Abstract
Recent advances in the quantitative assessment of viral burden, by permitting the extension of criteria applied to assess the efficacy of vaccines from all-or-none protection to diminution of the viral burden, may allow the identification of original immunogens of value in combined vaccines. Peptides corresponding to three domains of the envelope glycoproteins of feline immunodeficiency virus that are recognized during natural infection were used to immunize cats. After challenge with a primary isolate of feline immunodeficiency virus, the development of acute infection was monitored by quantitative assessment of the viral burden in plasma and tissues by competitive reverse transcription-PCR, by measurement of the humoral response developed to viral components, and by lymphocyte subset analysis. Whereas immunization with two peptides derived from the surface glycoprotein had no effect on the early course of infection, immunization with a peptide derived from the transmembrane glycoprotein delayed infection, as reflected by a diminished viral burden in the early phase of primary infection and delayed seroconversion. This peptide, located in the membrane-proximal region of the extracellular domain, has homology to an epitope of human immunodeficiency virus type 1 recognized by a broadly neutralizing monoclonal antibody. These results suggest that lentivirus transmembrane glycoproteins share a determinant in the juxtamembrane ectodomain which could be of importance in the design of vaccines against AIDS.
While it is likely that suppression of viral burden by both humoral and cellular immune responses contributes to the maintenance of a prolonged asymptomatic state during natural infection by the human, simian, and feline immunodeficiency viruses (HIV, SIV, and FIV), the immunological mechanisms underlying protection are as yet ill defined. This uncertainty constitutes the major obstacle to the rational design of AIDS vaccines. In practice, humoral immunity is frequently assessed by antibody-mediated neutralization of viral infectivity for cultured cells. When applied to strains of HIV type 1 (HIV-1) adapted for propagation in transformed T-cell lines, continuous epitopes sensitive to neutralizing antibodies were identified in the third variable region (V3) of the external glycoprotein, gp120 (33). However, neutralization of adapted virus by such antibodies has tended to be type specific (24, 49, 59) and largely ineffective against primary isolates (8, 43, 63, 65).
More recently, attention has been focused on the use of complex oligomeric envelope as an immunogen. Indeed, immunization with oligomeric envelope glycoproteins elicits broadly cross-reactive antibodies to discontinuous epitopes on gp120 and gp41, some of which are dependent on the oligomeric structure (9). Nevertheless, the concern exists that certain elements of the immune response, such as enhancing antibodies, could diminish the degree of protective immunity attainable after vaccination with envelope subunits (39, 42). The balance between neutralization and enhancement might explain, at least in part, the variable results of subunit vaccine trials performed in animals, ranging from protection to disease enhancement.
It is thus conceivable that protection afforded by immunization may be improved by the selective incorporation of particular envelope domains in lentivirus vaccines. Ideally, these immunogens would represent viral determinants of limited variability and their beneficial effect would be demonstrable against primary isolates. Due to the limitations of assays performed in vitro, protection may best be evaluated by immunization and virulent challenge in an animal model. In the past, vaccine protection was conventionally assessed by all-or-none infection of animals. No simple alternative method was available to quantify and compare vaccine efficacy in vivo. More recently, however, technological and conceptual developments in the quantitative analysis of viral burden have allowed rapid evaluation of the effect of therapeutic intervention. Such techniques may also be applied in vaccine trials, to define immunogens affecting viral burden in vivo upon challenge in animals. Thus, the same rationale that is used to optimize multitherapy may be applied to vaccines. The first step would be to define original immunogens that provide a degree of protection against an in vivo challenge in animal models. The second step would be to test whether protective immunity might be improved by a combination of such immunogens, which by themselves provide only partial protection.
We have previously defined nine domains comprising continuous B-cell epitopes of the envelope glycoproteins of FIV recognized during natural infection (51). Of these, five domains (SU1 to SU5) were localized in the surface glycoprotein and four (TM1 to TM4) were localized in the transmembrane glycoprotein. Three envelope domains (SU2, SU5, and TM3) that elicit antibodies in most infected cats were selected in the present study. The first domain, SU2, induces antibodies which neutralize viral infectivity for cultured cells (17, 36, 57). Analogy between the SU2 domain and the third variable (V3) region of HIV-1, beyond the induction of neutralizing antibodies, extends to involvement in tropism (32, 70, 72) and to structure (52). Immunization with a peptide derived from the SU2 domain, however, failed to afford protection against in vivo challenge (37). The second domain, SU5, corresponds to the fifth conserved region of gp120 of HIV-1 that elicits antibodies that cross-react with HLA class I (18) and has been hypothesized to be involved in antiviral protection (4, 5). Finally, the TM3 domain, located in the extracellular membrane-proximal domain of the transmembrane glycoprotein, corresponds spatially to a relatively well conserved region in gp41 of HIV-1 recognized by neutralizing antibodies in human sera (10). Moreover, a human monoclonal antibody directed against this region has potent neutralizing activity in vitro against primary isolates of HIV-1 (14, 54, 67).
In the present study, we have assessed whether any of these three envelope domains may elicit a degree of protection against challenge with a primary strain of FIV, as reflected by diminution of the viral burden during acute infection. The viral burden in plasma and tissues was assessed by competitive reverse transcription-PCR (RT-PCR). Whereas immunization with SU2 or SU5 had no effect on the early course of infection, immunization with the TM3 peptide delayed infection, as reflected by a diminished viral burden in the early phase of primary infection and by delayed seroconversion.
MATERIALS AND METHODS
Tissue culture.
The ID10 clone (48) of Crandell’s feline kidney cells (CrFK) was generously provided by R. Osborne (University of Glasgow, Bearsden, United Kingdom). The CrFK fibroblasts were cultivated in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum, 100 IU of penicillin per ml, and 100 μg of streptomycin per ml. The feline T-lymphoid cell line FL-4 (77), which is chronically infected with the Petaluma strain of FIV, was the generous gift of J. Yamamoto (University of Florida, Gainesville, Fla.). The FL-4 cell line was cultivated in RPMI 1640 with fetal calf serum and antibiotics as described for CrFK cells (complete RPMI). Feline peripheral blood mononuclear cells (PBMC) were isolated from the blood of specific-pathogen-free cats by density gradient centrifugation and activated for 3 days in complete RPMI containing 5 μg of concanavalin A per ml, 50 μM 2-mercaptoethanol (2-ME), and 10 mM HEPES.
Virus.
Stocks of the Petaluma (53) and Wo (45) isolates of FIV were derived from the supernatant of the FL-4 cell line and acutely infected feline PBMC, respectively. The Wo isolate has been passaged a limited number of times and only in feline PBMC.
Peptides.
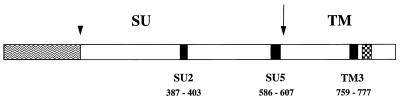
Peptides deduced from the envelope sequence of the Wo isolate (52) and coupled via the amino-terminal residue to keyhole limpet hemocyanin (KLH) were obtained from Neosystem (Strasbourg, France). The peptides corresponded to previously defined envelope domains containing continuous B-cell epitopes (51) and were as follows (residues affixed to facilitate coupling are underscored): YRAISSWKQRNRWEWRPD (SU2), CQVVKQPDYLVVPGEVMEYKPRR (SU5), and YKKGLQQLQEWEDWVGWIGN (TM3). The position of the peptides within the envelope sequence is shown in Fig. 1.
FIG. 1.
Diagrammatic representation of the envelope of FIV.
Sites of proteolytic cleavage eliminating an amino-terminal signal
peptide (arrowhead) and generating mature surface (SU) and
transmembrane (TM) envelope glycoproteins (arrow) are indicated.
Peptides SU2, SU5, and TM3 were used as immunogens. Positions
correspond to the amino acid sequence of the envelope glycoprotein of
the Wo strain. Symbols: ▪, peptides;
 , signal sequence;
, putative
membrane-spanning domain.
, signal sequence;
, putative
membrane-spanning domain.
Immunization and challenge.
Groups of four 4-month-old specific-pathogen-free cats (IFFA-CREDO) were immunized subcutaneously with 120 μg of FIV peptide (approximately 250 μg of KLH-coupled peptide) emulsified in complete Freund’s adjuvant. Four booster injections were administered subcutaneously in incomplete Freund’s adjuvant, generally at intervals of 2 to 3 weeks, although the interval between the last two injections was 7 weeks. As a negative control, four cats received the same regimen of inoculations containing adjuvant only. Development of the humoral response was monitored regularly. Cats were challenged 1 week after the final immunization by intraperitoneal inoculation of 10 50% cat infectious doses (CID50) of the Wo isolate of FIV. Blood was collected at weekly intervals for enumeration of lymphocyte subsets, isolation of virus, determination of viral RNA in plasma (competitive RT-PCR), and serological analyses. The cats were sacrificed five weeks after challenge. Lymphoid organs (spleen, thymus, and axillary lymph nodes) were removed and preserved in 10% buffered formalin solution for histological observation or frozen in liquid nitrogen for extraction of RNA.
Serological analyses.
The antibody response was assessed before challenge by enzyme-linked immunosorbent assay (ELISA) on immobilized envelope peptide and immunoprecipitation. After challenge, seroconversion was monitored by ELISA on immobilized envelope peptides and recombinant FIV capsid protein.
(i) ELISA.
ELISA on immobilized envelope peptides corresponding to the immunogenic domains SU2 and TM2 and on immobilized recombinant p25 (FIV capsid protein) expressed as a glutathione S-transferase fusion protein (56), the kind gift of O. Jarrett (University of Glasgow), was performed essentially as described by Avraméas et al. (1). Microplates (Immulon II; Dynatech) were coated with 0.5 μg of antigen per well for peptides and with 0.1 μg/well for p24. Assays were performed in duplicate, and the results are expressed as means. Antipeptide titers prior to challenge were expressed as the reciprocal of the last serum dilution yielding an absorbance at 405 nm greater than 0.1. After challenge, seroconversion with respect to envelope peptides and p24 was assessed at serum dilutions of 1/25 and 1/100, respectively. Absorbance values at 405 nm were corrected by subtraction of the absorbance values obtained upon binding to an irrelevant immobilized peptide. Assays were normalized with respect to binding of pooled sera of FIV-infected cats with a peptide representing the immunodominant domain (TM2) of the transmembrane glycoprotein of FIV (1).
(ii) Immunoprecipitation.
Sera collected on the day of challenge (at a dilution of 1/40) were used to immunoprecipitate envelope glycoproteins derived from metabolically labeled FL-4 cells and activated feline PBMC infected with the Wo strain of FIV, as described elsewhere (50).
Neutralization and reduction of viral infectivity assays.
Antibodies neutralizing the infection of CrFK by the Petaluma strain of FIV were detected as previously described (57). For the assay of the reduction of viral infectivity, serial dilutions (1/50, 1/100, 1/200, and 1/400) of a stock of the Wo isolate and a single dilution (1/5) of sera were prepared in complete RPMI containing 50 μM 2-ME and 10 mM HEPES. Feline serum was combined with an equal volume of serial dilutions of virus and incubated in a volume of 100 μl for 1 h at 37°C in quadruplicate in eight-strip cluster tubes (Costar). Mitogen-activated PBMC were adjusted to 4 × 106/ml in complete RPMI with 2-ME, HEPES, and 200 U of recombinant human interleukin 2 per ml, and 0.1 ml of the suspension (4 × 105 cells) was added to the tubes. Infection was allowed to proceed overnight. The virus inoculum was removed by washing the cells twice with 0.5 ml of complete RPMI. The cells were then resuspended in 0.2 ml of feline serum diluted in complete RPMI with 2-ME, HEPES, and 100 U of interleukin-2 per ml and transferred to wells of 96-well microtiter plates. Half the medium was replaced 4 days after infection. Aliquots of 10 μl were removed 7 days after infection for analysis of reverse transcriptase activity.
Lymphocyte subset analysis.
Counts of CD4+ lymphocytes were determined at regular intervals during immunization and acute infection. CD4+ lymphocytes were enumerated by flow cytometry with monoclonal antibodies obtained from Clinisciences.
Isolation of virus.
Plasma samples obtained from all cats after 2 and 3 weeks of infection were analyzed qualitatively by PBMC culture to verify that infection had occurred. Plasma viremia was quantified by limiting dilution in PBMC culture (27) at 3 weeks of infection for control and TM3 groups only, to compare the infectious titer with the RNA copy number. Infection was assessed by determination of p24 by ELISA (Petcheck; IDEXX) in culture supernatants 14 and 21 days after inoculation of various volumes (0.2, 2, 10, 40, 200, or 1000 μl) of plasma. The highest plasma dilution giving a positive culture was taken as the end point. Plasma viremia was expressed as 50% tissue culture infectious doses (TCID50)/ml plasma.
Preparation of RNA. (i) Plasma RNA.
Plasma was prepared from blood collected in heparin. Initially, the plasma was filtered (pore size, 0.45 μm) and cell-free RNA was extracted directly from filtered plasma with the viral RNA kit (Qiagen) and eluted in 50 μl of water as specified by the manufacturer. However, product formation after RT of competitor RNA and PCR amplification was frequently inhibited in the presence of RNA extracted from plasma in this manner, suggesting the presence of an inhibitory substance, which we presumed to be residual heparin. Amplification was substantially improved when the virus was isolated from plasma by ultracentrifugation before extraction. Therefore, when duplicate plasma samples were available, virus was sedimented from 140 μl of filtered plasma (213,000 × g for 20 min at 4°C) in duplicate and resuspended in 140 μl of RPMI 1640 prior to extraction of viral RNA with the viral RNA kit. When the quantification of RNA extracted directly from plasma was unavoidable, satisfactory results were obtained by diluting RNA by a factor of 1/10 before the RT step. RNA was aliquoted and stored at −80°C.
(ii) Tissue RNA.
Frozen splenic and thymic tissues were reduced to powder and immediately dispersed in lysis buffer, provided with the RNA extraction kit (RNeasy; Qiagen) by using a tissue homogenizer (Ultra-Turrax). Frozen axillary lymph nodes were dispersed directly in lysis buffer. RNA was subsequently extracted with the RNeasy kit as specified by the manufacturer. Contaminating DNA was removed by hydrolysis with DNase I (RQ1 RNase-free DNase; Promega).
Competitive RT-PCR.
Viral RNA in plasma and tissues was quantified by competitive RT-PCR. A conserved region of the gag gene of FIV was selected as the target sequence for RT and nested PCR amplification. Amplification of wild-type template yielded an initial product of 312 bp and a nested product of 165 bp, corresponding to nucleotides 1059 to 1370 and nucleotides 1157 to 1321, respectively, of the 34TF10 molecular clone (66).
(i) Molecular constructions.
The 312-bp target sequence was amplified from a plasmid (pKSgag) containing the entire gag gene of the Wo strain of FIV (52) by using a 5′ primer comprising the 5′ sequence of the target template and the restriction endonuclease site KpnI and a 3′ primer comprising the 3′ sequence of the target template and an XbaI site. The amplification product was subcloned into the corresponding sites of pBluescript KS+, yielding pBSQCgag.
To prepare a template for the synthesis of competitor RNA, a deletion of 31 nucleotides was introduced into the gag sequence by PCR. The 3′ portion of the wild-type sequence was amplified with a 5′ primer, SHΔWoG128, complementary to the natural HindIII site (nucleotides 1241 to 1246) and bearing a 31-nucleotide discontinuity, and a 3′ primer, RXWoG139, comprising the 3′ sequence of the target template and an XbaI site. Competitor templates have been prepared in a similar fashion by Pistello et al. (54) and Diehl et al. (19). Wild-type template, 10 ng of pBSQCgag, was amplified in a final volume of 100 μl containing 2.5 mM MgCl2, each deoxynucleoside triphosphate at 200 μM, 1× commercial buffer, and 2.5 U of Taq polymerase (both from Gibco BRL), and each primer at 0.2 μM. DNA was denatured at 94°C for 3 min, subjected to 30 amplification cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 30 s), and elongated at 72°C for 7 min. The sequences of the primers were as follows: SHΔWoG128, GGATGAAAGCTTAAAG/CCCCTGATGGTCCTAGAC (1235 to 1250/1282 to 1299); and RXWoG139, GCTCTAGATCTTGCTTCTGCTTGTTGTTCTTGAG (1370 to 1345). The amplification product was purified, digested with HindIII and XbaI, and substituted for the corresponding fragment of pBSQCgag, yielding pBSQCΔgag.
(ii) Synthesis of competitor RNA.
Competitor RNA was synthesized as the runoff transcription product of pBSQCΔgag linearized with XbaI by using T3 RNA polymerase (Promega Gemini kit). DNA template was hydrolyzed with RQ1 RNase-free DNase (Promega). Competitor RNA was purified by absorption to silica (RNeasy) and quantified by measurement of the absorbance at 260 nm. RNA was aliquoted and stored at −80°C.
(iii) Competitive RT-PCR.
Competitive RT-PCR and determination of the copy number of viral RNA were performed as described elsewhere (58).
Statistical analyses.
The cumulative area under the curve (16) was calculated for the plots of viral RNA copies per milliliter plasma against time after both 3 and 4 weeks of infection and compared by the Mann-Whitney test.
Sequence analysis of virus obtained postchallenge.
To determine whether immunization with the TM3 peptide had resulted in the selection of divergent virus, sequences of the TM3 domain were determined postchallenge for cats immunized with the TM3 peptide, a control cat, and the viral stock used for challenge. Viral RNA was isolated from plasma and subjected to RT as described above. Residues 8277 to 8912 (numbered according to the 34TF10 sequence) encompassing the TM3 domain were amplified by seminested PCR with Pwo DNA polymerase (Boehringer Mannheim). The primers were, in the first round of amplification, Spenew (5′-CATCAAGTACTAGTAATAGGATTAAA-3′) (8277 to 8302) and Asnde (5′-GATTTGATTCGAAATGGATTCATATGAC-3′) (8928 to 8901), and in the second round, Spenew and NdeWo (5′-GATTCATATGACATACCTTCCTCAAAG-5′) (8912 to 8886). The amplification products were purified and cloned by using the pCR-Script Amp SK (+) cloning kit (Stratagene). Positive clones were identified by hybridization, and five independent clones were sequenced for each cat at each time point examined.
RESULTS
Development of the humoral response to envelope peptides.
To assess the influence of humoral immunity to the envelope glycoproteins on the course of primary FIV infection, cats were immunized with peptides (Fig. 1) corresponding to envelope domains comprising continuous B-cell epitopes recognized during natural infection. All the immunized cats developed strong antibody responses, as determined by ELISA on envelope peptides used as immunogens (Table 1). On the day of challenge, titers (expressed as the logarithm of the reciprocal of serum dilution) ranged from 4.40 to 5.61. Recognition of envelope glycoproteins was assessed by immunoprecipitation of biosynthetically labelled envelope glycoprotein with sera collected on the day of challenge, as summarized in Table 1. Sera from all cats immunized with the SU5 peptide and three of four cats (cats 324, 325, and 327) immunized with the SU2 peptide immunoprecipitated envelope glycoproteins of the Petaluma strain from lysates of chronically infected FL-4 cells. Since the TM3 peptide, deduced from the env sequence from the Wo strain of FIV, differed in 4 of 19 residues from the Petaluma sequence, prechallenge sera of cats immunized with the TM3 peptide were tested by immunoprecipitation of envelope glycoproteins from lysates of PBMC acutely infected with the Wo strain of FIV. While sera from only two cats (cats 346 and 347) immunized with the TM3 peptide were observed to precipitate the Wo envelope, immunoprecipitation of viral envelope from acutely infected PBMC is generally less efficient than from chronically infected lymphoblastoid cell lines.
TABLE 1.
Antibody response to peptides during immunization
| Group and cat no. | Anti-peptide
titera on day
of:
|
Immunoprecipitationb on day of challenge | ||
|---|---|---|---|---|
| 1st boost | 2nd boost | Challenge | ||
| SU2 | ||||
| 324 | 4.71 | 5.01 | 5.61 | + |
| 325 | 5.01 | 5.31 | 5.01 | + |
| 326 | 4.40 | 4.71 | 5.31 | − |
| 327 | 5.31 | 5.01 | 4.71 | + |
| SU5 | ||||
| 328 | 5.01 | 5.31 | 4.71 | + |
| 329 | 5.31 | 5.31 | 5.01 | + |
| 340 | 4.71 | 5.01 | 5.01 | + |
| 341 | >5.61 | 5.31 | 5.01 | + |
| TM3 | ||||
| 346 | 4.71 | 4.71 | 4.40 | + |
| 347 | 5.01 | 5.31 | 4.71 | + |
| 348 | 5.31 | 5.01 | 4.40 | − |
| 349 | NDc | 3.20 | 4.40 | − |
The titer was expressed as the logarithm of the reciprocal of the last serum dilution for which the absorbance at 405 nm was greater than 0.1.
Immunoprecipitation of envelope glycoproteins from metabolically labeled FL-4 cells (SU2 and SU5 groups) or acutely infected PBMC (TM3 group).
ND, not done.
Activity of antibodies raised against envelope peptides in a biological assay.
To assess antibody function, feline sera raised against the SU2 peptide were assayed for antibodies neutralizing the infection of CrFK by the Petaluma strain of FIV. Only one cat (cat 327) immunized with the SU2 peptide developed neutralizing antibodies: the last doubling dilution reducing infection by at least 50% was 1/160. Sera of cats immunized with the SU5 and TM3 peptides did not neutralize the Petaluma strain, as measured in the CrFK cell line (data not shown). Due to the sequence diversity in the envelope glycoproteins of the Petaluma and Wo strains, notably in the TM3 domain, we also examined the functional activity of antibodies in a homologous assay. Since the Wo strain has not been adapted for passage in CrFK cells, the influence of antibodies on infection of feline PBMC was examined. In a reduction-of-infectivity assay with mitogen-activated PBMC, sera directed against the SU2 and TM3 peptides did not modify infection by the Wo strain (data not shown), despite the neutralizing activity which had been observed for one serum sample raised against the SU2 peptide when CrFK cells were used as the cellular substrate.
Isolation of FIV after viral challenge.
After challenge, all the cats became productively infected, as determined by successful isolation of FIV from plasma collected 2 to 3 weeks after challenge (data not shown).
Seroconversion after viral challenge.
After challenge with the Wo strain of FIV, most cats mounted a humoral response to viral components, as assessed by ELISA on SU2 and TM2 peptides, derived from the external and transmembrane glycoproteins, respectively, and on recombinant capsid protein (Table 2). The development of the antiviral humoral response was weak and delayed in three of four cats (cats 346, 348, and 349) immunized with the TM3 peptide compared with the response of the control cats. Five weeks after challenge, none of the cats immunized with the TM3 peptide had developed a detectable antibody response against the SU2 peptide whereas all the control cats had detectable responses. While most (11 of 12) cats that had been immunized with the SU2 or SU5 peptide or that had received only adjuvant developed a strong response to the TM2 peptide at 4 weeks postchallenge, all but one cat (cat 347) immunized with the TM3 peptide had weak or undetectable responses. Whereas at 5 weeks all the control cats had responded well to p24, one cat (cat 348) immunized with the TM3 peptide had not responded and, of the remaining three cats, two (cats 346 and 349) had developed only weak responses.
TABLE 2.
Seroconversion
| Group and cat no. | Binding of sera to viral components as a function of
time after challengea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SU2
|
TM2
|
p24
|
|||||||
| 3 wk | 4 wk | 5 wk | 3 wk | 4 wk | 5 wk | 3 wk | 4 wk | 5 wk | |
| SU2 | |||||||||
| 324 | —b | — | — | 0 | 2.1 | 2.5 | 0 | 0.2 | 1.9 |
| 325 | — | — | — | 0 | 1.4 | 2.1 | 0 | 0 | 0.2 |
| 326 | — | — | — | 0 | 1.3 | 2.1 | 0 | 0.2 | 1.1 |
| 327 | — | — | — | 0 | 2.2 | 2.3 | 0 | 0.4 | 1.3 |
| SU5 | |||||||||
| 328 | 0 | 1.9 | 2.4 | 2.3 | 2.9 | 3.0 | 0.1 | 2.7 | 2.9 |
| 329 | 0 | 0.5 | 0.7 | 0 | 2.8 | 3.1 | 0 | 1.0 | 2.2 |
| 340 | 0 | 0 | 0.1 | 0.1 | 2.4 | 2.8 | 0 | 0.2 | 0.8 |
| 341 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TM3 | |||||||||
| 346 | 0 | 0 | 0 | 0 | 0.1 | 1.9 | 0 | 0.1 | 0.6 |
| 347 | 0 | 0 | 0 | 1.2 | 2.4 | 2.5 | 0.2 | 2.3 | 2.3 |
| 348 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 349 | 0 | 0 | 0 | 0 | 0.5 | 2.3 | 0.1 | 0 | 0.1 |
| Adjuvant | |||||||||
| 354 | 0 | 2.4 | 3.0 | 0 | 2.6 | 3.0 | 0 | 0 | 2.6 |
| 355 | 0 | 0.2 | 0.2 | 0.1 | 2.3 | 3.1 | 0 | 0.1 | 2.0 |
| 356 | 0.4 | 0.3 | 0.3 | 2.2 | 3.0 | 3.1 | 0 | 0.5 | 1.4 |
| 357 | 0 | 0.2 | 1.0 | 0.2 | 2.9 | 3.1 | 0.1 | 0.9 | 2.5 |
Data were obtained by ELISA on envelope peptides SU2 and TM2 and on recombinant FIV capsid protein (p24) for serum dilutions of 1/25 (peptides) and 1/100 (p24) and are expressed as optical density as described in Materials and Methods.
—, Data not shown for the group of cats vaccinated with the SU2 peptide and thus seropositive prior to challenge.
Hematological alterations during acute infection.
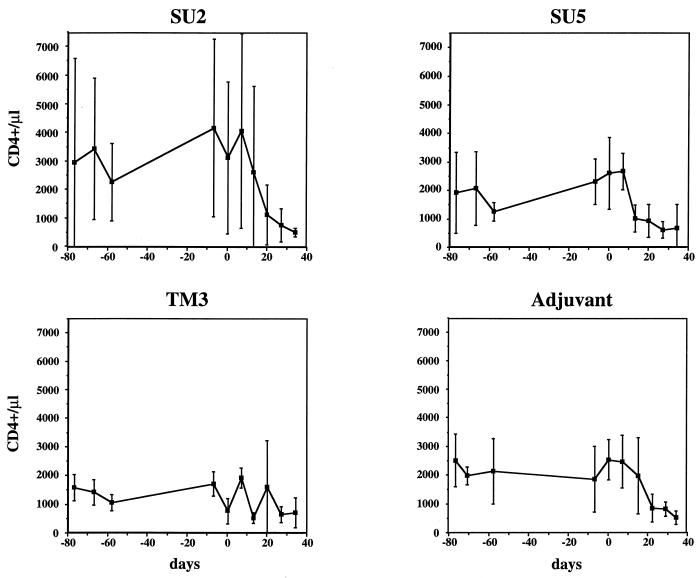
To assess the effect of immunization on pathology associated with primary infection, circulating CD4+ lymphocytes were enumerated before and after challenge with the Wo strain of FIV. Mean values for the number of circulating CD4+ lymphocytes during the immunization period and upon challenge are shown in Fig. 2. Although the trends were somewhat obscured by the large variation among cats within a given group, as well as in individual cats over time (data not shown), most cats immunized with the SU2 and SU5 peptides, as well as cats which had received only adjuvant, endured a progressive reduction in the number of CD4+ lymphocytes following infection (Fig. 2). By contrast, diminution in the population of CD4+ lymphocytes, despite considerable fluctuation, was not as marked in cats in the TM3 group (Fig. 2). Although mean CD4+ counts appeared to be low in the TM3 group on the day of challenge, cats with low CD4+ counts were also found in the other groups. No correlation was observed between the number of circulating CD4+ lymphocytes at the time of challenge and the subsequent course of infection.
FIG. 2.
Lymphocyte subset analysis. The number of circulating CD4+ lymphocytes during immunization and, following challenge (day 0), during acute infection are shown. Data are given as means. Error bars represent standard deviations.
Histology.
Histological analysis of the mesenteric lymph nodes and spleen revealed little difference among the groups of cats 5 weeks after challenge (data not shown). The most commonly encountered lesions of lymph nodes were follicular and paracortical lymphoid hyperplasia (in 14 of 16 and 13 of 16 cats, respectively). The histological lesions observed in the spleen, atrophy (five cases) or hyperplasia (one case) of the lymphoid sheath and ellipsoid hyperplasia (nine cases), were observed in all groups without distinction. Whereas no pathological changes were observed in thymic tissue of cats immunized with the TM3 peptide and control cats, three of eight cats immunized with the SU peptides displayed lesions; these included lymphoid hypoplasia with vanishing of the corticomedullary boundary (one case), infiltration of medullary tissue by lymphocytes (one case), and the presence of degenerating and necrotic foci (one case).
Viral burden in plasma as determined by competitive RT-PCR.
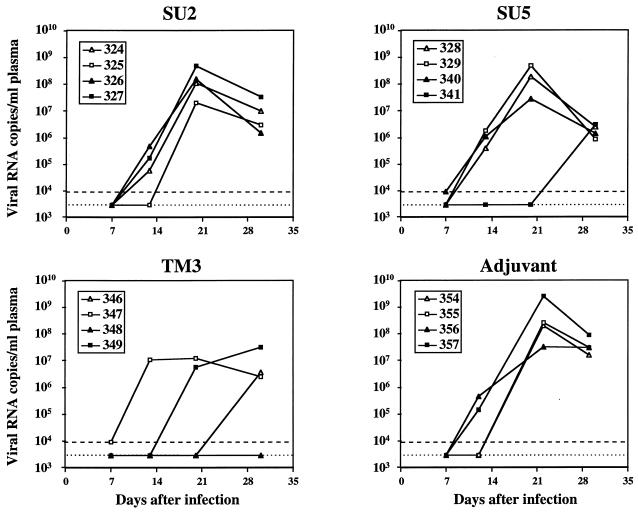
Since we did not expect to achieve sterilizing immunity by vaccination with single domains of the envelope glycoproteins, protection was assessed by quantitative analysis of the viral burden. A competitive RT-PCR was developed for the determination of viral RNA in plasma and tissues. The assay permits the detection of 10 copies of RNA in one reaction (data not shown), which sets the lower limit of detection at 1,430 RNA copies/ml of plasma when viral RNA is extracted from 140 μl of plasma. Upon challenge with 10 CID50 of the Wo isolate, viral RNA was detectable in plasma by RT-PCR as early as 2 weeks after infection. In cats that had received only adjuvant, the number of viral RNA copies generally peaked at 3 weeks and declined thereafter (Figure 3). The extent of viral dissemination, whether absolute values of viral RNA or the time course of infection was considered, was in good agreement with observations of Diehl et al. (19). The presence before challenge of antibodies directed against a domain known to elicit neutralizing antibodies, SU2, did not appear to alter the course of primary infection. In cats immunized with the SU5 peptide, acute infection of three cats followed kinetics similar to those of control cats while infection of one cat was delayed. The course of infection in cats immunized with the TM3 peptide was generally different from that in control cats. In three of four cats (cats 346, 348, and 349), infection was delayed. In fact, we did not detect viral RNA in the plasma of one cat (cat 348) during the period of measurement.
FIG. 3.
Viral burden in plasma during primary infection. The viral burden, as determined by competitive RT-PCR, is expressed as the number of copies of viral RNA per milliliter plasma. The dotted line, denoting plasma samples for which viral RNA was not detected, is placed at 2,860 copies/ml, which corresponds to the threshold of detection when plasma was diluted 1/2 (see Materials and Methods). Since plasma samples collected at 3 weeks had to be diluted 1/10 as a result of an inhibitory substance, the viral burden in the three 3-week samples for which no viral RNA was detected must be considered to be less than 14,300 copies/ml of plasma. Plasma samples for which viral RNA, although detected, was present at less than 30 copies per reaction are denoted by a dashed line, corresponding to 8,580 copies/ml of plasma.
We have analyzed the development of acute viremia by determining the cumulative area under the curve (16), a powerful method for the analysis of quantitative measurements made over time. This method permits the simultaneous consideration of both variation in the viral burden over time and the absolute burden attained. The comparison of the values obtained (by a nonparametric test) provides a single criterion by which a delay and/or a quantitative difference may be ascertained. We have used the Mann-Whitney test to compare the cumulative area under the curve of RNA copy number for the TM3 and control immunization groups at 3 and 4 weeks after challenge. The reduction in the cumulative viral burden observed in cats immunized with the TM3 peptide was significant at both 3 and 4 weeks (P = 0.02).
Plasma viremia.
For cats immunized with the TM3 peptide and control cats, the viral burden in plasma was assessed 3 weeks after challenge by isolation of virus. The number of copies of viral RNA determined by competitive RT-PCR is compared with plasma viremia determined by PBMC culture in Table 3. Immunization with the TM3 peptide prior to viral challenge resulted in a marked reduction in the levels of infectious virus in plasma early in primary infection. This reduction was of the same order as that observed in viral RNA copy numbers. These data confirm that the reduced levels of viral RNA observed for cats immunized with the TM3 peptide in comparison with control cats reflected reduction in the plasma titer of infectious virus. Moreover, comparison of these values by the Mann-Whitney test revealed that the reduction in the viral burden in the TM3 group was statistically significant whether RNA copy number (P = 0.02) or infectious titer (P = 0.017) was considered.
TABLE 3.
Plasma viremia 3 weeks after infection
| Group and cats no. | Plasma viremia by:
|
|
|---|---|---|
| RT-PCRa | PBMC cultureb | |
| TM3c | ||
| 346d | <1.43 × 104 | 1 |
| 347 | 1.19 × 107 | 1 × 102 |
| 348d | <1.43 × 104 | 1 |
| 349 | 5.56 × 106 | 1 |
| Adjuvant | ||
| 354 | 1.93 × 108 | 5 × 103 |
| 355 | 2.43 × 108 | 5 × 102 |
| 356 | 3.12 × 107 | 5 × 103 |
| 357 | 2.54 × 109 | 5 × 104 |
Plasma viremia as measured by competitive RT-PCR and expressed as the number of copies of viral RNA per milliliter of plasma.
Plasma viremia as measured by PBMC culture and expressed as TCID50 per milliliter of plasma.
The reduction in viral burden in the TM3 group was statistically significant whether RNA copy number (P = 0.02) or infectious titer (P = 0.017) was considered.
Since no viral RNA was detected at 3 weeks by RT-PCR for cats 346 and 348, the estimated threshold of detection for these samples is indicated (see the legend to Fig. 3).
Viral burden in tissues as determined by competitive RT-PCR.
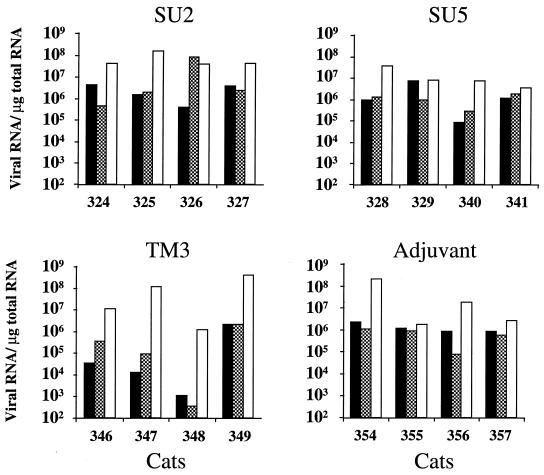
The viral burden in tissues was assessed 5 weeks after challenge by enumeration of the viral RNA copies in axillary lymph nodes, spleen, and thymus by competitive RT-PCR. Since the amplicon is located in the intron of subgenomic viral RNA, viral RNA copies enumerated in tissues represented full-length transcripts, either intracellular or encapsidated in extracellular viral particles. Viral RNA was detected in all tissues examined in all cats (Fig. 4). The levels of viral RNA in axillary lymph nodes were lower in three cats (cats 346, 348, and 349) immunized with the TM3 peptide than in cats immunized with the SU2 and SU5 peptides and in control cats. The level of viral RNA in the spleen was particularly low in one cat (cat 348) immunized with the TM3 peptide. In the thymus, however, the viral burden was similar in the four groups of cats and, generally speaking, higher than in the other tissues examined. A high viral burden in the thymus early in infection has also been observed by in situ hybridization (3). It is not clear whether the apparent preferential infection of thymic tissue reflects its richness in the cell types most prominently infected early in infection, a high capacity for trapping viral particles, or (since tissues were examined before the resolution of acute infection) the sequential nature of colonization.
FIG. 4.
Viral burden in lymphoid tissues. The viral burden in lymphoid tissues 5 weeks after challenge, as determined by competitive RT-PCR, is expressed as the number of copies of viral RNA per microgram of total RNA. Symbols: ▪, Axillary ganglion; , spleen; □, thymus.
Sequence analysis of virus postchallenge.
Since viral dissemination was rapid in one cat (cat 347) immunized with the TM3 peptide, we considered whether immunization with the TM3 peptide resulted in postchallenge selection of virus bearing divergent TM3 domain sequences, possibly as a consequence of heterogeneity in the viral inoculum. We examined TM3 domain sequences of virus isolated from a control cat, cats immunized with the TM3 peptide, and the challenge stock. Analysis of the viral inoculum and, at 4 weeks postinfection, plasma from two cats (cats 346 and 349) immunized with the TM3 peptide and one cat (cat 357) that had received only adjuvant revealed no polymorphism in primary structure. Furthermore, examination of the cat (cat 347) from the TM3 group in which infection was rapidly established revealed no evidence for selection of divergent TM3 sequences either 2 or 4 weeks after challenge. We were unable to detect viral RNA in the remaining cat (cat 348) in the TM3 group.
Comparison of the primary structures of FIV and HIV-1.
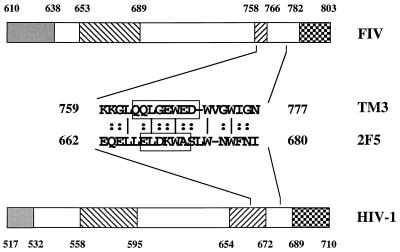
Alignment of amino acid sequences of HIV-1 and FIV transmembrane glycoproteins showed that the TM3 domain of the FIV envelope could be juxtaposed with a region of the HIV-1 envelope which comprises an epitope, ELDKWA, recognized by a broadly neutralizing human monoclonal antibody (14, 46, 55, 67). Comparison of the TM3 and HIV-1 domains revealed a degree of similarity in primary structure (5 of 20 TM3 residues identical) and analogy in the location of B-cell epitopes: the continuous epitope (QQLQEWED) of the TM3 domain best recognized during natural infection by FIV (1) may be closely aligned with the ELDKWA sequence (Fig. 5).
FIG. 5.
Diagrammatic representation of the ectodomain and membrane-spanning domain of the transmembrane glycoproteins of HIV-1 (LAI) and FIV (Wo) and alignment of the FIV TM3 peptide with an HIV-1 peptide comprising the 2F5 B-cell epitope. The TM3 epitope (QQLGEWED) best recognized during FIV infection and the 2F5 epitope (ELDKWA) are indicated. Symbols: ░⃞, fusion peptide; ▧, leucine zipper region; ▨, membrane-proximal α-helix; , membrane-spanning domain.
DISCUSSION
In this paper, we describe the influence of immunity to three domains of the viral envelope on acute infection by FIV. Immunization with two peptides derived from the surface glycoprotein did not alter the early course of infection. However, host immunity mounted against a peptide (TM3) derived from the extracellular membrane-proximal domain of the transmembrane glycoprotein delayed the dissemination of virus, as reflected by a reduced viral burden and reduced seroreactivity in the early phase of primary infection. The corresponding region of the HIV-1 envelope bears an epitope recognized by a human monoclonal antibody (2F5) that neutralizes primary and laboratory-adapted strains of HIV-1 from diverse clades (14, 46, 55, 67).
The induction of antibodies which neutralize viral infection in vitro is often a stated objective in efforts to achieve protective immunity. Nevertheless, the relationship between in vitro neutralization and in vivo protection is unresolved, owing, at least in part, to the diversity of neutralizing assays currently practiced. The extent of neutralization depends upon the assay conditions used, particularly on the cellular substrate used to measure residual infectivity and the passage history of the virus (2). Lentivirus adapted for propagation in cultured cells is more sensitive than primary isolates to neutralizing antibodies of diverse specificity (2, 43, 44, 65, 74), and this differential sensitivity is likely to reflect differences which are not purely quantitative (68). In the chimpanzee model, neutralization of challenge virus appeared to be correlated with protection against laboratory-adapted virus (6, 22) but not with protection against primary virus (7, 23). In macaques, protection against challenge with SIV has been correlated with the presence of antibodies that neutralize primary or heterologous pathogenic strains of SIV (12, 75) but not with antibodies that neutralize laboratory-adapted strains (15, 25, 31, 60). For FIV, protection against laboratory-adapted virus has sometimes but not always been correlated with neutralization of laboratory-adapted virus (28–30, 76, 78). Protection against a primary isolate of FIV has not consistently been associated with the induction of antibodies neutralizing laboratory-adapted or primary virus (40).
In the present study, induction of neutralizing antibodies following immunization with peptides coupled to KLH, whether assessed against laboratory-adapted or primary FIV isolates, was inefficient. Only one (cat 328) of four cats immunized with the SU2 peptide developed detectable levels of neutralizing antibodies when neutralization of laboratory-adapted virus was assessed in a fibroblastoid cell line. Viral dissemination in this cat was indistinguishable from that observed in control cats. Similar results have been obtained by others: while immunization with a peptide derived from the SU2 domain consistently elicited antibodies that neutralized virus adapted for culture in feline fibroblasts, such immunization not only failed to protect cats from infection but also may have augmented the viral burden during primary infection (37). By contrast, in the present study, infection was delayed in cats immunized with the TM3 peptide, although the sera of these cats did not reduce the infectivity of the homologous primary virus, as measured in mitogen-activated PMBC. Although the suppression of viral dissemination observed in vivo cannot be formally attributed to antibody, it is unlikely that a substantial level of cell-mediated immunity was generated by immunization with peptide emulsified in the adjuvant used (Freund’s adjuvant). Neutralizing antibodies may have been induced at concentrations which were ineffective when infection of highly activated cells by primary virus was considered. It is also conceivable that antibodies delayed acute FIV infection by mechanisms unrelated to neutralization, such as antibody-mediated cellular cytotoxicity or opsonization.
Transmembrane glycoproteins of retroviruses are thought to possess a common structural framework (21). The solution of the crystal structure of two peptides from the ectodomain of the transmembrane glycoprotein of HIV-1 has suggested that in oligomeric envelope, three membrane-proximal α-helices are packed against a coiled coil composed of three amphipathic α-helices from the amino-terminal portion of the transmembrane glycoprotein (11, 71). In view of its stability, this structure has been suggested to be the conformation of oligomeric transmembrane glycoproteins which is competent for membrane fusion. Peptides corresponding to the membrane-proximal α-helix are potent inhibitors of cell-cell fusion mediated by the HIV-1 envelope (34, 73). Such peptides may conceivably inhibit the association between amphipathic and membrane-proximal α-helices and interfere with the formation of the fusion-competent conformation (41). Similarly, peptides derived from the membrane-proximal region of the FIV transmembrane glycoprotein inhibited the replication of FIV in feline fibroblasts, although not in a feline lymphoblastoid cell line (38). Antibodies directed against amino-terminal and juxtamembrane peptides of HIV-1 have been suggested to be associated with protection from disease (35, 69). The HIV-1 epitope recognized by the monoclonal antibody 2F5 and the TM3 peptide lies at the carboxyl terminus of the juxtamembrane α-helix in the transmembrane glycoproteins of HIV-1 and FIV, respectively (Fig. 5). Binding of the 2F5 antibody may diminish the accessibility of the fusion domain of gp41 and some domains of gp120, including the CD4 binding site (47). Stoiber et al. (64) have shown that this antibody may inhibit the binding of complement factor H, a negative regulator of complement activation, to the envelope of HIV-1, thus enhancing complement-mediated lysis. While convergent data underscores the importance of the juxtamembrane domain to lentivirus envelope function, it is unclear how the immune response directed to the TM3 domain of FIV suppresses viral dissemination.
In animal models of lentivirus infection, passive immunity to defined B-cell epitopes has sometimes afforded protection or suppressed the viral burden. Passive immunity conferred by monoclonal antibodies directed against V3 loop peptides protected chimpanzees against challenge with a laboratory-adapted strain of HIV-1 (20). Notably, the administration of the 2F5 monoclonal antibody delayed viremia in chimpanzees infected with a primary strain of HIV-1 (13). Shafferman et al. (61) described a reduction in the viral burden in macaques after immunization with four envelope peptides expressed as fusion proteins. Suppression of the viral burden appeared to be associated with an antibody response to a peptide comprising the 2F5 epitope. Here we document a delay in lentivirus infection after active immunization with a single small antibody binding domain. That this delay occurred after infection with a primary virus representing a natural pathogen for the host species suggests that immunization with the TM3 peptide could contribute to the induction of immune responses which would limit viral dissemination after natural exposure. This peptide could be incorporated in a vaccine in association with viral subunits other than the envelope, or with envelope glycoproteins to improve the anti-envelope response to the TM3 domain. The humoral response to the TM3 domain of the FIV envelope and to the homologous domain of the HIV-1 envelope is weak during natural infection by FIV (1) and HIV-1 (10), respectively. We did not address the consequence of slowing viral dissemination during the primary phase of infection on disease progression. However, Hirsh et al. (26) have shown that induction of host immunity which reduces plasma viremia during acute infection, particularly when associated with sustained restriction of viral replication, was associated with long-term asymptomatic survival in the rhesus macaque model.
Whereas immunization with a 19-residue peptide from the transmembrane glycoprotein delayed the dissemination of FIV, accelerated infection after immunization with recombinant envelope glycoproteins (62) and genetic immunization with the env gene (58) has been described. It is possible that envelope glycoproteins induce immune responses with counteracting influences on viral dissemination. The present study suggests that domains which elicit predominately protective responses exist and that such domains may not always be predicted by functional assays conducted in vitro.
ACKNOWLEDGMENTS
We thank Dominique Costagliola for advice on statistical analyses and Colombe Chappey and Luc Camoin for assistance with sequence alignment. We are grateful to Karine Goude for determination of plasma viral titer and to Thierry Leste-Lasserre for analysis of env sequences during acute infection. We also thank Sylvie Manin and Jean-Pierre Legrand for performing hematological tests and lymphocyte subset analyses, and we thank Lydia Duarte, Corinne Melin, and Marie-Christine Romary for providing animal care and for assisting with veterinary procedures. We thank A. D. Strosberg for continuous support and Quentin Sattentau for having critically read the manuscript.
J.R. was supported by grants from the Agence Nationale de Recherches sur le SIDA and from Sidaction. This work was supported by the Ministère de la Recherche et de la Technologie (Saut Technologique 95T0007) and by a Biomed 2 grant from the European Economic Community.
REFERENCES
- 1.Avraméas A, Strosberg A D, Moraillon A, Sonigo P, Pancino G. Serological diagnosis of feline immunodeficiency virus (FIV) infection based on synthetic peptides from Env glycoproteins. Res Virol. 1993;144:209–218. doi: 10.1016/s0923-2516(06)80031-2. [DOI] [PubMed] [Google Scholar]
- 2.Baldinotti F, Matteucci D, Mazzetti P, Giannelli C, Bandecchi P, Tozzini F, Bendinelli M. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J Virol. 1994;68:4572–4579. doi: 10.1128/jvi.68.7.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beebe A M, Dua N, Faith T G, Moore P, Pedersen N, Dandekar S. Primary stage of feline immunodeficiency virus infection: viral dissemination and cellular targets. J Virol. 1994;68:3080–3091. doi: 10.1128/jvi.68.5.3080-3091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beretta A, Furci L, Burastero S, Cosma A, Dinelli M E, Lopalco L, De Santis C, Tambussi G, Carrow E, Sabbatani S, Clerici M, Lazzarin A, Siccardi A G. HIV-1-specific immunity in persistently seronegative individuals at high risk for HIV infection. Immunol Lett. 1996;51:39–43. doi: 10.1016/0165-2478(96)02553-9. [DOI] [PubMed] [Google Scholar]
- 5.Beretta A, Weiss S H, Rappocciolo G, Mayur R, De Santis C, Quirinale J, Cosma A, Robbioni P, Shearer G M, Berzofsky J A, Villa M L, Siccardi A G, Clerici M. Human immunodeficiency virus type 1 (HIV-1)-seronegative injection drug users at risk for HIV exposure have antibodies to HLA class I antigens and T cells specific for HIV envelope. J Infect Dis. 1996;173:472–479. doi: 10.1093/infdis/173.2.472. [DOI] [PubMed] [Google Scholar]
- 6.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 7.Berman P W, Murthy K K, Wrin T, Vennari J C, Cobb E K, Eastman D J, Champe M, Nakamura G R, Davison D, Powell M F, Bussiere J, Francis D P, Matthews T, Gregory T J, Obijeski J F. Protection of MN-rgp120-immunized chimpanzees from heterologous infection with a primary isolate of human immunodeficiency virus type 1. J Infect Dis. 1996;173:52–59. doi: 10.1093/infdis/173.1.52. [DOI] [PubMed] [Google Scholar]
- 8.Bou-Habib D M, Roderiquez G, Oravecz T, Berman P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broder C C, Earl P L, Long D, Abedon S T, Moss B, Doms R W. Antigenic implications of human immunodeficiency virus type 1 envelope quaternary structure: oligomer-specific and -sensitive monoclonal antibodies. Proc Natl Acad Sci USA. 1994;91:11699–11703. doi: 10.1073/pnas.91.24.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broliden P-A, von Gegerfelt A, Clapham P, Rosen J, Feniö E-M, Wahren B, Broliden K. Identification of human neutralization inducing regions of the human immunodeficiency virus type 1 envelope glycoproteins. Proc Natl Acad Sci USA. 1992;89:461–465. doi: 10.1073/pnas.89.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 12.Clements J E, Montelaro R C, Zink M C, Amedee A M, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conley A J, Kessler J A I, Boots L J, McKenna P M, Schleif W A, Emini E A, Mark G E I, Katinger H, Cobb E K, Lunceford S M, Rouse S R, Murthy K K. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J Virol. 1996;70:6751–6758. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conley A J, Kessler J A I, Boots L J, Tung J-S, Arnold B A, Keller P M, Shaw A R, Emini E A. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc Natl Acad Sci USA. 1994;91:3348–3352. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel M D, Mazara G P, Simon M A, Sehgal P K, Kodama T, Panicali D L, Desrosiers R C. High-titer immune responses elicited by recombinant vaccinia virus priming and particle boosting are ineffective in preventing virulent SIV infection. AIDS Res Hum Retroviruses. 1994;10:839–851. doi: 10.1089/aid.1994.10.839. [DOI] [PubMed] [Google Scholar]
- 16.Dawson J D. Comparing treatment groups on the basis of slopes, areas-under-the curve, and other summary measures. Drug Inf J. 1994;28:723–732. [Google Scholar]
- 17.de Ronde A, Stam J G, Boers P, Langedijk H, Meloen R, Hesselink W, Lian C E, Keldermans J M, van Vliet A, Verschoor E J, Horzinek M C, Egberink H F. Antibody response in cats to the envelope proteins of feline immunodeficiency virus: identification of an immunodominant neutralization domain. Virology. 1994;198:257–264. doi: 10.1006/viro.1994.1028. [DOI] [PubMed] [Google Scholar]
- 18.de Santis C, Lopalco L, Robbioni P, Longhi R, Rappocciolo G, Siccardi A G, Beretta A. Human antibodies to immunodominant C5 region of HIV-1 gp120 cross-react with HLA class 1 on activated cells. AIDS Res Hum Retroviruses. 1994;10:157–162. doi: 10.1089/aid.1994.10.157. [DOI] [PubMed] [Google Scholar]
- 19.Diehl L J, Mathiason-DuBard C K, O’Neil L, Hoover E A. Longitudinal assessment of feline immunodeficiency virus kinetics in plasma by use of a quantitative competitive reverse transcriptase PCR. J Virol. 1995;69:2328–2332. doi: 10.1128/jvi.69.4.2328-2332.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, Eichbereg J W, Murthy K K. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 21.Gallaher W R, Ball J M, Barry R F, Griffin M C, Montelaro R C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989;5:431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- 22.Girard M, Kieny M P, Pinter A, Barré-Sinoussi F, Nara P, Kolbe H, Kusumi K, Chaput A, Reinhart T, Muchmore E, Ronco J, Kaczorek M, Gomard E, Gluckman J C, Fultz P N. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girard M, Meignier B, Barré-Sinoussi F, Kieny M-P, Matthews T, Muchmore E, Nara P L, Wei Q, Rimsky L, Weinhold K, Fultz P N. Vaccine-induced protection of chimpanzees against infection by a heterologous human immunodeficiency virus type 1. J Virol. 1995;69:6239–6248. doi: 10.1128/jvi.69.10.6239-6248.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goudsmit J, Debouck C, Meloen R H, Smit L, Bakker M, Asher D M, Wolff A V, Gibbs C J, Gajdusek C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci USA. 1988;85:4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heeney J L, Van Els C, De Vries P, Ten Haft P, Otting N, Koornstra W, Boes J, Dubbes R, Niphuis H, Dings M, Cranage M, Norley S, Jonker M, Bontrop R E, Osterhaus A. Major histocompatibility complex class I-associated vaccine protection from simian immunodeficiency virus-infected peripheral blood cells. J Exp Med. 1994;180:769–774. doi: 10.1084/jem.180.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch V M, Fuerst T R, Sutter G, Carrol M W, Yang L C, Goldstein S, Piatak M, Jr, Elkins V R, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho D D, Moudgil T, Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989;321:1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- 28.Hosie M J, Dunsford T H, De Ronde A, Willett B J, Cannon C A, Neil J C, Jarrett O. Suppression of virus burden by immunization with feline immunodeficiency virus Env protein. Vaccine. 1996;14:405–411. doi: 10.1016/0264-410x(95)00193-5. [DOI] [PubMed] [Google Scholar]
- 29.Hosie M J, Flynn J N. Feline immunodeficiency virus vaccination: characterization of the immune correlates of protection. J Virol. 1996;70:7561–7568. doi: 10.1128/jvi.70.11.7561-7568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosie M J, Osborne R, Yamamoto J K, Neil J C, Jarrett O. Protection against homologous but not heterologous challenge induced by inactivated feline immunodeficiency virus vaccines. J Virol. 1995;69:1253–1255. doi: 10.1128/jvi.69.2.1253-1255.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulskotte E G, Geretti A M, Siebelink K H, Van A G, Cranage M, Rud E W, Norley S G, De V P, Osterhaus A D. Vaccine-induced virus-neutralizing antibodies and cytotoxic T cells do not protect macaques from experimental infection with simian immunodeficiency virus SIVmac32H (J5) J Virol. 1995;69:6289–6296. doi: 10.1128/jvi.69.10.6289-6296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–73. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 33.Javaherian K, Langlois A J, McDanal C, Ross K L, Eckler L I, Jellis C L, Profy A T, Rusche J R, Bolognesi D P, Putney S D, Matthews T J. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci USA. 1989;86:6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang S, Lin K, Strick N, Neurath A R. HIV-1 inhibition by a peptide. Nature. 1993;365:113. doi: 10.1038/365113a0. [DOI] [PubMed] [Google Scholar]
- 35.Klasse P J, Pipkorn R, Blomberg J. Presence of antibodies to a putatively immunodeficiency virus (HIV) envelope glycoprotein gp41 is strongly associated with health among HIV-positive subjects. Proc Natl Acad Sci USA. 1988;85:5225–5229. doi: 10.1073/pnas.85.14.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lombardi S, Garzelli C, La Rosa C, Zaccaro L, Specter S, Malvaldi G, Tozzini F, Esposito F, Bendinelli M. Identification of a linear neutralization site within the third variable region of the feline immunodeficiency virus envelope. J Virol. 1993;67:4742–4749. doi: 10.1128/jvi.67.8.4742-4749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lombardi S, Garzelli C, Pistello M, Massi C, Matteucci D, Baldinotti F, Cammarota G, Da Prato L, Bandecchi P, Tozzini F, Bendinelli M. A neutralizing antibody-inducing peptide of the V3 domain of feline immunodeficiency virus envelope glycoprotein does not induce protective immunity. J Virol. 1994;68:8374–8379. doi: 10.1128/jvi.68.12.8374-8379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lombardi S, Massi C, Indino E, La Rosa C, Mazzetti P, Falcone M L, Rovero P, Fissi A, Pieroni O, Bandecchi P, Esposito F, Tozzini F, Bendinelli M, Garzelli C. Inhibition of feline immunodeficiency virus infection in vitro by envelope glycoprotein synthetic peptides. Virology. 1996;220:274–284. doi: 10.1006/viro.1996.0315. [DOI] [PubMed] [Google Scholar]
- 39.Mascola J R, Mathieson B J, Zack P M, Walker M C, Halstead S B, Burke D S. Summary report: workshop on the potential risks of antibody-dependent enhancement in human HIV vaccine trials. AIDS Res Hum Retroviruses. 1993;9:1175–1184. doi: 10.1089/aid.1993.9.1175. [DOI] [PubMed] [Google Scholar]
- 40.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Zaccaro P, Bandecchi P, Tozzini F, Bendinelli M. Vaccination protects against in vivo-grown feline immunodeficiency virus even in the absence of detectable neutralizing antibodies. J Virol. 1996;70:617–622. doi: 10.1128/jvi.70.1.617-622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews T J, Wild C, Chen C-H, Bolognesi D P, Greenberg M L. Structural rearrangements in the transmembrane glycoprotein after receptor binding. Immunol Rev. 1994;140:93–104. doi: 10.1111/j.1600-065x.1994.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 42.Montefiori D C. Role of complement and Fc receptors in the pathogenesis of HIV-1 infection. Springer Semin Immunopathol. 1997;18:371–390. doi: 10.1007/BF00813504. [DOI] [PubMed] [Google Scholar]
- 43.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore, J. P., and D. D. Ho. 1995. HIV neutralization: the consequence of viral adaptation to growth on transformed T cells. AIDS 9(Suppl A):S117–S136. [PubMed]
- 45.Moraillon A, Barre-Sinoussi F, Parodi A, Moraillon R, Dauguet C. In vitro properties and experimental pathogenic effect of three feline immunodeficiency viruses (FIV) isolated from cats with terminal disease. Vet Microbiol. 1992;31:41–54. doi: 10.1016/0378-1135(92)90140-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Rüker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neurath A R, Strick N, Lin K, Jiang S. Multifaceted consequences of anti-gp41 monoclonal antibody 2F5 binding to HIV type 1 virions. AIDS Res Hum Retroviruses. 1995;11:687–696. doi: 10.1089/aid.1995.11.687. [DOI] [PubMed] [Google Scholar]
- 48.Osborne R, Rigby M, Siebelink K, Neil J C, Jarrett O. Virus neutralization reveals antigenic variation among feline immunodeficiency virus isolates. J Gen Virol. 1994;75:3641–3645. doi: 10.1099/0022-1317-75-12-3641. [DOI] [PubMed] [Google Scholar]
- 49.Palker T J, Clark M E, Langlois A J, Matthews T J, Weinhold K J, Randall R R, Bolognesi D P, Haynes B F. Type-specific neutralization of the HIV with antibodies to env encoded synthetic peptides. Proc Natl Acad Sci USA. 1988;85:1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pancino G, Castelot S, Sonigo P. Differences in feline immunodeficiency virus (FIV) host cell range correlate with envelope fusogenic properties. Virology. 1995;206:796–806. doi: 10.1006/viro.1995.1002. [DOI] [PubMed] [Google Scholar]
- 51.Pancino G, Chappey C, Saurin W, Sonigo P. B epitopes and selection pressures in feline immunodeficiency virus envelope glycoproteins. J Virol. 1993;67:664–672. doi: 10.1128/jvi.67.2.664-672.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pancino G, Fossati I, Chappey C, Castelot S, Hurtrel B, Moraillon A, Klatzmann D, Sonigo P. Structure and variations of feline immunodeficiency virus envelope glycoproteins. Virology. 1993;192:659–662. doi: 10.1006/viro.1993.1083. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen N C, Ho E W, Brown M L, Yamamoto J K. Isolation of T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 54.Pistello M, Menzo S, Giorgi M, Da Prato L, Cammarota G, Clementi M, Bendinelli M. Competitive polymerase chain reaction for quantitating feline immunodeficiency virus load in infected cat tissues. Mol Cell Probes. 1994;8:229–234. doi: 10.1006/mcpr.1994.1032. [DOI] [PubMed] [Google Scholar]
- 55.Purtscher M, Trkola A, Gruber G, Buchacher A, Predl R, Steindl F, Tauer C, Berger R, Barrett N, Jungbauer A, Katinger H. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1994;10:1651–1658. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 56.Reid G, Rigby M A, McDonald M, Hosie M J, Neil J C, Jarrett O. Immunodiagnosis of feline immunodeficiency virus infection using recombinant viral p17 and p24. AIDS. 1991;5:1477–1483. doi: 10.1097/00002030-199112000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Richardson J, Fossati I, Moraillon A, Castelot S, Sonigo P, Pancino G. Neutralization sensitivity and accessibility of continuous B cell epitopes of the feline immunodeficiency virus envelope. J Gen Virol. 1996;77:759–771. doi: 10.1099/0022-1317-77-4-759. [DOI] [PubMed] [Google Scholar]
- 58.Richardson J, Moraillon A, Baud S, Cuisinier A-M, Sonigo P, Pancino G. Enhancement of feline immunodeficiency virus (FIV) infection after DNA vaccination with the FIV envelope. J Virol. 1997;71:9640–9649. doi: 10.1128/jvi.71.12.9640-9649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rusche J R, Javaherian K, mcDonald C, Petro J, Lynn D L, Grimaila R, Langlois A, Gallo R C, Arthur L O, Fischinger P J, Bolognesi D P, Putney S D, Matthews T J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci USA. 1988;85:3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlienger K, Montefiori D C, Mancini M, Riviere Y, Tiollais P, Michel M L. Vaccine-induced neutralizing antibodies directed in part to the simian immunodeficiency virus (SIV) V2 domain were unable to protect rhesus macaques from SIV experimental challenge. J Virol. 1994;68:6578–6588. doi: 10.1128/jvi.68.10.6578-6588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shafferman A, Jahrling P B, Benveniste R E, Lewis M G, Phipps T J, Eden-McCutchan F, Sadoff J, Eddy G A, Burke D S. Protection of macaques with a simian immunodeficiency virus envelope peptide vaccine based on conserved human immunodeficiency virus type 1 sequences. Proc Natl Acad Sci USA. 1991;88:7126–7130. doi: 10.1073/pnas.88.16.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siebelink K H J, Tijhaar E, Huisman R C, Huisman W, de Ronde A, Darby I H, Francis M J, Rimmelzwaan G F, Osterhaus A D M E. Enhancement of feline immunodeficiency virus infection after immunization with envelope glycoprotein subunit vaccines. J Virol. 1995;69:3704–3711. doi: 10.1128/jvi.69.6.3704-3711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stamatatos L, Cheng-Mayer C. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J Virol. 1995;69:6191–6198. doi: 10.1128/jvi.69.10.6191-6198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoiber H, Pintér C, Siccardi A G, Clivio A, Dierich M P. Efficient destruction of human immunodeficiency virus in human serum by inhibiting the protective action of complement factor H and decay accelerating factor (DAF, CD55) J Exp Med. 1996;183:307–310. doi: 10.1084/jem.183.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan N, Sun Y, Li J, Hofman W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Talbott R L, Sparger E E, Lovelace K M, Fitch W M, Pedersen N C, Luciw P A, Elder J H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vancott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1390. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 69.Vanini S, Longhi R, Lazzarin A, Vigo E, Siccardi A G, Viale G. Discrete regions of HIV-1 gp41 defined by syncytia-inhibiting affinity-purified human antibodies. AIDS. 1993;7:167–174. doi: 10.1097/00002030-199302000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Verschoor E J, Boven L A, Blaak H, van Vliet A L W. A single mutation within the V3 envelope neutralization domain of feline deficiency virus determines its tropism for CRFK cells. J Virol. 1995;69:4752–4757. doi: 10.1128/jvi.69.8.4752-4757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 72.Westervelt P, Gendelman H E, Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci USA. 1991;88:3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wild C, Greenwell T, Matthews T. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res Hum Retroviruses. 1993;9:1051–1052. doi: 10.1089/aid.1993.9.1051. [DOI] [PubMed] [Google Scholar]
- 74.Wrin T, Loh T P, Vennari J C, Schuitemaker H, Nunberg J H. Adaptation to persistent growth in the H9 cell line renders a primary isolate of human immunodeficiency virus type 1 sensitive to neutralization by vaccine sera. J Virol. 1995;69:39–48. doi: 10.1128/jvi.69.1.39-48.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto J, Hohdatsu T, Olmsted R A, Pu R, Louie H, Zochlinski H A, Acevedo V, Johnson H M, Soulds G A, Gardner M B. Experimental vaccine protection against homologous and heterologous strains of feline immunodeficiency virus. J Virol. 1993;67:601–605. doi: 10.1128/jvi.67.1.601-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamamoto J K, Ackley C D, Zochlinski H, Louie H, Pembroke E, Torten M, Hansen H, Munn R, Okuda T. Development of IL-2-independent feline lymphoid cell lines chronically infected with feline immunodeficiency virus: importance for diagnostic reagents and vaccines. Intervirology. 1991;32:361–375. doi: 10.1159/000150220. [DOI] [PubMed] [Google Scholar]
- 78.Yamamoto J K, Okuda T, Ackley C D, Louie H, Pembroke E, Zochlinski, Munn R J, Gardner M B. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1991;7:911–922. doi: 10.1089/aid.1991.7.911. [DOI] [PubMed] [Google Scholar]