Abstract
Background:
The association between prenatal household air pollution (HAP) exposure and childhood blood pressure (BP) is unknown.
Objective:
Within the Ghana Randomized Air Pollution and Health Study (GRAPHS) we examined time-varying associations between a) maternal prenatal and b) first-year-of-life HAP exposure with BP at 4 years of age and, separately, whether a stove intervention delivered prenatally and continued through the first year of life could improve BP at 4 years of age.
Methods:
GRAPHS was a cluster-randomized cookstove intervention trial wherein pregnant women were randomized to one of two stove interventions: a) a liquefied petroleum gas (LPG) stove or improved biomass stove, or b) control (open fire cooking). Maternal HAP exposure over pregnancy and child HAP exposure over the first year of life was quantified by repeated carbon monoxide (CO) measurements; a subset of women () also performed one prenatal and one postnatal personal fine particulate matter () measurement. Systolic and diastolic BP (SBP and DBP) were measured in 4-y-old children along with their exposure (). We examined the effect of the intervention on resting BP -scores. We also employed reverse distributed lag models to examine time-varying associations between a) maternal prenatal and b) first-year-of-life HAP exposure and resting BP -scores. Among those with measures, we examined associations between and resting BP -scores. Sex-specific effects were considered.
Results:
Intention-to-treat analyses identified that DBP -score at 4 years of age was lower among children born in the LPG arm (LPG ; 95% CI: , ) as compared with those in the control arm, and females were most susceptible to the intervention. Higher CO exposure in late gestation was associated with higher SBP and DBP -score at 4 years of age, whereas higher late-first-year-of-life CO exposure was associated with higher DBP -score. In the subset with measurements, higher maternal postnatal exposure was associated with higher SBP -scores.
Discussion:
These findings suggest that prenatal and first-year-of-life HAP exposure are associated with child BP and support the need for reductions in exposure to HAP, with interventions such as cleaner cooking beginning in pregnancy. https://doi.org/10.1289/EHP13225
Introduction
Blood pressure (BP), a critical component of cardiovascular health (CVH), may be programmed early in life.1 Evidence from longitudinal cohort studies suggests that children with higher BP are at increased risk for higher BP in adulthood,2–4 as well as risk for indicators of cardiovascular disease (CVD), such as subclinical atherosclerosis.5–7 Worldwide, CVD is a leading cause of morbidity and mortality. In Africa, CVD is accounting for an increasingly greater proportion of total deaths (18% in 2019 compared with 13% in 2009).8 Despite this disease burden, most evidence informing our understanding of modifiable risk factors for poorer CVH and CVD risk comes from high-income countries where risk factors differ from resource-poor settings. Better characterization of factors in low- and middle-income countries (LMICs) that increase risk of poorer CVH and interventions to reduce risk is imperative.
In most of sub-Saharan Africa, including in Ghana, of the population burns biomass and other solid fuels to meet their daily cooking and heating needs,9 resulting in high household air pollution (HAP) exposures.10 Worldwide, deaths are attributed to HAP every year, with the highest proportion of HAP-related deaths (45%) attributable to CVD.11 The effect of HAP exposure in early life on childhood BP is poorly described, but evidence from studies focusing on prenatal ambient air pollution exposure supports a plausible link.12,13 Mechanistically, pollutants may cause an imbalance of the autonomic nervous system (ANS) with higher sympathetic and lower parasympathetic tone.14,15 In addition, evidence suggests that oxidative stress, inflammation, and endothelial dysfunction contribute to increased arterial vasoconstrictor responsiveness, leading to elevated BP.15–17 A few studies suggest sex-specific cardiovascular effects of prenatal air pollution exposure,18,19 but the evidence is mixed.12,13
Early life interventions that improve childhood BP, and thus CVH programming, could be efficient and powerful tools to address the growing burden of CVD in LMICs.20 Supporting this concept, studies have examined the impact of early life nutritional interventions on BP, finding that those interventions have had a sustained effect on BP after the intervention ended.20 For instance, results from the Special Turku Coronary Risk Factor Intervention Project for Children (STRIP) study found that a dietary intervention started in infancy lowered systolic and diastolic BP (SBP and DBP) throughout childhood21 and was effective in the prevention of metabolic syndrome between 15 and 20 years of age.22
To evaluate the effect of HAP exposure in early life on childhood BP, we leveraged a Ghanaian pregnancy cohort derived from the Ghana Randomized Air Pollution and Health Study (GRAPHS). GRAPHS primary outcomes were birth weight and childhood pneumonia risk, and these findings have been reported elsewhere.23,24 GRAPHS was a cluster-randomized cookstove intervention trial wherein pregnant women were randomized to one of two cookstove interventions: a) a liquefied petroleum gas (LPG) stove or improved biomass stove, or b) control (open fire cooking).25 GRAPHS also measured maternal exposure to HAP as represented by carbon monoxide (CO) at four time points over pregnancy. In a randomly selected subset of children, GRAPHS continued longitudinal follow-up through 4 years of child age, when resting BP was measured. We first examined whether a stove intervention delivered prenatally and continued through the first year of life could improve resting BP at 4 years of age in an intention-to-treat analysis. We next examined time-varying associations between maternal prenatal and child first-year-of-life (hereafter, first year of life) CO exposures, and, in a subset pre- and postnatal exposures, as well as age 4-y resting SBP and DBP. Finally, we explored sex-specific effects.
Methods
Study Participants
GRAPHS was a cluster-randomized cookstove intervention trial carried out in the Bono East region of Ghana and has been described in detail elsewhere.25,26 Briefly, 1,414 nonsmoking pregnant women were enrolled prior to ultrasound-confirmed27 24th week of gestation from communities in the Kintampo North Municipality and Kintampo South District of Ghana between June 2013 and June 2015. Pregnant women in the intervention arms received either an LPG stove or two improved biomass stoves; those in the LPG arm were provided with free fuel from enrollment through the index child’s first birthday. The primary outcomes of GRAPHS were birth weight and severe pneumonia risk, which have been reported elsewhere.23,24,26 In 2017, additional funding was obtained to continue longitudinal follow-up of mother–child pairs with continued exposure and health phenotyping assessments,28 including resting BP at 4 years of age. We intentionally oversampled children from the control and LPG study arms because the effect of the improved biomass stove intervention on exposure reduction was minimal.29 Table S1 demonstrates baseline characteristics among the children enrolled as compared with the children who were not enrolled in the follow-up cohort. Children who completed the age 4-y study visit with valid BP measures are included in the present analyses. Procedures were approved by the Kintampo Health Research Centre (KHRC) Institutional Ethics Committee (IRB 2017-31), the Ghana Health Service Ethics Review Committee, and institutional review boards at Columbia University (IRB-AAAR4373) and Icahn School of Medicine at Mount Sinai (STUDY-17-01265). Informed consent was obtained from all mothers.
Stove Interventions
As previously described,25,30 GRAPHS included two stove interventions, an LPG stove and an improved biomass stove, that were delivered prenatally and supported until the index infant was 1 year of age. Women randomized to the control arm continued to cook with traditional, open fire stoves. In the LPG intervention arm, households received one two-burner LPG cookstove, two LPG cylinders, and LPG cylinder refills as needed. In the improved biomass stove arm, households received two BioLite Home Stoves (BioLite Inc.). The improved biomass stove allowed continued use of solid fuels, but the stove design improves heat transfer efficiency and theoretically increases combustion efficiency via a thermoelectric-powered fan circulating air through the combustion chamber.31 All study households received mosquito bed nets and health insurance. Fieldworkers visited each household weekly to ascertain maternal health over pregnancy and infant health following delivery, as well as to facilitate stove and mosquito net repairs. As previously reported, the LPG intervention resulted in a 47% [95% confidence interval (CI): 36, 56%] reduction in average maternal CO exposure between pregnancy and the first year of life as compared with control, which corresponds to an absolute reduction of (95% CI: 0.28, 0.75 ppm lower).29 In the post-intervention period (1 y from birth), the of the maternal personal exposure was in the LPG arm and in the control arm. When adjusting for monitoring device wearing (the number of hours the exposure monitoring device was worn by the participant during each monitoring session), this 32% difference between arms increased to 50%.29,32 There was no significant difference in CO or exposure between the improved biomass and control arms.
At the child age 4-y visit, we queried mothers on household cooking practices over the past week, specifically, “I want to ask some questions about cooking [morning, afternoon, or evening] meals for the household over the past week. What stove did you use most often to prepare the [morning, afternoon, or evening] meal?” Categorical responses included the following: open mokyia (stone/mud/clay), closed mokyia (stone/mud/clay), metal mokyia, sawdust stove, improved wood stove, coal pot, kerosene stove, LPG stove, electric stove, other, and not applicable (NA)-no meal cooked. The Fisher exact test was used to examine differences in cooking stove by study arm.
Prenatal and Infant CO Exposures
As previously described,29 maternal CO exposure was assessed at four time points over pregnancy (with the first assessment taking place at the time of enrollment and the three others spaced at 3-wk intervals), and the first-year-of-life CO exposure was assessed at three time points over the child’s first year of life (when the child was 1 month old, 4 months old, and 1 year old) using the Lascar EL-CO-USB Data Logger (Lascar Electronics). Each monitoring session lasted 72 h. The devices were programmed to record CO concentrations every 10 s. The devices report concentrations between 0 and 1,000 ppm and have a manufacturer-reported precision of . Participants were asked to wear the personal monitor except while sleeping or bathing, during which times they were told to keep the monitor nearby and off the floor. The Lascar monitors were exposed to certified span gas ( CO in zero air) at the KHRC laboratory every 6 wk to quantify responses and adjust field values. Additional quality assurance/quality control (QA/QC) checks on the functioning of the CO monitors were made based on run time and visual inspection of each deployment, following GRAPHS protocols.29 As with prior analyses,23,24,30 data used in this analysis was restricted to the first 48 h of each 72-h deployment and passed all QA/QC checks (including deployment duration, visual validity, and correction factor confidence).29 Deployments deemed invalid based on these checks were excluded. For each CO measurement, we assigned the gestational or infant age at the time of measurement using the recorded date of each CO measurement and ultrasound-derived gestational dates or date of birth, respectively.
Exposure Measurements
As previously reported,29 a convenience sample subset of pregnant women performed one 72-h personal, prenatal exposure measurement and, subsequently, mothers performed one 72-h personal, postnatal measurement over the infant’s first year of life, given that the monitors were assumed to be too bulky for the infants to wear. At child age 4-y, children performed one 48-h personal exposure measurement ending the day of or prior to BP measurement. All measurements were performed using the MicroPEM (RTI International), which provides real-time particle monitoring using a light-scattering nephelometer and gravimetric analysis via a Teflon filter (Pall Biotech) for integrated analysis, as well as an accelerometer to assess wearing compliance. The MicroPEM was run following the manufacturer’s specifications at a 50% duty cycle (30 s on, 30 s off) and flow rate of 0.4 LPM. Pre and post sampling, filters were stored at 4°C and shipped on ice (post sampling only) between Columbia University and KHRC for pre and post weighing.29 Baseline adjustment pre and post sampling was performed using a high-efficiency particulate air filter, and weights were field-blank adjusted as described by Chillrud et al.29 The net filter weight of was used to adjust the mean nephelometer response over the entire deployment period. All measurements were also adjusted for field and laboratory blanks. Maternal deployments lasting were removed from the data analyses, and the mean of the first 48 h was used in the analyses. Similarly, age 4-y child deployments were included if they lasted at least 24 h, and the mean of the first 24 h was used in analyses. These QC measures explain the sample size reductions when we include in the models.
BP Measurements
Child resting BP was measured at an age 4-y visit using the oscillometric and digital OMRON BP742N BP monitor (OMRON Healthcare) per study clinical protocol. Following a 10-min period of seated rest in a standard wooden clinic chair, trained fieldworkers measured SBP and DBP using an appropriately fitted pediatric BP cuff on the left arm. We performed two repeated BP measurements spaced apart by 5 min of rest. We averaged these two measurements and performed a -score transformation by subtracting the sample mean and dividing by the sample SD. The -score of average SBP and DBP measurements were used in analyses.
Covariates
To aid in isolating the direct association between HAP exposure and child BP beyond the cluster-randomized nature of the cookstove intervention (which itself then may largely determine HAP exposures), we assembled a range of variables that could plausibly covary with both the exposure and outcome. These variables were selected using directed acyclic graph theory.33 Information on maternal ethnicity (categorical variable) and secondhand smoke exposure, defined as a smoking household member (categorical variable; yes vs. no), were collected through questionnaires during the GRAPHS enrollment visit. Ethnicity is expected to be a confounder because it predicts cooking practices (which are associated with HAP exposure) and food choices (which are associated with BP). Numerical labels were employed to represent ethnic groups owing to discrimination and privacy concerns. As previously described,30 questionnaires assessed household characteristics, which were enumerated as counts and used to generate a household asset index, a measure of relative household wealth.34 Maternal SBP and DBP were measured once at enrollment following 10 min of seated rest and using an oscillometric BP machine. Child sex (female vs. male) was determined at birth from the birth record or, in the event of a home birth, the mother. At the age 4-y visit, we measured child weight (Seca Clara 803 Digital Scale) and height (Seca 213 Portable Stadiometer) in duplicate to calculate child body mass index (BMI; continuous variable in kilograms per meter squared). REDCAP boundaries flagged implausible values; during data analysis, anthropometry data distributions were examined and fieldworker manual entry books were referenced to confirm or correct entries.
Statistical Analyses
We first examined differences in personal exposure measurements by study arm by clustered Wilcoxon rank sum testing among all study children with valid age 4-y measures. We then performed unadjusted intention-to-treat using generalized linear regression to examine whether the GRAPHS stove arm (LPG or improved biomass as compared with control) was associated with BP -scores at 4 years of age. We explored sex-specific effects, first, by introducing a study arm (control, LPG, improved biomass) sex interaction term in the main regression models, and, second, by examining associations in models stratified by child sex. Given prior associations, a sensitivity model additionally adjusted for maternal BP at the time of enrollment. All models included cluster-robust standard errors (SEs) at the level of the community (intervention cluster).
We next employed distributed lag models (DLMs), a data-driven statistical method, to examine associations between prenatal and first-year-of-life CO exposures, considered separately, and BP -scores.35 DLMs yield time-varying estimates of underlying exposure–response relationships while adjusting for both exposures at other time points and covariates. This approach has been extensively applied to studies examining the effects of prenatal environmental exposures on child health outcomes.36,37 Traditional DLMs require each participant to have an exposure measure at each time point (corresponding, in our case, to a given week either over gestation or over the first year of life); however, recently developed statistical methods have extended this model by interchanging the outcome and exposure and employing a functional spline model with time-varying coefficients (e.g., a reversed DLM; rDLM) to allow for exposure measurements occurring at differing time points over gestation or the first year of life.35,38 Using the gamm4 package,39 we implemented rDLMs with cubic splines; penalty selection parameter was done using the default restricted maximum likelihood. Along with point estimates from these models, we report -values from -tests on smooth terms, which are joint tests for equality to zero for all the coefficients making up a single spline term.
We examined the distribution of gestational and infant age in weeks for all available CO measurements (Figure S1A,B). We performed separate rDLMs for prenatal and first-year-of-life CO exposures, which were standardized using the interquartile range (IQR; the range comprised between the 25th and 75th percentile of the distribution). Leveraging the rDLM framework, we examined associations between repeated CO exposures and age 4-y resting SBP and DBP -scores, considered separately. For each regression, we plotted the rDLM model output with 95% CIs to demonstrate the time-varying associations. A sensitive window of exposure was identified where the estimated CIs did not include zero. All models included a random effect for the participant to account for the repeated CO measurements. Multivariable model 1 adjusted for child sex and BMI, maternal ethnicity and enrollment BP, secondhand tobacco smoke exposure, and household asset index. Multivariable model 2 additionally adjusted for child age 4-y exposure, and we included inverse probability weights to account for the age 4-y exposure measurements available in only a subset. Models investigating the association between first-year-of-life CO exposure and age-y 4 BP additionally adjusted for maternal prenatal average CO exposure to isolate the effect of first-year-of-life CO exposure. Sex-specific effects were explored through stratified models using multivariable model 1.
measurements were available at only three time points, and only a subset had all three measurements. Therefore, we used generalized linear regression to examine associations between all available pre- and postnatal exposure and BP -scores, modeled per IQR increase, in the subset of participants with these measures. Specifically, we performed bivariate analyses and multivariable analyses with models 1 and 2 as above. Point estimates were considered significant if the associated -value was .
Analyses were run in R [version 4.2.2 (“Innocent and Trusting”); R Development Core Team]. The clusrank package40 was used to perform the clustered Wilcoxon rank sum test. The sandwich package41 was used for estimating cluster-robust SEs. The rDLM framework was implemented using the gamm4 package.39
Results
Cohort characteristics, which were similar for participants included in the longitudinal cohort (Table S2) compared with those who were not (Table S3), are presented in Table 1. Of the children recruited into the longitudinal cohort study, (96%) had resting BP data. Two hundred ninety (43%) children were born to mothers randomized to the control arm, 124 (19%) to the improved biomass arm, and 255 (38%) to the LPG arm. Of these, (99%) had at least one valid maternal prenatal CO measurement and (81%) had at least one valid first-year-of-life CO measurement. Repeated CO measures were common for both maternal prenatal and first-year-of-life periods. For example, (41%) children with valid maternal prenatal CO had at least three prenatal CO measures and (36%) had two prenatal measures, whereas (13%) infants with valid first-year-of-life CO had three first-year-of-life CO measures and (38%) had two first-year-of-life CO measures. Among those with repeated CO measurements, the repeated prenatal and first-year-of-life measures were moderately correlated (correlation of each measure with individual’s average, prenatal ; child ). Approximately half of the cohort had a valid maternal prenatal (, 55%) and maternal postnatal (, 54%) measurement. Of the 692 children who performed age 4-y personal exposure monitoring, 494 (71%) had valid 24-h estimates and 396 (57%) had valid 48-h estimates following QA/QC protocols. Average prenatal and first-year-of-life CO exposures were not correlated with the age 4-y 24-h exposure (prenatal , ; postnatal , ); a complete correlation matrix of exposure measures is presented in Figure S2. Exposure results for the original GRAPHS cohort are described elsewhere.29
Table 1.
GRAPHS cohort characteristics, women and children (), Ghana.
| Variable | Overall () |
Males () |
Females () |
|---|---|---|---|
| Intervention arm [ (%)] | |||
| Control | 290 (43.3) | 133 (40.3) | 157 (46.3) |
| Improved biomass | 124 (18.5) | 71 (21.5) | 53 (15.7) |
| LPG | 255 (38.1) | 126 (38.2) | 129 (38.1) |
| Ethnicity [ (%)]a | |||
| 1 | 98 (14.6) | 47 (14.2) | 51 (15) |
| 2 | 97 (14.5) | 50 (15.2) | 47 (13.9) |
| 3 | 444 (66.4) | 218 (66.1) | 226 (66.7) |
| 4 | 30 (4.5) | 15 (4.5) | 15 (4.4) |
| Secondhand smoke exposure [ (%)] | |||
| Yes | 132 (19.7) | 67 (20.3) | 65 (19.2) |
| No | 537 (80.3) | 263 (79.7) | 274 (80.8) |
| Asset index [median (IQR)] | ( to 0.80) | ( to 0.94) | ( to 0.7) |
| Child age [y; median (IQR)] | 4.0 (3.9–4.2) | 4.0 (3.9–4.2) | 4.0 (3.9–4.2) |
| Child sex [ (%)] | |||
| Male | 330 (49.3) | — | — |
| Female | 339 (50.7) | — | — |
| Child BMI [; median (IQR)] | 15.1 (14.4–16.0) | 15.4 (14.6–16.2) | 14.9 (14.3–15.8) |
| Missing [ (%)] | 2 (0.3) | 0 (0) | 2 (0.6) |
| SBP [mmHg; median (IQR)] | 90 (84–96) | 91 (85–97) | 88.5 (83.3–95) |
| DBP [mmHg; median (IQR)] | 63.5 (58.5–68) | 62.8 (58.5–69) | 63.5 (59–68) |
| Maternal prenatal 48-h CO average [ppm; median (IQR)]b | 0.97 (0.6–1.56) | 0.95 (0.59–1.52) | 1.01 (0.61–1.61) |
| Missing [ (%)] | 36 (5.4) | 19 (5.8) | 17 (5.0) |
| Child postnatal 48-h CO average [ppm; median (IQR)]c | 0.49 (0.23–0.99) | 0.48 (0.22–0.99) | 0.5 (0.24–1) |
| Missing [ (%)] | 129 (19.3) | 63 (19.1) | 66 (19.5) |
| Maternal prenatal 48-h [; median (IQR)]d | 58.1 (38.5–91.3) | 55.7 (37.8–92.1) | 63.1 (42–85.4) |
| Missing [ (%)] | 301 (45.0) | 139 (42.1) | 162 (47.8) |
| Maternal postnatal 48-h [; median (IQR)]d | 55 (37–80.5) | 54.4 (34.6–77.2) | 56.2 (37.8–84.1) |
| Missing [ (%)] | 310 (46.3) | 151 (45.8) | 159 (46.9) |
| Child age 4-y 24-h [; median (IQR)] | 55.9 (34.3–84.9) | 60 (34.6–86.2) | 53.8 (34.1–82.8) |
| Missing [ (%)] | 196 (29.3) | 100 (30.3) | 96 (28.3) |
Note: —, not applicable; BMI, body mass index; CO, carbon monoxide; DBP, diastolic blood pressure; GRAPHS, Ghana Randomized Air Pollution and Health Study; IQR, interquartile range; LPG, liquefied petroleum gas; , fine particulate matter (PM with an aerodynamic diameter of ); SBP, systolic blood pressure.
Numerical labels were employed owing to discrimination and privacy concerns.
Prenatal maternal personal CO exposure was measured in parts per million at four time points. Prenatal average CO exposures include pre- and post-intervention exposure measurements.
Postnatal child personal CO exposure was measured in parts per million at three time points.
Pre- and postnatal maternal exposure measurements were performed once in each time point.
Approximately half (, 51%) of the children were female, and the median age at BP measurement was 4.0 y (IQR: 3.9–4.2). The repeated systolic and diastolic measures within individuals were correlated ( and , respectively). Median SBP and DBP were (IQR: 84–96) and (IQR: 59–68), respectively. At 4 years of child age, cooking habits were collected on households (Table S4). Across all meals, no mother reported using the LPG stove, which was not unexpected given that the trial had ended roughly 3 y prior and thus families no longer had access to free LPG cylinder refills. The open mokyia stove (i.e., the traditional stone, mud, or clay open fire stove) was the primary stove used almost exclusively across all former study participants. Among children who had valid age 4-y 24-h or 48-h measurements there was no difference in exposure by study arm [24-h median (IQR): control (38.1–90.6); improved biomass (41.1–95.7); LPG (29.6–73.8); clustered Wilcoxon rank sum . 48-h median (IQR): control (37.6–87.4); improved biomass (42.8–104.4); LPG (31.9–73.8); clustered Wilcoxon rank sum ].
Estimated Associations between Stove Intervention Assignment and BP at 4 Years of Age
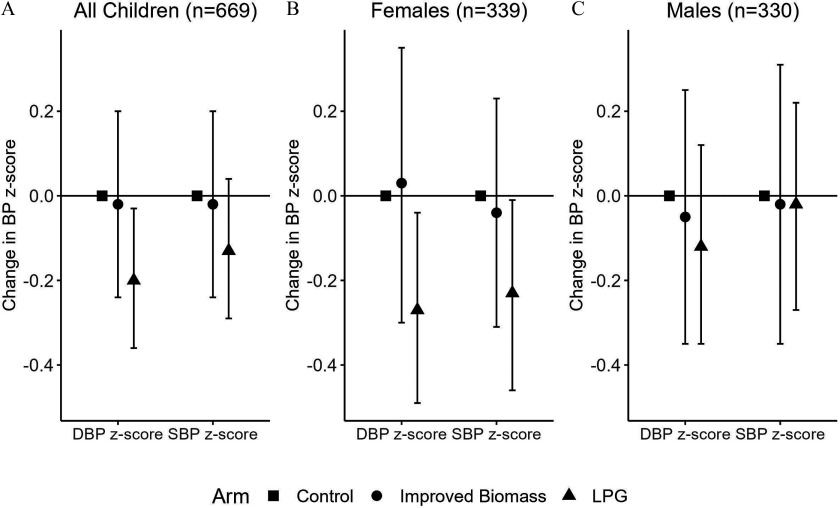
In intention-to-treat models (), we observed that children born to mothers randomized to the LPG arm had lower DBP -scores (LPG ; 95% CI: , ; ) as compared with control (Figure 1; Table S5). In millimeters mercury, this reduction is equivalent on average to a (95% CI: 0.25, 2.95), or 3%, reduction in DBP at 4 years of age. No association was seen between the improved biomass stove intervention and SBP or DBP. Sensitivity analysis additionally adjusting for maternal BP () found that children in the LPG arm had lower DBP -scores (LPG ; 95% CI: , ; ) and suggested lower SBP -scores (LPG ; 95% CI: , 0.02; ) as compared with control (Figure S3).
Figure 1.
Systolic and diastolic blood pressure (SBP and DBP) -scores by GRAPHS stove intervention arm. This figure shows the intention-to-treat associations between GRAPHS stove intervention arm and SBP and DBP -scores at 4 years of age, using cluster-robust generalized linear regression models. In GRAPHS, pregnant women were randomized to open fire (control), liquefied petroleum gas (LPG), or improved biomass stoves that were supported over pregnancy and through the index child’s first year of life. Resting SBP and DBP were measured at the child age 4-y visit, 3 y after support for the intervention ended. The term was only significant for DBP -score in females in the improved biomass arm (); all other terms were . Numeric values can be found in Table S3. Note: GRAPHS, Ghana Randomized Air Pollution and Health Study; LPG, liquefied petroleum gas.
In sex-stratified models, we observed that females born to mothers randomized to the LPG arm had lower DBP -scores (LPG ; 95% CI: , ) and lower SBP -scores (LPG ; 95% CI: , ) as compared with control (; Table 2). In millimeters mercury, this reduction is equivalent on average to a (95% CI: 0.33, 4.04) or 4% reduction in DBP and a (95% CI: 0.09, 4.06) or 2% reduction in SBP at 4 years of age. There was no difference in SBP or DBP among females in the improved biomass arm as compared with control. Among males (), no differences in SBP or DBP in either arm were identified as compared with control. Sensitivity models additionally adjusting for maternal BP did not substantively alter these findings (Figure S3).
Table 2.
Associations between pre- and postnatal maternal exposures (per interquartile range increase) and child age 4-y blood pressure in the GRAPHS intervention study, Ghana.
| Outcomes | Prenatal | Postnatal | ||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | -Value | 95% CI | -Value | |||||
| Bivariate model | ||||||||
| SBP -score | 370 | 0.06 | , 0.18 | 0.38 | 359 | 0.09 | , 0.22 | 0.16 |
| DBP -score | 370 | 0.11 | , 0.24 | 0.08 | 359 | 0.11 | , 0.23 | 0.11 |
| Multivariable model 1 | ||||||||
| SBP -score | 237 | 0.02 | , 0.17 | 0.77 | 237 | 0.18 | 0.02, 0.33 | 0.03 |
| DBP -score | 237 | 0.07 | , 0.23 | 0.35 | 237 | 0.11 | , 0.26 | 0.18 |
| Multivariable model 2 | ||||||||
| SBP -score | 165 | , 0.15 | 0.92 | 165 | , | 0.03 | ||
| DBP -score | 165 | , 0.07 | 0.24 | 165 | , 0.09 | 0.33 | ||
Note: Bivariate model considered pre- and postnatal maternal separately. Multivariable model 1 considered both prenatal and child age 1-y maternal , maternal BP on enrollment (systolic or diastolic depending on the child BP end point), child sex, child age 4-y BMI, ethnicity, asset index, and secondhand smoke exposure. Multivariable model 2 is multivariable model 1 plus additional adjustment for child age 4-y exposure measurement. The pre- and postnatal IQRs were 52.8 and , respectively. BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; GRAPHS, Ghana Randomized Air Pollution and Health Study; IQR, interquartile range; , fine particulate matter (PM with an aerodynamic diameter of ); SBP, systolic blood pressure.
Estimated Time-Varying Effect of Maternal Prenatal CO Exposure on BP at 4 Years of Age
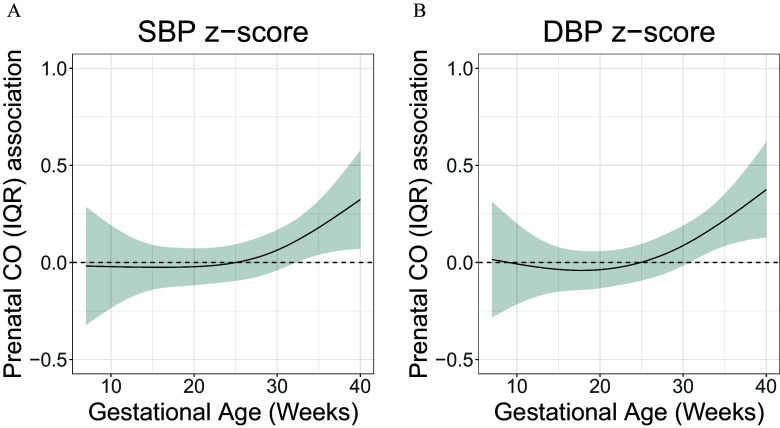
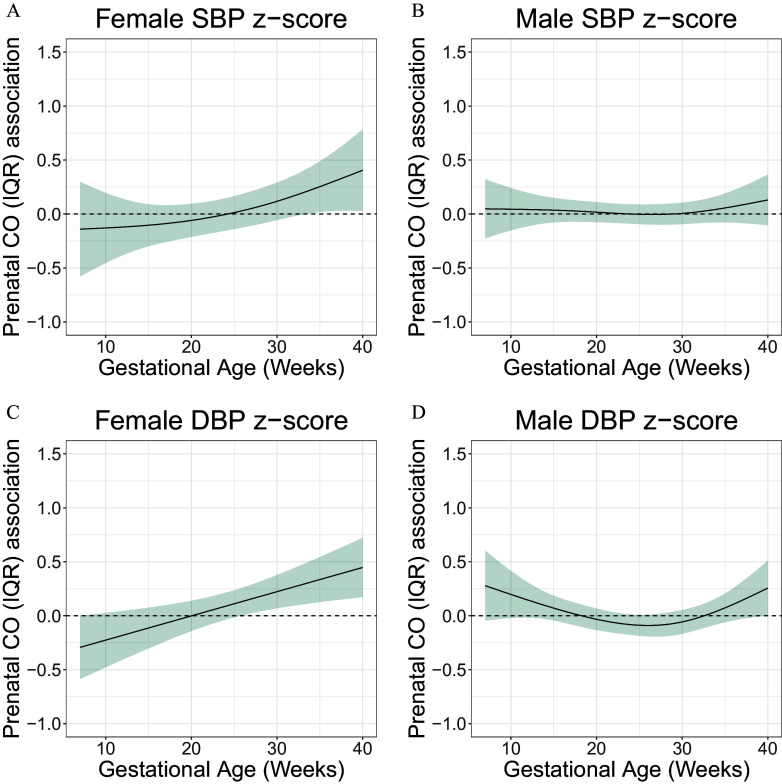
Using multivariable rDLM models (model 1 observations from children; model 2 observations from children), we found that CO exposure in late gestation (second half of the third trimester) was positively associated with SBP -score (multivariable model 1 , multivariable model 2 ). Multivariable model 2 identified a sensitive window between 31 and 40 wk, where higher prenatal CO exposure was positively associated with SBP -score (Figure 2A; Figure S4). Similarly, we found that CO exposure in late gestation was positively associated with DBP -score (multivariable model 1 , multivariable model 2 ). Both models identified a sensitive window of exposure in late gestation (multivariable model 1: 31–40 wk of gestation; multivariable model 2: 29–40 wk of gestation), where CO exposure was positively associated with DBP -score (Figure 2B; Figure S4). In sex-specific analyses, age 4-y females ( observations from children; ) whose mothers were exposed to higher CO exposure during late gestation had higher DBP -scores (Figure 3C). We did not observe an association between maternal prenatal CO exposure and SBP in males ( observations from children; ) or females (Figure 3A,B).
Figure 2.
Time-varying associations between prenatal household air pollution exposure, as represented by personal carbon monoxide (CO), and resting (A) systolic blood pressure (SBP) and (B) diastolic BP (DBP) -scores at 4 years of age in the GRAPHS intervention study, Ghana. The multivariable model 1 ( observations from children) adjusted for child sex and BMI; maternal ethnicity, secondhand tobacco smoke exposure and enrollment BP; and household asset index. The y-axis represents the time-varying association between BP -score and an interquartile (IQR) increase in CO exposure; the x-axis depicts gestational age in weeks at CO measurement. The solid line shows the predicted estimate, and the shaded area represents the 95% CI. A sensitive window was identified when the CIs did not include zero. Data are included in Excel Table S1. Note: BMI, body mass index; CI, confidence interval; GRAPHS, Ghana Randomized Air Pollution and Health Study.
Figure 3.
Sex-specific associations between prenatal household air pollution exposure, as represented by personal maternal carbon monoxide (CO) measurements, and resting diastolic blood pressure (DBP) and systolic BP (SBP) at 4 years of age in children in the GRAPHS intervention study, Ghana. This figure demonstrates the association between maternal personal CO exposure measurements over pregnancy and SBP and DBP at 4 years of age assuming week-specific effects among females [(A,C) observations from children] and males [(B,D) observations from children]. The multivariable model 1 adjusted for child BMI; maternal ethnicity, secondhand tobacco smoke exposure, and enrollment BP; and household asset index. The y-axis represents the time-varying association between BP -score and an interquartile (IQR) increase in CO exposure; the x-axis depicts gestational age in weeks at CO measurement. The solid line shows the predicted estimate, and the shaded area represents the 95% CI. A sensitive window was identified when the CIs did not include zero. Data are included in Excel Table S2. Note: BMI, body mass index; CI, confidence interval; GRAPHS, Ghana Randomized Air Pollution and Health Study.
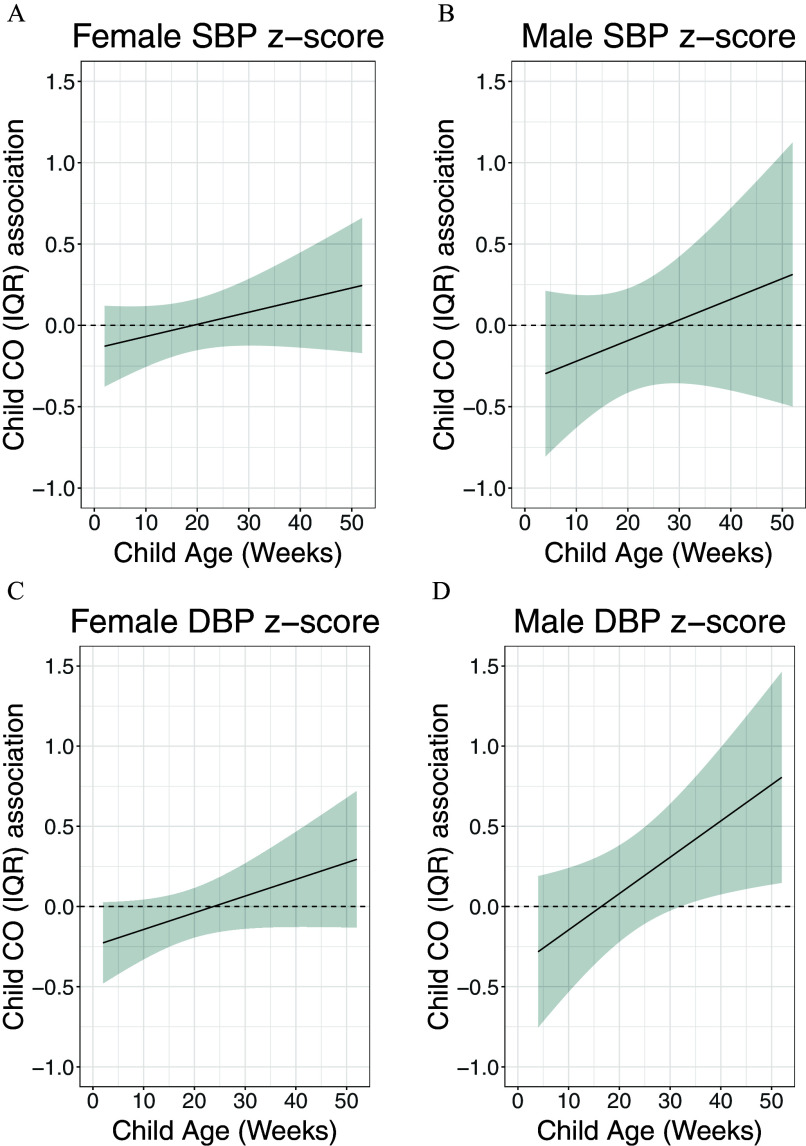
Estimated Time-Varying Effect of First-Year-of-Life CO Exposure on BP at 4 Years of Age
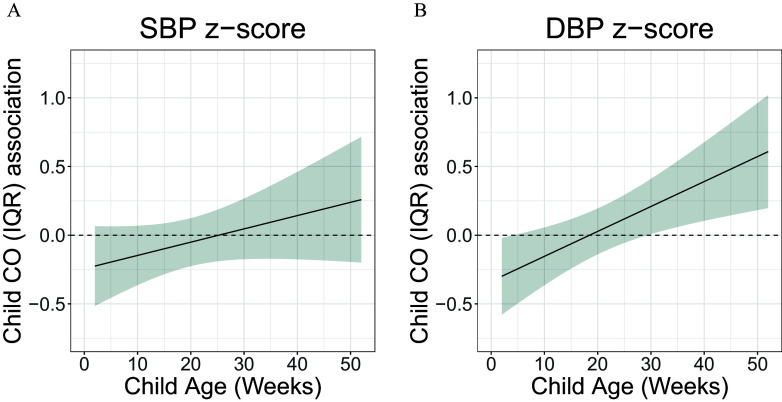
Using multivariable rDLM models (model 1 observations from children; model 2 observations from children), we identified a positive association between first-year-of-life CO exposure and DBP -score in multivariable model 1 () with a sensitive window between 30 and 40 wk (hereafter referred to as late first year of life); however, this association was not identified in multivariable model 2 (; Figure 4B; Figure S5). No association was identified between first-year-of-life CO exposure and SBP (Figure 4A; Figure S5). Among males only, we observed a trend for association between child CO exposure and DBP -score ( observations from children; ); no other sex-specific associations were identified with child CO exposure (Figure 5A–D).
Figure 4.
Time-varying associations between child household air pollution exposure, as represented by maternal personal carbon monoxide (CO), and resting (A) systolic blood pressure (SBP) and (B) diastolic BP (DBP) -scores at 4 years of age in children in the GRAPHS intervention study, Ghana. The multivariable model 1 ( observations from children) adjusted for child sex and BMI; maternal ethnicity, secondhand tobacco smoke exposure, average prenatal CO exposure and enrollment BP; and household asset index. The y-axis represents the time-varying association between BP -score and an interquartile (IQR) increase in CO exposure; the x-axis depicts gestational age in weeks at CO measurement. The solid line shows the predicted estimate, and the shaded area represents the 95% CI. A sensitive window was identified when the CIs did not include zero. Data are included in Excel Table S3. Note: BMI, body mass index; CI, confidence interval; GRAPHS, Ghana Randomized Air Pollution and Health Study.
Figure 5.
Sex-specific associations between child household air pollution exposure, as represented by maternal personal carbon monoxide (CO), and resting diastolic BP (DBP) and systolic BP (SBP) at 4 years of age in children in the GRAPHS intervention study, Ghana. This figure demonstrates the association between child personal carbon monoxide exposure and SBP and DBP at 4 years of age assuming week-specific effects among [(A,C) females and (B,D) males, observations from children)]. The multivariable model 1 adjusted for child BMI; maternal ethnicity, secondhand tobacco smoke exposure, average prenatal CO exposure and enrollment BP; and household asset index. The y-axis represents the time-varying association between BP -score and an interquartile (IQR) increase in CO exposure; the x-axis depicts gestational age in weeks at CO measurement. The solid line shows the predicted estimate, and the shaded area represents the 95% CI. A sensitive window was identified when the CIs did not include zero. Data are included in Excel Table S4. Note: BMI, body mass index; CI, confidence interval; GRAPHS, Ghana Randomized Air Pollution and Health Study.
Estimated Associations between Maternal Pre- and Postnatal and BP at 4 Years of Age
Using multivariable model 1 () considering both maternal pre- and postnatal but not additionally adjusting for age 4-y exposure, we found no association between maternal prenatal exposure and SBP or DBP -scores (Table 2). Higher maternal postnatal exposure was associated with higher SBP -score (; 95% CI: 0.02, 0.33, per IQR increase in exposure; Table 2). No association was identified between maternal postnatal exposure and DBP -score. In models additionally adjusting for age 4-y exposure (), we found that higher maternal postnatal exposure was positively but not statistically significantly associated with SBP -score (; 95% CI: , 0.32; ) per IQR increase in exposure.
Discussion
By combining evidence from a randomized controlled LPG intervention begun prenatally and continued through the first year of life and exposure–response analyses, these data provide evidence for the harmful effects of HAP exposure on child BP beginning prenatally. Specifically, this work provides evidence that a prenatally delivered LPG stove intervention with previously demonstrated exposure reductions29 is associated with lower DBP at 4 years of age, 3 y after the intervention support ended and at a time when no household reported LPG stove use. Further, we find that higher late gestation exposures are associated with higher SBP and DBP at 4 years of age and late-first-year-of-life CO exposures are associated with higher DBP at 4 years of age. In a limited subset of children with exposures, we found evidence suggesting an association between higher maternal postnatal exposure and higher SBP. Taken together, these results suggest that early life HAP exposure negatively impacts BP, with implications for life course CVD risk, and they establish the potential for cleaner-burning stove interventions to reduce risks.
Establishing and maintaining ideal CVH in early life is critical; earlier development of CVD risk factors is associated with higher risk for subsequent CVD.42–44 Barker et al. documented that lower weights at birth and at 1 year of age were associated with higher risk of CVD death, highlighting the importance of early life exposures on programming adult CVD risk.45 Childhood BP tracks into adulthood and is associated with CVD risk. Childhood BP has been linked to future target-organ damage, including carotid intima-media thickness (cIMT), pulse-wave velocity reflecting arterial stiffness, and left ventricular mass.7,46–48 Subclinical atherosclerosis has been observed at autopsy in children as young as 2 years of age, and more extensive lesions were associated with more risk factors.49,50 CVH in adolescence has been associated with adult cardiac structure and function.6 These data warrant continued cohort follow-up to characterize BP over childhood and understand whether these associations persist into later childhood.
A growing literature supports an association between HAP exposure and CVH51–53; our results extend this evidence to include prenatal and early childhood associations. Inefficient biomass stoves emit high levels of a complex mixture of pollutants, many of which have known cardiometabolic effects (e.g., , CO, black carbon).54–56 Cross-sectional studies and those documenting cookstove type by questionnaire suggest associations with CVD risk in adults.52,57 For example, the Shanghai Putuo study found that reported solid fuel use was associated with higher odds for self-reported hypertension, coronary heart disease, and diabetes as compared with never users.58 However, studies directly measuring personal exposure remain limited. Our group and others have found associations between higher short-term HAP exposure and BP in adult women.59,60 Kanagasabai et al. found that higher annual exposure was associated with higher SBP and DBP and greater cIMT.53 A cross-sectional HAP study from Honduras reported associations with exposures and CVD risk factors in adults, including metabolic syndrome.57 To our knowledge, only one cross-sectional study has examined associations between HAP and BP in children,51 although ambient air pollution studies do support this association.61 Our results thus extend these prior findings to observe that higher HAP exposures, particularly during the prenatal period, are associated with higher SBP and DBP in early childhood.
Prior evidence of the effect of a stove intervention on BP is mixed and limited to adults. For example, with the RESPIRE trial, McCracken et al. found that the improved stove intervention was associated with lower DBP.62 Conversely, in the multicounty Household Air Pollution Intervention Network (HAPIN) trial, Ye et al. reported higher SBP and DBP in the LPG intervention arm as compared with control.63 We now report that a prenatally introduced LPG stove supported through child age 1 year, but with no evidence of continued use at 4 years of age or exposure differential between groups, was associated with a , or 3%, reduction in DBP relative to children whose mothers used a traditional open fire stove throughout pregnancy. In females, the LPG intervention was associated with a , or 4%, reduction in DBP and a , or 2%, reduction in SBP as compared with control. These findings are similar in magnitude to the effect of dietary interventions on BP in adults. For example, a systematic review and meta-analysis of 24 nutritional intervention trials in adults with and without hypertension at baseline found that the overall pooled net effect of diet on DBP was .64 Although on the individual level these reductions may appear small, on the population level they may translate to large reductions in CVD risk even though, to our knowledge, population-wide studies of BP and CVD health in LMICs are as yet unavailable. Still, the Framingham Heart Study and the National Health and Nutrition Examination Survey II found that among US White, normotensive adults 35–64 years of age, an average reduction in DBP would result in a 17% decrease in hypertension prevalence, a 6% reduction in coronary heart disease risk and a 15% reduction in risk of stroke and transient ischemic attacks.65 These effects may be further amplified in a resource-poor setting, where limited access to preventive medicine and therapeutics restrict the detection and treatment of hypertension.66 Continued follow-up of the cohort to understand whether these associations persist into later childhood and adulthood is warranted.
Despite our LPG intervention ending upon the index child’s first birthday, we observed lower DBP at 4 years of age, suggesting a lasting effect of the early life stove intervention. These results are supported by early life nutrition intervention studies that also demonstrated a sustained effect on BP into later life. For example, in STRIP, a nutrition intervention study of 1,000 infants who were followed until 20 years of age, investigators observed a reduction in the prevalence of metabolic syndrome among control vs. intervention participants driven by reductions in BP.22 In a second study, Dutch newborn infants were randomized to low- vs. normal-sodium formula. At 25-wk of age, infants in the low-sodium arm had on average lower SBP as compared with those in the control arm.67 Investigators recontacted a subset of the study participants at 15 years of age and found adolescents who had been in the low-sodium arm had on average lower SBP than those who had been in the control arm.68 Taken together with our exposure–response analyses, these data suggest that the early life period may be a critical window of cardiovascular developmental plasticity when interventions to improve CVH may have sustained effects. These health findings, identified years after an originally null randomized controlled trial, underscore the importance of working alongside community and policy partners to ensure that study results can meaningfully drive public health policy. These results identify pregnancy and early childhood as critical windows where HAP exposure programs future CVH and justify continued investments in cleaner cooking and novel ways to ensure that vulnerable communities have access to cleaner cooking fuels and technology.
Time-varying analyses allowed for the identification of two sensitive windows: one in late pregnancy, and the other close to the child’s first birthday. Although the parasympathetic nervous system rapidly develops from 25 wk gestation to birth,69,70 parasympathetic tone starts dominating basal fetal cardiovascular function at wk gestation.69,71 Animal studies suggest that insults that occur during this period of pregnancy lead to changes in the renin–angiotensin system that can alter kidney development.72 Further, maternal exposure to air pollutants could induce maternal oxidative stress and inflammation, provoking an imbalance in the ANS and increasing arterial vasoconstriction,73,74 with impacts on these interrelated systems in the developing fetus.75 Maternal HAP exposure could also impact placental functioning in several ways, including thrombotic placental lesions,76 placental inflammation,77 and abnormal placental vascularization.78 Sex differences in these pathways have been observed, which may partly explain sex differences in disease outcomes and severity later in life.72 Similarly to maternal pathways, air pollution exposure in childhood may trigger systemic inflammation and oxidative stress, which can affect vascular function.79 Our results pointing to sensitive windows in both the prenatal and late-first-year-of-life period suggests that repeated injury may occur and warrants further investigation.
Continued follow-up of the cohort with tracking of BP over childhood will be important to understand how BP at 4 years of age relates to BP over childhood. DBP reflects resting vascular tone; higher DBP suggests higher peripheral resistance. Higher DBP in adults, for example, in persons with isolated diastolic hypertension, is associated with higher risk for CVD events, heart failure, atrial fibrillation, and chronic kidney diseases.80 Although less extensively studied, DBP in childhood correlates with adolescent or young adult DBP and has also been found to predict CVD risk.81 Among hypertensive youth, elevated peripheral DBP was superior to SBP in predicting future CVD.82 Similarly, Flint et al. found that DBP independently influenced cardiovascular outcomes.83 In the Fels Longitudinal Study, DBP in adolescents was associated with 1.9 times the risk for hypertension at 35 years of age.84
Our intention-to-treat results suggest that females may be more susceptible to the effect of HAP exposure on BP programming than males. This finding is consistent with our prior work, which found female infants were more susceptible to HAP exposure for a number of health outcomes, including lung function,85 pneumonia risk,24 and some metrics of infant growth,30 as well as a biomarker of oxidative stress.30,86 Prior studies have reported a cardiovascular effect of air pollution exposure by sex with mixed results.18 A study by Curto et al. in peri-urban India concluded that higher within-village exposure was associated with higher SBP among women compared with men. In that study, the association for DBP was also positive but did not reach statistical significance.87 Although mechanisms underlying sex-specific effects are hypothetical, heightened vulnerability of hypothalamic–pituitary–adrenal axis development in females in response to prenatal stressors has been identified.88 Supporting this, one study identified associations between exposure over pregnancy and disrupted cardiac vagal tone in infancy, with exploratory analyses suggesting that females who are more highly exposed prenatally may have a reduced ability to recover from a stressor as compared with males.89
We note several study strengths. We leveraged the well-characterized GRAPHS cohort, derived from a cluster-randomized cookstove intervention delivered prenatally and continued through the first year of life. GRAPHS also included repeated personal exposure assessments over pregnancy and postnatally at 4 years of age (Table S6). Current follow-up included resting BP at 4 years of age, a valid measure of childhood CVH. Ultrasound-established gestational dating allowed us to accurately identify the gestational age of each prenatal and child CO exposure measurement, thus allowing us to employ advanced statistical models to identify sensitive windows of exposure. Questionnaires captured individual- and household-level covariates.
We also acknowledge limitations. Funding limitations only allowed continued longitudinal follow-up of a subset of the GRAPHS cohort. BP measurements predominantly occurred in the morning, although we did not record the exact time nor activity or diet in the 30–60 min before measurement. However, we hypothesize that these data would be nondifferentially associated with exposure or intervention arm, thus resulting in measurement noise that would bias to the null. It would have also been beneficial to take more BP measurements, as well as BP measurements, on consecutive days but the latter proved unfeasible from a fieldwork perspective. Further, GRAPHS primary exposure measure was CO; we lacked measurements on the entire cohort and repeated measurements over the intervention period. measurements of the mother were taken as a proxy for infant measurements, introducing exposure misclassification bias. Finally, although the rDLM models allowed us to circumvent the issue of unaligned exposure measurements, they did not provide standard association estimates owing to the reverse temporality of the outcome–exposure relationship and they did not clearly account for confounding given that the right-hand side included both outcome and adjustment variables.
Conclusion
In summary, we found that children born to mothers randomized to the LPG arm of the GRAPHS cookstove intervention had lower DBP at 4 years of age and exposure–response analyses support an association between early life HAP exposure and BP at 4 years of age. These findings support the need for reductions in exposure to HAP, beginning in pregnancy.
Supplementary Material
Acknowledgments
We are grateful to the community leaders in the study area, the study mothers and their infants who participated in the study, and the Ghana Health Service facilities in the Kintampo North Municipality and Kintampo South District.
The Ghana Randomized Air Pollution and Health Study (GRAPHS) was supported by the National Institute of Environmental Health Sciences (NIEHS; R01 ES019547, R01 ES026991, R01 ES034433, P30ES023515, and P30 ES009089), the Fogarty Institute (R21 TW010957), the Thrasher Research Fund, and the Clean Cooking Alliance. A.G.L. was additionally supported by the National Heart, Lung and Blood Institute K23 HL135349. M.D. was additionally supported by the Columbia World Project, Combating Household Air Pollution With Clean Energy. During the preparation of this manuscript, E.C. was supported by NIEHS R01ES032242 and 5U2CES026555-03.
Trial registration number: NCT01335490.
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.Chen X, Wang Y. 2008. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation 117(25):3171–3180, PMID: , 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suh I, Nam CM, Jee SH, Kim SI, Lee KH, Kim HC, et al. 1999. Twelve-year tracking of blood pressure in Korean school children: the Kangwha Study. Yonsei Med J 40(4):383–387, PMID: , 10.3349/ymj.1999.40.4.383. [DOI] [PubMed] [Google Scholar]
- 3.Bao W, Threefoot SA, Srinivasan SR, Berenson GS. 1995. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens 8(7):657–665, PMID: , 10.1016/0895-7061(95)00116-7. [DOI] [PubMed] [Google Scholar]
- 4.Naidoo S, Kagura J, Fabian J, Norris SA. 2019. Early life factors and longitudinal blood pressure trajectories are associated with elevated blood pressure in early adulthood. Hypertension 73(2):301–309, PMID: , 10.1161/HYPERTENSIONAHA.118.11992. [DOI] [PubMed] [Google Scholar]
- 5.Davis PH, Dawson JD, Riley WA, Lauer RM. 2001. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine Study. Circulation 104(23):2815–2819, PMID: , 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 6.Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N, et al. 2003. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA 290(17):2277–2283, PMID: , 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 7.Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, et al. 2010. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation 122(24):2514–2520, PMID: , 10.1161/CIRCULATIONAHA.110.966465. [DOI] [PubMed] [Google Scholar]
- 8.GBD 2019 Diseases and Injuries Collaborators. 2020. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258):1204–1222, PMID: , 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Prüss-Ustün A, et al. 2013. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environ Health Perspect 121(7):784–790, PMID: , 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HEI (Health Effects Institute). 2020. State of Global Air 2020. https://www.stateofglobalair.org/sites/default/files/documents/2020-10/soga-2020-report.pdf [accessed 21 October 2020].
- 11.WHO (World Health Organization). 2023. Household air pollution and health. [Fact sheet.]. https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health [accessed 14 March 2024].
- 12.van Rossem L, Rifas-Shiman SL, Melly SJ, Kloog I, Luttmann-Gibson H, Zanobetti A, et al. 2015. Prenatal air pollution exposure and newborn blood pressure. Environ Health Perspect 123(4):353–359, PMID: , 10.1289/ehp.1307419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Mueller NT, Wang H, Hong X, Appel LJ, Wang X. 2018. Maternal exposure to ambient particulate matter ≤ 2.5 µm during pregnancy and the risk for high blood pressure in childhood. Hypertension 72(1):194–201, PMID: , 10.1161/HYPERTENSIONAHA.117.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez CM, Hazari MS, Farraj AK. 2015. Role of autonomic reflex arcs in cardiovascular responses to air pollution exposure. Cardiovasc Toxicol 15(1):69–78, PMID: , 10.1007/s12012-014-9272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seaton A, MacNee W, Donaldson K, Godden D. 1995. Particulate air pollution and acute health effects. Lancet 345(8943):176–178, PMID: , 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 16.Elder A, Oberdörster G. 2006. Translocation and effects of ultrafine particles outside of the lung. Clin Occup Environ Med 5(4):785–796, PMID: , 10.1016/j.coem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Miller MR. 2014. The role of oxidative stress in the cardiovascular actions of particulate air pollution. Biochem Soc Trans 42(4):1006–1011, PMID: , 10.1042/BST20140090. [DOI] [PubMed] [Google Scholar]
- 18.Shin HH, Parajuli RP, Gogna P, Maquiling A, Dehghani P. 2021. Pollutant-sex specific differences in respiratory hospitalization and mortality risk attributable to short-term exposure to ambient air pollution. Sci Total Environ 755(pt 2):143135, PMID: , 10.1016/j.scitotenv.2020.143135. [DOI] [PubMed] [Google Scholar]
- 19.Ni Y, Szpiro AA, Young MT, Loftus CT, Bush NR, LeWinn KZ, et al. 2021. Associations of pre- and postnatal air pollution exposures with child blood pressure and modification by maternal nutrition: a prospective study in the CANDLE cohort. Environ Health Perspect 129(4):047004, PMID: , 10.1289/EHP7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillman MW. 2015. Primordial prevention of cardiovascular disease. Circulation 131(7):599–601, PMID: , 10.1161/CIRCULATIONAHA.115.014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niinikoski H, Jula A, Viikari J, Rönnemaa T, Heino P, Lagström H, et al. 2009. Blood pressure is lower in children and adolescents with a low-saturated-fat diet since infancy: the Special Turku Coronary Risk Factor Intervention Project. Hypertension 53(6):918–924, PMID: , 10.1161/HYPERTENSIONAHA.109.130146. [DOI] [PubMed] [Google Scholar]
- 22.Nupponen M, Pahkala K, Juonala M, Magnussen CG, Niinikoski H, Rönnemaa T, et al. 2015. Metabolic syndrome from adolescence to early adulthood: effect of infancy-onset dietary counseling of low saturated fat: the Special Turku Coronary Risk Factor Intervention Project (STRIP). Circulation 131(7):605–613, PMID: , 10.1161/CIRCULATIONAHA.114.010532. [DOI] [PubMed] [Google Scholar]
- 23.Quinn AK, Adjei IA, Ae-Ngibise KA, Agyei O, Boamah-Kaali EA, Burkart K, et al. 2021. Prenatal household air pollutant exposure is associated with reduced size and gestational age at birth among a cohort of Ghanaian infants. Environ Int 155:106659, PMID: , 10.1016/j.envint.2021.106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinney PL, Asante KP, Lee AG, Ae-Ngibise KA, Burkart K, Boamah-Kaali E, et al. 2021. Prenatal and postnatal household air pollution exposures and pneumonia risk: evidence from the Ghana Randomized Air Pollution and Health Study. Chest 160(5):1634–1644, PMID: , 10.1016/j.chest.2021.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jack DW, Asante KP, Wylie BJ, Chillrud SN, Whyatt RM, Ae-Ngibise KA, et al. 2015. Ghana Randomized Air Pollution and Health Study (GRAPHS): study protocol for a randomized controlled trial. Trials 16:420, PMID: , 10.1186/s13063-015-0930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack DW, Ae-Ngibise KA, Gould CF, Boamah-Kaali E, Lee AG, Mujtaba MN, et al. 2021. A cluster randomised trial of cookstove interventions to improve infant health in Ghana. BMJ Global Health. 6(8):e005599, PMID: , 10.1136/bmjgh-2021-005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boamah EA, Asante K, Ae-Ngibise K, Kinney PL, Jack DW, Manu G, et al. 2014. Gestational age assessment in the Ghana Randomized Air Pollution and Health Study (GRAPHS): ultrasound capacity building, fetal biometry protocol development, and ongoing quality control. JMIR Res Protoc 3(4):e3797, PMID: , 10.2196/resprot.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaali S, Jack DW, Prah RKD, Chillrud SN, Mujtaba MN, Kinney PL, et al. 2022. Poor early childhood growth is associated with impaired lung function: evidence from a Ghanaian pregnancy cohort. Pediatr Pulmonol 57(9):2136–2146, PMID: , 10.1002/ppul.26015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chillrud SN, Ae-Ngibise KA, Gould CF, Owusu-Agyei S, Mujtaba M, Manu G, et al. 2021. The effect of clean cooking interventions on mother and child personal exposure to air pollution: results from the Ghana Randomized Air Pollution and Health Study (GRAPHS). J Expo Sci Environ Epidemiol 31(4):683–698, PMID: , 10.1038/s41370-021-00309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boamah-Kaali E, Jack DW, Ae-Ngibise KA, Quinn A, Kaali S, Dubowski K, et al. 2021. Prenatal and postnatal household air pollution exposure and infant growth trajectories: evidence from a rural Ghanaian pregnancy cohort. Environ Health Perspect 129(11):117009, PMID: , 10.1289/EHP8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jetter J, Ebersviller S. 2016. Test Report, BioLite Home Stove with Wood Fuel, Air Pollutant Emissions and Fuel Efficiency. EPA/625/R-16/001. https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NRMRL&dirEntryId=318121 [accessed 29 July 2021].
- 32.Gould CF, Mujtaba MN, Yang Q, Boamah-Kaali E, Quinn AK, Manu G, et al. 2023. Using time-resolved monitor wearing data to study the effect of clean cooking interventions on personal air pollution exposures. J Expo Sci Environ Epidemiol 33(3):386–395, PMID: , 10.1038/s41370-022-00483-0. [DOI] [PubMed] [Google Scholar]
- 33.Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology 10(1):37–48, PMID: , 10.1097/00001648-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Gunnsteinsson S, Labrique AB, West KP Jr, Christian P, Mehra S, Shamim AA, et al. 2010. Constructing indices of rural living standards in northwestern Bangladesh. J Health Popul Nutr 28(5):509–519, PMID: , 10.3329/jhpn.v28i5.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bello GA, Arora M, Austin C, Horton MK, Wright RO, Gennings C. 2017. Extending the distributed lag model framework to handle chemical mixtures. Environ Res 156:253–264, PMID: , 10.1016/j.envres.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee AG, Cowell W, Kannan S, Ganguri HB, Nentin F, Wilson A, et al. 2020. Prenatal particulate air pollution and newborn telomere length: effect modification by maternal antioxidant intakes and infant sex. Environ Res 187:109707, PMID: , 10.1016/j.envres.2020.109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horton MK, Hsu L, Claus Henn B, Margolis A, Austin C, Svensson K, et al. 2018. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int 121(pt 1):148–158, PMID: , 10.1016/j.envint.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen YH, Ferguson KK, Meeker JD, McElrath TF, Mukherjee B. 2015. Statistical methods for modeling repeated measures of maternal environmental exposure biomarkers during pregnancy in association with preterm birth. Environ Health 14:9, PMID: , 10.1186/1476-069X-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood S, Scheipl F. 2020. gamm4: generalized additive mixed models using ‘mgcv’ and ‘lme4.’ https://cran.r-project.org/web/packages/gamm4/index.html [accessed 24 April 2023].
- 40.Wang W, Jiang Y, Lee MLT, Yan J. 2022. clusrank: Wilcoxon rank tests for clustered data. https://cran.r-project.org/web/packages/clusrank/index.html [accessed 24 April 2023].
- 41.Zeileis A, Lumley T, Graham N, Koell S. 2022. sandwich: robust covariance matrix estimators. https://cran.r-project.org/web/packages/sandwich/index.html [accessed 24 April 2023].
- 42.Yano Y, Reis JP, Lewis CE, Sidney S, Pletcher MJ, Bibbins-Domingo K, et al. 2020. Association of blood pressure patterns in young adulthood with cardiovascular disease and mortality in middle age. JAMA Cardiol 5(4):382–389, PMID: , 10.1001/jamacardio.2019.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laitinen TT, Pahkala K, Magnussen CG, Viikari JSA, Oikonen M, Taittonen L, et al. 2012. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation 125(16):1971–1978, PMID: , 10.1161/CIRCULATIONAHA.111.073585. [DOI] [PubMed] [Google Scholar]
- 44.Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, et al. 2018. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with cardiovascular events later in life. JAMA 320(17):1774–1782, PMID: , 10.1001/jama.2018.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. 1989. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298(6673):564–567, PMID: , 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koivistoinen T, Hutri-Kähönen N, Juonala M, Aatola H, Kööbi T, Lehtimäki T, et al. 2011. Metabolic syndrome in childhood and increased arterial stiffness in adulthood: the Cardiovascular Risk in Young Finns Study. Ann Med 43(4):312–319, PMID: , 10.3109/07853890.2010.549145. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Li S, Ulusoy E, Chen W, Srinivasan SR, Berenson GS. 2004. Childhood adiposity as a predictor of cardiac mass in adulthood: the Bogalusa Heart Study. Circulation 110(22):3488–3492, PMID: , 10.1161/01.CIR.0000149713.48317.27. [DOI] [PubMed] [Google Scholar]
- 48.Koskinen JS, Kytö V, Juonala M, Viikari JSA, Nevalainen J, Kähönen M, et al. 2020. Childhood risk factors and carotid atherosclerotic plaque in adulthood: the Cardiovascular Risk in Young Finns Study. Atherosclerosis 293:18–25, PMID: , 10.1016/j.atherosclerosis.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 49.Berenson GS, Srinivasan SR, Bao W, Newman WP III, Tracy RE, Wattigney WA. 1998. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 338(23):1650–1656, PMID: , 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 50.Newman WP III, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, et al. 1986. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med 314(3):138–144, PMID: , 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 51.Baumgartner J, Zhang Y, Schauer JJ, Ezzati M, Patz JA, Bautista LE. 2012. Household air pollution and children’s blood pressure. Epidemiology 23(4):641–642, PMID: , 10.1097/EDE.0b013e3182593fa9. [DOI] [PubMed] [Google Scholar]
- 52.Baumgartner J, Carter E, Schauer JJ, Ezzati M, Daskalopoulou SS, Valois MF, et al. 2018. Household air pollution and measures of blood pressure, arterial stiffness and central haemodynamics. Heart 104(18):1515–1521, PMID: , 10.1136/heartjnl-2017-312595. [DOI] [PubMed] [Google Scholar]
- 53.Kanagasabai T, Xie W, Yan L, Zhao L, Carter E, Guo D, et al. 2022. Household air pollution and blood pressure, vascular damage, and subclinical indicators of cardiovascular disease in older Chinese adults. Am J Hypertens 35(2):121–131, PMID: , 10.1093/ajh/hpab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ljungman PLS, Andersson N, Stockfelt L, Andersson EM, Nilsson Sommar J, Eneroth K, et al. 2019. Long-term exposure to particulate air pollution, black carbon, and their source components in relation to ischemic heart disease and stroke. Environ Health Perspect 127(10):107012, PMID: , 10.1289/EHP4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C, Yin P, Chen R, Meng X, Wang L, Niu Y, et al. 2018. Ambient carbon monoxide and cardiovascular mortality: a nationwide time-series analysis in 272 cities in China. Lancet Planet Health 2(1):e12–e18, PMID: , 10.1016/S2542-5196(17)30181-X. [DOI] [PubMed] [Google Scholar]
- 56.Brook RD, Newby DE, Rajagopalan S. 2018. Air pollution and cardiometabolic disease: an update and call for clinical trials. Am J Hypertens 31(1):1–10, PMID: , 10.1093/ajh/hpx109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajkumar S, Young BN, Clark ML, Benka-Coker ML, Bachand AM, Brook RD, et al. 2019. Household air pollution from biomass-burning cookstoves and metabolic syndrome, blood lipid concentrations, and waist circumference in Honduran women: a cross-sectional study. Environ Res 170:46–55, PMID: , 10.1016/j.envres.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee MS, Hang JQ, Zhang FY, Dai HL, Su L, Christiani DC. 2012. In-home solid fuel use and cardiovascular disease: a cross-sectional analysis of the Shanghai Putuo study. Environ Health 11(1):18, PMID: , 10.1186/1476-069X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quinn AK, Ae-Ngibise KA, Kinney PL, Kaali S, Wylie BJ, Boamah E, et al. 2017. Ambulatory monitoring demonstrates an acute association between cookstove-related carbon monoxide and blood pressure in a Ghanaian cohort. Environ Health 16(1):76, PMID: , 10.1186/s12940-017-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, et al. 2011. Indoor air pollution and blood pressure in adult women living in rural China. Environ Health Perspect 119(10):1390–1395, PMID: , 10.1289/ehp.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bilenko N, van Rossem L, Brunekreef B, Beelen R, Eeftens M, Hoek G, et al. 2015. Traffic-related air pollution and noise and children’s blood pressure: results from the PIAMA birth cohort study. Eur J Prev Cardiol 22(1):4–12, PMID: , 10.1177/2047487313505821. [DOI] [PubMed] [Google Scholar]
- 62.McCracken JP, Smith KR, Díaz A, Mittleman MA, Schwartz J. 2007. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect 115(7):996–1001, PMID: , 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye W, Steenland K, Quinn A, Liao J, Balakrishnan K, Rosa G, et al. 2022. Effects of a liquefied petroleum gas stove intervention on gestational blood pressure: intention-to-treat and exposure-response findings from the HAPIN trial. Hypertension 79(8):1887–1898, PMID: , 10.1161/HYPERTENSIONAHA.122.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gay HC, Rao SG, Vaccarino V, Ali MK. 2016. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension 67(4):733–739, PMID: , 10.1161/HYPERTENSIONAHA.115.06853. [DOI] [PubMed] [Google Scholar]
- 65.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. 1995. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med 155(7):701–709, PMID: , 10.1001/archinte.1995.00430070053006. [DOI] [PubMed] [Google Scholar]
- 66.Nyaaba GN, Masana L, de-Graft Aikins A, Beune E, Agyemang C. 2020. Factors hindering hypertension control: perspectives of front-line health professionals in rural Ghana. Public Health 181:16–23, PMID: , 10.1016/j.puhe.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Hofman A, Hazebroek A, Valkenburg HA. 1983. A randomized trial of sodium intake and blood pressure in newborn infants. JAMA 250(3):370–373, PMID: , 10.1001/jama.1983.03340030030023. [DOI] [PubMed] [Google Scholar]
- 68.Geleijnse JM, Hofman A, Witteman JCM, Hazebroek AAJM, Valkenburg HA, Grobbee DE. 1997. Long-term effects of neonatal sodium restriction on blood pressure. Hypertension 29(4):913–917, PMID: , 10.1161/01.hyp.29.4.913. [DOI] [PubMed] [Google Scholar]
- 69.Cerritelli F, Frasch MG, Antonelli MC, Viglione C, Vecchi S, Chiera M, et al. 2021. A review on the vagus nerve and autonomic nervous system during fetal development: searching for critical windows. Front Neurosci 15:721605, PMID: , 10.3389/fnins.2021.721605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Longin E, Gerstner T, Schaible T, Lenz T, König S. 2006. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J Perinat Med 34(4):303–308, PMID: , 10.1515/JPM.2006.058. [DOI] [PubMed] [Google Scholar]
- 71.Gagnon R, Campbell K, Hunse C, Patrick J. 1987. Patterns of human fetal heart rate accelerations from 26 weeks to term. Am J Obstet Gynecol 157(3):743–748, PMID: , 10.1016/s0002-9378(87)80042-x. [DOI] [PubMed] [Google Scholar]
- 72.Moritz KM, Cuffe JSM, Wilson LB, Dickinson H, Wlodek ME, Simmons DG, et al. 2010. Review: sex specific programming: a critical role for the renal renin–angiotensin system. Placenta 31 Suppl:S40–S46, PMID: , 10.1016/j.placenta.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Giorgini P, Di Giosia P, Grassi D, Rubenfire M, Brook RD, Ferri C. 2016. Air pollution exposure and blood pressure: an updated review of the literature. Curr Pharm Des 22(1):28–51, PMID: , 10.2174/1381612822666151109111712. [DOI] [PubMed] [Google Scholar]
- 74.Brook RD, Rajagopalan S. 2009. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens 3(5):332–350, PMID: , 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 75.Gold DR, Zanobetti A. 2018. Do maternal air pollution exposures have long-lasting influences on child blood pressure? Hypertension 72(1):56–58, PMID: , 10.1161/HYPERTENSIONAHA.118.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wylie BJ, Matechi E, Kishashu Y, Fawzi W, Premji Z, Coull BA, et al. 2017. Placental pathology associated with household air pollution in a cohort of pregnant women from Dar es Salaam, Tanzania. Environ Health Perspect 125(1):134–140, PMID: , 10.1289/EHP256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erickson AC, Arbour L. 2014. The shared pathoetiological effects of particulate air pollution and the social environment on fetal-placental development. J Environ Public Health 2014:901017, PMID: , 10.1155/2014/901017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hettfleisch K, Bernardes LS, Carvalho MA, Pastro LDM, Vieira SE, Saldiva SRDM, et al. 2017. Short-term exposure to urban air pollution and influences on placental vascularization indexes. Environ Health Perspect 125(4):753–759, PMID: , 10.1289/EHP300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang M, Chen J, Yang Y, Yuan H, Huang Z, Lu Y. 2021. Effects of ambient air pollution on blood pressure among children and adolescents: a systematic review and meta‐analysis. J Am Heart Assoc 10(10):e017734, PMID: , 10.1161/JAHA.120.017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yano Y, Kim HC, Lee H, Azahar N, Ahmed S, Kitaoka K, et al. 2022. Isolated diastolic hypertension and risk of cardiovascular disease: controversies in hypertension - pro side of the argument. Hypertension 79(8):1563–1570, PMID: , 10.1161/HYPERTENSIONAHA.122.18459. [DOI] [PubMed] [Google Scholar]
- 81.Urbina EM, Khoury PR, Bazzano L, Burns TL, Daniels S, Dwyer T, et al. 2019. Relation of blood pressure in childhood to self-reported hypertension in adulthood. Hypertension 73(6):1224–1230, PMID: , 10.1161/HYPERTENSIONAHA.118.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, et al. 2001. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 103(9):1245–1249, PMID: , 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 83.Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, et al. 2019. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med 381(3):243–251, PMID: , 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 84.Beckett LA, Rosner B, Roche AF, Guo S. 1992. Serial changes in blood pressure from adolescence into adulthood. Am J Epidemiol 135(10):1166–1177, PMID: , 10.1093/oxfordjournals.aje.a116217. [DOI] [PubMed] [Google Scholar]
- 85.Lee AG, Kaali S, Quinn A, Delimini R, Burkart K, Opoku-Mensah J, et al. 2019. Prenatal household air pollution is associated with impaired infant lung function with sex-specific effects. Evidence from GRAPHS, a cluster randomized cookstove intervention trial. Am J Respir Crit Care Med 199(6):738–746, PMID: , 10.1164/rccm.201804-0694OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaali S, Jack D, Delimini R, Hu L, Burkart K, Opoku-Mensah J, et al. 2018. Prenatal household air pollution alters cord blood mononuclear cell mitochondrial DNA copy number: sex-specific associations. Int J Environ Res Public Health 16(1):26, PMID: , 10.3390/ijerph16010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Curto A, Wellenius GA, Milà C, Sanchez M, Ranzani O, Marshall JD, et al. 2019. Ambient particulate air pollution and blood pressure in peri-urban India. Epidemiology 30(4):492–500, PMID: , 10.1097/EDE.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gifford RM, Reynolds RM. 2017. Sex differences in early-life programming of the hypothalamic–pituitary–adrenal axis in humans. Early Hum Dev 114:7–10, PMID: , 10.1016/j.earlhumdev.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 89.Cowell WJ, Brunst KJ, Malin AJ, Coull BA, Gennings C, Kloog I, et al. 2019. Prenatal exposure to PM2.5 and cardiac vagal tone during infancy: findings from a multiethnic birth cohort. Environ Health Perspect 127(10):107007, PMID: , 10.1289/EHP4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.