Abstract
Replication-deficient adenovirus used in humans for gene therapy induces a strong immune response to the vector, resulting in transient recombinant protein expression and the blocking of gene transfer upon a second administration. Therefore, in this study we examined in detail the capsid-specific humoral immune response in sera of patients with lung cancer who had been given one dose of a replication-defective adenovirus. We analyzed the immune response to the three major components of the viral capsid, hexon (Hx), penton base (Pb), and fiber (Fi). A longitudinal study of the humoral response assayed on adenovirus particle-coated enzyme-linked immunosorbent assay plates showed that patients had preexisting immunity to adenovirus prior to the administration of adenovirus–β-gal. The level of the response increased in three patients after adenovirus administration and remained at a maximum after three months. One patient had a strong immune response to adenovirus prior to treatment, and this response was unaffected by adenovirus administration. Sera collected from the patients were assayed for recognition of each individual viral capsid protein to determine more precisely the molecular basis of the humoral immune response. Clear differences existed in the humoral response to the three major components of the viral capsid in serum from humans. Sequential appearance of these antibodies was observed: anti-Fi antibodies appeared first, followed by anti-Pb antibodies and then by anti-Hx antibodies. Moreover, anti-Fi antibodies preferentially recognized the native trimeric form of Fi protein, suggesting that they recognized conformational epitopes. Our results showed that sera with no neutralizing activity contained only anti-Fi antibodies. In contrast, neutralizing activity was only obtained with sera containing anti-Fi and anti-Pb antibodies. More importantly, we showed that anti-native Fi and anti-Pb antibodies had a synergistic effect on neutralization. The application of these conclusions to human gene therapy with recombinant adenovirus should lead to the development of strategies to overcome the formation of such neutralization antibodies, which have been shown to limit the efficacy of gene transfer in humans.
Many studies with animal models have indicated that the replication-defective recombinant adenovirus (Rec-Ad) is useful for in vivo gene transfer because it allows expression of recombinant proteins in dividing and resting cells (17, 22, 30). Rec-Ad has been used in phase I gene therapy clinical trials in patients with cystic fibrosis and lung cancer (19, 27). However, studies have shown that cellular and humoral immune responses to the vector and transgene product were involved in the transient recombinant gene expression observed in Rec-Ad-injected hosts (11, 26, 32, 33). In phase I gene therapy clinical trials in patients with lung cancer, we showed that one patient who already had high levels of neutralizing antibodies prior to Rec-Ad administration did not develop an immune response to the transgene product (4). This is of interest because Rosenecker et al. showed that cystic fibrosis patients have high levels of specific anti-Ad antibodies, suggesting a high prevalence of Ad infection in these patients (18). Thus, preexisting neutralizing antibodies reactive to surface epitopes of the virion may affect expression of the transgene product in gene therapy. Moreover, other groups have also reported that the formation of neutralizing antibodies may prevent gene transfer when a second injection of Rec-Ad is given (34, 35). The mechanism by which antibodies neutralize adenovirus is still poorly understood. Therefore, analysis of neutralizing antibodies that recognize viral proteins is necessary to shed some light on the functional basis of neutralization.
The Ad viral capsid is composed of three major types of proteins: hexons (Hx) (130 kDa), penton bases (Pb) (82 kDa), and fibers (Fi) (62 kDa). Five Pb subunits (82 kDa) form a Pb capsomere, which is linked to the trimeric Fi by noncovalent bonds (9). Three minor proteins, IIIa, VIII, and IX, are thought to stabilize the particle. The entry of human Ad into host cells involves the interaction of virus particles with two separate cell receptors. The initial binding of the virus to recently identified cell receptors is mediated by Fi protein (1, 8, 24). The subsequent event of virus infection is mediated by Pb protein binding to integrins, promoting virus internalization and/or penetration (10, 14, 29).
In this study, we examine the temporal recognition of the three major molecular components of the adenovirus capsid (Hx, Pb, and Fi) in sera of patients with lung cancer who were given a single intratumoral administration of Rec-Ad. We also show that the recognition of the three major capsid proteins differed among patients. Synergistic recognition of viral capsid proteins led to the emergence of neutralizing antibodies.
MATERIALS AND METHODS
Patients and clinical protocol.
The gene therapy approach has been described in detail elsewhere (4, 27). Briefly, Rec-Ad containing lacZ (Ad–β-gal) was administered locally by fiberoptic bronchoscopy to patients with advanced lung cancer. The patients received no chemotherapy before the administration of Ad–β-gal, but standard chemotherapy began 3 days after administration. Cohorts of patients received a single dose of 109 PFU (patients 1 through 4 [Pt-1 through Pt-4]), 108 PFU (Pt-5 through Pt-7), or 107 PFU (Pt-8 through Pt-10) of Ad–β-gal. Sera were collected on day 0 (before treatment) and on days 8, 30, 60, and 90 after the administration of Ad–β-gal.
Virus.
Rec-Ad was constructed from a modified adenovirus type 5 (Ad5) genome with the entire E1 and E3 regions deleted (23). The proteins of 19, 11.6, 10.4, 14.5, and 14.7 kDa are not synthesized, because the whole E3 region has been deleted. Almost all of these proteins are involved in modulation of the immune system (20). The Rous sarcoma virus promoter was used to drive transcription of the lacZ transgene. lacZ encodes the bacterial β-galactosidase (β-gal). Rec-Ad was prepared by infecting the 293 cell line and was purified twice by centrifugation through a CsCl gradient. The virus preparation was subjected to titer determination by a limiting-dilution plaque assay on 293 cells. The virus stock was supplied by Transgène (Strasbourg, France).
Anti-Ad antibodies detected by enzyme-linked immunosorbent assay.
Polystyrene plates (Nunc, Roskilde, Denmark) were coated by incubation with 107 PFU of Rec-Ad per well at 4°C overnight. Saturation was performed with a solution of phosphate-buffered saline (PBS) containing 0.1% Tween 20 and 3% bovine serum albumin (3). Dilutions of sera were incubated in the coated wells at 4°C overnight, and bound antibodies were detected with alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG) (1/5,000; Sigma-Aldrich Chimie). The phosphatase activity was measured with 4-methylumbelliferyl phosphate as the substrate (Sigma-Aldrich Chimie) and with fluorescence read at 360/460 nm in a Cytofluor 2300 apparatus (Millipore, Saint Quentin, France). To compare specific anti-Ad antibodies between humans, and since almost all humans have been in contact with this virus, we used serum from primates that cross-reacted with goat anti-human Ig as a negative control in each experiment. We have shown that preimmune serum from macaques does not react with Ad before immunization. Since the preimmune serum from macaques gave the same fluorescence as the fluorescence obtained in the absence of serum, the data are expressed as fluorescence units corrected for background fluorescence in the absence of serum.
Recombinant baculovirus producing Ad2 Pb and Ad5 Fi proteins.
The recombinant proteins described previously were produced by infecting Sf9 cells with the recombinant Ad2 PbFL571 (12), which codes for Pb protein, or Ad5 FiFL581 (8), which codes for fiber protein. Recombinant baculovirus Ad5 Fi-AT386 was also produced; this protein corresponds to the Fi knob domains of the Fi protein and includes the last shaft repeat. The last repeat was found to be essential for stable trimerization of the knob domain (8). The infection was performed in complete TC-100 medium (TC-100 medium [Gibco-BRL] supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 1 mM l-glutamine, and 4% heat-inactivated fetal calf serum). The cells were harvested 24 h postinfection by centrifugation for 10 min at 1,000 × g. The cell pellet was washed twice with PBS, resuspended in lysis buffer (1% Nonidet P-40, 1 mM EDTA, 150 mM NaCl, 10 mM Tris-HCl [pH 8.0]) containing protease inhibitors (phenylmethylsulfonyl fluoride, pepstatin A, leupeptin, and aprotinin), and lysed by incubation at 4°C for 30 min. The cytoplasmic proteins were then collected by centrifugation for 30 min at 100,000 × g. The extracted proteins were analyzed by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), nondenaturing SDS-PAGE (NDS-PAGE), and Western blotting (see above).
Gel electrophoresis and Western blotting.
SDS-PAGE was performed on 10% acrylamide gels (Bio-Rad, Ivry Sur Seine, France). Samples (10 μl, containing 107 PFU of Ad particles) were denatured by boiling in Laemmli buffer (62.5 mM Tris-HCl, 2% SDS, 25% glycerol, 0.01% bromophenol blue [Bio-Rad]) prior to electrophoresis. NDS-PAGE differed from SDS-PAGE in that the proteins were not denatured by boiling prior to electrophoresis; the samples were mixed with native buffer (62.5 mM Tris-HCl, 40% glycerol, 0.01% bromophenol blue; Bio-Rad). The trimeric full length of the Fi protein (Ad5 FiFL581) was visible in NDS-PAGE gels as a band of 180 kDa, and the trimeric Fi knob domains (Ad5 Fi-AT386) were visible as a band of 60 kDa (8, 15). To obtain the monomeric form of the full-length Fi protein or the knob domains of Fi, SDS-PAGE analysis was used; the proteins were 62 and 20 kDa, respectively. In SDS-PAGE gels, the Pb proteins (Ad2 PbFL571) were visible as a band of 82 kDa. After migration, the proteins were transferred to nitrocellulose membranes in 25 mM Tris–192 mM glycine buffer, containing 20% ethanol, at 100 V for 1 h with a mini-Trans-Blot system (Bio-Rad). The blots were saturated for 1 h with PBS–0.1% Tween 20 containing 5% milk. Immunoblotting was performed for Rec-Ad particles, Pb, and Fi, using different human sera and rabbit and mouse control sera. Goat anti-human total Ig and IgM (The Binding Site, Birmingham, United Kingdom), horseradish peroxidase (HRP)-labeled anti-goat IgG conjugate (The Binding Site), and a chemiluminescent substrate (luminol; Amersham, Les Ulis, France) were used to detect the reaction.
Ad neutralization assay.
Permissive 293 cells were distributed in 96-well Costar plates at 20,000 cells/well and cultured in complete Dulbecco’s modified Eagle’s medium (Gibco-BRL) for 24 h. An Ad–β-gal viral suspension (0.3 PFU/ml) was incubated with decomplemented diluted serum samples for 1 h at room temperature. This mixture (100 μl) was incubated with the 293 cells for 1 h at 37°C; 100 μl of complete Dulbecco’s modified Eagle’s medium was then added, and the cells were incubated at 37°C overnight. The cells were washed with 200 μl of PBS and incubated with 100 μl of lysis buffer (6 mM Na2HPO4, 4 mM NaH2PO4, 10 mM KCl, 0.1 mM MgSO4, 50 mM β-mercaptoethanol, 0.5% Triton X-100) containing 17 μg of a fluorescent β-gal substrate (4-methylumbelliferyl-β-d-galactoside [Sigma-Aldrich Chimie]) for 30 min to 1 h at 37°C. The resulting fluorescence was measured with a Cytofluor 2300 apparatus. Sera containing neutralizing antibodies were shown by the absence of β-gal activity in 293 lysates.
Exhaustion of anti-Pb and anti-Fi antibodies.
Human serum diluted 1/100 and decomplemented for 30 min at 56°C was exhausted from anti-Pb antibodies by incubating the serum on a nitrocellulose membrane containing the recombinant protein (Ad2 PbFL571). The serum was passed across the membrane six times, overnight for the first exhaustion and for 1 h for the five overexhaustions. For anti-Fi exhaustion, serum was mixed with the membrane containing the native or denatured form of the Fi protein (Ad5 FiFL581). One-quarter of the exhausted serum was used in an Ad neutralization assay (see above).
Purification of anti-Pb and anti-Fi antibodies.
The serum diluted 1/100 and decomplemented for 30 min at 56°C was mixed overnight at 4°C with a nitrocellulose membrane containing Pb or Fi protein. After incubation, the membranes were intensively washed with a PBS–0.1% Tween 20 solution. The antibodies were eluted with 480 μl of an elution buffer (0.1 M glycine-HCl [pH 2.7]) for a few seconds, and the solution was neutralized with 20 μl of 1 M Tris-HCl (pH 9). The purified antibodies (1/10 of the eluted antibodies) were tested in a neutralization assay.
Densitometry.
The film obtained from Western blot experiments was scanned with a scanner, and the signal intensity was measured with the Image Quant program (Molecular Dynamics).
RESULTS
Humoral response to a single intratumoral administration of Rec-Ad particles in patients.
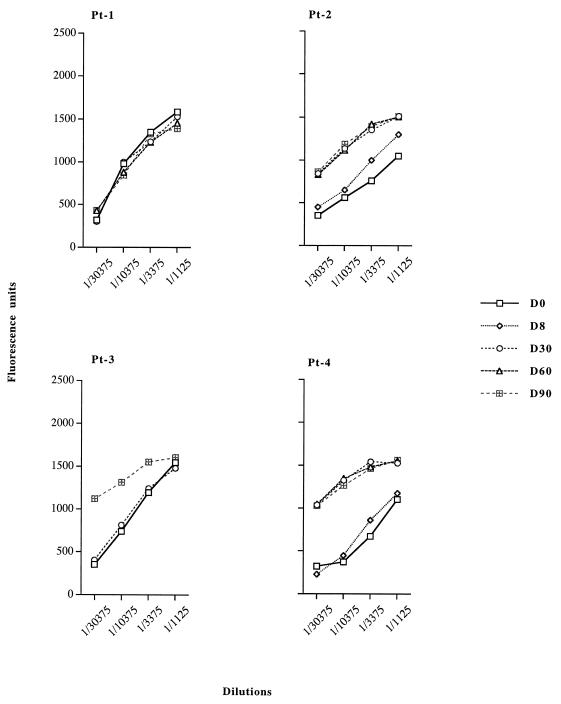
Blood samples were collected from four patients (Pt-1 through Pt-4) with lung cancer given 109 PFU of Ad–β-gal on day 0 (pretreatment) and on days 30, 60, and 90 (postadministration) and were assayed for anti-Ad antibodies by enzyme-linked immunosorbent assay (Fig. 1). A longitudinal study of the anti-Ad humoral response showed that all the patients had a preexisting immune response to Ad prior to administration. The level of specific IgG to Ad in Pt-1 was high before treatment and was unaffected by Ad–β-gal administration. In the other patients, the level of anti-Ad IgG increased strongly after administration of Ad–β-gal and remained maximal 3 months later, as shown by assays on Rec-Ad particle-coated plates. Although Rec-Ad did not enable proliferation to occur (because of deletions in E1A and E1B), the single intratumoral injection of Ad produced very strong humoral immune responses.
FIG. 1.
Specific IgG response to Rec-Ad particles. Humans blood samples were collected 8 (D8), 30 (D30), 60 (D60), and 90 (D90) days after Rec-Ad administration. Serum dilutions were tested on Rec-Ad-coated plates (107 PFU/well). Data are expressed as fluorescence units corrected for background fluorescence in the absence of serum. Sera collected from humans on day 0 (D0) indicated the immunization state against Ad before the injection.
Humoral response to viral capsid proteins.
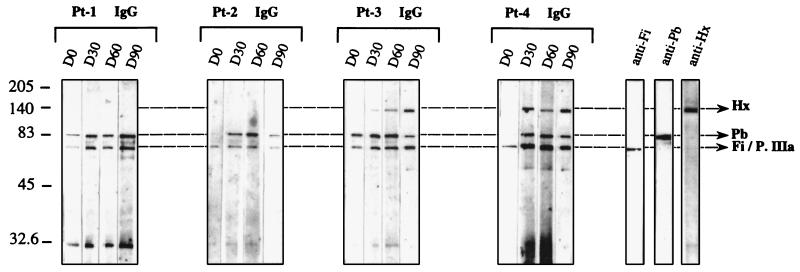
The molecular basis of the humoral immune response was examined in greater detail by assaying sera collected from patients for antiviral protein antibodies by Western blotting with the entire Rec-Ad particle. This analysis revealed the recognition of each individual viral capsid protein, unlike results obtained with Rec-Ad particle-coated plates, which showed only an overall response to the vector. The results from four patients (Pt-1 through Pt-4) are presented in detail (Fig. 2), but the experiments were also performed with sera from six other patients. There were clear differences in humoral responses to the three major components (Hx, Pb, and Fi) of the viral capsid in the sera. The protein identified as Fi protein may be the penton-associated protein (66 kDa) called protein IIIa (9), in which case the Fi protein was associated with protein IIIa (Fi/P.IIIa). Anti-Fi/P.IIIa and anti-Pb antibodies were detected in the sera of Pt-1 and Pt-3 before administration of Rec-Ad (Fig. 2). Serum collected on day 0 from three other patients gave the same results (Pt-6 through Pt-8 [data not shown]). Serum collected on day 0 from Pt-2 and Pt-4 contained only specific anti-Fi/P.IIIa IgG antibodies (Fig. 2), as did serum from two other patients (Pt-9 and Pt-10).
FIG. 2.
Immunoblot pattern of serum specific for Ad capsid components. Rec-Ad (107 PFU/well) was deposited, and the viral proteins were separated by SDS-PAGE. Immunoblots were incubated with the different sera, collected on day 0 (D0) to day 90 (D90), diluted at 1/100, and washed with HRP-labeled anti-human Ig conjugate. The capsid proteins Hx, Pb, and Fi are indicated. The controls contained rabbit anti-Fi, rabbit anti-Pb, and mouse anti-Hx antibodies.
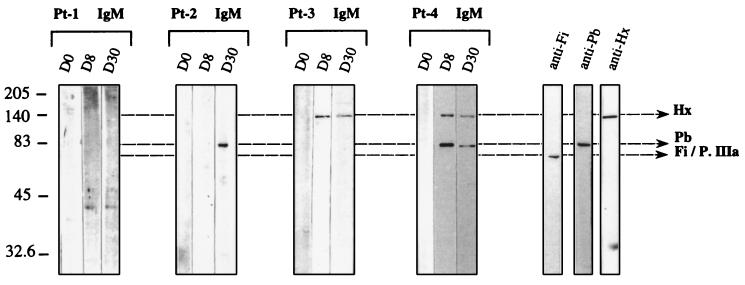
Serum collected from Pt-1 before the administration of Rec-Ad contained IgG antibodies that recognized the Fi/P.IIIa and Pb capsid proteins, and the injection did not modify the recognition of viral proteins. Sera collected from three patients (Pt-6 through Pt-8) before or after the administration of 108 or 107 PFU of Ad–β-gal contained IgG that recognized the two major capsid components, as did serum from Pt-1 (data not shown). Sera collected from Pt-2 through Pt-4 on day 30 after the injection showed a change in the pattern of recognition of capsid components. Pt-2 had anti-Fi/P.IIIa and anti-Pb antibodies after treatment. Another patient (Pt-9) had the same recognition profile as Pt-2 (data not shown). Pt-3 and Pt-4 developed humoral responses to all the major molecular components of the Ad capsid (Fi/P.IIIa, Pb, and Hx). Specific anti-Pb IgM was detected on day 8 (Pt-4) and on day 30 (Pt-2) in Pt-2 and Pt-4. We also found specific anti-Hx IgM on days 8 and 30 in serum from Pt-3 and Pt-4 (Fig. 3). All the patients had previously been exposed to a related human Ad because their sera contained anti-Fi antibodies. However, new contact with the Ad after intratumoral administration could induce IgM antibodies against viral proteins. The failure to detect Hx and Pb antibodies in some patients before the administration of Ad–β-gal can be an indication of a decayed immunity.
FIG. 3.
Pattern of IgM specific for Ad capsid components. Sera collected on different days (D0, D8, and D30) and diluted at 1/100 were tested for the presence of IgM antiviral proteins after immunoblotting of Rec-Ad (107 PFU/well). After overnight incubation of immunoblots with the diluted sera, the proteins were detected with sheep anti-human IgM (1/500) and HRP-labeled anti-sheep IgG conjugate (1/2,000).
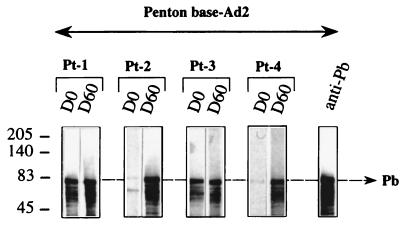
Recognition of recombinant Pb protein.
To confirm the absence of recognition of Pb protein by sera from Pt-2 and Pt-4 before injection and to ensure that it was not due to a limiting quantity of antigenic Pb proteins, recombinant Ad-2 Pb proteins were used in a Western blot analysis (Fig. 4). Note that the baculovirus recombinant Pb used came from Ad2 (12), whereas the recombinant Ad injected was Ad5, but these two proteins had 99% identity. The sera from Pt-1 and Pt-3 contained anti-Pb antibodies before injection (Fig. 4); these antibodies were also detected in the sera from three other patients (Pt-6 through Pt-8 [data not shown]). The absence of anti-Pb IgG antibodies from the sera of Pt-2 and Pt-4 was confirmed in this experiment and was also found with Pt-5 (data not shown). Three other patients (Pt-2, Pt-3, and Pt-6) who were tested for their capacity to recognize the recombinant Pb protein gave the same result as Pt-2 and Pt-4. Densitometry analysis with the Image Quant program (Molecular Dynamics) determined a significant difference between positive and negative sera in terms of Pb protein recognition. This result also showed that anti-Pb antibodies recognized the monomeric form of the Pb protein.
FIG. 4.
Immunoblot pattern of serum specific for Pb. Suspensions of recombinant Pb protein (10 μl) harvested from infected Sf9 cells with the recombinant Ad2 PbFL571 were separated by SDS-PAGE. Sera (diluted 1/100) collected from patients at different time points (day 0 [D0] and day 60 [D60]) were tested and revealed with a sheep HRP-labeled anti-human Ig conjugate (1/2,000).
Recognition of native trimeric recombinant Fi protein.
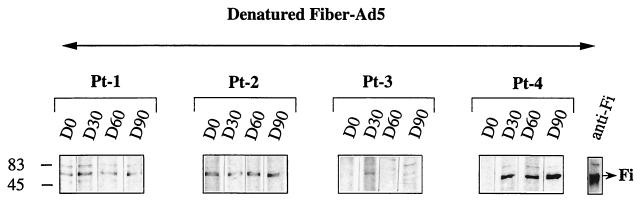
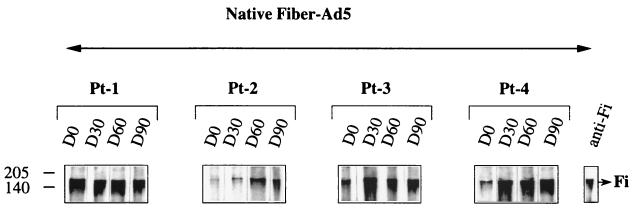
To precisely identify and determine the quality of the anti-Fi antibody response in human serum, the recombinant baculovirus Ad5 Fi was used in Western blots. To obtain monomeric Fi protein (62 kDa), SDS-PAGE analysis was required. Figure 5 shows that anti-Fi antibodies from sera of Pt-1 through Pt-3 weakly recognized the monomeric form of the Fi proteins (62 kDa) before the treatment. After administration of Rec-Ad, only serum from Pt-4 strongly recognized the denatured form of the Fi protein. The trimeric nature of the Fi synthesized in vitro was conserved, as shown in NDS-PAGE analyses (12, 15). The Fi trimers were visible as a 180-kDa band. Figure 6 shows that anti-Fi antibodies from sera of patients preferentially recognized the native trimeric form of the fiber protein. Pt-1 and Pt-3 presented a high level of anti-native Fi antibodies on day 0 (as did Pt-6 through Pt-8 [data not shown]). The sera of Pt-2 and Pt-4 recognized the native Fi proteins weakly (as did sera of Pt-9 and Pt-10). Injection led to maturation of the humoral response, and the amount of anti-native Fi antibodies increased with time and become maximal on days 30 (Pt-4) and 60 (Pt-2). The sera from Pt-9 and Pt-10 also contained anti-native Fi antibodies on day 30 after the injection, but the serum from patient 5 did not contain anti-Fi antibodies (data not shown).
FIG. 5.
Immunoblot pattern of serum specific for monomeric Fi protein. Suspensions (20 μl) of recombinant Fi protein (Ad5 FiFL581) were separated by SDS-PAGE. Sera (collected on day 0 [D0] to day 90 [D90]) were tested at a dilution of 1/100, and Fi was detected with HRP-labeled anti-human Ig conjugate (1/2,000). The monomeric form of the Fi protein was detected as a band of 62 kDa.
FIG. 6.
Immunoblot pattern of serum specific for trimeric Fi protein. Suspensions (20 μl) of recombinant Fi protein (Ad5 FiFL581) were separated by NDS-PAGE. Sera (collected on day 0 [D0] to day 90 [D90]) were tested as described in the legend to Fig. 4. The trimeric form of the Fi protein was detected as a band of 180 kDa.
Recognition of native trimeric Fi knob domains.
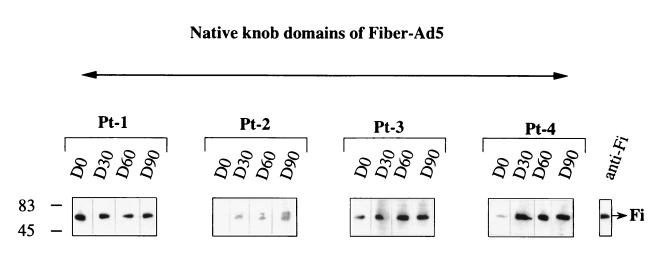
The part of the native Fi protein recognized by human serum was identified with a recombinant baculovirus Ad5 Fi-AT386 corresponding to the Fi knob domains of the Fi protein, which included the last shaft repeat. The last repeat was found to be essential for stable trimerization of the knob domain. The trimeric Fi knob domains were visible as a band of 60 kDa on NDS-PAGE (8). Figure 7 shows that anti-Fi antibodies from the sera recognized the native trimeric form of the Fi knob domains. There was also a direct correlation between the capacity to recognize the native trimeric form of the Fi protein and recognition of the native trimeric knob of the Fi protein. The results showed that anti-Fi antibodies recognizing conformational epitopes were essentially directed against the C-terminal part of the protein, i.e., the Fi knob domain.
FIG. 7.
Immunoblot pattern of serum specific for trimeric Fi knob domains. Suspensions (20 μl) of recombinant Ad5 Fi knob domains (Ad5 Fi-AT386) were separated by NDS-PAGE. The size of the protein was 60 kDa. The sera were tested as described in the legend to Fig. 4. D0 to D90, days 0 to 90, respectively.
Induction of neutralizing antiadenovirus antibodies after a single administration of Ad–β-gal.
Sera taken from patients before and after the Ad–β-gal administration were examined for neutralizing antibodies to Ad. Table 1 presents significant results obtained from sera of patients. The sera from Pt-1 and Pt-3 contained significant amounts of neutralizing antibodies prior to the Rec-Ad administration. In addition, the sera from three other patients (Pt-6 through Pt-8) had significant neutralizing activity (data not shown). Sera from Pt-2, Pt-4, and Pt-5 contained no neutralizing activity on day 0, like sera from Pt-9 and Pt-10 (data not shown). After the treatment, sera from all patients tested except one (Pt-5) had neutralizing antibodies. The administration of Ad–β-gal acted like a boost for patients whose serum contained significant amounts of neutralizing antibodies before the treatment, and the titers of these antibodies increased. The results also showed a direct relation between neutralization and the level of recognition of the Pb and the native Fi proteins (Table 2). Patients with neutralizing antibodies before the Rec-Ad injection (Pt-1 and Pt-3) recognized the two capsid proteins. The appearance of neutralizing antibodies in Pt-2 and Pt-4 was directly related to the presence of antiviral capsid protein antibodies. Pt-5, who did not develop neutralizing antibodies, did not recognize the two viral proteins. In conclusion, the greater the neutralizing activity, the better the recognition of the Pb protein and the native trimeric form of the Fi protein.
TABLE 1.
Anti-Ad neutralizing antibodies in sera of humans injected with Rec-Ad
| Time of collectiona | % Neutralizationb in serum from:
|
||||
|---|---|---|---|---|---|
| Pt-1c | Pt-2c | Pt-3c | Pt-4c | Pt-5d | |
| D0 | 100 | 0 | 42 | 0 | 0 |
| D30 | 100 | 50 | 100 | 40 | 0 |
| D60 | 100 | 52 | 100 | 20 | 0 |
Sera of patients were collected before (D0) and on days 30 (D30) and 60 (D60) after a single injection of Rec-Ad.
Neutralization obtained with a 1/200 serum dilution.
Given 109 PFU of Ad–β-gal.
Given 108 PFU of Ad–β-gal.
TABLE 2.
Relation between neutralization and the recognition of Pb and native Fi proteins
| Patient | Time of collectiona | % Neutralizationb | Recognition of:
|
|
|---|---|---|---|---|
| Pb | Native Fi | |||
| Pt-1c | D0 | 100 | ++++ | ++++ |
| D30 | 100 | ++++ | ++++ | |
| D60 | 100 | ++++ | ++++ | |
| Pt-2c | D0 | 0 | +/− | + |
| D30 | 50 | +++ | +++ | |
| D60 | 52 | +++ | +++ | |
| Pt-3c | D0 | 42 | ++++ | +++ |
| D30 | 100 | ++++ | ++++ | |
| D60 | 100 | ++++ | ++++ | |
| Pt-4c | D0 | 0 | +/− | ++ |
| D30 | 40 | ++ | ++++ | |
| D60 | 20 | ++ | ++++ | |
| Pt-5d | D0 | 0 | − | +/− |
| D30 | 0 | − | +/− | |
| D60 | 0 | +/− | + | |
D0, before injection of Rec-Ad; D30 and D60, 30 and 60 days after injection of Rec-Ad.
Neutralization obtained at 1/200 serum dilution.
Given 109 PFU of Ad–β-gal.
Given 108 PFU of Ad–β-gal.
Direct involvement of anti-Pb antibodies in neutralizing activity.
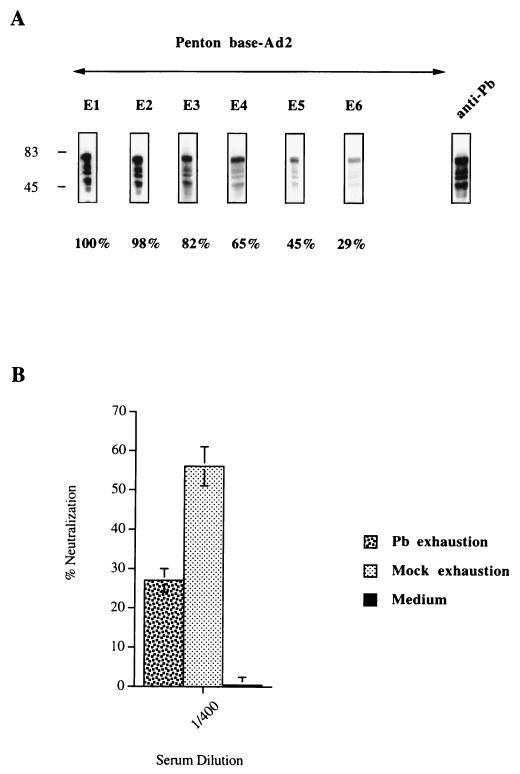
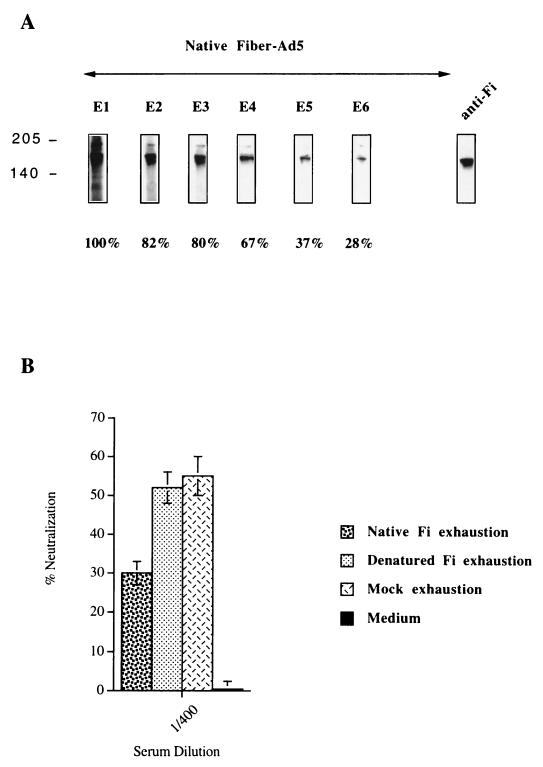
The differences observed in neutralizing activity may be due to differences in the ability of sera to recognize capsid proteins. Patient sera containing anti-Pb antibodies (Pt-1) were used to test the capacity of these antibodies to directly affect the neutralization activity observed. Serum diluted 1/100 was partially exhausted of anti-Pb antibodies by sequential incubation with Pb proteins. Figure 8A shows the sequential exhaustion of anti-Pb antibodies after six different contacts with the Pb protein. The decrease in the signal was determined by densitometry with the Image Quant program; 71% of the signal was lost after the final exhaustion. The depleted serum was then tested for its capacity to neutralize the virus. The diluted serum partially exhausted of anti-Pb antibodies (1/400) had lost much of its capacity to neutralize the virus compared to the control (Fig. 8B).
FIG. 8.
Role of anti-Pb antibodies in neutralizing activity. (A) The recombinant Pb proteins were separated by SDS-PAGE. The immunoblot was sequentially incubated with serum (Pt-1, day 60) diluted 1/100. Sequential exhaustion on the Pb protein was detected with HRP-labeled anti-human Ig conjugate. The percentage of anti-Pb antibodies exhausted from the serum was determined after quantification by the Image Quant program. Densitometry analysis was done in the linear response range of the film. After the final exhaustion (E6), we detected only 29% of the initial signal. (B) The partially exhausted serum (1/400) was tested for its capacity to neutralize the viral particle. The same serum was sequentially exhausted on mock proteins (six times) and was tested in the same way for the presence of anti-Ad neutralizing antibodies. The neutralizing activities are given as percentages and are the means and standard deviations of three individual triplicate experiments.
Direct involvement of anti-native trimeric Fi antibodies in neutralizing activity.
The same serum from Pt-1 containing anti-Fi antibodies was used to test the capacity of these antibodies to play a direct role in neutralizing activity. Human serum diluted 1/100 was partially exhausted of anti-Fi antibodies by sequential incubation with Fi proteins. The depletion was performed by NDS-PAGE to conserve the native trimeric form of the Fi protein and by SDS-PAGE to produce the Fi monomeric form. Figure 9A shows the sequential exhaustion, on native trimeric Fi protein, of diluted serum after six independent contacts with the native protein. The loss of the signal was measured by densitometry and indicated partial (but significant) exhaustion of anti-trimeric Fi antibodies. Figure 9B shows the percent neutralization obtained with serum exhausted on the native trimeric form or the monomeric form of Fi protein and on mock protein. The partial depletion (72%) of antibodies recognizing the native trimeric form of Fi proteins significantly decreased the neutralizing activity.
FIG. 9.
Role of conformational anti-Fi antibodies in neutralizing activity. (A) The recombinant Fi proteins were separated by NDS-PAGE to conserve the trimeric form of the protein. The diluted serum (1/100) from patient 1 at D60 was sequentially incubated (six times) on immunoblots. Sequential exhaustion on the Fi protein was detected with HRP-labeled anti-human Ig conjugates. The percentage of the decrease in the initial signal was determined by densitometry analysis done in the linear response range of the film. (B) The neutralizing activity toward Ad in serum partially exhausted of anti-native Fi antibodies was determined with serum diluted at 1/400. Likewise, the serum from Pt-1 was sequentially exhausted on denatured Fi proteins (SDS-PAGE) and on mock proteins. The exhausted serum was used to determine the neutralizing activity. The neutralizing activities are given as percentages and are the means and standard deviations of three individual triplicate experiments.
Synergistic effect of anti-Fi and anti-Pb antibodies on viral neutralization.
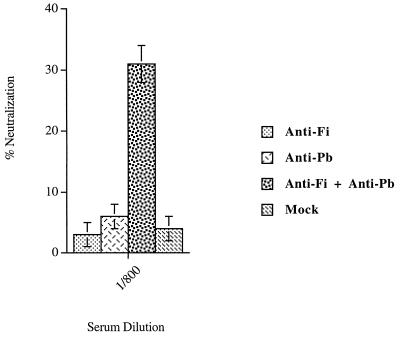
The direct effect of anti-Fi and anti-Pb antibodies on neutralizing activity was determined with the two kinds of antibodies purified independently from sera from Pt-1 and Pt-3 (day 30). The anti-Fi antibodies recognizing conformational epitopes were obtained by passing the serum (diluted 1/100) over a nitrocellulose membrane containing the native trimeric form of the Fi protein. The anti-Pb antibodies were similarly purified from the same diluted serum by using a membrane containing the recombinant Pb protein. The purified antibodies were eluted from the membrane, and one-quarter of the purified antibody solution was used in an Ad neutralization assay. We showed that to obtain viral neutralization, a mixture of anti-Fi and anti-Pb antibodies from Pt-1 serum was necessary (Fig. 10). This result was confirmed with the purified anti-Fi and anti-Pb antibodies from the serum of Pt-3 (data not shown). The results demonstrated a synergistic effect between the conformational anti-Fi antibodies and the anti-Pb antibodies in the Ad-neutralizing activity.
FIG. 10.
Synergistic effect of anti-native Fi and Pb antibodies on neutralizing activity. The anti-native Fi and anti-Pb antibodies were purified after incubation of serum from Pt-1 (diluted 1/100) on immunoblots containing trimeric native Fi proteins or Pb proteins. The purified antibodies were used to determine the neutralizing activity. Human anti-native Fi antibodies (anti-Fi), anti-Pb antibodies (anti-Pb), and the mixture of the two antibodies (anti-Fi + anti-Pb) were used in the neutralization experiments. A negative control experiment was performed with mock proteins. The same serum was incubated on immunoblots containing mock proteins. After being washed, the membrane was incubated with an elution solution. The solution (mock) was tested for neutralizing activity. The neutralizing activities are given as percentages and are the means and standard deviations of two individual triplicate experiments.
DISCUSSION
In this study, we examined in detail the Ad-specific humoral immune response induced in patients with lung cancer given a single dose of Rec-Ad by fiberoptic bronchoscopy (18). Assays of the anti-Ad response before the administration of Ad–β-gal, on Rec-Ad particle-coated plates, showed that the patients had preexisting immunity to adenovirus. After Rec-Ad administration, three patients developed strong immunity to Ad, which remained at a maximum after 3 months. Pt-1 had strong immunity to Ad before the administration of Rec-Ad, and this was unaffected by the treatment. Although Rec-Ad did not replicate, a single administration of Ad induced a very strong humoral immune response. This result is consistent with our previous findings in animal models (3).
Recognition of the different capsid proteins was analyzed by Western blotting to determine more precisely the molecular basis of the humoral immune response. This analysis made it possible to detect the recognition of each individual viral capsid protein, unlike the results obtained with Rec-Ad particle-coated plates, which showed an overall response to the vector. It is difficult to differentiate between Fi protein (62 kDa) and protein IIIa (66 kDa) recognition by the sera. Before administration of Ad–β-gal, sera from Pt-1 and Pt-3 recognized two capsid proteins, Fi/P.IIIa and Pb, and sera from Pt-2 and Pt-4 recognized only the Fi/P.IIIa protein. Sera from Pt-2, Pt-3, and Pt-4 gave a complex response after injection. Specific anti-Pb IgG appeared in Pt-2 and Pt-4 sera, and specific anti-Hx IgG antibodies appeared in Pt-3 and Pt-4 sera. The heterogeneity of the response to the capsid proteins was also assayed with the sera of six other patients (data not shown). Molecular analysis was required to better understand the humoral immune response to capsid components, because of this complex response. Therefore, recombinant Pb and Fi proteins were used. The use of recombinant Fi protein made it possible to differentiate between Fi and P.IIIa recognition by the sera. More importantly, the results of this study demonstrate that anti-Fi antibodies were composed essentially of antibodies recognizing conformational epitopes of the trimeric form of the Fi protein. There was a direct correlation between the capacity of a serum to recognize the native trimeric form of the Fi protein and the recognition of the native trimeric knob of the Fi protein. Thus, the anti-Fi antibodies recognizing conformational epitopes were essentially directed toward the C-terminal part of the protein, i.e., the knob domain, which is directly implicated in cell receptor recognition (8). There were clear differences in the humoral responses of patients to the three major components of the viral capsid. The sera collected at various times from patients given Rec-Ad had more complex responses to viral antigens than were previously found in mice (3); anti-Fi antibodies appeared first, followed by anti-Pb antibodies, and anti-Hx antibodies appeared last. Most human sera recognized only two major capsid proteins, the native trimeric Fi protein and the monomeric Pb protein.
Neutralizing activity was measured in vitro before and after Rec-Ad administration. Pt-1 and Pt-3 had anti-Ad neutralizing antibodies on day 0, whereas no activity was detectable in the other three patients (Pt-2, Pt-4, and Pt-5). After administration of Ad–β-gal, all the patients except Pt-5 had neutralizing activity in their serum. The differences in neutralizing activity were due to differences in their ability to recognize the capsid proteins. Some studies suggest that anti-Hx antibodies have a greater neutralizing effect whereas anti-Fi antibodies only prevent attachment, and any neutralization appears to be due to aggregation (16, 25, 31). Our results show that before Ad administration, the sera of two patients (Pt-2 and Pt-4) with only anti-Fi antibodies had no neutralizing activity whereas the sera of two other patients (Pt-1 and Pt-3), with high levels of anti-Fi and anti-Pb antibodies, had significant neutralizing activity. After administration, the Fi and Pb capsid proteins were also recognized by sera from Pt-2 and Pt-4, and the sera of these patients contained neutralizing activity. Similar results were obtained with sera from nine other patients (data not shown).
To confirm the importance of anti-Pb and anti-Fi antibodies in the neutralizing activity observed, we exhausted the serum of one of these. The partial exhaustion of anti-Pb or anti-Fi antibodies resulted in a decrease in neutralization activity. Only anti-Fi antibodies that recognized conformational epitopes of the native trimeric Fi proteins were implicated. Our results also show that anti-Fi and anti-Pb antibodies have a synergistic effect in neutralization. These components are essential for virus infection, because the Fi proteins are implicated in virus attachment and the Pb protein is involved in internalization of the virus by binding to the cell integrin receptor.
The binding of Pb to cell integrins can be inhibited by soluble synthetic Arg-Gly-Asp (RGD) peptides. The RGD sequence is conserved within a highly variable region in the Pb protein of four Ad serotypes. The RGD epitope has recently been shown to escape antibody neutralization (21). Steric hindrance from the Fi and a few bound IgG molecules probably prevents IgG binding to all RGD sites on the Pb within the intact virus (21). Greber et al. showed that penetration of Ad particles into cells requires a stepwise disassembly program (7). The Fi proteins are released, the Pb proteins are dissociated, and the other proteins are degraded or shed. These authors also showed that, although dissociated from the rest of the virus, the Fi proteins remained associated with the cell membrane. They proposed that the Fi proteins released from the Ad particle are essential for virus endocytosis, after the Pb-integrin interaction (7). Boudin and Boulanger showed that Ad penton capsomers could be dissociated into its two constituents, Pb and Fi. This Ad penton capsomer dissociation was obtained with the anti-Fi antibody but not with the anti-Pb antibody (2). In conclusion, these observations suggest that the Ad particles may be uncoated by strong Fi-antibody or Fi-receptor interactions, leading to release of the Fi proteins and making the Pb proteins accessible to anti-Pb neutralizing antibodies.
Since the Rec-Ad was administered directly into the patients’ lungs, it should be interesting to determine mucosal immunity. It has previously been shown that lymphocytes obtained from bronchoalveolar lavage secrete both interleukin-2 and interleukin-4, indicating that Th1 and Th2 cells are activated in the airways (33). The profile of cytokines produced by bronchoalveolar lymphocytes before and after Rec-Ad administration should help us to better understand the local tumor-specific immune response in these patients.
Mucosal antibodies to Ad and transgene product should also be induced in the lungs of these patients, because it had been shown that recombinant Ad administered intranasally to mice induces secretory IgA specific for the transgene product (5). Gallichan et al. also showed that mucosal immunization of mice with a recombinant Ad induces both mucosal and systemic immune responses. Similarly, Van Ginkel et al. showed that intratracheal administration of Ad–β-gal results in high levels of systemic IgG and mucosal IgA antibodies to Ad and β-gal product. The sera collected from these mice had neutralization activity (28). In preliminary experiments, we detected neutralizing antibodies and IgG antibodies recognizing Ad capsid components in the bronchoalveolar lavage fluid of these patients. Thus, the levels of neutralizing antibodies and the specific antiviral antigen IgG in the serum seem to reflect the humoral response in bronchoalveolar lavage fluid. The mucosal IgA response is also a critical point and is still under investigation.
In conclusion, since the formation of neutralizing antibodies may prevent gene transfer when recombinant Ad is administered (4, 34, 35), immunomodulation of the immune system could be a strategy to modify the Ad immune response. We have shown that the transgene product expression can be prolonged by treating the rodents with cyclosporin (6). Llan et al. showed that oral tolerization to Ad antigens permits long-term expression of the transgene product when Rec-Ad vectors are used (13). The development of anti-Ad neutralizing antibodies and cytotoxic lymphocytes was markedly inhibited in the tolerant rats, but not in the control rats. A better understanding of the mechanisms by which antibodies neutralize Ad should permit us to modulate the immune system and adapt it for gene therapy.
ACKNOWLEDGMENTS
We thank Transgène (Strasbourg, France) for providing Ad–β-gal, and we thank C. Trancrede, P. Saulnier, and E. Gautier for providing the human sera.
This study was supported by grants from the Institut National de la Santé et la Recherche Médical (INSERM), from the Association pour la Recherche contre le Cancer (ARC), La Ligue contre le cancer, the CRTG from Institut Cochin de Génétique Moléculaire (ICGM), and the Association Française contre les myopathies (AFM). D. Godfrin is a recipient of a Ministère de la Recherche et la Technologie (MRT) doctoral fellowship, and H. Gahéry-Ségard is supported by a fellowship from ECS (Ensemble Contre le SIDA, Sidaction).
REFERENCES
- 1.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 2.Boudin M-L, Boulanger P. Antibody-triggered dissociation of adenovirus penton capsomer. Virology. 1981;113:781–786. doi: 10.1016/0042-6822(81)90208-7. [DOI] [PubMed] [Google Scholar]
- 3.Gahéry-Ségard H, Juillard V, Gaston J, Lengagne R, Pavirani A, Boulanger P, Guillet J-G. Humoral response to the capsid components of recombinant adenoviruses: routes of immunization modulate virus-induced Ig subclass shifts. Eur J Immunol. 1997;27:653–659. doi: 10.1002/eji.1830270312. [DOI] [PubMed] [Google Scholar]
- 4.Gahéry-Ségard H, Molinier-Frenkel V, Le Boulaire C, Saulnier P, Opolon P, Lengagne R, Gautier E, Le Cesne A, Zitvogel L, Venet A, Schatz C, Courtney M, Le Chevallier T, Tursz T, Guillet J-G, Farace F. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J Clin Invest. 1997;100:2218–2226. doi: 10.1172/JCI119759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallichan W S, Johnson D C, Graham F L, Rosenthal K L. Mucosal immunity and protection after intranasal immunization with recombinant adenovirus expressing herpes simplex virus glycoprotein B. J Infect Dis. 1993;168:622–629. doi: 10.1093/infdis/168.3.622. [DOI] [PubMed] [Google Scholar]
- 6.Gilgenkrantz H, Duboc D, Juillard V, Couton D, Pavirani A, Guillet J-G, Briand P, Kahn A. Transient expression of genes transferred in vivo into heart using first-generation adenoviral vectors: role of the immune response. Hum Gene Ther. 1995;6:1265–1274. doi: 10.1089/hum.1995.6.10-1265. [DOI] [PubMed] [Google Scholar]
- 7.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 8.Hong S S, Karayan L, Tournier J, Curiel D T, Boulanger P A. Adenovirus type 5 knob binds to MHC class I α2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz M S. Adenoviridae and their replication. In: Fields B N, editor. Virology. New York, N.Y: Raven Press; 1990. pp. 1679–1721. [Google Scholar]
- 10.Huang S, Endo R I, Nemerow G R. Upregulation of integrins avb3 and avb5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juillard V, Villefroy P, Godfrin D, Pavirani A, Venet A, Guillet J-G. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur J Immunol. 1995;25:3467–3473. doi: 10.1002/eji.1830251239. [DOI] [PubMed] [Google Scholar]
- 12.Karayan L, Gay B, Gerfaux J, Boulanger P A. Oligomerization of recombinant penton base of adenovirus type 2 and its assembly with fiber in baculovirus-infected cells. Virology. 1994;202:782–795. doi: 10.1006/viro.1994.1400. [DOI] [PubMed] [Google Scholar]
- 13.Llan Y, Prakash R, Davidson A, Jona V, Droguett G, Hortwitz M S, Roy Chowdhury N, Roy Chowdhury J. Oral tolerization to adenoviral antigens permits long-term gene expression using recombinant adenoviral vectors. J Clin Invest. 1997;99:1098–1106. doi: 10.1172/JCI119238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novelli A, Boulanger P A. Deletion analysis of functional domains in baculovirus-expressed adenovirus type 2 fiber. Virology. 1991;185:365–376. doi: 10.1016/0042-6822(91)90784-9. [DOI] [PubMed] [Google Scholar]
- 16.Philipson L, Lonberg-Holm K, Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968;2:1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quantin B, Perricaudet L D, Tajbakhsh S, Mandel J-L. Adenovirus as an expression vector in muscle cells in vivo. Proc Natl Acad Sci USA. 1992;89:2581–2584. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenecker J, Harms K-H, Bertele R M, Pohl-Koppe A, Mutius E V, Adam D, Nicolai T. Adenovirus infection in cystic fibrosis patients: implications for the use of adenoviral vectors for gene transfer. Infection. 1996;24:5–8. doi: 10.1007/BF01780642. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld M A, Yoshimura K, Trapnell B C, Yoneyama K, Rosenthal E R, Dalemans W, Fukayama M, Bargon J, Stier L E, Stratford-Perricaudet L, Perricaudet M, Guggino W B, Pavirani A, Lecocq J-P, Crystal R G. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 20.Sparer T E, Tripp R A, Dillehay D L, Hermiston T W, Wold W S M, Gooding L R. The role of human adenovirus early region 3 proteins (gp19K, 10.4K, 14.5K, and 14.7K) in a murine pneumonia model. J Virol. 1996;70:2431–2439. doi: 10.1128/jvi.70.4.2431-2439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart P L, Chiu C Y, Huang S, Muir T, Zhoa Y, Chait B, Mathias P, Nemerow G R. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO J. 1997;16:1189–1198. doi: 10.1093/emboj/16.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stratford-Perricaudet L D, Levrero M, Chasse J F, Perricaudet M, Briand P. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther. 1990;1:241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- 23.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toogood C I A, Crompton J, Hay R T. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J Gen Virol. 1992;73:1429–1435. doi: 10.1099/0022-1317-73-6-1429. [DOI] [PubMed] [Google Scholar]
- 26.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 27.Tursz T, Le Cesne A, Baldeyrou P, Gautier E, Opolon P, Schatz C, Pavirani A, Courtney M, Lamy D, Ragot T, Saulnier P, Andremont A, Monier R, Perricaudet M, Le Chevalier T. Phase I study of a recombinant adenoviral-mediated gene transfer in lung cancer patients. J Natl Cancer Inst. 1997;88:1857–1863. doi: 10.1093/jnci/88.24.1857. [DOI] [PubMed] [Google Scholar]
- 28.Van Ginkel F W, Liu C, Simecka J W, Dong J Y, Greenway T, Frizzell R A, Kiyono H, McGhee J R, Pascual D W. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and β-galactosidase. Hum Gene Ther. 1995;6:695–903. doi: 10.1089/hum.1995.6.7-895. [DOI] [PubMed] [Google Scholar]
- 29.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 30.Wilson J M. Vehicles for gene therapy. Nature. 1993;365:691–692. doi: 10.1038/365691a0. [DOI] [PubMed] [Google Scholar]
- 31.Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988;62:2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Hildegund C J E, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Trinchieri G, Wilson J M. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-890. [DOI] [PubMed] [Google Scholar]
- 35.Zabner J, Ramsey B W, Meeker D P, Aitken M L, Balfour R, Gilson R L, Launspach J, Moscicki R A, Richards S M, Standaert T A, Williams-Warren J, Wadsworth S C, Smith A, Welsh M J. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J Clin Invest. 1996;97:1504–1511. doi: 10.1172/JCI118573. [DOI] [PMC free article] [PubMed] [Google Scholar]