Abstract
Poverty is strongly associated with all-cause and chronic obstructive pulmonary disease (COPD) mortality. Less is known about the contribution of poverty to spirometrically defined chronic airflow obstruction (CAO) – a key characteristic of COPD. Using cross-sectional data from an asset-based questionnaire to define poverty in 21 sites of the Burden of Obstructive Lung Disease study, we estimated the risk of CAO attributable to poverty. Up to 6% of the population over 40 years had CAO attributable to poverty. Understanding the relationship between poverty and CAO might suggest ways to improve lung health, especially in low- and middle-income countries.
Introduction
Poverty is an important risk factor for COPD prevalence and mortality, particularly in low- and middle-income countries (LMICs).1 In a previous report of 12 Burden of Obstructive Lung Disease (BOLD) study sites, we found that this association is also true between spirometrically-defined post-bronchodilator chronic airflow obstruction (CAO) and individual wealth defined from household assets.2 Here, we extend the investigation of the association between CAO and poverty to 21 BOLD sites and assess the population impact of poverty on CAO using site-specific population attributable risk (PAR) estimates.
Methods
Data on ownership of household assets were collected from 14,611 adults (≥40 years) from 21 sites of the population-based BOLD study, between March 2010 and December 2016 (Table S1). We calculated a wealth score (0–10) based on household assets to estimate each participant’s household wealth (Supplementary Methods, Figure S1). We defined poverty as a wealth score lower than or equal to seven.3 We assessed the association of poverty with the forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio and with CAO (FEV1/FVC < lower limit of normal (LLN)).4 We used a Bayesian hierarchical model to account for the multi-level structure of the data (Supplementary Methods).5 Identification of potential confounders was based on previous findings and graphically represented in directed acyclic diagrams prior to analysis (Supplementary Methods). We adjusted for age and sex in the analysis of FEV1/FVC (Model 1, Figure S2a), but not in the analysis of CAO, as age and sex are already accounted for by the LLN. We did not adjust for other risk factors in the main analysis as these were most likely on the causal pathway between poverty and CAO. However, in secondary analyses we further adjusted for education (Model 2, Figure S2b), and for smoking pack-years, exposure to a dusty job, passive smoking, body mass index (BMI), childhood hospitalisation due to respiratory infection, family history of respiratory disease and history of tuberculosis (Model 3, Figure S2c). These analyses were repeated using wealth score as a continuous measure.
We estimated the site-specific PAR of CAO attributable to poverty in all sites, except for Saudi Arabia (Riyadh), where the prevalence of poverty was 0. PAR estimates are reported with a 95% credible interval (95%CrI).
Results
CAO prevalence ranged from 3.1% in Saudi Arabia (Riyadh) to 16.4% in India (Kashmir) (Table S2). The mean wealth score was 6.3, ranging from just below 1 in Malawi (Chikwawa) to almost 10 in Saudi Arabia (Riyadh). The overall prevalence of poverty was 55%, with almost 100% in Malawi (Chikwawa). The prevalence of poverty was consistently high in LMICs (Table S2).
Per each unit increase in wealth score, the FEV1/FVC increases by 0.30% (95%Crl: 0.19% to 0.41%) and the risk of CAO is reduced by 11% (RR=0.89; 95%CrI: 0.86 to 0.92) (Table 1, Main model). Using wealth score as a binary variable to define poverty, poorer individuals had on average 1.19% lower FEV1/FVC (95%Crl: −1.61% to −0.77%) and 57% greater risk of CAO (RR=1.57; 95%Crl: 1.32 to 1.87), compared with wealthier individuals (Table 1). In the secondary analysis, further adjusting for education (Table 1, Model 2) and for all other considered potential confounders (Table S3, Model 3) did not materially change the association of wealth score or poverty with either FEV1/FVC or CAO. There was no substantial heterogeneity across sites in the associations of wealth score or poverty with either FEV1/FVC or CAO (Figures S3-S6).
Table 1.
Association of wealth score and poverty with FEV1/FVC (%) and chronic airflow obstruction (CAO).
| Outcome variable | FEV1/FVC (%) | CAO (FEV1/FVC<LLN) | ||
|---|---|---|---|---|
| Model 1 (main model) | Model 2 | Model 1 (Main model) | Model 2 | |
| Variable | Coeff (95% Crl) | Coeff (95% Crl) | RR (95% Crl) | RR (95% Crl) |
| Wealth score | 0.30 (0.19, 0.41) | 0.26 (0.16, 0.36) | 0.89 (0.86, 0.92) | 0.93 (0.89, 0.96) |
| Poverty | −1.19 (−1.61, −0.77) | −1.03 (−1.42, −0.63) | 1.57 (1.32, 1.87) | 1.34 (1.12, 1.61) |
FEV1: forced expiratory volume in one second; FVC: forced vital capacity; LLN: lower limit of normal; wealth score: calculated from ownership of a range of assets; Poverty: defined as a wealth score lower than 8; Education: the highest level of education completed. Model 1 (main model), accounts for age and sex. Model 2, accounts for age, sex and education
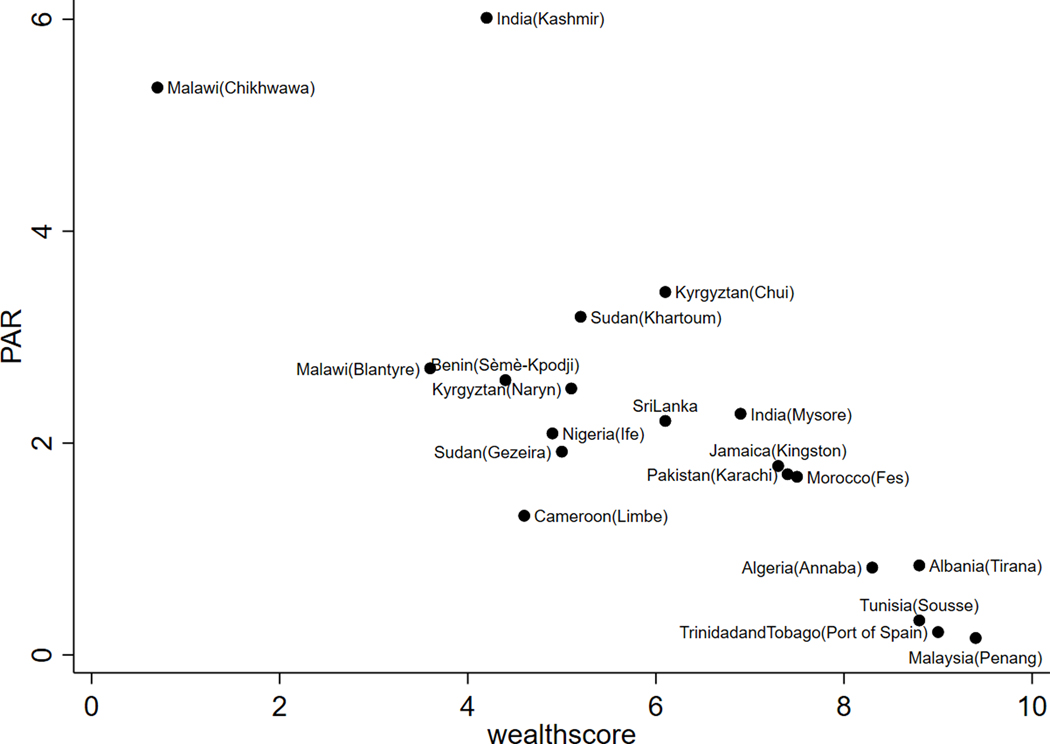
The highest PAR for CAO due to poverty was in India (Kashmir) (PAR=6.1%; 95%CrI: 3.65% to 8.59%) and the lowest in Malaysia (Penang) (PAR=0.16%, 95%CrI: 0.03% to 0.41%) (Model 1, Table S4). PAR estimates were inversely related to the wealth score (Figure 1). The PAR results from Models 2 and 3 are reported in Figure S7.
Figure 1.
PAR for CAO due to poverty against wealth score. CAO, chronic airflow obstruction; PAR, population attributable risk.
Discussion
We have confirmed the association between poverty and CAO previously found in a subset of our BOLD study,2 and we have shown that up to 6% of the population have CAO attributable to poverty.
Our results are consistent with findings reported by Grigsby et al. using data from LMICs, where they used monthly household income as a proxy measure for poverty.6 Raju et al. have also observed an association between self-reported COPD and household income in the USA.7 Previous findings of the BOLD study suggest that poor education, an indicator of socioeconomic deprivation, is the second most important risk factor for CAO after smoking.5 Using ecological data from the Global Burden of Disease study, we have previously reported a strong association between COPD mortality and poverty defined by Gross National Income per capita.8
The reduction in the RR observed in our secondary analyses adjusting for education and, subsequently, for other risk factors including low BMI, is possibly due to the mediating effect of these factors, with adjustment for them leading to the underestimation of the impact of poverty on CAO.
The association between poverty and CAO could be due to limited access to healthcare services, poor nutrition, including foetal programming during gestation, low birth weight, poor living conditions, overcrowding, and water supply/sanitation for poorer people, particularly in LMICs.9
We did not adjust for solid fuel use because this was not associated with CAO in the BOLD study.10 Although meta-analyses have been interpreted as showing an association between airflow obstruction and solid fuel use, including for instance that of Smith et al.,11 they show very high heterogeneity across studies (I2 between 67% and 98%) and strong evidence of publication bias, thus not supporting a causal association.
One of the strengths of BOLD is the use of a standardised protocol across study sites. Spirometry was performed post-bronchodilator, and its quality was assured with rigorous training and regular checks of all lung function curves. Poverty is not a one-dimensional concept and understanding the nature of its relationship with CAO requires further investigation. The observed reduction of RR when adjusting for education suggests that education may partly mediate the effects of poverty on lung health. Nevertheless, the association with poverty is clear and suggests that poverty reduction itself is still important.
Supplementary Material
Acknowledgements
The authors thank the participants and field workers of this study for their time and cooperation, and the BOLD (Burden of Obstructive Lung Disease) Coordinating Centre members for their technical and scientific support.
Funding:
The work is supported by Wellcome Trust grant 085790/Z/08/Z for the Burden of Obstructive Lung Disease (BOLD) study. The initial BOLD program was funded in part by unrestricted educational grants to the Operations Center in Portland, Oregon from Altana, Aventis, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Novartis, Pfizer, Schering-Plough, Sepracor, and the University of Kentucky (Lexington, KY). A full list of local funders can be found at https://www.boldstudy.org.
Footnotes
Competing Interests:
The authors have no conflict of interest to disclose.
The BOLD (Burden of Obstructive Lung Disease) Collaborative Research Group members
Albania: Hasan Hafizi (PI), Anila Aliko, Donika Bardhi, Holta Tafa, Natasha Thanasi, Arian Mezini, Alma Teferici, Dafina Todri, Jolanda Nikolla, and Rezarta Kazasi (Tirana University Hospital Shefqet Ndroqi, Albania); Algeria: Hamid Hacene Cherkaski (PI), Amira Bengrait, Tabarek Haddad, Ibtissem Zgaoula, Maamar Ghit, Abdelhamid Roubhia, Soumaya Boudra, Feryal Atoui, Randa Yakoubi, Rachid Benali, Abdelghani Bencheikh, and Nadia Ait-Khaled (Faculté de Médecine Annaba, Service de Epidémiologie et Médecine Préventive, El Hadjar, Algeria); Benin: Herve Lawin (PI), Arsene Kpangon, Karl Kpossou, Gildas Agodokpessi, Paul Ayelo, Benjamin Fayomi (Unit of Teaching and Research in Occupational and Environmental Health, University of Abomey Calavi, Cotonou, Benin); Cameroon: Bertrand Mbatchou (PI), Atongno Humphrey Ashu (Douala General Hospital, Douala, Cameroon); India: Mahesh Rao (PI) (JSS Medical College, Mysuru, India); Parvaiz A Koul (PI), Sajjad Malik, Nissar A Hakim, and Umar Hafiz Khan (Sher-i-Kashmir Institute of Medical Sciences, Srinagar, J&K, India); Jamaica: Althea Aquart-Stewart (PI), Akosua Francia Aikman (University of the West Indies, Kingston, Jamaica); Kyrgyzstan: Talant M. Sooronbaev (PI), Bermet M. Estebesova, Meerim Akmatalieva, Saadat Usenbaeva, Jypara Kydyrova, Eliza Bostonova, Ulan Sheraliev, Nuridin Marajapov, Nurgul Toktogulova, Berik Emilov, Toktogul Azilova, Gulnara Beishekeeva, Nasyikat Dononbaeva, and AijamalTabyshova (Pulmunology and Allergology Department, National Centre of Cardiology and Internal Medicine, Bishkek, Kyrgyzstan); Malawi: Kevin Mortimer (PI), Wezzie Nyapigoti, Ernest Mwangoka, Mayamiko Kambwili, Martha Chipeta, Gloria Banda, Suzgo Mkandawire, and Justice Banda (the Malawi Liverpool Wellcome Trust, Blantyre, Malawi); Malaysia: Li-Cher Loh (PI), Abdul Rashid, and Siti Sholehah (RCSI & UCD Malaysia Campus, Penang, Malaysia); Morocco: Mohamed C Benjelloun (PI), Chakib Nejjari, Mohamed Elbiaze, and Karima El Rhazi (Laboratoire d’épidémiologie, Recherche Clinique et Santé Communautaire, Fès, Morocco); Nigeria: Daniel Obaseki (PI), Gregory Erhabor, Olayemi Awopeju, and Olufemi Adewole (Obafemi Awolowo University, Ile-Ife, Nigeria); Pakistan: Asaad A. Nafees (PI) Muhammad Irfan, Zafar Fatmi, Aysha Zahidie, Natasha Shaukat and Meesha Iqbal (Aga Khan University, Karachi, Pakistan); Saudi Arabia: M. Al Ghobain (PI), H. Alorainy (PI), E. El-Hamad, M. Al Hajjaj, A. Hashi, R. Dela, R. Fanuncio, E. Doloriel, I. Marciano, and L. Safia (Saudi Thoracic Society, Riyadh, Saudi Arabia); Sri Lanka: Kirthi Gunasekera (PI), Rajitha Wickremasinghe (Medical Research Institute, Central Chest Clinic, Colombo, Sri Lanka); Sudan: Asma Elsony (PI), Hana A. Elsadig, Nada Bakery Osman, Bandar Salah Noory, Monjda Awad Mohamed, Hasab Alrasoul Akasha Ahmed Osman, Namarig Moham ed Elhassan, Abdel Mu’is El Zain, Marwa Mohamed Mohamaden, Suhaiba Khalifa, Mahmoud Elhadi, Mohand Hassan, and Dalia Abdelmonam (the Epidemiological Laboratory, Khartoum, Sudan); Trinidad and Tobago: Terence Seemungal (PI), Fallon Lutchmansingh, Liane Conyette (University of the West Indies, St. Augustine, Trinidad and Tobago); Tunisia: Imed Harrabi (PI), Myriam Denguezli, Zouhair Tabka, Hager Daldoul, Zaki Boukheroufa, Firas Chouikha, and Wahbi Belhaj Khalifa (University Hospital Farhat Hached, Faculté de Médecine, Sousse, Tunisia); United Kingdom: Peter GJ Burney (PI), Anamika Jithoo, Louisa Gnatiuc, Hadia Azar, Jaymini Patel, Caron Amor, James Potts, Michael Tumilty, and Fiona McLean, Risha Dudhaiya, Andre Amaral (National Heart and Lung Institute, Imperial College London, London, UK);
References
- 1.Burney P, Jithoo A, Kato B, et al. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty--a BOLD analysis. Thorax 2014;69(5):465–73. doi: 10.1136/thoraxjnl-2013-204460 [published Online First: 20131218] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townend J, Minelli C, Mortimer K, et al. The association between chronic airflow obstruction and poverty in 12 sites of the multinational BOLD study. Eur Respir J 2017;49(6):1601880. doi: 10.1183/13993003.01880-2016 [published Online First: 20170601] [DOI] [PubMed] [Google Scholar]
- 3.Townend J, Minelli C, Harrabi I, et al. Development of an international scale of socio-economic position based on household assets. Emerg Themes Epidemiol 2015;12:13. doi: 10.1186/s12982-015-0035-6 [published Online First: 20150922] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiefer EM, Hankinson JL, Barr RG. Similar relation of age and height to lung function among Whites, African Americans, and Hispanics. Am J Epidemiol 2011;173(4):376–87. doi: 10.1093/aje/kwq417 [published Online First: 20110117] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burney P, Patel J, Minelli C, et al. Prevalence and Population-Attributable Risk for Chronic Airflow Obstruction in a Large Multinational Study. Am J Respir Crit Care Med 2021;203(11):1353–65. doi: 10.1164/rccm.202005-1990OC [published Online First: 20201110] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grigsby M, Siddharthan T, Chowdhury MAH, et al. Socioeconomic status and COPD among low- and middle-income countries. Int J Chronic Obstr 2016;11:2497–507. doi: 10.2147/Copd.S111145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raju S, Keet CA, Paulin LM, et al. Rural Residence and Poverty Are Independent Risk Factors for Chronic Obstructive Pulmonary Disease in the United States. Am J Respir Crit Care Med 2019;199(8):961–69. doi: 10.1164/rccm.201807-1374OC [published Online First: 2018/11/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burney PG, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990–2010. Eur Respir J 2015;45(5):1239–47. doi: 10.1183/09031936.00142414 [published Online First: 20150402] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meghji J, Mortimer K, Agusti A, et al. Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet 2021;397(10277):928–40. doi: 10.1016/S0140-6736(21)00458-X [published Online First: 20210222] [DOI] [PubMed] [Google Scholar]
- 10.Amaral AFS, Patel J, Kato BS, et al. Airflow Obstruction and Use of Solid Fuels for Cooking or Heating: BOLD Results. Am J Respir Crit Care Med 2018;197(5):595–610. doi: 10.1164/rccm.201701-0205OC [published Online First: 20170912] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith KR, Bruce N, Balakrishnan K, et al. Millions Dead: How Do We Know and What Does It Mean? Methods Used in the Comparative Risk Assessment of Household Air Pollution. Annu Rev Public Health 2014;35:185–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.