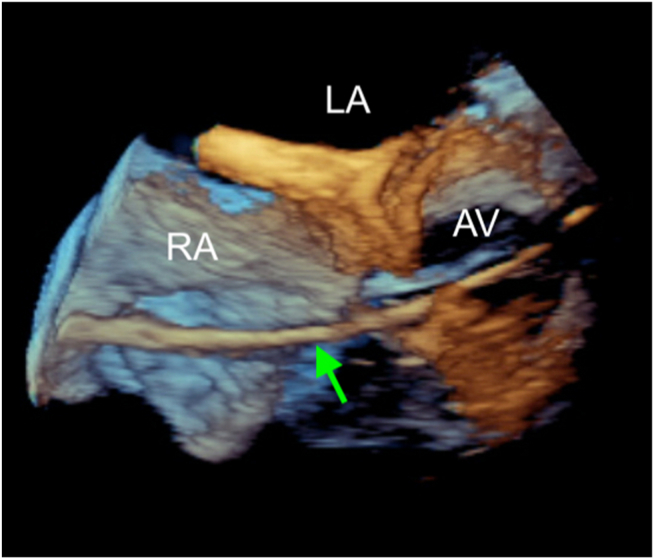
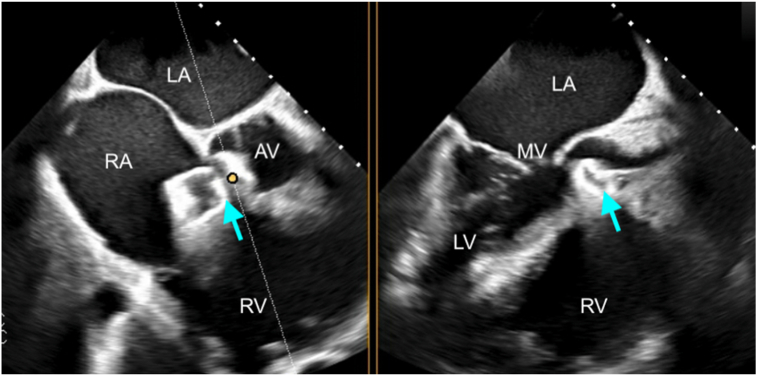
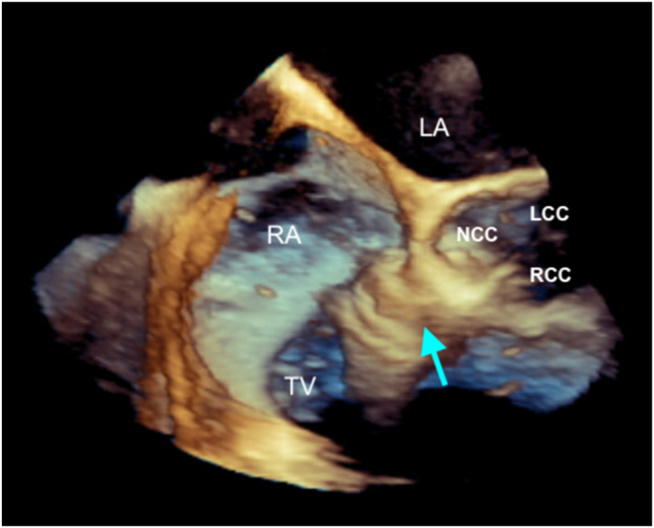
Graphical abstract
Highlights
-
•
SOVA is a rare cardiac anomaly.
-
•
Ruptured SOVA carries a high mortality rate.
-
•
SOVA often coexists with other congenital lesions, most commonly VSD and bicuspid AV.
-
•
Ruptured SOVA needs repair; percutaneous repair is a safe alternative to surgery.
-
•
Echo plays a vital role in both diagnosing SOVA and guiding percutaneous closure.
Introduction
Sinus of Valsalva aneurysm (SOVA) is a rare cardiac anomaly that can remain clinically silent for years. The most feared complication, SOVA rupture, carries a high mortality rate unless diagnosed and treated in a timely manner. Surgical repair has traditionally been the treatment of choice. However, percutaneous device closure of a ruptured SOVA has become a feasible alternative to surgical repair for select patients. Here we present a patient with a ruptured SOVA and demonstrate the importance of echocardiography in the diagnosis and procedural management of this complex lesion.
Case Presentation
A 31-year-old man with a history of heavy alcohol use and obesity class III (body mass index, 54) presented to the emergency department with new onset of progressive lower extremity and scrotal edema, as well as dyspnea on exertion for 2 weeks. Their vital signs were blood pressure 109/62 mm Hg, sinus tachycardia at a heart rate of 120 bpm, and SpO2 96% on room air. However, physical examination was notable for a loud machinery continuous murmur and thrill at the left sternal edge, with chest x-ray revealing cardiomegaly and pulmonary edema. Transthoracic echocardiogram (TTE) demonstrated a severely dilated right ventricle (RV) with moderately reduced systolic function and severe tricuspid regurgitation (TR) secondary to a ruptured SOVA into the right atrium (RA) with continuous left-to-right flow (Figure 1, Video 1). There was mild aortic regurgitation (AR), and the aortic root was mildly dilated (4.3 cm) at the level of the sinuses. The left ventricle (LV) was moderately dilated with moderately reduced systolic function. The patient’s obesity, biventricular dysfunction, and severely reduced functional capacity placed them at high risk for surgical repair; therefore percutaneous closure of this ruptured SOVA was pursued.
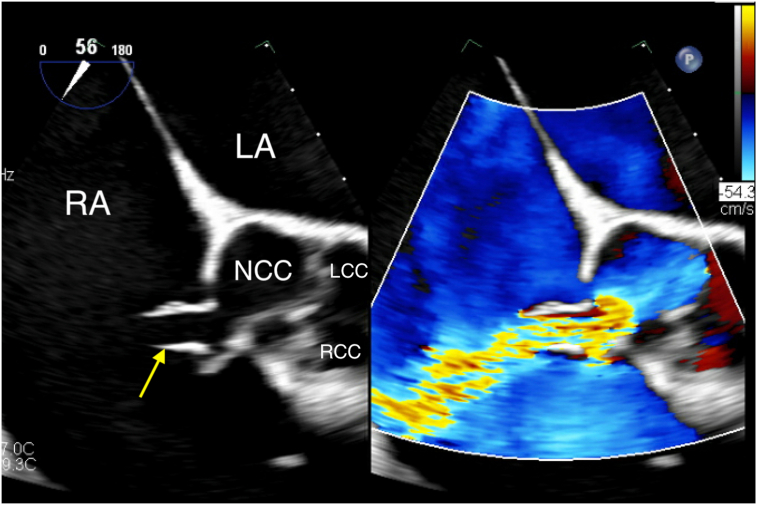
Figure 1.
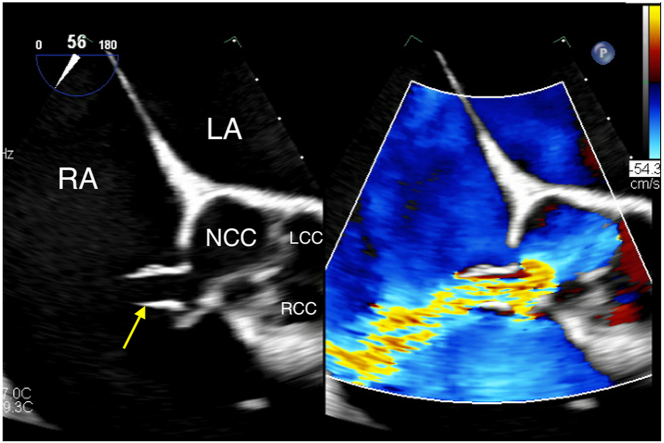
Two-dimensional TTE, parasternal short-axis view in diastole, without (left) and with (right) color-flow Doppler, demonstrates the aortic valve (AV) and the elongated body of the ruptured SOVA (arrows) arising from the noncoronary sinus extending into the RA, above the plane of the tricuspid valve (TV). LA, Left atrium; LCC, left coronary cusp; NCC, noncoronary cusp; RCC, right coronary cusp.
In the catheterization lab, the patient tolerated induction of general anesthesia with special attention paid to the diastolic blood pressure to minimize exacerbation of coronary hypoperfusion in the setting of an aorta-to-RA shunt. Transesophageal echocardiography (TEE) confirmed the preoperative diagnosis of a ruptured SOVA. On two-dimensional (2D) imaging, the midesophageal 4-chamber view demonstrated a small “windsock” aneurysm protruding into the RA (Figure 2, Video 2). Biplane imaging localized the rupture to the noncoronary sinus of Valsalva in close proximity to the right and noncoronary commissures (Figure 3). Color-flow Doppler showed a distinct, high-velocity jet from the aortic sinus into the RA, above the plane of the tricuspid valve annulus and separate from the severe central TR jet (Figure 4, Video 3). Continuous-wave Doppler through this jet showed blood flow during both systole and diastole (Figure 5). Three-dimensional (3D) imaging further demonstrated the classic windsock appearance of the aneurysm sac extending into the RA (Figure 6, Video 4).
Figure 2.
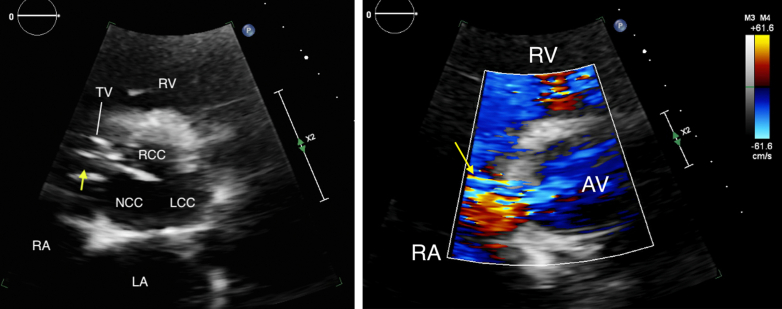
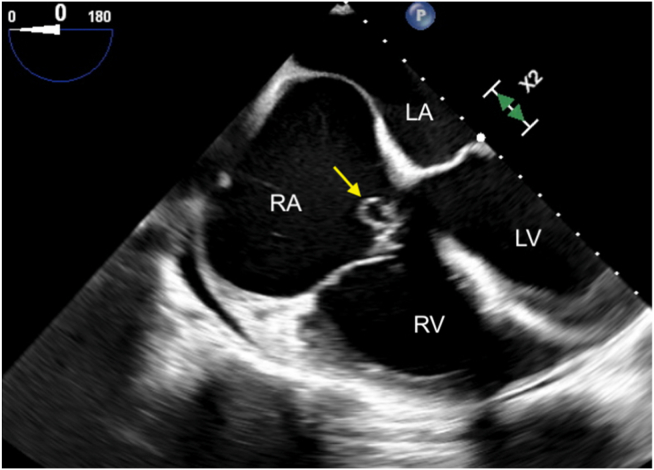
Two-dimensional TEE, midesophageal 4-chamber view (0°) in systole, demonstrates the ruptured SOVA (arrow) protruding into the RA, just above the tricuspid valve annulus. LA, Left atrium.
Figure 3.
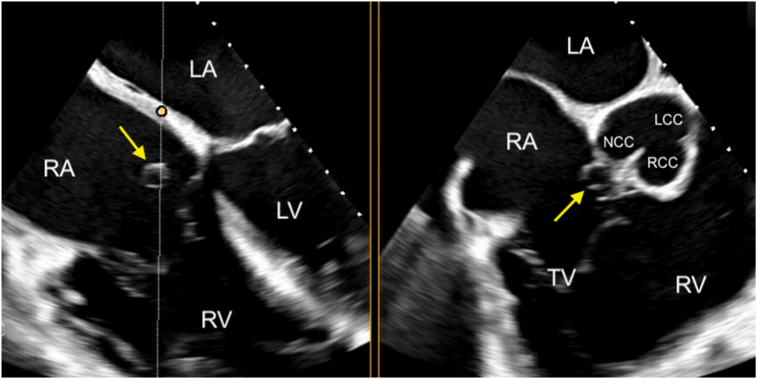
Two-dimensional TEE, midesophageal biplane imaging (dotted white line with yellow circle) through the SOVA (arrows), 4-chamber (left) and basal short-axis (right) displays in diastole, demonstrates the SOVA is located at the noncoronary cusp of the aortic valve, in close proximity to the non- and right aortic commissure.
Figure 4.
Two-dimensional TEE, midesophageal aortic valve short-axis view (56°) in diastole, without (left) and with (right) color-flow Doppler, demonstrates a distinct, high-velocity horizontal jet arising from the noncoronary sinus, through the ruptured SOVA (arrow) into the RA.
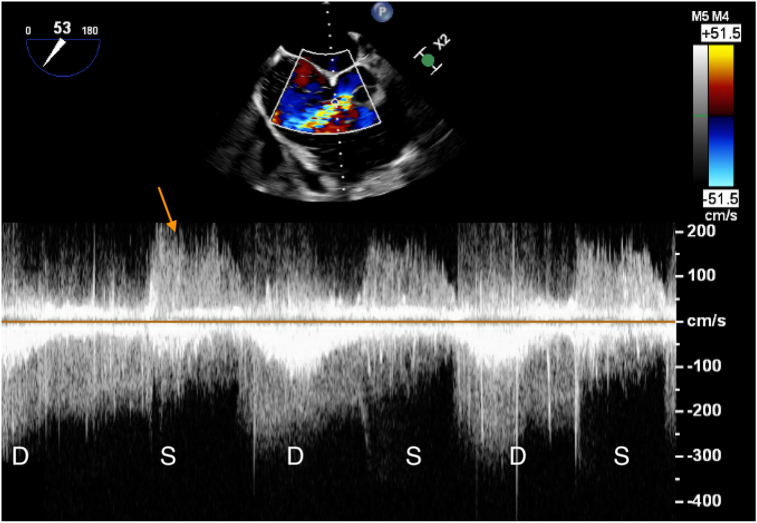
Figure 5.
Two-dimensional TEE, midesophageal aortic valve short-axis view (53°) with color-flow Doppler-guided continuous-wave Doppler misaligned through the color jet from the ruptured SOVA. The flow is high velocity (despite the misalignment) and continuous in systole (S) and diastole (D) but accentuates in diastole. The arrow points to the contaminating TR signal in systole.
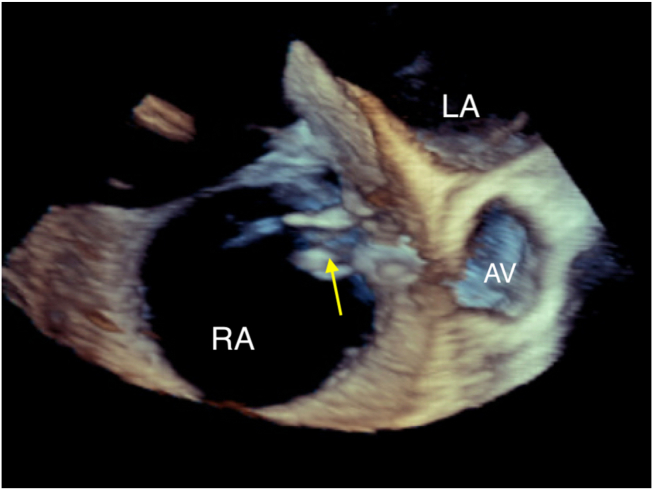
Figure 6.
Three-dimensional TEE, volume-rendered, basal short-axis display, demonstrates the classic windsock appearance of the aneurysm sac (arrow) with its tail in the RA.
Aortic root angiography was consistent with the TEE diagnosis, and a transaortic approach to device closure was planned. The size of the defect was carefully measured in 2D and 3D TEE, and closure devices were chosen accordingly. A guidewire was passed through the ruptured noncoronary sinus into the RA followed by a long delivery sheath, all with fluoroscopic and real-time TEE guidance using live 3D and biplane modes (Figures 7 and 8). During the first attempt, a 10 × 8 mm Amplatzer Duct Occluder was delivered in an antegrade fashion. Fluoroscopy and TEE confirmed that the device was appropriately seated and occluded the communication. However, there was unwanted capture of nearby aortic leaflet resulting in worsening AR. During the second attempt, a 20 mm Amplatzer AVP II device was selected and advanced in a retrograde fashion with deployment of the first disk and the central body in the RA and the second disk in the noncoronary sinus of Valsalva, again under fluoroscopic and real-time TEE guidance (Figures 9 and 10, Video 5). It was verified to be in an appropriate location and released. On TEE, there was no residual flow through the device (Video 6). No changes in the severity of AR or TR were seen compared to the preprocedural, baseline TEE. Biventricular systolic function remained abnormal, but unchanged, and no new pericardial effusion was observed at the end of the procedure. Follow-up TTE at 6 months showed that the device was well seated with complete occlusion of the ruptured SOVA. Left ventricular systolic function had recovered to normal and with diuresis, TR had improved from severe to mild. Clinically, the patient was more responsive to diuretic therapy, was walking daily, and had improved energy levels, suggesting better functional capacity and fewer symptoms after closure of the ruptured SOVA. The investigation of the etiology of the patient’s SOVA was negative for chest trauma or infection. Given the associated mildly dilated aortic root, the patient was referred for genetic evaluation for connective tissue disease but was lost to follow-up.
Figure 7.
Intraprocedural 2D TEE, midesophageal biplane imaging (dotted white line with yellow circle) through the ruptured SOVA (yellow arrows), aortic valve short-axis (left) and long-axis (right) diastolic displays, demonstrates the guidewire (green arrows) being passed retrogradely from the aorta, through the noncoronary sinus into the RA.
Figure 8.
Real-time 3D TEE, volume-rendered short-axis view at the level of the aortic valve demonstrates the guidewire (arrow) being passed through the ruptured SOVA retrogradely into the RA.
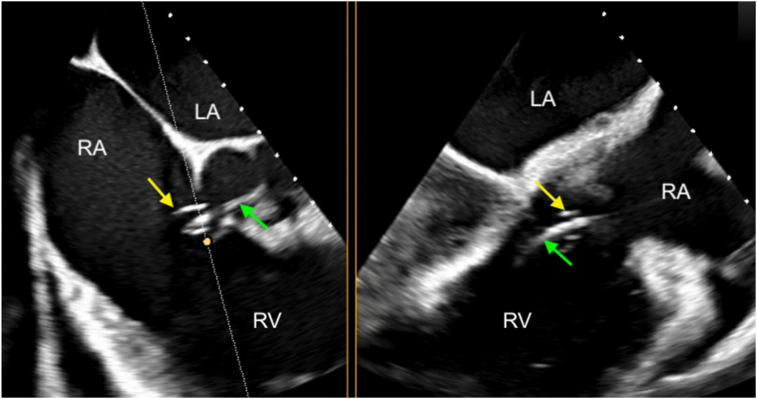
Figure 9.
Intraprocedural 2D TEE, midesophageal biplane imaging (dotted white line with yellow circle) through the closure device, short-axis (left) and long-axis (right) displays, demonstrates the Amplatzer AVPII device (arrows) being deployed with the first disk and the central body in the RA and the second disk in the noncoronary sinus. MV, mitral valve.
Figure 10.
Three-dimensional TEE, volume-rendered short-axis view at the level of the aortic valve demonstrates the closure device (arrow) seated between the RA and noncoronary cusp, with the first disk and the central body in the RA and the second disk in the noncoronary sinus of Valsalva.
Discussion
Sinus of Valsalva aneurysm is a rare cardiac anomaly characterized by thin-walled outpouching of the aortic sinus into the neighboring cardiac chambers. The aneurysm sac often appears elongated and fingerlike due to chronic high aortic pressure on the weakened tissue. When ruptured, it assumes the shape of a windsock on TTE. Sinus of Valsalva aneurysm can be acquired through chest trauma, infection (endocarditis, syphilis), vasculitis, or connective tissue disorder (Marfan, Ehlers-Danlos, Loeys-Dietz). However, in most cases, SOVA is thought to be congenital due to the absence of the elastic lamellae of the aortic media resulting in failure of fusion between the aortic media and annulus.1 Epidemiologic studies suggest that the incidence of congenital SOVA ranges from 0.1% to 3.5%, with a 4:1 male predominance and incidence of rupture 5 times higher in East Asian patients than in Westerners.2, 3, 4 Congenital SOVA most commonly originates from the right coronary sinus (70%-80%), followed by the noncoronary sinus (20%-30%), with <5% arising from the left coronary sinus.5 Sakakibara and Konno subsequently classified the anatomic origin of SOVA and respective protrusion sites into 5 categories known as the Sakakibara classification, which can be simplified into the following: type I, rupture into the RV just beneath the pulmonary valve; type II, rupture into or just beneath the crista supraventricularis of the RV; type III, rupture into the RA or RV near or at the tricuspid annulus; type IV, rupture into the RA; and type V, other rare conditions, such as rupture into the LV, left atrium, pericardium, or pulmonary artery.6,7
An important consideration for congenital SOVA is its association with other congenital cardiac defects, the most common being ventricular septal defects (VSDs), with an incidence between 12% and 60%.3,5 Aortic valve structural anomalies such as cusp prolapse (with or without VSD) and bicuspid aortic valve are found in 10% to 20% of patients.5,8 Other less commonly associated defects include atrial septal defects, patent ductus arteriosus, coarctation of the aorta, and pulmonic or subaortic stenosis.9 The types of VSD in SOVA follow a similar geographic distribution, with supracristal VSD most commonly seen in East Asians and membranous VSD seen in Caucasians.9 One possible explanation for the close association between VSD and right and noncoronary SOVA is that they stem from the same embryological defect of incomplete fusion between the aortopulmonary and interventricular septums, the base of which becomes the right and noncoronary sinuses.10 Given the high prevalence of VSD with SOVA, it is important to distinguish the 2 anomalies. Doppler waveform pattern can be useful since the continuous left-to-right shunting of a ruptured SOVA often produces a high-velocity jet that is accentuated in diastole, whereas an uncomplicated VSD typically demonstrates high-velocity flow mainly in systole. Another distinguishing feature is that a membranous VSD is usually located lower (between LV and RV), whereas SOVA rupture is higher (between aorta and RV or RA). Therefore, on TEE it requires one to sweep from upper to mid esophagus in 45° or looking at 120° midesophagus and distinguish location between the aortic root and LV outflow tract for SOVA versus VSD. An abdominal aortic Doppler pattern of diastolic flow reversal can aid the diagnosis of SOVA in the absence of severe AR. Finally, high spatial resolution 3D TEE images can also help distinguish these 2 entities.
One important diagnostic challenge that frequently accompanies and complicates the pathology and presentation of a ruptured SOVA is AR, with an incidence between 24% and 45%.4,8 In addition, there was a highly significant correlation between AR and the presence of an associated subarterial VSD.8 When all 3 entities, ruptured SOVA, VSD, and severe AR, coexist, the combined systolic VSD flow and diastolic AR flow could mimic the color-flow Doppler pattern of a ruptured SOVA, creating a diagnostic conundrum. In such a case, the subtleties in Doppler waveform pattern could help distinguish them. Shunting due to SOVA rupture begins in mid diastole and gradually increases toward end diastole, whereas AR usually begins in early diastole and continues throughout diastole in a decrescendo fashion.11
The difficulty with diagnosing ruptured SOVA is mainly due to the highly variable clinical presentation. Symptoms range from sudden cardiac death, malignant arrhythmias, and acute coronary ischemia, to insidious onset of heart failure, to being completely asymptomatic.12 Once a ruptured SOVA is on the differential, diagnosis can be made via echocardiogram, which has replaced cardiac catheterization as the first-line imaging modality. In a single-center study of 212 surgical patients with ruptured SOVA, compared against surgical results, the sensitivity, specificity, and accuracy of echocardiographic diagnosis of SOVA were 93.9%, 99.9%, and 99.8%, respectively.13 If patients are stable, tomographic imaging with cardiac computed tomography and cardiovascular magnetic resonance imaging can be helpful for accurate assessment of the entire thoracoabdominal aorta and can further assist surgical/interventional planning.
Once a SOVA ruptures, the mean survival without intervention is 3.9 years, so repair is always indicated. Surgical repair has been the mainstay treatment since it was established in 1957. Several studies have reported acceptable short-term risks and favorable outcomes of percutaneous closure for carefully selected patients.14 Selection bias prohibits a direct comparison between groups of patients undergoing surgical versus percutaneous closure, but a review of 34 studies detailing the percutaneous approach in 136 patients showed that mild early residual shunting either remained stable or had completely disappeared by early follow-up.15
As illustrated by this patient, TEE plays a critical role in visualizing the defect as well as guiding percutaneous repair of the ruptured SOVA. Important advances in 3D TEE technology have enabled the creation of real-time 3D images with high temporal and spatial resolution that clearly demonstrate the complex spatial relationships between the ruptured SOVA and its surrounding structures. They also allow accurate and effective tracking and navigation of wires and devices through these nuanced structures. Furthermore, TEE is critical in assessing the repair success and providing immediate evaluation of procedural complications, which may result from device impingement on surrounding structures such as the aortic valve or tricuspid valve leaflets. Rare complications include device thrombosis, device embolization, cardiac perforation, coronary embolism or impingement, and conduction abnormalities such as complete atrioventricular block, all of which can be readily assessed on TEE. Currently, there are no surveillance imaging recommendations specifically made for SOVAs. A multimodality imaging approach can be taken based on each individual case. In patients with adequate transthoracic imaging windows, TTE could be the modality of choice for serial follow-up to evaluate ventricular function, TR, aortic root size, AR, and residual leaks. When tomographic imaging is indicated, cardiovascular magnetic resonance imaging or cardiac computed tomography may be considered.
Conclusion
In conclusion, ruptured SOVA is a rare congenital anomaly that can present with a wide clinical spectrum and requires physicians to have a high index of suspicion. Echocardiography offers excellent diagnostic sensitivity and specificity. In addition, as percutaneous device closure is becoming more widely employed, a clear understanding of the echocardiographic features of rupture of SOVA and possible procedure complications is crucial in periprocedural management.
Consent Statement
Complete written informed consent was obtained from the patient (or appropriate parent, guardian, or power of attorney) for the publication of this study and accompanying images.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Acknowledgments
We thank Xiu Tang for acquisition of the transthoracic echo images.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2023.12.006.
Supplementary Data
Two-dimensional TTE, parasternal short-axis view without (left) and with (right) color-flow Doppler, zoom-in view on the aortic valve in short axis. The body of the ruptured SOVA is seen arising from the noncoronary cusp extending into the RA. Color flow is continuous through the SOVA but accentuates in diastole.
Two-dimensional TEE, midesophageal 4-chamber view, demonstrates a small flailing SOVA in the RA, just above the tricuspid valve annulus. The RA and RV are dilated. The interatrial septum bulges toward the left, indicating relatively elevated right-sided pressure. Both the RV and LV have moderately reduced systolic function.
Two-dimensional TEE, midesophageal short-axis view at the aortic valve, without (left) and with (right) color-flow Doppler, demonstrates 2 distinct color jets. One is more horizontal with a continuous jet (accentuated during diastole) from the ruptured SOVA into the RA. The other is more perpendicular and central representing the systolic TR jet into the RA.
Three-dimensional TEE, volume-rendered, basal short-axis view of the ruptured SOVA, which nicely demonstrates the classic windsock appearance of the aneurysm sac with its tail in the RA.
Intraprocedural 2D TEE, midesophageal short-axis view at the aortic valve, demonstrates that the closure device is well seated across the RA and the noncoronary sinus. The tricuspid valve leaflets and the aortic valve cusps are all moving freely without impingement by the device.
Two-dimensional TEE, midesophageal short-axis view with color-flow Doppler, demonstrates that the closure device has successfully captured the SOVA jet. There is minimal color flow across the device itself. The severity of the central TR jet remains the same as precatheter TR.
References
- 1.Edwards J.E., Burchell H.B. The Pathological anatomy of deficiencies between the aortic root and the heart, including aortic sinus aneurysms. Thorax. 1957;12:125–139. doi: 10.1136/thx.12.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babacan K.M., Tasdemir O., Zengin M., Karagöz H.Y., Zorlutuna Y.I., Özer C., et al. Fistulous communication of aortic sinuses into the cardiac chambers. Jpn Heart J. 1986;27:865–870. doi: 10.1536/ihj.27.865. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi K., Sasaki N., Matsuura Y., Uemura R. Surgical correction of aneurysm of the sinus of Valsalva: a report of forty-five consecutive patients including eight with total replacement of the aortic valve. Am J Cardiol. 1969;23:180–191. doi: 10.1016/0002-9149(69)90065-4. [DOI] [PubMed] [Google Scholar]
- 4.Chu S.-H., Hung C.-R., How S.-S., Chang H., Wang S.-S., Tsai C.-H., et al. Ruptured aneurysms of the sinus of Valsalva in oriental patients. J Thorac Cardiovasc Surg. 1990;99:288–298. [PubMed] [Google Scholar]
- 5.Takach T.J., Reul G.J., Duncan J.M., Cooley D.A., Livesay J.J., Ott D.A., et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68:1573–1577. doi: 10.1016/s0003-4975(99)01045-0. [DOI] [PubMed] [Google Scholar]
- 6.Sakakibara S., Konno S. Congenital aneurysm of the sinus of Valsalva anatomy and classification. Am Heart J. 1962;63:405–424. doi: 10.1016/0002-8703(62)90287-9. [DOI] [PubMed] [Google Scholar]
- 7.Xin-jin L., Xuan L., Bo P., Hong-wei G., Wei W., Shou-jun L., et al. Modified Sakakibara classification system for ruptured sinus of Valsalva aneurysm. J Thorac Cardiovasc Surg. 2013;146:874–878. doi: 10.1016/j.jtcvs.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 8.Azakie A., David T.E., Peniston C.M., Rao V., Williams W.G. Ruptured sinus of Valsalva aneurysm: early recurrence and fate of the aortic valve. Ann Thorac Surg. 2000;70:1466–1470. doi: 10.1016/s0003-4975(00)01734-3. [DOI] [PubMed] [Google Scholar]
- 9.Feldman D.N., Roman M.J. Aneurysms of the sinuses of Valsalva. Cardiology. 2006;106:73–81. doi: 10.1159/000092635. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg N., Krasnow N. Sinus of Valsalva aneurysms. Clin Cardiol. 1990;13:831–836. doi: 10.1002/clc.4960131204. [DOI] [PubMed] [Google Scholar]
- 11.Guo D.W., Cheng T.O., Lin M.L., Gu Z.Q. Aneurysm of the sinus of Valsalva: a roentgenologic study of 105 Chinese patients. Am Heart J. 1987;114:1169–1177. doi: 10.1016/0002-8703(87)90193-1. [DOI] [PubMed] [Google Scholar]
- 12.Golzari M., Riebman J.B. The four seasons of ruptured sinus of Valsalva aneurysms: case presentations and review. Heart Surg. Forum. 2004;7:E577–E583. doi: 10.1532/HSF98.20041128. [DOI] [PubMed] [Google Scholar]
- 13.Cheng T.O., Yang Y.-L., Xie M.-X., Wang X.-F., Dong N.-G., Su W., Lü Q., et al. Echocardiographic diagnosis of sinus of Valsalva aneurysm: a 17-year (1995–2012) experience of 212 surgically treated patients from one single medical center in China. Int J Cardiol. 2014;173:33–39. doi: 10.1016/j.ijcard.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Yang K., Luo X., Tang Y., Hu H., Sun H. Comparison of clinical results between percutaneous closure and surgical repair of ruptured sinus of Valsalva aneurysm. Catheter Cardiovasc Interv. 2021;97:E354–E361. doi: 10.1002/ccd.29216. [DOI] [PubMed] [Google Scholar]
- 15.Kuriakose E.M., Bhatla P., McElhinney D.B. Comparison of reported outcomes with percutaneous versus surgical closure of ruptured sinus of Valsalva aneurysm. Am J Cardiol. 2015;115:392–398. doi: 10.1016/j.amjcard.2014.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE, parasternal short-axis view without (left) and with (right) color-flow Doppler, zoom-in view on the aortic valve in short axis. The body of the ruptured SOVA is seen arising from the noncoronary cusp extending into the RA. Color flow is continuous through the SOVA but accentuates in diastole.
Two-dimensional TEE, midesophageal 4-chamber view, demonstrates a small flailing SOVA in the RA, just above the tricuspid valve annulus. The RA and RV are dilated. The interatrial septum bulges toward the left, indicating relatively elevated right-sided pressure. Both the RV and LV have moderately reduced systolic function.
Two-dimensional TEE, midesophageal short-axis view at the aortic valve, without (left) and with (right) color-flow Doppler, demonstrates 2 distinct color jets. One is more horizontal with a continuous jet (accentuated during diastole) from the ruptured SOVA into the RA. The other is more perpendicular and central representing the systolic TR jet into the RA.
Three-dimensional TEE, volume-rendered, basal short-axis view of the ruptured SOVA, which nicely demonstrates the classic windsock appearance of the aneurysm sac with its tail in the RA.
Intraprocedural 2D TEE, midesophageal short-axis view at the aortic valve, demonstrates that the closure device is well seated across the RA and the noncoronary sinus. The tricuspid valve leaflets and the aortic valve cusps are all moving freely without impingement by the device.
Two-dimensional TEE, midesophageal short-axis view with color-flow Doppler, demonstrates that the closure device has successfully captured the SOVA jet. There is minimal color flow across the device itself. The severity of the central TR jet remains the same as precatheter TR.