, Hemmingsson, & Allebeck, 2015a; Sjölund, Hemmingsson, Gustafsson, & Allebeck, 2015b; Wennberg, Andersson, & Bohman, 2002; Zettergren & Bergman, 2014), and similar trends are typically seen elsewhere (Barr, Silberg, Dick, & Maes, 2018; Müller et al., 2013; Rogne, Pedersen, & Von Soest, 2021). In two twin studies, the heritability of alcohol consumption was moderated by SES such that heritability was lower in subjects with higher SES (Davis & Slutske, 2018; Hamdi, Krueger, & South, 2015).
We seek to expand on these two twin studies in four important ways. First, we examined AUD rather than alcohol consumption. Second, instead of using an overt measure such as SES, we instead look at the genetic propensity to high educational attainment. This permits us to model directly the interaction at a genetic level, an approach rarely implemented in the past. Third, we added a second substance use disorder, DUD, to see if the findings generalize. Finally, instead of using twin models, we take advantage of a recently developed method to estimate genetic risks or aptitudes from extended pedigrees in a Swedish national sample – the family genetic risk score (FGRS) (Kendler et al., In press; Kendler, Ohlsson, Sundquist, & Sundquist, 2021a, 2021b). So, our specific question is whether the genetic risks for AUD and DUD are moderated by the GAEA. In line with prior work, we predict that, if moderation occurs, high levels of GAEA will attenuate the impact of genetic liability on the risk for AUD or DUD.
Methods
We collected information on individuals from Swedish population-based registers with national coverage linking each person’s unique personal identification number, which for confidentiality, was replaced with a serial number by Statistics Sweden. This study was approved by the Regional Ethical Review Board of Lund (No. 2008/409, 2012/795, and 2016/679).
Our database consisted of all individuals born in Sweden from 1960 to 1980 of parents themselves born in Sweden. In the database, we included date of first registration for AUD and DUD utilizing ICD-8, 9, 10 codes from Swedish national primary care, specialist, and hospital registries as well as criminal registers and prescribed drug registers. We also included individual educational attainment (see Appendix Table 1 for full definitions). In the database, individual FGRS for the two disorders as well as a GAEA were included. Similar to prior studies (Kendler et al., In press; Kendler et al., 2021a, 2021b), the FGRS and GAEA were based on selected 1st, 2nd, 3rd, 4th, and 5th degree relatives to the probands with a mean of 40.1 relatives per proband. Briefly (see Appendix Table 2 for details), we first calculated the morbid risk for the phenotype in our sample of relatives based on age at first registration and then we transformed the binary trait into an underlying liability distribution, with the threshold that divides the population into those unaffected and affected for the disorder. Thereafter, we calculated the mean z-score for relatives with the disorder and the mean z-score for individuals without the disorder. For first-degree relatives, we also multiplied the z-score with a factor that sought to subtract the influence of cohabitation separately for siblings and parent–offspring pairs, thereby helping to remove from the FGRS any residual shared-environmental effect. For parent–offspring pairs, this was calculated by comparing the resemblance, by logistic regression, for father–offspring pairs where the biological father sired and raised his child (i.e. a father in an intact family) to the resemblance between children and their not-lived-with fathers, that is, those who sired their off-spring but never lived with or near them when they were growing up. We have examined such not-lived-with fathers in several prior extended adoption studies (e.g. Kendler et al., 2015a 2015b). For sibling pairs, we compared the resemblance in half-sibs who were v. were not reared together. As seen in Table 2 step 3 in the Appendix, the correction factors – the degree of the resemblance for our individual diagnoses that was retained after discounting the effect of shared environment equaled 0.99 (AUD) and 0.92 (DUD) for parent–offspring pairs and 0.69 (AUD) and 0.52 (DUD) for sibling pairs. Within each type of relative, we then had two components: the sum of the z-score and the total weighted number of relatives. These two components were weighted according to the genetic resemblance to the proband. For each proband, we summed the two components across all groups of relatives and used the quotient between the two components. Finally, to obtain the individual FGRS, we multiplied the quotient with a shrinkage factor based on the variance of the z-score across all relatives, the variance in the mean z-score across all probands, and the number of weighted number of relatives for each proband. So that the FGRSs would be more comparable across traits and to reduce the effect of register coverage, we standardized the FGRS by year of birth and county of residence into a z-score with mean = 0 and s.d. = 1. The calculation for GAEA followed the same principles but was simpler as we summed the weighted (by genetic resemblance) educational attainment across all relatives. The educational attainment was discounted for the effect of shared environment by a factor of 0.82 for parents and 0.73 for siblings. The weighted sum was then further weighted by number of relatives and standardized as described above.
To investigate whether GAEA moderates the impact of the familial genetic risk, we used Aalen’s linear hazards model (Aalen, 1989). Follow-up time in months was measured from age 15 until time of first registration for AUD (DUD), death, emigration, or end of follow-up (31 December 2017). In the first model, aside from year of birth and sex, we include FGRSAUD (DUD) and the GAEA as well as their interaction. The results from this model are presented as the excess number of cases per 10 000 person-years, and the interaction is measured on the additive scale as we have defended previously (Kendler & Gardner, 2010). To investigate the degree to which the interaction is a result of the effect of GAEA on educational attainment, we fitted three additional models, where the follow-up time started at age 25 so as to increase the chance that individuals had largely completed their education. Individuals who were registered for AUD or DUD prior to age 25 were excluded. Model A is the same model as described above, while model B also includes a main effect for educational attainment and model C further includes two additional two-way interaction terms [between educational attainment and FGRSAUD (FGRSDUD), and between educational attainment and GAEA] as well as a three-way interaction term between FGRSAUD (FGRSDUD), GAEA, and educational attainment. In all models, we investigated the proportionality assumption and there were no major violations. Statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute, 2012) and the R-package Timereg in R (Martinussen & Scheike, 2006; Scheike & Zhang, 2011; Team, 2020).
Results
Descriptive statistics
Table 1 provides descriptive statistics for the AUD and DUD. AUD had a modestly higher population prevalence (5.4%) than DUD (3.4%), with the expected male excess in both disorders. In affected individuals, the genetic risk for each of the disorders was more strongly elevated for DUD (+0.72 s.d.) than for AUD (+0.58 s.d.). The average GAEA was substantially below the population mean for individuals affected with DUD (−0.40 s.d.) and AUD (−0.34 s.d.).
Table 1.
Key Descriptive Statistics for Our Sample of Individuals with the Swedish General Population with Alcohol Use Disorder (AUD) and Drug Use Disorder (DUD)
| AUD | No AUD | |
|---|---|---|
| N (%) | 100,054 (5.4%) | 1,762,381 (94.6%) |
| Familial Genetic Risk Score for AUD (FGRSAUD) (Mean, SD) | 0.58 (1.4) | −0.03 (1.0) |
| Genetic Aptitude for Educational Attainment (GAEA) (Mean, SD) | −0.34 (0.9) | 0.02 (1.0) |
| Year of Birth (Mean, SD) | 1968 (5.9) | 1970 (5.9) |
| Males | 71.8% | 50.1% |
| Educational Attainment (EA) (Mean, SD) | −0.50 (0.9) | 0.03 (1.0) |
| DUD | No DUD | |
| N (%) | 62,776 (3.4%) | 1,802,486 (96.6%) |
| Familial Genetic Risk Score for DUD (FGRSDUD) (Mean, SD) | 0.72 (1.6) | −0.02 (1.0) |
| Genetic Aptitude for Educational Attainment (GAEA)(Mean, SD) | −0.40 (0.9) | 0.01 (1.0) |
| Year of Birth (Mean, SD) | 1970 (6.3) | 1969 (5.9) |
| Males | 64.5% | 50.8% |
| Educational Attainment (EA) (Mean, SD) | −0.66 (0.8) | 0.02 (1.0) |
Model fitting: primary models
We first predicted risk for AUD controlling for sex and year of birth (Table 2). The model contained both the linear effects of the FGRS for AUD and the GAEA and their interaction. As expected, the FGRS for AUD had a strong and substantial effect on AUD risk while GAEA had a protective effect, approximately half as strong. Most importantly, we found a robust negative interaction between the FGRSAUD and the GAEA (Fig. 1a). For those with a high GAEA [light blue line (very light grey) at the bottom of the figure], the slope of increased risk with increasing FGRSAUD was relatively flat – that is, a large increase in genetic risk produced only a modest increase in affected cases. However, this slope got steeper as the GAEA got lower. At the lowest level of GAEA, the same change in FGRSAUD risk produced more than three times as many affected individuals as seen with those at the highest level of GAEA.
Table 2.
Results from Aalen’s linear Hazard models (Beta Coefficients equaling excess number of cases per 10,000 person years) and 95% CIs
| Alcohol Use Disorder | Drug Use Disorder | |||
|---|---|---|---|---|
| Beta Coefficient | Z-value | Beta Coefficient | Z-value | |
| Familial Genetic Risk Score (FGRS) | 6.32 (6.21; 6.43) | 64.9 | 4.65 (4.56, 4.74) | 59.4 |
| Genetic Aptitude for Educational Attainment (GAEA) | −2.90 (2.83; 2.97) | 44.1 | −2.08 (2.03; 2.13) | 46.4 |
| Year of Birth | 0.11 (0.10; 0.12) | 8.8 | 0.43 (0.42; 0.44) | 24.4 |
| Male vs Female | 10.10 (9.95; 10.20) | 68.9 | 4.08 (3.97; 4.19) | 25.7 |
| FGRS* GAEA | −1.93 (1.83; 2.03) | 32.9 | −1.58 (1.50; 1.66) | 30.2 |
Fig. 1.
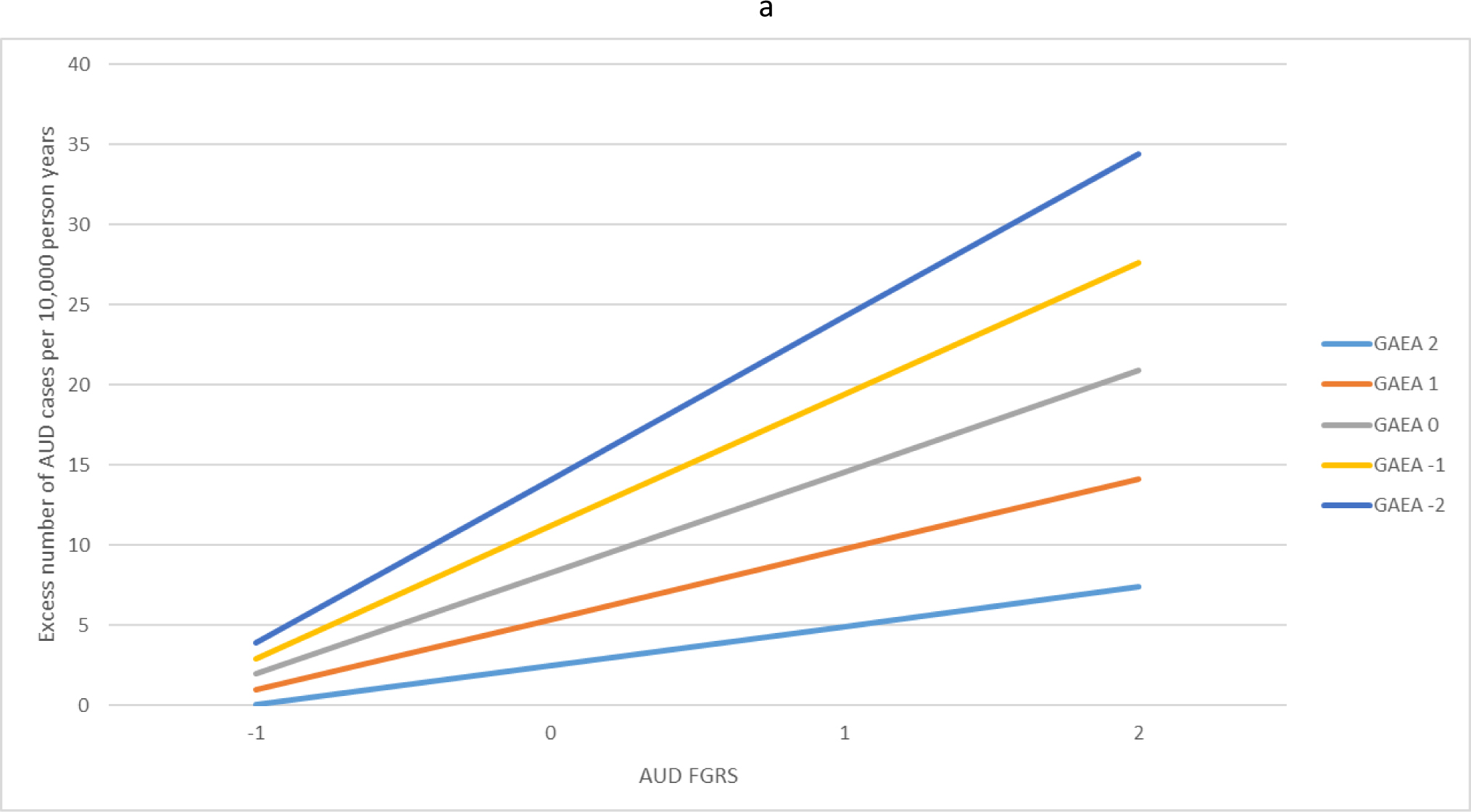
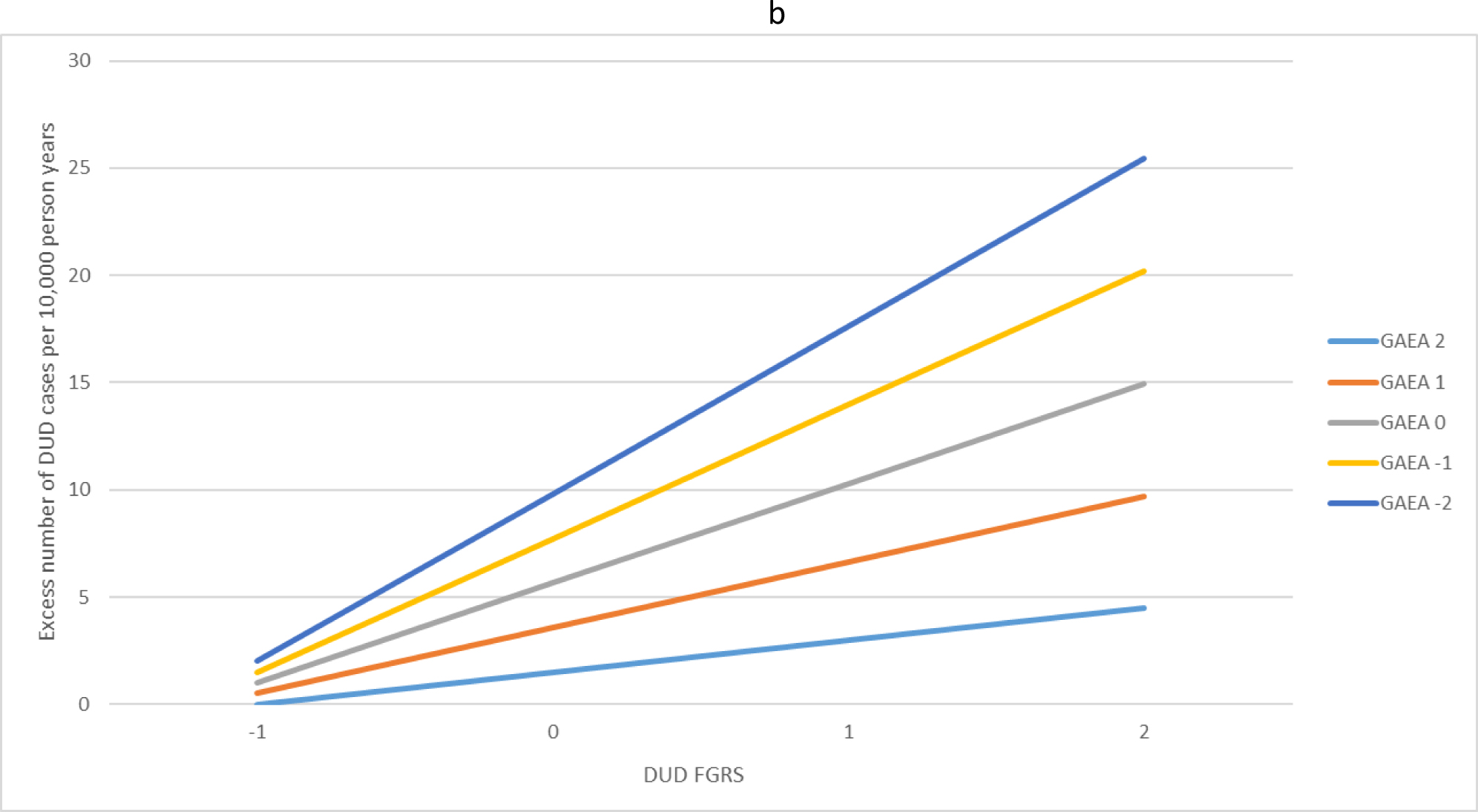
(a) The interaction effects in the prediction of alcohol use disorder (AUD) between the family genetic risk score for alcohol use disorder and the genetic aptitude for educational attainment. The x-axis is the level of the family genetic risk score for AUD in standard deviation units. The y-axis is the excess number of AUD cases predicted per 10 000 person years. The colored (grey-scale) lines reflect the level of the genetic aptitude for educational attainment in standard deviation units. For example, the light blue (very light grey) line at the bottom reflects a quite high genetic aptitude for educational attainment (+2 s.d.) while the dark blue line (very dark grey) at the top of the figure reflects a quite low genetic aptitude for educational attainment (−2 s.d.). (b) The interaction effects in the prediction of drug use disorder (DUD) between the family genetic risk score for DUD and the genetic aptitude for educational attainment. The x-axis is the level of the family genetic risk score for DUD in standard deviation units. The y-axis is the excess number of DUD cases predicted per 10 000 person years. The colored lines reflect the level of the genetic aptitude for educational attainment in standard deviation units. For example, the light blue (very light grey) line at the bottom reflects a quite high genetic aptitude for educational attainment (+2 s.d.) while the dark blue line (very dark grey) at the top of the figure reflects a quite low genetic aptitude for educational attainment (−2 s.d.).
For DUD, both the main effect of the FGRSDUD and the GAEA were slightly smaller than seen with AUD as was the interaction between them (Table 2) but the pattern of their interaction, as illustrated in Fig. 1b, was very similar to that seen for AUD.
Model fitting with educational attainment
GAEA appeared to strongly moderate the impact of the genetic risk for AUD and DUD. However, this moderation by GAEA could be largely driven by the impact of GAEA on educational attainment itself. To investigate this, we fitted three models to our original sample from which we censored individuals with a registration of AUD/DUD prior to <age 25 to insure they had achieved their final level of educational attainment (Table 3). Model A is the same model fitted to our entire sample in Table 2. The results were similar although the strength of the interaction effects between the FGRS and GAEA declined for both AUD and DUD suggesting that these interactions are strongest in the early onset cases censored from this sample.
Table 3.
Results from Three Aalen's linear Hazard models (Beta Coefficients (excess number of cases per 10,000 person years) and 95% CIs With the Latter Two Including Educational Attainment First as a Main Effect and then in Two and Three-Way Interactions*
| AUD | DUD | |||
|---|---|---|---|---|
| Beta Coefficient | Z-value | Beta Coefficient | Z-value | |
| Model A | ||||
| FGRS | 4.25 (2.16; 3.34) | 70.5 | 3.58 (3.50; 3.66) | 67.2 |
| GAEA | −1.64 (−1.70; −1.58) | 41.3 | −1.67 (−1.72; −1.62) | 48.5 |
| Year of Birth | 0.13 (0.12; 0.14) | 15.1 | 0.23 (0.22; 0.23) | 26.5 |
| Male vs Female | 5.81 (5.69; 5.93) | 49.7 | 2.89 (2.79; 2.99) | 30.4 |
| FGRS * GAEA | −1.06 (−1.14; −0.98) | 24.8 | −1.24 (−1.32; −1.16) | 28.3 |
| Model B | ||||
| FGRS | 4.08 (3.99; 4.17) | 68.4 | 3.45 (3.37; 3.53) | 66.3 |
| GAEA | −0.41 (−0.47; −0.41) | 10.8 | −0.36 (−0.41; −0.31) | 11.3 |
| Year of Birth | 0.13 (0.12; 0.14) | 14.4 | 0.23 (0.22; 0.24) | 25.1 |
| Male vs Female | 5.81 (5.69; 5.93) | 46.0 | 2.87 (2.77; 2.97) | 27.8 |
| FGRS * GAEA | −1.04 (−1.12; −0.96) | 24.4 | −1.22 (−1.30; −1.14) | 28.3 |
| EA | −2.93 (−2.99; −2.87) | 61.3 | −3.08 (−3.14; −3.02) | 64.0 |
| Model C | ||||
| FGRS | 3.80 (3.71; 3.89) | 61.1 | 2.97 (2.88; 3.06) | 57.3 |
| GAEA | −0.52 (−0.59; −0.45) | 12.6 | −0.53 (−0.59; −0.47) | 14.5 |
| Year of Birth | 0.13 (0.12; 0.14) | 14.1 | 0.22 (0.21; 0.23) | 24.8 |
| Male vs Female | 5.81 (5.69; 5.93) | 46.6 | 2.87 (2.77; 2.97) | 28.5 |
| FGRS * GAEA | −0.39 (−0.48; −0.30) | 8.2 | −0.39 (−0.48; −0.30) | 8.4 |
| EA | −2.99 (−3.06; −2.92) | 60.0 | −3.12 (−3.18; −3.06) | 63.4 |
| FGRS* EA | −1.61 (−1.71; −1.51) | 30.4 | −2.01 (−2.11; −1.91) | 39.6 |
| GAEA * EA | 0.57 (0.51; 0.63) | 18.1 | 0.80 (0.74; 0.85) | 28.0 |
| FGRS* GAEA*EA | 0.30 (0.22; 0.38) | 7.3 | 0.50 (0.42; 0.58) | 11.9 |
FGRS = Familial genetic Risk Score; GAEA = Genetic Aptitude for Educational Attainment; EA = Educational Attainment; Cases with registrations prior to age 25 are excluded (NAUD = 31,851 (31.8%); NDUD = 12,516 (19.9%))
Model B included a main effect for educational attainment which robustly predicted risk for AUD and DUD. Importantly, the magnitudes of the interaction between GAEA and the FGRSAUD, and FGRSDUD observed in this model were nearly identical to those seen in model A. These results indicate that little of the observed interaction between GAEA and the genetic risks for AUD and DUD was mediated through actual educational attainment.
Model C contained all the parameters in model B to which we added a three-way interaction between the relevant FGRS, GAEA, and educational attainment. This interaction was significant for both AUD and DUD. We illustrate this interaction in Fig. 2a and b presenting the results obtained for individuals with high educational attainment (+1 s.d.), mean educational attainment, and very low educational attainment (−2 s.d.). The pattern was similar across both AUD and DUD. The largest interaction between GAEA and FGRS was seen in those with very low educational attainment. The interaction was present but attenuated for those who obtained a mean level of education. However, for those at high educational attainment, the interaction effects disappeared.
Fig. 2.
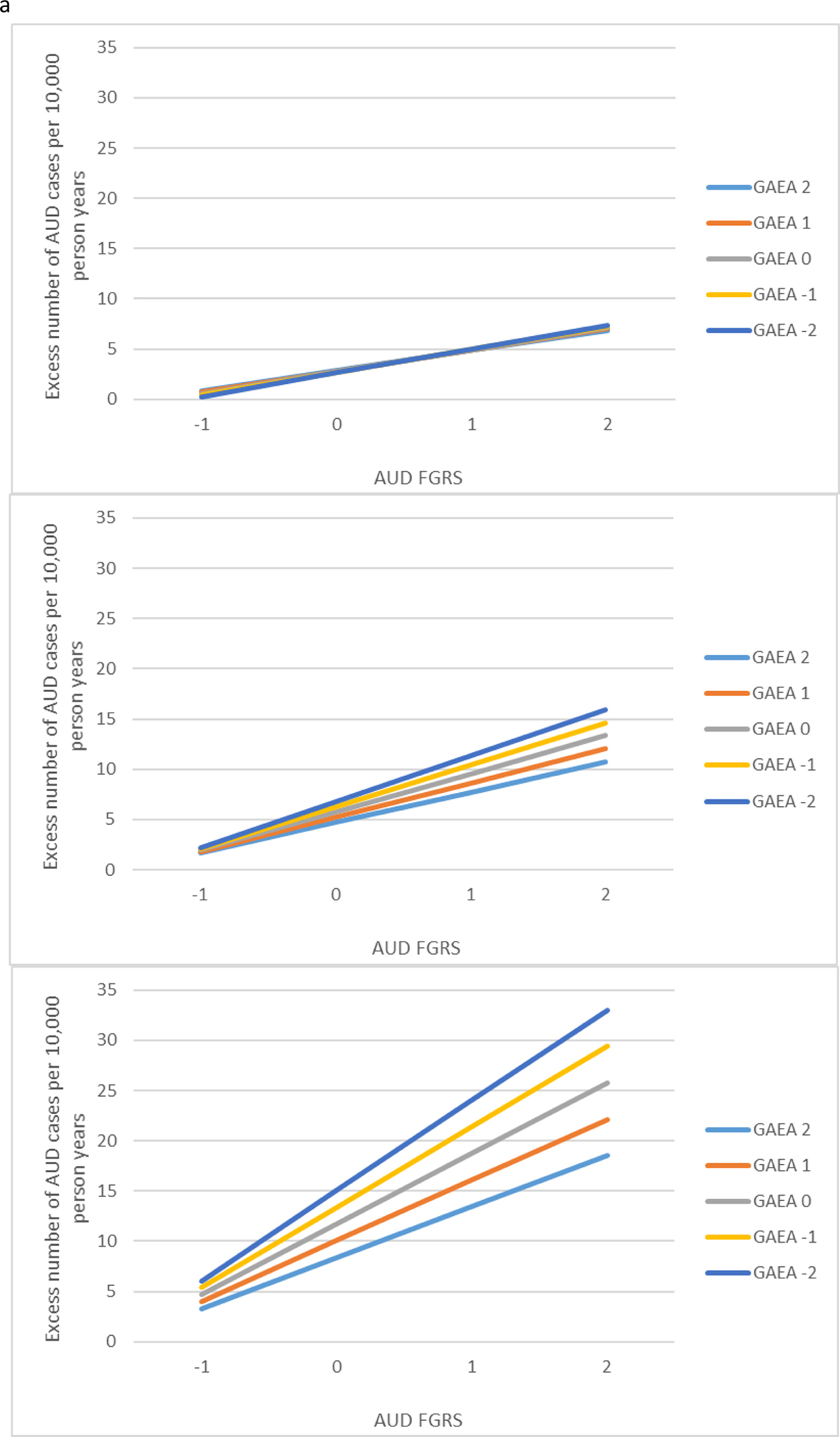
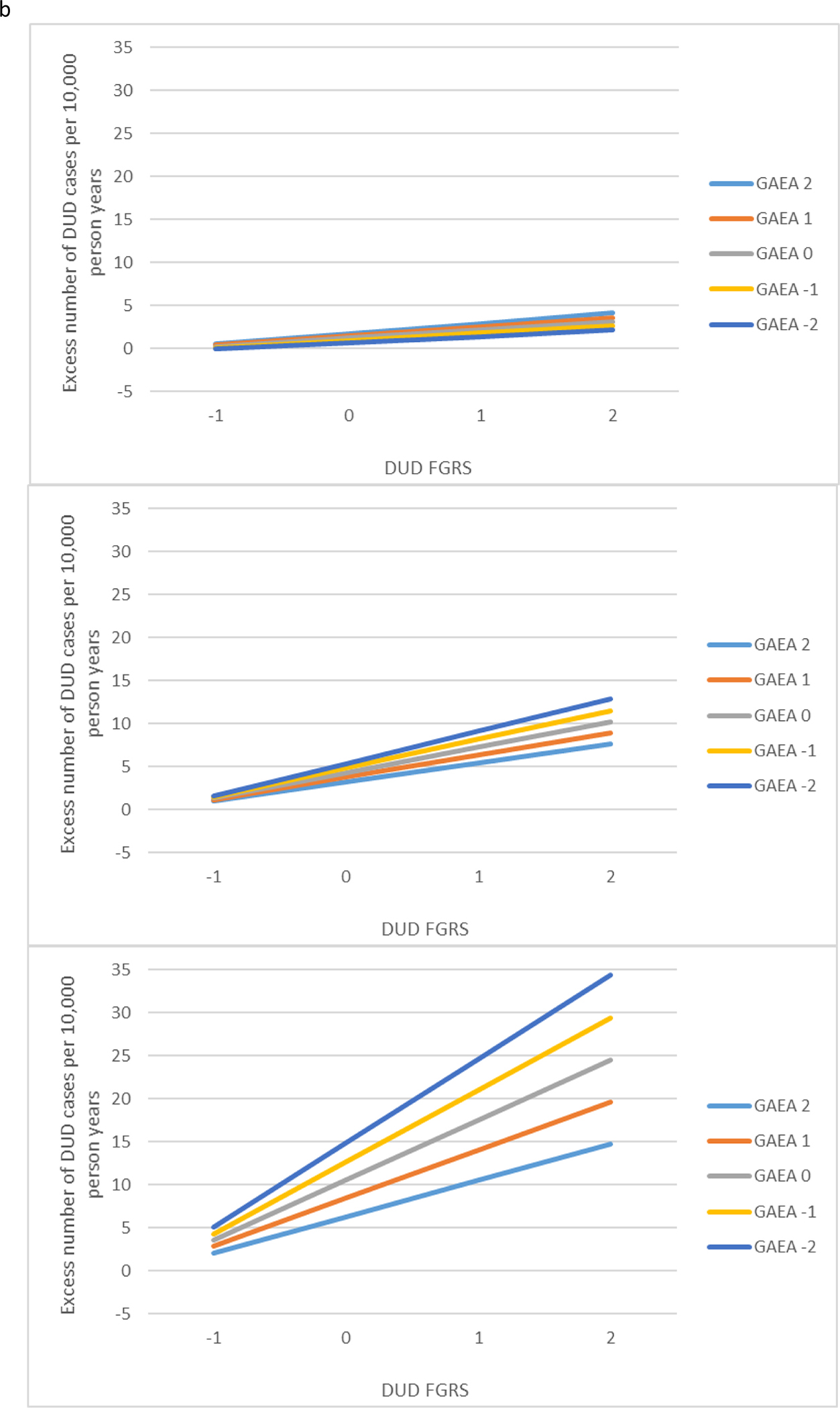
(a) Results of a three-way interaction analysis for the prediction of alcohol use disorder (AUD) from the family genetic risk score for AUD, the genetic aptitude for educational attainment and the actual level of achieved educational attainment. Each of the three panels is configured like that of Fig. 1a. The top panel then depicts the interaction between the family genetic risk score for AUD and the genetic aptitude for educational attainment in the prediction of AUD in individuals with high levels of achieved educational attainment (+1 s.d.). The middle figure presents the same analyses for those with an average level of achieved educational attainment. The lowest figure presents the same analyses for those very low levels of achieved educational attainment (−2 s.d.). (b) Results of a three-way interaction analysis for the prediction of drug use disorder (DUD) from the family genetic risk score for DUD, the genetic aptitude for educational attainment and the actual level of achieved educational attainment. Each of the three panels is configured like that of Fig. 1b. The top panel then depicts the interaction between the family genetic risk score for DUD and the genetic aptitude for educational attainment in the prediction of DUD in individuals with high levels of achieved educational attainment (+1 s.d.). The middle figure presents the same analyses for those with an average level of achieved educational attainment. The lowest figure presents the same analyses for those very low levels of achieved educational attainment (−2 s.d.).
Discussion
The goal of this paper was to determine whether the GAEA moderates the impact of the genetic risks for AUD and DUD on rates of, respectively, AUD and DUD. We addressed this question with three analyses. We review them in turn.
First, in a national sample of individuals affected with AUD and DUD, we found, consistent with prior studies of the phenotype of educational attainment (Davis & Slutske, 2018; Hamdi et al., 2015), that high GAEA attenuated the impact of genetic liability on the risk of both AUD and DUD. This effect was clearly seen in the slope of the FGRS curves depicted in Fig. 1. For both AUD and DUD, the slope of the curve was shallowest for those with the highest GAEA and became progressively steeper as the GAEA became lower.
Second, while the Swedish registry does not contain the fine-grained phenotypic assessments that would permit us to test the specific mechanisms through which the GAEA protected against the genetic risk for AUD and DUD, we could evaluate one important hypothesis – the degree to which the protective effect of GAEA results from its impact on the actual level of educational attainment. Contrary to our expectation, our analyses demonstrated that almost none of the moderating effects of GAEA on genetic risk for AUD and DUD was mediated through the completed years of education. Instead, the resilience to genetic risk for AUD and DUD associated with a high GAEA likely results from the direct effects of GAEA.
We would speculate that the direct protective effect of GAEA on genetic risk for AUD and DUD likely involves its impact on cognition, personality, and development. A number of studies have used twin and molecular-genetic strategies to disentangle the components of the GAEA. Consistent with prior studies (Belsky et al., 2016; Johnson, Deary, & Iacono, 2009; Tambs et al., 1989), Krapohl et al. (2014) found that the strongest component of the heritability of EA is intelligence. That high levels of general cognitive functioning could mediate the protective effect of GAEA in our Swedish sample is supported by a range of studies showing that high IQ in Sweden is related to lower levels of problematic drinking and/or the negative consequences thereof (Müller et al., 2013; Sjolund, Allebeck, & Hemmingsson, 2012; Sjölund et al., 2015a; Zettergren & Bergman, 2014). Other more specific cognitive processes could also be involved in protective effects such as working memory (Ellingson, Fleming, Vergés, Bartholow, & Sher, 2014).
However, Krapohl et al. (2014) also found that substantial proportions of the genetic propensity to high educational attainment resulted from the personality trait of self-efficacy. Other studies have shown that a polygenic risk score (PRS) for educational attainment predicts high levels of conscientiousness, agreeableness (Smith-Woolley, Selzam, & Plomin, 2019), and self-control (Belsky et al., 2016), personality traits which, along with the correlated constructs of self-efficacy and constraint, predict low levels of impulsivity, risk taking, and substance misuse (Belcher, Volkow, Moeller, & Ferre, 2014; Bogg & Roberts, 2004; Kendler et al., 1999; Settles et al., 2012; Tang, Posner, Rothbart, & Volkow, 2015).
Finally, strong academic performance in adolescence, predicted by GAEA, is associated with reduced risks for psychoactive substance use and subsequent AUD and DUD (Fothergill et al., 2008; Gauffin, Vinnerljung, Fridell, Hesse, & Hjern, 2013; Schulenberg, Bachman, O’Malley, & Johnston, 1994). Evidence that this relationship is likely causal comes from individual and school-based interventions for children and adolescents showing that improving academic performance results in lower psychoactive substance use (Eggert, Thompson, Herting, Nicholas, & Dicker, 1994; Fletcher, Bonell, Sorhaindo, & Strange, 2009; Lewis et al., 2012). Furthermore, recent prospective cohort studies employing instrumental variable and co-relative designs have shown that high academic performance was likely causally related to a lowered risk for both DUD (Kendler et al., 2018b) and AUD (Kendler et al., 2020). These findings are consistent with the predictions of social control (Hirschi, 1969) and social development theories (Kellam et al., 2008), which posit that students who succeed academically develop positive attachments to school. These attachments tend to facilitate a commitment to a prosocial lifestyle which in turn reduces the risk for AUD or DUD. By contrast, those who perform poorly in school are more likely to adopt antisocial lifestyles including an elevated risk for AUD and DUD. Thus, high GAEA would predict a developmental pathway marked by academic achievement which leads to the adoption of prosocial attitudes which could protect again the impact of high genetic risk for DUD or AUD.
Our third analysis examined whether the magnitude of the interactions between GAEA and FGRSAUD and FGRSDUD depended on the level of educational attainment. We found a robust three-way interaction which revealed a much stronger moderation of the genetic risk for AUD and DUD by GAEA in individuals with low v. high educational attainment. That is, much of the buffering by GAEA of the impact of elevated genetic risk for AUD and DUD in the Swedish population occurs in those with low levels of education. If targeted interventions could be developed on the basis of our findings with the goal of reducing future risk for substance use disorders, such interventions would have the highest impact if directed to those with high familial risk for AUD and/or DUD and low educational attainment.
Limitations
These findings should be viewed in the context of six potential methodological limitations. First, our results are dependent on the quality of the diagnosis of AUD and DUD in Swedish registries. While such registry data have important advantages (e.g. no refusals or reporting biases), it will not identify all the same cases as an interview-based assessment. Our affected subjects are likely, on average, more severely ill than those meeting DSM-5 criteria (American Psychiatric Association, 2013) at interview, although our lifetime prevalence of AUD and DUD is only moderately lower than those identified in nearby Norway (Kringlen, Torgersen, & Cramer, 2001). The validity of our definitions is supported by the high rates of concordance observed across our ascertainment methods (Kendler, Lönn, Salvatore, Sundquist, & Sundquist, 2018a; Kendler et al., 2015a, 2015b). Furthermore, the pattern of resemblance in relatives for AUD and DUD seen in Sweden is similar to those found in other samples based on personal interviews (Prescott & Kendler, 1999; Tsuang et al., 1996).
Second, our FGRS, used previously in this journal (Kendler et al., In press), assesses genetic risk on the basis of the aggregation of disorders in close and distant biological relatives and is not equivalent to a molecular PRS. Our corrections for shared environmental effects with parents and siblings are approximate. We also correct for year of birth to control for possible cohort effects and county of birth control for regional variations in diagnostic or policing practices. Appendix Table 3 shows that our final genetic risk scores are not highly sensitive to key steps in their calculation, as their deletion produces results that correlate highly with those from the full model and have similar predictive power. We show (Appendix Fig. 1) that the FGRS for DUD and AUD is relatively stable across regions within Sweden and have found, when controlling for pedigree structure, that the FGRS correlates very highly with a recently published quantitative family-history score using a different analytic approach (Hujoel, Gazal, Loh, Patterson, & Price, 2020).
We also explored, in several different analyses, the impact of the degree of relatives included in the FGRS calculation. In Appendix Table 4, we show the high correlations in the FGRS reported in the MS for 1st through 5th degree relatives with those obtained by eliminating more distant relatives and in Appendix Table 5, show the polychoric correlation between AUD/DUD and FGRSAUD, FGRSDUD, and FGRSEDU as a function of the breadth of relatives considered. The results are relatively stable until we trim down to only 1st and 2nd degree relatives. In Table 6, we recalculate our main results with various trimmings of the classes of relatives considered which shows that our findings are not sensitive to the particular set of relatives that contribute to the FGRS.
Third, as in other samples (Compton, Thomas, Stinson, & Grant, 2007; Kessler et al., 1997), we observe substantial comorbidity between AUD and DUD. We therefore re-ran our key results in four groups: AUD with DUD only cases censored, DUD with AUD cases only censored, AUD with comorbid DUD-AUD cases censored, and DUD with comorbid DUD-AUD cases censored. As seen in Appendix Table 7 and Fig. 2, the broad pattern of moderation seen in our main analyses was evident in all four of these groups.
Fourth, would our findings differ if analyzed with a more standard multiplicative Cox model, rather than the additive model employed? We present the results for AUD and DUD using such a model in Appendix Table 8 and Fig. 3a and b. The same pattern of results is observed.
Fifth, ADHD diagnoses are genetically correlated with risk for substance use disorders (Hicks, Iacono, & McGue, 2012; Kendler & Myers, 2014) and might contribute to their association with GAEA, but were not considered here.
Finally, there is substantial current interest in the relationship of the genetic influences on AUD and alcohol consumption (e.g. Kranzler et al., 2019). While it would therefore be of interest to see if we obtained similar or different results for the interactions of GAEA on genetic control of alcohol consumption as we do for FGRSAUD, no registry data are available in Sweden for individual alcohol consumption levels.
Conclusions
The genetic propensity to high educational attainment substantially moderates genetic risk for both AUD and DUD. The impact of the genetic liability to AUD and DUD on the risk of illness is substantially attenuated in those with high v. low GAEA. This effect is not appreciably mediated by the level of educational attainment. Rather, it likely arises because individuals with high GAEA tend to have higher levels of intelligence, conscientiousness, self-control, and follow a developmental pathway that includes good school performance leading to prosocial attitudes. All these factors, in aggregate, seem to protect even those at high genetic risk, from developing AUD and DUD. The protective effects of a genetic propensity to high educational attainment on risk for substance use disorders are particularly strong in those with low educational attainment. These naturalistic findings could form the basis of prevention efforts in high-risk youth. Our findings are also of theoretical interest and encourage further investigations, including those using polygenic risks scores, that examine the degree to which genetic liabilities for psychiatric and substance use disorders moderate the impact on one another on disorder risk.
Supplementary Material
Financial support.
Grant Support:
This project was supported by grants R01AA023534 and R01DA030005 from the National Institutes of Health and from the Swedish Research Council (2016-01176), as well as Avtal om Läkarutbildning och Forskning (ALF) funding from Region Skåne. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of interest. None.
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721005134.
Access to data and data analysis.
Kristina Sundquist had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Aalen OO (1989). A linear regression model for the analysis of life times. Statistics in Medicine, 8(8), 907–925. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2678347. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: Fifth edition, DSM-5. Washington, DC: American Psychiatric Association. [Google Scholar]
- Baker LA, Treloar SA, Reynolds CA, Heath AC, & Martin NG (1996). Genetics of educational attainment in Australian twins: Sex differences and secular changes. Behavior Genetics, 26(2), 89–102. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8639155. [DOI] [PubMed] [Google Scholar]
- Barr PB, Silberg J, Dick DM, & Maes HH (2018). Childhood socioeconomic status and longitudinal patterns of alcohol problems: Variation across etiological pathways in genetic risk. Social Science & Medicine, 209, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, Volkow ND, Moeller FG, & Ferre S (2014). Personality traits and vulnerability or resilience to substance use disorders. Trends in Cognitive Sciences, 18(4), 211–217. doi: S1364-6613(14)00027-8 [pii]; 10.1016/j.tics.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Corcoran DL, Domingue B, Harrington H, Hogan S, … Williams BS (2016). The genetics of success: How single-nucleotide polymorphisms associated with educational attainment relate to life-course development. Psychological Science, 27(7), 957–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogg T, & Roberts BW (2004). Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin, 130(6), 887. [DOI] [PubMed] [Google Scholar]
- Branigan AR, McCallum KJ, & Freese J (2013). Variation in the heritability of educational attainment: An international meta-analysis. Social Forces, 92(1), 109–140. 10.1093/sf/sot076. [DOI] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, … Neale BM (2015). An atlas of genetic correlations across human diseases and traits. Nature Genetics, 47(11), 1236–1241. doi: ng.3406 [pii]; 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, & Grant BF (2007). Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry, 64(5), 566–576. doi: 64/5/566 [pii]; 10.1001/archpsyc.64.5.566 [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium (2013). Genome-wide analysis identifies loci with shared effects on five major psychiatric disorders. Lancet (London, England), 381(9875), 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium (2019). Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell, 179(7), 1469–1482.e1411. doi: 10.1016/j.cell.2019.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CN, & Slutske WS (2018). Socioeconomic status and adolescent alcohol involvement: Evidence for a gene–environment interaction. Journal of Studies on Alcohol and Drugs, 79(5), 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert LL, Thompson EA, Herting JR, Nicholas LJ, & Dicker BG (1994). Preventing adolescent drug abuse and high school dropout through an intensive school-based social network development program. American Journal of Health Promotion, 8(3), 202–215. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10172017. [DOI] [PubMed] [Google Scholar]
- Ellingson JM, Fleming KA, Vergés A, Bartholow BD, & Sher KJ (2014). Working memory as a moderator of impulsivity and alcohol involvement: Testing the cognitive-motivational theory of alcohol use with prospective and working memory updating data. Addictive Behaviors, 39(11), 1622–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A, Bonell C, Sorhaindo A, & Strange V (2009). How might schools influence young people’s drug use? Development of theory from qualitative case-study research. Journal of Adolescent Health, 45(2), 126–132. doi: S1054-139X(09)00051-2 [pii]; 10.1016/j.jadohealth.2008.12.021 [DOI] [PubMed] [Google Scholar]
- Fothergill KE, Ensminger ME, Green KM, Crum RM, Robertson J, & Juon HS (2008). The impact of early school behavior and educational achievement on adult drug use disorders: A prospective study. Drug and Alcohol Dependence, 92(1–3), 191–199. doi: S0376-8716(07)00291-8 [pii]; 10.1016/j.drugalcdep.2007.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauffin K, Vinnerljung B, Fridell M, Hesse M, & Hjern A (2013). Childhood socio-economic status, school failure and drug abuse: A Swedish national cohort study. Addiction, 108(8), 1441–1449. doi: 10.1111/add.12169 [DOI] [PubMed] [Google Scholar]
- Hamdi NR, Krueger RF, & South SC (2015). Socioeconomic status moderates genetic and environmental effects on the amount of alcohol use. Alcoholism: Clinical and Experimental Research, 39(4), 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Iacono WG, & McGue M (2012). Index of the transmissible common liability to addiction: Heritability and prospective associations with substance abuse and related outcomes. Drug and Alcohol Dependence, 123(Suppl. 1), S18–S23. doi: S0376-8716(11)00554-0[pii]; 10.1016/j.drugalcdep.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi T (1969). Causes of delinquency. Berkeley, CA: University of California Press. [Google Scholar]
- Hujoel ML, Gazal S, Loh P-R, Patterson N, & Price AL (2020). Liability threshold modeling of case–control status and family history of disease increases association power. Nature Genetics, 52(5), 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, Deary IJ, & Iacono WG (2009). Genetic and environmental transactions underlying educational attainment. Intelligence, 37(5), 466–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellam SG, Brown CH, Poduska JM, Ialongo NS, Wang W, Toyinbo P, … Wilcox HC (2008). Effects of a universal classroom behavior management program in first and second grades on young adult behavioral, psychiatric, and social outcomes. Drug and Alcohol Dependence, 95 (Suppl 1), S5–S28. doi: S0376-8716(08)00019-7 [pii]; 10.1016/j.drugalcdep.2008.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Roysamb E, Neale MC, & Reichborn-Kjennerud T (2011). The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. American Journal of Psychiatry, 168(1), 29–39. doi: appi.ajp.2010.10030340 [pii]; 10.1176/appi.ajp.2010.10030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, & Gardner CO (2010). Interpretation of interactions: Guide for the perplexed. British Journal of Psychiatry, 197(3), 170–171. doi: 10.1192/bjp.bp.110.081331 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Ji J, Edwards AC, Ohlsson H, Sundquist J, & Sundquist K (2015a). An extended Swedish national adoption study of alcohol use disorder. JAMA Psychiatry, 72(3), 211–218. doi: 2088151 [pii]; 10.1001/jamapsychiatry.2014.2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Lönn SL, Salvatore J, Sundquist J, & Sundquist K (2018a). The origin of spousal resemblance for alcohol use disorder. JAMA Psychiatry, 75(3), 280–286. doi: doi: 10.1001/jamapsychiatry.2017.4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, & Myers J (2014). The boundaries of the internalizing and externalizing genetic spectra in men and women. Psychological Medicine, 44(3), 647–655. doi: 10.1017/S0033291713000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, & Prescott CA (1999). A population-based twin study in women of smoking initiation and nicotine dependence. Psychological Medicine, 29(2), 299–308. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10218922. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Fagan AA, Lichtenstein P, Sundquist J, & Sundquist K (2018b). Academic achievement and drug abuse risk assessed using instrumental variable analysis and co-relative designs. JAMA Psychiatry, 75(11), 1182–1188. doi: 2697855 [ pii]; 10.1001/jamapsychiatry.2018.2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Fagan AA, Lichtenstein P, Sundquist J, & Sundquist K (2020). Nature of the causal relationship between academic achievement and the risk for alcohol use disorder. Journal of Studies on Alcohol and Drugs, 81(4), 446–453. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32800080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Maes HH, Sundquist K, Lichtenstein P, & Sundquist J (2015b). A population-based Swedish twin and sibling study of cannabis, stimulant and sedative abuse in men. Drug and Alcohol Dependence, 149, 49–54. doi: 10.1016/j.drugalcdep.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Mościcki EK, Sundquist J, Edwards AC, & Sundquist K (In press; ). Genetic liability to suicide attempt, suicide death and psychiatric and substance use disorders on the risk for suicide attempt and suicide death: A Swedish national study. Psychological Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist J, & Sundquist K (2021a). Family genetic risk scores and the genetic architecture of major affective and psychotic disorders in a Swedish national sample. JAMA Psychiatry, 78, 735–743. doi: 10.1001/jamapsychiatry.2021.0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist J, & Sundquist K (2021b). The patterns of family genetic risk scores for eleven major psychiatric and substance use disorders in a Swedish national sample. Translational Psychiatry, 11(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, & Anthony JC (1997). Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry, 54(4), 313–321. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9107147. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Zhou H, Kember RL, Smith RV, Justice AC, Damrauer S, … Reid J (2019). Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nature Communications, 10(1), 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapohl E, Rimfeld K, Shakeshaft NG, Trzaskowski M, McMillan A, Pingault J-B, … Dale PS (2014). The high heritability of educational achievement reflects many genetically influenced traits, not just intelligence. Proceedings of the National Academy of Sciences, 111(42), 15273–15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringlen E, Torgersen S, & Cramer V (2001). A Norwegian psychiatric epidemiological study. American Journal of Psychiatry, 158(7), 1091–1098. doi: 10.1176/appi.ajp.158.7.1091 [DOI] [PubMed] [Google Scholar]
- Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, … Cesarini D (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics, 50(8), 1112–1121. doi: 10.1038/s41588-018-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KM, Bavarian N, Snyder FJ, Acock A, Day J, DuBois DL, … Flay BR (2012). Direct and mediated effects of a social-emotional and character development program on adolescent substance use. International Journal of Emotional Education 4(1), 56–78. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24308013. [PMC free article] [PubMed] [Google Scholar]
- Martinussen T, & Scheike TH (2006). Dynamic regression models for survival data (2006 edition ed.). New York, NY: Springer. [Google Scholar]
- Müller M, Kowalewski R, Metzler S, Stettbacher A, Rössler W, & Vetter S (2013). Associations between IQ and alcohol consumption in a population of young males: A large database analysis. Social Psychiatry and Psychiatric Epidemiology, 48(12), 1993–2005. [DOI] [PubMed] [Google Scholar]
- Prescott CA, & Kendler KS (1999). Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry, 156(1), 34–40. doi: 10.1176/ajp.156.1.34 [DOI] [PubMed] [Google Scholar]
- R Core Team (2020). R: A language and environment for statistical computing. Retrieved from https://www.R-project.org/.
- Rogne AF, Pedersen W, & Von Soest T (2021). Intelligence, alcohol consumption, and adverse consequences. A study of young Norwegian men. Scandinavian Journal of Public Health, 49(4), 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff DB, Kaminsky ZA, McIntosh AM, Smith GD, & Lohoff FW (2020). Educational attainment reduces the risk of suicide attempt among individuals with and without psychiatric disorders independent of cognition: A bidirectional and multivariable Mendelian randomization study with more than 815000 participants. Translational Psychiatry, 10(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute, Inc. (2012). SAS/STAT® Online Documentation, Version 9.4. Cary, N.C.: SAS Institute, Inc. In. (Reprinted from: Not in File). [Google Scholar]
- Scheike TH, & Zhang MJ (2011). Analyzing competing risk data using the R timereg package. Journal of Statistical Software, 38(2). Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22707920. [PMC free article] [PubMed] [Google Scholar]
- Schulenberg J, Bachman JG, O’Malley PM, & Johnston LD (1994). High school educational success and subsequent substance use: A panel analysis following adolescents into young adulthood. Journal of Health and Social Behavior, 35(1), 45–62. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8014429. [PubMed] [Google Scholar]
- Settles RE, Fischer S, Cyders MA, Combs JL, Gunn RL, & Smith GT (2012). Negative urgency: A personality predictor of externalizing behavior characterized by neuroticism, low conscientiousness, and disagreeableness. Journal of Abnormal Psychology, 121(1), 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolund S, Allebeck P, & Hemmingsson T (2012). Intelligence quotient (IQ) in adolescence and later risk of alcohol-related hospital admissions and deaths – 37-year follow-up of Swedish conscripts. Addiction, 107(1), 89–97. doi: 10.1111/j.1360-0443.2011.03544.x [DOI] [PubMed] [Google Scholar]
- Sjölund S, Hemmingsson T, & Allebeck P (2015a). IQ and level of alcohol consumption – findings from a national survey of Swedish conscripts. Alcoholism: Clinical and Experimental Research, 39(3), 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölund S, Hemmingsson T, Gustafsson J-E, & Allebeck P (2015b). IQ and alcohol-related morbidity and mortality among Swedish men and women: The importance of socioeconomic position. Journal of Epidemiology and Community Health, 69(9), 858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Woolley E, Selzam S, & Plomin R (2019). Polygenic score for educational attainment captures DNA variants shared between personality traits and educational achievement. Journal of Personality and Social Psychology, 117(6), 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambs K, Sundet JM, Magnus P, & Berg K (1989). Genetic and environmental contributions to the covariance between occupational status, educational attainment, and IQ: A study of twins. Behavior Genetics, 19(2), 209–222. [DOI] [PubMed] [Google Scholar]
- Tang Y-Y, Posner MI, Rothbart MK, & Volkow ND (2015). Circuitry of self-control and its role in reducing addiction. Trends in Cognitive Sciences, 19(8), 439–444. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, … Eaves L (1996). Genetic influences on DSM-III-R drug abuse and dependence: A study of 3,372 twin pairs. American Journal of Medical Genetics, 67 (5), 473–477. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8886164. [DOI] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, & Kendler KS (2015). The heritability of alcohol use disorders: A meta-analysis of twin and adoption studies. Psychological Medicine, 45(5), 1061–1072. doi: S0033291714002165 [pii]; 10.1017/S0033291714002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg P, Andersson T, & Bohman M (2002). Psychosocial characteristics at age 10; differentiating between adult alcohol use pathways: A prospective longitudinal study. Addictive Behaviors, 27(1), 115–130. [DOI] [PubMed] [Google Scholar]
- Zettergren P, & Bergman LR (2014). Adolescents with high IQ and their adjustment in adolescence and midlife. Research in Human Development, 11(3), 186–203. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Kristina Sundquist had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.


