Abstract
The incidence and prevalence of non‐alcoholic fatty liver disease (NAFLD) have been steadily increasing worldwide, with a huge societal and economic burden. Recently, NAFLD and non‐alcoholic steatohepatitis have been renamed and redefined as metabolic dysfunction associated steatotic liver disease (MASLD) and steatohepatitis (Metabolic Dysfunction Associated Steatohepatitis (MASH)), which result from an imbalance between metabolic and inflammatory stress (mainly as a consequence of adipose tissue dysfunction and insulin resistance) and the defence and repair mechanisms of the steatotic liver. Once MASLD progresses to end‐stage of liver disease, treatment efficacy becomes limited and may require liver transplantation. Early detection and intervention are crucial. Lifestyle modification is consequently the cornerstone of its management. Timely consideration of bariatric surgeries should be given to patients meeting specific criteria. A multidisciplinary approach is warranted, starting from the concept that MASLD/MASH is at the centre of the cardiovascular‐liver‐metabolic syndrome. In some cases, pharmacological treatment can complement lifestyle modification. Several drugs used to treat the cardiometabolic co‐morbidities have some potential efficacy in slowing Down disease progression, and some have demonstrated efficacy on histological endpoints that are likely to translate into long‐term clinical benefits. Optimising the use of these drugs within their licenced indications is thus paramount for patients with MASLD. Several MASH‐specific drugs are on the horizon and are likely to enrich our therapeutic armamentarium in the near future, particularly in non‐cirrhotic stages of the disease. Much work still needs to be done to understand the specific features of MASH cirrhosis and develop efficacious treatments for this disease stage.
Keywords: management, MASH, MASLD, metabolic dysfunction associated steatotic liver disease, metabolic syndrome lifestyle, NAFLD, NASH, therapy
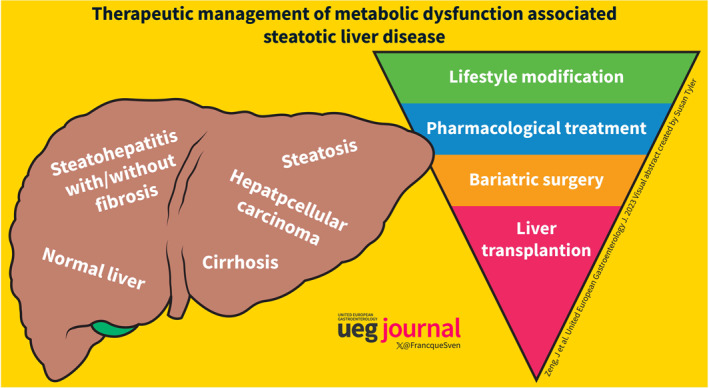
INTRODUCTION AND BASIC CONCEPTS
Non‐alcoholic fatty liver disease (NAFLD) refers to the accumulation of triglycerides in hepatocytes in the absence of classic causes of steatosis. 1 It covers a spectrum from simple steatosis to non‐alcoholic steatohepatitis (NASH), and then cirrhosis and hepatocellular carcinoma (HCC). 2 NAFLD has emerged as the leading cause of chronic liver diseases, and NAFLD‐related cirrhosis and HCC are becoming major indications for liver transplantation worldwide. 3 The terms NAFLD and NASH do not indicate the cause of the disease, and the exclusionary nature of the definition does not allow the diagnosis of its co‐existence with other chronic liver diseases, although this is frequently encountered in daily practice. Moreover, the majority of patients with NAFLD have cardiometabolic risk factors; insulin resistance, visceral adiposity, and adipose tissue dysfunction are pivotal pathophysiology of NAFLD/NASH. 4 Therefore, a new nomenclature was introduced. Patients with steatosis or steatohepatitis and the presence of at least one cardiometabolic risk factor are now diagnosed as metabolic dysfunction associated steatotic liver disease (MASLD) or steatohepatitis (Metabolic Dysfunction Associated Steatohepatitis (MASH)). 5 Over 95% of patients with NAFLD have MASLD, while the small group of non‐MASLD NAFLD patients requires further study to understand the underlying causes. The new nomenclature offers a solution for the co‐existence of metabolic risk factors with other causes of liver disease, most notably the co‐existence with excessive alcohol consumption (metabolic and alcohol‐related liver disease, MetALD).
This review hence focuses on MASLD, whether alone or in the context of MetALD. The pathophysiology is complex but some global concepts are helpful to understand the management. MASLD is driven by ‘metabolic overload’, resulting from excess calory intake not counterbalanced by physical activity. Whether this metabolic overload leads to metabolic stress, depends on the individual's ability to cope with it. 6 This ability, amongst others determined by adipose tissue expandability (Figure 1), is influenced by many factors (i.e, genetic, epigenetic, environmental, age, and gender). When the capacity of coping with the ‘metabolic overload’ is exceeded, ectopic lipid accumulation can occur as well as adipose tissue dysfunction that will add a metabolic‐inflammatory stress impacting several end‐organs, including the liver. 7 Whether this ectopic lipid accumulation (which by itself is not per se harmful) and this metabolic‐inflammatory stress will then result in MASH, not only depends on the nature and magnitude of these stressors, but also on the ability of the liver to cope with it (Figure 2). The liver is equipped with defence and repair mechanisms and their efficacy is an important determinant of the ultimate liver damage. All these different contributing elements results in important inter‐individual differences and notorious patient heterogeneity. 6 This oversimplification of the complicated pathophysiology of MASLD/MASH highlights the importance of a holistic multidisciplinary approach that takes into account both liver‐centred approaches and approaches that take into account the extrahepatic drivers as well as consequences of MASLD/MASH.
FIGURE 1.
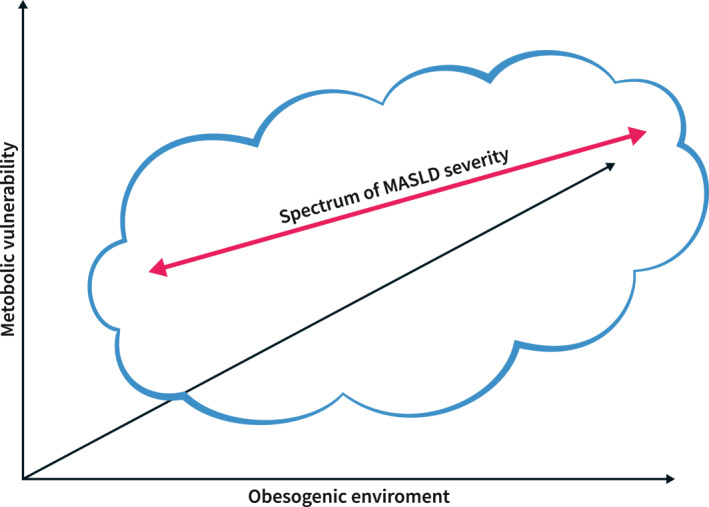
Metabolic dysfunction associated steatotic liver disease is a heterogeneous disease. Disease severity increases with more severe metabolic pressure, depending on the body's capacity to cope with an obesogenic environment. The armamentarium of the liver to deal with damage and inflammation adds to the inter‐individual variability. The contribution of all these factors explains the variability of liver disease severity in relation to metabolic risk factors.
FIGURE 2.
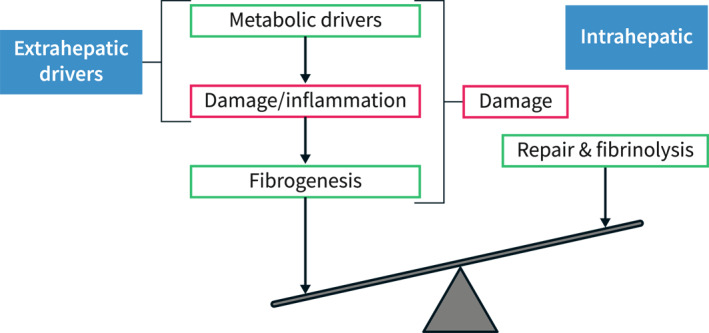
Schematic concept of progression of disease in Metabolic Dysfunction Associated Steatotic Liver Disease. Disease progression depends intrahepatically on the balance between pro‐fibrogenic mechanisms and defence/repair mechanisms. Fibrosis is driven by upstream processes of damage and inflammation, which result from a metabolic pressure. An efficacious management of Metabolic Dysfunction Associated Steatohepatitis most likely needs to tackle these upstream drivers of disease to be successful. Adapted from. 1
It is well‐known that MASLD not only leads to end‐stage liver disease but also has clinical implications on extrahepatic tissues with reduced quality of life 7 , 8 . Cardiovascular disease (CVD) is the most common cause of death in patients with MASLD, and emerging evidence suggests that MASLD contribute independently to CVD development, highlighting the need to consider CVD prevention in the management of MASLD. MASLD also increases the risk of type 2 diabetes, chronic kidney disease, and non‐hepatic malignancy, calling again for a more holistic approach that views MASLD/MASH as an important driving force of a multisystemic disorder (Figure 3), rather than an isolated liver disease. 9 Conducting a comprehensive evaluation is thus vital for the assessment of MASLD and MetALD, as well as for identifying potential comorbidities. Recent guidance papers provide key details on the management of metabolic and cardiovascular co‐morbidities in the context of MASLD/MASH. 10 , 11
FIGURE 3.
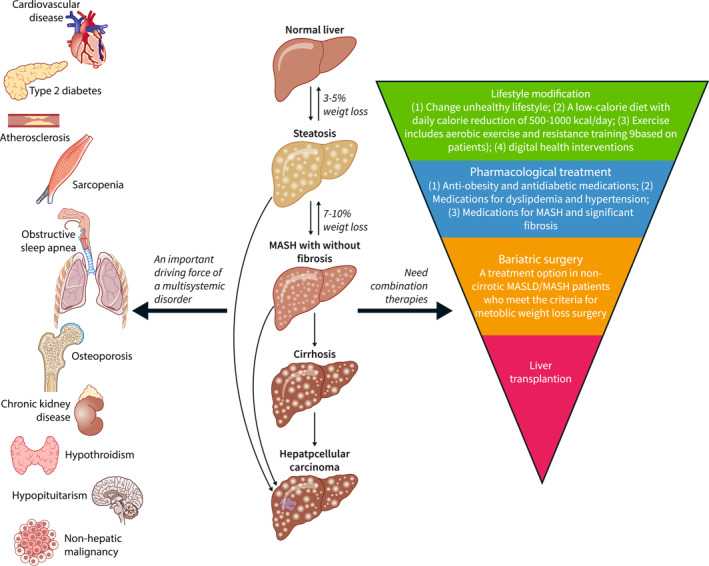
Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD) as an important driving force of multisystemic disorders and its management. MASLD covers a spectrum from simple steatosis to Metabolic Dysfunction Associated Steatohepatitis (MASH), and then cirrhosis and hepatocellular carcinoma. It is well‐known that MASLD not only leads to end‐stage liver disease but also has clinical implications on extrahepatic tissues with reduced quality of life, including increasing the risk of type 2 diabetes, chronic kidney disease, and non‐hepatic malignancy. Given its pathophysiology, the management of the underlying metabolic drivers of the disease by lifestyle modification and weight loss is the cornerstone of the treatment of MASLD/MASH. For MASLD patients with overweight/obesity, a weight loss of 3%–5% can reduce steatosis, while a 7% weight loss can lead to MASH regression, and a 10% weight loss may result in fibrosis regression. Some drugs already on the marked for the treatment of co‐morbidities have shown some efficacy. Liver transplantation and bariatric surgery should be performed in some patients with good indications. Combination therapies targeting multiple pathways and the integration of digital health interventions hold potential for enhancing the efficacy and safety of MASLD treatments.
CURRENT NON‐PHARMACOLOGICAL MANAGEMENT OPTIONS
Lifestyle modification and weight management
The cornerstone of MASLD/MASH management is lifestyle management, which can reduce the metabolic overload and improve the adipose tissue dysfunction and the resulting metabolic‐inflammatory stress, allowing intrahepatic repair mechanisms to operate (Figure 2). It has been clearly demonstrated that weight loss improves liver histology, whether by lifestyle change or by bariatric surgery. 12 For MASLD patients with overweight/obesity, a weight loss of 3%–5% can reduce steatosis, while a 7% weight loss can lead to MASH regression, and a 10% weight loss may result in fibrosis regression (Figure 3). 13 Even lean MASLD patients can benefit from a subtle weight loss. 14 It is worth noting that MASLD patients are at an increased risk of sarcopenia, and sarcopenia can exacerbate the progression of liver disease towards fibrosis and significant cirrhosis. During the weight loss, attention is required to ensure adequate protein intake and the incorporation of resistance exercise to prevent the further loss of muscle mass.
Public education and awareness campaigns are essential to highlight the detrimental effects of obesity and sedentary behaviour on health for MASLD patients. 12 Given that smoking and alcohol consumption act as synergistic factors promoting MASLD progression and increasing the risk of various malignancies, patients with MASLD need to quit smoking and limit alcohol intake. Strict abstinence from alcohol is required for patients with significant fibrosis or cirrhosis. Although daily consumption of three or more cups of coffee is associated with a reduced risk of MASLD progression and HCC, the protective effects of green tea and black tea on MASLD require further research for confirmation. Multidisciplinary collaboration involving clinical nutritionists, exercise rehabilitation therapists, and psychological counsellors, along with digital therapies using smartphone apps, 15 , 16 , 17 can provide patients with personalised diet and exercise prescriptions to enhance the enthusiasm and persistence of lifestyle intervention.
Diet and exercise
Foods rich in saturated fats, cholesterol, refined carbohydrates, sugary beverages, red meat, and highly processed foods, which are energy‐dense or have inflammatory potential, are closely associated with the development of MASLD/MASH. Conversely, a healthy diet index, Dietary Approaches to Stop Hypertension diet score, Mediterranean‐style diet score, and foods high in antioxidant capacity can reduce the risk of MASLD. 18 There is a dose‐response relationship between the extent of dietary calorie restriction and weight loss, as well as improvements in the liver function test in MASLD. Gradual weight loss achieved by reducing daily calorie intake by 500–1000 kcal can improve insulin resistance, lower liver enzyme levels, and reduce liver fat content. Low‐carbhybrate diets, low‐fat diets, intermittent fasting, and the Mediterranean diet all have potential metabolic, cardiovascular, and liver benefits. Therefore, clinical nutritionists should formulate personalised dietary prescriptions for MASLD patients based on their comorbidities and preferences, focusing on controlling energy intake, and adjusting dietary composition.
Exercise with or without diet has an effect on the improvement of muscle function and the muscle‐liver axis. 19 There is no consensus on the superiority of aerobic exercise and resistance training; both are independently associated with weight loss, reduction of liver fat, and other metabolic benefits. Further details regarding exercise for MASLD patients are available elsewhere. 13 , 15 Resistance exercise is particularly suitable for patients with musculoskeletal problems, poor baseline health, or cardiopulmonary health contraindications to aerobic exercise.
Bariatric surgery
Bariatric surgery has proven to be an effective long‐term weight loss method with a reduction in mortality from CVD and malignancy in morbidly obese patients. It is also effective for the improvement of metabolic dysfunction and related‐steatohepatitis and fibrosis. Therefore, current guidelines recommend considering bariatric surgery as a treatment option in non‐cirrhotic MASLD/MASH patients who meet the criteria for metabolic weight loss surgery. 20 The latest systematic reviews demonstrate that performing bariatric surgery in severe obese patients with compensated cirrhosis and portal hypertension appears to be safe and is associated with acceptable perioperative and long‐term outcomes. 21 , 22 Further research is required to determine whether the efficacy of bariatric surgery is solely mediated by weight loss or if surgery‐induced hormonal changes also play a role. Well‐controlled prospective studies are also required to evaluate the effects of bariatric surgery on specific subpopulations and its long‐term impact on cirrhosis and HCC in patients with MASLD. It is noteworthy that bariatric surgery might increase the risk of postoperative alcoholic cirrhosis.
CURRENT PHARMACOLOGICAL OPTIONS
As a non‐pharmacological approach can already have a substantial benefit, it is important to first define the potential indications for a MASLD/MASH‐specific pharmacological treatment, given that any drug comes with safety and tolerability concerns. It has been demonstrated that, although steatohepatitis drives disease progression, fibrosis is the most critical predictor of prognosis, both in terms of liver disease and overall (mainly CVD) mortality. 23 This is probably because the extent of liver fibrosis reflects not only the underlying disease activity but also the insufficient counterbalancing by defence and repair mechanisms, thereby determining the individual's overall vulnerability. Therefore, treatment indication for at risk MASH is considered when a NAFLD Activity Score is at least four using the NASH Clinical Research Network scoring system, or an Activity of at least two using the Steatosis‐Activity‐Fibrosis scoring system, along with a significant fibrosis. 24 This diagnosis needs a liver biopsy for an accurate assessment, but the research on non‐invasive testing will hopefully provide tools to accurately make this diagnosis without the need for histology in the near future.
Also, the goals of treatment need to be defined. MASLD/MASH is a slowly progressing disease, making it challenging to establish short‐term clinical goals, especially in non‐cirrhotic patients. Although it still needs to be proven that this will translate into long‐term clinical benefits, histological improvement is likely to reflect a positive change in the natural history of the disease. Therefore, the traditional endpoints for assessing treatment efficacy include the resolution of steatohepatitis without worsening of fibrosis, or regression of fibrosis without worsening of steatohepatitis, and more recently, a combined resolution of steatohepatitis and regression of fibrosis. 25
Based on these criteria, only a few drugs currently on the market for indications other than MASH have demonstrated efficacy, and none have completed a Phase 3 trial to support their use for fibrotic MASH (Figure 4). Glucagon‐like peptide (GLP‐1) is an incretin hormone regulating satiety, gastric emptying, and glycaemic control. GLP‐1 receptor agonists (GLP‐1RA) are approved for the treatment of diabetes and obesity. A small trial with liraglutide 25 and a large Phase 2 trial with semaglutide 26 showed the beneficial effects of both drugs on the resolution of MASH. However, despite their considerable impact on MASH and, in case of semaglutide, a treatment duration of 1.5 years, there was no significant regression of fibrosis. Since there are no GLP‐1Rs in the liver, this improvement is likely attributed solely to the amelioration in the metabolic‐inflammatory disease drivers. The absence of direct intrahepatic anti‐inflammatory and anti‐fibrogenic effects likely explains the absence of a strong signal on fibrosis regression within the timeframe of the study. While the Phase 3 trial of semaglutide is ongoing (Table 1), the use of GLP‐1RA in patients with diabetes or obesity and indicators of fibrotic MASH has been incorporated into the recent guidelines of the American Association of Clinical Endocrinology, the first non‐hepatology scientific society to issue recommendations on the management of MASLD/MASH. 20 Side effects, mainly gastro‐intestinal in nature, may limit patient tolerability. The improvement in the cardiometabolic risk factors that translate into proven cardiovascular benefit (albeit not specifically studied in MASH patients) of this class of drugs, is an aspect worth considering, given the aforementioned link with CVD.
FIGURE 4.
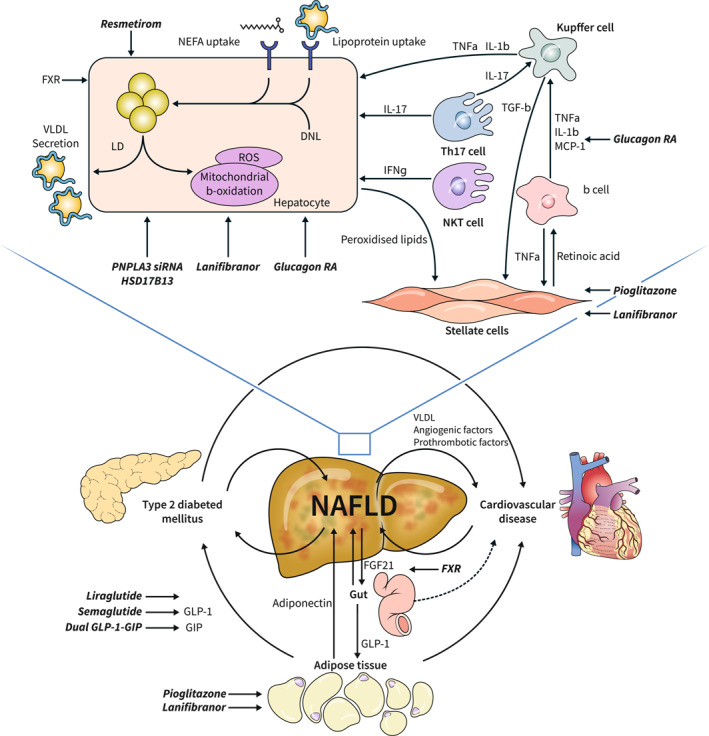
The complex pathophysiology of Metabolic Dysfunction Associated Steatohepatitis (MASH). MASH is driven by the complex interplay of metabolic, inflammatory and fibrogenic processes. Within the liver, hepatocytes (and its intracellular organelles, most notably mitochondria), play an important role, alongside the stellate cells and several resident and infiltrating immune cells of different populations. The liver is at the centre of an important crosstalk between the liver, the adipose tissue, the gut the pancreas and the cardiovascular system, 2 , 8 with MASH being an important driver of complex vicious circles involving many organs. Drugs/pathways that have been tested in MASH or that are under development, mentioned and/or referenced in the text are depicted anchoring point in the complex pathophysiology of MASH. DNL, de novo lipogenesis; FGF21, fibroblast growth factor 21; FXR, farnesoid receptor X; GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon‐like peptide 1; HSD17B13, Hydroxysteroid 17β dehydrogenase; IFNγ, interferon gamma; IL1‐β, interleukin 1 beta; IL‐6, interleukin 6; IL‐17, interleukin 17; LD, lipid droplets; MCP‐1, monocyte chemoattractant protein 1; NEFA, non‐esterified fatty acids; NKT cell, natural killer T cell; PNPLA3, patatin‐like phospholipase domain‐containing protein 3; RA, receptor agonist; ROS, reactive oxygen species; siRNA, small interfering RNA; Th17, T helper 17 cell; TGFβ, tumour growth factor beta; TNFα, tumour necrosis factor alpha; VLDL, very low density lipoproteins. Source: Figure adapted from 2 (courtesy J. Haas) and Ref 8 .
TABLE 1.
Drugs with ongoing or upcoming phase 3 and 4 clinical trials for NAFLD/NASH.
| Drug name | Targets and mechanism | Trial identifiers | Indication | Aim | Starting year | Phase and status | Sponsor |
|---|---|---|---|---|---|---|---|
| Belapectin | Galectin‐3 (Gal‐3) inhibitor | NAVIGATE (NCT04365868) | Patients with NASH cirrhosis and clinical signs of portal hypertension but without oesophageal varices at baseline | Evaluating the efficacy and safety of belapectin for the prevention of esophageal varices in NASH cirrhosis | 2020 | Phase 2b/3 | Galectin Therapeutics Inc. |
| Dulaglutide | GLP‐1R agonist | REALIST (NCT03648554) | T2DM with NASH | Researching an effect of GLP‐1 agonist on liver steatosis | 2019 | Phase 4 | Central Hospital, Nancy, France |
| Empagliflozin | SLGT2 inhibitor | NCT04642261 | NAFLD without DM | Effect of empagliflozin on liver fat in non‐diabetic patients | 2021 | Phase 4 | The University of Hong Kong |
| Erugliflozin | SGLT2 inhibitor | Ertu‐NASH (NCT05644717) | T2DM with NAFLD/NASH | Effect of erugliflozin on liver fat, liver fibrosis and glycemic control in type II DM patients with NASH/NAFLD | 2022 | Phase 4 | Getz Pharma |
| Lanifibranor (IVA337) | panPPAR agonist | NATiV3 (NCT04849728) | NASH and liver fibrosis histological stage F2 or F3 | Evaluating efficacy and safety of lanifibranor followed by an active treatment extension in adult patients with NASH and fibrosis stages F2 and F3 | 2021 | Phase 3 | Inventiva Pharma |
| MSDC‐0602K | Thiazolidinedione (TZD) insulin sensitizers | NCT03970031 | Subjects with pre‐T2DM or T2DM and evidence of NAFLD/NASH | Assess glycemic control and cardiovascular outcomes in patients with Pre‐T2D or T2D and NAFLD/NASH | 2022 | Phase 3 | Cirius Therapeutics, Inc. |
| Resmetirom (MGL‐3196) a | Selective THRβ agonist | MAESTRO‐NAFLD1 (NCT04197479) | NAFLD | Evaluate safety and biomarkers of resmetirom in NAFLD | 2019 | Phase 3 (completed) | Madrigal pharmaceuticals, Inc. |
| MAESTRO‐NASH (NCT03900429) | Patients with NASH and fibrosis | Evaluate the efficacy and safety of MGL‐3196 (resmetirom) in patients with NASH and fibrosis | 2019 | Phase 3 (completed) | |||
| MAESTRO‐NAFLD‐OLE (NCT04951219) | NAFLD | Evaluate safety and biomarkers of resmetirom in patients with NAFLD | 2021 | Phase 3 | |||
| MAESTRO‐NASH‐OUTCOMES (NCT05500222) | Patients with well‐compensated NASH cirrhosis | Evaluate the effect of resmetirom on clinical outcomes in patients with well‐compensated NASH cirrhosis | 2022 | Phase 3 | |||
| Saroglitazar | Dual PPAR agonist (a PPAR‐α/γ agonist) | NCT05872269 | NAFLD with comorbidities (either obesity, T2DM, dyslipidemia or metabolic syndrome) | Evaluate the safety and effectiveness of saroglitazar 4 mg in patients with NAFLD with comorbidities | 2023 | Phase 4 | Zydus Lifesciences Limited |
| Semaglutide | GLP‐1R agonist | ESSENCE (NCT04822181) | NASH | Study on whether semaglutide works in people with non‐alcoholic steatohepatitis | 2021 | Phase 4 | Novo Nordisk A/S |
Abbreviations: GLP‐1R, glucagon‐like peptide‐1 receptor; NAFLD, non‐alcoholic fatty liver disease; NASH, non‐alcoholic steatohepatitis; PPAR, peroxisome proliferators‐activated receptor; SGLT2, sodium‐glucose cotransporter‐2; T2DM, type 2 diabetes mellitus; THRβ, thyroid hormone receptor‐β agonist.
Resmetirom (MGL‐3196) has met the aforementioned endpoints in a Phase 3 clinical trial (interim analysis), which showed statistically significant benefits on fibrosis regression as well as NASH resolution, along with several other markers of efficacy, and a favourable safety and tolerability profile.
The peroxisome proliferator‐activated receptor (PPAR) gamma agonist pioglitazone is another compound with histologically proven benefit. PPARs are nuclear receptors, consisting of three isotypes expressed differentially across a wide range of tissues and cell types. 27 They are key regulators of energy handling, bile acid metabolism, inflammation, and fibrogenesis. PPAR gamma is the primary PPAR in adipose tissue and quiescent stellate cells. Pioglitazone induces resolution of MASH and a decrease in the mean fibrosis stage, although it does not reach the endpoint of one‐stage fibrosis regression 28 (Figure 4). This goes along with improved glycaemic control, a better lipid profile, and enhanced cardiovascular outcomes, mainly atherosclerotic CVD. 29 It substantially improves adipose tissue function, as reflected by an adiponectin increase. 30 The improvement in adipose tissue function leads to increased storage capacity and expandability of the adipose tissue, resulting in weight gain, that at first sight might seem contradictory. It is, however, a shift from visceral to subcutaneous adipose tissue that explains the weight gain, along with metabolic, cardiovascular, and liver improvement. 30 Fluid retention and cardiac decompensation in patients with pre‐existing cardiac insufficiency warrant caution, as well as an increased risk of bone fractures. Based on all these data, pioglitazone has been included in the recent American Association of Clinical Endocrinology guidelines within its licenced indications. 20
Vitamin E, as an anti‐oxidant and potentially other modes of action, has proven efficacy on steatohepatitis in non‐cirrhotic non‐diabetic MASH patients and can be considered for use in these patients. 31 However, its side effects, including a potential increase in overall mortality, hemorrhagic cerebral stroke, and prostate cancer, limit its routine use. Of note, drugs used to treat some of the co‐morbidities of MASLD also have data supporting some evidence of liver benefit. Aspirin and other anti‐aggregant treatments, most probably by acting on the endothelium‐thrombocyte interaction and the role of thrombocytes in MASH progression, may reduce liver fat content and long‐term disease progression. 32 Data on MASH cirrhosis specifically are not available, but the potential benefit of these compounds in reducing the risk of decompensation in cirrhosis might also hold true in MASH cirrhosis. Statins have an impact on endothelial function and endothelial insulin resistance and showed clear benefits in pre‐clinical studies, but there are very few clinical data. 33 Metformin did not show a benefit in terms of liver histology, but data indicate that it lowers the risk of HCC development. 34 The presence of arterial hypertension has been associated with more severe fibrosis and angiotensin receptor antagonists as well as endothelin receptor antagonists may show some benefit. 35 All these drugs are probably not powerful enough to induce MASH resolution or fibrosis alleviation, but might, as long‐term background treatment, have a subtle added benefit that helps slow down liver disease progression. Drugs used in the treatment of some co‐morbidities might have benefits and delay hepatic decompensation, although this remains to be proven. Trials in patients with MASH cirrhosis have been negative so far, except for belapectin, a galactin‐3 inhibitor, that might prevent the development of varices. 36
PHARMACOLOGICAL TREATMENT ON THE HORIZON
Over a decade of clinical trials involving many drugs, no drug is currently approved for the treatment of MASH. The reasons for this are outlined elsewhere. 37 Currently, only two drugs have met the aforementioned endpoints in Phase 3 clinical trials: the farnesoid receptor X agonist obeticholic acid 38 (which will not be developed further as a MASH treatment as it was not granted accelerated approval by the Food & Drug Administration), and more recently, the thyroid hormone receptor beta agonist resmetirom. 39 The latter showed statistically significant benefits on fibrosis regression as well as NASH resolution, along with several other markers of efficacy, and a favourable safety and tolerability profile. Other promising drugs, based on Phase 2 data, include the panPPAR agonist lanifibranor, 40 the GLP‐1RA receptor agonist semaglutide, 26 both in ongoing Phase 3 trials (Table 1), as well as the fibroblast growth factor 21 agonist pegozafermin 41 that recently reported beneficial effects on histological endpoints and will enter Phase 3. Additionally, there are other promising drugs such as dual and triple GLP‐1/glucagon/glucose‐dependent insulinotropic polypeptide agonists that, on top of inducing significant weight loss (some of them comparable to bariatric surgery), may have direct intrahepatic effects, theoretically resulting in a more pronounced effect on fibrosis compared to GLP‐1RA alone. 37 Gene targeting therapy, for example, on PNPLA3 or on HSD17B13, represents other interesting avenues currently explored in the quest for an efficacious MASH drug. 42 The anchor points of these drugs are depicted in Figure 4.
LIVER TRANSPLANTATION
Liver transplantation should be considered in MASLD patients with decompensated cirrhosis and/or HCC, as their post‐transplant complication rates, overall survival, and graft survival are equivalent to that of other end‐stage liver diseases in appropriately selected patients. 43 However, careful attention must be paid to the thorough evaluation and appropriate treatment of the cardiometabolic comorbidities in the pre‐ and post‐transplantation phase, which might hamper the eligibility of liver transplantation and influence the post‐transplantation outcomes. Morbid obesity alone does not constitute a contraindication for liver transplantation. This situation represents a new surgical reality of both liver transplant and bariatric surgery. The majority of papers on bariatric surgery after liver transplantation could be explained because of the commodity of the flow of these patients. 44 In these clinical settings, the management becomes particularly challenging and requires a multidisciplinary approach in specialised high‐volume centres.
CONCLUSION
Given its pathophysiology, the management of the underlying metabolic drivers of the disease by lifestyle modification and weight loss is the cornerstone of the treatment of MASLD/MASH. Some drugs already on the marked for the treatment of co‐morbidities have shown some efficacy and their use should be optimised, within their licenced indications. MASH‐specific drugs are on the horizon and will likely change our management in the near future. MASH cirrhosis is an area of particular concern, as treatment is more challenging and drug development is lacking in compared to non‐cirrhotic MASH. In addition, liver transplantation and bariatric surgery should be performed in some patients with good indication. Future research should focus on optimising lifestyle intervention strategies, improving adherence and success rates, exploring the role of new weight‐loss medications, and identifying effective weight loss surgical methods for MASH patients. Combination therapies targeting multiple pathways and the integration of digital health interventions hold potential for enhancing the efficacy and safety of MASLD treatments.
CONFLICT OF INTEREST STATEMENT
SF holds a senior clinical investigator fellowship from the Research Foundation Flanders (FWO) (1802154N). His institution has received grants from Astellas, Falk Pharma, Genfit, Gilead Sciences, GlympsBio, Janssens Pharmaceutica, Inventiva, Merck Sharp & Dome, Pfizer, and Roche. He has acted as consultant for Abbvie, Actelion, Aelin Therapeutics, AgomAb, Aligos Therapeutics, Allergan, Alnylam, Astellas, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristoll‐Meyers Squibb, CSL Behring, Coherus, Echosens, Dr. Falk Pharma, Eisai, Enyo, Galapagos, Galmed, Genetech, Genfit, Genflow Biosciences, Gilead Sciences, Intercept, Inventiva, Janssens Pharmaceutica, PRO.MED.CS Praha, Julius Clinical, Madrigal, Medimmune, Merck Sharp & Dome, Mursla Bio, NGM Bio, Novartis, Novo Nordisk, Promethera, Roche, Siemens Healthineers. He has been a lecturer for Abbvie, Allergan, Bayer, Eisai, Genfit, Gilead Sciences, Janssens Cilag, Intercept, Inventiva, Merck Sharp & Dome, Novo Nordisk, Promethera, and Siemens Healthineers.
Zeng J, Fan J‐G, Francque SM. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024;12(2):177–86. 10.1002/ueg2.12525
Contributor Information
Jian‐Gao Fan, Email: fanjiangao@xinhuamed.com.cn, Email: fattyliver2004@126.com.
Sven M. Francque, Email: sven.francque@uza.be, Email: sven.francque@uantwerpen.be.
DATA AVAILABILITY STATEMENT
No data are available.
REFERENCES
- 1. Francque SM, Marchesini G, Kautz A, Walmsley M, Dorner R, Lazarus JV, et al. Non‐alcoholic fatty liver disease: a patient guideline. JHEP Rep. 2021;3(5):100322. 10.1016/j.jhepr.2021.100322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haas JT, Francque S, Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol. 2016;78(1):181–205. 10.1146/annurev-physiol-021115-105331 [DOI] [PubMed] [Google Scholar]
- 3. Yilmaz Y, Toraman AE, Alp C, Doğan Z, Keklikkiran C, Stepanova M, et al. Impairment of patient‐reported outcomes among patients with non‐alcoholic fatty liver disease: a registry‐based study. Aliment Pharmacol Ther. 2023;57(2):215–223. 10.1111/apt.17301 [DOI] [PubMed] [Google Scholar]
- 4. Ayonrinde OT. Historical narrative from fatty liver in the nineteenth century to contemporary NAFLD – reconciling the present with the past. JHEP Rep. 2021;3:100261. 10.1016/j.jhepr.2021.100261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi‐society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–1556. 10.1016/j.jhep.2023.06.003 [DOI] [PubMed] [Google Scholar]
- 6. Francque S. Towards precision medicine in non‐alcoholic fatty liver disease. Rev Endocr Metab Disord. 2023;24(5):885–899. 10.1007/s11154-023-09820-6 [DOI] [PubMed] [Google Scholar]
- 7. Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. 2019;1(4):312–328. 10.1016/j.jhepr.2019.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Francque SM, van der Graaff D, Kwanten WJ. Non‐alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol. 2016;65(2):425–443. 10.1016/j.jhep.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 9. Schattenberg JM, Allen AM, Jarvis H, Zelber‐Sagi S, Cusi K, Dillon JF, et al. A multistakeholder approach to innovations in NAFLD care. Commun Med. 2023;3:1. 10.1038/s43856-022-00228-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pais R, Cariou B, Noureddin M, Francque S, Schattenberg JM, Abdelmalek MF, et al. A proposal from the Liver Forum for the management of comorbidities in nonalcoholic steatohepatitis therapeutic trials. J Hepatol. 2023;79(3):829–841. 10.1016/j.jhep.2023.03.014 [DOI] [PubMed] [Google Scholar]
- 11. Glass O, Filozof C, Noureddin M, Berner‐Hansen M, Schabel E, Omokaro SO, et al. Standardisation of diet and exercise in clinical trials of NAFLD‐NASH: recommendations from the Liver Forum. J Hepatol. 2020;73(3):680–693. 10.1016/j.jhep.2020.04.030 [DOI] [PubMed] [Google Scholar]
- 12. Younossi ZM, Zelber‐Sagi S, Henry L, Gerber LH. Lifestyle interventions in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2023;20(11):708–722. 10.1038/s41575-023-00800-4 [DOI] [PubMed] [Google Scholar]
- 13. Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2021;160(3):912–918. 10.1053/j.gastro.2020.11.051 [DOI] [PubMed] [Google Scholar]
- 14. Long MT, Noureddin M, Lim JK. AGA clinical practice update: diagnosis and management of nonalcoholic fatty liver disease in lean individuals: expert review. Gastroenterology. 2022;163(3):764–774.e1. 10.1053/j.gastro.2022.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan JG, Wei L, Zhuang H. National workshop on fatty liver and alcoholic liver disease, Chinese society of hepatology, Chinese medical association; fatty liver disease expert committee, Chinese medical doctor association. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig. 2019;20(4):163–173. 10.1111/1751-2980.12685 [DOI] [PubMed] [Google Scholar]
- 16. Mazzotti A, Caletti MT, Brodosi L, Di Domizio S, Forchielli ML, Petta S, et al. An internet‐based approach for lifestyle changes in patients with NAFLD: two‐year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155–1163. 10.1016/j.jhep.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 17. Expert Committee of Fatty Liver Prevention and Treatment Fund of China Health Promotion Foundation . Chronic Disease Management Branch of China Medical Biotechnology Association. Brief version of health management service package of metabolic dysfunction associated fatty liver disease. Chin J Health Manage. 2023;17:169–179. [Google Scholar]
- 18. Zelber‐Sagi S, Grinshpan LS, Ivancovsky‐Wajcman D, Goldenshluger A, Gepner Y. One size does not fit all; practical, personal tailoring of the diet to NAFLD patients. Liver Int. 2022;42(8):1731–1750. 10.1111/liv.15335 [DOI] [PubMed] [Google Scholar]
- 19. Zelber‐Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, et al. Effect of resistance training on non‐alcoholic fatty‐liver disease a randomized‐clinical trial. World J Gastroenterol. 2014;20(15):4382–4392. 10.3748/wjg.v20.i15.4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American association of clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and Endocrinology clinical settings: Co‐sponsored by the American association for the study of liver diseases (AASLD). EndocrPract. 2022;28(5):528–562. 10.1016/j.eprac.2022.03.010 [DOI] [PubMed] [Google Scholar]
- 21. Manzano‐Nunez R, Rivera‐Esteban J, Comas M, Angel M, Flores V, Bañares J, et al. Outcomes of patients with severe obesity and cirrhosis with portal hypertension undergoing bariatric surgery: a systematic review. Obes Surg 2023;33(1):224–233. , 10.1007/s11695-022-06362-9 [DOI] [PubMed] [Google Scholar]
- 22. Wang G, Huang Y, Yang H, Lin H, Zhou S, Qian J. Impacts of bariatric surgery on adverse liver outcomes: a systematic review and meta‐analysis. Surg Obes Relat Dis. 2023;19(7):717–726. 10.1016/j.soard.2022.12.025 [DOI] [PubMed] [Google Scholar]
- 23. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology. 2017;65(5):1557–1565. 10.1002/hep.29085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loomba R, Ratziu V, Harrison SA, McFarlane SC, Tamaki N, et al. NASH clinical trial design international working group. Expert panel review to compare FDA and EMA guidance on drug development and endpoints in nonalcoholic steatohepatitis. Gastroenterology. 2022;162(3):680–688. 10.1053/j.gastro.2021.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet. 2016;387(10019):679–690. 10.1016/S0140-6736(15)00803-X [DOI] [PubMed] [Google Scholar]
- 26. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo‐controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–1124. 10.1056/NEJMoa2028395 [DOI] [PubMed] [Google Scholar]
- 27. Francque S, Szabo G, Abdelmalek MF, Byrne CD, Cusi K, Dufour J‐F, et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator‐activated receptors. Nat Rev Gastroenterol Hepatol. 2021;18(1):24–39. 10.1038/s41575-020-00366-5 [DOI] [PubMed] [Google Scholar]
- 28. Mantovani A, Byrne CD, Targher G. Efficacy of peroxisome proliferator‐activated receptor agonists, glucagon‐like peptide‐1 receptor agonists, or sodium‐glucose cotransporter‐2 inhibitors for treatment of non‐alcoholic fatty liver disease: a systematic review. Lancet Gastroenterol Hepatol. 2022;7(4):367–378. 10.1016/S2468-1253(21)00261-2 [DOI] [PubMed] [Google Scholar]
- 29. Liu J, Wang L‐N. Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack. Cochrane Database Syst Rev. 2023;1(1):CD010693. 10.1002/14651858.CD010693.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gastaldelli A, Sabatini S, Carli F, Gaggini M, Bril F, Belfort‐DeAguiar R, et al. PPAR‐γ‐induced changes in visceral fat and adiponectin levels are associated with improvement of steatohepatitis in patients with NASH. Liver Int. 2021;41(11):2659–2670. 10.1111/liv.15005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pacana T, Sanyal AJ. Vitamin E and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15(6):641–648. 10.1097/MCO.0b013e328357f747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25(4):641–655. 10.1038/s41591-019-0379-5 [DOI] [PubMed] [Google Scholar]
- 33. Ng CH, Teng ML, Chew NW, Chan KE, Yong JN, Quek J, et al. Statins decrease overall mortality and cancer related mortality but are underutilized in NAFLD: a longitudinal analysis of 12,538 individuals. Expert Rev Gastroenterol Hepatol. 2022;16(9):895–901. 10.1080/17474124.2022.2119128 [DOI] [PubMed] [Google Scholar]
- 34. Zeng RW, Yong JN, Tan DJH, Fu CE, Lim WH, Xiao J, et al. Meta‐analysis: chemoprevention of hepatocellular carcinoma with statins, aspirin and metformin. Aliment Pharmacol Ther. 2023;57(6):600–609. 10.1111/apt.17371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van der Graaff D, Chotkoe S, De Winter B, De Man J, Casteleyn C, Timmermans J‐P, et al. Vasoconstrictor antagonism improves functional and structural vascular alterations and liver damage in rats with early NAFLD. JHEP Rep. 2022;4(2):100412. 10.1016/j.jhepr.2021.100412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chalasani N, Abdelmalek MF, Garcia‐Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, et al. Effects of belapectin, an inhibitor of galectin‐3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158(5):1334–1345.e5. 10.1053/j.gastro.2019.11.296 [DOI] [PubMed] [Google Scholar]
- 37. Francque S, Ratziu V. Future treatment options and regimens for nonalcoholic fatty liver disease. Clin Liver Dis. 2023;27(2):429–449. 10.1016/j.cld.2023.01.010 [DOI] [PubMed] [Google Scholar]
- 38. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non‐alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo‐controlled phase 3 trial. Lancet. 2019;394(10215):2184–2196. 10.1016/S0140-6736(19)33041-7 [DOI] [PubMed] [Google Scholar]
- 39. Harrison S, Bedossa P, Guy C, Schattenberg J, Loomba R, Taub R, et al. Primary results from MAESTRO‐NASH a pivotal phase 3 52‐week serial liver biopsy study in 966 patients with NASH and fibrosis. J Hepatol. 2023:78:GS‐001. [Google Scholar]
- 40. Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, et al. A randomized, controlled trial of the pan‐PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385(17):1547–1558. 10.1056/NEJMoa2036205 [DOI] [PubMed] [Google Scholar]
- 41. Loomba R, Sanyal AJ, Kowdley KV, Bhatt DL, Alkhouri N, Frias JP, et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N Engl J Med. 2023;389(11):998–1008. 10.1056/NEJMoa2304286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lindén D, Romeo S. Therapeutic opportunities for the treatment of NASH with genetically validated targets. J Hepatol. 2023;79(4):1056–1064. 10.1016/j.jhep.2023.05.007 [DOI] [PubMed] [Google Scholar]
- 43. Yong JN, Lim WH, Ng CH, Tan DJH, Xiao J, Tay PWL, et al. Outcomes of nonalcoholic steatohepatitis after liver transplantation: an updated meta‐analysis and systematic review. Clin Gastroenterol Hepatol 2023;21(1):45–54.e6. 10.1016/j.cgh.2021.11.014 [DOI] [PubMed] [Google Scholar]
- 44. de Barros F, Cardoso Faleiro Uba PH. Liver transplantation and bariatric surgery: a new surgical reality: a systematic review of the best time for bariatric surgery. Updates Surg 2021;73(5):1615–1622. 10.1007/s13304-021-01106-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.


