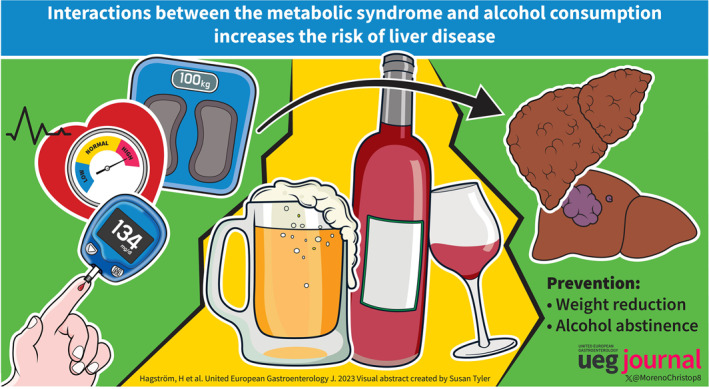
Abstract
Alcohol‐related liver disease (ALD) and non‐alcoholic fatty liver disease (NAFLD, recently renamed metabolic dysfunction‐associated steatotic liver disease [MASLD]) share many features, including certain pathophysiological mechanisms, susceptibility genes, and histological lesions. However, the natural history of the two diseases, studied separately, is significantly different, with ALD being associated with a higher risk of cirrhosis and liver‐related mortality. Moreover, evidence suggests an interactive effect between ALD and metabolic risk factors that are associated with NAFLD on the risk of progressive fibrosis and development of cirrhosis. Patients with both a high consumption of alcohol and metabolic risk factors, such as obesity or diabetes, should therefore be considered a particularly high‐risk group for cirrhosis. Additional studies regarding the efficacy of screening for advanced liver fibrosis or cirrhosis in these risk groups are needed. The most effective and established method for reducing the risk of progression in ALD is alcohol abstinence, whereas weight loss is effective in NAFLD. In this narrative review, we introduce the reader to the literature of the field and present key studies showing this interactive effect.
Keywords: alcohol‐related liver disease, cirrhosis, epidemiology, NAFLD, natural history
INTRODUCTION
Alcohol‐related liver disease (ALD) and non‐alcoholic fatty liver disease (NAFLD) are by far the two most common liver diseases in most countries. 1 , 2 , 3 Fortunately, not all people who drink alcohol or all patients with NAFLD, develop progressive fibrosis, cirrhosis, or hepatocellular carcinoma (HCC). 4 , 5 , 6 There is a growing understanding that a combination of ALD and NAFLD might act synergistically on the risk of developing progressive liver fibrosis. This interaction requires attention from policymakers and health care professionals who meet these patients in their everyday care.
DEFINITIONS OF LIVER DISEASE RELATED TO ALCOHOL, OBESITY, AND DIABETES
Since alcohol, obesity, and type 2 diabetes mellitus (T2DM) are independent risk factors for developing liver disease, 7 , 8 it is important to know how synchronous diabetes or obesity might modify the risk of developing more severe forms of liver disease in people with a high consumption of alcohol. Likewise, it is important to know how the consumption of alcohol might modify the risk of progressive liver disease in people with obesity and T2DM who are likely to have NAFLD. When investigating the risk of liver‐related outcomes based on alcohol consumption in obese and diabetic patients, many studies have investigated patients with NAFLD, which is closely related to these conditions. The definition of hepatic steatosis in general is a finding of more than 5% of hepatocytes having steatosis on a liver biopsy, although steatosis is more frequently diagnosed with radiological modalities such as findings of a bright liver in comparison with the kidneys on ultrasound or reduced attenuation of the liver on computerized tomography. 9
Internationally, a daily intake of less than 20 g of alcohol in women, and less than 30 g in men, is usually recommended to define the presence of NAFLD and rule out ALD in the setting of hepatic steatosis. 10 However, some countries and organizations recommend an even lower threshold or total abstinence. 11 ALD is generally considered to be the diagnosis if the patient has an alcohol consumption above the recommended limits and other causes of liver disease have ruled out. 12 If alcohol consumption is within the recommended limits, NAFLD should be considered after ruling out other causes of liver disease. 13 These cut‐offs are rather arbitrary since it is unlikely that there is a true biological effect at exactly 20 or 30 g of alcohol consumption, where, for example, men consuming 29 g are at no risk of ALD, whereas those drinking 31 g should only be considered to have ALD. Further, it remains a central problem that it is difficult to measure alcohol consumption objectively, and definitions of ALD differ between studies, which must be taken into consideration when interpreting the available evidence.
EPIDEMIOLOGY OF ALCOHOL‐RELATED LIVER DISEASE
Approximately 80% of adults in Europe, the United States (US), and similar countries drink alcohol sometime in their life, and up to 60% of adults are active drinkers. 14 The estimated consumption among active drinkers globally is 33 g of pure alcohol per day or 15 L of pure alcohol annually, but there are large differences between and within countries. 14 Approximately 90% of patients who drink more than 60 g of alcohol daily, which equals five or six units depending on the definition of a unit, are considered to have liver steatosis, but only around 20%–30% develop advanced fibrosis or cirrhosis. 4 , 5 , 6 , 15 The prevalence of ALD is estimated at around 5% of the global population, although estimates vary considerably. 16
If an individual who drinks fewer than 14 drinks per week is used as a reference, the hazard ratio (HR) for incident cirrhosis associated with drinking 14–28 drinks per week is about 2.3 for men and 3.5 for women, and the HR associated with drinking 28–42 drinks per week is 7.0 and 16.2 for men and women, respectively. 17 Long‐term follow‐up of 3453 patients with biopsy‐proven ALD showed that 5‐year cumulative mortality was 40.9% in patients with ALD compared with 5.8% in the matched reference population with a near five‐fold increase in death. 18 Among the 3453 biopsy‐proven ALD individuals in the study, the risk of death gradually increased from 28% in those with normal liver function, 27% in those with steatosis, 34% in those with steatohepatitis to 47% in those with cirrhosis. Liver‐related deaths accounted for 43% of total deaths with a 168‐fold increased risk of liver‐related death that was significantly increased in all ALD subgroups compared with their respective reference individuals, except for those with normal liver on biopsy. Given the large population at risk, ALD is attributable to roughly half of all deaths related to cirrhosis, corresponding to 607,000 deaths per year globally. 14 Apart from the total amount of alcohol ingested, drinking patterns also seem to affect the risk, and heavy episodic drinking has been associated with an increased risk of ALD and mortality. 19
EPIDEMIOLOGY OF LIVER DISEASE IN OBESITY
Overweight and obesity, defined as a body mass index (BMI) ≥25 and ≥30 kg/m2, respectively, have a prevalence of 39% globally. In total numbers, this equals to around 1.9 billion overweight people, of which 650 million are obese. 20 In the United States alone, the prevalence of obesity is 35% and is expected to reach 45% by 2030. 21 Based on previous studies, approximately 70% of people with obesity have NAFLD, with an even higher proportion in those that undergo bariatric surgery (90%). 22 , 23 The duration of obesity likely affects the risk of liver disease. A population‐based study of 1.2 million men showed that a high BMI during late adolescence was associated with an increased risk of severe liver disease later in life 7 (Table 1). Similar findings have been seen in young women. 31 One meta‐analysis reported a modest increase in the rate of developing severe liver disease (1.20) in people with obesity compared to those without this condition 24 (Table 1).
TABLE 1.
Key studies on associations between risk factors and liver‐related outcomes.
| Reference | Setting and design | N patients | Key exposure | Key outcome | Key finding |
|---|---|---|---|---|---|
| Hagström 7 | Sweden, cohort study | 1.2M men | Body mass index | Liver‐related outcomes from national registers | Higher BMI early in life associates with higher risk of cirrhosis. Further increase if presence of diabetes |
| Jarvis et al. 24 | Multiple countries, meta‐analysis | 22.8M | Body composition including BMI, diabetes | Liver‐related outcomes | Meta‐analysis finding an association between obesity (HR = 1.2) and diabetes type 2 (HR = 2.3) with future cirrhosis |
| Ajmera et al. 25 | US, cohort study | 285 patients with biopsy‐proven NAFLD with at least 2 biopsies | Questionnaire‐defined alcohol consumption | Biopsy‐defined improvement of hepatic steatosis and NASH | Modest alcohol use was associated with less improvement in steatosis as well as lower odds of NASH resolution, compared with no use of alcohol |
| Åberg et al. 26 | Finland, cohort study | 6732 | Alcohol use and metabolic risk factors | Liver‐related outcomes from national registers | Interaction between alcohol use and type 2 diabetes, higher risk for liver‐related outcomes |
| Israelsen et al. 27 | Denmark, cross‐sectional | 325 patients with alcohol‐related liver disease | Metabolic and genetic parameters | Hepatic fibrosis defined by liver biopsy | Insulin resistance strongest risk factor for hepatic fibrosis in patients with ALD |
| Whitfield et al. 28 | UK, case‐control study | 1293 cases with ALD‐cirrhosis and 754 controls with similar alcohol consumption but no cirrhosis | Alcohol use, life‐style and metabolic risk factors | Cirrhosis defined as by clinical records | Patients with cirrhosis more commonly had type 2 diabetes and higher BMI |
| Sahlman et al. 29 | Finland, cohort study | 41,260 | Patterns of alcohol consumption and metabolic, lifestyle‐related, and anthropometric parameters | Liver‐related outcomes from national registers | Strong synergism between alcohol and central obesity on the risk of liver‐related outcomes |
| Liu et al. 30 | UK, cohort study | 1.2M women | BMI and questionnaire‐defined alcohol consumption | Hospital admissions for cirrhosis | Alcohol and higher BMI interactively increases risk for cirrhosis. 17% of cirrhosis cases attributable to obesity, 42% to alcohol |
Abbreviations: ALD, alcohol‐related liver disease; BMI, body mass index; HR, hazard ratio; NAFLD, non‐alcoholic fatty liver disease; NASH, non‐alcoholic steatohepatitis; T2DM, type 2 diabetes mellitus; UK, United Kingdom; US, United States.
EPIDEMIOLOGY OF LIVER DISEASE IN TYPE 2 DIABETES MELLITUS
T2DM is also a disease of global concern with a prevalence of 10% in 2014 and is expected to increase to 13% by 2030. The prevalence of diabetes is associated with that of obesity and follows the increase in obesity prevalence closely. 21 Diabetes is associated with NAFLD, since it is estimated that 55%–70% of patients with T2DM have NAFLD. 22 , 32 T2DM has also been shown to increase the risk of severe liver disease. 33 When screened for liver disease by MRI, almost 64% of patients with T2DM had liver steatosis, and 6% had signs of cirrhosis. 34 An increased severity of liver disease in patients with T2DM and NAFLD was also reported in a meta‐analysis in which 37% were estimated to have non‐alcoholic steatohepatitis (NASH), and 17% had advanced fibrosis. 35 That is of prognostic significance as the stage of fibrosis is the best predictor of severe liver disease and overall mortality in patients with NAFLD. 36 , 37 A recent individual participant‐level data meta‐analysis including 2016 NAFLD patients clearly indicated that NAFLD patients with diabetes have significantly higher risks of hepatic decompensation and HCC at 1, 3, and 5 years compared with NAFLD patients without diabetes. 38
ALCOHOL CONSUMPTION AS A RISK FACTOR IN NAFLD
ALD and NAFLD have many similarities, including certain pathophysiological mechanisms, susceptibility genes, and histological features, and patients often share common clinical risk factors for disease progression. Evidence regarding the risk of developing liver disease or worsening of known liver disease in people who consume low to moderate amounts of alcohol is inconsistent. A study performed in patients with NAFLD found that a moderate consumption of alcohol, defined as less than two drinks daily, was associated with less reduction of steatosis and less resolution of NASH in paired liver biopsies compared to patients who were abstinent from alcohol 25 (Table 1). In patients with metabolic risk factors correlated with NAFLD, alcohol was a significant independent predictor of liver‐related outcomes along with smoking, age, waist circumference, and insulin resistance 26 (Table 1). A study in 8300 patients with known liver steatosis reported a dose‐dependent increase in the risk of incident advanced liver disease in people who consumed more than 10 g of alcohol per day. 39 A Mendelian randomization study used a mutation in the alcohol dehydrogenase gene as a proxy for long‐term alcohol consumption in patients with NAFLD. Patients with the mutation reported less consumption of alcohol and did not have higher stages of fibrosis or a higher prevalence of steatohepatitis on biopsy compared with patients without this mutation. 40
Binge‐drinking has also been shown to be a risk factor for progression to advanced liver disease. In patients with biopsy‐proven NAFLD, an association between heavy episodic drinking and progression of fibrosis was observed, 41 and the same result was reported in a population‐based study where increased risk of decompensated liver disease was observed in patients with weekly and monthly binge drinking. 42 Furthermore, binge‐drinking and other behaviors associated with alcohol overconsumption have been shown to be associated with increased risk of cirrhosis in healthy young adults later in life. 43 Collectively, these data suggest that the amount of drinking and drinking patterns affect the risk of fibrosis progression in NAFLD. The complex interaction between alcohol consumption and metabolic syndrome has been recently and extensively reported. 44
Controversially, several cross‐sectional studies have reported a reduced risk of advanced liver disease in patients with fatty liver who drink low to moderate amounts of alcohol compared to abstainers. 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 However, there was a risk of bias in these studies due to methodological issues. For example, patients with known manifest advanced liver disease were included, and there was a risk of misclassification bias in patients who reported current total abstinence, since many of these patients could previously have been drinking larger amount of alcohol, so called “sick quitters.” 53 Interestingly, one of the studies also measured phosphatidyl ethanol, a validated marker for recent alcohol consumption. 54 , 55 , 56 Patients who had reported low to moderate alcohol consumption had an elevated phosphatidyl ethanol >0.3 μmol/L in 11% of the cases, indicating a larger consumption than reported. 50 This is suggestive of either recall bias or dishonesty in the reported alcohol consumption. Similar findings were also made in an Austrian study where 29% of patients with presumed NAFLD and self‐reported low alcohol consumption had elevated biomarkers of alcohol consumption, highly suggestive of under‐reporting. 57
INTERACTIONS BETWEEN ALCOHOL AND DIABETES
A high chronic consumption of alcohol is associated with increased insulin resistance and the development of T2DM. 58 People with an alcohol use disorder (AUD) have a higher risk of T2DM compared with people in the general population. 59 This is most likely due to the progression of insulin resistance seen in patients with ALD, where the insulin resistance is more severe in more advanced stages of fibrosis 27 (Table 1). Progression to cirrhosis is more common in patients with diabetes and high BMI than in people without these conditions who drink the same high amount of alcohol 28 (Table 1).
Even without known alcohol consumption, patients with diabetes and severe insulin resistance progress to fibrosis at a higher rate than diabetic patients with other metabolic profiles, 60 and an increased blood glucose level is associated with increased fibrosis. 61 Therefore, it is not surprising that a combination of alcohol and diabetes could have a synergistic effect on the risk of liver disease. This synergistic effect was reported in a population‐based study of 6700 people with a follow‐up time of 12 years. People with diabetes and high alcohol consumption had a 20‐fold increased rate of progression to liver cancer, hospital admission due to liver disease, or liver‐related mortality compared with people with little or no alcohol consumption and no diabetes. In contrast, there was a 3.5‐fold increase in patients with high alcohol consumption alone, and a 2.5‐fold increase in patients with diabetes alone 26 (Table 1). Another study reported a similar interactive effect where patients with liver steatosis and the metabolic syndrome, who drank excessive amounts of alcohol, had an increased mortality risk compared to people who did not drink excessive amounts of alcohol or did not have the metabolic syndrome. 62 The metabolic syndrome is closely related to insulin resistance, and diabetes was the only component in the metabolic syndrome that was significantly associated with increased mortality in this cohort. 62 In a recent French study that analyzed data from two hospital‐based cohorts of approximately 50,000 patients with T2DM, the attributable fractions of alcohol use disorders, non‐metabolic liver‐related risk factors, and obesity to the liver burden were 55%, 14%, and 7%, respectively. 63 Recently, in a cohort of 1068 patients with diabetes, severe insulin‐resistant diabetes (SIRD) and excessive alcohol consumption were associated with significant fibrosis and the highest risk of liver‐related events (Otero‐Sanchez et al., Journal of Hepatology Reports 2023, in press). The results identified a group of T2DM patients with a particularly high risk of severe liver disease, that is, SIRD and alcohol use disorders. Furthermore, patients with T2DM who carry mutations in the PNPLA3 gene have more severe insulin resistance compared with patients with T2DM that do not carry this genotype. 64 In patients with ALD, the presence of increased insulin resistance and a mutation in PNPLA3 is associated with more advanced stages of fibrosis and, together with active alcohol consumption, is an independent risk factor for inflammatory activity in the liver, 27 (Table 1) a main driver of fibrogenesis. Hence, similar mutations are seen in patients with hepatic steatosis due to NAFLD and ALD, suggesting a shared genetic risk profile for disease progression. Taken together, considering the metabolic changes in ALD and diabetes, and the combined effect of alcohol and insulin resistance, screening for insulin resistance and diabetes in patients with manifest ALD might be valuable in clinical practice.
INTERACTIONS BETWEEN ALCOHOL AND OBESITY
Pure ethanol contains 7 kcal/gram and heavy drinking is associated with weight gain. 65 The opposite effect with weight loss is usually seen in patients with severe AUD and in cirrhotic patients with sarcopenia. 66 Consumption of 500 mL of beer daily has a weak association with weight gain, whereas a low consumption of wine has been associated with a possible protective effect on weight gain. 67 , 68 Careful interpretation of such cross‐sectional studies must be made, since residual confounding factors, such as a better diet and more exercise, may be present in those consuming wine. As mentioned above, obesity is defined by a BMI of 30 kg/m2 or more, but the adipose tissue differently distributed in the body and a high waist‐hip ratio are better predictors of liver disease than a high BMI, suggesting that it is more important to consider abdominal obesity than only BMI. 69 This was recently also suggested in a study where the waist‐hip ratio was superior to blood‐based scores such as the FIB‐4 and the NAFLD fibrosis score for predicting the presence of hepatic fibrosis in a low‐prevalence population. 70 Longitudinal studies in a Finnish cohort reported an interaction between high waist circumference or high waist‐hip ratio and alcohol consumption over 210 g per week in men and over 140 g per week in women with an increased risk of liver‐related outcomes 26 , 29 (Table 1). Weekly binge drinking in patients with the metabolic syndrome has also been shown to have a synergistic effect on the risk of developing advanced liver disease with complications such as ascites, esophageal variceal bleeding, and hepatic encephalopathy. 42 Similar findings were reported in almost 10,000 British men over 29 years of follow‐up. An increased risk of death from liver disease was observed if drinking was >15 drinks per week across all BMI categories, but the risk in obese men was even greater. 71 However, a recent publication assessing the incidence of cirrhosis morbidity for participants of the United Kingdom Biobank study identified excessive alcohol intake and obesity as independent risk factors for cirrhosis morbidity but found no additive interaction between alcohol and obesity. 72
Abdominal obesity is further associated with insulin resistance, an important driver of liver disease, by increasing lipolysis from adipose tissue and shunting free fatty acids to the liver. 73 This could, in part, explain why estimations of pathologic fat distribution show a stronger association than BMI with the development of liver disease. In patients with biopsy‐proven steatosis due to ALD, independent risk factors of alcohol‐associated hepatitis and cirrhosis included being overweight for more than 10 years, female sex, and the duration of a risky consumption of alcohol. 74 There were similar findings in women who consumed more than 150 g of alcohol per week, and the risk of developing cirrhosis was higher if they were obese compared to non‐obese women 30 (Table 1).
NEW STEATOTIC LIVER DISEASE NOMENCLATURE AND THE CONCEPT OF MetALD
Recently, a Delphi consensus panel proposed a new nomenclature and diagnostic criteria for fatty liver disease, which were endorsed by European association for the study of the liver and American association for the study of liver diseases. 75 The main aim of this new nomenclature was to avoid the stigmatizing terms “alcoholic” and “fatty.” Excessive fat accumulation in the liver is now defined as “steatotic liver disease” (SLD). SLD is subclassified in 3 main groups: steatosis due to metabolic dysfunction, now termed Metabolic‐ Associated Steatotic Liver Disease, steatosis in the context of ALD, and a group in which metabolic and alcohol risk factors coexist, termed MetALD. Criteria to be defined as MetALD group are a weekly alcohol intake range from 140 to 350 g for females (average daily alcohol intake 20–50 g) and from 210 to 420 g for males (average daily intake 30–60 g), and the presence of only one (or more) cardiometabolic factor. Although patients with MetALD are frequently seen in clinical practice, this term raises a lot of questions among the scientific community.
Although alcohol thresholds of 20–50 g for female and 30–60 g for male are termed “moderate” in the new SLD nomenclature, WHO classified such consumption as important due to the drastic increase in the risk of liver and non‐liver related death. 76 Moreover, this terminology does not consider drastic differences in terms of natural history and liver disease progression. A study comparing the long‐term prognosis in patients with biopsy‐proven NAFLD and ALD reported the occurrence of cirrhosis in 21% of cases in ALD, and only 1% of cases in NAFLD. 77 In addition, a significant proportion of patients classified as “MetALD” have AUD, thereby implicating the need for a specific management of these patients, as recommended by the scientific addictology societies. The introduction of this new nomenclature, particularly the “MetALD” group, will require future prospective studies in order to better define the natural history of this group and to define the more important factors associated with liver‐related events and mortality, in order to define prioritization in the management of these patients.
RISK OF HEPATOCELLULAR CARCINOMA IN PATIENTS WITH ALCOHOL‐RELATED LIVER DISEASE AND OBESITY OR DIABETES
HCC is the most common primary liver malignancy and the third most common cause of cancer‐related death. 78 ALD contributes to nearly a third of HCC cases worldwide and is the main driver of liver carcinogenesis in North America and many Western countries. 79 Several clinical features have been identified in cohort studies as risk factors for alcohol‐related HCC and include older age, male sex, and liver fibrosis, with most cases developing at the cirrhosis stage. Recently, genome‐wide association studies have identified PNPLA3, TM6SF2, HSD17B13, and WNT3A‐WNT9A as important risk loci for alcohol‐related HCC. 80 Currently, NAFLD‐related HCC accounts for 1%–38% of the HCC burden in different countries/regions. 81 Among patients with NAFLD‐associated cirrhosis, the estimated annual incidence of HCC ranges from 0.5% to 2.6%. Less frequently, NAFLD‐related HCC has also been reported in observational studies in patients with noncirrhotic liver at rates between 0.1 and 0.8 per 1000 patient‐years. 82 Several studies have identified diabetes mellitus as a strong risk factor for HCC in patients with NAFLD with and without cirrhosis. 83 , 84 Obesity is also an independent risk factor of HCC. 85 , 86 Several studies have provided strong evidence that obesity, especially early age onset, impacts HCC development with an associated increase in mortality. 7 , 82 , 87 In patients with both alcohol and obesity as risk factors, there seems to be a synergistic effect regarding the risk of HCC. 88 , 89 The effect is also present in patients with diabetes and high alcohol consumption. 42 , 90 , 91 However, there are limited data on the association of mild to moderate alcohol intake with HCC risk in NAFLD. Recent studies have suggested that mild alcohol intake increases the risk of HCC in patients with NAFLD. 92
RISK REDUCTION OF PROGRESSIVE LIVER DISEASE IN PATIENTS WITH ALCOHOL‐RELATED LIVER DISEASE, OBESITY AND DIABETES
Apart from the use of corticosteroids in alcohol‐related hepatitis, no disease modifying drug is approved in ALD or NAFLD, but several important precautions have been suggested to reduce the risk of disease progression. A weight loss of more or equal to 10% in patients with NAFLD who received advice on lifestyle changes reduced fibrosis in 45% of patients, resolved NASH in 90% and improved NAS‐scores on liver biopsies. 93 A dose‐response effect of these outcomes was also seen in patients with a weight loss less than 10%. Weight loss and changes in metabolism by bariatric surgery have also been associated with a reduced risk of progression to cirrhosis, HCC, or liver‐related mortality compared to non‐surgical treatment. 94 Furthermore, secondary prevention by improved treatment of metabolic risk factors other than obesity in the metabolic syndrome is also important. The prescence of hypertension, dyslipidemia, or T2DM adds to the risk of progression to cirrhosis or HCC in patients with NAFLD with each additional metabolic risk factor. 95
Alcohol abstinence is of utmost importance to reduce the risk of progression to cirrhosis and its complications in ALD. In a study investigating factors of long‐term survival in patients with early compensated (fibrosis stage 3 and 4) and decompensated ALD showed that abstinence from alcohol significantly reduced liver‐related mortality in both groups. 96 Given the more progressive nature of ALD compared to NAFLD, 77 a primary focus on alcohol cessation should be made to prevent the progression to liver‐related morbidity and mortality in patients with the metabolic syndrome that consume excessive amounts of alcohol, even though the treatment of metabolic risk factors should not be forgotten.
SUMMARY
Excessive alcohol consumption and metabolic risk factors are the main causes of chronic liver disease worldwide. Current evidence suggests a multiplicative interaction between ALD and metabolic risk factors associated with NAFLD on the risk of developing cirrhosis. Patients with both a high consumption of alcohol and obesity or diabetes should therefore be considered as a risk group for cirrhosis and HCC. Additional studies regarding the efficacy of screening in such risk groups are needed.
CONFLICT OF INTEREST STATEMENT
None.
ACKNOWLEDGMENTS
The authors acknowledge the contribution of a medical writer, Sandy Field, PhD, for English language editing and formatting of this manuscript. Hagström was supported by grants from The Swedish Research Council, The Swedish Cancer Society, the Radiumhemmet Research Foundation and Region Stockholm. Hagströms’ institution has received research grants from Astra Zeneca, EchoSens, Gilead, Intercept, MSD and Pfizer unrelated to the current manuscript.
Hagström H, Hegmar H, Moreno C. Interactions between the metabolic syndrome and alcohol consumption increases the risk of liver disease. United European Gastroenterol J. 2024;12(2):168–176. 10.1002/ueg2.12524
Guarantor of article: Hannes Hagström and Christophe Moreno. All authors approved the final version of the article, including the authorship list.
Contributor Information
Hannes Hagström, Email: hannes.hagstrom@ki.se.
Christophe Moreno, Email: Christophe.moreno@hubruxelles.be.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851–861. 10.1016/s2468-1253(22)00165-0 [DOI] [PubMed] [Google Scholar]
- 2. Dang K, Hirode G, Singal AK, Sundaram V, Wong RJ. Alcoholic liver disease epidemiology in the United States: a retrospective analysis of 3 US databases. Am J Gastroenterol. 2020;115(1):96–104. 10.14309/ajg.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 3. Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59(1):160–168. 10.1016/j.jhep.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 4. Sørensen TI, Orholm M, Bentsen KD, Høybye G, Eghøje K, Christoffersen P. Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet. 1984;2(8397):241–244. 10.1016/s0140-6736(84)90295-2 [DOI] [PubMed] [Google Scholar]
- 5. Leevy CM. Fatty liver: a study of 270 patients with biopsy proven fatty liver and review of the literature. Medicine (Baltim). 1962;41:249–276. [DOI] [PubMed] [Google Scholar]
- 6. Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346(8981):987–990. 10.1016/s0140-6736(95)91685-7 [DOI] [PubMed] [Google Scholar]
- 7. Hagström H, Tynelius P, Rasmussen F. High BMI in late adolescence predicts future severe liver disease and hepatocellular carcinoma: a national, population‐based cohort study in 1.2 million men. Gut. 2018;67(8):1536–1542. 10.1136/gutjnl-2016-313622 [DOI] [PubMed] [Google Scholar]
- 8. Hagström H, Hemmingsson T, Discacciati A, Andreasson A. Alcohol consumption in late adolescence is associated with an increased risk of severe liver disease later in life. J Hepatol. 2018;68(3):505–510. 10.1016/j.jhep.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 9. Rinella ME, Neuschwander‐Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797–1835. 10.1097/hep.0000000000000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysus study group. Gut. 1997;41(6):845–850. 10.1136/gut.41.6.845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–1035. 10.1016/s0140-6736(18)31310-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thursz M, Gual A, Lackner C, Mathurin P, Moreno C, Spahr L, et al. EASL clinical practice guidelines: management of alcohol‐related liver disease. J Hepatol. 2018;69(1):154–181. 10.1016/j.jhep.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 13. Liver EAftSot, Diabetes EAftSo, Obesity EAftSo . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization . Global status report on alcohol and health 2018. World Health Organization; 2019. [Google Scholar]
- 15. O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14–32; quiz 3. 10.1038/ajg.2009.593 [DOI] [PubMed] [Google Scholar]
- 16. Niu X, Zhu L, Xu Y, Zhang M, Hao Y, Ma L, et al. Global prevalence, incidence, and outcomes of alcohol related liver diseases: a systematic review and meta‐analysis. BMC Publ Health. 2023;23(1):859. 10.1186/s12889-023-15749-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Askgaard G, Grønbæk M, Kjær MS, Tjønneland A, Tolstrup JS. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol. 2015;62(5):1061–1067. 10.1016/j.jhep.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 18. Hagström H, Thiele M, Roelstraete B, Söderling J, Ludvigsson JF. Mortality in biopsy‐proven alcohol‐related liver disease: a population‐based nationwide cohort study of 3453 patients. Gut. 2021;70(1):170–179. 10.1136/gutjnl-2019-320446 [DOI] [PubMed] [Google Scholar]
- 19. Barrio E, Tomé S, Rodríguez I, Gude F, Sánchez‐Leira J, Pérez‐Becerra E, et al. Liver disease in heavy drinkers with and without alcohol withdrawal syndrome. Alcohol Clin Exp Res. 2004;28(1):131–136. 10.1097/01.alc.0000106301.39746.eb [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization . Obesity and overweight 2021. [cited 2022 May 8]. Available from https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight
- 21. Ampofo AG, Boateng EB. Beyond 2020: modelling obesity and diabetes prevalence. Diabetes Res Clin Pract. 2020;167:108362. 10.1016/j.diabres.2020.108362 [DOI] [PubMed] [Google Scholar]
- 22. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–2682. 10.1002/hep.30251 [DOI] [PubMed] [Google Scholar]
- 23. Machado M, Marques‐Vidal P, Cortez‐Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600–606. 10.1016/j.jhep.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 24. Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. Metabolic risk factors and incident advanced liver disease in non‐alcoholic fatty liver disease (NAFLD): a systematic review and meta‐analysis of population‐based observational studies. PLoS Med. 2020;17(4):e1003100. 10.1371/journal.pmed.1003100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ajmera V, Belt P, Wilson LA, Gill RM, Loomba R, Kleiner DE, et al. Among patients with nonalcoholic fatty liver disease, modest alcohol use is associated with less improvement in histologic steatosis and steatohepatitis. Clin Gastroenterol Hepatol. 2018;16(9):1511–1520.e5. 10.1016/j.cgh.2018.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Åberg F, Helenius‐Hietala J, Puukka P, Färkkilä M, Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018;67(6):2141–2149. 10.1002/hep.29631 [DOI] [PubMed] [Google Scholar]
- 27. Israelsen M, Juel HB, Detlefsen S, Madsen BS, Rasmussen DN, Larsen TR, et al. Metabolic and genetic risk factors are the strongest predictors of severity of alcohol‐related liver fibrosis. Clin Gastroenterol Hepatol. 2020. [DOI] [PubMed] [Google Scholar]
- 28. Whitfield JB, Masson S, Liangpunsakul S, Mueller S, Aithal GP, Eyer F, et al. Obesity, diabetes, coffee, tea, and cannabis use alter risk for alcohol‐related cirrhosis in 2 large cohorts of high‐risk drinkers. Am J Gastroenterol. 2021;116(1):106–115. 10.14309/ajg.0000000000000833 [DOI] [PubMed] [Google Scholar]
- 29. Sahlman P, Nissinen M, Puukka P, Jula A, Salomaa V, Männistö S, et al. Genetic and lifestyle risk factors for advanced liver disease among men and women. J Gastroenterol Hepatol. 2020;35(2):291–298. 10.1111/jgh.14770 [DOI] [PubMed] [Google Scholar]
- 30. Liu B, Balkwill A, Reeves G, Beral V. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. Bmj. 2010;340(mar11 1):c912. 10.1136/bmj.c912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagström H, Höijer J, Andreasson A, Bottai M, Johansson K, Ludvigsson JF, et al. Body mass index in early pregnancy and future risk of severe liver disease: a population‐based cohort study. Aliment Pharmacol Ther. 2019;49(6):789–796. 10.1111/apt.15162 [DOI] [PubMed] [Google Scholar]
- 32. Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212–1218. 10.2337/dc06-2247 [DOI] [PubMed] [Google Scholar]
- 33. El‐Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–468. 10.1053/j.gastro.2003.10.065 [DOI] [PubMed] [Google Scholar]
- 34. Arab JP, Barrera F, Gallego C, Valderas JP, Uribe S, Tejos C, et al. High prevalence of undiagnosed liver cirrhosis and advanced fibrosis in type 2 diabetic patients. Ann Hepatol. 2016;15(5):721–728. [DOI] [PubMed] [Google Scholar]
- 35. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol. 2019;71(4):793–801. 10.1016/j.jhep.2019.06.021 [DOI] [PubMed] [Google Scholar]
- 36. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy‐proven NAFLD. J Hepatol. 2017;67(6):1265–1273. 10.1016/j.jhep.2017.07.027 [DOI] [PubMed] [Google Scholar]
- 37. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology. 2020;158(6):1611–1625.e12. 10.1053/j.gastro.2020.01.043 [DOI] [PubMed] [Google Scholar]
- 38. Huang DQ, Noureddin N, Ajmera V, Amangurbanova M, Bettencourt R, Truong E, et al. Type 2 diabetes, hepatic decompensation, and hepatocellular carcinoma in patients with non‐alcoholic fatty liver disease: an individual participant‐level data meta‐analysis. Lancet Gastroenterol Hepatol. 2023;8(9):829–836. 10.1016/s2468-1253(23)00157-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Åberg F, Puukka P, Salomaa V, Männistö S, Lundqvist A, Valsta L, et al. Risks of light and moderate alcohol use in fatty liver disease: follow‐up of population cohorts. Hepatology. 2020;71(3):835–848. 10.1002/hep.30864 [DOI] [PubMed] [Google Scholar]
- 40. Sookoian S, Flichman D, Castaño GO, Pirola CJ. Mendelian randomisation suggests no beneficial effect of moderate alcohol consumption on the severity of nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2016;44(11‐12):1224–1234. 10.1111/apt.13828 [DOI] [PubMed] [Google Scholar]
- 41. Ekstedt M, Franzén LE, Holmqvist M, Bendtsen P, Mathiesen UL, Bodemar G, et al. Alcohol consumption is associated with progression of hepatic fibrosis in non‐alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44(3):366–374. 10.1080/00365520802555991 [DOI] [PubMed] [Google Scholar]
- 42. Åberg F, Helenius‐Hietala J, Puukka P, Jula A. Binge drinking and the risk of liver events: a population‐based cohort study. Liver Int. 2017;37(9):1373–1381. 10.1111/liv.13408 [DOI] [PubMed] [Google Scholar]
- 43. Hagström H, Hemmingsson T, Discacciati A, Andreasson A. Risk behaviors associated with alcohol consumption predict future severe liver disease. Dig Dis Sci. 2019;64(7):2014–2023. 10.1007/s10620-019-05509-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Åberg F, Byrne CD, Pirola CJ, Männistö V, Sookoian S. Alcohol consumption and metabolic syndrome: clinical and epidemiological impact on liver disease. J Hepatol. 2023;78(1):191–206. 10.1016/j.jhep.2022.08.030 [DOI] [PubMed] [Google Scholar]
- 45. Hamaguchi M, Kojima T, Ohbora A, Takeda N, Fukui M, Kato T. Protective effect of alcohol consumption for fatty liver but not metabolic syndrome. World J Gastroenterol. 2012;18(2):156–167. 10.3748/wjg.v18.i2.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hiramine Y, Imamura Y, Uto H, Koriyama C, Horiuchi M, Oketani M, et al. Alcohol drinking patterns and the risk of fatty liver in Japanese men. J Gastroenterol. 2011;46(4):519–528. 10.1007/s00535-010-0336-z [DOI] [PubMed] [Google Scholar]
- 47. Kwon HK, Greenson JK, Conjeevaram HS. Effect of lifetime alcohol consumption on the histological severity of non‐alcoholic fatty liver disease. Liver Int. 2014;34(1):129–135. 10.1111/liv.12230 [DOI] [PubMed] [Google Scholar]
- 48. Mitchell T, Jeffrey GP, de Boer B, MacQuillan G, Garas G, Ching H, et al. Type and pattern of alcohol consumption is associated with liver fibrosis in patients with non‐alcoholic fatty liver disease. Am J Gastroenterol. 2018;113(10):1484–1493. 10.1038/s41395-018-0133-5 [DOI] [PubMed] [Google Scholar]
- 49. Moriya A, Iwasaki Y, Ohguchi S, Kayashima E, Mitsumune T, Taniguchi H, et al. Roles of alcohol consumption in fatty liver: a longitudinal study. J Hepatol. 2015;62(4):921–927. 10.1016/j.jhep.2014.11.025 [DOI] [PubMed] [Google Scholar]
- 50. Hagström H, Nasr P, Ekstedt M, Kechagias S, Önnerhag K, Nilsson E, et al. Low to moderate lifetime alcohol consumption is associated with less advanced stages of fibrosis in non‐alcoholic fatty liver disease. Scand J Gastroenterol. 2017;52(2):159–165. 10.1080/00365521.2016.1239759 [DOI] [PubMed] [Google Scholar]
- 51. Dunn W, Sanyal AJ, Brunt EM, Unalp‐Arida A, Donohue M, McCullough AJ, et al. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non‐alcoholic fatty liver disease (NAFLD). J Hepatol. 2012;57(2):384–391. 10.1016/j.jhep.2012.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patel PJ, Smith D, Connor JP, Horsfall LU, Hayward KL, Hossain F, et al. Alcohol consumption in diabetic patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol. 2017;2017:7927685–7927688. 10.1155/2017/7927685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hayashi PH, Harrison SA, Torgerson S, Perez TA, Nochajski T, Russell M. Cognitive lifetime drinking history in nonalcoholic fatty liver disease: some cases may be alcohol related. Am J Gastroenterol. 2004;99(1):76–81. 10.1046/j.1572-0241.2003.04013.x [DOI] [PubMed] [Google Scholar]
- 54. Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C. PHosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol‐dependent patients. Alcohol Alcohol. 2006;41(4):431–437. 10.1093/alcalc/agl027 [DOI] [PubMed] [Google Scholar]
- 55. Kechagias S, Dernroth DN, Blomgren A, Hansson T, Isaksson A, Walther L, et al. Phosphatidylethanol compared with other blood tests as a biomarker of moderate alcohol consumption in healthy volunteers: a prospective randomized study. Alcohol Alcohol. 2015;50(4):399–406. 10.1093/alcalc/agv038 [DOI] [PubMed] [Google Scholar]
- 56. Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47(5):552–557. 10.1093/alcalc/ags065 [DOI] [PubMed] [Google Scholar]
- 57. Staufer K, Huber‐Schönauer U, Strebinger G, Pimingstorfer P, Suesse S, Scherzer TM, et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non‐alcoholic fatty liver disease. J Hepatol. 2022;77(4):918–930. 10.1016/j.jhep.2022.04.040 [DOI] [PubMed] [Google Scholar]
- 58. Kim SJ, Kim DJ. Alcoholism and diabetes mellitus. Diabetes Metab J. 2012;36(2):108–115. 10.4093/dmj.2012.36.2.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leong C, Bolton JM, Ekuma O, Prior HJ, Singal D, Nepon J, et al. Association of alcohol use disorder on alcohol‐related cancers, diabetes, ischemic heart disease and death: a population‐based, matched cohort study. Addiction. 2022;117(2):368–381. 10.1111/add.15646 [DOI] [PubMed] [Google Scholar]
- 60. Zaharia OP, Strassburger K, Strom A, Bönhof GJ, Karusheva Y, Antoniou S, et al. Risk of diabetes‐associated diseases in subgroups of patients with recent‐onset diabetes: a 5‐year follow‐up study. Lancet Diabetes Endocrinol. 2019;7(9):684–694. 10.1016/s2213-8587(19)30187-1 [DOI] [PubMed] [Google Scholar]
- 61. Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, et al. Risk factors of fibrosis in alcohol‐induced liver disease. Hepatology. 2002;35(3):635–638. 10.1053/jhep.2002.31782 [DOI] [PubMed] [Google Scholar]
- 62. Younossi ZM, Stepanova M, Ong J, Yilmaz Y, Duseja A, Eguchi Y, et al. Effects of alcohol consumption and metabolic syndrome on mortality in patients with nonalcoholic and alcohol‐related fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(8):1625–1633.e1. 10.1016/j.cgh.2018.11.033 [DOI] [PubMed] [Google Scholar]
- 63. Mallet V, Parlati L, Martinino A, Scarano Pereira JP, Jimenez CN, Sakka M, et al. Burden of liver disease progression in hospitalized patients with type 2 diabetes mellitus. J Hepatol. 2022;76(2):265–274. 10.1016/j.jhep.2021.09.030 [DOI] [PubMed] [Google Scholar]
- 64. Zaharia OP, Strassburger K, Knebel B, Kupriyanova Y, Karusheva Y, Wolkersdorfer M, et al. Role of patatin‐like phospholipase domain‐containing 3 gene for hepatic lipid content and insulin resistance in diabetes. Diabetes Care. 2020;43(9):2161–2168. 10.2337/dc20-0329 [DOI] [PubMed] [Google Scholar]
- 65. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4(1):122–130. 10.1007/s13679-014-0129-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Parker R, Kim SJ, Gao B. Alcohol, adipose tissue and liver disease: mechanistic links and clinical considerations. Nat Rev Gastroenterol Hepatol. 2018;15(1):50–59. 10.1038/nrgastro.2017.116 [DOI] [PubMed] [Google Scholar]
- 67. Bendsen NT, Christensen R, Bartels EM, Kok FJ, Sierksma A, Raben A, et al. Is beer consumption related to measures of abdominal and general obesity? A systematic review and meta‐analysis. Nutr Rev. 2013;71(2):67–87. 10.1111/j.1753-4887.2012.00548.x [DOI] [PubMed] [Google Scholar]
- 68. Sayon‐Orea C, Martinez‐Gonzalez MA, Bes‐Rastrollo M. Alcohol consumption and body weight: a systematic review. Nutr Rev. 2011;69(8):419–431. 10.1111/j.1753-4887.2011.00403.x [DOI] [PubMed] [Google Scholar]
- 69. Andreasson A, Carlsson AC, Önnerhag K, Hagström H. Waist/hip ratio better predicts development of severe liver disease within 20 Years than body mass index: a population‐based cohort study. Clin Gastroenterol Hepatol. 2017;15(8):1294–1301.e2. 10.1016/j.cgh.2017.02.040 [DOI] [PubMed] [Google Scholar]
- 70. Graupera I, Thiele M, Serra‐Burriel M, Caballeria L, Roulot D, Wong GL, et al. Low accuracy of FIB‐4 and NAFLD fibrosis scores for screening for liver fibrosis in the population. Clin Gastroenterol Hepatol. 2022;20(11):2567–2576.e6. 10.1016/j.cgh.2021.12.034 [DOI] [PubMed] [Google Scholar]
- 71. Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. Bmj. 2010;340(mar11 1):c1240. 10.1136/bmj.c1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Innes H, Crooks CJ, Aspinall E, Card TR, Hamill V, Dillon J, et al. Characterizing the risk interplay between alcohol intake and body mass index on cirrhosis morbidity. Hepatology. 2022;75(2):369–378. 10.1002/hep.32123 [DOI] [PubMed] [Google Scholar]
- 73. Åberg F, Färkkilä M, Männistö V. Interaction between alcohol use and metabolic risk factors for liver disease: a critical review of epidemiological studies. Alcohol Clin Exp Res. 2020;44(2):384–403. 10.1111/acer.14271 [DOI] [PubMed] [Google Scholar]
- 74. Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25(1):108–111. 10.1053/jhep.1997.v25.pm0008985274 [DOI] [PubMed] [Google Scholar]
- 75. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi‐society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–1556. 10.1016/j.jhep.2023.06.003 [DOI] [PubMed] [Google Scholar]
- 76. Moreno C, Sheron N, Tiniakos D, Lackner C, Mathurin P. "Dual aetiology fatty liver disease": a recently proposed term associated with potential pitfalls. J Hepatol. 2021;74(4):979–982. 10.1016/j.jhep.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 77. Dam‐Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sørensen TI, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53(5):750–755. 10.1136/gut.2003.019984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7(1):6. 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- 79. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3(12):1683–1691. 10.1001/jamaoncol.2017.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Trépo E, Caruso S, Yang J, Imbeaud S, Couchy G, Bayard Q, et al. Common genetic variation in alcohol‐related hepatocellular carcinoma: a case‐control genome‐wide association study. Lancet Oncol. 2022;23(1):161–171. 10.1016/s1470-2045(21)00603-3 [DOI] [PubMed] [Google Scholar]
- 81. Huang DQ, El‐Serag HB, Loomba R. Global epidemiology of NAFLD‐related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18(4):223–238. 10.1038/s41575-020-00381-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shah PA, Patil R, Harrison SA. NAFLD‐related hepatocellular carcinoma: the growing challenge. Hepatology. 2023;77(1):323–338. 10.1002/hep.32542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology. 2020;71(3):907–916. 10.1002/hep.30858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alexander M, Loomis AK, van der Lei J, Duarte‐Salles T, Prieto‐Alhambra D, Ansell D, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real‐world study of 18 million patients in four European cohorts. BMC Med. 2019;17(1):95. 10.1186/s12916-019-1321-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta‐analysis. Diabetes Metab Res Rev. 2012;28(2):109–122. 10.1002/dmrr.1291 [DOI] [PubMed] [Google Scholar]
- 86. Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60(1):110–117. 10.1016/j.jhep.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 87. Hassan MM, Abdel‐Wahab R, Kaseb A, Shalaby A, Phan AT, El‐Serag HB, et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology. 2015;149(1):119–129. 10.1053/j.gastro.2015.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Loomba R, Yang HI, Su J, Brenner D, Barrett‐Connor E, Iloeje U, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177(4):333–342. 10.1093/aje/kws252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42(2):218–224. 10.1016/j.jhep.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 90. Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36(5):1206–1213. 10.1053/jhep.2002.36780 [DOI] [PubMed] [Google Scholar]
- 91. Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the. US Cancer. 2004;101(5):1009–1017. 10.1002/cncr.20427 [DOI] [PubMed] [Google Scholar]
- 92. Kimura T, Tanaka N, Fujimori N, Sugiura A, Yamazaki T, Joshita S, et al. Mild drinking habit is a risk factor for hepatocarcinogenesis in non‐alcoholic fatty liver disease with advanced fibrosis. World J Gastroenterol. 2018;24(13):1440–1450. 10.3748/wjg.v24.i13.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, Torres‐Gonzalez A, Gra‐Oramas B, Gonzalez‐Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378.e5; quiz e14‐5. 10.1053/j.gastro.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 94. Aminian A, Al‐Kurd A, Wilson R, Bena J, Fayazzadeh H, Singh T, et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy‐proven nonalcoholic steatohepatitis. JAMA. 2021;326(20):2031–2042. 10.1001/jama.2021.19569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology. 2020;71(3):808–819. 10.1002/hep.31014 [DOI] [PubMed] [Google Scholar]
- 96. Lackner C, Spindelboeck W, Haybaeck J, Douschan P, Rainer F, Terracciano L, et al. Histological parameters and alcohol abstinence determine long‐term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66(3):610–618. 10.1016/j.jhep.2016.11.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


