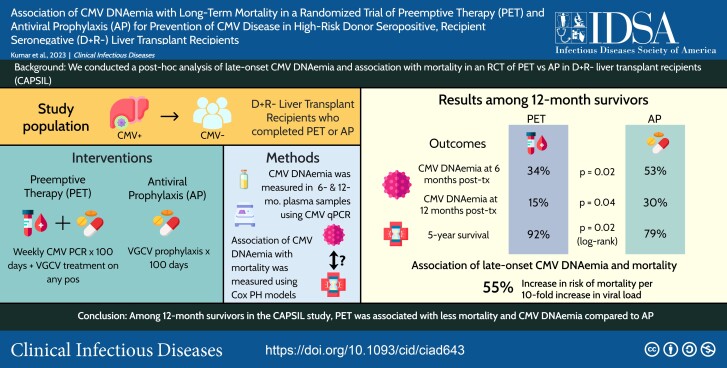
Abstract
In a post-hoc analysis of the association of CMV DNAemia with long-term mortality in a randomized trial of CMV preemptive therapy vs. antiviral prophylaxis in D+R- liver transplant recipients, post-intervention CMV DNAemia was associated with increased mortality after adjusting for study arm.
Keywords: liver transplant, immunocompromised host, viral infections, cytomegalovirus, antiviral therapy
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/association-of-cmv-dnaemia-with-long-term-mortality-in-a-randomized-trial-of-preemptive-therapy-pet-and-antiviral-prophylaxis-ap-for-prevention-of-cmv-disease-in-high-risk-donor-seropositive-recipient-se-2480b1a4-e573-43a6-973b-94ea264eed04
In a National Institutes of Health–sponsored, multicenter, randomized trial (Cytomegalovirus [CMV] Antiviral Prevention Strategies in D + R– Liver Transplant [CAPSIL]), we recently demonstrated that preemptive therapy (PET) was superior to antiviral prophylaxis (AP) for prevention of CMV disease in high-risk donor-seropositive, recipient-seronegative (D + R–) liver transplant recipients (LTxRs) [1]. Other clinical outcomes by 12 months, including overall long-term survival, were similar between groups at a median follow-up of 3.2 years after transplant. All analyses in the primary trial were conducted within the intent-to-treat population. To further explore long-term mortality among those who completed the primary trial intervention of PET or AP (per-protocol analysis), we performed a post hoc landmark analysis at 100 days post-transplant, which is the duration of the primary study intervention excluding participants who did not complete their assigned intervention for any reason. We further assessed the association between late-onset CMV DNAemia at 6 and 12 months and long-term mortality using a landmark analysis among survivors by 12 months post-transplant.
METHODS
Study Design
This study was a post hoc analysis of the CAPSIL study that compared PET and AP in D + R– LTxRs. The full study details are provided in the primary study [1]. Patients randomized to the PET arm were monitored weekly for CMV DNAemia using CMV polymerase chain reaction (PCR) through day 100 and treated for any level of DNAemia until resolution. The prophylaxis arm consisted of 100 days of prophylaxis dose valganciclovir (900 mg daily). Post-intervention CMV monitoring and treatment were done at clinical discretion. Plasma specimens were prospectively collected at 6 and 12 months post-transplant for all patients and cryopreserved for future analysis.
Post Hoc Sensitivity Analysis of Long-Term Mortality With PET vs AP Among Survivors by Day 100 Post-Transplant
Post hoc Kaplan–Meier survival analyses were conducted in the per-protocol population from the CAPSIL trial [1] using a landmark of 100 days for inclusion, the duration of the CMV prevention intervention. A total of 12 participants were excluded from this analysis population (Supplementary Figure 1). Differences in mortality between arms were assessed using the log-rank test, with a P value < .05 considered significant.
Association Between CMV DNAemia and Long-Term Mortality Among Survivors by 12 Months Post-Transplant
Plasma CMV DNAemia at 6 and 12 months post-transplant was retrospectively measured in stored samples from participants who survived 12 months post-transplant using quantitative PCR. The proportion of patients with CMV DNAemia at each time point was compared between the 2 arms using a 2-sample χ 2 test. The distribution of CMV viral loads was compared at each point using the Mann–Whitney U test. Kaplan–Meier curves were constructed and analyzed for participants who survived to 12 months post-transplant. Since previous work has shown that Cox proportional hazards (PH) models incorporating internal longitudinal measurements as time-dependent covariates are mathematically unsound and may produce spurious correlations [2, 3], we used time-independent Cox PH models to determine the association of maximum CMV viral load with mortality in this population. Covariates included in the Cox PH model were the maximum viral load from the 6- and 12-month post-transplant samples calculated as log10 (maximum CMV viral load + 1) and treatment arm coded as a binary variable with AP as the referent group (0). Multiple imputation using chained equations was used to impute missing CMV DNAemia values for Cox PH analysis, and a sensitivity analysis using a complete case approach was conducted. Analyses were conducted using the tidyverse, mice, survival, and survminer packages in R (v 4.2.3).
RESULTS
Comparison of Survival Between PET and AP Arms Using Landmark Kaplan–Meier Analyses
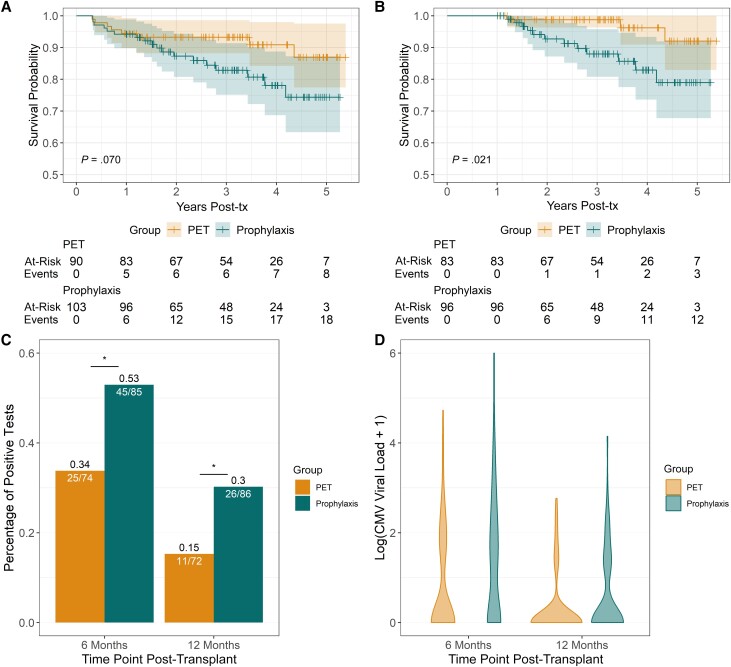
Of the 205 randomized participants in the original trial, 193 (94%) completed the 100-day intervention. In this group, estimated survival at 5 years post-transplant was 87% in the PET group vs 74% in the AP group (log-rank P = .07; Figure 1A). There were 179 of 205 participants (87%) who completed their assigned intervention and survived to 12 months post-transplant; estimated survival was 92% in the PET group vs 79% in the AP group by 5 years post-transplant. Survival was significantly higher among participants in the PET group compared with the AP group (log-rank P = .021; Figure 1B). The reported causes of death for patients who survived 1-year post-transplant are provided in Supplementary Table 1.
Figure 1.
Comparisons of mortality in participants who completed the intervention period and late-onset CMV DNAemia in donor-seropositive, recipient-seronegative liver transplant recipients. A, Kaplan–Meier survival curve of participant who survived and completed the assigned intervention. B, Kaplan–Meier survival curve of participants who survived to 12 months post-transplant. C, Comparison of percent of participants who developed CMV DNAemia at 6 months and 12 months post-transplant. Analysis was limited to participants who survived to 12 months post-transplant. D, Violin plots of CMV DNAemia showing distribution of log10 (CMV viral load) at 6 and 12 months post-transplant in participants who survived to 12 months post-transplant. Abbreviations: CMV, cytomegalovirus; PET, preemptive therapy; tx, treatment.
CMV DNAemia at 6 Months and 12 Months Post-Transplant Between PET and AP Arms
The proportion with CMV DNAemia was significantly lower in the PET vs AP arm at both 6 months (P = .02) and 12 months (P = .04) post-transplant (Figure 1C). The distribution of CMV viral loads was significantly different at both time points between the 2 arms (P = .02 at 6 months post-transplant and P = .03 at 12 months post-transplant, Figure 1D). Further details are provided in Supplementary Table 2.
Association of CMV DNAemia With Mortality Among 12-Month Survivors Between PET and AP
Maximum viral load at either 6 or 12 months was significantly associated with mortality after 1 year (adjusted hazards ratio [aHR], 1.55; 95% confidence interval [CI], 1.09–2.2; P = .03), while the study arm was not (aHR, 0.33; 95% CI, 0.09–1.23; P = .12). The results using a complete case approach were similar for both maximum viral load (aHR, 1.57; P = .01) and study arm (aHR, 0.36; P = .12).
DISCUSSION
In this post hoc landmark analysis of long-term survival in the CAPSIL trial, long-term mortality was significantly lower in the PET arm compared with the AP arm among 12-month survivors. Both the proportion with late-onset CMV DNAemia and the CMV viral load at 6 and 12 months post-transplant was lower in the PET group, and CMV viral load was associated with increased long-term mortality even when controlling for CMV prevention strategy.
In 2 meta-analyses of PET vs AP in LTxRs, no difference in survival was reported, but both included few studies with follow-up beyond 1 year post-transplant [4, 5]. In contrast, our results that demonstrate improved survival with PET compared with AP are consistent with the long-term follow-up results from a prior randomized trial of PET and AP in kidney transplant recipients [6]. Though our findings from the CAPSIL study mirror the results of the kidney transplant trial, there were significant differences between the 2 studies (type of organ transplant, CMV serostatus, duration of follow-up), so results of our post hoc analyses should be interpreted cautiously and considered hypothesis-generating. These preliminary findings provide compelling rationale for future studies to assess long-term survival in comparative trials of PET and AP.
We identified a quantitative relationship between CMV DNAemia at 6 and 12 months post-transplant and increased long-term mortality. Every 10-fold increase in maximum CMV viral load was associated with a 55% increased risk of subsequent mortality, even after controlling for CMV prevention strategy (PET or AP). This raises the hypothesis that CMV DNAemia may potentially mediate long-term mortality in D + R– LTxRs through previously proposed mechanisms [7, 8], as reported in observational studies in kidney and hematopoietic stem cell transplant settings [9, 10]. If this association is confirmed, characterizing the underlying mechanisms should be a priority of future studies.
In the CAPSIL study, PET was associated with increased CMV-specific immunity compared with AP at the end of the 100-day intervention [1]. The finding of decreased viremia in the PET arm at 6 and 12 months post-transplant is compatible with the hypothesis that the improved CMV-specific immunity with PET may better control CMV DNAemia long-term. CMV DNAemia has been linked to increased inflammation, alloimmune responses, and immune senescence [7], which may lead to increased risk of mortality and underlie the improved long-term survival with PET compared with AP that we identified.
Strengths of the study include the prospective, randomized design of the trial and the analytic approaches used. We acknowledge potential limitations. Although limiting the analysis to the per-protocol population provides an estimate of the difference in outcomes between successful treatments, it can introduce attrition bias and complicate generalization of findings to real-world situations where differences in feasibility of the treatment may impact success. There was a significant loss to follow-up over time, limiting confidence around survival estimates at later time points. Further, the overall number of deaths was relatively low. Because of the stochastic nature of CMV replication, episodes of DNAemia could have been missed, leading to misclassification since CMV DNAemia measurements were limited to 2 time points. However, because of the randomized trial study design, there would have had to be differential misclassification between arms to affect the results, which seems unlikely. Additionally, these results in liver transplant may not be generalizable to other solid organ transplant recipients, although similar findings have been reported in other organ transplant settings [9–11].
In summary, this post hoc analysis of a multicenter, randomized trial of PET vs AP for CMV disease prevention demonstrated improved long-term survival with PET in high-risk CMV D + R– LTxRs and an association of CMV DNAemia with worse long-term survival. These findings identify testable hypotheses and provide compelling rationale for a large head-to-head trial of PET vs AP that includes long-term follow-up for mortality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Lakshin Kumar, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Sayan Dasgupta, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Cristina Murray-Krezan, Division of General Internal Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Nina Singh, Department of Medicine, VA Pittsburgh Healthcare System and University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Robert M Rakita, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Cynthia E Fisher, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Ajit P Limaye, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Notes
Financial support. This work was funded by the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH; grant U01 AI63098 to C. E. F and A. P. L.).
References
- 1. Singh N, Winston DJ, Razonable RR, et al. Effect of preemptive therapy vs antiviral prophylaxis on cytomegalovirus disease in seronegative liver transplant recipients with seropositive donors: a randomized clinical trial. JAMA 2020; 323:1378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med 2018; 6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Austin PC, Latouche A, Fine JP. A review of the use of time-varying covariates in the Fine-Gray subdistribution hazard competing risk regression model. Stat Med 2020; 39:103–13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mumtaz K, Faisal N, Husain S, Morillo A, Renner EL, Shah PS. Universal prophylaxis or preemptive strategy for cytomegalovirus disease after liver transplantation: a systematic review and meta-analysis. Am J Transplant 2015; 15:472–81. [DOI] [PubMed] [Google Scholar]
- 5. Yadav DK, Adhikari VP, Yadav RK, et al. Antiviral prophylaxis or preemptive therapy for cytomegalovirus after liver transplantation?: a systematic review and meta-analysis. Front Immunol 2022; 13:953210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spinner ML, Saab G, Casabar E, Bowman LJ, Storch GA, Brennan DC. Impact of prophylactic versus preemptive valganciclovir on long-term renal allograft outcomes. Transplantation 2010; 90:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis 2016; 214 Suppl 2(Suppl 2):S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pickering H, Sen S, Arakawa-Hoyt J, et al. NK and CD8+ T cell phenotypes predict onset and control of CMV viremia after kidney transplant. JCI Insight 2021; 6:e153175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Selvey LA, Lim WH, Boan P, et al. Cytomegalovirus viraemia and mortality in renal transplant recipients in the era of antiviral prophylaxis. Lessons from the western Australian experience. BMC Infect Dis 2017; 17:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 2016; 3:e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith JM, Corey L, Bittner R, et al. Subclinical viremia increases risk for chronic allograft injury in pediatric renal transplantation. J Am Soc Nephrol 2010; 21:1579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.