Abstract
Background
Prostate cancer is affecting males globally, with several complications. Zinc can play roles in cancers. We aimed to clarify the association between zinc levels or intake with prostate cancer development.
Methods
We searched PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science until May 1, 2023. We included case-controls and cross-sectionals that measured zinc level and/or intake in patients with prostate cancer or cohorts that evaluated the association between zinc and prostate cancer development. Studies that did not have a healthy control group were excluded. Joanna Briggs Institute was used for quality assessment. Publication bias was evaluated using Egger’s and Begg’s tests and funnel plot.
Results
Overall, 52 studies (n = 44 case controls, n = 4 cohorts, and n = 4 cross sectionals) with a total number of 163909 participants were included. Serum (standardized mean difference (SMD): -1.11; 95% confidence interval (CI): -1.67, -0.56), hair (SMD: -1.31; 95% CI: -2.19, -0.44), and prostatic fluid or tissue zinc levels (SMD: -3.70; 95% CI: -4.90, -2.49) were significantly lower in prostate cancer patients. There were no significant differences in nail zinc level and zinc intake between those with prostate cancer and healthy controls. There was no publication bias except for serum and hair zinc levels based on Begg’s and Egger’s tests, respectively. The mean risk of bias scores were 4.61 in case-controls, eight in cohorts, and seven in cross-sectionals.
Conclusions
Overall, high zinc levels might have a protective role in prostate cancer, which can be used as a therapeutic or preventive intervention. Future large-scale studies are needed to confirm the association.
Introduction
Prostate cancer was the second cancer with the highest incidence among males in 2019 globally [1]. In 2020, approximately 1.41 million individuals were newly diagnosed with prostate cancer and it led to more than 375 thousand deaths worldwide [1]. Moreover, complications like urinary tract symptoms have a significant impact on the physical and mental well-being of patients [2–4]. Genetic and environmental factors play roles in prostate cancer development so the disparities in prostate cancer incidence worldwide suggest that dietary factors may contribute to these variations. However, the specific components of the diet that contribute to this phenomenon have not been identified [5, 6].
Zinc has been identified as a dietary factor that may play a protective role in prostate cancer [7]. The levels of zinc are tightly controlled as it is involved in many physiological processes [8]. Zinc accumulates in the prostate ten times higher than any other tissue and this accumulation plays a vital role in maintaining the overall health of the prostate gland [9, 10]. Epidemiological studies have shown that there is a notable decrease in serum zinc levels in different types of cancer, such as head and neck, breast, gastrointestinal tract, female genital tract, gallbladder, lung, and thyroid cancers [11–13].
While experimental data has supported the beneficial impact of zinc in prostate cancer, various epidemiological studies have yielded conflicting results [14, 15]. Some studies have found that zinc intake reduces the likelihood of developing prostate cancer and its mortality rate [16–18]. Conversely, other studies have linked high zinc consumption to advanced prostate cancer [19]. However, several observations have indicated that dietary or supplemental zinc intake is not associated with the risk of prostate cancer or its progression [20–22]. Previous meta-analysis studies have been conducted on this topic [23–25]. However, considering that their search dates back to years ago and more recent studies with larger sample sizes have been conducted [26, 27], we would like to update them and also consider their limitations in the current study. In this regard, a case-control study and meta-analysis was conducted in 2016, whereas it did not perform quality assessment [24]. Also, its search date went back to 2016 and only included 17 studies [24]. In another meta-analysis which searched the literature up to 2015, the association between serum zinc and different prostate diseases were evaluated, although it was not focused on prostate cancer [28]. The meta-analyses by Stratton et al. and Gumulec et al. also evaluated the broad range of different types of supplemental vitamins and minerals or assessed zinc effects on different types of epithelial malignancies [23, 25]. Overall, the previous studies are out-of-dated or did not evaluate the effects of zinc concentrations in different samples on prostate cancer. So, our study considered zinc levels in various specimens, such as blood serum, prostate tissue, nail, and scalp hair, as well as zinc supplementation and dietary intake. Zinc level in each one is profoundly measured and correlated to the risk of prostate cancer in various studies, but none of the studies have gathered all in a systematic review. Herein, we aimed to conduct a systematic review and meta-analysis to evaluate the association between zinc levels or intake and prostate cancer development.
Materials and methods
This systematic review and meta-analysis was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration ID CRD42023439347.
Search strategy and study selection
We searched PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science up to May 1, 2023. We used the following terms: ("zinc" OR "zinc compounds" OR "Zn" OR "zinc citrate" OR "zinc sulfate") AND ("prostatic neoplasm*" OR "prostate cancer" OR "prostate malignancy") (S1 Table). No filters on any search field, such as language and study types were implemented. Backward and forward citation searching were performed. Also, the first 300 results of the Google Scholar search engine were evaluated as the grey literature search [30].
The inclusion criteria consisted of case-controls, cohorts, and analytical cross-sectionals on patients with prostate cancer of any stage which evaluated the effects of zinc supplementation or zinc levels on prostate cancer. The outcome of interest was the occurrence of prostate cancer. The studies should have reported or provided calculable odds ratio (OR), hazard ratio (HR), or relative risk (RR) with a 95% confidence interval (CI).
The exclusion criteria were studies that included patients with types of cancers other than prostate cancer, studies reporting levels of vitamins/minerals other than zinc, and studies reporting outcomes other than prostate cancer occurrence (e.g., mortality and survival). Also, in vitro and in vivo studies, animal studies, case reports, case series, editorials, commentaries, letters, review articles, notes, news, book chapters, meta-analyses, and re-analyses of previously published articles were excluded. The records were imported and deduplicated using the EndNote software (Clarivate Analytics). The records were divided into two groups, and two different pairs of reviewers (AASAK & RE–ZG & SSN) screened each one independently by title and abstract search in the first step. Then, the full texts of the studies from the previous screening were evaluated. Any disagreements were resolved by discussing and consulting with the lead investigator (SAN).
Data extraction
A form in Microsoft Office Excel was used for data extraction. Two pairs of independent researchers (AASAK & RE–ZG & SSN) extracted the following information from each study: first author name; year of publication; study design; country where the research was conducted; study population; definition of case or exposure groups and controls; age range and mean age in cases and controls; follow-up duration; source of sampling; comorbidities; smoking, alcohol consumption, and other risk factors; prostate cancer ascertainment; and methods of zinc measurement. Any discrepancies were resolved by discussing or consulting with a third author.
Quality assessment
The included studies were divided into two groups and two different pairs of reviewers (AASAK & RE–ZG & SSN) assessed the quality of each one independently using the Joanna Briggs Institute (JBI), critical appraisal tools for case controls, cohorts, and analytical cross-sectionals [31]. A third reviewer (SAN) was consulted if there were any discrepancies.
Statistical analysis
The pooled ORs with 95% CIs for dichotomous data and the pooled standardized mean difference (SMD) with 95% CIs for continuous data were assessed using random or fixed-effects models. The random-effects model was used because of the estimated methodological heterogeneity of the true effect sizes. The between-study heterogeneity was assessed by Cochran’s Q and the I-square statistic. I-square values of more than 50% were considered high heterogeneity [32]. Stratified analysis was done for SMD calculation according to the source of zinc sampling. This method was conducted to consider the effects of zinc level in different types of samples or zinc intake. The median and interquartile range were converted to mean and standard deviation for SMD calculation [33]. Publication bias was evaluated using Egger’s and Begg’s tests as well as the funnel plot (p<0.05 was considered indicative of statistically significant publication bias as well as funnel plot asymmetry) [34]. If the results of Egger’s and Begg’s tests were incoherent, the trim-and-fill method was used to find probable missing studies [35, 36]. The funnel plot was not used for publication bias assessment in analysis with fewer than ten studies [37].
Results
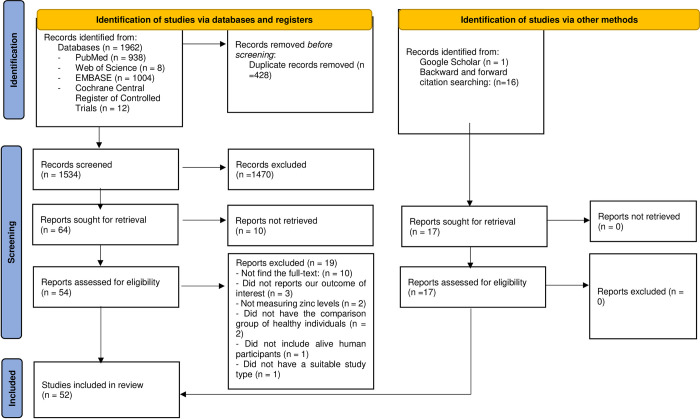
A total of 1962 hits were found through online database searching. After removing duplicated results, 1524 studies were evaluated in the title and abstract screening, and 1470 records were excluded in this step. Then, 54 studies were evaluated in full-text reviewing. We could not find the full text of ten studies [38–47], three did not report our outcomes of interest [48–50], two were excluded due to not measuring zinc levels [51, 52], two did not have the comparison group of healthy individuals [53, 54], one did not include alive human participants [55], and one did not have a suitable study type [56]. Overall, 35 studies were included in the full-text review [19, 21, 22, 26, 57–87]. Moreover, 16 studies were found through backward [20, 24, 88–98] and forward citation searching [99–101], and one study from searching Google Scholar [102]. Finally, 52 articles were included in this systematic review, of which forty-four were case-controls [19, 21, 22, 24, 57–65, 67, 69–73, 75–82, 84–92, 94, 95, 97–102], four were cohorts [20, 26, 66, 74], and four were analytical cross-sectional studies [68, 83, 93, 96]. Overall, 50 studies were included in quantitative synthesis and two in qualitative synthesis (Fig 1).
Fig 1. Study selection process.
Study characteristics
Twelve studies were conducted in the United States [19–21, 24, 26, 66, 72–75, 80, 84], nine in Nigeria [57, 59, 68, 76–78, 83, 88, 94], five in Turkey [58, 70, 79, 85, 89], four in China [91–93, 96], three in India [61, 65, 69], two in Saudi Arabia [82, 95], two in Russia [86, 87], two in Germany [62, 63], one study in each other countries, such as Poland [60], Venezuela [90], Spain [67], Sudan [99], Singapore [102], Iraq [100], Serbia [97], United Kingdom [98], Iran [101], Malaysia [71], Italy [64], Sweden [22], and Pakistan [81]. The follow-up duration of cohort studies ranged from seven to about 28 years [20, 26, 66, 74]. In 36 studies, prostate cancer was confirmed by a histopathological examination [19, 20, 22, 24, 26, 57–59, 61–64, 67–69, 71–74, 76–78, 80, 82–89, 92, 97, 100–102]; in two studies it was confirmed by prostate-specific antigen (PSA) test [81, 94], and in one study it was confirmed by clinical data and current international clinical staging method [93]. In other 13 studies, prostate cancer diagnosis method was not described [21, 60, 65, 66, 70, 75, 79, 90, 91, 95, 96, 98, 99] (Table 1 and S2 Table).
Table 1. Study characteristics.
| First Author | Publication year | Country | Study design | Total participants | Total mean (SD) age | Zinc measurement method | Source of sampling |
|---|---|---|---|---|---|---|---|
| Yiwen Zhang [26] | 2022 | United States | Cohort | 47240 | 66.14 (0.27) | NA | FFQ |
| Mehmet Kaba [70] | 2014 | Turkey | Case-control | 62 | 64.06 (1.31) | AAS | Serum |
| KS Adedapo [88] | 2012 | Nigeria | Case-control | 120 | 66.85 (1.66) | AAS using a direct method | Serum |
| Collins Amadi [57] | 2020 | Nigeria | Case-control | 440 | 69.35 (0.35) | AAS using a direct method | Serum |
| Katarzyna Białkowska [60] | 2018 | Poland | Case-control | 394 | 77 | ICP-MS technique | Serum |
| Ayşe Eken [89] | 2016 | Turkey | Case-control | 131 | 61.27 (7.14) | AAS with a Zeaman Background Correction, flame atomic absorption spectrometer | Serum |
| Yenny Gómez [90] | 2007 | Venezuela | Case-control | 40 | 54.29 (NA) | ETA-AAS | Prostatic fluid |
| Jingkang Guo [91] | 2007 | NA | Case-control | 115 | NA | ICP-MS | Scalp hair |
| Enrique Gutiérrez-González [67] | 2018 | Spain | Case-control | 1961 | 65.95 (0.25) | Dietary zinc intake estimated | FFQ |
| Martin Igbokwe [68] | 2021 | Nigeria | Cross-sectional | 82 | 71.78 (1.05) | PIXE | Toe-nail |
| Lina Mustafa khedir Abdelmajid [99] | 2022 | Sudan | Case-control | 60 | NA | AAS | Serum |
| Alan R. Kristal [20] | 2010 | United States | Cohort | 9559 | 62.77(0.38) | FFQ and a structured supplement-use questionnaire | FFQ |
| Marion M. Lee [92] | 1998 | China | Case-control | 398 | NA | FFQ and face-to-face interviews | FFQ |
| Xiao-Meng Li [93] | 2005 | China | Cross-sectional | 3940 | 68.47 (1.05) | Deproteinization method using a Perkin-Elmer 503 AAS | Serum |
| Jue Tao Lim [102] | 2019 | Singapore | Case-control | 255 | NA | ICP-MS | Serum |
| Abeer M. Mahmoud [24] | 2016 | United States | Case-control | 208 | 66.30 (0.24) | Block FFQ | FFQ |
| Rana Kareem Mohammed [100] | 2015 | Iraq | Case-control | 50 | NA | AAS | Serum |
| Augusta Chinyere Nsonwu-Anyanwu [76] | 2022 | Nigeria | Case-control | 90 | 36.76 (2.04) | Wet acid digestion method | Serum |
| Wasiu Eniola Olooto [78] | 2021 | Nigeria | Case-control | 75 | NA | AAS | Serum |
| Bridget Obiageli Onyema-iloh [94] | 2014 | Nigeria | Case-control | 100 | NA | AAS | Serum |
| Saleh A. K. Saleh [95] | 2017 | Saudi Arabia | Case-control | 174 | 69.10 (1.70) | ICP-MS | Scalp hair |
| Chao Tan [96] | 2011 | China | Cross-sectional | 113 | NA | ICP-MS | Scalp hair |
| H.D. Vlajinac [97] | 1997 | Serbia | Case-control | 303 | 71.16 (NA) | Measurements of consumption using standard cups, spoons, and slices | Zinc intake |
| Victor C. Wakwe [83] | 2019 | Nigeria | Cross-sectional | 440 | 69.35 (0.38) | AAS | Serum |
| Elizabeth G. Willden [98] | 1975 | United Kingdom | Case-control | 92 | NA | AAS | Serum |
| Hasan Yari [101] | 2015 | Iran | Case-control | 72 | 65.30 (1.20) | Polarography | Serum |
| Vladimir Zaichick [86] | 2019 | Russia | Case-control | 146 | 61.32 (2.94) | EDXRF | Prostatic fluid |
| Michael F. Leitzmann [74] | 2003 | United States | Cohort | 46974 | 54.34 (0.64) | Zinc intake | FFQ |
| J.O.Ogunlewe [77] | 1989 | Nigeria | Case-control | 127 | 63.81 (2.34) | AAS | Serum and prostatic tissue |
| T.Goel [65] | 2006 | India | Case-control | 80 | NA | AAS | Serum |
| Ahmet Aydin [58] | 2006 | Turkey | Case-control | 85 | 65.44 (1.37) | Flame AAS | Serum |
| V.YE.Zaichick [87] | 1996 | Russia | Case-control | 91 | 56.18 (7.90) | XRF | Prostatic fluid |
| D.W.West [84] | 1991 | United States | Case-control | 1037 | NA | FFQ | FFQ |
| Song-Yi Park [21] | 2013 | United States | Case-control | 1175 | 68.97 (0.09) | ICP-MS | Serum |
| Alejandro Gonzalez [66] | 2009 | United States | Cohort | 35242 | NA | Zinc intake | FFQ |
| Laurence N. Kolonel [72] | 1988 | United States | Case-control | 1351 | NA | FFQ | FFQ |
| A. Feustel [62] | 1986 | Germany | Case-control | 147 | 53.70 (NA) | Flame AAS | Serum and erythrocytes |
| Golgis Karimi [71] | 2012 | Malaysia | Case-control | 100 | 72.05 (0.35) | ICP-MS | Hair and nail |
| Silvano Gallus[64] | 2007 | Italy | Case-control | 2745 | 62.20 (NA) | Zinc intake | FFQ |
| Elizabeth A. Platz [80] | 2002 | United States | Case-control | 342 | 66.03 (0.05) | Furnace AAS and flame AAS | Nail |
| M. Jain [69] | 1994 | India | Case-control | 50 | NA | AAS | Serum and prostatic tissues |
| Alan R. Kristal [73] | 1999 | United States | Case-control | 1363 | NA | Zinc intake | FFQ |
| M. I. Yilmaz [85] | 2004 | Turkey | Case-control | 121 | 65.01 | Flame AAS | Serum |
| Habibe Ozmen [79] | 2006 | Turkey | Case-control | 41 | 69.32 (3.10) | AAS | Serum |
| Swen-Olof Andersson [22] | 1996 | Sweden | Case-control | 1056 | 70.65 (0.05) | Zinc intake | FFQ |
| Muhammad Abdul Qayyum [81] | 2014 | Pakistan | Case-control | 394 | 51.70 (NA) | AAS | Serum, scalp hair, and nail |
| Pamela Christudoss [61] | 2011 | India | Case-control | 83 | NA | AAS | Serum and tissue |
| Saleh A.K. Saleh [82] | 2020 | Saudi Arabia | Case-control | 92 | 67.17 (1.04) | ICP-MS | Serum |
| Onyinyechi Bede-Ojimadu [59] | 2023 | Nigeria | Case-control | 273 | NA | ICP-MS | Urine and serum |
| Lois D. Mcbean [75] | 1974 | United States | Case-control | 95 | 49.00 (NA) | AAS | Serum |
| A.Feustel [63] | 1989 | Germany | Case-control | 75 | 68.75 (NA) | Flame AAS | Serum |
| Yuqing Zhang [19] | 2009 | United States | Case-control | 4110 | 60.15 (1.48) | Supplementation use using a structured questionnaire | FFQ |
Abbreviations: NA: not available; AAS: atomic absorption spectrometry; FFQ: food frequency questionnaire; ICP-MS: inductively coupled plasma mass spectroscopy; PIXE: Particle induced X-ray emission; EDXRF: energy dispersive X-ray fluorescent; XRF: X-ray fluorescence.
The total number of participants was 163909 which ranged from 40 to 47240 participants in individual studies with mean age ranging from 36.76 to 77 years. Atomic absorption spectrometry (AAS) was the most common method for zinc measurement. The sources of sampling were different which included serum [21, 57–63, 65, 69, 70, 75–79, 81–83, 85, 88, 89, 93, 94, 98–102], erythrocyte [62], prostatic fluid or tissue [61, 69, 77, 86, 87, 90], hair [71, 81, 91, 95, 96], and nail [68, 71, 80, 81]. Also, eleven studies used a questionnaire to assess levels of zinc intake [19, 20, 24, 26, 64, 66, 67, 73, 74, 84, 92]. Out of these 11 studies, eight studies grouped patients based on a zinc level threshold [19, 20, 26, 64, 66, 73, 74, 84], hence using odds ratio to report results; and four studies compared zinc intake levels using a mean intake measurement [24, 67, 74, 92] (Table 1 and S2 Table).
Meta-analysis and publication bias
Serum zinc level
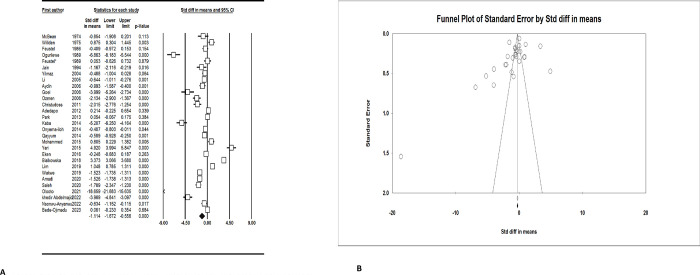
Serum zinc level was significantly lower in prostate cancer patients (SMD: -1.11; 95% CI: -1.67, -0.56) (Fig 2A). There was a significant publication bias according to the Begg’s test (p = 0.03) and funnel plot, however, no significant publication bias was found in the Egger’s test (p = 0.15) (Fig 2B).
Fig 2.
A. Forest plot of the standardized mean difference for the association between prostate cancer and serum zinc level. B. Funnel plot for serum zinc level and prostate cancer.
Zinc intake
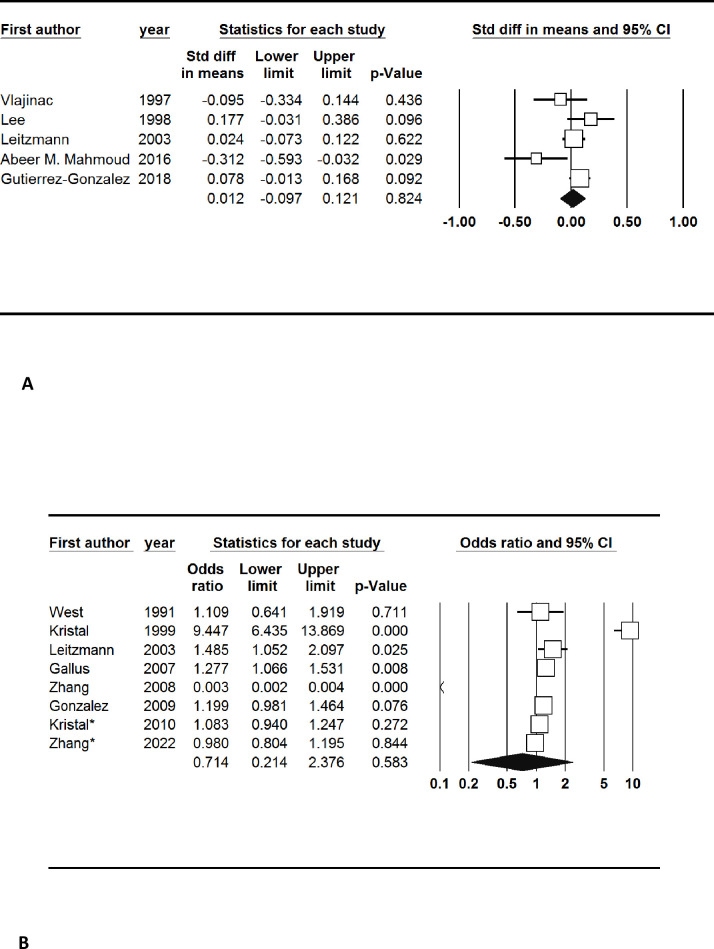
Five studies reported the mean levels of zinc intake per day. The pooled results showed that zinc intake was non-significantly higher in prostate cancer patients (SMD: 0.01, 95% CI: -0.10, 0.12). There was also no publication bias for the measurement of serum zinc intake (Begg’s test: 0.22 and Egger’s test: 0.35) (Fig 3A).
Fig 3.
A. Forest plot of the standardized mean difference for the association between prostate cancer and zinc intake. B. Forest plot of the odds ratio for the association between prostate cancer and zinc intake.
Eight studies categorized the participants based on the levels of zinc intake and reported the number of participants in each category. There was no significant association between zinc intake and prostate cancer (OR: 0.71; 95% Cl: 0.21, 2.38). Also, there was no publication bias (Begg’s test: 0.54 and Egger’s test: 0.68) (Fig 3B).
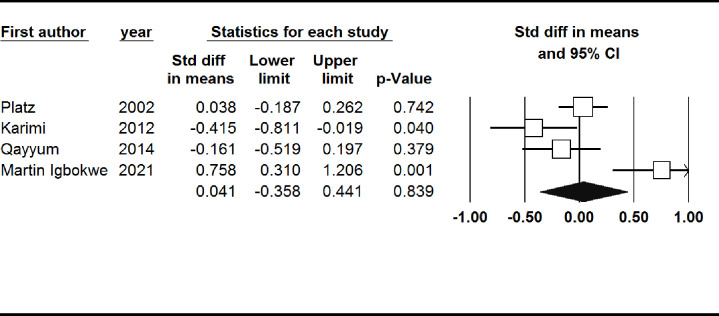
Nail zinc level
Nail zinc level was non-significantly higher in patients with prostate cancer (SMD: 0.04, 95% CI: -0.36, 0.44). There was also not a significant publication bias (Begg’s test: 1.00, Egger’s test: 0.85) (Fig 4).
Fig 4. Forest plot of the standardized mean difference for the association between prostate cancer and nail zinc level.
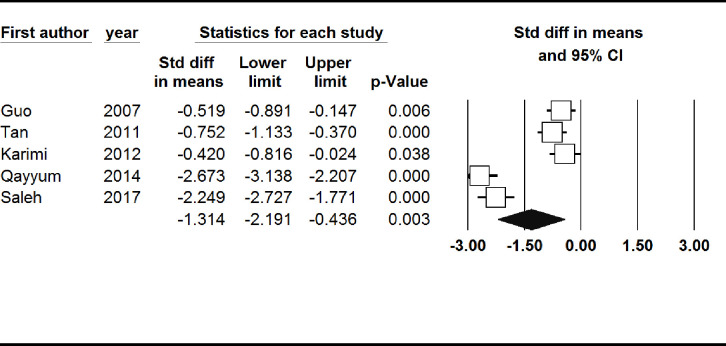
Hair zinc level
Hair zinc level was significantly lower in patients with prostate cancer (SMD: -1.31, 95% CI: -2.19, -0.44). There was not a significant publication bias based on the Begg’s test (p = 0.46), while the Egger’s test showed a significant publication bias (p = 0.02) (Fig 5).
Fig 5. Forest plot of the standardized mean difference for the association between prostate cancer and hair zinc level.
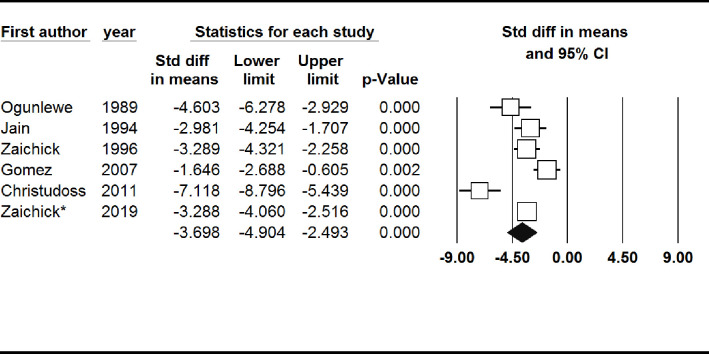
Prostatic fluid or tissue zinc level
Prostatic fluid or tissue zinc level was significantly lower in patients with prostate cancer (SMD: -3.70, 95% CI: -4.90, -2.49). Also, no significant publication bias was found (Begg’s test: 0.26 and Egger’s test: 0.23) (Fig 6).
Fig 6. Forest plot of the standardized mean difference for the association between prostate cancer and prostatic fluid or tissue zinc level.
Quality assessment
The mean quality assessment score in case-control studies was 4.61 which ranged from one to eight. Out of 44 case-control studies, one had an overall score of one [94], four had an overall score of two [75, 90, 91, 98], eight had an overall score of three [58, 61, 62, 65, 70, 73, 99, 100], seven had an overall score of four [60, 63, 69, 77, 85, 87, 88], seven had an overall score of five [22, 57, 59, 64, 78, 79, 84], thirteen had an overall score of six [24, 67, 71, 72, 76, 81, 82, 86, 92, 95, 97, 101, 102], three had an overall score of seven [19, 21, 89], and one had an overall score of eight [80]. The selection of control and non-response rates had the lowest score (S3 Table).
The mean risk of bias in cohort studies was eight which ranged from seven to nine. Out of four cohort studies, two had an overall score of seven [26, 74] and two had an overall score of nine [20, 66]. Ascertainment of exposure, outcome, and adequacy of follow-up were the domains with the highest risk of bias (S4 Table).
The mean risk of bias in cross-sectional studies was seven which ranged from six to eight. Out of four cross-sectional studies, two had an overall score of six [93, 96] and two had an overall score of eight [68, 83]. The sample size and non-respondents domains had the highest risk of bias (S5 Table).
Discussion
Our findings showed that in individuals with prostate cancer, zinc levels of serum, hair, and prostatic fluid or tissue were significantly lower than controls, while no notable differences were found in nail zinc levels and zinc intake.
Numerous studies with a similar design to our study confirmed this finding that patients with prostate cancer have lower serum zinc levels. In this regard, a meta-analysis of ten studies revealed significantly lower serum zinc levels in patients with prostate cancer compared with controls (SMD: −0.94; 95% CI: −1.57, −0.32) [28]. Consistent with our findings, another meta-analysis of 114 studies manifested decreased serum zinc levels in prostate cancer patients (SMD: -1.08; 95% CI: −1.33, −0.82) [25]. It also appears to be similar in other cancers. For instance, a meta-analysis on bladder cancer patients including six studies showed significantly lower serum zinc levels (three studies, SMD: −1.07; 95% CI: −1.49, −0.66) compared with controls [103]. In breast cancer, a meta-analysis of 36 studies with 5747 females showed lower serum zinc levels in cancer patients compared to healthy controls (SMD: -1.20; 95% CI: -1.74, -0.66) [104].
Regarding zinc intake, it showed no significant differences between prostate cancer patients and healthy controls in our study. Another dose-response meta-analysis study showed similar findings (OR: 1.07; 95% CI: 0.98, 1.16) [24]. However, this study was performed on African Americans, and based on studies, black men pose a higher risk of prostate cancer compared to other ethnicities [105]. In addition, zinc intake was self-reported which can explain the differences found between the studies [24]. Yet, this finding is contrary to most of the literature mentioning the anti-tumor effects of zinc supplementation in prostate cancer aside from many other types of cancer [106]. This could bring up a debate on the beneficial dosage of zinc supplements. The controversy on whether excessive dosage would benefit prostate cancer led to studies like a 30-year follow-up study of Zhang et al., which pointed out the risk of lethal prostate cancer with 75 mg/day or more than 15 years of zinc supplementation [26]. However, one of our included studies 19 years before the aforementioned study, followed 46974 health professionals for 14 years and delineated that 100 mg/day of supplemental zinc was a cut-off for the risk of prostate cancer compared to control (RR: 2.29; 95% CI: 1.06, 4.95) [74]. Another systematic review showed that high zinc intake in patients with advanced prostate cancer could be protective in a dose-response manner [107]. As for the risk of advanced prostate disease, it is noteworthy to mention in the study of Gonzalez et al. on 35242 participants, the overall prostate cancer risk was not related to supplemental zinc intake for 10 years (adjusted HR: 0.82; 95% CI: 0.58, 1.14 for >15 mg/day vs. nonuse). However, due to the necessity of the PSA test in detecting the early stage of the disease and since they did not have access to this test, they could not assess the early stages of the disease. Contrary to the findings of zinc supplementation, Gonzalez et al. claimed that the dietary intake of zinc was not associated with the development of prostate cancer [66]. However, this was not the case in other studies done in Hawaii and Italy [64, 72]. As well as zinc supplementation, dietary zinc itself can play a role in preventing prostate cancer. High dietary zinc intake can be associated with reduced mortality in prostate cancer patients after the diagnosis [108]. However, in vivo studies suggest that an optimal dose of zinc intake in the diet is the best amount for protecting against prostate tumors, as both lower and higher than normal levels can lead to carcinogenesis [109]. It is worth mentioning that this association is stronger in advanced prostate cancers [64]. Inconsistent data on the effective dose of zinc can be attributed to the factors affecting the absorption of zinc, such as phytic acid in the vegetables which can inhibit zinc absorption. In addition, when zinc levels are excessively high, its absorption is reduced [66].
However, zinc effects on cancer are tissue-specific and differ amongst various cancers [110]. For example, contrary to prostate cancer, a meta-analysis of 19 studies with 400000 participants showed that higher zinc intake reduced the risk of digestive tract carcinomas particularly colorectal cancers [111].
Zinc has a higher concentration in healthy prostate tissue compared to other tissues. However, in prostate cancer, levels of zinc declined significantly [7]. Accordingly, our results showed significantly lower zinc levels of prostatic fluid and tissue in prostate cancer patients compared to controls (SMD: -3.70, 95% CI: -4.90, -2.49) just like serum zinc levels. It is justifiable because, for the most part, zinc levels in epithelial cells of prostate tissue are highest in a healthy individual, whereas the carcinogenesis process causes depletion in them. This shows the necessity of zinc for the physiologic function of the prostate [28]. One explanation for the lower levels of zinc in prostate cancer tissue would be that cells lose their ability to accumulate zinc when normal cells that produce citrate transform into malignant cells that oxidize citrate [7]. Accumulated zinc blocks the oxidation of citrate in the prostate which is the main component of the prostatic fluid [112]. Zinc has regulatory effects on cell proliferation by modulating DNA synthesis. It has positive effects on DNA maintenance and can prevent DNA damage by affecting DNA polymerase [7]. As well as this, zinc can block the proliferation of prostate cancer cells and stop them at the G2/M checkpoint in the cell cycle. Also, zinc causes up-regulation of genes such as p21 which helps with the growth inhibition of prostate cancer cells. Zinc plays a protective role by blocking the NF-κB function that would induce the production of angiogenic and metastatic factors like matrix metalloproteinase 9, vascular endothelial growth factor, and interleukin-6. Moreover, zinc reduces hypoxia-inducible factor-1α in prostate cancer cells and can have apoptotic effects by activating caspase-3 and caspase-9, and increasing levels of Bax protein, a pro-apoptotic protein; hence elevating the Bax/Bcl-2 ratio [112]. However, its apoptotic effects are only limited to those cell lines that have not lost their ability to accumulate intracellular zinc. Zinc has anti-oxidant effects in tissues; from lung microsomes to prostate mitochondria, it removes free radicals and prevents production of them [113]. Zinc is enormously involved in the immune system as well and its depletion results in an imbalance of T-cell functions and cytokines release. It is also indicated that zinc transporters play a necessary role in its homeostasis, hence leading to cancer when dysregulated [110].
Sample preparation can have a significant effect on measuring zinc levels. Our included studies involved samples extracted from the serum, prostatic tissue, nails, and hair. Different methods are employed for the sampling process. Variability in sampling procedures might affect the results; however, the studies included have not indicated evidence surrounding the different sample preparations. The most common way of sampling was blood sampling with 5–10 ml of venous blood, mainly from the antecubital region. Then, it was transferred to anti-septic tubes to avoid any probable contamination. The crucial steps in the prevention of contamination with zinc when collecting the samples were taken based on the International Zinc Nutrition Consultative Group [114]. The blood sampling was mostly done in the morning after a complete night of fasting [83]. Having the specimens stored at -20°C, the next step was centrifuging and digesting by adding nitric acid-perchloric acid to them. Then, samples were cooled down and diluted with distilled water [81]. In the end, measuring zinc level was usually done by atomic spectrophotometry, which can be done through the direct method discussed by Smith et al. [115].
For hair samples, the most routine method was collecting a specific length of hair from the scalp by cutting with scissors, for instance, 3.5 cm long. The samples were then stored and purified by washing. After shortening the length, they were mixed with a detergent solution and shaken thoroughly. Digestion was done by adding acids as mentioned above [95]. Nail samples were washed, purified, digested, and finally prepared in the same way [81]. The nail samples could also be digested with a microwave digestion system [80].
A simple protocol was utilized in a study to prepare prostate tissues for Laser Ablation inductively coupled plasma mass spectrometry (ICP-MS) imaging instead of fresh-frozen sampling, formalin fixation, and formalin-fixed paraffin embedding. The two last techniques mentioned result in massive washouts of the target elements which could lead to false negative results [71, 116]. Notably, the elemental data related to hair samples should be normalized in a way that every element be in the range of 0–1 [91].
ICP-MS is a kind of mass spectrometry that allows for the measuring of metals in low concentrations. It has higher sensitivity and accuracy compared to the AAS technique but can interfere with many species when compared to other types of mass spectrometry. For instance, microbes in the glassware, plasma argon, and air leaks through orifices can be named as interfering substances [117]. While the analytical techniques in the measurement of zinc levels vary among the studies included in our review, it is noteworthy that most of them are conducted through the two abovementioned techniques. More precisely, 24 studies used the AAS technique, nine used ICP-MS, and others used various techniques, such as particle-induced X-ray emission, wet acid digestion, and energy-dispersive X-ray fluorescence. Overall, we did not come across any attributable effects of variability in methods used in different studies, meaning that none of the studies mentioned any significant impact of analytical methods on different outcomes.
In other sources of sampling, although hair zinc levels were significantly lower in prostate cancer patients (SMD: -1.31, 95% CI: -2.19, -0.44), there were no significant changes in nail zinc levels. Likewise, another study on prostate carcinoma evaluating trace elements including zinc in hair samples of 18 prostate cancer patients demonstrated significantly lower hair zinc levels in patients [43]. In other tumors like nasopharyngeal cancer, patients had lower zinc levels in hair samples than controls [118]. Similarly, a meta-analysis of seven studies assessing hair zinc levels in breast cancer patients proved lower hair zinc levels in breast cancer patients compared to controls (SMD: −1.99; 95% CI: −3.46, −0.52) [119].
On the whole, the comprehensive results from our study seem to be of pivotal importance, specifically for physicians and policymakers to target zinc for prostate cancer prevention on a global scale. As an example, a systematic review of 23 studies with 1230 patients of mainly head and neck cancer under treatment of zinc concomitant with chemoradiotherapy, speculated that zinc could help reduce mucositis associated with radiotherapy [120]. As many in vivo studies have demonstrated the effective and therapeutic role of zinc administration on prostate cancer models on murine [121–123], perhaps zinc supplements can be considered as a chemo-preventive agent in prostate cancer. Despite this, the issues associated with the bioavailability and toxicity of its routine supplement use should be noticed and need further study [110].
It is an updated systematic review and meta-analysis of the most recent studies that evaluated the effects of zinc on prostate cancer. It has a large sample size and includes studies on zinc levels of different sources as well as zinc supplementation. Nevertheless, we acknowledge that it has several limitations. Although we used random-effect models and stratified analysis due to the high heterogeneity among the included studies, the results should be interpreted with caution because we could not find the probable cause of heterogeneity. Also, we could not access to full texts of ten studies despite contacting the corresponding authors. As well as this, serum zinc levels could fluctuate based on the circadian rhythm [124]; thus, it was not a reliable tool, mainly because research groups had not considered this when assessing serum zinc levels. Studies could miss the diagnosis of prostate cancer due to a lack of symptoms [66]. Also, using more reliable biological samples like toenail zinc levels that can better indicate chronic exposures to zinc, plus repeated testing and collecting of samples, would present higher quality evidence for similar studies [24]. Moreover, we evaluated and compared total zinc concentrations, while free zinc and bound zinc were not assessed. Future primary studies should report other types of zinc status. It should be considered in the interpretation of the results that not only zinc supplementation but also diet itself can influence the zinc levels.
Conclusions
It appears that serum zinc level is an important factor in the development of prostate cancer. As a result of the inconsistent studies mentioned in the literature, we need more thorough investigations to be able to suggest zinc supplementations as a preventive or therapeutic option for prostate cancer and gain a better insight into this critical question.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur Urol. 2020;77(1):38–52. doi: 10.1016/j.eururo.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 3.Brookman-May SD, Campi R, Henríquez JDS, Klatte T, Langenhuijsen JF, Brausi M, et al. Latest Evidence on the Impact of Smoking, Sports, and Sexual Activity as Modifiable Lifestyle Risk Factors for Prostate Cancer Incidence, Recurrence, and Progression: A Systematic Review of the Literature by the European Association of Urology Section of Oncological Urology (ESOU). Eur Urol Focus. 2019;5(5):756–87. doi: 10.1016/j.euf.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Cao G, Wu F, Wang Y, Liu Z, Hu H, et al. Global Burden of Prostate Cancer and Association with Socioeconomic Status, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study. Journal of Epidemiology and Global Health. 2023;13(3):407–21. doi: 10.1007/s44197-023-00103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall JR. Diet and prostate cancer prevention. World J Urol. 2012;30(2):157–65. doi: 10.1007/s00345-011-0810-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253–6. doi: 10.1017/S002966510800712X [DOI] [PubMed] [Google Scholar]
- 7.To PK, Do MH, Cho JH, Jung C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int J Mol Sci. 2020;21(8). doi: 10.3390/ijms21082991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86(4):521–34. doi: 10.1007/s00204-011-0775-1 [DOI] [PubMed] [Google Scholar]
- 9.Zaichick V, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29(5):565–74. doi: 10.1007/BF02552202 [DOI] [PubMed] [Google Scholar]
- 10.Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7(2):111–7. doi: 10.1038/sj.pcan.4500712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grattan BJ, Freake HC. Zinc and cancer: implications for LIV-1 in breast cancer. Nutrients. 2012;4(7):648–75. doi: 10.3390/nu4070648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alam S, Kelleher SL. Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients. 2012;4(8):875–903. doi: 10.3390/nu4080875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John E, Laskow TC, Buchser WJ, Pitt BR, Basse PH, Butterfield LH, et al. Zinc in innate and adaptive tumor immunity. J Transl Med. 2010;8:118. doi: 10.1186/1479-5876-8-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem. 1997;272(46):28875–81. doi: 10.1074/jbc.272.46.28875 [DOI] [PubMed] [Google Scholar]
- 15.Costello LC, Liu Y, Zou J, Franklin RB. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J Biol Chem. 1999;274(25):17499–504. doi: 10.1074/jbc.274.25.17499 [DOI] [PubMed] [Google Scholar]
- 16.Ito Y, Suzuki K, Sasaki R, Otani M, Aoki K. Mortality rates from cancer or all causes and SOD activity level and Zn/Cu ratio in peripheral blood: population-based follow-up study. J Epidemiol. 2002;12(1):14–21. doi: 10.2188/jea.12.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu T, Sempos CT, Freudenheim JL, Muti P, Smit E. Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann Epidemiol. 2004;14(3):195–201. doi: 10.1016/S1047-2797(03)00119-4 [DOI] [PubMed] [Google Scholar]
- 18.Leone N, Courbon D, Ducimetiere P, Zureik M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17(3):308–14. doi: 10.1097/01.ede.0000209454.41466.b7 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Coogan P, Palmer JR, Strom BL, Rosenberg L. Vitamin and mineral use and risk of prostate cancer: the case-control surveillance study. Cancer causes & control: CCC. 2009;20(5):691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristal AR, Arnold KB, Neuhouser ML, Goodman P, Platz EA, Albanes D, et al. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2010;172(5):566–77. doi: 10.1093/aje/kwq148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SY, Wilkens LR, Morris JS, Henderson BE, Kolonel LN. Serum zinc and prostate cancer risk in a nested case-control study: The multiethnic cohort. The Prostate. 2013;73(3):261–6. doi: 10.1002/pros.22565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson SO, Wolk A, Bergström R, Giovannucci E, Lindgren C, Baron J, et al. Energy, nutrient intake and prostate cancer risk: a population-based case-control study in Sweden. International journal of cancer. 1996;68(6):716–22. doi: [DOI] [PubMed] [Google Scholar]
- 23.Stratton J, Godwin M. The effect of supplemental vitamins and minerals on the development of prostate cancer: a systematic review and meta-analysis. Family Practice. 2011;28(3):243–52. doi: 10.1093/fampra/cmq115 [DOI] [PubMed] [Google Scholar]
- 24.Mahmoud AM, Al-Alem U, Dabbous F, Ali MM, Batai K, Shah E, et al. Zinc Intake and Risk of Prostate Cancer: Case-Control Study and Meta-Analysis. PloS one. 2016;11(11):e0165956. doi: 10.1371/journal.pone.0165956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumulec J, Masarik M, Adam V, Eckschlager T, Provaznik I, Kizek R. Serum and tissue zinc in epithelial malignancies: A meta-analysis. PLoS ONE. 2014;9(6). doi: 10.1371/journal.pone.0099790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Song M, Mucci LA, Giovannucci EL. Zinc supplement use and risk of aggressive prostate cancer: a 30-year follow-up study. European Journal of Epidemiology. 2022;37(12):1251–60. doi: 10.1007/s10654-022-00922-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutiérrez-González E, Castelló A, Fernández-Navarro P, Castaño-Vinyals G, Llorca J, Salas-Trejo D, et al. Dietary Zinc and Risk of Prostate Cancer in Spain: MCC-Spain Study. Nutrients. 2019;11(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Wu Q, Hu X, Dong X, Wang L, Liu Q, et al. Comparative study of serum zinc concentrations in benign and malignant prostate disease: A Systematic Review and Meta-Analysis. Scientific reports. 2016;6:25778. doi: 10.1038/srep25778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Systematic Reviews. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haddaway NR, Collins AM, Coughlin D, Kirk S. The Role of Google Scholar in Evidence Reviews and Its Applicability to Grey Literature Searching. PLOS ONE. 2015;10(9):e0138237. doi: 10.1371/journal.pone.0138237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Institute JB. Critical Appraisal Tools JBIUoA, South Australia; 2021 [Available from: https://jbi.global/critical-appraisal-tools.
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 33.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology. 2014;14(1):135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 35.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 36.Duval S, Tweedie R. A Nonparametric "Trim and Fill" Method of Accounting for Publication Bias in Meta-Analysis. Journal of the American Statistical Association. 2000;95(449):89–98. [Google Scholar]
- 37.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 38.Al-Timimi RJM. Serum concentration of CU, ZN and SE in malignant and benign prostatic lesions. Pakistan Journal of Biotechnology. 2018;15(3):635–9. [Google Scholar]
- 39.Altaf M, Ul Haque S, Inayat ur R. Low serum zinc and higher risk of Ca prostate in local population study. Medical Forum Monthly. 2010;21(8):7–9. [Google Scholar]
- 40.Chirulescu Z, Chiriloiu C, Suciu A, Pîrvulescu R. Variations of zinc, calcium and magnesium in normal subjects and in patients with neoplasias. Medecine interne. 1987;25(4):257–61. [PubMed] [Google Scholar]
- 41.Ji K, Zhang L, Shao Y, Tian Y, Liang Z, Liu Y, et al. Significance of trace elements copper and zinc change in the serum of patients with prostate cancer. Chinese Journal of Andrology. 2007;21(5):9–10+4. [Google Scholar]
- 42.Maganto E, Gil A, Mateos JA. Serum zinc and prostate cancer. Actas Urologicas Espanolas. 1982;6(6):347–52. [PubMed] [Google Scholar]
- 43.Ouyang SY, Li SL. [Investigation of trace elements in hair of patients with prostate carcinoma, benign prostatic hypertrophy, and normal controls]. Hunan yi ke da xue xue bao = Hunan yike daxue xuebao = Bulletin of Hunan Medical University. 2000;25(3):279–80. [PubMed] [Google Scholar]
- 44.Rahman MT, Mumu MA, Kabir Y, Choudhury MM, Saiedullah M. Serum zinc level and prostatic lesion. Mymensingh medical journal: MMJ. 2012;21(4):679–83. [PubMed] [Google Scholar]
- 45.Rekha C, Praveena V. Serum zinc and copper levels in benign and malignant lesions of prostate. International Journal of Pharma and Bio Sciences. 2014;5(3):B926–B30. [Google Scholar]
- 46.Sanada S, Ogura K, Kiriyama T, Yoshida O. Serum copper and zinc levels in patients with malignant neoplasm of the urogenital tract. Acta Urologica Japonica. 1985;31(8):1299–316. [PubMed] [Google Scholar]
- 47.Schneider HJ, Anke M. The mineral content of the human scalp hair in various diseases. Z Ges Inn Med. 1966;21(24):802–6. [PubMed] [Google Scholar]
- 48.Bataineh ZM, Bani Hani IH, Al-Alami JR. Zinc in normal and pathological human prostate gland. Saudi medical journal. 2002;23(2):218–20. [PubMed] [Google Scholar]
- 49.Maddalone MG, Oderda M, Mengozzi G, Gesmundo I, Novelli F, Giovarelli M, et al. Urinary Zinc Loss Identifies Prostate Cancer Patients. Cancers. 2022;14(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sapota A, Darago A, Taczalski J, Kilanowicz A. Disturbed homeostasis of zinc and other essential elements in the prostate gland dependent on the character of pathological lesions. Biometals: an international journal on the role of metal ions in biology, biochemistry, and medicine. 2009;22(6):1041–9. doi: 10.1007/s10534-009-9255-y [DOI] [PubMed] [Google Scholar]
- 51.Adaramoye OA, Akinloye O, Olatunji IK. Trace elements and vitamin E status in Nigerian patients with prostate cancer. African health sciences. 2010;10(1):2–8. [PMC free article] [PubMed] [Google Scholar]
- 52.Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT. A case-control study of diet and prostate cancer. British journal of cancer. 1997;76(5):678–87. doi: 10.1038/bjc.1997.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abhishek A, Singh V, Sinha RJ, Ansari NG, Siddiqe MKJ, Verma M, et al. To study the relationship between cadmium, zinc and mtDNA copy number in North Indian patients suffering from prostate cancer: A case control study. African Journal of Urology. 2017;23(2):126–32. [Google Scholar]
- 54.Habib FK, Mason MK, Smith PH, Stitch SR. Cancer of the prostate: early diagnosis by zinc and hormone analysis? British journal of cancer. 1979;39(6):700–4. doi: 10.1038/bjc.1979.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaichick S, Zaichick V. Trace elements of normal, benign hypertrophic and cancerous tissues of the human prostate gland investigated by neutron activation analysis. Applied radiation and isotopes: including data, instrumentation and methods for use in agriculture, industry and medicine. 2012;70(1):81–7. doi: 10.1016/j.apradiso.2011.08.021 [DOI] [PubMed] [Google Scholar]
- 56.Lu Y, Su H, Wang Y, Li H. Micronutrients and risks of three main urologic cancers: A mendelian randomization study. Frontiers in nutrition. 2023;10:1016243. doi: 10.3389/fnut.2023.1016243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amadi C, Aleme BM. The Prevalence of Zinc Deficiency among Men with and without Prostate Cancer in Port Harcourt, Nigeria. Nutrition and cancer. 2020;72(6):1018–25. doi: 10.1080/01635581.2019.1664600 [DOI] [PubMed] [Google Scholar]
- 58.Aydin A, Arsova-Sarafinovska Z, Sayal A, Eken A, Erdem O, Erten K, et al. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clinical biochemistry. 2006;39(2):176–9. doi: 10.1016/j.clinbiochem.2005.11.018 [DOI] [PubMed] [Google Scholar]
- 59.Bede-Ojimadu O, Nnamah N, Onuegbu J, Grant-Weaver I, Barraza F, Orakwe J, et al. Cadmium exposure and the risk of prostate cancer among Nigerian men: Effect modification by zinc status. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements (GMS). 2023;78:127168. doi: 10.1016/j.jtemb.2023.127168 [DOI] [PubMed] [Google Scholar]
- 60.Białkowska K, Marciniak W, Muszyńska M, Baszuk P, Gupta S, Jaworska-Bieniek K, et al. Association of zinc level and polymorphism in MMP-7 gene with prostate cancer in Polish population. PLoS One. 2018;13(7):e0201065. doi: 10.1371/journal.pone.0201065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christudoss P, Selvakumar R, Fleming JJ, Gopalakrishnan G. Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian journal of urology: IJU: journal of the Urological Society of India. 2011;27(1):14–8. doi: 10.4103/0970-1591.78405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feustel A, Wennrich R. Zinc and cadmium plasma and erythrocyte levels in prostatic carcinoma, BPH, urological malignancies, and inflammations. The Prostate. 1986;8(1):75–9. doi: 10.1002/pros.2990080109 [DOI] [PubMed] [Google Scholar]
- 63.Feustel A, Wennrich R, Schmidt B. Serum-Zn-levels in prostatic cancer. Urological research. 1989;17(1):41–2. doi: 10.1007/BF00261049 [DOI] [PubMed] [Google Scholar]
- 64.Gallus S, Foschi R, Negri E, Talamini R, Franceschi S, Montella M, et al. Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur Urol. 2007;52(4):1052–6. doi: 10.1016/j.eururo.2007.01.094 [DOI] [PubMed] [Google Scholar]
- 65.Goel T, Sankhwar SN. Comparative study of zinc levels in benign and malignant lesions of the prostate. Scandinavian journal of urology and nephrology. 2006;40(2):108–12. doi: 10.1080/00365590500368922 [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez A, Peters U, Lampe JW, White E. Zinc intake from supplements and diet and prostate cancer. Nutrition and cancer. 2009;61(2):206–15. doi: 10.1080/01635580802419749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gutiérrez-González E, Castelló A, Fernández-Navarro P, Castaño-Vinyals G, Llorca J, Salas D, et al. Dietary Zinc and Risk of Prostate Cancer in Spain: MCC-Spain Study. Nutrients. 2018;11(1). doi: 10.3390/nu11010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Igbokwe M, Salako A, Badmus T, Obiajunwa E, Olasehinde O, Igbokwe C, et al. Tissue Zinc Concentration in Prostate Cancer: Relationship with Prostate Specific Antigen and Gleason Score in a Cohort of Nigerian Men. Asia Pacific Journal of Cancer Biology. 2021;6(2):147–53. [Google Scholar]
- 69.Jain M, Sharma K, Sharma VP. Serum and tissue levels of zinc, copper, magnesium and retinol in prostatic neoplasms. Indian Journal of Clinical Biochemistry. 1994;9(2):106–8. [Google Scholar]
- 70.Kaba M, Pirincci N, Yuksel MB, Gecit I, Gunes M, Ozveren H, et al. Serum levels of trace elements in patients with prostate cancer. Asian Pacific journal of cancer prevention: APJCP. 2014;15(6):2625–9. [DOI] [PubMed] [Google Scholar]
- 71.Karimi G, Shahar S, Homayouni N, Rajikan R, Abu Bakar NF, Othman MS. Association between trace element and heavy metal levels in hair and nail with prostate cancer. Asian Pacific journal of cancer prevention: APJCP. 2012;13(9):4249–53. [DOI] [PubMed] [Google Scholar]
- 72.Kolonel LN, Yoshizawa CN, Hankin JH. Diet and prostatic cancer: a case-control study in Hawaii. Am J Epidemiol. 1988;127(5):999–1012. doi: 10.1093/oxfordjournals.aje.a114903 [DOI] [PubMed] [Google Scholar]
- 73.Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1999;8(10):887–92. [PubMed] [Google Scholar]
- 74.Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. Zinc supplement use and risk of prostate cancer. Journal of the National Cancer Institute. 2003;95(13):1004–7. doi: 10.1093/jnci/95.13.1004 [DOI] [PubMed] [Google Scholar]
- 75.McBean LD, Smith JC Jr, Berne BH, Halsted JA. Serum zinc and alpha2 macroglobulin concentration in myocardial infarction, decubitus ulcer, multiple myeloma, prostatic carcinoma, Down’s syndrome and nephrotic syndrome. Clinica Chimica Acta. 1974;50(1):43–51. doi: 10.1016/0009-8981(74)90076-x [DOI] [PubMed] [Google Scholar]
- 76.Nsonwu-Anyanwu AC, Icha BE, Nsonwu MC, William MI, Emughupogh KS, Usoro CAO. Assessment of Essential and Non-essential Elements as Risk Evaluation Indices in Men with Prostate Cancer in Calabar South-South Nigeria. Middle East Journal of Cancer. 2022;13(2):285–92. [Google Scholar]
- 77.Ogunlewe JO, Osegbe DN. Zinc and cadmium concentrations in indigenous blacks with normal, hypertrophic, and malignant prostate. Cancer. 1989;63(7):1388–92. doi: [DOI] [PubMed] [Google Scholar]
- 78.Olooto WE, Oyelekan AA, Adewole OO, Fajobi AO, Adedo AA, Olasimbo O. Serum gonadotropins, cortisol, PSA, and micronutrient levels among men with prostate carcinoma. African Journal of Urology. 2021;27(1). [Google Scholar]
- 79.Ozmen H, Erulas FA, Karatas F, Cukurovali A, Yalcin O. Comparison of the concentration of trace metals (Ni, Zn, Co, Cu and Se), Fe, vitamins A, C and E, and lipid peroxidation in patients with prostate cancer. Clinical chemistry and laboratory medicine. 2006;44(2):175–9. doi: 10.1515/CCLM.2006.032 [DOI] [PubMed] [Google Scholar]
- 80.Platz EA, Helzlsouer KJ, Hoffman SC, Morris JS, Baskett CK, Comstock GW. Prediagnostic toenail cadmium and zinc and subsequent prostate cancer risk. The Prostate. 2002;52(4):288–96. doi: 10.1002/pros.10115 [DOI] [PubMed] [Google Scholar]
- 81.Qayyum MA, Shah MH. Comparative study of trace elements in blood, scalp hair and nails of prostate cancer patients in relation to healthy donors. Biological trace element research. 2014;162(1–3):46–57. doi: 10.1007/s12011-014-0123-4 [DOI] [PubMed] [Google Scholar]
- 82.Saleh SAK, Adly HM, Abdelkhaliq AA, Nassir AM. Serum Levels of Selenium, Zinc, Copper, Manganese, and Iron in Prostate Cancer Patients. Current urology. 2020;14(1):44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wakwe VC, Odum EP, Amadi C. The impact of plasma zinc status on the severity of prostate cancer disease. Investigative and clinical urology. 2019;60(3):162–8. doi: 10.4111/icu.2019.60.3.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer causes & control: CCC. 1991;2(2):85–94. doi: 10.1007/BF00053126 [DOI] [PubMed] [Google Scholar]
- 85.Yilmaz MI, Saglam K, Sonmez A, Gok DE, Basal S, Kilic S, et al. Antioxidant system activation in prostate cancer. Biological trace element research. 2004;98(1):13–9. doi: 10.1385/BTER:98:1:13 [DOI] [PubMed] [Google Scholar]
- 86.Zaichick V, Zaichick S. Using prostatic fluid levels of zinc to iron concentration ratio in non-invasive and highly accurate screening for prostate cancer. International Journal of Medical Sciences. 2019;6(11):24–31. [Google Scholar]
- 87.Zaichick VY, Sviridova TV, Zaichick SV. Zinc concentration in human prostatic fluid: normal, chronic prostatitis, adenoma and cancer. Int Urol Nephrol. 1996;28(5):687–94. doi: 10.1007/BF02552165 [DOI] [PubMed] [Google Scholar]
- 88.Adedapo KS, Arinola OG, Shittu OB, Kareem OI, Okolo CA, Nwobi LN. Diagnostic value of lipids, total antioxidants, and trace metals in benign prostate hyperplasia and prostate cancer. Niger J Clin Pract. 2012;15(3):293–7. doi: 10.4103/1119-3077.100623 [DOI] [PubMed] [Google Scholar]
- 89.Eken A, Kaya E, nluEndirlik B, Erdem O, Akay C, Ozgok Y. Evaluation of trace element levels in patients with prostate cancer, benign prostatic hyperplasia and chronic prostatitis. Gulhane Medical Journal. 2016;58:1. [Google Scholar]
- 90.Gómez Y, Arocha F, Espinoza F, Fernández D, Vásquez A, Granadillo V. [Zinc levels in prostatic fluid of patients with prostate pathologies]. Invest Clin. 2007;48(3):287–94. [PubMed] [Google Scholar]
- 91.Guo J, Deng W, Zhang L, Li C, Wu P, Mao P. Prediction of prostate cancer using hair trace element concentration and support vector machine method. Biological trace element research. 2007;116(3):257–72. doi: 10.1007/BF02698010 [DOI] [PubMed] [Google Scholar]
- 92.Lee MM, Wang RT, Hsing AW, Gu FL, Wang T, Spitz M. Case-control study of diet and prostate cancer in China. Cancer causes & control: CCC. 1998;9(6):545–52. [DOI] [PubMed] [Google Scholar]
- 93.Li XM, Zhang L, Li J, Li Y, Wang HL, Ji GY, et al. Measurement of serum zinc improves prostate cancer detection efficiency in patients with PSA levels between 4 ng/mL and 10 ng/mL. Asian J Androl. 2005;7(3):323–8. doi: 10.1111/j.1745-7262.2005.00044.x [DOI] [PubMed] [Google Scholar]
- 94.Onyema-Iloh O, Meludu S, Iloh E, Nnodim J, Onyegbule O, Mykembata B. Biochemical changes in some trace elements, antioxidant vitamins and their therapeutic importance in prostate cancer patients. Asian Journal of Medical Sciences. 2014;6. [Google Scholar]
- 95.Saleh S, Adly H, Nassir A. Altered Trace Elements Levels in Hair of Prostate Cancer Patients. Journal of Cancer Science & Therapy. 2017;09. [Google Scholar]
- 96.Tan C, Chen H. Screening of prostate cancer by analyzing trace elements in hair and chemometrics. Biological trace element research. 2011;144(1–3):97–108. doi: 10.1007/s12011-011-9038-5 [DOI] [PubMed] [Google Scholar]
- 97.Vlajinac HD, Marinković JM, Ilić MD, Kocev NI. Diet and prostate cancer: a case-control study. Eur J Cancer. 1997;33(1):101–7. doi: 10.1016/s0959-8049(96)00373-5 [DOI] [PubMed] [Google Scholar]
- 98.WILLDEN EG, Robinson M. Plasma zinc levels in prostatic disease. British Journal of Urology. 1975;47(3):295–9. [DOI] [PubMed] [Google Scholar]
- 99.khedir Abdelmajid LM, Hessen RIE, Dafalla AM, Hassan MI, Mohammed YA. Serum Zinc and Copper Levels among Patients with Prostatic Cancer Attending National Cancer Institute, Gezira University, Sudan. Sudan Medical Laboratory Journal. 2022;10(2):69–77. [Google Scholar]
- 100.Mohammed RK. Evaluation of Copper and Zinc in Sera of Iraqi Male Patients with Prostate Cancer in Baghdad City. Iraqi National Journal Of Chemistry. 2015;15(3). [Google Scholar]
- 101.Yari H, Mohseni M, Vardi R, Alizadeh AM, Mazloomzadeh S. Copper, Lead, Zinc and Cadmium levels in serum of prostate cancer patients by polarography in Iran. J Chem Pharmaceut Res. 2015;7(2):403–8. [Google Scholar]
- 102.Lim JT, Tan YQ, Valeri L, Lee J, Geok PP, Chia SE, et al. Association between serum heavy metals and prostate cancer risk–A multiple metal analysis. Environment International. 2019;132:105109. doi: 10.1016/j.envint.2019.105109 [DOI] [PubMed] [Google Scholar]
- 103.Mao S, Huang S. Zinc and Copper Levels in Bladder Cancer: A Systematic Review and Meta-Analysis. Biological trace element research. 2013;153(1):5–10. doi: 10.1007/s12011-013-9682-z [DOI] [PubMed] [Google Scholar]
- 104.Feng Y, Zeng J-W, Ma Q, Zhang S, Tang J, Feng J-F. Serum copper and zinc levels in breast cancer: A meta-analysis. Journal of Trace Elements in Medicine and Biology. 2020;62:126629. doi: 10.1016/j.jtemb.2020.126629 [DOI] [PubMed] [Google Scholar]
- 105.Tsodikov A, Gulati R, de Carvalho TM, Heijnsdijk EAM, Hunter-Merrill RA, Mariotto AB, et al. Is prostate cancer different in black men? Answers from 3 natural history models. Cancer. 2017;123(12):2312–9. doi: 10.1002/cncr.30687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463(2):211–7. doi: 10.1016/j.abb.2007.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahmoud AM, Al-alem U, Shah E, Batai K, Dabbous F, Ali M, et al. Zinc and prostate cancer: A systematic review. Cancer Research. 2013;73(8). [Google Scholar]
- 108.Epstein MM, Kasperzyk JL, Andrén O, Giovannucci EL, Wolk A, Håkansson N, et al. Dietary zinc and prostate cancer survival in a Swedish cohort. Am J Clin Nutr. 2011;93(3):586–93. doi: 10.3945/ajcn.110.004804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zinc Dietary and Prostate Cancer in the TRAMP Mouse Model. Journal of Medicinal Food. 2010;13(1):70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang J, Zhao H, Xu Z, Cheng X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer biology & medicine. 2020;17(3):612–25. doi: 10.20892/j.issn.2095-3941.2020.0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li P, Xu J, Shi Y, Ye Y, Chen K, Yang J, et al. Association between zinc intake and risk of digestive tract cancers: a systematic review and meta-analysis. Clin Nutr. 2014;33(3):415–20. doi: 10.1016/j.clnu.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 112.Kolenko V, Teper E, Kutikov A, Uzzo R. Zinc and zinc transporters in prostate carcinogenesis. Nature reviews Urology. 2013;10(4):219–26. doi: 10.1038/nrurol.2013.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ho E, Song Y. Zinc and prostatic cancer. Current Opinion in Clinical Nutrition & Metabolic Care. 2009;12(6):640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lönnerdal B, et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25(1 Suppl 2):S99–203. [PubMed] [Google Scholar]
- 115.Smith JC Jr, Butrimovitz GP, Purdy WC. Direct measurement of zinc in plasma by atomic absorption spectroscopy. Clinical Chemistry. 1979;25(8):1487–91. [PubMed] [Google Scholar]
- 116.Buchholz R, Krossa S, Andersen MK, Holtkamp M, Sperling M, Karst U, et al. A simple preparation protocol for shipping and storage of tissue sections for laser ablation-inductively coupled plasma-mass spectrometry imaging. Metallomics. 2022;14(3). doi: 10.1093/mtomcs/mfac013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sudhakar P, Latha P, Reddy PV. Chapter 17—Analytical techniques. In: Sudhakar P, Latha P, Reddy PV, editors. Phenotyping Crop Plants for Physiological and Biochemical Traits: Academic Press; 2016. p. 137–49. [Google Scholar]
- 118.Leung PL, Huang HM. Analysis of trace elements in the hair of volunteers suffering from naso-pharyngeal cancer. Biological trace element research. 1997;57(1):19–25. doi: 10.1007/BF02803866 [DOI] [PubMed] [Google Scholar]
- 119.Wu X, Tang J, Xie M. Serum and hair zinc levels in breast cancer: a meta-analysis. Scientific reports. 2015;5(1):12249. doi: 10.1038/srep12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoppe C, Kutschan S, Dörfler J, Büntzel J, Büntzel J, Huebner J. Zinc as a complementary treatment for cancer patients: a systematic review. Clinical and Experimental Medicine. 2021;21(2):297–313. doi: 10.1007/s10238-020-00677-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shah MR, Kriedt CL, Lents NH, Hoyer MK, Jamaluddin N, Klein C, et al. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. Journal of experimental & clinical cancer research: CR. 2009;28(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prasad AS, Mukhtar H, Beck FW, Adhami VM, Siddiqui IA, Din M, et al. Dietary zinc and prostate cancer in the TRAMP mouse model. Journal of medicinal food. 2010;13(1):70–6. doi: 10.1089/jmf.2009.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ko YH, Woo YJ, Kim JW, Choi H, Kang SH, Lee JG, et al. High-dose dietary zinc promotes prostate intraepithelial neoplasia in a murine tumor induction model. Asian J Androl. 2010;12(2):164–70. doi: 10.1038/aja.2009.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Couturier E, van Onderbergen A, Bosson D, Neve J. Circadian variations in plasma zinc and cortisol in man. J Trace Elem Electrolytes Health Dis. 1988;2(4):245–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.