Abstract
INTRODUCTION
Approximately 20–40% of kidney cancer patients treated for localized disease experience post-surgical recurrence. Several prognostic models exist to help clinicians determine the risk of distant recurrence, but these models vary in criteria and endpoints. We aimed to examine the recurrence rate and clinicopathologic factors as predictors of recurrence in high-risk renal cell carcinoma (RCC) patients.
METHODS
We conducted a single-center, retrospective chart review of pT3 RCC patients who underwent a nephrectomy between January 2000 and December 2015. Patients registered in clinical trials for adjuvant therapy and those with fewer than three years of followup were excluded. Kaplan-Meier survival analysis and univariate and multivariate Cox regression were performed to identify the rate and predictors of disease recurrence.
RESULTS
Eighty-eight pT3 RCC patients were included, and 39 patients had recurrence with a median of 23.5 months (range 1.6–127.5). Nine patients had disease recurrence beyond 58 months. Kaplan-Meier log-rank tests identified patients with negative surgical margins and low Fuhrman nuclear grades had greater recurrence-free survival. Univariate Cox regression revealed positive surgical margins, high Fuhrman nuclear grade, and large tumor sizes were significant predictors. In the multivariate Cox regression model, high Fuhrman nuclear grade and positive surgical margins were significant predictors of recurrence.
CONCLUSIONS
Disease recurrence occurred in 44% of pT3-staged patients. High Fuhrman nuclear grade and positive surgical margins were associated with time to recurrence. Physicians should use prognostic models to facilitate conversations about disease recurrence and continue to monitor high-risk patients beyond the recommended five-year followup period. We recommend monitoring pT3 resected patients for up to 10 years post-surgery.
INTRODUCTION
Kidney cancer cases continue to increase worldwide; 431 288 new cases were diagnosed in 2020,1 accounting for 3% of all reported human cancers worldwide.2 Renal cell carcinoma (RCC) is the most common form of kidney cancer, accounting for 90% of all cases.3–5 Localized RCC is often managed through the surgical intervention of partial or radical nephrectomy.5 Despite surgery being the most effective treatment option, post-surgical disease recurrence is observed in 20–40% of patients treated for localized disease.6
Identifying the risk for recurrence is valuable for counselling and scheduling followup surveillance for patients.7 Multiple prognostic and risk stratification models and nomograms are available to help clinicians predict RCC post-surgical outcomes in nonmetastatic patients.8–15 The most commonly used models include the Kattan postoperative RCC predictive nomogram, 7 the UISS postoperative prognostic RCC model based on UCLA risk group stratification,8 the Leibovich RCC model for the prediction of progression after radical nephrectomy for patients with clear-cell RCC (ccRCC),9 the Mayo D-SSIGN model for postoperative cancer-specific survival following radical nephrectomy for ccRCC,10 and the Karakiewicz RCC cancer-specific survival nomogram.13
Additionally, a recent prognostic model was developed using the ASSURE randomized trial data to predict oncologic outcomes for non-metastatic high-risk RCC cases.16 All of these models differ in the prognostic variables used to determine the risk of recurrence, which include pre-and postoperative factors such as histology, TNM staging, tumor size and grade, necrosis, and lymph node status, Fuhrman grade, Eastern Cooperative Oncology Group (ECOG ) score, and symptoms.8–16 These models aim to predict that patients with primary ≥pT3 may be at an intermediate to high risk for post-surgical recurrence; however, as these models differ in criteria and endpoints, the rate of recurrence often varies based on the model used,14 leading to variation in the literature.
Following partial nephrectomy (PN) or radical nephrectomy (RN ), recurrence rates remain high at 7%, 26%, and 39% for pT1, pT2, and pT3 stage disease, respectively. 17 Using predictive models to accurately assess the risk for RCC recurrence at any stage is useful. This can help identify patients who may benefit from adjuvant therapy in a clinical trial, as well as close monitoring to improve oncologic outcomes;18,19 however, for pT3 disease, the available evidence concerning predictors of distant recurrence is divergent in the literature.20,21
To further examine recurrence rate and clinicopathologic factors as predictors of distant recurrence in patients with pT3 RCC, we evaluated patients who had PN or RN between 2000 and 2015 at our academic tertiary care center.
METHODS
Patient selection
After obtaining approval from the Hamilton Integrated Research Ethics Board (Project #12654), we performed a retrospective electronic chart review of all PN and RN cases completed between January 1, 2000 and December 31, 2015 at our center. The date range was chosen to allow evaluation of recurrence for up to at least five years post-nephrectomy. Patients were eligible for inclusion if they had a pT3 non-metastatic RCC (nmRCC) tumor removed during the study period and were ≥18 years of age at the time of surgery. This included patients with pT1a cases that were upstaged to pT3a. Patients were excluded if they were part of a registered clinical trial for adjuvant therapy, received preoperative chemotherapy, underwent any ablative therapy, were followed up for fewer than three years at our center, had positive lymph node involvement or bilateral renal masses, had unavailable pathologic data, age <18, patients with non-RCC histology, or had a previous history of invasive kidney cancer. Followup data were collected until June 30, 2022.
Variables and outcome measures
Baseline variables were extracted, including age, birth-assigned sex, and date of surgery, while postoperative parameters included type of surgery (PN or RN ), RCC histology, tumor size and stage, Fuhrman nuclear grade, number of lymph nodes resected, surgical margin status (positive or negative), the status of recurrence (yes/no), recurrence date, months until the first recurrence, and metastatic disease sites. The variables ‘type of surgery’, ‘RCC histology’, ‘surgical margin status’, ‘tumor stage’, and ‘the status of recurrence’ were operationalized as categorical variables in this study. The Fuhrman nuclear grade variable was treated as an ordinal variable in the study. The variable was categorized according to the grade assigned in the pathology report. A grade of 4 was designated as the most severe. Additionally, tumor size, number of lymph nodes resected, and months until the first recurrence were captured as continuous variables.
The primary outcome measure was time to progression, with progression defined as local recurrence in either kidney or regional or distant metastases. The secondary outcome was to identify prognostic variables for recurrence.
Statistical analysis
Baseline and postoperative characteristics were evaluated using descriptive statistics: means ± standard deviations (SD ) for continuous variables with normal distribution and medians with interquartile range (IQR) for continuous variables with non-normal distribution means; numbers (%) for categorical data; univariate and multivariate binary logistic regression models were used to evaluate prognostic factors for recurrence. Given the low sample size of patients with disease recurrence (n=39), only three independent variables were used for multivariate analysis.22 A multivariate analysis was conducted with the three significant variables from the univariate analysis. Kaplan-Meier analysis was used to estimate the survival probabilities and the log-rank test was used to determine the significance of recurrence-free survival (RFS) based on prognostic variables. Hazard ratios (HR s) were evaluated using the Cox proportional hazards model. Statistical significance was set at p<0.05 and all statistical tests were conducted using IBM SPSS Statistics v.28 (Armonk, NY, U.S.).
RESULTS
Patients
With a computerized search, we identified 273 cases that were classified as pT3 or higher at our institution. A total of 88 patients who underwent PN or RN at our center between January 1, 2000, and December 31, 2015, met the inclusion criteria. The demographic and clinicopathologic characteristics of all patients are summarized in Table 1.
Table 1.
Clinicopathologic characteristics of patients
| Variables | Overall (n=88) | Recurrence (n=39) | No recurrence (n=49) |
|---|---|---|---|
|
| |||
| Median age, years | 64±12.3 | 64±11.59 | 66±13 |
|
| |||
| Sex | |||
| Male | 59 (67%) | 29 (74.4%) | 30 (61.2%) |
| Female | 29 (33%) | 10 (25.6%) | 19 (38.8%) |
|
| |||
| Surgical approach | |||
| Open radical nephrectomy | 20 (22.7%) | 10 (25.6%) | 10 (20.4%) |
| Laparoscopic radical nephrectomy | 62 (70.5%) | 28 (71.8%) | 34 (69.4%) |
| Open partial nephrectomy | 2 (2.3%) | 0 | 2 (4.1%) |
| Laparoscopic partial nephrectomy | 4 (4.5%) | 1 (2.6%) | 3 (6.1%) |
|
| |||
| Pathological subtype | |||
| Clear-cell RCC | 74 (84.1%) | 34 (87.2%) | 40 (81.6%) |
| Chromophobe RCC | 4 (4.5%) | 0 | 4 (8.2%) |
| Papillary RCC (type 2) | 3 (3.4%) | 2 (5.1%) | 1 (2%) |
| Other (e.g., sarcomatoid, RCC unclassified, papillary RCC type not specified) | 7 (8%) | 3 (7.7%) | 4 (8.2%) |
|
| |||
| pT classification | |||
| T3a | 66 (75%) | 26 (66.7%) | 40 (81.6%) |
| T3b | 8 (9.1%) | 6 (15.4%) | 2 (4.1%) |
| T3c | 1 (1.1%) | 0 | 1 (2%) |
| T3 undefined | 13 (14.8%) | 7 (17.9%) | 6 (12.2%) |
|
| |||
| Fuhrman nuclear grade | |||
| 1 | 4 (4.5%) | 1 (2.6%) | 3 (6.1%) |
| 2 | 45 (51.1%) | 18 (46.2%) | 27 (55.1%) |
| 3 | 27 (30.7%) | 13 (33.3%) | 14 (28.6%) |
| 4 | 7 (8%) | 7 (17.9%) | 0 |
| Not classified | 5 (5.7%) | 0 | 5 (10.2%) |
|
| |||
| Surgical margin | |||
| Positive | 7 (8%) | 6 (15.4%) | 1 (2%) |
| Negative | 81 (92%) | 33 (84.6%) | 48 (98%) |
| Median largest tumor size (IQR) | 7.1 (5, 9.5) | 8 (6, 10) | 7 (4, 9.5) |
|
| |||
| Mean number of lymph nodes resected | 2.3±4.2 | 3.1±5.1 | 1.7±3.7 |
IQR: interquartile range; RCC: renal cell carcinoma.
Of the 88 patients, 74 (84.1%) had ccRCC histology, four (4.5%) had chromophobe RCC (ChRCC), three (3.4%) had papillary RCC type 2 (pRCC), and seven (8%) were unclassified pRCC or an unclassified RCC (with or without sarcomatoid change). RCC recurrence was found in 39 (44.3%) patients, of which 29 (74.4%) were male, 28 (71.8%) underwent laparoscopic RN, 34 (87.2%) had ccRCC histology, 26 (66.7%) had pT3a disease, and the mean age was 62.9 (±11.59). Six (15.4%) of the patients that experienced recurrence had positive surgical margins. The median time to recurrence was 23.5 months (range 1.6–127.5, IQR 5.9, 58.2), with nine patients experiencing recurrence past the 58-month postoperative mark.
A total of 13 patients had metastasis at one site, while 26 had multiple metastatic sites. Metastases were observed in the lung, abdomen (e.g., liver, pancreas, duodenum), bone, brain, and thyroid. Out of the total of 39 patients, two experienced localized recurrence in the renal bed or fossa, whereas the remaining 37 patients had distant recurrence.
Evaluation of prognostic factors for disease recurrence
Univariate and multivariate logistic regression models were computed to identify prognostic factors for disease recurrence (Table 2). The univariate logistic regression analysis found positive surgical margins (odds ratio [OR ] 8.73, 95% confidence interval [CI] 1–75.9, p=0.05) and a high Fuhrman nuclear grade (OR 2.41, 95% CI 1.22–4.74, p=0.011), as well as large tumor sizes (OR 1.14, 95% CI 1–1.3, p=0.047) were significant predictors of disease recurrence.
Table 2.
Binary logistic regression to evaluate prognostic factors for recurrence
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
|
| ||||
| Age | 0.99 (0.96, 1.03) | 0.813 | ||
|
| ||||
| Sex | ||||
| Male | 1.84 (0.73, 4.61) | 0.195 | ||
| Female | Reference | Reference | ||
|
| ||||
| Pathologic subtype | ||||
| Clear-cell RCC | 1.53 (0.47, 5.00) | 0.482 | ||
| Other (e.g., chromophobe, sarcomatoid, RCC unclassified, papillary RCC) | Reference | Reference | ||
|
| ||||
| Surgical approach | ||||
| Partial nephrectomy | Reference | Reference | ||
| Radical nephrectomy | 4.32 (0.48, 38.6) | 0.191 | ||
| Largest tumor size | 1.14 (1, 1.3) | 0.047 | 1.1 (0.95, 1.26) | 0.215 |
| Number of lymph nodes resected | 1.08 (0.97, 1.21) | 0.150 | ||
|
| ||||
| pT classification | ||||
| T3a and T3 undefined | Reference | Reference | ||
| T3b and T3c | 0.36 (0.084, 1.54) | 0.168 | ||
| Fuhrman nuclear grade | 2.41 (1.22, 4.74) | 0.011 | 2.18 (1.04, 4.54) | 0.038 |
|
| ||||
| Surgical margin | ||||
| Positive | 8.73 (1.00, 75.9) | 0.050 | 7.05 (0.77, 64.95) | 0.085 |
| Negative | Reference | Reference | Reference | Reference |
|
| ||||
| Goodness of fit - Hosmer and Lemeshow test | X2, DF | p | ||
| 8.594, 8 | 0.378 | |||
CI: confidence interval; RCC: renal cell carcinoma.
The multivariate analysis identified a high Fuhrman nuclear grade (OR 2.18, 95% CI 1.04, 4.54, p=0.038) as a significant predictor for disease recurrence when adjusted.
Recurrence-free survival (RFS)
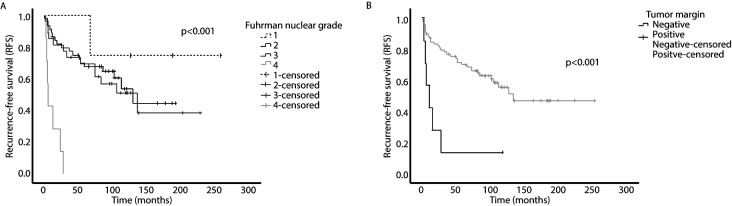
Kaplan-Meier analyses showed patients with negative surgical margins and low Fuhrman nuclear grades had greater RFS (Figures 1A and 1B). Kaplan-Meier analyses revealed no significant association between RFS and prognostic factors, including birth-assigned sex (p=0.115), pT staging (p=0.232), surgical approach (p=0.276), or pathologic subtype (p=0.343).
Figure 1.
Kaplan-Meier survival curve for (A) Fuhrman nuclear grade; and (B) tumor margin.
Subsequently, univariate Cox regression revealed a high Fuhrman nuclear grade (HR 2.27, 95% CI 1.40, 3.68, p<0.001) positive surgical margins (HR 4.60, 95% CI 1.89, 11.23, p<0.001), and large tumor sizes (HR 1.09, 95% CI 1.00, 1.19, p=0.004) were associated with worse RFS (Table 3). Multivariate analysis found high Fuhrman nuclear grade (HR 2.12, 95% CI 1.30, 3.47, p=0.003) and positive surgical margins (HR 4.23, 95% CI 1.71, 10.46, p=0.002) were significant factors for disease recurrence.
Table 3.
Cox regression predicting recurrence-free survival
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
|
| ||||
| Age | 0.99 (0.97, 1.02) | 0.936 | ||
|
| ||||
| Sex | ||||
| Male | 1.77 (0.86, 3.65) | 0.120 | ||
| Female | Reference | Reference | ||
|
| ||||
| Pathologic subtype | ||||
| Clear-cell RCC | 1.57 (0.61, 4.03) | 0.347 | ||
| Other (e.g., chromophobe, sarcomatoid, RCC unclassified, papillary RCC) | Reference | Reference | ||
|
| ||||
| Surgical approach | ||||
| Partial nephrectomy | Reference | Reference | ||
| Radical nephrectomy | 1.05 (0.39, 20.9) | 0.298 | ||
| Largest tumor size | 1.09 (1.00, 1.19) | 0.044 | 1.06 (0.97, 1.15) | 0.240 |
| Number of lymph nodes resected | 1.03 (0.97, 1.09) | 0.235 | ||
|
| ||||
| pT classification | ||||
| T3a and T3 undefined | Reference | Reference | ||
| T3b and T3c | 1.69 (0.71, 4.05) | 0.238 | ||
| Fuhrman nuclear grade | 2.27 (1.40, 3.68) | <0.001 | 2.12 (1.30, 3.47) | 0.003 |
|
| ||||
| Surgical margin | ||||
| Positive | 4.60 (1.89, 11.23) | <0.001 | 4.23 (1.71, 10.46) | 0.002 |
| Negative | Reference | Reference | Reference | Reference |
|
| ||||
| Goodness of fit | X2, DF | p | p | |
| 19.64, 3 | <0.001 | |||
CI: confidence interval; RCC: renal cell carcinoma.
DISCUSSION
The rate and predictors of disease recurrence post-surgical resection in pT3 RCC patients is not well-defined in the literature. Our study examined recurrence rate and clinical-pathologic predictors associated with disease recurrence in 88 patients with pT3 RCC. Our results demonstrated that positive surgical margins and high Fuhrman nuclear grade significantly impact the time to recurrence for patients with pT3 stage disease.
Similar to our findings, other studies have reported high Fuhrman nuclear grade to be associated with recurrence for patients with pT3 stage disease.16,23–25 Recently, a prognostic model for predicting disease recurrence for high-risk localized RCC was developed using the ASSURE clinical trial data. The model identified six factors (vascular invasion, histology, tumor size, grade and necrosis, nodal disease) that significantly impact disease recurrence.16 In comparison to our findings, positive surgical margins were not a significant predictor in this analysis. This predictor could be surgeon-specific or due to the inclusion of patients with T1b or T2 staging.
Correa et al developed this prognostic model to help clinicians provide an accurate risk assessment for patients, as previous models were created using retrospective data.16 Although, the generalizability of this prognostic model might be challenging, especially for this population. Clinical trials for adjuvant therapy often have strict inclusion and exclusion criteria, which may not be representative of the true patient population.26 Additionally, patients that do meet the inclusion criteria for these trials may not be interested in partaking.
Given this, prognostic models such as the ASSURE trial tool should be used as a decision-aid tool. Clinicians can use this tool to determine the individualized risk of recurrence and facilitate conversations about surveillance and potential clinical trials or treatments with patients.
Clinical guidelines currently recommend that patients with pT3 stage non-metastatic disease are followed for surveillance (routine imaging, blood work) for up to five years post-surgical resection; however, routine followup beyond five years is at the discretion of the treating physician. 4,27,28 Our results revealed median survival time for
patients with pT3-stage non-metastatic disease was 23.5 months, although, 10% (n=9) of our sample experienced disease recurrence past 58 months of surgical resection. These findings highlight important implications for physicians providing followup care for post-surgical resection. Physicians can identify whether the patient is eligible for any adjuvant therapy clinical trials. A survey by KCCure identified that RCC patients are interested in and willing to use adjuvant therapy to achieve recurrence-free survival and overall survival.29 Alternatively, physicians should continue to monitor patients with pT3 stage disease beyond 60 months or provide strong recommendations for primary care providers to continue routine imaging beyond the five-year mark.
Limitations
Our study has some limitations. Our institution is a tertiary care centre, where patients may receive surgery and continue followup with their community urologist or primary care physician. Given this, we had to exclude many patients, as we were unable to obtain at least three years of followup data. Additionally, our institution actively participates as a site for large clinical trials in adjuvant therapy. To ensure patients receive optimal care, a significant number of pT3 stage patients are enrolled in these trials, causing them to be excluded from this retrospective analysis. The pT3 cohort is defined by the pathologic stage and the authors are aware of the work by Bonsib and Taneja et al.30,31
An internal analysis at our institution of 912 ccRCC (accessioned 2013–2020) suggests some inconsistency in the pathology call at the pT2x-pT3 interface and a relatively high call rate of pT2x.32 Preliminary data from a national kidney cancer collaboration (Canadian Kidney Cancer Information System) suggests this quality issue is present in a majority of participating (Canadian) institutions and requires further research to be fully understood and addressed. Due to this, our sample size remains quite low.
Lastly, we may have omitted some critical predictors in disease recurrences, such as necrosis or vascular invasion.
CONCLUSIONS
Disease recurrence was found in 44.3% of patients in our sample, with a median recurrence-free survival of 23.5 months. High Fuhrman nuclear grade and positive surgical margins were clinicopathologic predictors associated with time to recurrence. Physicians should continue to monitor patients with pT3 stage disease beyond the five-year mark. We recommend monitoring pT3 resected patients up to 10 years post-surgery.
KEY MESSAGES
■ 44% of pT3-staged RCC patients at a single center experienced disease recurrence.
■ Prognostic models should be used to facilitate conversations about disease recurrence.
■ Clinicians should continue to monitor high-risk patients beyond the recommended 5-year followup period.
ACKNOWLEDGEMENT
(on behalf of Shipra Taneja): This paper is dedicated to Dr. Anil Kapoor, whose unwavering support has been instrumental in fostering my research career. Thank you for the numerous opportunities, guidance, and encouragement throughout this journey. You will be greatly missed but never forgotten.
Footnotes
This paper has been peer-reviewed.
COMPETING INTERESTS: Dr. Shayegan has received grants/honoraria from Abbvie, Astellas, Bayer, Janssen, Knight, Pfizer, TerSera, and Tolmar; and participated in the CREST clinical trial supported by Pfizer. All other authors do not report any competing personal or financial interests related to this work.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Hotte SJ, Kapoor A, Basappa NS, et al. Management of advanced kidney cancer: Kidney cancer research network of Canada (KCRNC) consensus update 2019. Can Urol Assoc J. 2019;13:343–54. doi: 10.5489/cuaj.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: The 2019 update. Eur Urol. 2019;75:799–810. doi: 10.1016/j.eururo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Sharma T, Tajzler C, Kapoor A. Is there a role for adjuvant therapy after surgery in “high risk for recurrence” kidney cancer? An update on current concepts. Curr Oncol. 2018;25:e444–53. doi: 10.3747/co.25.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–52. doi: 10.1016/S0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 7.Blackmur JP, Gaba F, Fernando D, et al. Leibovich score is the optimal clinico-pathological system associated with recurrence of non-metastatic clear cell renal cell carcinoma. Urol Oncol. 2021;39:438e11–438.e21. doi: 10.1016/j.urolonc.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Kattan MW, Reuter V, Motzer RJ, et al. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63–7. doi: 10.1016/S0022-5347(05)66077-6. [DOI] [PubMed] [Google Scholar]
- 9.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. JCO. 2001;19:1649–57. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 10.Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma. Cancer. 2003;97:1663–71. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 11.Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: The SSIGN score. J Urol. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 12.Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173:48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 13.Speed JM, Trinh Q-D, Choueiri TK, et al. Recurrence in localized renal cell carcinoma: A systematic review of contemporary data. Curr Urol Rep. 2017;18:15. doi: 10.1007/s11934-017-0661-3. [DOI] [PubMed] [Google Scholar]
- 14.Karakiewicz PI, Briganti A, Chun FK-H, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25:1316–22. doi: 10.1200/JCO.2006.06.1218. [DOI] [PubMed] [Google Scholar]
- 15.Kapoor A, Gharajeh A, Sheikh A, et al. Adjuvant and neoadjuvant small-molecule targeted therapy in high-risk renal cell carcinoma. Curr Oncol. 2009;16:S60–6. doi: 10.3747/co.v16i0.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correa AF, Jegede O, Haas NB, et al. Predicting renal cancer recurrence: Defining limitations of existing prognostic models with prospective trial-based validation. J Clin Oncol. 2019;37:2062–71. doi: 10.1200/JCO.19.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chin AI, Lam JS, Figlin RA, et al. Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev Urol. 2006;8:1–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Patel HD, Puligandla M, Shuch BM, et al. The future of perioperative therapy in advanced renal cell carcinoma: how can we PROSPER? Future Oncol. 2019;15:1683–95. doi: 10.2217/fon-2018-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klatte T, Rossi SH, Stewart GD. Prognostic factors and prognostic models for renal cell carcinoma: A literature review. World J Urol. 2018;36:1943–52. doi: 10.1007/s00345-018-2309-4. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T, Miyake M, Hori S, et al. Clinical significance of tumor size, pathological invasion sites including urinary collecting system and clinically detected renal vein thrombus as predictors for recurrence in pT3a localized renal cell carcinoma. Diagnostics (Basel) 2020;10:154. doi: 10.3390/diagnostics10030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leopold Z, Srivastava A, Singer EA. Predictors of recurrence for T3a RCC: A recurring conundrum. Diagnostics (Basel) 2020;10:983. doi: 10.3390/diagnostics10110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bujang MA, Sa’at N, Sidik TMITAB, et al. Sample size guidelines for logistic regression from observational studies with large population: emphasis on the accuracy between statistics and parameters based on real life clinical data. Malays J Med Sci. 2018;25:122–30. doi: 10.21315/mjms2018.25.4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang T-W, Cheng W-M, Fan Y-H, et al. Predictive factors for disease recurrence in patients with locally advanced renal cell carcinoma treated with curative surgery. J Chin Med Assoc. 2021;84:405–9. doi: 10.1097/JCMA.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 24.Hakam N, Abou Heidar N, Khabsa J, et al. Does a positive surgical margin after nephron sparing surgery affect oncological outcome in renal cell carcinoma?: A systematic review and meta-analysis. Urology. 2021;156:e30–9. doi: 10.1016/j.urology.2021.04.058. [DOI] [PubMed] [Google Scholar]
- 25.Shah PH, Moreira DM, Okhunov Z, et al. Positive surgical margins increase risk of recurrence after partial nephrectomy for high-risk renal tumors. J Urol. 2016;196:327–34. doi: 10.1016/j.juro.2016.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart EA, Bradshaw CP, Leaf PJ. Assessing the generalizability of randomized trial results to target populations. Prev Sci. 2015;16:475–85. doi: 10.1007/s11121-014-0513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell SC, Uzzo RG, Karam JA, et al. Renal mass and localized renal cancer: Evaluation, management, and followup: AUA guideline: Part II. J Urol. 2021;206:209–18. doi: 10.1097/JU.0000000000001912. [DOI] [PubMed] [Google Scholar]
- 28.Kassouf W, Monteiro LL, Drachenberg DE, et al. Canadian Urological Association guideline for followup of patients after treatment of non-metastatic renal cell carcinoma. Can Urol Assoc J. 2018;12:231–8. doi: 10.5489/cuaj.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battle D, Jonasch E, Hammers HJ, et al. Patients perspectives on adjuvant therapy in renal cell carcinoma. JCO. 2018;36:644. doi: 10.1200/JCO.2018.36.6_suppl.644. [DOI] [Google Scholar]
- 30.Bonsib SM. T2 clear-cell renal cell carcinoma is a rare entity: A study of 120 clear-cell renal cell carcinomas. J Urol. 2005;174:1199–202. doi: 10.1097/01.ju.0000173631.01329.1f. [DOI] [PubMed] [Google Scholar]
- 31.Taneja K, Arora S, Rogers CG, et al. Pathological staging of renal cell carcinoma: A review of 300 consecutive cases with emphasis on retrograde venous invasion. Histopathology. 2018;73:681–91. doi: 10.1111/his.13672. [DOI] [PubMed] [Google Scholar]
- 32.Bonert M, Nikzad N, El-Shinnawy I, et al. Tumor stage by size and pathologist in clear-cell renal cell carcinoma from synoptic reports: Estimating rates by tumor size and a case for mandated reviews USCAP 2022 abstracts: Quality and patient safety. Mod Pathol. 2022;35:1473–522. doi: 10.1038/s41379-022-01050-6. [DOI] [PubMed] [Google Scholar]