Abstract
Background
Recent studies have shown a decrease in CD4 count during adolescence in young people with perinatally acquired human immunodeficiency virus (HIV, PHIV).
Methods
Young people with PHIV in the United Kingdom, followed in the Collaborative HIV Paediatric Study who started antiretroviral therapy (ART) from 2000 onward were included. Changes in CD4 count over time from age 10 to 20 years were analyzed using mixed-effects models, and were compared to published CD4 data for the gerneral population. Potential predictors were examined and included demographics, age at ART start, nadir CD4 z score (age-adjusted) in childhood, and time-updated viral load.
Results
Of 1258 young people with PHIV included, 669 (53%) were female, median age at ART initiation was 8.3 years, and the median nadir CD4 z score was −4.0. Mean CD4 count was higher in young people with PHIV who started ART before age 10 years and had a nadir CD4 z score ≥−4; these young people with PHIV had a decline in CD4 count after age 10 that was comparable to that of the general population. Mean CD4 count was lower in young people with PHIV who had started ART before age 10 and had a nadir CD4 z score <−4; for this group, the decline in CD4 count after age 10 was steeper over time.
Conclusions
In children, in addition to starting ART at an early age, optimizing ART to maintain a higher CD4 z score during childhood may be important to maximizing immune reconstitution later in life.
Keywords: CD4 T cell, perinatal, HIV, child, adult
Young people with human immunodeficiency virus who started antiretroviral therapy before age 10 years and had a nadir CD4 z score <-4 in childhood, had a decline in CD4 count after age 10 steeper than that of the general population.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/evolution-of-cd4-t-cell-count-with-age-in-a-cohort-of-young-people-growing-up-with-perinatally-acquired-hiv-e628fcfc-4e7a-4d1f-b878-ebf12b31f134
In 2021, there were an estimated 1.7 million adolescents with human immunodeficiency virus (HIV) worldwide [1]. Due to the availability of effective antiretroviral therapy (ART), children living with perinatally acquired HIV (PHIV) are surviving into adulthood [2]. However, some studies have shown poor health outcomes for young people with PHIV compared with younger children and adults with HIV, including lower levels of virological suppression, worse immunological status, poor growth, and lower retention in care [3–7].
Current guidelines state that ART should be initiated immediately for all individuals with HIV [8], and initial CD4 recovery following the start of ART in children has been well described [9]. The few studies that have examined long-term CD4 evolution have shown a decrease in CD4 count during adolescence [7, 10, 11]. However, the reasons for this decline remain unclear, including how much is part of the natural decline in CD4 count seen after infancy in the general population [12]. Previous studies have predicted that children who start ART at an early age are likely to follow a trajectory of CD4 that is expected in the general population [13, 14], and higher CD4 counts before/at ART initiation have been associated with higher long-term CD4 [13–16] and a better CD4 response after treatment interruptions [17].
Our aim in this analysis was to examine changes in and predictors of CD4 over time in young people with PHIV in the United Kingdom and to compare the changes to published data on CD4 in young people in the general population. A previous analysis specifically explored CD4 changes before and after transition to adult care in young people who had been followed in the Collaborative HIV Paediatric Study (CHIPS) and subsequently transitioned to adult clinics in the UK Collaborative HIV Cohort (UK CHIC) Study [10] and did not compare the results to the general population. Here, we included all young people followed in CHIPS and linked updated datasets from the 2 studies, resulting in a larger cohort of young people with PHIV and with longer follow-up through pediatric and adult HIV care than previously.
METHODS
CHIPS is a national observational cohort of children with HIV in the United Kingdom and Ireland [18]. In brief, all infants born to women with HIV and all children aged <16 years at HIV diagnosis in the United Kingdom and Ireland are reported to the Integrated Screening Outcomes Surveillance Service (formerly the National Study of HIV in Pregnancy and Childhood). Children diagnosed with HIV were reported to CHIPS and followed from first presentation in pediatric care until the child transitioned to adult care. Follow-up data were collected annually until March 2021. The UK CHIC Study was a multicenter study of adults with HIV aged ≥16 years attending 1 of 21 collaborating adult outpatient clinics across the United Kingdom [19]. Routinely collected clinical information was submitted annually (until September 2019) from participating clinics to the study database. Both studies had National Health Service Research Ethics approval.
In this analysis, young people followed in CHIPS with documented PHIV who started ART during or after 2000 with at least 1 CD4 measurement at age ≥10 years were included. They were linked to the UK CHIC database using their date of birth and sex as well as Soundex (an indexing system that encodes surnames), clinic name/clinic number, or the young person’s initials. Data used for the linkage were stored in a secure location with restricted access. Young people were grouped by age at the start of ART and nadir CD4 z score (age-adjusted using CD4 counts) in childhood [20] to create 6 groups (A: started ART at age ≤5 years and nadir CD4 z score <−4, B: started ART at age ≤5 years and nadir CD4 z score ≥−4, C: started ART at age >5 to <10 years and nadir CD4 z score <−4, D: started ART at age >5 to <10 years and nadir CD4 z score ≥−4, E: started ART at age ≥10 years and nadir CD4 z score <−4, and F: started ART at age ≥10 years and nadir CD4 z score ≥−4). Nadir CD4 z score in childhood was defined as the lowest CD4 z score before age 10; for young people without CD4 measurements before age 10, it was defined as the CD4 z score at the start of ART (closest measurement within 6 months of starting ART).
Data were analyzed using Stata version 17.0 (Stata Corp, College Station, TX). Characteristics of the young people included in the analysis with non-missing values were summarized using proportions, medians, and interquartile range (IQR). Data were missing for <10% of young people for each variable unless specified. Immunodeficiency was categorized based on the World Health Organization definition for children aged >5 years using CD4 count [21].
Changes in CD4 count over time were analyzed using mixed-effects models, allowing for multiple CD4 measurements per young person. All available CD4 measurements from CHIPS and UK CHIC were analyzed from age 10 years to age 20 years. Time since age 10 was modeled using both linear and quadratic variables that were included in all models. Person-level random effects were included for intercept and slopes (unstructured covariance matrix). The effect of predictors of changes in CD4 over time was explored and included the following variables: sex, ethnicity, country of birth, year of birth, age at ART start and nadir CD4 z score groups, transitioned to adolescent/adult care, and suppressed viral load <400 copies/mL within 6 months of the CD4 measurement (time-updated); ART regimen was not included as a predictor. Variables were included in the multivariable model using backward elimination, and a P value <.05 was considered statistically significant. Interactions between time/time squared since age 10 and each variable were added to the multivariable model if the interaction P value was <.05. The statistical significance of predictors in the multivariate model are reported in the results section as P values adjusted for the presence of the other variables in the final model. Due to changes in the frequency of CD4 measurements over time, a variable for time since previous CD4 measurement (≤4 months vs >4 months) was included in a sensitivity analysis. Additional sensitivity analyses included fitting the multivariable model separately to young people linked and not linked to UK CHIC.
Published data on CD4 counts in young people aged 10 to 20 years in the general population were identified using PubMed. Relevant search terms included lymphocyte, subset/subpopulation, reference/control value/range, child/childhood, and adolescent. Reference and cited-by lists from all articles found to be relevant were also searched.
RESULTS
Of 1258 young people with PHIV included in the analysis, 53% were female and 84% were of Black ethnicity (Table 1). The median age at ART initiation was 8.3 years, and the median nadir CD4 z score in childhood was −4.0. Most young people had no or mild immunodeficiency at age 10 (88%). Only 66% (612 of 932) of young people with available data had a suppressed viral load <400 copies/mL at age 10 (592 of 693 [85%] for those who started ART before age 10). At last follow-up, 797 (63%) had transitioned to adolescent/adult care and 464 (37%) were linked to UK CHIC. Of those not linked, 389 (49%) were still being followed in CHIPS, 355 (45%) had transitioned to an adult clinic not participating in UK CHIC, 40 (5%) were lost to follow-up, and 10 (1%) had died. Twenty-four percent (304) had ever had an AIDS-defining diagnosis; of those, 75 had their first AIDS event after age 10, 59 of whom had started ART aged ≥10. Sixteen (1%) had died at a median age of 19 years (2 within 1 year of diagnosis), with 13 starting ART at age ≥10.
Table 1.
Characteristics of the 1258 Young People Included in the Analysis
| Total (n = 1258) | Linked to UK Collaborative HIV Cohort (n = 464) | |
|---|---|---|
| Characteristic | n (%) or Median (IQR) | |
| Female sex | 669 (53) | 245 (53) |
| Black ethnicity | 1047 (84) | 396 (86) |
| Born outside United Kingdom/Ireland | 775 (62) | 312 (67) |
| Year of birth | ||
| Up to 1996 | 381 (30) | 233 (50) |
| 1997 to 2000 | 429 (34) | 208 (45) |
| 2001 onward | 448 (36) | 23 (5) |
| Year of starting ART | ||
| 2000 to 2004 | 442 (35) | 210 (45) |
| 2005 to 2009 | 468 (37) | 165 (36) |
| 2010 onward | 348 (28) | 89 (19) |
| Age started ART, y | 8.3 (3.5 to 12.1) | 10.3 (6.5 to 13.2) |
| ≤5 | 406 (32) | 83 (18) |
| >5 and <10 | 352 (28) | 140 (30) |
| ≥10 | 500 (40) | 241 (52) |
| Nadir CD4 z scorea | −4.0 (−5.9 to −2.5) | −4.3 (−6.8 to −2.8) |
| <−4 | 588 (49) | 237 (54) |
| ≥−4 | 602 (51) | 202 (46) |
| Age started ART (y) and nadir CD4 z scorea | ||
| A: Started ART ≤5 y of age and nadir CD4 z score <−4 | 138 (12) | 34 (8) |
| B: Started ART ≤5 y of age and nadir CD4 z score ≥−4 | 259 (22) | 46 (10) |
| C: Started ART >5 to <10 y of age and nadir CD4 z score <−4 | 211 (18) | 89 (20) |
| D: Started ART >5 to <10 y of age and nadir CD4 z score ≥−4 | 115 (10) | 44 (10) |
| E: Started ART ≥10 y of age and nadir CD4 z score <−4 | 239 (20) | 114 (26) |
| F: Started ART ≥10 y of age and nadir CD4 z score ≥−4 | 228 (19) | 112 (26) |
| Median (IQR) age (y) started ART within age started ART and nadir CD4 z score groupsa | ||
| A: Started ART ≤5 y of age and nadir CD4 z score <−4 | 2.5 (0.7 to 3.8) | 3.4 (2.4 to 4.0) |
| B: Started ART ≤5 y of age and nadir CD4 z score ≥−4 | 1.2 (0.3 to 2.8) | 2.4 (1.6 to 3.4) |
| C: Started ART >5 to <10 y of age and nadir CD4 z score <−4 | 7.9 (6.8 to 8.9) | 8.2 (7.3 to 9.1) |
| D: Started ART >5 to <10 y of age and nadir CD4 z score ≥−4 | 6.9 (5.8 to 8.0) | 6.9 (5.9 to 7.8) |
| E: Started ART ≥10 y of age and nadir CD4 z score <−4 | 12.6 (11.3 to 14.2) | 12.5 (11.2 to 14.2) |
| F: Started ART ≥10 y of age and nadir CD4 z score ≥−4 | 13.1 (11.6 to 14.8) | 13.7 (12.0 to 15.6) |
| CD4 count at age 10 y (cells/mm3, within ±6 m)b | 724 (480 to 998) | 621 (405 to 910) |
| Immunodeficiency at age 10 y (within ±6 m)b | ||
| None or not significant (≥500 cells/mm3) | 685 (73) | 207 (63) |
| Mild (≥350 to <500 cells/mm3) | 144 (15) | 65 (20) |
| Advanced (≥200 to <350 cells/mm3) | 86 (9) | 39 (12) |
| Severe (<200 cells/mm3) | 28 (3) | 17 (5) |
| CD4 z score at age 10 y (within ±6 m)b | −1.3 (−2.9 to −0.2) | −1.9 (−3.6 to −0.5) |
| Viral load <400 copies/mL at age 10 y (within ±6 m)c | 612 (66) | 174 (54) |
| Known to have transitioned to adolescent/adult care | 797 (63) | 442 (95) |
| Ever CDC class C (AIDS) diagnosis | 304 (24) | 115 (25) |
| Ever CDC class C (AIDS) at age 10 y | 228 (18) | 70 (15) |
| First CDC class C (AIDS) event after age 10 yd | 75 (6) | 44 (9) |
| A: Started ART ≤5 y of age and nadir CD4 z score <−4 | 2 (0.2) | 1 (0.2) |
| B: Started ART ≤5 y of age and nadir CD4 z score ≥−4 | 1 (0.1) | 1 (0.2) |
| C: Started ART >5 to <10 y of age and nadir CD4 z score <−4 | 2 (0.2) | 1 (0.2) |
| D: Started ART >5 to <10 y of age and nadir CD4 z score ≥−4 | 3 (0.3) | 3 (0.7) |
| E: Started ART ≥10 y of age and nadir CD4 z score <−4 | 38 (3) | 21 (5) |
| F: Started ART ≥10 y of age and nadir CD4 z score ≥−4 | 21 (2) | 13 (3) |
| Age at first CDC class C (AIDS) event (y) | 3.4 (0.6 to 10.0) | 7.8 (2.7 to 12.4) |
| Diede | 16 (1) | 6 (1) |
| C: Started ART >5 to <10 y of age and nadir CD4 z score <−4 | 1 (0.1) | … |
| D: Started ART >5 to <10 y of age and nadir CD4 z score ≥−4 | 1(0.1) | … |
| E: Started ART ≥10 y of age and nadir CD4 z score <−4 | 8 (0.7) | 4 (0.9) |
| F: Started ART ≥10 y of age and nadir CD4 z score ≥−4 | 5 (0.4) | 2 (0.4) |
| Age when died, y | 18.8 (15.2 to 22.7) | 22.7 (20.3 to 25.2) |
| Years since diagnosis when died, y | 10.6 (6.0 to 14.8) | 11.7 (10.0 to 14.8) |
Abbreviations: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus; IQR, interquartile range.
aNadir CD4 z score was defined as the lowest CD4 z score before age 10 years (n = 945); for young people without CD4 measurements before age 10, nadir CD4 z score was defined as CD4 z score at start of ART (within 6 months; n = 245). Unknown for 68 young people.
bUnknown for 315 young people.
cUnknown for 326 young people.
dNadir CD4 z score unknown for 8 young people with first CDC class C (AIDS) event after age 10 years.
eAll died after age 10. Nadir CD4 z score unknown for 1 young person who died.
A total of 26 270 CD4 measurements from 1996 to 2020 were available for analysis. Young people had a median (IQR) of 2.8 (2.1 to 3.3) CD4 measurements per year (only 24 [2%] had only 1 CD4 measurement), and the median age at the last CD4 measurement was 17.8 years (IQR, 16.0 to 19.3). Of the 258 young people who were immunosuppressed (<500 cells/mm3) at age 10, 42 of 63 (67%) with a CD4 measurement at age 20 (within ±6 months) were still immunosuppressed at age 20. In multivariable analysis, mean CD4 count at age 10 differed by age at the start of ART and nadir CD4 z score, with young people who had started ART at a younger age and had a higher nadir CD4 z score in childhood having higher CD4 counts at age 10 (P < .001; Table 2). Young people who were female (P < .001), of non-Black ethnicity (P = .002), born in later calendar years (P < .001), and virally suppressed (P < .001) also had higher mean CD4 counts at age 10.
Table 2.
Univariate and Multivariable Predictors of CD4 Count Over Time From Age 10 Years to Age 20 Years
| Univariablea | Multivariable (n = 1181) | |||||
|---|---|---|---|---|---|---|
| Predictor | Coefficient | 95% CI | P Value | Coefficient | 95% CI | P Value |
| Constant | … | … | … | 664.1 | 588.2 to 739.9 | … |
| Main effects | … | … | … | … | … | … |
| Time since age 10 (per 1-y increase after age 10) | −13.1 | −16.3 to −9.9 | <.001 | 1.4 | −10.8 to 13.6 | .824 |
| Time since age 10 squared (per year squared increase after age 10) | −1.7 | −2.1 to −1.4 | <.001 | −3.4 | −4.6 to −2.2 | <.001 |
| Female (vs male) | 21.6 | −7.9 to 51.1 | .151 | 67.9 | 31.2 to 104.5 | <.001 |
| Non-Black ethnicity (vs Black ethnicity) | 96.5 | 56.5 to 136.6 | <.001 | 52.3 | 18.8 to 85.8 | .002 |
| Born abroad (vs born in United Kingdom/Ireland) | −54.1 | −84.6 to −23.6 | .001 | … | … | … |
| Year of birth (per 1-y increase since 1985) | 23.5 | 20.4 to 26.6 | <.001 | 6.7 | 3.4 to 10.0 | <.001 |
| Age started ART (y) and nadir CD4 z score | … | … | <.001 | … | … | <.001 |
| A: Started ART ≤5 y of age and nadir CD4 z score <−4 | 0.0 | … | … | 0.0 | … | … |
| B: Started ART ≤5 y of age and nadir CD4 z score ≥−4 | 165.6 | 117.5 to 213.8 | … | 197.3 | 132.5 to 262.0 | … |
| C: Started ART >5 to <10 y of age and nadir CD4 z score <−4 | −94.0 | −143.1 to −44.8 | … | −97.7 | −165.0 to −30.4 | … |
| D: Started ART >5 to <10 y of age and nadir CD4 z score ≥−4 | 44.2 | −13.3 to 101.7 | … | 132.4 | 54.9 to 209.9 | … |
| E: Started ART ≥10 y of age and nadir CD4 z score <−4 | −264.8 | −312.7 to −216.9 | … | −533.6 | −604.8 to −462.4 | … |
| F: Started ART ≥10 y of age and nadir CD4 z score ≥−4 | −95.4 | −143.7 to −47.1 | … | −137.6 | −206.8 to −68.4 | … |
| Known to have transitioned to adolescent/adult care (vs not known to have transitioned) | −129.6 | −160.6 to −98.7 | <.001 | … | … | … |
| Viral suppression (time updated, within ±6 m of CD4 count) | … | … | <.001 | … | … | <.001 |
| Suppressed, <400 copies/mL | 0.0 | … | … | 0.0 | … | … |
| Not suppressed, ≥400 copies/mL | −149.8 | −156.1 to −143.6 | … | −78.6 | −96.5 to −60.7 | … |
| Interactions with time/time squared since age 10 | … | … | … | … | … | … |
| Sex (time) | … | … | … | … | … | <.001 |
| Male | … | … | … | 0.0 | … | … |
| Female | … | … | … | −21.6 | −29.9 to −13.4 | … |
| Sex (time squared) | … | … | … | … | … | <.001 |
| Male | … | … | … | 0.0 | … | … |
| Female | … | … | … | 1.9 | 1.2 to 2.7 | … |
| Age started ART (y) and nadir CD4 z score (time) | … | … | … | … | … | <.001 |
| A: Started ART ≤5 y of age and nadir CD4 z score <−4 | … | … | … | 0.0 | … | … |
| B: Started ART ≤5 y of age and nadir CD4 z score ≥−4 | … | … | … | −33.5 | −48.8 to −18.3 | … |
| C: Started ART >5 to <10 y of age and nadir CD4 z score <−4 | … | … | … | 3.8 | −10.8 to 18.3 | … |
| D: Started ART >5 to <10 y of age and nadir CD4 z score ≥−4 | … | … | … | −35.7 | −53.0 to −18.3 | … |
| E: Started ART ≥10 y of age and nadir CD4 z score <−4 | … | … | … | 102.8 | 86.9 to 118.7 | … |
| F: Started ART ≥10 y of age and nadir CD4 z score ≥−4 | … | … | … | 18.0 | 2.5 to 33.5 | … |
| Age started ART (y) and nadir CD4 z score (time squared) | … | … | … | … | … | <.001 |
| A: Started ART ≤5 y of age and nadir CD4 z score <−4 | … | … | … | 0.0 | … | … |
| B: Started ART ≤5 y of age and nadir CD4 z score ≥−4 | … | … | … | 3.5 | 2.0 to 5.1 | … |
| C: Started ART >5 to <10 y of age and nadir CD4 z score <−4 | … | … | … | 0.9 | −0.5 to 2.3 | … |
| D: Started ART >5 to <10 y of age and nadir CD4 z score ≥−4 | … | … | … | 3.5 | 1.8 to 5.1 | … |
| E: Started ART ≥10 y of age and nadir CD4 z score <−4 | … | … | … | −4.7 | −6.1 to −3.2 | … |
| F: Started ART ≥10 y of age and nadir CD4 z score ≥−4 | … | … | … | 1.4 | 0.0 to 2.9 | … |
| Viral suppression (time updated, within ±6 m of CD4 count) (time) | … | … | … | … | … | <.001 |
| Suppressed, <400 copies/mL | … | … | … | 0.0 | … | … |
| Not suppressed, ≥400 copies/mL | … | … | … | −26.5 | −34.4 to −18.6 | … |
| Viral suppression (time updated, within ±6 m of CD4 count) (time squared) | … | … | … | … | … | <.001 |
| Suppressed, <400 copies/mL | … | … | … | 0.0 | … | … |
| Not suppressed, ≥400 copies/mL | … | … | … | 2.1 | 1.3 to 2.9 | … |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval.
aNumber of young people in the univariable models, n = 1258; except for ethnicity, n = 1249; country of birth, n = 1256; years on ART at age 10 and nadir CD4 z score, n = 1190; and viral suppression, n = 1257.
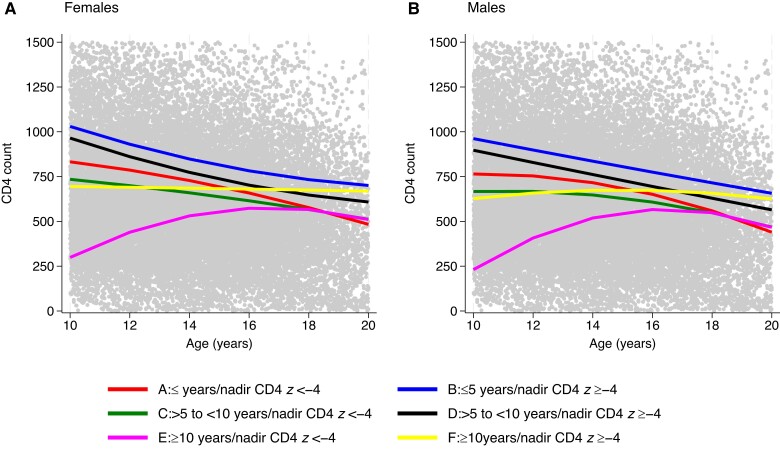
In multivariable analysis, CD4 counts over time also differed by age at the start of ART and nadir CD4 z score and by sex (P < .001 for all time variables; Table 2). Figure 1 shows the predicted association between CD4 over time (age 10 to 20 years) and age at the start of ART/nadir CD4 z score groups for modeled females (Figure 1A) and males (Figure 1B) of Black ethnicity and born in 2000 with a suppressed viral load <400 copies/mL (time-updated). At age 10, mean CD4 count was highest in young people who had started ART before age 10 and had a higher nadir CD4 z score, with a decline in CD4 count after age 10 that slowed over time in females (Figure 1A, groups B and D; Supplementary Table 1A) and was more linear in males (Figure 1B, groups B and D; Supplementary Table 1B). Mean CD4 count at age 10 was lower in young people who had started ART before age 10 and had a lower nadir CD4 z score (Figure 1A and 1B, groups A and C; Supplementary Table 1A and 1B); the decline in CD4 over time was steeper than in those who had a higher nadir CD4 z score (groups B and D).
Figure 1.
Predicted mean CD4 counts over time for young people with perinatal human immunodeficiency virus of Black ethnicity born in 2000 with suppressed viral load (time updated) by age at the start of antiretroviral therapy/nadir CD4 z score groups.
At age 10, young people who had started ART at age ≥10 years and had a higher nadir CD4 z score (group F) had a mean CD4 count that was similar to that of group C; however, the CD4 count remained stable in group F over time with a predicted average of 650 cells/mm3 for both males and females (Figure 1B; Supplementary Table 1A and 1B). At age 10, mean CD4 count was lowest in young people who had started ART at age ≥10 and had a lower nadir CD4 z score (group E). In this group, CD4 increased over time until approximately age 16 years, plateaued, and then decreased, with a similar trend in males and females.
There was no evidence that mean CD4 counts differed over time by ethnicity or year of birth. On average, being virally unsuppressed was associated with having greater decreases or smaller increases in CD4 over time than being suppressed (P < .001). There was no evidence of an effect of time since last CD4 measurement on CD4 trends over time (P = .168), and fitting separate multivariable models to young people linked and not linked to UK CHIC found comparable results to the overall multivariable model (data not shown).
Published data from 9 studies (7 in Europe or the United States) of CD4 counts in the general population were identified [22–30] (Table 3). All studies were in young people without any known medical conditions except for 1 study that included young people admitted to the hospital for presurgery screenings for malformations, trauma, or benign diseases [24] and 1 that included young people with bleeding disorders but no lymphocyte abnormalities [25].
Table 3.
Published Data on CD4 Count in Young People in the General Population
| Averagea CD4 Count (cells/mm3) At Age (y) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Age Range | Ethnicity | Gender | 10 | 12 | 14 | 16 | 18 | 20 |
| Comans-Bitter et al [22], 1997 | Netherlands | 0 to adults 5–10 y; n = 35 10–16 y; n = 23 adults; n = 51 |
NG | NG | 1000 (300–2000)b | 800 (400–2100)b | 700 (300–1400)b | |||
| Huenecke et al [23], 2008 | Germany | 2 m to 40 y 4–10 y; n = 31 10–18 y; n = 10 adults; n = 20 |
NG | 35% female | 986c (499–1588) | 954c (483–1537) | 939c (475–1512) | 931c (471–1500) | 928c (469–1494) | 926c (468–1491) |
| Tosato et al [24], 2015 | Italy | 0 to 18 y 6–12 y; n = 56 12–18 y; n = 20 |
85% White | 42% female | 1030 (646–1515) | 887 (610–1446) | … | |||
| Bofill et al [25], 1992 | United Kingdom | 1 to 79 y 9–10 y; n* = 25 11–79 y; n* = 600 |
NG | NG | 980d (243) | 830d (288) | ||||
| Valiathian et al [26], 2014 | United States | 12 to 67 y 12–18 y; n = 50 |
NG | 61% female | … | 920 (467–1563)e | … | |||
| Shearer et al [27], 2003 | United States | 0 to 18 y 6–12 y; n = 90 12–18 y; n = 90 |
53% African American | 48% female | 980 (650–1500) | 840 (530–1300) | … | |||
| Rudy et al [28], 2002 | United States | 14 to 20 y 14 y; n* = 3(m), 5(f) 16 y; n* = 13(m),55(f) 18 y; n* = 36(m), 108(f) 20 y; n* = 11(m),35(f) |
63% African American | 77% female | … | … | m: 833d (143) f: 779d (243) | m: 632d (107) f: 866d (287) | m: 753d (207) f: 817d (273) | m: 712d (192) f: 881d (319) |
| Mandala et al [29], 2010 | Malawi | 0 to 92 y 5–10 y; n = 52 10–15 y; n = 49 15–20 y; n = 51 |
African | 53% female | 1200 (800–2100) | 1100 (800–1700) | 900 (600–1200) | |||
| de Moraes-Pinot et al [30], 2014 | Brazil | 0 to 49 y 6–12 y; n = 50 12–18 y; n = 50 19–48 y; n = 51 |
NG | 43% female | 858 (566–1298) | 847 (640–1279) | 813 (487–1141) | |||
Abbreviations: f, female; m, male; n, number of participants (except for * where n is number of tests (number of participants was not given); NG, not given.
aMedian and 10th and 90th percentiles in parenthesis, unless otherwise stated.
b5th and 95th percentiles.
cPredicted values from exponential model and 90% lower and upper bounds in parenthesis.
dMean and standard deviation in parenthesis.
eRange.
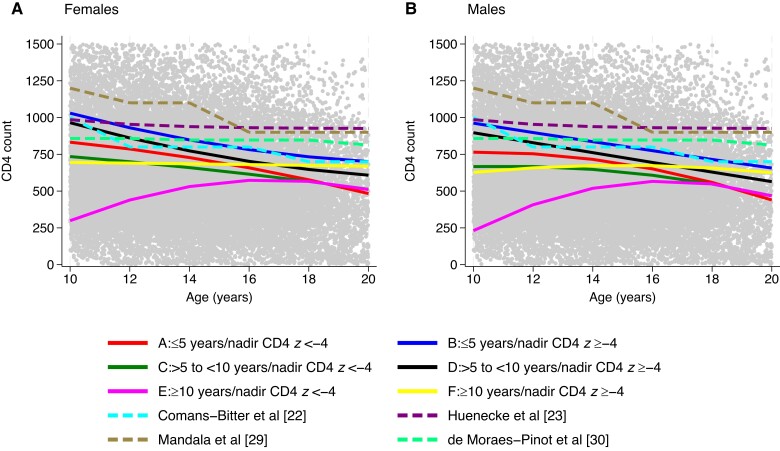
Figure 2 shows published data from 4 studies that included young people aged 10 to 20 years and had 3 or more age groups in that range [22, 23, 29, 30]. At age 10, the predicted mean CD4 count in young people in our study who started ART before age 10 and had a higher nadir CD4 z score (groups B and D) was similar to CD4 counts in young people in studies from the Netherlands (Comans–Bitter et al [22]) and Germany (Huenecke et al [23]), with the predicted decline in CD4 over time in groups B and D comparable to the Comans-Bitter study but faster than the Huenecke study. By age 20 years, young people who had a lower nadir CD4 z score, regardless of the age at which they started ART (groups A, C, and E), had a predicted mean CD4 count lower than that observed in all 4 studies of young people in the general population.
Figure 2.
Predicted mean CD4 counts over time for young people with perinatal human immunodeficiency virus of Black ethnicity born in 2000 with suppressed viral load (time-updated) by age at the start of antiretroviral therapy/nadir CD4 z score groups compared with published data on CD4 count in young people in the general population.
DISCUSSION
We found that changes in CD4 count over time in a cohort of young people with PHIV varied by age at ART start and nadir CD4 z score in childhood. For young people who started ART before the age of 10, CD4 count decreased from age 10. For those with a higher nadir CD4 z score, the predicted decline in some subgroups was comparable to published data of CD4 counts in young people in the general population. However, for those who had a lower nadir CD4 z score, the decline was steeper, and results from our model, assuming, on average, viral suppression <400 copies/mL over time, predicted average CD4 counts to approach mild immunodeficiency (350–500 cells/mm3) by age 20 years in some subgroups with characteristics associated with lower mean CD4 counts.
In our previous study, we found a decline in CD4 count over time in 271 young people in the period before transition to adult care that continued after transition in some groups. However, we were unable to assess the trends by age at ART start and nadir CD4 due to limited sample size [10]. This current analysis builds on our previous work and highlights the long-term impact of these factors and how the CD4 trajectory compares to the general population in Europe.
Rodriguez et al [11] found that in 132 young people who started ART at a median age of 5.7 years in Peru, CD4 count (unadjusted for viral load) decreased from age 5 to 18 years and that the decline was faster after age 13 years. A global cohort collaboration [7] also found that CD4 count (unadjusted for viral load) decreased from age 10 to 17 years in 19 557 young people with PHIV who started ART at a median age of 6.9 years in 46 countries worldwide, with similar trends by sex and geographical region.
The decline in CD4 count observed in our study in young people who started ART before the age of 5 years and had a higher nadir CD4 z score in childhood appears to mirror the decline in CD4 observed in least 1 study in young people in the general population. However, due to the small sample size, there is large variation in the median estimate. Mean CD4 counts also followed a similar trajectory over time for young people who started ART at ages between 5 and 10 years and had a higher nadir CD4 z score; at age 20, mean CD4 counts were predicted to be higher than for those who had a lower nadir CD4 z score and started ART at any age.
For young people who started ART before age 10 and had a lower nadir CD4 z score in childhood, the decline in CD4 was steeper. Children with HIV have a profound capacity for immune recovery following ART, as thymic output is high in infancy [31]. However, our study implies that immune reconstitution may not only rely on early age of ART initiation but also on the immune system’s ability to maintain a normal level of CD4 T-cell numbers during childhood. Previous studies have suggested the importance of a high thymic output and diverse T-cell receptor repertoire in maintaining viral suppression as well as contributing to high CD4 later in life [32, 33]. It is reassuring that there were few AIDS events or deaths in young people who started ART before age 10 in our study. However, future research is needed to determine whether the steeper decline in CD4 counts seen in young people with a lower nadir CD4 during childhood results in different clinical outcomes during adulthood compared with the general population.
We found that changes in CD4 count over time differed by sex, with females having higher mean CD4 counts than males in early and late adolescence. Previous studies in children [34, 35] and adults [36, 37] have suggested that females have a better immunological response than males after ART initiation, and Rudy et al [28] found that females in the general population without HIV infection had higher CD4 counts than males.
One limitation of our study is that we were unable to examine the effects of adherence to ART on CD4 evolution, as adherence information was not available. However, we adjusted our analysis for time-updated viral load as a surrogate. Another limitation is that we did not adjust for ART regimen, and young people who started ART in earlier calendar years and on older regimens may have contributed more CD4 data over time than those on newer regimens. However, we included only young people who started ART from 2000 onward, when effective ART was available for children. Also, findings from young people in our study who started ART at older ages may not be relevant to infants starting ART today. Last, young people lost to follow-up or who died before age 10 would not have contributed to the analysis, and some young people in CHIPS transitioned to non-UK CHIC clinics and would not have contributed CD4 data after transition. Young people participating in CHIPS had high retention in pediatric care and low mortality rates [3], and sensitivity analyses fitting the model to young people linked and not linked to UK CHIC found comparable results.
We have shown that for young people with PHIV and who started ART before age 10, having a lower nadir CD4 z score in childhood was associated with a decline in CD4 count over time. This suggests that in children, in addition to starting ART at an early age, optimizing ART to maintain good levels of immune function may be important to maximize immune reconstitution later in life. Young people who start ART before age 10 with a higher nadir CD4 z score during childhood are likely to achieve CD4 levels during adolescence and early adulthood that are comparable to those of young people in the general population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Collaborative HIV Paediatric Study (CHIPS)
CHIPS Steering Committee: Hermione Lyall (chair), Alasdair Bamford, Karina Butler, Katja Doerholt, Conor Doherty, Caroline Foster, Julia Kenny, Nigel Klein, Gillian Letting, Paddy McMaster, Fungai Murau, Edith Nsangi, Katia Prime, Andrew Riordan, Fiona Shackley, Delane Shingadia, Sharon Storey, Gareth Tudor-Williams, Anna Turkova, Steve Welch. MRC Clinical Trials Unit (MRC CTU): Intira Jeannie Collins, Claire Cook, Siobhan Crichton, Donna Dobson, Keith Fairbrother, Diana M. Gibb, Ali Judd, Marthe Le Prevost, Nadine Van Looy.
Integrated Screening Outcome Surveillance Service (ISOSS), University College London (UCL): Helen Peters, Kate Francis, Claire Thorne.
Hospitals participating in CHIPS in 2019/2020: University Hospitals Birmingham National Health Service (NHS) Foundation Trust, Birmingham: L. Thrasyvoulou, S. Welch; Brighton and Sussex University Hospitals NHS Trust, Brighton: K. Fidler; University Hospitals Bristol NHS Foundation Trust, Bristol: J. Bernatoniene, F. Manyika; Calderdale and Huddersfield NHS Foundation Trust, Halifax: G. Sharpe; Derby Teaching Hospitals NHS Foundation Trust, Derby: B. Subramaniam; Glasgow Royal Hospital for Children, Glasgow: R. Hague, V. Price; Great Ormond Street Hospital for Children NHS Foundation Trust, London: J. Flynn, A. Cardoso, M. Abou–Rayyah, N. Klein, A. Bamford, D. Shingadia, K. Grant; Oxford University Hospitals NHS Foundation Trust, Oxford: S. Yeadon, S. Segal; King’s College Hospital NHS Foundation Trust, London: S. Hawkins; Leeds Teaching Hospitals NHS Trust, Leeds: M. Dowie; University Hospitals of Leicester NHS Trust, Leicester: S. Bandi, E. Percival; Luton and Dunstable Hospital NHS Foundation Trust, Luton: M. Eisenhut, K. Duncan; Milton Keynes General University Hospital NHS Foundation Trust, Milton Keynes: L. Anguvaa, L. Wren; Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle: T. Flood, A. Pickering; Pennine Acute Hospitals NHS Trust, Manchester: P. McMaster, C. Murphy; North Middlesex University Hospital NHS Trust, London: J. Daniels, Y. Lees; Northampton General Hospital NHS Trust, Northampton: F. Thompson; London North West Healthcare NHS Trust, Middlesex: A. Williams, B. Williams, S. Pope; Barts Health NHS Trust, London: Dr S. Libeschutz; Nottingham University Hospitals NHS Trust, Nottingham: L. Cliffe, S. Southall; Portsmouth Hospitals NHS Trust, Portsmouth: A. Freeman; Raigmore Hospital, Inverness: H. Freeman; Royal Belfast Hospital for Sick Children, Belfast: S. Christie; Royal Berkshire NHS Foundation Trust, Reading: A. Gordon; Royal Children’s Hospital, Aberdeen: D. Rosie Hague, L. Clarke; Royal Edinburgh Hospital for Sick Children, Edinburgh: L. Jones, L. Brown; Royal Free NHS Foundation Trust, London: M. Greenberg; Alder Hey Children’s NHS Foundation Trust, Liverpool: C. Benson, A. Riordan; Sheffield Children’s NHS Foundation Trust, Sheffield: L. Ibberson, F. Shackley; University Hospital Southampton NHS Foundation Trust, Southampton: S. Patel, J. Hancock; St George’s University Hospitals NHS Foundation Trust, London: K. Doerholt, K. Prime, M. Sharland, S. Storey; Imperial College Healthcare NHS Trust, London: E. G. H. Lyall, C. Foster, P. Seery, G. Tudor-Williams, N. Kirkhope, S. Raghunanan; Guy’s and St Thomas’ NHS Foundation Trust, London: Dr J. Kenny, A. Callaghan; University Hospitals of North Midlands NHS Trust, Stoke On Trent: A. Bridgwood, P. McMaster; University Hospital of Wales, Cardiff: J. Evans, E. Blake; NHS Frimley Health Foundation Trust, Slough: A. Yannoulias.
UK Collaborative HIV Cohort (UK CHIC)
UK CHIC Steering Committee: Jonathan Ainsworth, Sris Allan, Jane Anderson, Ade Apoola, David Chadwick, Duncan Churchill, Valerie Delpech, David Dunn, Ian Fairley, Ashini Fox, Richard Gilson, Mark Gompels, Phillip Hay, Rajesh Hembrom, Teresa Hill, Margaret Johnson, Sophie Jose, Stephen Kegg, Clifford Leen, Dushyant Mital, Mark Nelson, Hajra Okhai, Chloe Orkin, Adrian Palfreeman, Andrew Phillips, Deenan Pillay, Ashley Price, Frank Post, Jillian Pritchard, Caroline Sabin, Achim Schwenk, Anjum Tariq, Roy Trevelion, Andy Ustianowski, John Walsh.
UK CHIC Central Co-ordination: UCL, London (David Dunn, Teresa Hill, Hajra Okhai, Andrew Phillips, Caroline Sabin); Medical Research Council Clinical Trials Unit at UCL (MRC CTU at UCL), London (Nadine van Looy, Keith Fairbrother).
UK CHIC Participating Centers: Barts Health NHS Trust, London (Chloe Orkin, Janet Lynch, James Hand); Brighton and Sussex University Hospitals NHS Trust (Duncan Churchill, Stuart Tilbury, Elaney Youssef, Duncan Churchill); Chelsea and Westminster Hospital NHS Foundation Trust, London (Mark Nelson, Richard Daly, David Asboe, Sundhiya Mandalia); Homerton University Hospital NHS Trust, London (Jane Anderson, Sajid Munshi); King's College Hospital NHS Foundation Trust, London (Frank Post, Ade Adefisan, Chris Taylor, Zachary Gleisner, Fowzia Ibrahim, Lucy Campbell); South Tees Hospitals NHS Foundation Trust, Middlesbrough (David Chadwick, Kirsty Baillie); Mortimer Market Centre, UCL, London (Richard Gilson, Ian Williams); North Middlesex University Hospital NHS Trust, London (Jonathan Ainsworth, Achim Schwenk, Sheila Miller, Chris Wood); Royal Free NHS Foundation Trust/UCL, London (Margaret Johnson, Mike Youle, Fiona Lampe, Colette Smith, Rob Tsintas, Clinton Chaloner, Caroline Sabin, Andrew Phillips, Teresa Hill, Hajra Okhai); Imperial College Healthcare NHS Trust, London (John Walsh, Nicky Mackie, Alan Winston, Jonathan Weber, Farhan Ramzan, Mark Carder); Lothian University Hospitals NHS Trust, Edinburgh (Clifford Leen, Andrew Kerr, David Wilks, Sheila Morris); North Bristol NHS Trust (Mark Gompels, Sue Allan); University Hospitals of Leicester NHS Trust, Leicester (Adrian Palfreeman, Adam Lewszuk); Lewisham and Greenwich NHS Trust, Woolwich (Stephen Kegg, Victoria Ogunbiyi, Sue Mitchell), St. George's Healthcare NHS Trust (Phillip Hay, Christopher Hunt, Olanike Okolo, Benjamin Watts); York Teaching Hospital NHS Foundation Trust (Ian Fairley, Sarah Russell-Sharpe, Olatunde Fagbayimu); University Hospitals Coventry and Warwickshire NHS Trust, Coventry (Sris Allan, Debra Brain); Royal Wolverhampton Hospitals NHS Trust, Wolverhampton (Anjum Tariq, Liz Radford, Sarah Milgate); Ashford and St. Peter's Hospitals NHS Foundation Trust, Chertsey (Jillian Pritchard, Shirley Cumming, Claire Atkinson); Milton Keynes Hospital NHS Foundation Trust (Dushyant Mital, Annie Rose, Jeanette Smith); Pennine Acute Hospitals NHS Trust (Andy Ustianowski, Cynthia Murphy, Ilise Gunder); Nottingham University Hospitals NHS Trust (Ashini Fox, Howard Gees, Gemma Squires, Laura Anderson), Kent Community Health NHS Foundation Trust (Rajesh Hembrom, Serena Mansfield, Lee Tomlinson, Christine LeHegerat, Roberta Box, Tom Hatton, Doreen Herbert), Newcastle upon Tyne Hospitals NHS Foundation Trust (Ashley Price, Ian McVittie, Victoria Murtha, Laura Shewan); Derby Teaching Hospitals NHS Foundation Trust (Ade Apoola, Zak Connan, Luke Gregory, Kathleen Holding, Victoria Chester, Trusha Mistry, Catherine Gatford); Public Health England, London (Valerie Delpech); i-Base (Roy Trevelion).
We acknowledge members of the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Blood Borne and Sexually Transmitted Infections at UCL Steering Committee: Professor Caroline Sabin (HPRU Director), Dr John Saunders (Public Health England Lead), Professor Catherine Mercer, Dr Hamish Mohammed, Professor Greta Rait, Dr Ruth Simmons, Professor William Rosenberg, Dr Tamyo Mbisa, Professor Rosalind Raine, Dr Sema Mandal, Dr Rosamund Yu, Dr Samreen Ijaz, Dr Fabiana Lorencatto, Dr Rachel Hunter, Dr Kirsty Foster, and Dr Mamoona Tahir.
Disclaimer. The views expressed here are those of the authors and not necessarily those of the NIHR, the Department of Health and Social Care, or UK Health Security Agency (HSA).
Data sharing statement. Requests for data can be initiated by contacting mrcctu.datareleaserequest@ucl.ac.uk for CHIPS and Caroline Sabin (c.sabin@ucl.ac.uk), the UK CHIC principal investigator for UK CHIC.
Financial support. CHIPS funding: This work was supported by NHS England (London Specialised Commissioning Group) and received additional support from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, GSK, Gilead Sciences, Janssen, and Roche. The Medical Reasearch Council Clinical Trials Unit at UCL is supported by the Medical Research Council (grant MC_UU_00004/03). UK CHIC funding: This work was supported by the MRC, UK (G0000199, G0600337, G0900274, and M004236). The research was supported by the NIHR, Health Protection Research Unit in Blood Borne and Sexually Transmitted Infections at UCL in partnership with the UK HSA. I. J. C. reports support for this work from AbbVie, ViiV Healthcare, and Gilead (all payments to institution).
Contributor Information
Hannah Castro, Institute of Clinical Trials and Methodology, Medical Research Council Clinical Trials Unit at University College London, University College London, London, United Kingdom.
Caroline Sabin, Institute for Global Health, University College London, London, United Kingdom; National Institute for Health and Care Research, Health Protection Research Unit in Blood Borne and Sexually Transmitted Infections at University Colllege London, University College London, London, United Kingdom.
Intira Jeannie Collins, Institute of Clinical Trials and Methodology, Medical Research Council Clinical Trials Unit at University College London, University College London, London, United Kingdom.
Hajra Okhai, Institute for Global Health, University College London, London, United Kingdom.
Katrine Schou Sandgaard, Department of Pediatrics and Adolescent Medicine, Aarhus University Hospital, Aarhus, Denmark.
Katia Prime, Department of Genitourinary Medicine, St George’s University Hospitals National Health Service Foundation Trust, London, United Kingdom.
Caroline Foster, Department of Paediatric Infectious DIseases, Imperial College Healthcare National Health Service Trust, London, United Kingdom.
Marthe Le Prevost, Institute of Clinical Trials and Methodology, Medical Research Council Clinical Trials Unit at University College London, University College London, London, United Kingdom.
Siobhan Crichton, Institute of Clinical Trials and Methodology, Medical Research Council Clinical Trials Unit at University College London, University College London, London, United Kingdom.
Nigel Klein, Infection, Immunity and Inflammation, University College London, Great Ormond Street Institute of Child Health, London, United Kingdom.
Ali Judd, Institute of Clinical Trials and Methodology, Medical Research Council Clinical Trials Unit at University College London, University College London, London, United Kingdom.
Collaborative HIV Paediatric Study:
Hermione Lyall, Alasdair Bamford, Karina Butler, Katja Doerholt, Conor Doherty, Caroline Foster, Julia Kenny, Nigel Klein, Gillian Letting, Paddy McMaster, Fungai Murau, Edith Nsangi, Katia Prime, Andrew Riordan, Fiona Shackley, Delane Shingadia, Sharon Storey, Gareth Tudor-Williams, Anna Turkova, Steve Welch, Intira Jeannie Collins, Claire Cook, Siobhan Crichton, Donna Dobson, Keith Fairbrother, Diana M Gibb, Ali Judd, Marthe Le Prevost, Nadine Van Looy, Helen Peters, Kate Francis, Claire Thorne, L Thrasyvoulou, S Welch, K Fidler, J Bernatoniene, F Manyika, G Sharpe, B Subramaniam, R Hague, V Price, J Flynn, A Cardoso, M Abou–Rayyah, N Klein, A Bamford, D Shingadia, K Grant, S Yeadon, S Segal, S Hawkins, M Dowie, S Bandi, E Percival, M Eisenhut, K Duncan, L Anguvaa, L Wren, T Flood, A Pickering, P McMaster, C Murphy, J Daniels, Y Lees, F Thompson, A Williams, B Williams, S Pope, Dr S Libeschutz, L Cliffe, S Southall, A Freeman, H Freeman, S Christie, A Gordon, L Jones, L Brown, M Greenberg, C Benson, A Riordan, L Ibberson, F Shackley, S Patel, J Hancock, K Doerholt, K Prime, M Sharland, S Storey, E G H Lyall, C Foster, P Seery, G Tudor-Williams, N Kirkhope, S Raghunanan, Dr J Kenny, A Callaghan, A Bridgwood, P McMaster, J Evans, E Blake, and A Yannoulias
UK Collaborative HIV Cohort Study:
Jonathan Ainsworth, Sris Allan, Jane Anderson, Ade Apoola, David Chadwick, Duncan Churchill, Valerie Delpech, David Dunn, Ian Fairley, Ashini Fox, Richard Gilson, Mark Gompels, Phillip Hay, Rajesh Hembrom, Teresa Hill, Margaret Johnson, Sophie Jose, Stephen Kegg, Clifford Leen, Dushyant Mital, Mark Nelson, Hajra Okhai, Chloe Orkin, Adrian Palfreeman, Andrew Phillips, Deenan Pillay, Ashley Price, Frank Post, Jillian Pritchard, Caroline Sabin, Achim Schwenk, Anjum Tariq, Roy Trevelion, Andy Ustianowski, John Walsh, David Dunn, Teresa Hill, Hajra Okhai, Andrew Phillips, Caroline Sabin, Nadine van Looy, Keith Fairbrother, Chloe Orkin, Janet Lynch, James Hand, Duncan Churchill, Stuart Tilbury, Elaney Youssef, Duncan Churchill, Mark Nelson, Richard Daly, David Asboe, Sundhiya Mandalia, Jane Anderson, Sajid Munshi, Frank Post, Ade Adefisan, Chris Taylor, Zachary Gleisner, Fowzia Ibrahim, Lucy Campbell, David Chadwick, Kirsty Baillie, Richard Gilson, Ian Williams, Jonathan Ainsworth, Achim Schwenk, Sheila Miller, Chris Wood, Margaret Johnson, Mike Youle, Fiona Lampe, Colette Smith, Rob Tsintas, Clinton Chaloner, Caroline Sabin, Andrew Phillips, Teresa Hill, Hajra Okhai, John Walsh, Nicky Mackie, Alan Winston, Jonathan Weber, Farhan Ramzan, Mark Carder, Clifford Leen, Andrew Kerr, David Wilks, Sheila Morris, Mark Gompels, Sue Allan, Adrian Palfreeman, Adam Lewszuk, Stephen Kegg, Victoria Ogunbiyi, Sue Mitchell, Phillip Hay, Christopher Hunt, Olanike Okolo, Benjamin Watt, Ian Fairley, Sarah Russell-Sharpe, Olatunde Fagbayimu, Sris Allan, Debra Brain, Anjum Tariq, Liz Radford, Sarah Milgate, Jillian Pritchard, Shirley Cumming, Claire Atkinson, Dushyant Mital, Annie Rose, Jeanette Smith, Andy Ustianowski, Cynthia Murphy, Ilise Gunder, Ashini Fox, Howard Gees, Gemma Squires, Laura Anderson, Rajesh Hembrom, Serena Mansfield, Lee Tomlinson, Christine LeHegerat, Roberta Box, Tom Hatton, Doreen Herbert, Ashley Price, Ian McVittie, Victoria Murtha, Laura Shewan, Ade Apoola, Zak Connan, Luke Gregory, Kathleen Holding, Victoria Chester, Trusha Mistry, Catherine Gatford, Valerie Delpech, and Roy Trevelion
Notes
Author Contributions. H. C., N. K., C. S., A. J., and H.O. substantially contributed to the concept and design of the study. All authors substantially contributed to the acquisition, analysis, or interpretation of the data. S. C., M. L. P., and H. O. acquired and verified the data. H. C. performed the analysis. H. C., N. K., C. S., A. J., I. J. C., S. C., K. S. S., K. P., and C. F. interpreted the data. H. C. drafted the article, with major contributions from C. S., A. J., I. J. C., S. C., and K. S. S. All authors revised the manuscript for important intellectual content, approved the final version for publication, and are accountable for all aspects of the work.
References
- 1. UNICEF data . Available at: https://data.unicef.org/topic/hivaids/adolescents-young-people/. Accessed 1 December 2022.
- 2. Slogrove AL, Schomaker M, Davies M-A, et al. The epidemiology of adolescents living with perinatally acquired HIV: a cross-region global cohort analysis. PLoS Med 2018; 15:e1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chappell E, Lyall H, Riordan A, et al. The cascade of care for children and adolescents with HIV in the UK and Ireland, 2010 to 2016. J Int AIDS Soc 2019; 22:e25379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weijsenfeld AM, Smit C, Wit FWNM, et al. Long-term virological treatment outcomes in adolescents and young adults with perinatally and non-perinatally acquired human immunodeficiency virus. Open Forum Infect Dis 2022; 9:ofac561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ritchwood TD, Malo V, Jones C, et al. Healthcare retention and clinical outcomes among adolescents living with HIV after transition from pediatric to adult care: a systematic review. BMC Public Health 2020; 20:1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Pregnancy and Paediatric HIV Cohort Collaboration Study Group . Height and timing of growth spurt during puberty in young people living with vertically acquired HIV in Europe and Thailand. AIDS 2019; 33:1897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jesson J, Crichton S, Quartagno M. Growth and CD4 patterns of adolescents living with perinatally acquired HIV worldwide, a CIPHER cohort collaboration analysis. J Int AIDS Soc 2022; 25:e25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization, 2021. [PubMed] [Google Scholar]
- 9. Lewis J, Payne H, Walker AS, et al. Thymic output and CD4 T-cell reconstitution in HIV-infected children on early and interrupted antiretroviral treatment: evidence from the Children with HIV Early Antiretroviral Therapy Trial. Front Immunol 2017; 8:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Judd A, Collins IJ, Parrott F, et al. Growing up with perinatal HIV: changes in clinical outcomes before and after transfer to adult care in the UK. J Int AIDS Soc 2017; 20(Suppl 3):21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodriguez CA, Kolevic L, Ramos A, et al. Lifetime changes in CD4 T-cell count, viral load suppression and adherence among adolescents living with HIV in urban Peru. Pediatr Infect Dis J 2020; 39:54–6. [DOI] [PubMed] [Google Scholar]
- 12. Wade AM, Ades AE. Incorporating correlations between measurements into the estimation of age-related reference ranges. Stat Med 1998; 17:1989–2002. [DOI] [PubMed] [Google Scholar]
- 13. Picat MQ, Lewis J, Musiime V, et al. Predicting patterns of long-term CD4 reconstitution in HIV-infected children starting antiretroviral therapy in sub-Saharan Africa: a cohort-based modelling study. PLoS Med 2013; 10:e1001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schröter J, Anelone AJN, de Boer RJ; EPIICAL Consortium . Quantification of CD4 recovery in early-treated infants living with HIV. J Acquir Immune Defic Syndr 2022; 89:546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewis J, Walker AS, Castro H, et al. Age and CD4 count at initiation of antiretroviral therapy in HIV-infected children: effects on long-term T-cell reconstitution. J Infect Dis 2012; 205:548–56. [DOI] [PubMed] [Google Scholar]
- 16. Krogstad P, Patel K, Karalius B, et al. Incomplete immune reconstitution despite virologic suppression in HIV-1 infected children and adolescents. AIDS 2015; 29:683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Pregnancy and Paediatric HIV Cohort Collaboration Study Group in EuroCoord . CD4 recovery following antiretroviral treatment interruptions in children and adolescents with HIV infection in Europe and Thailand. HIV Med 2019; 20:456–72. [DOI] [PubMed] [Google Scholar]
- 18. Gibb DM, Duong T, Tookey PA, et al. Decline in mortality, AIDS, and hospital admissions in perinatally HIV-1 infected children in the United Kingdom and Ireland. Br Med J 2003; 327:1019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. UK Collaborative HIV Cohort Steering Committee . The creation of a large UK-based multicentre cohort of HIV-infected individuals: the UK collaborative HIV cohort (UK CHIC) study. HIV Med 2004; 5:115–24. [DOI] [PubMed] [Google Scholar]
- 20. Wade AM, Ades AE. Age related reference ranges: significance test for models and confidence intervals for centiles. Stat Med 1994; 13:2359–67. [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization . WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: World Health Organization, 2007. [Google Scholar]
- 22. Comans-Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr 1997; 130:388–93. [DOI] [PubMed] [Google Scholar]
- 23. Huenecke S, Behl M, Fadler C, et al. Age-matched lymphocyte subpopulation reference values in childhood and adolescence: application of exponential regression analysis. Eur J Haematol 2008; 80:532–9. [DOI] [PubMed] [Google Scholar]
- 24. Tosato F, Bucciol G, Pantano G, et al. Lymphocytes subsets reference values in childhood. Cytometry A 2015; 87:81–5. [DOI] [PubMed] [Google Scholar]
- 25. Bofill M, Janossy G, Lee CA, et al. Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis. Clin Exp Immunol 1992; 88:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valiathan R, Deeb K, Diamante M, Ashman M, Sachdeva N, Asthana D. Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology 2014; 219:487–96. [DOI] [PubMed] [Google Scholar]
- 27. Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol 2003; 112:973–80. [DOI] [PubMed] [Google Scholar]
- 28. Rudy BJ, Wilson CM, Durako S, Moscicki AB, Muenz L, Douglas SD. Peripheral blood lymphocyte subsets in adolescents: a longitudinal analysis from the REACH project. Clin Diagn Lab Immunol 2002; 9:959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mandala WL, MacLennan JM, Gondwe EN, Ward SA, Molyneux ME, MacLennan CA. Lymphocyte subsets in healthy Malawians: implications for immunologic assessment of HIV infection in Africa. J Allergy Clin Immunol 2010; 125:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Moraes-Pinto MI, Ono E, Santos-Valente EC, et al. Lymphocyte subsets in human immunodeficiency virus-unexposed Brazilian individuals from birth to adulthood. Mem Inst Oswaldo Cruz 2014; 109:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandgaard KS, Lewis J, Adams S, Klein N, Callard R. Antiretroviral therapy increases thymic output in children with HIV. AIDS 2014; 28:209–14. [DOI] [PubMed] [Google Scholar]
- 32. Sandgaard KS, Margetts B, Attenborough T, et al. Plasticity of the immune system in children following treatment interruption in HIV-1 infection. Front Immunol 2021; 12:643189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sandgaard KS, Gkouleli T, Attenborough T, et al. The importance of taking ART appropriately in children and adolescents with HIV-1 to reach the highest capacity of immune function later in life. Front Immunol 2022; 13:860316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mori M, Adland E, Paioni P, et al. Sex differences in antiretroviral therapy initiation in pediatric HIV infection. PLoS One 2015; 10:e0131591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruel TD, Zanoni BC, Ssewanyana I, et al. Sex differences in HIV RNA level and CD4 cell percentage during childhood. Clin Infect Dis 2011; 53:592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novelli S, Delobel P, Bouchaud O, et al. Enhanced immunovirological response in women compared to men after antiretroviral therapy initiation during acute and early HIV-1 infection: results from a longitudinal study in the French ANRS Primo cohort. J Int AIDS Soc 2020; 23:e25485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Means AR, Risher KA, Ujeneza EL, Maposa I, Nondi J, Bellan SE. Impact of age and sex on CD4+ cell count trajectories following treatment initiation: an analysis of the Tanzanian HIV treatment database. PLoS One 2016; 11:e0164148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.