Abstract
Background
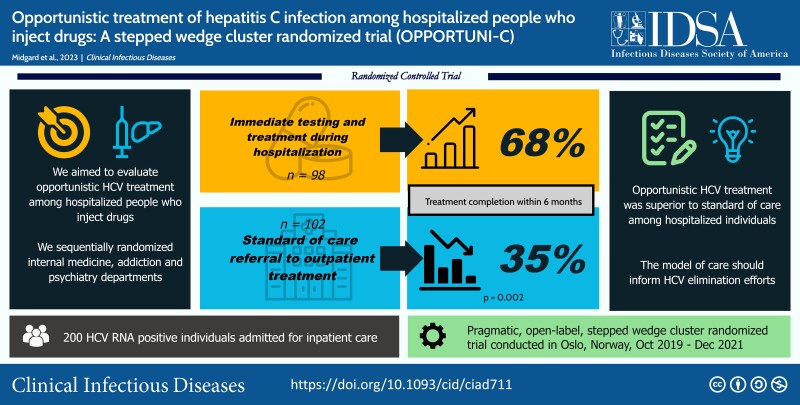
We aimed to evaluate the efficacy of opportunistic treatment of hepatitis C virus (HCV) infection among hospitalized people who inject drugs (PWID).
Methods
We performed a pragmatic, stepped wedge cluster randomized trial recruiting HCV RNA positive individuals admitted for inpatient care in departments of internal medicine, addiction medicine, and psychiatry at three hospitals in Oslo, Norway. Seven departments were sequentially randomized to change from control conditions (standard of care referral to outpatient care) to intervention conditions (immediate treatment initiation). The primary outcome was treatment completion, defined as dispensing the final package of the prescribed treatment within six months after enrolment.
Results
A total of 200 HCV RNA positive individuals were enrolled between 1 October 2019 and 31 December 2021 (mean age 47.4 years, 72.5% male, 60.5% injected past 3 months, 20.4% cirrhosis). Treatment completion was accomplished by 67 of 98 (68.4% [95% confidence interval {CI}: 58.2–77.4]) during intervention conditions and by 36 of 102 (35.3% [95% CI: 26.1–45.4]) during control conditions (risk difference 33.1% [95% CI: 20.0–46.2]; risk ratio 1.9 [95% CI: 1.4–2.6]). The intervention was superior in terms of treatment completion (adjusted odds ratio [aOR] 4.8 [95% CI: 1.8–12.8]; P = .002) and time to treatment initiation (adjusted hazard ratio [aHR] 4.0 [95% CI: 2.5–6.3]; P < .001). Sustained virologic response was documented in 60 of 98 (61.2% [95% CI: 50.8–70.9]) during intervention and in 66 of 102 (64.7% [95% CI: 54.6–73.9]) during control conditions.
Conclusions
An opportunistic test-and-treat approach to HCV infection was superior to standard of care among hospitalized PWID. The model of care should be considered for broader implementation.
Clinical Trials Registration. NCT04220645
Keywords: hepatitis C virus, people who inject drugs, model of care, pragmatic clinical trial, stepped wedge cluster randomized trial
Opportunistic HCV treatment among hospitalized people who inject drugs was superior to a referral-based standard of care in terms of treatment initiation and completion. The intervention was effective in all subgroups. Mortality was driven by liver cancer and renal failure.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/opportunistic-treatment-of-hepatitis-c-infection-among-hospitalized-people-who-inject-drugs-opportuni-c-a-stepped-wedge-cluster-randomized-trial
(See the Editorial Commentary by Rowan and Wyles on pages 591–3.)
In Western Europe and North America 65%–80% of the hepatitis C virus (HCV) disease burden is attributable to injecting drug use [1]. People who inject drugs (PWID) therefore represent a priority population for testing and treatment to reach the World Health Organization's goal of eliminating HCV infection as a major public health threat within 2030 [2].
Despite direct-acting antiviral (DAA) therapy being safe and effective among PWID [3–9], treatment uptake in this population remains suboptimal [10, 11]. One of the critical obstacles to HCV care among PWID is the lack of treatment models adapted to marginalized individuals [12]. The current standard of care, involving referral of patients to specialist care at hospital outpatient clinics, is of limited value due to lack of retention in care [13, 14].
Although PWID are at high risk of hospitalization for skin and soft tissue infections and other drug-related harms [15, 16], hospital admissions are not sufficiently utilized for HCV testing and treatment [17]. An almost 4-fold increased risk of all-cause hospitalization has been shown among people with HCV infection in the United States [18], further highlighting the potential role of hospitalization as a venue for HCV treatment. Except for one small observational study reporting 66% treatment uptake among eligible inpatients in Australia [19] and one qualitative study addressing the concept [20], no study has assessed opportunistic HCV treatment among hospitalized individuals.
OPPORTUNI-C aimed to evaluate the efficacy of immediate testing and treatment of HCV infection among PWID admitted for inpatient care in internal medicine, addiction medicine, and psychiatry departments. To evaluate this intervention at the level of health service delivery, we used a stepped wedge cluster randomized trial design [21]. This design was chosen to facilitate a gradual and “naturalistic” implementation and to avoid contamination of the intervention and disappointment effects in unexposed clusters. We hypothesized that hospitalizations represent opportunities to engage PWID in HCV care more effectively than a referral-based standard of care.
METHODS
Study Design
OPPORTUNI-C was a pragmatic, open-label, multicenter, stepped wedge cluster randomized trial. The design involved a sequential rollout of the intervention over 8 time periods. Seven departments (clusters) of internal medicine (n = 3), addiction medicine (n = 2), and psychiatry (n = 2) at 3 hospitals in Oslo, Norway, were assigned to change from control (standard of care) to intervention conditions in a random order until all clusters were exposed to the intervention (Supplementary Figure 1). The study protocol has been published previously [22] and a full methods description is available in the Supplementary materials and the Statistical Analysis Plan. The study was registered with ClinicalTrials.gov (NCT04220645) on 1 October 2019.
The trial commenced on 1 October 2019, and the planned duration of each period was 2 months. Enrollment was affected by the coronavirus disease 2019 (COVID-19) pandemic, and the trial was temporarily stopped for 1 month during the first Norwegian lockdown in April 2020. As subsequent enrollment was almost halved, we increased the duration of the remaining 5 periods to reach the recruitment target.
The study was approved by the Regional Committee for Medical Research Ethics in Norway on 3 March 2019 (reference number 2019–128). The study was conducted according to the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guideline. Written, informed consent was obtained from all participants.
Participants
Participant inclusion criteria were (1) age > 18 years, (2) current HCV infection, defined as detectable HCV RNA, (3) admitted for inpatient care in one of the clusters, and (4) able to provide informed written consent. Participants were ineligible only if they (1) had ongoing HCV treatment, (2) were pregnant or breastfeeding, or (3) did not provide or withdrew their consent.
Screening for HCV infection was done according to usual practice and as soon as possible after admission. Following identification of any HCV RNA positive individual, the local microbiology department alerted a local investigator who obtained informed consent and facilitated enrollment in cooperation with the clinical staff.
Randomization
Allocation was computer-generated and stratified according to expected cluster size (small, medium, large) to keep high HCV prevalence clusters separated regarding the timing of the intervention. The sequences were prepared by a statistician not involved in enrollment and kept in closed opaque envelopes. Concealment of a new step in the sequence was made available to the researchers on the day of transition and immediately disclosed to the clinical staff at the relevant cluster.
Procedures
During intervention conditions, all participants were offered immediate HCV assessment and treatment initiation during hospitalization or as soon as possible after discharge. The intervention was delivered by the local investigator in cooperation with the responsible inpatient physician. Briefly, it comprised the following components: (1) Liver disease staging based on transient elastography or FIB-4 index; (2) Pre-treatment counseling at the discretion of the treating physician; (3) DAA treatment initiation following Norwegian HCV treatment recommendations, typically with oral fixed-dose pan-genotypic combinations sofosbuvir/velpatasvir for 12 weeks or glecaprevir/pibrentasvir for 8 weeks; (4) Individualized follow-up at the discretion of the treating physician, with support from the local low-threshold HCV clinic [9] or other facilities, as needed.
During control conditions, all enrolled participants were referred for outpatient HCV care following discharge in accordance with the established standard of care for hospitalized individuals.
Participants did not complete a conventional case report form, but key background variables were summarized in a standardized inclusion template in the electronic patient files at enrollment.
Outcomes
The primary outcome was treatment completion, defined as dispensing the final 4-week package of the prescribed DAAs from the pharmacy within 6 months after enrollment. Failure to accomplish the primary outcome was noted either if no treatment had been dispensed (ie, loss to follow-up or other reasons), if treatment had been dispensed but completed later than six months after enrolment (ie, delayed treatment), or if the final package had not been dispensed (ie, treatment discontinuation).
Secondary outcomes were treatment initiation and sustained virologic response (SVR). Treatment initiation was defined as dispensing the first package of DAAs within 6 months after enrolment. SVR was defined as undetectable HCV RNA at least 4 weeks after the estimated date of end of treatment (SVR ≥ 4). Failure to achieve SVR was noted either if HCV RNA was detectable following end of treatment (ie, virologic failure), if no samples were available for SVR assessment (ie, loss to follow-up), or if no DAAs were dispensed (ie, no treatment).
Data on treatment completion and treatment initiation were extracted retrospectively by review of the “core medical record” in the electronic patient files 6 months after enrolment of the final participant. This record contains complete prescription and dispensation data from pharmacies nationwide within the previous 3 years. Data on SVR, baseline variables, and causes of death were obtained by retrospective review of the electronic hospital files and microbiology files from local and collaborating laboratories. No measures of adherence or records of protocol deviations were recorded.
Statistical Methods
To show a 30% difference in effect size (60% intervention vs 30% control) for the primary outcome, with 85% power and 5% significance level, assuming a large intra-cluster correlation coefficient of 0.2, we planned to recruit on average 4 participants per cluster per period for a total of 224 participants (Supplementary Table 1).
Trial data are reported as mean (SD), median (interquartile range), or N (%) as appropriate. Data analysis followed an intention-to-treat principle according to cluster allocation regardless of what occurred, with no account of protocol non-adherence.
Outcomes are reported as proportions, risk differences, and risk ratios with 95% exact confidence intervals (CI). We analyzed treatment completion using mixed-effects logistic regression adjusted for calendar time with cluster as random effect, according to the Hussey and Hughes model [23]. We analyzed treatment initiation using Cox regression adjusted for calendar time with cluster as a shared frailty factor [24]. Time at risk for each participant was from the date of enrolment until the date of treatment, death, or 6 months after enrollment, whatever came first. Effect estimates are reported as adjusted odds ratios (aOR) or adjusted hazard ratios (aHR), and superiority of the intervention is claimed if a 2-sided P- value under the null hypothesis is < .05 in favor of the intervention.
We performed subgroup analyses using intervention × subgroup interaction according to pre-specified variables and post hoc analyses of mortality using Cox regression. As robustness analyses, we did a permutation test for the primary outcome with 10 000 random permutations of the cluster allocation [25] and analyzed the secondary outcome using a clustered sandwich estimator [26].
All analyses were performed using STATA 17 (College Station, Texas, USA) with the sample size calculation using the steppedwedge package [27].
RESULTS
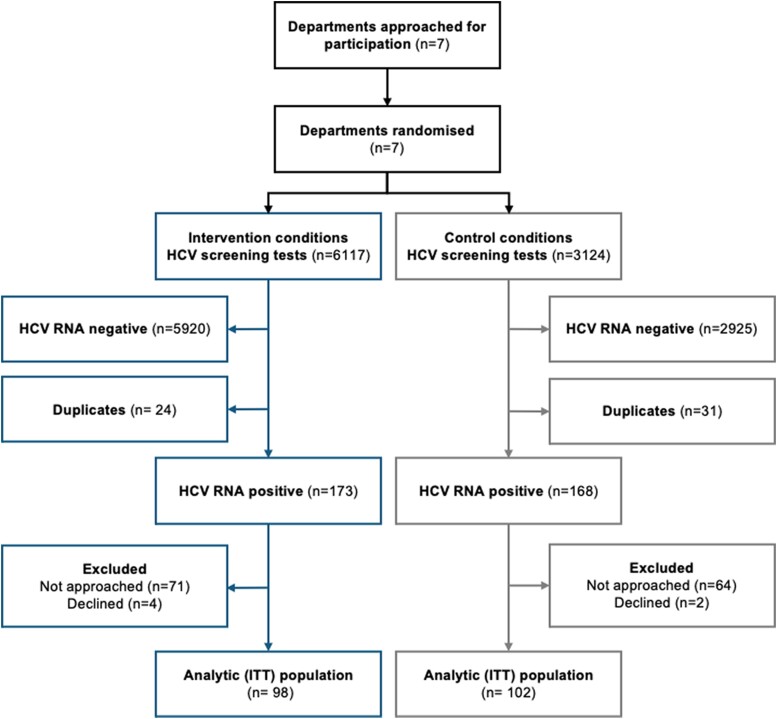
Between 1 October 2019 and 31 December 2021, 200 individuals were enrolled in clusters of internal medicine (n = 107), addiction medicine (n = 65), and psychiatry (n = 28). A total of 9241 HCV screening tests were performed over the 8 time periods of the trial (Supplementary Table 2). Of 341 HCV RNA positive individuals identified, 141 (41.3%) were not included; 135 were not approached due to early discharge before potential enrolment, 6 were not willing to participate, whereas none were ineligible due to ongoing HCV treatment or pregnancy/breastfeeding (Figure 1). Despite stable screening activity, viremic rates and enrollment rates declined during the trial (Supplementary Figures 2 and 3).
Figure 1.
Flow of clusters and participants in OPPORTUNI-C by intervention condition. Abbreviations: HCV, hepatitis C virus; ITT, intention to treat.
Baseline characteristics were similar during intervention and control conditions (Table 1) and throughout the trial (Supplementary Table 3). The mean age was 47.4 years, the majority were male, and most had injected drugs in the previous 3 months. Liver cirrhosis was diagnosed in one-fifth of participants, and hepatocellular carcinoma was detected in 9 participants. Individuals enrolled in internal medicine were older and had more comorbidities than those enrolled in addiction medicine and psychiatry (Supplementary Table 4). The spectrum of discharge diagnoses is shown in Supplementary Table 5.
Table 1.
Baseline Characteristics Summarized by Total and Intervention Condition
| Variable | Total (n = 200) |
Intervention (n = 98) |
Control (n = 102) |
|---|---|---|---|
| Age, mean (SD) | 47.4 (12.7) | 48.0 (13.0) | 46.8 (12.5) |
| Age groups | |||
| 20–29 | 21 (10.5) | 11 (11.2) | 10 (9.8) |
| 30–39 | 40 (20.0) | 16 (16.3) | 24 (23.5) |
| 40–49 | 51 (25.5) | 21 (21.4) | 30 (29.4) |
| 50–59 | 52 (26.0) | 33 (33.7) | 19 (18.6) |
| 60–80 | 36 (18.0) | 17 (17.4) | 19 (18.6) |
| Sex | |||
| Male | 145 (72.5) | 69 (70.4) | 76 (74.5) |
| Female | 55 (27.5) | 29 (29.6) | 26 (25.5) |
| Housing status | |||
| Rented/owned accommodation | 124 (62.0) | 64 (65.3) | 60 (58.8) |
| Drug rehabilitation institution | 10 (5.0) | 7 (7.1) | 3 (2.9) |
| Low-threshold institution | 28 (14.0) | 10 (10.2) | 18 (17.7) |
| Prison | 1 (0.5) | 0 (0) | 1 (1.0) |
| Homeless/on the street | 37 (18.5) | 17 (17.4) | 20 (19.6) |
| Source of incomea | |||
| Part- or full-time job | 26 (13.1) | 14 (14.3) | 12 (11.9) |
| Welfare pension | 116 (58.6) | 61 (62.9) | 55 (54.5) |
| Social | 52 (26.3) | 20 (20.6) | 32 (31.7) |
| Other | 4 (2.0) | 2 (2.1) | 2 (2.0) |
| History of injecting drug use | |||
| Yes | 183 (91.5) | 86 (87.8) | 97 (95.1) |
| No | 17 (8.5) | 12 (12.2) | 5 (4.9) |
| Recent (past 3 m) injecting drug use | |||
| Yes | 121 (60.5) | 58 (59.2) | 63 (61.8) |
| No | 79 (39.5) | 40 (40.8) | 39 (38.2) |
| Recent sharing of injecting equipmentb | |||
| Yes | 34 (28.1) | 13 (22.4) | 21 (33.3) |
| No | 54 (44.6) | 30 (51.7) | 24 (38.1) |
| Unknown | 33 (27.3) | 15 (24.9) | 18 (28.6) |
| Preferred injected drugc | |||
| Heroin | 114 (64.8) | 54 (64.3) | 60 (65.2) |
| Amphetamines | 51 (29.0) | 25 (29.8) | 26 (28.3) |
| Other/mixed | 11 (6.3) | 5 (6.0) | 6 (6.5) |
| Current opioid agonist therapy | |||
| Yes | 90 (45.0) | 38 (38.8) | 52 (51.0) |
| No | 110 (55.0) | 60 (61.2) | 50 (49.0) |
| Opioid agonist therapy drugd | |||
| Methadone | 48 (53.3) | 21 (55.3) | 27 (51.9) |
| Buprenorphine | 36 (40.0) | 14 (36.8) | 22 (42.3) |
| Buprenorphine-naloxone | 2 (2.2) | 0 (0) | 2 (3.9) |
| Other | 4 (4.4) | 3 (7.9) | 1 (1.9) |
| Stage of liver diseasee | |||
| Mild or no liver fibrosis | 102 (52.0) | 51 (52.0) | 51 (52.0) |
| Intermediate fibrosis | 54 (27.6) | 25 (25.5) | 29 (29.6) |
| Compensated cirrhosis | 21 (10.7) | 14 (14.3) | 7 (7.1) |
| Decompensated cirrhosis | 19 (9.7) | 8 (8.2) | 11 (11.2) |
| FIB-4 index, mean (SD)f | 2.72 (5.95) | 2.85 (7.69) | 2.60 (3.43) |
| Hepatocellular carcinoma | |||
| Yes | 9 (4.5) | 5 (5.1) | 4 (3.9) |
| No or not assessed | 191 (95.5) | 93 (94.9) | 98 (96.1) |
| Renal function | |||
| eGFR >60 mL/min/1.73 m2 | 188 (94.0) | 89 (90.8) | 99 (97.1) |
| eGFR 30–59 mL/min/1.73 m2 | 8 (4.0) | 6 (6.1) | 2 (2.0) |
| eGFR <30 mL/min/1.73 m2 | 4 (2.0) | 3 (3.1) | 1 (1.0) |
| HIV coinfection | |||
| Yes | 6 (3.0) | 3 (3.1) | 3 (2.9) |
| No | 169 (84.5) | 86 (87.8) | 83 (81.4) |
| Not assessed | 25 (12.5) | 9 (9.2) | 16 (15.7) |
| HBV coinfection (HBsAg+) | |||
| Yes | 1 (0.5) | 1 (1.0) | 0 (0) |
| No | 180 (90.0) | 93 (94.9) | 87 (85.3) |
| Not assessed | 19 (9.5) | 4 (4.1) | 15 (14.7) |
| HCV genotype | |||
| Genotype 1 | 47 (23.5) | 16 (16.3) | 31 (30.4) |
| Genotype 2 | 7 (3.5) | 1 (1.0) | 6 (5.9) |
| Genotype 3 | 48 (24.0) | 20 (20.4) | 28 (27.5) |
| Genotype 4–6 | 6 (3.0) | 2 (2.0) | 4 (3.9) |
| Not genotyped | 92 (46.0) | 59 (60.2) | 33 (32.4) |
| Days of hospitalization, median (IQR) | 6 (3–13) | 5 (2–13) | 7 (4–13) |
| Main discharge diagnosis | |||
| Drug related | 93 (46.5) | 39 (39.8) | 54 (52.9) |
| Infectious diseases | 33 (16.5) | 22 (22.5) | 11 (10.8) |
| Gastroenterology/hepatology | 24 (12.0) | 10 (10.2) | 14 (13.7) |
| Mental health | 15 (7.5) | 9 (9.2) | 6 (5.9) |
| Cardiopulmonary | 14 (7.0) | 8 (8.2) | 6 (5.9) |
| Alcohol related | 7 (3.5) | 4 (4.1) | 3 (2.9) |
| Other | 14 (7.0) | 6 (6.1) | 8 (7.8) |
| Charlson comorbidity index | |||
| 0–1 | 101 (50.5) | 41 (41.8) | 60 (58.2) |
| 2–3 | 43 (21.5) | 30 (30.6) | 13 (12.8) |
| 4–5 | 33 (16.5) | 16 (16.3) | 17 (16.7) |
| ≥6 | 23 (11.5) | 11 (11.2) | 12 (11.8) |
| Charlson comorbidity index, mean (SD) | 2.6 (2.3) | 2.8 (2.4) | 2.5 (2.2) |
Numbers are shown as n (%) unless otherwise indicated. Missing values are excluded from percentages.
Abbreviations: FIB-4, fibrosis-4; GFR, glomerular filtration rate; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation.
aMissing data for 2 participants (1 intervention, 1 control).
bAmong those with recent (past 3 m) injecting drug use.
cAmong those with a history of injecting drug use; missing data for 7 participants (2 intervention, 5 control).
dAmong those with current opioid agonist therapy.
eBased on liver stiffness measurements in 86, FIB-4 index in 107, and imaging in 3 participants; missing data for 4 control participants.
fAmong 193 participants with an available FIB-4 index.
Treatment completion within 6 months (Table 2) was accomplished by 67 of 98 (68.4% [95% CI: 58.2–77.4]) during intervention conditions and by 36 of 102 (35.3% [95% CI: 26.1–45.4]) during control conditions (risk difference 33.1% [95% CI: 20.0–46.1]; risk ratio 1.9 [95% CI: 1.4–2.6]). In mixed-effects logistic regression, the intervention was superior to standard of care (aOR 4.8 [95% CI: 1.8–12.8]; P = .002) with no significant effect of secular trends (aOR 1.0 [95% CI: .95–1.06]). Cluster effects were moderate (SD 0.16 [95% CI: .01–1.69]) with an intra-cluster correlation coefficient of 0.046 (95% CI: .005–.339). The permutation test confirmed the result (P = .008).
Table 2.
Overview of Primary and Secondary Outcomes Summarized by Total and Intervention Condition
| Total (n = 200) |
Intervention (n = 98) | Control (n = 102) |
Risk Difference | Risk Ratio (95% CI) | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Treatment completion within 6 months | |||||
| N | 103 | 67 | 36 | ||
| % (95% CI) | 51.5 (44.3–58.6) | 68.4 (58.2–77.4) | 35.3 (26.1–45.4) | 33.1 (20.0–46.1) | 1.9 (1.4–2.6) |
| Failure to accomplish completion, n (%) | |||||
| Delayed treatment initiation | 39 (19.5) | 7 (7.1) | 32 (31.4) | NA | NA |
| Treatment discontinuation | 13 (6.5) | 10 (10.2) | 3 (2.9) | ||
| Short life expectancy or death | 15 (7.5) | 6 (6.1) | 9 (8.8) | ||
| Declined treatment | 2 (1.0) | 1 (1.0) | 1 (1.0) | ||
| Prolonged treatment | 1 (0.5) | 1 (1.0) | 0 (0) | ||
| Loss to follow-up | 27 (13.5) | 6 (6.1) | 21 (20.6) | ||
| Secondary outcomes | |||||
| Treatment initiation within 6 months | |||||
| n | 131 | 84 | 47 | ||
| % (95% CI) | 65.5 (58.5–72.1) | 85.7 (77.2–92.0) | 46.1 (36.2–56.2) | 39.6 (27.7–51.5) | 1.9 (1.5–2.3) |
| Treatment initiation within data lock | |||||
| n | 159 | 87 | 72 | ||
| % (95% CI) | 79.5 (73.2–84.9) | 88.8 (80.8–94.3) | 70.6 (60.7–79.2) | 18.2 (7.4–29.0) | 1.3 (1.1–1.5) |
| SVR ≥4 | |||||
| n | 126 | 60 | 66 | ||
| % (95% CI) | 63.0 (55.9–69.7) | 61.2 (50.8–70.9) | 64.7 (54.6–73.9) | −3.5 (−16.9–9.9) | 0.95 (.76–1.2) |
| SVR ≥12 | |||||
| n | 104 | 51 | 53 | ||
| % (95% CI) | 52.0 (44.8–59.1) | 52.0 (41.7–62.2) | 52.0 (41.8–62.0) | 0.0 (−0.14–0.14) | 1.00 (.77–1.3) |
| Failure to accomplish SVR ≥4, n (%) | |||||
| Virologic failure | 8 (4.0) | 6 (6.1) | 2 (2.0) | NA | NA |
| Loss to follow-up (missing SVR data) | 25 (12.5) | 21 (21.4) | 4 (3.9) | ||
| No treatment | 41 (20.5) | 11 (11.2) | 30 (29.4) |
Abbreviations: CI, confidence interval; NA, not applicable; SVR, sustained virologic response.
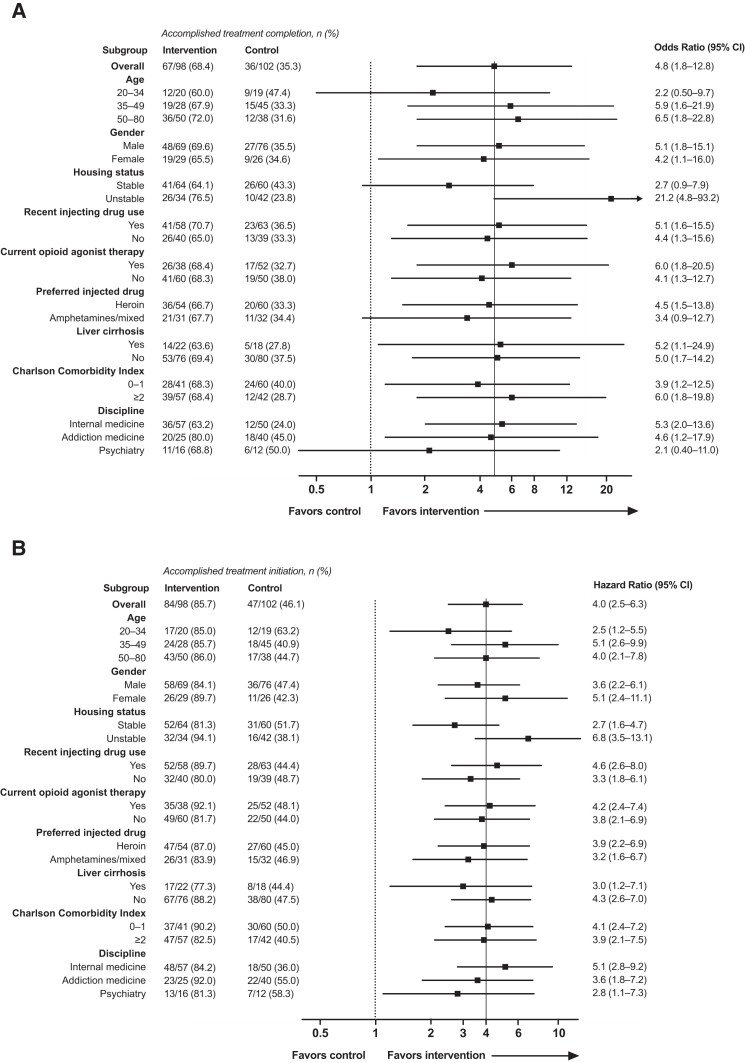
Retention in care at 6 months was higher during intervention than during control conditions but the difference was less prominent at data lock (Supplementary Figure 4). Most received pan-genotypic treatment with sofosbuvir/velpatasvir (55.3%) or glecaprevir/pibrentasvir (26.2%), and one-half of treatments during intervention conditions were self-administered without aid from other services (Supplementary Table 6). Subgroup analysis (Figure 2A) indicated a homogeneous intervention effect but with a signal that the intervention had a stronger impact among those with unstable housing (interaction P = .004).
Figure 2.
Subgroup analyses of (A) the primary outcome (treatment completion within 6 months) and (B) the secondary outcome (treatment initiation within 6 months). Abbreviation: CI, confidence interval.
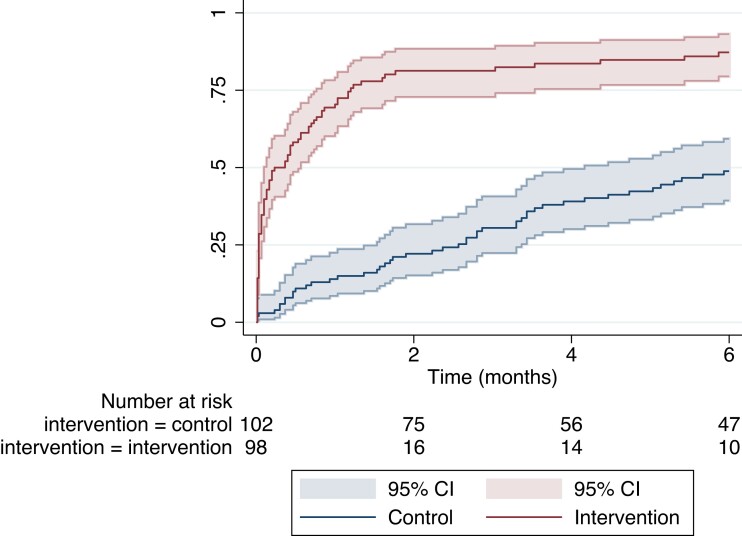
Treatment initiation within 6 months (Table 2; Supplementary Table 6) was accomplished by 84 of 98 (85.7% [95% CI: 77.2–92.0]) during intervention conditions and by 47 of 102 (46.1% [95% CI: 36.2–56.2]), during control conditions (risk difference 39.6% [95% CI: 27.7–51.5]; risk ratio 1.9 [95% CI: 1.5–2.3]). The hazard of treatment (Figure 3) was significantly higher during intervention compared to control conditions (aHR 4.0 [95% CI: 2.5–6.3]; P < .001) with no significant effect of secular trends (aHR 1.0; 95% CI: .98–1.03). Robustness analysis confirmed the result (aHR 4.0 [95% CI: 2.4–6.5]; P < .001). The intervention effect was homogeneous across subgroups (Figure 2B).
Figure 3.
Kaplan–Meier plot of time to treatment initiation. Red lines represent the proportion of participants in intervention conditions and blue lines represent the proportion of participants in control conditions. Shaded areas indicate 95% confidence intervals.
SVR ≥4 (Table 2) was documented in 60 of 98 (61.2% [95% CI: 50.8–70.9]) during intervention conditions and in 66 of 102 (64.7% [95% CI: 54.6–73.9]) during control conditions (risk difference −3.5% [95% CI: −16.9–9.9]; risk ratio 0.95 [95% CI: .76–1.2]). Failure to achieve SVR was largely explained by loss to follow-up (ie, missing data) during intervention and by lack of treatment during control conditions (Table 2). Among 141 participants with complete dispensation within data lock, 120 (85.1%) achieved SVR ≥4 (Supplementary Table 7).
Post hoc analysis of mortality (Supplementary Table 8) showed that 24 participants (12.0%) died during the study period. The main causes of death were liver-related (n = 7) or due to end-stage renal disease (n = 6), and no deaths were related to HCV treatment. Of 9 participants with hepatocellular carcinoma, 6 were advanced cases who died within 2 months after enrollment. There was a trend of higher mortality during intervention (11.5/100 PY [95% CI: 6.3–19.2]) compared to control conditions (4.8/100 PY [95% CI: 2.3–8.9]), but Cox regression showed no significant effect of the intervention (aHR 2.0; 95% CI: .7–5.5; P = .19) and no significant effect of secular trends (aHR 1.0; 95% CI: .93–1.1; P = .89).
DISCUSSION
Opportunistic HCV treatment among hospitalized PWID was superior to a referral-based standard of care in terms of treatment completion and treatment initiation. The results could change clinical practice and health policy internationally and should inform HCV elimination efforts among PWID.
Treatment efficacy was lower than in previous studies of HCV treatment among PWID [3–9, 28]. Our results are more in line with a recent trial from the United States, reporting rates of treatment initiation, completion, and SVR of 83%, 68%, and 61% in intention-to-treat analysis [29]. This probably reflects the pragmatic features of both trials, enabling recruitment of more marginalized individuals than in previous studies. In the present study, failure to accomplish the primary outcome was largely explained by loss to follow-up and delayed treatments, and consistent with literature [3], rates of treatment discontinuation were low. Although the benefit of early treatment was limited by relatively low SVR, it could be explained by a higher proportion of missing data during intervention conditions.
The superiority of the intervention was driven by a considerably shorter time to treatment during intervention conditions. This is of clinical and public health significance because persisting viraemia can lead to onward HCV transmission among those with ongoing risk behaviors. Although treatment uptake was slower during control conditions, it increased after the protocol-specified 6 months follow-up and toward data lock. We observed that these individuals had often been engaged in low-threshold HCV treatment in the City of Oslo and had not received specialist care at the hospital outpatient clinics as planned. The intervention effect may therefore be underestimated compared to settings without access to similar low-threshold care.
Subgroup analysis also favored the intervention among the most marginalized individuals. Notably, the intervention seemed more effective among those with unstable housing. For individuals at risk of loss to outpatient follow-up due to homelessness (ie, lack of contact address), the intervention may have enabled retention in care by linkage to low-threshold facilities.
Despite stable screening rates, viremic rates declined in all clusters during the trial, particularly following the COVID-19 pandemic. Due to the natural dominance of intervention observations arising from later calendar times inherent to the stepped wedge design, the viremic prevalence was 50% lower during intervention conditions than during control conditions. Although the declining rates could be attributed to the pandemic, it may also reflect the marked decline in HCV RNA prevalence reported among PWID in Oslo [30].
Mortality was higher than reported in previous HCV treatment studies among PWID [3], which largely have comprised of community dwelling individuals recruited from the outpatient setting. Mortality was mainly driven by underlying chronic diseases, reflecting recruitment of an acutely hospitalized and ageing population with a high prevalence of advanced liver disease and renal disease. The potential trend in increased mortality during intervention conditions could be explained by the small sample and 3 cases of suicide in the intervention group but also due to recruitment of an increasingly marginalized population in a period where Norway was approaching HCV elimination.
To our knowledge, this is the first controlled study to evaluate an opportunistic HCV treatment model among hospitalized individuals. Key strengths relate to the pragmatic features of the trial, including (1) broad recruitment of marginalized individuals, (2) the use of clinical infrastructures with minimal research-specific frameworks, (3) extraction of routinely collected data without the need for individual follow-up, and (4) an intention-to-treat principle for data analysis. Although the stepped wedge design is unconventional with numerous methodological complexities, it is a pragmatic design considered appropriate for evaluation of health delivery interventions with political, logistical, and statistical advantages over an individual-randomized or a parallel cluster randomized design [21].
Together, these features have ensured representativeness of the study population, eliminated the impact of loss to follow-up and generated optimal conditions for generalizability at a low cost. Taken a step further, the approach could serve as a model for addressing other health problems among PWID opportunistically. However, the intervention relied on the unrestricted access to DAAs across healthcare settings that is available in Norway. Implementation would be more difficult in countries where treatment access is restricted by health insurance authorization and in countries where hospital formularies may be restricted to selected medications.
The trial had several limitations. First, because recruitment was done with study personnel and participants knowing the treatment allocation, potentially influencing screening activity and participation, the trial is at risk of selection bias between the intervention conditions. Second, the stepped wedge design is associated with potential confounding with time, an effect that may have been augmented by the COVID-19 pandemic and subsequent trial prolongation. However, we found no evidence that underlying secular trends had influenced the intervention effect. Third, the estimated effect sizes are imprecise. This could have been improved by increasing the number of participating clusters instead of increasing cluster size. Finally, the primary outcome remains a proxy. However, our data largely validate registry-based proxies as a pragmatic correlate for cure in marginalized populations. Given that good results of DAA treatment have been shown with suboptimal adherence [6, 31] or treatment shortened to four weeks [32], we expect that high SVR rates have been achieved also among those with missing data. Thus, virologic cure is probably underestimated among intervention participants.
In conclusion, we provide evidence that opportunistic HCV treatment is superior to a referral-based standard of care among hospitalized individuals. Hospitalizations should be utilized for testing and treatment of HCV infection and the model of care could represent a key strategy moving forward in the global response to the HCV epidemic.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Håvard Midgard, Department of Gastroenterology, Oslo University Hospital, Oslo, Norway; Department of Infectious Diseases, Akershus University Hospital, Lørenskog, Norway.
Kristian Braathen Malme, Department of Infectious Diseases, Akershus University Hospital, Lørenskog, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Charlotte Meinich Pihl, Department of Medicine, Lovisenberg Diaconal Hospital, Oslo, Norway; Unger-Vetlesen Institute, Lovisenberg Diaconal Hospital, Oslo, Norway.
Riikka Mari Berg-Pedersen, Department of Addiction Medicine, Oslo University Hospital, Oslo, Norway.
Lars Tanum, Department for Research and Development in Mental Health, Akershus University Hospital, Lørenskog, Norway; Faculty of Health Sciences, Oslo Metropolitan University, Oslo, Norway.
Ingvild Klundby, Department of Microbiology, Oslo University Hospital, Oslo, Norway.
Anne Haug, Department of Acute Medicine, Oslo University Hospital, Oslo, Norway.
Ida Tveter, Department of Infectious Diseases, Oslo University Hospital, Oslo, Norway.
Ronny Bjørnestad, ProLAR Nett, Søgne, Norway.
Inge Christoffer Olsen, Department of Research Support for Clinical Trials, Oslo University Hospital, Oslo, Norway.
Ane-Kristine Finbråten, Department of Medicine, Lovisenberg Diaconal Hospital, Oslo, Norway; Unger-Vetlesen Institute, Lovisenberg Diaconal Hospital, Oslo, Norway.
Olav Dalgard, Department of Infectious Diseases, Akershus University Hospital, Lørenskog, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Notes
Author Contributions. Conception and design: O. D., H. M., A.-K. F., I. C. O. Collection and assembly of data: H. M., O. D., A.-K. F., K. B. M., C. M. P., R. M., B.-P., A. H., I. T., I. K. Analysis and interpretation of data: H. M., O. D., A.-K. F., K. B. M., R. B. L. T., I. C. O.. Drafting of the article: H. M.. Critical revision for important intellectual content: H. M., O. D., A.-K. F., K. B. M., C. M. P., R. M. B.-P., A. H., I. T., L. T., I. K., R. B., I. C. O. Final approval of the article: H. M., O. D., A.-K. F., K. B. M., C. M. P., R. M. B.-P., A. H., I. T., L. T., I. K., R. B., I. C. O. Statistical expertise: I. C. O., H. M. Obtaining of funding: O. D., H. M., A.-K. F. Administrative, technical, or logistic support: A, U., H. R.. H. M., K. B. M., A.-K. F., and O. D. have directly accessed and verified the underlying data reported in the manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors had access to all the data and accept the responsibility to submit for publication. No compensation was provided to the authors. An earlier version of this manuscript was posted as a preprint on 8 December 2022 on the following SSRN sever: https://ssrn.com/abstract=4293865 or http://dx.doi.org/10.2139/ssrn.4293865
Acknowledgments. The authors thank all study participants for their contribution to research and wishes to acknowledge the following members of the study group:
Protocol steering committee: O. D., H. M., A.-K. F., K. B. M., L. T., R. B., I. C. O.
User involvement: R. B., proLAR Nett, Søgne.
Akershus University Hospital: O. D., H. M., K. B. M., L. T., H. R., M. H., K. B., L. Z., D. E. H., T. L., F. R., S. K., T. H. F.
Oslo University Hospital: H. M., A. H., I. T., R. M. B.-P., I. K., . S.-J., I. C. O., S. L. M., H. E., L. L., D. N., B.-M. B., A. L.
Lovisenberg Diaconal Hospital: A.-K. F., C. M. P., H. L., H. A., I. G., L. R., H. M. N., A. N., A. T., J. V., A. U.
Financial support. This work was supported by a research grant from the Southern and Eastern Norway Regional Health Authority (grant number 2020011). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
References
- 1. Degenhardt L, Charlson F, Stanaway J, et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis 2016; 16:1385–98. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021—accountability for the global health sector strategies 2016–2021: actions for impact. Available at: https://www.who.int/publications/i/item/9789240027077. 2021. Accessed on 17 Sept 2023.
- 3. Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2018; 3:754–67. [DOI] [PubMed] [Google Scholar]
- 4. Dore GJ, Altice F, Litwin AH, et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016; 165:625–34. [DOI] [PubMed] [Google Scholar]
- 5. Grebely J, Conway B, Cunningham EB, et al. Paritaprevir, ritonavir, ombitasvir, and dasabuvir with and without ribavirin in people with HCV genotype 1 and recent injecting drug use or receiving opioid substitution therapy. Int J Drug Policy 2018; 62:94–103. [DOI] [PubMed] [Google Scholar]
- 6. Grebely J, Dalgard O, Conway B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol 2018; 3:153–61. [DOI] [PubMed] [Google Scholar]
- 7. Akiyama MJ, Norton BL, Arnsten JH, Agyemang L, Heo M, Litwin AH. Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy: a randomized controlled trial. Ann Intern Med 2019; 170:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fadnes LT, Aas CF, Vold JH, et al. Integrated treatment of hepatitis C virus infection among people who inject drugs: a multicenter randomized controlled trial (INTRO-HCV). PLoS Med 2021; 18:e1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Midgard H, Ulstein K, Backe O, et al. Hepatitis C treatment and reinfection surveillance among people who inject drugs in a low-threshold program in Oslo, Norway. Int J Drug Policy 2021; 96:103165. [DOI] [PubMed] [Google Scholar]
- 10. Bartlett SR, Yu A, Chapinal N, et al. The population level care cascade for hepatitis C in British Columbia, Canada as of 2018: impact of direct acting antivirals. Liver Int 2019; 39:2261–72. [DOI] [PubMed] [Google Scholar]
- 11. Valerio H, Alavi M, Law M, et al. High hepatitis C treatment uptake among people with recent drug dependence in New South Wales, Australia. J Hepatol 2021; 74:293–302. [DOI] [PubMed] [Google Scholar]
- 12. Day E, Hellard M, Treloar C, et al. Hepatitis C elimination among people who inject drugs: challenges and recommendations for action within a health systems framework. Liver Int 2019; 39:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McDonald SA, Hutchinson SJ, Innes HA, et al. Attendance at specialist hepatitis clinics and initiation of antiviral treatment among persons chronically infected with hepatitis C: examining the early impact of Scotland's hepatitis C Action Plan. J Viral Hepat 2014; 21:366–76. [DOI] [PubMed] [Google Scholar]
- 14. Wade AJ, Doyle JS, Gane E, et al. Outcomes of treatment for hepatitis C in primary care, compared to hospital-based care: a randomized, controlled trial in people who inject drugs. Clin Infect Dis 2020; 70:1900–6. [DOI] [PubMed] [Google Scholar]
- 15. Wheeler A, Valerio H, Cunningham EB, et al. Prevalence and factors associated with hospitalisation for bacterial skin infections among people who inject drugs: the ETHOS Engage Study. Drug Alcohol Depend 2022; 237:109543. [DOI] [PubMed] [Google Scholar]
- 16. Hope VD, Ncube F, Parry JV, Hickman M. Healthcare seeking and hospital admissions by people who inject drugs in response to symptoms of injection site infections or injuries in three urban areas of England. Epidemiol Infect 2015; 143:120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valerio H, Alavi M, Law M, et al. Opportunities to enhance linkage to hepatitis C care among hospitalized people with recent drug dependence in New South Wales, Australia: a population-based linkage study. Clin Infect Dis 2021; 73:2037–44. [DOI] [PubMed] [Google Scholar]
- 18. Teshale EH, Xing J, Moorman A, et al. Higher all-cause hospitalization among patients with chronic hepatitis C: the Chronic Hepatitis Cohort Study (CHeCS), 2006–2013. J Viral Hepat 2016; 23:748–54. [DOI] [PubMed] [Google Scholar]
- 19. Chiong F, Post J. Opportunistic assessment and treatment of people with hepatitis C virus infection admitted to hospital for other reasons: a prospective cohort study. Int J Drug Policy 2019; 65:50–5. [DOI] [PubMed] [Google Scholar]
- 20. Levander XA, Vega TA, Seaman A, Korthuis PT, Englander H. Exploring how hospitalization can alter hepatitis C virus treatment prioritization and trajectories in people who use drugs: a qualitative analysis. Subst Abus 2022; 43:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ 2015; 350:h391. [DOI] [PubMed] [Google Scholar]
- 22. Midgard H, Finbraten AK, Malme KB, et al. Opportunistic treatment of hepatitis C virus infection (OPPORTUNI-C): study protocol for a pragmatic stepped wedge cluster randomized trial of immediate versus outpatient treatment initiation among hospitalized people who inject drugs. Trials 2020; 21:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hemming K, Taljaard M, Forbes A. Analysis of cluster randomised stepped wedge trials with repeated cross-sectional samples. Trials 2017; 18:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer, 2000. [Google Scholar]
- 25. Wang R, De Gruttola V. The use of permutation tests for the analysis of parallel and stepped-wedge cluster-randomized trials. Stat Med 2017; 36:2831–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc 1989; 84:1074–8. [Google Scholar]
- 27. Hemming K, Girling A. A menu-driven facility for power and detectable-difference calculations in Stepped-Wedge Cluster-Randomized Trials. Stata J 14 2014; 2:363–80. [Google Scholar]
- 28. Midgard H, Bjornestad R, Egeland M, et al. Peer support in small towns: a decentralized mobile hepatitis C virus clinic for people who inject drugs. Liver Int 2022; 42:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Litwin AH, Lum PJ, Taylor LE, et al. Patient-centered models of hepatitis C treatment for people who inject drugs: a multicentre, pragmatic randomised trial. Lancet Gastroenterol Hepatol 2022; 7:1112–27. [DOI] [PubMed] [Google Scholar]
- 30. Opheim E, Dalgard O, Ulstein K, et al. Towards elimination of hepatitis C in Oslo: Cross-sectional prevalence studies among people who inject drugs. Int J Drug Policy 2023; In Press. [DOI] [PubMed] [Google Scholar]
- 31. Norton BL, Akiyama MJ, Arnsten JH, Agyemang L, Heo M, Litwin AH. High HCV cure rates among people who inject drugs and have suboptimal adherence: a patient-centered approach to HCV models of care. Int J Drug Policy 2021; 93:103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ovrehus ALH, Krarup H, Birkemose I, et al. Four weeks of ledipasvir/sofosbuvir and ribavirin with or without pegylated interferon for chronic hepatitis C in non-cirrhotic people who inject drugs. A randomized trial. J Hepatol 2018; 68:840–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.