Abstract
Purpose
To examine the impact of the national poultry housing order the UK government introduced on 7 November 2022 on the spreading of the avian influenza virus among poultry premises.
Methods
A longitudinal design with 15 weeks of infected poultry specialist incidence rates per 100 poultry specialists during the 2022/23 winter for 8 English regions. A multilevel regression model was used to analyse repeated measurements. Time was level-1 unit and regions level-2 unit resulting in 120 observations. Random intercept models included interactions between housing order and weekly infected wild birds, poultry density, or weekly average temperatures divided into terciles. In models where these variables were not included as an interaction term they were introduced as confounders.
Results
After the introduction of the housing order, it took 3 weeks for a considerable reduction in poultry specialist incidence rates. Reduction in incidence rates was strongest in regions with highest poultry density, from 1.27 (95%CI 0.99 to 1.56) to 0.30 (95%CI 0.09 to 0.52). Considerable reductions were also seen in regions with most detected infected wild birds.
Conclusion
The housing order was successful in reducing infected poultry specialist incidence rates three weeks after its introduction. Strongest impact in regions with highest poultry density.
Keywords: Virus surveillance, avian influenza, outbreak, epidemiology, housing order, England
Highlights
-
•
Weekly regional incidence rates of avian influenza infected poultry specialists calculated from 26/09/2022 till 16/01/2023.
-
•
Multilevel regression models applied to evaluate the national poultry housing order introduced on 7 November 2022.
-
•
Within 3 weeks after the introduction of the poultry housing order the average incidence rate had dropped considerably.
-
•
The strongest reduction in incidence rates was seen in regions with highest poultry density.
-
•
A considerable reduction in incidence rates was also seen in regions with most infected wild birds.
1. Introduction
Worldwide there is a great concern about the spreading of avian influenza (AI) virus as it seems to spread more easily in wild birds than ever before. The spreading of AI already caused thousands of outbreaks in poultry premises in dozens of countries increasing the opportunity for spillover into people [1,2]. In the UK, an unprecedented outbreak of highly pathogenic avian influenza (HPAI) of the subtype H5N1 in wild birds has taken place since October 2021 [3]. In 2021 and 2022, this subtype of the AI virus infected dozens of poultry premises according to reports from Department for Environment, Food and Rural Affairs (DEFRA) [4]. Since all the poultry of infected premises are required to be killed, recently this had resulted in a serious shortage of eggs in the UK [5]. Furthermore, this virus was detected in twenty mammals and in one human [3,6]. Moreover, AI is spreading to places and in times not seen before [7]. Mandatory housing measures for all poultry and captive birds were introduced on 12 October 2022 in the hotspot areas Norfolk, Suffolk, and parts of Essex in the region East of England. On 7 November 2022, the UK government announced the introduction of a poultry housing order across the whole of England to reduce the threat of the AI virus. This policy intervention included to keep poultry indoors and to follow stringent biosecurity measures such as restricted access to premises, changing clothes and footwear before entering bird enclosures, and regularly disinfecting vehicles to reduce the risk of spreading of AI virus.1
The introduction of the national poultry housing order resulted in an overall decline in the number of infected premises over time, though there was huge regional variation according to the UK Health Security Agency (UKHSA) in their briefings on AI and the risk to human health [3,8]. This study focuses on a few environmental factors potentially driving regional spreading of the AI virus [9,10]. Migrating wild birds are a main driver infecting poultry premises [11,12]. Though the poultry housing order did not reduce much the number of infected wild birds according to the UKHSA briefings [3,8] − and the risk of AI infection from wild birds remained high, this order may have had a bigger impact in reducing the risk of infections among poultry premises in areas with higher numbers of infected wild birds.
Furthermore, premises in regions with higher poultry density are at higher risk of being infected with the AI virus [13,14]. This could be a result of more transport, trading, and cross-premises visits in these areas which was limited by the poultry housing order. This order may have then resulted in a stronger drop in infected premises in regions with higher poultry density.
Daily temperature could be an environmental driver as it may impact aggregation of (infected) wild bird assisting spreading of the AI virus. Besides, the AI virus may survive longer under certain temperatures increasing the likelihood of this virus to be spread. Therefore, variation in regional daily temperature could be related to differences in regional spreading of the AI virus among poultry premises [15,16]. The poultry housing order may have reduced the difference in risk between regions differing in temperature by limiting infections of poultry through transport and trade or wild birds. This study aims to examine the spreading of the AI virus among poultry specialists before and after the introduction of the poultry housing order in relation to the described environmental factors to better understand the impact of outbreak interventions on reducing the spreading of the AI virus.
2. Material and methods
2.1. Weekly incidence rates of infected poultry specialists per 100 poultry specialists
The underlying data in the two published UKHSA briefings [3,8] on AI includes the weekly number of infected poultry premises for each English region for the period end of September 2022 to mid-February 2023, a substantial part of the 2022–23 AI season.2 The focus is on the nearly 2500 poultry specialists as they are big premises containing the vast majority of all poultry in England. Adding the number of poultry specialists in each region taken from published statistics by DEFRA3 allowed us to calculate weekly incidence rates of infected poultry specialists per 100 poultry specialists, the outcome variable in this study. Weekly incidence rates were calculated till mid-January 2023 as thereafter there were a few consecutive weeks without infected poultry specialists. Incidence rates of infected poultry specialists were not calculated for London as there are no poultry specialists in that region.
2.2. Weekly number of detected infected wild birds
The weekly number of detected infected wild birds for each of the English regions are also published in UKHSA's briefings [3,8], but were only available from 3 October 2022 onwards. These figures were divided into terciles: tercile 1 indicates less than two infected wild birds, tercile 2 between two and four infected wild birds, and tercile 3 more than four infected wild birds.
2.3. Poultry density
To calculate regional poultry density, the total number of poultry in a region was divided by poultry specialists' total hectares in a region in 2021, both taken from published statistics by DEFRA.4 Poultry density was divided into terciles: tercile 1 indicates the least densely regions and tercile 3 the most densely regions.
2.4. Weekly average temperatures
Average daily temperature on the Celsius scale in each region for the period under study were provided by the Met Office.5 To account for difference in daily temperature between northern, central, and southern England, temperatures in regions North West, North East, and Yorkshire and the Humber were taken to determine weekly terciles for low, average, and high temperatures in northern England by month to account for seasonal changes. Temperatures in the regions East Midlands, West Midlands, and East of England were taken to determine these terciles for central England, and temperatures in the regions South West, South East, and London for southern England.
2.5. Methods of analysis
A multilevel regression model was used to analyse repeated measurements [17] in 8 English regions (East Midlands, West Midlands, East of England, South West, South East, North West, North East, and Yorkshire and the Humber) for 15 consecutive weeks starting in the week commencing 3 October 2022 up until the week commencing 9 January 2023. Time was the level-1 unit and regions the level-2 unit resulting in 120 observations. Three random intercept models with predictor variables were run with MLwiN in Stata [18]. The first model included an interaction between poultry housing order and weekly number of infected wild birds, the second model an interaction between poultry housing order and poultry density, and the third model an interaction between poultry housing order and weekly temperatures; weekly number of infected wild birds, poultry density, and weekly temperatures were all divided into terciles while poultry housing order was a binary variable. In the models where weekly number of infected wild birds, poultry density, or weekly temperatures were not included as an interaction term they were introduced as confounders and standardized around the mean.
3. Results
3.1. Trend in incidence rate of infected poultry specialists
Table 1 shows for each week whether there was a national poultry housing order and provides for each week the mean, minimum and maximum incidence rates of infected poultry specialists.
Table 1.
Descriptive weekly statistics, 8 English regions.
| Incidence rate infected poultry specialists Mean (min, max) |
Number Infected birds Mean (min, max) |
Temperature Celsius Mean (min, max) |
Poultry density per ha2 Mean (min, max) |
|
|---|---|---|---|---|
| w/c 26/09/2022 | 0.17 (0.0–1.06) | – | 12.02 (10.89–13.72) | 1689 (983–2828) |
| w/c 03/10/2022 | 0.16 (0.0–0.86) | 1.5 (0–7) | 13.35 (12.34–14.53) | 1698 (983–2828) |
| w/c 10/10/2022 | 0.49 (0.0–3.24) | 3.63 (0–9) | 11.43 (10.16–13.76) | 1698 (983–2828) |
| w/c 17/10/2022 | 0.67 (0.0–3.57) | 3.88 (1–7) | 13.33 (11.62–15.85) | 1698 (983–2828) |
| w/c 24/10/2022 | 0.46 (0.0–1.65) | 0.75 (0–2) | 14.28 (12.79–16.2) | 1698 (983–2828) |
| w/c 31/10/2022 | 0.46 (0.0–1.87) | 3.63 (0–6) | 10.35 (9.10–12.31) | 1698 (983–2828) |
| w/c 07/11/2022 | 0.30 (0.0–0.72) | 3.5 (1–6) | 12.24 (10.97–13.34) | 1698 (983–2828) |
| w/c 14/11/2022 | 0.34 (0.0–2.13) | 6.5 (1–13) | 9.00 (8.01–10.89) | 1698 (983–2828) |
| w/c 21/11/2022 | 0.11 (0.0–0.33) | 3.5 (1–9) | 8.13 (6.39–10.07) | 1698 (983–2828) |
| w/c 28/11/2022 | 0.06 (0.0–0.48) | 2.38 (0–5) | 4.89 (3.58–6.74) | 1698 (983–2828) |
| w/c 05/12/2022 | 0.06 (0.0–0.49) | 2.0 (0–6) | 1.59 (0.25–3.25) | 1698 (983–2828) |
| w/c12/12/2022 | 0.15 (0.0–0.67) | 3.12 (0–11) | −0.46 (−2.81–1.99) | 1698 (983–2828) |
| w/c 19/12/2022 | 0 (0.0–0.0) | 2.5 (0–5) | 7.53 (6.31–9.37) | 1698 (983–2828) |
| w/c 26/12/2022 | 0.03 (0.0–0.24) | 2.25 (0–6) | 7.47 (5.63–9.56) | 1698 (983–2828) |
| w/c 02/01/2023 | 0.03 (0.0–0.24) | 4.5 (0–14) | 8.07 (6.77–9.57) | 1698 (983–2828) |
| w/c 09/01/2023 | 0.08 (0–0.43) | 2 (0–4) | 7.67 (6.44–9.21) | 1698 (983–2828) |
w/c = week commencing.
The descriptive statistics in Table 1 show that it took three weeks for the nation-wide average incidence rate of infected poultry specialists to drop considerably after the introduction of the poultry housing order on 7 November; respectively from 0.46 in the week commencing 31 October (a week before the poultry housing order was introduced) to 0.11 in the week commencing 21 November. The week including Christmas Day (the week commencing 19 December) is the only week in our period under study without any infected poultry specialists. After the week commencing on 9 January no infected poultry specialists were reported for a few weeks in a row.
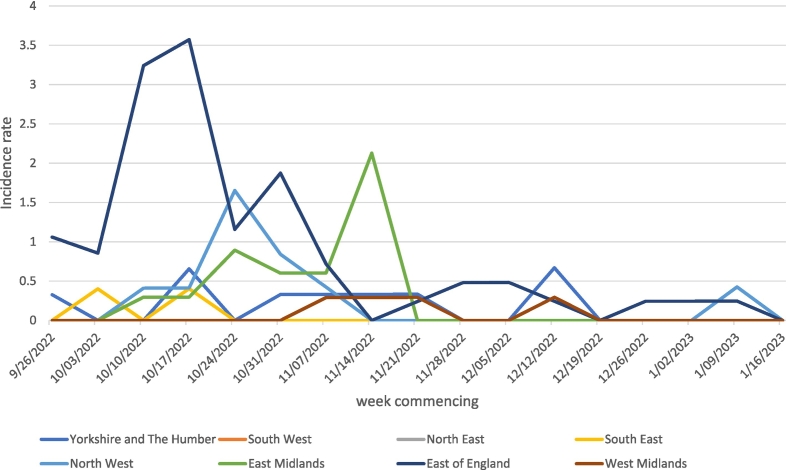
The weekly incidence rates of infected poultry specialists for each region are plotted in Fig. 1. The region East of England was a big hotspot in October while the region North West peaked at the end of that month. Remarkably, the region East Midlands peaked mid-November, after the introduction of the national poultry housing order.
Fig. 1.
Weekly incidence rates of infected poultry specialists per 100 poultry specialists for 8 English regions, 26 September 2022 to 23 January 2023.
3.2. National poultry housing order and environmental factors
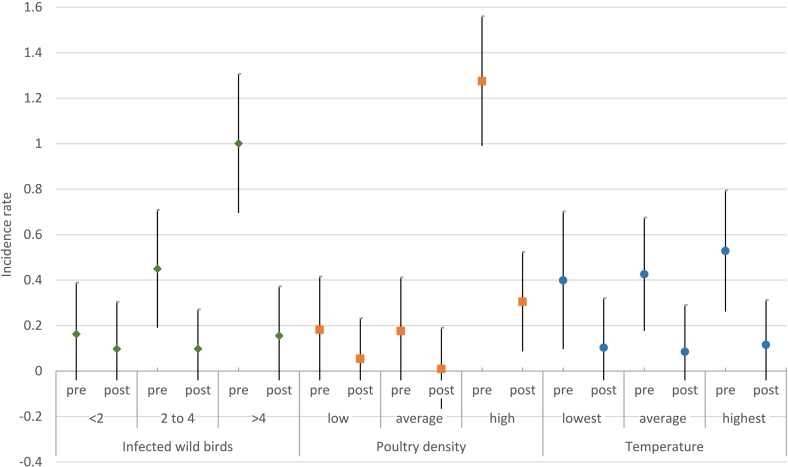
The multilevel regression analyses showed firstly that before the introduction of the national poultry housing order, incidence rates of infected poultry specialists were highest in regions with the most weekly detected infected wild birds as displayed in Fig. 2, 1.00 (95% confidence interval (95%CI) 0.70 to 1.31). After the introduction of the national poultry housing order, this rate dropped considerably to 0.16 (95%CI -0.06 to 0.37). A sensitivity analysis with an interaction between poultry housing order and detected infected wild birds in the previous week showed a similar result (Fig. S1). Secondly, before the national poultry housing order, the incidence rate of infected poultry specialists was much higher in regions with highest poultry density (1.27, 95%CI 0.99 to 1.56); thereafter the incidence rate dropped considerably (0.30, 95%CI 0.09 to 0.52). Thirdly, before the national poultry housing order the incidence rate of infected poultry specialists in regions with highest weekly temperature (0.53, 95%CI 0.26 to 0.79) was similar to that in regions with average or lowest weekly temperature. After the introduction of the national poultry housing order the incidence rate in regions with highest weekly temperature dropped to 0.11 (95%CI -0.08 to 0.31); the incidence rate in regions with average or lowest weekly temperature dropped to a similar level.
Fig. 2.
Adjusted estimates of B-coefficients (95% confidence interval) from multilevel regression models for the association between introduction of national poultry housing order and weekly incidence rates of avian influenza infected poultry specialists per 100 specialists, 3 October 2022 to 16 January 2023 (n = 120), split according to level of infected wild birds, poultry density, and daily temperature.
4. Discussion
Evaluating the effectiveness of the national poultry housing intervention to slow down the spreading of the AI virus among poultry specialists is crucial given the major implications for the agricultural and trade sectors, the high mortality among certain bird species, and the potential risk of transmission to humans [5]. This study combined (publicly) available longitudinal data from UKHSA and DEFRA allowing to calculate weekly incidence rates of infected poultry specialists during the winter of 2022/23. As far as we know, this has not been done in other publicly available reports or studies on spreading of AI among poultry premises in the UK. Other reports mentioned East of England region as a hotspot of infected poultry premises [3]. Calculating and plotting the incidence rates of infected poultry specialists over time showed the North West region as another but smaller hotspot. The region East Midlands peaked after the introduction of the national poultry housing order and might indicate the implementation of the housing order was delayed in this region.
The calculated incidence rates of infected poultry specialists allowed us to better indicate when infections among poultry specialists dropped. The calculated incidence rates of infected poultry specialists show that infections among poultry specialists strongly declined after the introduction of the national poultry housing order suggesting that this intervention had a strong impact on this decline. The national poultry housing order, though, had no immediate but a delayed effect of about three weeks before incidence rates dropped considerably on average nation-wide. This might be longer than expected since the incubation period of AI is usually estimated to be around five days.6 It could be that it took some time before poultry farmers and traders implemented the biosecurity measures included in the housing order. An alternative explanation could be that poultry farmers and traders implemented some additional preventative measures a couple of weeks later. Surveys and interviews among poultry farmers and traders could shed more light on the implementation of mandatory biosecurity measures and additional protection measures.
It might be noteworthy that the first week with no reported infected poultry specialists is the week including Christmas Day (the week commencing 19 December). As it seems unlikely that an infected poultry specialist was not reported or recorded, it may be the result of fewer visits and travel just before and during Christmas.
This study linked regional data from different institutions such as UKHSA, DEFRA, and Met Office into one database. The created longitudinal database allowed us to apply a multilevel approach to further evaluate the national poultry housing order on the spreading of the AI virus among poultry specialists. It examined incidence rates of infected poultry specialists before and after the national poultry housing order related to key environmental factors such as infected wild birds, poultry density, and daily temperature. The data on daily temperature, poultry density, and infected wild birds at regional level did not allow us to exclude the hotspot areas in the East of England region where mandatory housing measures were introduced a few weeks before the national poultry housing order.
Detecting AI infected wild birds is subject to dynamic surveillance policies and thresholds for collection [3]. Using other data such as information on local wetlands might be an alternative for the number of detected infected wild birds or could be an underlying factor to take into account. Wade, Ashton-Butt, Scott et al. suggest other measurements to detect and count infected wild birds such as utilisation and expansion of hunter harvested AI surveillance network [19]. Furthermore, some wild bird species may have developed antibodies overtime which could have influenced the observed and recorded numbers in some areas [20].
This study's findings on the impact of the English poultry housing order may also be of interest to the international community given the worldwide concern of the spread of the AI virus. As past influenza pandemics originated from animal reservoirs it is crucial to take actions to prevent the AI virus from becoming a future pandemic [21]. These further actions should be part of the so-called One Health approach to control AI benefitting animal health and public health as these are interrelated [1,22]. The most expedient way to address the One Health principle is to control poultry infections [23]. Evaluation of interventions such as the national poultry housing order are therefore critical. It increases our understanding of the impact of such an intervention and may enhance further interventions and actions to slow down the spreading of the AI virus among poultry premises and thereby reduce human exposure to this virus.
5. Conclusion
A longitudinal study design with weekly incidence rates of infected poultry specialists between October 2022 and mid-January 2023 for English regions was used to evaluate the national poultry housing order introduced on 7 November 2022. This housing order had no immediate but a delayed effect of about three weeks before the average nation-wide incidence rate of infected specialists considerably dropped. Results from multilevel regression models showed the strongest reduction in incidence rates of infected poultry specialists in regions with highest poultry density. A considerable reduction was also seen in regions with weekly highest detected infected wild birds. This might indicate that the national poultry housing order protected against infections from wild birds. Regional daily temperature was weaker associated with differences in incidence rates and in reductions in these rates after the introduction of the national poultry housing order. This might indicate that temperature is a less important environmental factor in the UK in the spreading of the AI among poultry specialists.
Ethical approval
Ethical approval was not required as this study made use of publicly available data.
Funding
None.
CRediT authorship contribution statement
Peter Tammes: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing.
Declaration of competing interest
None Declared
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dialog.2024.100165.
Avian influenza: Housing order to be introduced across England: https://www.gov.uk/government/news/avian-influenza-housing-order-to-be-introduced-across-england (accessed 25 March 2023).
See: https://www.gov.uk/government/publications/avian-influenza-influenza-a-h5n1-technical-briefings (accessed 2 March 2023) and https://www.gov.uk/animal-disease-cases-england?disease_type%5B%5D=bird-flu (accessed 19 November 2023).
See published statistics by DEFRA in data series ‘Region’: https://www.gov.uk/government/statistical-data-sets/structure-of-the-agricultural-industry-in-england-and-the-uk-at-june (accessed 5 March 2023).
See note 3 for data source.
Historic monthly weather data can be found on https://www.metoffice.gov.uk/research/climate/maps-and-data/historic-station-data (accessed 14 March 2023). After a request, the Met Office National Meteorological Archive provided us with daily weather information for each region on 16 March 2023.
European Centre for Disease Prevention and Control: https://www.ecdc.europa.eu/en/zoonotic-influenza/facts/faq-avian-influenza#:∼:text=The%20introduction%20of%20avian%20influenza,to%20be%20around%20five%20 days.
Appendix A. Supplementary data
Supplementary Figure S1
Data availability
This study made use of publicly available data and the sources are provided in the article. Met Office provided data on daily temperatures and will be shared upon reasonable request to the corresponding author.
References
- 1.Mahase E. World must prepare for next “inevitable” pandemic, flu expert warns. BMJ. 2023;381:391. doi: 10.1136/bmj.p931. [DOI] [PubMed] [Google Scholar]
- 2.Miller B.J. Unprecedented bird flu outbreaks concern scientists. Nature. 2022;606:18–19. doi: 10.1038/d41586-022-01338-2. [DOI] [PubMed] [Google Scholar]
- 3.UK Health Security Agency . UK Health Security Agency; London: 21 December 2022. Investigation into the risk to human health of avian influenza (influenza A H5N1) in England: Technical briefing 1. [Google Scholar]
- 4.Freath L., Bacigalupo S., Brown I., Banyard A., Pacey A., Gale P., et al. Department for EnvironmentFood Rural Affairs, Outbreak Assessment. Vol. 32. 2022, 1 September. Highly pathogenic avian influenza (HPAI) in the UK and Europe; pp. 1–19. [Google Scholar]
- 5.Haider N., Kock R., Zumla A., Lee S.S. Consequences and Global Risks of Highly Pathogenic Avian Influenza outbreaks in poultry in the United Kingdom. Int J Infect Dis. 2023;129:162–164. doi: 10.1016/j.ijid.2023.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Oliver I., Roberts J., Brown C.S., Byrne A.M., Mellon D., Hansen R.D., et al. A case of avian influenza A (H5N1) in England, January 2022. Eurosurveillance. 2022;27(5):2200061. doi: 10.2807/1560-7917.ES.2022.27.5.2200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahase E. H5N1: Do we need to worry about the latest bird flu outbreaks? BMJ. 2023;380:401. doi: 10.1136/bmj.p401. [DOI] [PubMed] [Google Scholar]
- 8.UK Health Security Agency . UK Health Security Agency; London: 23 February 2023. Investigation into the risk to human health of avian influenza (influenza A H5N1) in England: technical briefing 2. [Google Scholar]
- 9.Si Y., de Boer W.F., Gong P. Different environmental drivers of highly pathogenic avian influenza H5N1 outbreaks in poultry and wild birds. PloS One. 2013;8(1) doi: 10.1371/journal.pone.0053362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert M., Pfeiffer D.U. Risk factor modelling of the spatio-temporal patterns of highly pathogenic avian influenza (HPAIV) H5N1: a review. pat Spatiotemp Epidemiol. 2012;3(3):173–183. doi: 10.1016/j.sste.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das Gupta S., Barua B., Fournié G., Hoque M.A., Henning J. Village and farm-level risk factors for avian influenza infection on backyard chicken farms in Bangladesh. Sci Rep. 2022;12(1):13009. doi: 10.1038/s41598-022-16489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velkers F.C., Manders T.T., Vernooij J.C., Stahl J., Slaterus R., Stegeman J.A. Association of wild bird densities around poultry farms with the risk of highly pathogenic avian influenza virus subtype H5N8 outbreaks in the Netherlands, 2016. Transbound Emerg Dis. 2021;68(1):76–87. doi: 10.1111/tbed.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Food Safety Authority, European Centre for Disease Prevention Control, European Union Reference Laboratory for Avian Influenza, Adlhoch C., Fusaro A., Gonzales J.L., et al. Avian influenza overview December 2020–February 2021. EFSA J. 2021;19(3) doi: 10.2903/j.efsa.2021.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Food Safety Authority, European Centre for Disease Prevention Control, European Union Reference Laboratory for Avian influenza, Adlhoch C., Fusaro A., Gonzales J.L., et al. 2022. Avian influenza overview December 2021–March 2022. Report No.: 1831-4732 Contract No.: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reperant L.A., Fučkar N.S., Osterhaus A.D., Dobson A.P., Kuiken T. Spatial and temporal association of outbreaks of H5N1 influenza virus infection in wild birds with the 0 C isotherm. PLoS Pathog. 2010;6(4) doi: 10.1371/journal.ppat.1000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C.-M., Lin S.-H., Chen Y.-C., Lin K.C.-M., Wu T.-S.J., King C.-C. Temperature drops and the onset of severe avian influenza A H5N1 virus outbreaks. PloS One. 2007;2(2) doi: 10.1371/journal.pone.0000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snijders T., Bosker R. 1999. Multilevel modeling: An introduction to basic and advanced multilevel modeling. London. [Google Scholar]
- 18.Leckie G.B., Charlton C.M. Runmlwin: a program to run the MLwiN multilevel modeling software from within Stata. J Stat Softw. 2013;52(11) [Google Scholar]
- 19.Wade D., Ashton-Butt A., Scott G., Reid S.M., Coward V., Hansen R.D., et al. High pathogenicity avian influenza: targeted active surveillance of wild birds to enable early detection of emerging disease threats. Epidemiol Infect. 2023;151 doi: 10.1017/S0950268822001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caliendo V., Leijten L., van de Bildt M.W., Poen M.J., Kok A., Bestebroer T., et al. Long-term protective effect of serial infections with H5N8 highly pathogenic avian influenza virus in wild ducks. J Virol. 2022;96(18) doi: 10.1128/jvi.01233-22. e01233-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuiken T., Fouchier R.A., Koopmans M.P. Being ready for the next influenza pandemic? Lancet Infect Dis. 2023;23(4):398–399. doi: 10.1016/S1473-3099(23)00117-2. [DOI] [PubMed] [Google Scholar]
- 22.Leung K., Lam T.T.Y., Wu J.T. Controlling avian influenza. BMJ. 2023;380:560–561. doi: 10.1136/bmj.p560. [DOI] [PubMed] [Google Scholar]
- 23.Verhagen J.H., Fouchier R.A., Lewis N. Highly pathogenic avian influenza viruses at the wild–domestic bird interface in Europe: Future directions for research and surveillance. Viruses. 2021;13(2):2012–2046. doi: 10.3390/v13020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Data Availability Statement
This study made use of publicly available data and the sources are provided in the article. Met Office provided data on daily temperatures and will be shared upon reasonable request to the corresponding author.