Abstract
Six genes, including UL32, have been implicated in the cleavage and packaging of herpesvirus DNA into preassembled capsids. We have isolated a UL32 insertion mutant which is capable of near-wild-type levels of viral DNA synthesis; however, the mutant virus is unable to cleave and package viral DNA, consistent with the phenotype of a previously isolated temperature-sensitive herpes simplex virus type 1 mutant, tsN20 (P. A. Schaffer, G. M. Aron, N. Biswal, and M. Benyesh-Melnick, Virology 52:57–71, 1973). A polyclonal antibody which recognizes UL32 was previously used by Chang et al. (Y. E. Chang, A. P. Poon, and B. Roizman, J. Virol. 70:3938–3946, 1996) to demonstrate that UL32 accumulates predominantly in the cytoplasm of infected cells. In this report, a functional epitope-tagged version of UL32 showed that while UL32 is predominantly cytoplasmic, some nuclear staining which colocalizes with the major DNA binding protein (ICP8, UL29) in replication compartments can be detected. We have also used a monoclonal antibody (5C) specific for the hexon form of major capsid protein VP5 to study the distribution of capsids during infection. In cells infected with wild-type KOS (6 and 8 h postinfection), 5C staining patterns indicate that capsids are present in nuclei within replication compartments. These results suggest that cleavage and packaging occur in replication compartments at least at 6 and 8 h postinfection. Cells infected with the UL32 mutant exhibit a hexon staining pattern which is more diffusely distributed throughout the nucleus and which is not restricted to replication compartments. We propose that UL32 may play a role in “bringing” preassembled capsids to the sites of DNA packaging and that the failure to localize to replication compartments may explain the cleavage/packaging defect exhibited by this mutant. These results suggest that the UL32 protein is required at a step distinct from those at which other cleavage and packaging proteins are required and may be involved in the correct localization of capsids within infected cells.
During infection of cells with herpes simplex virus type 1 (HSV-1), the large concatemeric products of DNA replication are cleaved to unit length and packaged into preassembled capsids. Capsids are icosahedral structures composed of 150 hexons and 12 pentons. Three types of capsids (A, B, and C) can be isolated from infected cells by velocity centrifugation (20). C capsids contain the viral DNA genome; B capsids contain the scaffolding protein; and A capsids contain neither DNA nor the scaffolding protein. Pulse chase experiments with another alphaherpesvirus, equine herpesvirus 1, indicate that at least some B capsids can package DNA and mature into infectious virions, while A capsids cannot (46). By analogy with the bacteriophages, these results suggest that B capsids represent procapsids which are intermediates in the packaging process. However, a new intermediate in the assembly process has recently been identified (41, 62). These newly identified capsid forms observed in in vitro assembly extracts have the same protein content as B capsids but are more spherical; these capsids are unstable and adopt the more angular form characteristic of B capsids after prolonged incubation in vitro. These results suggest that the unstable spherical forms may represent the true procapsid intermediate (41, 62).
In many bacteriophages, the procapsid contains at least three essential components: an icosahedrally arranged protein shell, an internal scaffold, and a dodecameric ring called the portal vertex through or around which the phage DNA is taken up (8, 11, 18). For HSV-1, the outer shell is composed of four proteins: the major capsid protein, VP5; a small protein bound to hexons, VP26; and a triplex structure made up of heterotrimers of VP19C and VP23 (reviewed in reference 56). VP24, VP21, and VP22a are found in the interior of the capsid and are encoded by overlapping genes UL26 and UL26.5; VP21 and VP22a are present in B but not A or C capsids and are considered to make up the internal scaffold (reviewed in reference 56). Although bacteriophages contain a portal vertex, no such structure has been observed in HSV-1 capsids. Whether the herpesviruses have a unique portal vertex through which viral DNA is taken up is unclear; it is possible that this type of unique vertex is only needed in viruses which have a tail. Capsids indistinguishable from those isolated from HSV-1-infected cells have been observed in extracts from insect cells infected with recombinant baculoviruses bearing HSV-1 capsid genes (42, 60). Therefore, it is clear that these proteins are sufficient for capsid assembly in vitro; however, it is not known whether capsids formed in vitro are competent for DNA uptake. It is possible that minor components of capsids play important roles in genome encapsidation.
In addition to the capsid proteins, at least six genes are essential for the encapsidation of viral DNA: the UL6, UL15, UL25, UL28, UL32, and UL33 genes. Temperature-sensitive (ts) strains with mutations in these genes have similar phenotypes, in that viral DNA can be replicated but not cleaved and packaged (1, 2, 4, 6, 48, 51, 54, 55, 66). Strains with null mutations in the UL6, UL15, UL25, UL28, and UL33 genes have been isolated and characterized, thereby confirming the roles of these genes in cleavage and packaging (5, 27, 37, 45, 59, 68). Despite the identification of these required genes, the mechanism by which viral DNA is cleaved and packaged is not understood, nor has the role of any of the gene products been determined. The UL6 and UL25 proteins have been detected in A, B, and C capsids as well as in virions (3, 28, 37, 44); however, the precise role of these two proteins in capsids remains to be determined.
A ts UL32 mutant, tsN20, defective in cleavage and packaging, has been reported previously (51). Because mutants with lesions resulting in temperature sensitivity are often prone to problems associated with incomplete penetrance at the nonpermissive temperature, we isolated a UL32 insertion mutant, hr64. Characterization of hr64 confirms that UL32 is essential for cleavage and packaging. Previous studies demonstrated that UL32 localizes to the cytoplasm of infected cells (13). We have used a functional epitope-tagged version of UL32 to confirm that in infected cells, this protein is mainly cytoplasmic, although some nuclear staining was observed.
HSV-1 DNA replication occurs in globular nuclear domains termed “replication compartments” initially identified by ICP8 (UL29) staining patterns in an immunofluorescence assay (49). All seven replication proteins have now been localized within replication compartments (10, 24, 29–31, 43) as has regulatory protein ICP4 (26, 50). Ward et al. have recently reported that at late times after infection (18 h), capsids accumulate in the nucleus in regions distinct from replication compartments (64). These authors suggest that these regions represent assembly stations in which DNA is packaged. We report herein, however, that at 6 and 8 h postinfection, capsids colocalize with ICP8 in replication compartments. This suggests that at these early times, cleavage and packaging occur within replication compartments. Furthermore, we report that in cells infected with the UL32 mutant virus, capsids are distributed throughout the nucleus, accumulating in regions outside the replication compartments. This suggests that UL32 may play a role in the efficient localization of capsids in infected cells.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney cells (Vero; American Type Culture Collection, Rockville, Md.) were propagated and maintained as previously described (65). HSV-1 strain KOS was used as the wild-type virus and the parental virus for the isolation of the UL32 host range mutant. tsN20, a ts UL32 mutant, was generously provided by Priscilla A. Shaffer (University of Pennsylvania, Philadelphia). hr74 containing a lacZ insertion in the UL6 gene was previously described (27). KUL25NS, a mutant with an in-frame stop codon in the UL25 gene, was generously provided by Fred Homa (Pharmacia and Upjohn, Kalamazoo, Mich.) (37).
Plasmids.
The pAPV vector contains the inducible HSV-1 promoter from the ICP6 gene and the simian virus 40 (SV40) polyadenylation sequence on either side of a BamHI site into which genes to be expressed can be cloned. pAPV was generated by ligating the HindIII-XhoI fragment containing the ICP6 promoter into pUC118 digested with HindIII-SalI. A BamHI-to-BclI fragment from SV40 containing the poly(A) site was then inserted at the BamHI site. pAPVUL6, containing the UL6 sequences from nucleotide (nt) 15103 to nt 17323, under the control of the ICP6 promoter, was generously provided by Fred Homa. p1BD1 contains the BglII D fragment of HSV-1 (14). p205, containing the UL31, UL32, and UL33 genes, was obtained by subcloning the EcoRV-to-HpaI fragment (nt 63987 to nt 70315) from p1BD1 into SmaI-digested pUC119. pAPVUL32, containing the UL32 gene under the control of the ICP6 promoter, was generated in several steps. A BamHI fragment from p205 containing the sequence of the UL32 gene (nt 68885 to nt 70168) encoding the N-terminal portion of UL32 was inserted at the BamHI site of pAPV to generate pAPVUL32 GP. To position the UL32 gene initiation codon (nt 69159) downstream from the ICP6 promoter, pAPVUL32 GP was digested with XbaI, which releases a fragment from nt 69247 to nt 70168, and religated to generate pAPVUL32 F. A BamHI fragment containing the UL32 gene sequences (nt 68338 to nt 66238) was inserted into the unique BamHI site of pAPVUL32 F to generate pAPVUL32 S. A BstEII-to-SalI fragment (nt 68938 to nt 67771) was subsequently inserted into pAPVUL32 S digested with BstEII-SalI to obtain the final construct, pAPVUL32. In pAPVEEUL32 the coding sequence for the EE epitope was fused in frame to that for the N terminus of the UL32 protein in pAPV as follows. A SmaI-to-BamHI fragment containing UL32 gene sequences (nt 69144 to nt 68885) from pAPVUL32 was ligated with pUC119 digested with SmaI and BamHI to generate p32 S/B. Two annealed synthetic complementary oligonucleotides containing coding sequences for the EE epitope (EEYMPME) and the four amino acids of UL32 upstream of the position corresponding to the SmaI site (nt 69144) were inserted in p32 S/B digested with EcoRI and SmaI. Oligonucleotide 5′-GGGGGGCGAAGTTGCCATTTCCATTGGCATATA TTCTCCATGG-3′ and its complement were designed to yield a blunt SmaI site and a cohesive EcoRI site on annealing. Nucleotides corresponding to the EE epitope are underlined. The resulting plasmid, pEE32S/B, was subsequently digested with NcoI and BstEII to obtain a fragment that contained the coding sequence for the EE epitope in frame with the N terminus of UL32; this fragment was subsequently inserted into pAPVUL32 digested with NcoI and BstEII to generate pAPVEEUL32. pUL32lacZ, containing the UL32 gene disrupted by the lacZ cassette at the codon for amino acid 274, was constructed in two steps. A KpnI-to-XbaI fragment containing the UL32 gene sequences (nt 66660 to nt 69247) was inserted into XbaI-KpnI sites of pUC119 to obtain pUC UL32. The ICP6::lacZ cassette was subsequently inserted in pUC UL32 at the BamHI site (nt 68338) (Fig. 1). The direction of transcription of the lacZ gene in pUL32lacZ is inverted with respect to the direction of transcription of the UL32 gene.
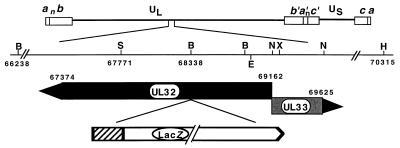
FIG. 1.
Physical map of the region of the HSV genome containing the UL32 gene. The region of HSV-1 DNA spanning the UL32 and UL33 open reading frames has been expanded to show the cleavage sites for BamHI (B), SalI (S), XbaI (X), NcoI (N), HpaI (H), and BstEII (E) and the start (nt 69162) and the stop (nts 67374 and 69625) sites for UL32 and UL33, respectively. On the last line is a diagram of the insertion of the ICP6::lacZ cassette in UL32 at the BamHI site (nt 68338). ▨, ICP6 promoter. UL, unique long region; US, unique short region.
pGST-UL6, containing the coding sequence for the C-terminal region of UL6 (amino acid residues 381 to 676) fused with the glutathione S-transferase (GST) gene, was constructed as follows. The Asp718 fragment (nt 16273 to 17793) containing the sequence of the UL6 gene encoding the C-terminal portion of UL6 was inserted into Asp718-digested pUC119. This fragment was released by digestion with BamHI and EcoRI and cloned in frame with the GST gene sequence into plasmid pGEX-2T (Pharmacia) digested with BamHI-EcoRI. pAPVAU1-UL6 contains the UL6 gene, whose product has an epitope tag at its N terminus. Plasmid pNN9 containing two copies of the 5.9-kb BamHI K fragment from strain KOS cloned into pUC19 was generously provided by Mark Challberg (National Institutes of Health, Bethesda, Md.).
Isolation of UL32 cell lines.
To generate cell lines containing the UL32 gene under the control of inducible promoter ICP6, Vero cells were cotransfected with pSV2neo and pAPVUL32 as described previously (21). In brief, pSV2neo (2 μg) and pAPVUL32 (18 μg) were coprecipitated in a final volume of 1 ml and mixed with freshly trypsinized Vero cells in suspension (25). Two weeks after transfection, G418-resistant colonies were collected and screened for their ability to support the growth of a ts UL32 mutant (tsN20) at the nonpermissive temperature of 39.5°C.
Marker transfer.
Marker transfer experiments were performed as previously described (23, 71). Transfections were carried out in the UL32-permissive cell line with linearized pUL32lacZ and infectious wild-type (KOS) DNA at a molar ratio of 10:1. Cells (1.5 × 106) were transfected with 0.4 μg of the linearized plasmid and 0.8 μg of infectious DNA.
Transient complementation test.
Transient complementation was performed as previously described (35).
DNA isolation, Southern blotting, and PFGE.
Infectious viral DNA was prepared as previously described (72). Cellular DNA was isolated as previously described (67). For DNA analysis by Southern blot hybridization, virion or infected-cell DNA was digested with restriction endonucleases as directed by the manufacturer, subjected to agarose gel electrophoresis, and transferred to Gene Screen Plus nylon membranes (New England Nuclear Corp., Boston, Mass.) as recommended by the supplier. DNA fragments to be used as probes were labeled as described previously (19) by the random hexamer primed method (Boehringer Mannheim). Pulsed-field gel electrophoresis (PFGE) was performed as previously described (34).
DNase I protection.
Vero cells on a 60-mm-diameter plate were infected at a multiplicity of infection (MOI) of 10 PFU/cell with wild-type KOS or mutant viruses. At the end of the 1-h adsorption period the cells were washed three times with medium and overlayed with complete medium. Time zero DNA is DNA from cellular extracts collected immediately after the 1-h adsorption period. The DNase protection experiment was performed as previously described (68).
Analysis of viral capsids and virions.
HSV-1 viral particles were isolated as described by Sherman and Bachenheimer (55) with some modifications. At 18 h postinfection cells were scraped and collected by low-speed centrifugation (4°C, 10 min), resuspended in phosphate buffer (40 mM phosphate [pH 7.4], 0.15 M NaCl) with 1% Nonidet P-40 (NP-40), and treated with a probe sonicator. The total cellular lysate was layered onto 15-to-45% (wt/wt in phosphate buffer) sucrose gradients and centrifuged at 35,000 rpm for 30 min in an SW41 rotor. To prepare large quantities of capsids, 40 10-ml-diameter plates of 60 to 70% confluent monolayers of Vero cells were infected at an MOI of 6 PFU/cell for 24 h. Infected cells were lysed with 50 ml of phosphate buffer with 1% NP40. After low-speed centrifugation, the pellet was resuspended in 20 ml of TEN buffer (20 mM Tris-HCl [pH 7.4], 1 mM EDTA, 0.5 M NaCl) and frozen and thawed three times. A probe sonicator was used to clarify the extract (1 min) before it was layered on a 35% (wt/wt) sucrose cushion, which was centrifuged at 19,600 rpm in an SW41 rotor for 1 h. The pellet containing the capsids was carefully resuspended with 400 μl of TEN–NP-40 containing 5 mM MgCl and 10 mM dithiothreitol and layered on a 15-to-45% sucrose gradient. Capsid bands were visualized by light scattering upon illumination with a halogen fiber optic lamp. Bands corresponding to A, B, and C capsids were removed from each gradient by side puncture with an 18-gauge needle on a 5-ml syringe. Each band was diluted fivefold with phosphate buffer and pelleted by centrifugation at 35,000 rpm in an SW41 rotor for 1 h. Alternatively, the collected band was diluted 1:3 in phosphate buffer and layered on a second sucrose gradient for further purification. The pellets were resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and analyzed by Western blotting. Mature virions were prepared as previously described (58).
Antibodies.
pGST-UL6 described above was used to transform UT481 cells. Expression of the fusion protein was induced by 0.4 M IPTG (isopropyl-β-d-thiogalactopyranoside) when the culture reached an A600 of 0.4 at 37°C. SDS-PAGE was used to visualize a protein of the expected size in the IPTG-induced bacterial culture which was not present in the uninduced culture. Cells were lysed with a French press apparatus. The vast majority (90%) of the expressed protein was present in the insoluble fraction of the lysed cells. This protein was purified by SDS-PAGE with a 10% preparative gel. After staining with Coomassie blue and destaining, gel slices containing the GST-UL6 protein were excised, washed in distilled water, and sent to HRP Inc. (Denver, Pa.) for antiserum production in rabbits (α-UL6). The UL32 polyclonal antibody was generously donated by Bernard Roizman (University of Chicago, Chicago, Ill.) (13). Anti-EE ascites was previously described (30). Anti-ICP8 was generously provided by William Ruyechan (State University of New York at Buffalo, Buffalo) (53). Monoclonal antibody 5C was generously provided by Jay Brown (University of Virginia Health Science Center, Charlottesville) (63).
Detection of proteins by immunoblotting.
Vero cells were plated onto 60-mm-diameter tissue culture dishes. At 18 h postinfection, the cells were processed as previously described (35). Samples were subjected to SDS–10% PAGE, and the proteins were transferred for 2 h onto an Immobilon-P membrane (Millipore, Bedford, Mass.) in 25 mM Tris–192 mM glycine–20% methanol (61). The filters were incubated in TBST (10 mM Tris [pH 8.0], 150 mM NaCl, 0.1% Tween 20) containing 20% fetal calf serum and 1% bovine serum albumin for 1 h at room temperature and were incubated overnight at 4°C with the α-UL6 antiserum, diluted 1:1,500 in TBST. After five washes in TBST, the filters were incubated in TBST containing goat anti-rabbit immunoglobulin G–alkaline phosphatase conjugate (Promega, Madison, Wis.). Alkaline phosphatase color development was carried out as previously described (72). For UL32 protein detection, the blot was incubated in 5% dry milk in phosphate-buffered saline (PBS) for 1 h at room temperature and incubated with a 1:500 dilution of the UL32 antibody for 1 h. After four washes with PBS-dry milk suspension the blot was incubated for 1 h at room temperature with secondary antibody and developed as described above. Alternatively, the enhanced chemiluminescence (ECL) detection system (Amersham, Arlington Heights, Ill.) was used as recommended by the supplier.
Indirect immunofluorescence.
The ICP6 promoter can be induced with either of the two transactivators ICP0 and VP16 (17, 22, 57). Vero cells were cotransfected (21) with equal amounts of pAPVEEUL32 and pVP16 (33) and grown on coverslips for 24 h, or cells were transfected with pAPVEEUL32 and infected 18 h later for 6 h prior to fixation. Cells were fixed and processed for immunofluorescence as previously described (30). In these experiments the EE monoclonal antibody was diluted 1:500 and anti-ICP8 was used at 1:400. Cells infected with the wild-type KOS strain or the mutant viruses were fixed and processed as described above. In these experiments monoclonal antibody 5C was used at 1:1,000. All double-labeled cells were checked for bleed-through staining and cross-reactivity by treatment with only a single primary antibody and both secondary antibodies.
Imaging.
Cells stained for immunofluorescence were imaged on a Zeiss Axiovert 135 laser scanning microscope (confocal) equipped with a ZeissX630 Plan Neofluar objective. Collected images were arranged and labeled with a Silicon Graphics workstation equipped with Adobe Photoshop version 3.0 software. To obtain the three-dimensional images of replication compartment and capsid staining patterns, a series of two-dimensional slices (Z series) 0.3 μ thick were imaged on the confocal microscope. Collected Z series images were subsequently analyzed and displayed by the Voxel View 2.5 program.
RESULTS
To propagate a potentially lethal mutation in the UL32 gene, it was first necessary to isolate a complementing cell line. Vero cells were transfected with pAPVUL32 and pSV2neo as described under Materials and Methods. Ten G418-resistant cell lines were isolated, and the one which could support the growth of tsN20 at the nonpermissive temperature most efficiently was designated 158. To isolate a viral mutant containing a disrupted UL32 gene, a plasmid containing the ICP6::lacZ cassette inserted at the codon for amino acid 274 within the UL32 gene was used for marker transfer. The permissive cell line, 158, was cotransfected with infectious KOS DNA and pUL32lacZ as described under Materials and Methods. Three blue plaques were purified and one, designated hr64, was chosen for further study. We confirmed by Southern blot analysis that DNA from hr64 contains the ICP6::lacZ cassette at the expected position (data not shown).
Characterization of the UL32 mutant.
The UL32 mutant was then examined for its ability to form plaques on permissive and nonpermissive cells (Vero and 158). hr64 was unable to form plaques on Vero cells, but it could efficiently form plaques on 158 cells (titer on 158 cells, 5 × 107 PFU/ml). The ability to form plaques on 158 cells but not on Vero cells indicates that hr64 carries a defective UL32 gene. This result was further confirmed in a transient complementation assay; Vero cells were transfected with pAPVUL32, which expresses wild-type UL32, and superinfected with hr64. pAPVUL32, but not a control plasmid containing the UL6 gene under the control of the ICP6 promoter, was able to complement the growth of hr64 (data not shown). In sum, these results indicate that the mutation in hr64 lies within the UL32 gene.
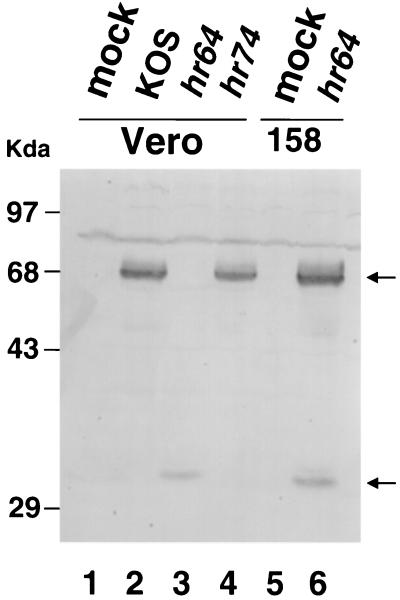
In addition, hr64 was tested for expression of the UL32 protein during infection of permissive and nonpermissive cells. The UL6 mutant hr74 was used as a control in these experiments. Total cell extracts from infected cells were subjected to Western blot analysis with the polyclonal UL32 antiserum as described under Materials and Methods. Figure 2 clearly shows that a band of 68 kDa corresponding to the UL32 protein is present in KOS- and in hr74-infected cells (Fig. 2, lanes 2 and 4, respectively). On the other hand, the 68-kDa protein was not detected in mock- or hr64-infected Vero cells (Fig. 2, lanes 1 and 3, respectively). A smaller band of 30 kDa is present in hr64-infected Vero cells (Fig. 2, lane 3). Since the lacZ gene in hr64 was inserted at the codon for amino acid 274, it is likely that this 30-kDa band represents the N-terminal portion of UL32. This truncated UL32 protein is not sufficient for viral growth, as demonstrated by the inability of hr64 to form plaques on Vero cells (see above). Furthermore, the behavior of hr64 on the complementing cell line (see below) suggests that the truncated form does not exhibit any inhibitory effect. When 158 cells were infected with the mutant hr64, two bands corresponding to the full-length UL32 protein and to the truncated version were observed (Fig. 2, lane 6). Thus, we confirm that hr64 does not express the full-length UL32 protein in nonpermissive Vero cells.
FIG. 2.
Immunoblot of extracts from KOS- and hr64-infected cells. Monolayers of Vero and 158 cells were infected with either KOS, hr74, or hr64 (5 PFU/cell) or were mock infected. Extracts were prepared at 18 h postinfection as described under Materials and Methods and resolved by SDS-PAGE. Proteins were revealed by Western blotting with a UL32 polyclonal antibody and alkaline phosphatase-conjugated secondary antibodies. Lane 1, mock-infected Vero cell extracts; lane 2, KOS-infected Vero cell extracts; lane 3, hr64-infected Vero cell extracts; lane 4, hr74-infected Vero cell extracts; lane 5, mock-infected 158 cell extracts; lane 6, hr64-infected 158 cell extracts.
The UL32 mutant can synthesize viral DNA but is defective for cleavage.
We tested the ability of hr64 to synthesize and process viral DNA. DNA extracts from infected cells were assayed for hybridization to an HSV DNA probe as previously described (72); hr64 synthesizes viral DNA at near-wild-type levels (data not shown). To measure genomic cleavage activity during viral infection, we tested for the presence of genomic termini in DNA extracted from hr64- and UL6 mutant hr74-infected cells at 18 h postinfection. BamHI digested-DNA from hr64-, hr74-, and KOS-infected Vero and 158 cells was subjected to Southern blot analysis. As shown in Fig. 3A, only KOS-infected Vero and 158 cells (lanes 1 and 2, respectively) and hr64-infected 158 cells (lane 6) contain genomic termini, represented by the BamHI fragments S and Q. In contrast, Vero and 158 cells infected with hr74 (lanes 3 and 4, respectively) and Vero cells infected with hr64 (lane 5) contain only DNA corresponding to the L-S junction fragment (designated SQ), indicating that these viruses do not produce genomic termini. The amounts of junction fragment are comparable in all three lanes, confirming that viral DNA synthesis is unaffected in mutants hr74 and hr64.
FIG. 3.
Southern blot analysis of total DNA from KOS-, hr74-, and hr64-infected Vero cells. (A) Vero cells were infected with the indicated virus at 5 PFU per cell. At 18 h postinfection, DNA was isolated as described under Materials and Methods and digested with BamHI. The blotted DNA was hybridized with a 32P-labeled probe containing the BamHI SQ fragment. SQ represents viral DNA junctions, and S and Q represent viral DNA termini. Lanes 1, 3, and 5, total DNA from KOS-, hr74-, and hr64-infected Vero cells, respectively; lanes 2, 4, and 6, total DNA from KOS-, hr74-, and hr64-infected 158 cells, respectively. (B) Vero cells were infected at 5 PFU per cell. At 18 h postinfection the cells were collected in low-melting-point agarose. Agarose blocks were introduced into the well of a 1.3% pulsed-field gel. The complex high-molecular-weight DNA which does not enter the gel is labeled “well,” and unit-length monomeric DNA is labeled “152Kb.” Lane 1, total DNA from KOS-infected Vero cells; lane 2, total DNA from hr64-infected Vero cells; lane 3, total DNA from hr64-infected 158 cells.
To confirm the inability of hr64 to carry out genomic cleavage, we analyzed the behavior of viral DNA from mutant and wild-type viruses by PFGE. During replication, viral DNA accumulates as concatemeric complex intermediates that are unable to enter a pulsed-field gel (well DNA), while mature monomeric viral DNA (152 kb) is well resolved (7, 34, 38, 52, 70). Figure 3B shows a Southern blot analysis of a pulsed-field gel of viral DNA prepared from KOS-infected Vero cells (lane 1) or hr64-infected Vero or 158 cells (lanes 2 and 3, respectively). Monomeric viral DNA (152 kb) is present in Vero cells infected with KOS and not in Vero cells infected with hr64 virus. Monomeric viral DNA is present in 158 cells infected with the hr64 virus. We conclude that the UL32 insertion mutant synthesizes wild-type levels of viral DNA but fails to process the DNA into monomeric units.
The UL32 mutant is defective for encapsidation.
Once replicating DNA has been cleaved and packaged, a DNase-resistant band representing a monomer unit of viral DNA can be detected in extracts of infected cells. DNA extracts from cells infected with KOS or hr64 for different times were treated with DNase I as described under Materials and Methods. Figure 4 (lanes 1 to 6) shows that in KOS-infected Vero cells, DNase-resistant monomeric DNA can be detected as early as 6 h postinfection. On the other hand, no DNase-resistant DNA was observed in cells infected with hr64 (Fig. 4, lanes 7 to 12). The faint band observed in all the lanes (lanes 8 to 12) is comparable to the band present at time zero (lane 7), indicating that these bands represent input viral DNA.
FIG. 4.
Southern blot analysis of DNase-treated DNA from KOS- and hr64-infected Vero cells. Total DNA extracts from KOS or hr64 were treated with DNase I and subsequently digested with BamHI as described under Materials and Methods. The samples were collected at the end of a 1-h incubation time (time zero) and at 2, 4, 6, 8, and 16 h postinfection.
By analogy with other cleavage/packaging mutants, cells infected with the UL32 mutant would be expected to exhibit an altered pattern of capsid formation compared to the wild type. After lysis and sonication of infected cells in 1% NP40, whole-cell extracts were analyzed by sucrose gradient sedimentation. KOS-infected cells exhibit three types of capsids (A, B, and C), while UL32 mutant virus-infected cells produce only B capsids (data not shown). This result is consistent with a defect in cleavage and packaging.
UL32 is not detected in capsids or in virions.
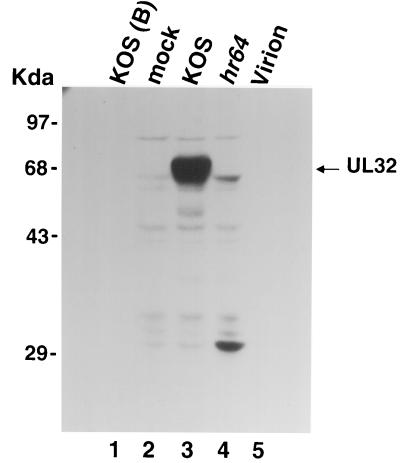
Cleavage/packaging proteins UL6 and UL25 are associated with A, B, and C capsids found in infected cells (28, 37, 44); UL6 and UL25 are also found in complete virions (3, 28, 44). In order to determine whether UL32 is also associated with capsids and/or virions, we prepared B capsids and mature virions and analyzed by Western blotting as described under Materials and Methods. Figure 5 shows that UL32 is not detected in B capsids or virions from KOS-infected cells (Fig. 5, lanes 1 and 5, respectively). As expected, UL32 (marked by an arrow) can easily be detected in KOS-infected cell extracts (Fig. 5, lane 3) but not in mock- or hr64-infected cell extracts (Fig. 5, lanes 2 and 4). The band, just below the band marked by an arrow, present in hr64-infected Vero cells is not specific since a similar band is also present in mock-infected cells. Thus, it appears that UL32 is not present in B capsids or in virions, although at this point we cannot rule out the possibility that UL32 is present at too low a level to be detectable with the antibody used.
FIG. 5.
UL32 can be detected in KOS-infected cell extracts but not in B capsid or virions. B capsids from KOS-infected Vero cells were collected from a sucrose gradient and purified on a second gradient as described under Materials and Methods. Monolayers of Vero cells were infected with either KOS or hr64 (5 PFU/cell) or were mock infected. Extracts were prepared at 18 h postinfection as described under Materials and Methods. Virions were prepared as described under Materials and Methods. Proteins were revealed by ECL Western blotting with UL32 polyclonal antibodies (13) and horseradish peroxidase-labeled secondary antibodies. Lane 1, B capsids from KOS-infected Vero cells; lane 2, mock-infected Vero cell extracts; lane 3, KOS-infected Vero cell extracts; lane 4, hr64-infected Vero cell extracts; lane 5, virion from KOS-infected Vero cells.
Expression and capsid localization of other cleavage/packaging proteins in cells infected with the UL32 mutant virus.
Since mutations in the cleavage and packaging genes produce similar phenotypes, we were interested in whether the expression of one viral gene affects the expression of the others. Western blot analysis indicates that the levels of UL6, UL15, UL25, and UL28 proteins, as well as levels of protease and scaffold proteins, in Vero cells infected with a UL32 mutant are similar to those seen in wild-type infections (data not shown). This result indicates that the expression of these proteins is not dependent on the presence of a functional UL32. Likewise, we asked whether the presence of UL6 and UL25 in capsids was dependent on the expression of UL32. Therefore, hr64-infected Vero cells were collected at 20 h postinfection and B capsids were prepared as described under Materials and Methods. Figure 6A shows that the UL6 protein is present in B capsids from both KOS- and hr64-infected cells (Fig. 6A, lanes 1 and 2, respectively). As expected, the UL6 protein can be detected in KOS-infected cell extracts and in virions from KOS-infected cells (Fig. 6A, lanes 4 and 6) but not in mock- or hr74-infected cell extracts (Fig. 6A, lanes 3 and 5, respectively). Similar results were obtained with the UL25 polyclonal antibody, as shown in Fig. 6B. B capsids collected from KOS- and hr64-infected cells contain UL25 protein (Fig. 6B, lanes 3 and 4) as do KOS-infected cell extracts (Fig. 6B, lane 2) and virions from KOS-infected cells (Fig. 6B, lane 6). On the other hand UL25 is not detected in mock-infected cell extracts or in extracts from cells infected with virus lacking UL25 (Fig. 6B, lanes 1 and 5, respectively). The presence of UL6 and UL25 in B capsids in cells infected with viruses lacking UL32 indicates that the accumulation of UL6 and UL25 in B capsids is not dependent on the expression of full-length UL32.
FIG. 6.
UL6 and UL25 are present in B capsids from wild-type- and hr64-infected cells. The presence or absence of UL6 and UL25 in KOS- and hr64-infected Vero cell extracts, in B capsids from KOS- and hr64-infected cells, and in virions from KOS-infected Vero cells was determined as described under Materials and Methods. Proteins were revealed by Western blotting with α-UL6 antibodies and alkaline phosphatase-conjugated secondary antibodies (A) or by ECL Western blotting with a UL25 polyclonal antibody and horseradish peroxidase-labeled secondary antibodies (B). Panel A lanes: 1, B capsids from KOS-infected Vero cells; 2, B capsids from hr64-infected Vero cells; 3, mock-infected Vero cell extracts; 4, KOS-infected Vero cell extracts; 5, hr74-infected Vero cell extracts; 6, virions from KOS-infected Vero cells (this lane was developed for a longer time to visualize the band). Panel B lanes: 1, mock-infected Vero cell extracts; 2, KOS-infected Vero cell extracts; 3, B capsids from KOS-infected Vero cells; 4, B capsids from hr64-infected Vero cells; 5, extracts from Vero cells infected with virus lacking UL25; 6, virions from KOS-infected Vero cells.
Intracellular localization of UL32.
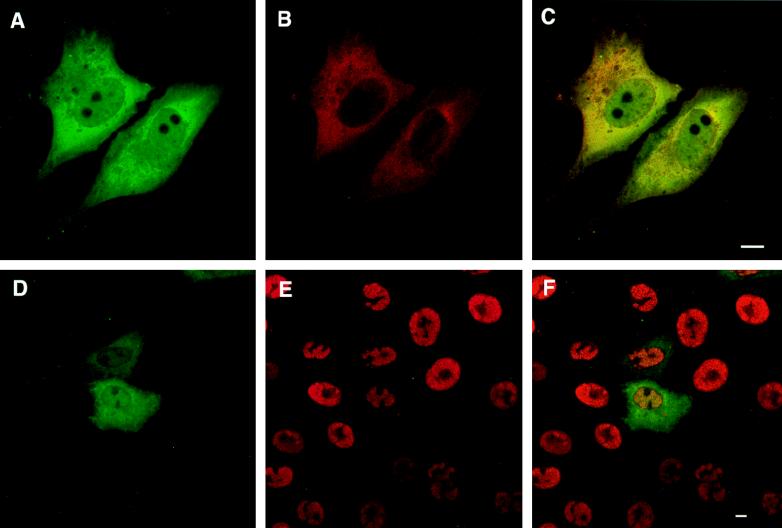
Previous studies have shown that in infected cells, the UL32 protein was found predominantly in the cytoplasm (13). Although in that report a small amount of nuclear staining was also observed at 18 h postinfection, we were intrigued by the unexpected distribution of a protein that has a role in cleavage and packaging. We constructed an epitope-tagged version of UL32 containing the EE epitope (EEUL32) in frame with the N-terminal portion of the UL32 protein. The plasmid encoding this construct, pAPVEEUL32, was found to be able to complement UL32 mutant virus hr64 as well as the wild type in a transient complementation assay (data not shown). This indicates that EEUL32 is functional. Vero cells were transfected with pAPVEEUL32 and a transactivator and processed for immunofluorescence with the monoclonal EE antibody and the polyclonal UL32 antibody as described under Materials and Methods (Fig. 7A and B, respectively). Although both antibodies are presumably detecting the UL32 gene product, the staining patterns differ somewhat. The monoclonal antibody clearly detects UL32 in the cytoplasm and in the nucleus (Fig. 7A); the polyclonal antibody detects UL32 mainly in the cytoplasm with very faint staining in the nucleus (Fig. 7B). In Fig. 7C, a merged image is shown. Control experiments indicate that the EE antibody does not react with untransfected cells (data not shown). It is possible that the EE antibody reaction with its epitope is much stronger than that of the polyclonal serum with UL32; alternatively, the polyclonal antibody may not recognize nuclear UL32 as efficiently. In any case, we have demonstrated that UL32, when expressed only with transactivator VP16, accumulates in both the nucleus and the cytoplasm. In contrast, in cells transfected with a plasmid bearing a tagged version of the UL6 gene, another cleavage/packaging gene, UL6 can efficiently localize to the nucleus on its own (32).
FIG. 7.
Localization of UL32 in cells transfected with pAPVEEUL32. Cells shown in panels A to C were transfected with pAPVUL32 and pVP16 as described under Materials and Methods. Cells shown in panels D to F were transfected with pAPVEEUL32 and superinfected with hr64. In panels A and B, green represents staining with the EE monoclonal antibody and red represents staining with the UL32 polyclonal antibody, respectively. In panel D green represents staining with the EE monoclonal antibody, and in panel E red represents staining for ICP8. Panels C and F show the merged images of the staining patterns. Bars = 10 μm.
In order to determine the localization of UL32 in infected cells, Vero cells were transfected with pAPVEEUL32 and superinfected with hr64 at an MOI of 10 PFU/cell (Fig. 7D to F). Although most cells will be infected under these conditions, only a fraction of cells are expected to be transfected. To ensure that all the transfected cells were also infected, cells were double stained with the EE antibody and with a polyclonal antibody directed against ICP8 (anti-ICP8), the major DNA-binding protein of HSV-1. ICP8 is found in globular nuclear domains termed replication compartments, in which viral DNA synthesis is known to occur (49). In Fig. 7, panel D shows the EE staining pattern, panel E shows the ICP8 staining pattern, and panel F shows the merged image. ICP8 staining in panel E indicates that most of the cells are infected; characteristic replication compartments are observed. Figure 7D shows that in cells that are transfected and infected, the EE staining (UL32 protein) is predominantly in the cytoplasm. Nevertheless, in these cells, some EE staining is seen in the nucleus, in a pattern that colocalizes with that of ICP8 (Fig. 7F). Cells that do not react with the EE antibody are cells which are infected but not transfected (Fig. 7D and E); the lack of reactivity in these cells indicates that the antibody does not cross-react with other viral proteins. In sum, although in infected cells most of the UL32 protein localizes to the cytoplasm, the UL32 protein in the nucleus appears to be present in replication compartments.
Capsids localize to replication compartments.
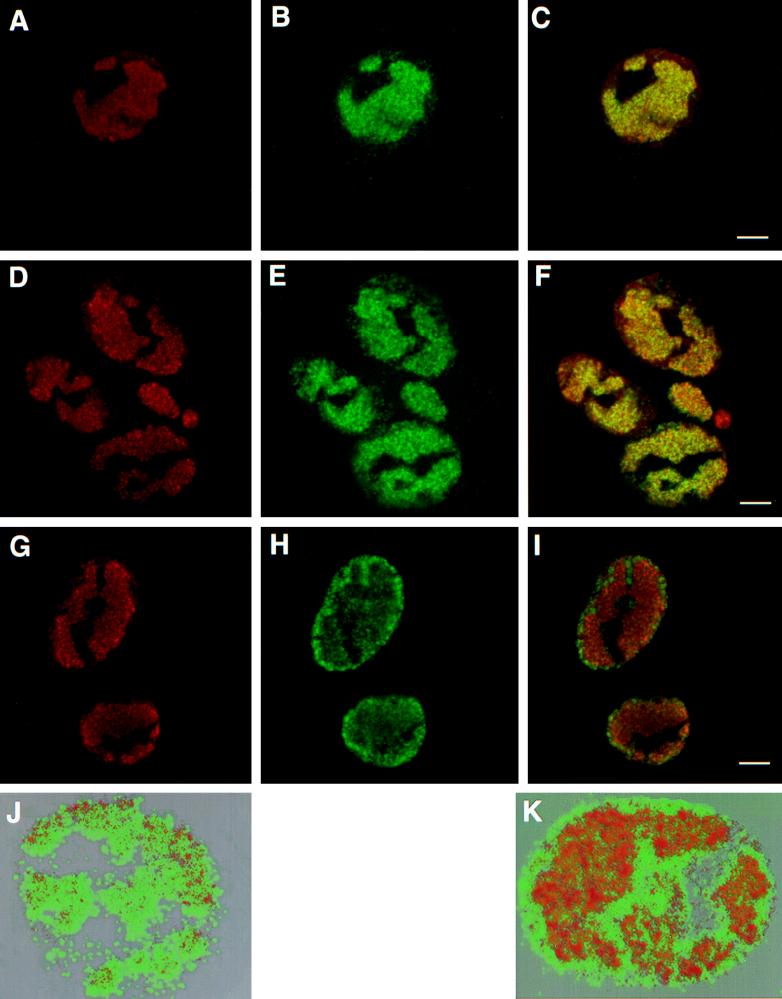
Ward et al. have proposed that at late times after infection, some capsid and tegument proteins assemble in discrete nuclear structures at the borders of replication compartments and that these sites represent sites of capsid assembly and encapsidation (64). Since we and others (70) have shown that encapsidation begins as early as 6 h postinfection (Fig. 4), we decided to look at the localization of capsids at earlier times. Furthermore, at 6 and 8 h postinfection, the cells are still well attached to the coverslips and cellular structures are more recognizable than at later times. Three monoclonal antibodies (8F5, 5C, and 3B) which recognize distinct epitopes of major capsid protein VP5 on the capsid itself have been reported (63); however, they do not recognize VP5 in a Western blot (40a, 63). These conformational epitopes have been used previously as probes for assembled capsid structures (36); monoclonal antibodies 8F5 and 5C recognize hexons, while 3B is specific for pentons.
In this study, assembled capsids were stained with monoclonal antibody 5C, which recognizes hexons within preassembled capsids. Vero cells were infected with KOS, the UL6 mutant (hr74), or the UL32 mutant (hr64) for 6 h at an MOI of 10 PFU/cell and stained with monoclonal antibody 5C to label hexon-containing capsids. Figure 8B and E show that in Vero cells infected with KOS or hr74, hexon staining is restricted to regions resembling replication compartments, well-defined structures in which DNA synthesis occurs (49). Vero cells infected with hr64 (Fig. 8H), on the other hand, exhibit an altered pattern of hexon staining in which capsids are distributed throughout the nucleus, with some accumulation at the periphery of the nucleus. In order to determine the localization of capsids with respect to replication compartments, the same Vero cells infected with KOS, the UL6 mutant (hr74), or the UL32 mutant (hr64) were also labeled with the ICP8 polyclonal antibody (Fig. 8A, D, and G, respectively). The replication compartments seen at 6 h postinfection are large globular domains that take up a large portion of the nucleus; however, as has been seen before, replication compartments do not occupy the entire nucleus (Fig. 8A, D, and G). ICP8 staining is not seen in the nucleolus, nor does it extend to the periphery of the nucleus. These patterns are consistent with previous reports by de Bruyn Kops and Knipe (16). When the staining patterns of ICP8 and monoclonal antibody 5C are merged, it becomes apparent that in KOS- and hr74-infected Vero cells, hexon staining is indeed restricted to the replication compartments (Fig. 8C and F, respectively). The demonstration that in cells infected with wild-type virus for 6 h capsids colocalize with replication compartments suggests that cleavage and packaging occur in these structures.
FIG. 8.
Replication compartments and capsid localization. Vero cells were infected with KOS (A to C and J), hr74 (D to F), or hr64 (G to I and K) for 6 h and stained with anti-ICP8 polyclonal antibodies (A, D, and G) and 5C monoclonal antibodies (B, E, and H). Panels C, F, and I represent the merged images of staining patterns with ICP8 and 5C antibodies. Panels J and K represent three-dimensional reconstructions from Z series images of KOS- and hr64-infected Vero cells obtained with the Voxel View program as described under Materials and Methods. In a control experiment, mock-infected cells were treated with primary and secondary antibodies, and infected cells were treated with the secondary antibodies alone; in neither case was any cross-reactivity for anti-ICP8 or monoclonal antibody 5C observed (data not shown). Bars = 10 μm.
On the other hand, hr64-infected cells exhibit a very different staining pattern (Fig. 8G to I). As mentioned above, these cells contain replication compartments similar to those seen in wild-type- or hr74-infected cells; however, the merged image (Fig. 8I) indicates that the distribution of hexon-containing capsids is not restricted to replication compartments. Hexon staining is observed throughout the nucleus and is present in the areas, especially around the periphery of the nucleus, in which replication compartments are not seen (Fig. 8H and I). To confirm that hexon staining is not restricted to the replication compartments, we used the Voxel View program to create three-dimensional images of replication compartment and capsid staining patterns. Figure 8J and K show Vero cells infected with KOS and the UL32 mutant, hr64, respectively. The green regions represent hexon-containing capsids, while the red regions represent replication compartments. In KOS-infected cells (Fig. 8J), the green and red regions colocalize. In hr64-infected cells (Fig. 8K) some of the green color is observed within replication compartments (red); however, a significant portion of the green is visible outside the red regions. The image reconstructions confirm our previous observations that in Vero cells infected with the UL32 mutant, capsids are not restricted to replication compartments. When hr64 was used to infect 158 cells, the complementing cell line expressing the UL32 protein, the hexon staining pattern was found to resemble that of wild-type virus (data not shown), confirming that the alteration in capsid staining in hr64-infected Vero cells is due to the defect in the UL32 gene.
DISCUSSION
We report the construction and characterization of a UL32 insertion mutant, hr64, which fails to form plaques on Vero cells and which is defective for cleavage and packaging. Both the growth phenotype and the cleavage and packaging defects are corrected when hr64 is grown on the complementing cell line which expresses only the UL32 gene. These results confirm previous results with ts mutant tsN20 (55). While two other cleavage/packaging gene products (UL6 and UL25) appear to be associated with A, B, and C capsids and with virions, in our hands UL32 has not been detected in B capsids or in virions. On the other hand, UL6 and UL25 are still present in B capsids made in cells infected with the UL32 mutant, indicating that UL32 is not required for the correct localization of UL6 and UL25 in the capsids.
Chang et al. (13) previously reported that UL32 can be detected primarily in the cytoplasm of infected cells. Using an epitope-tagged version of this protein, we were able to detect some UL32 staining in the nuclei of infected cells in addition to the cytoplasmic staining. The UL32 which is in the nucleus appears to localize to replication compartments. Furthermore, in cells transfected with the UL32 gene, both nuclear and cytoplasmic staining was observed, indicating that no other cleavage/packaging or capsid proteins are required for the cytoplasmic and nuclear localization patterns. Finding UL32 in the nucleus is consistent with its putative role in cleavage and packaging. The reason for the presence of UL32 in the cytoplasm is not understood; however, it may indicate that UL32 carries out more than one function. It is thus possible that the role of nuclear UL32 is in capsid localization and that the role of cytoplasmic UL32 is performed at a later step in infection, such as egress of the capsid once it buds from the nucleus. Ward et al. previously reported that another cleavage/packaging protein, UL15, also localizes to replication compartments in infected cells, and we have recently confirmed this finding (64, 69). Furthermore, UL6 has also been observed in replication compartments (47).
The presence of UL6, UL15, and UL32 in replication compartments prompted us to ask whether cleavage and packaging occurs in replication compartments or at another adjacent site as previously suggested by Ward et al. for late times after infection. Using a hexon-specific antibody we observed capsid staining which colocalizes completely with replication compartments at 6 and 8 h postinfection. Since DNase-protected DNA can be detected as early as 6 h postinfection, we conclude that at these early times, packaging is likely to occur within replication compartments. In support of this conclusion, a recent report by Phelan et al. (47) demonstrates that capsid proteins and the HSV-1 DNA polymerase also colocalize at 8 h postinfection. We also analyzed capsid distribution at 16 h postinfection; at this time KOS-infected Vero cells exhibit several different staining patterns (data not shown). In some cells, hexon staining is present in aggregates that resemble the “assemblon” structures described by Ward et al. (64); however, in other cells hexon staining was diffuse. Ward et al. have proposed that the overlap of replication compartments with these sites of capsid accumulation may represent sites at which cleavage and packaging occur (64). While we cannot rule out that some packaging occurs outside replication compartments at late times postinfection, we favor a model in which cleavage and packaging occur within replication compartments. Our model is based on our observations that (i) some cells do not contain assemblon structures even at 16 h postinfection; (ii) at 6 h postinfection none of the cells contain assemblons in spite of the fact that we can detect significant levels of cleavage and packaging; and (iii) hexon-containing capsid staining occurs within replication compartments at 6 h postinfection. It is possible that assemblons which are only observed at late times postinfection represent dead-end accumulations of capsids, which are not destined for the cleavage and packaging processes.
The most striking feature of the UL32 mutant phenotype is the alteration of capsid distribution. Whereas in cells infected with wild-type virus and a virus with a mutant version of cleavage and packaging gene UL6, capsids localize exclusively to replication compartments, cells infected with the UL32 mutant exhibit an unusual capsid-staining pattern in which capsids are not restricted to replication compartments. Instead, many capsids accumulate at the nuclear periphery in areas which are devoid of ICP8. We thus conclude that UL32 may play a role in the correct localization of capsids after their assembly, a role in the packaging process which is distinct from that played by UL6. The DNA bacteriophages provide useful models for the process of genome encapsidation in the eukaryotic viruses. By analogy with these better-studied viruses, we might expect herpesviruses to encode a terminase complex responsible for translocation and cleavage of concatemeric DNA (reviewed in reference 9). In lambda phage, the gpF1 protein influences the efficiency of the terminase (12) and in combination with the terminase may serve to facilitate capsid interactions with DNA (40). We are intrigued by the possibility that UL32 may play a role similar to that of gpF1 in facilitating the interaction between capsids and DNA by bringing capsids to replication compartments. Our observations raise the possibility that UL32 may be expected to interact with capsids. In this report, however, we were not able to detect UL32 in purified B capsids or in virions. It is possible that UL32 interacts only transiently with capsids in a way that is not detected under the conditions used in this study. In fact, if UL32 plays a role similar to that played by the gpF1 protein of the lambda phage, one might expect a transient interaction with capsids and with the terminase. Although the HSV terminase has not been definitively identified, it has been suggested that UL15 may form part of a viral terminase complex (6, 15, 68). Recent work in our laboratory suggests that UL15 may exhibit a transient association with capsids; we can detect UL15 primarily in B capsids and in much lesser amounts in C capsids (69). It is possible that UL32 also plays a transient role in interactions between capsids, terminase, and viral genomes. Further experimentation will be required to test these proposals.
The apparent colocalization of ICP8 and capsids in replication compartments deserves further comment. Replication compartments are large globular domains in which several viral processes are known to occur. In addition to the seven viral replication proteins and several cellular replication proteins which are now known to colocalize within replication compartments, ICP4 and several cleavage and packaging proteins are also observed within these structures. The relationship between these various processes is not well understood. It is worthwhile to note that there are viral processes which occur in the nucleus outside the replication compartments. For example, although we believe that encapsidation occurs within replication compartments, our preliminary evidence suggests that capsids themselves may assemble at discrete sites outside replication compartments and subsequently move to replication compartments prior to packaging (28). One model, which is consistent with all our data, is that UL32 facilitates the interaction between capsids which may be assembled outside replication compartments, viral genomes which are presumably generated within the replication compartments, and the cleavage/packaging machinery. In cells infected with UL32 mutant virus, some capsids are seen within replication compartments, but a large number accumulate in spaces outside the replication compartments. This may indicate that the appropriate interactions between capsids and the cleavage/packaging machinery leading to the initiation of the encapsidation process cannot occur in this mutant. In fact, the absence of A and C capsids in UL32 mutant-infected cells indicates that encapsidation was not even attempted in these cells. In bacteriophage T4, packaging has been shown to compete with the replication and recombination machinery (39). It is possible that in HSV-1-infected cells, various competing reactions will determine whether viral DNA undergoes replication, recombination, or cleavage and packaging.
Two major conclusions can be made from the data presented in this paper: (i) in contrast to the results of a recent study by Ward et al. (64), we believe that cleavage and packaging are likely to occur within replication compartments at least at early times postinfection; (ii) the UL32 gene product acts at a unique step in the cleavage/packaging process and likely plays a role in the efficient localization of capsids to replication compartments. We propose that the inability of capsids to localize to replication compartments may be responsible for the cleavage/packaging defect of the UL32 mutant.
ACKNOWLEDGMENTS
We thank the members of our laboratory and Fred Homa for critically reviewing the manuscript. We thank Kevin Nawotka for the construction of pAPV plasmid and Fred Homa for pAPV UL6. We are grateful to Bernard Roizman for providing the UL32 antiserum, Bill Ruyechan for providing ICP8 antiserum (anti-ICP8), Jay Brown for providing monoclonal antibody 5C, and Fred Homa for UL25 antiserum and the KUL25NS virus. We especially thank Susan Krueger and Frank Morgan for assistance with confocal imaging.
This study was supported by National Institutes of Health grant AI 37549.
REFERENCES
- 1.Addison C, Rixon F J, Palfreyman J W, O’Hara M, Preston V G. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology. 1984;138:246–259. doi: 10.1016/0042-6822(84)90349-0. [DOI] [PubMed] [Google Scholar]
- 2.Addison C, Rixon F J, Preston V G. Herpes simplex virus type 1 UL28 gene product is important for the formation of mature capsids. J Gen Virol. 1990;71:2377–2384. doi: 10.1099/0022-1317-71-10-2377. [DOI] [PubMed] [Google Scholar]
- 3.Ali M A, Forghani B, Cantin E M. Characterization of an essential HSV-1 protein encoded by the UL25 gene reported to be involved in virus penetration and capsid assembly. Virology. 1996;216:278–283. doi: 10.1006/viro.1996.0061. [DOI] [PubMed] [Google Scholar]
- 4.Al-Kobaisi M F, Rixon F J, McDougall I, Preston V G. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology. 1991;180:380–388. doi: 10.1016/0042-6822(91)90043-b. [DOI] [PubMed] [Google Scholar]
- 5.Baines J D, Cunningham C, Nalwanga D, Davison A. The UL15 gene of herpes simplex virus type 1 contains within its second exon a novel open reading frame that is translated in frame with the UL15 gene product. J Virol. 1997;71:2666–2673. doi: 10.1128/jvi.71.4.2666-2673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baines J D, Poon A P, Rovnak J, Roizman B. The herpes simplex virus 1 UL15 gene encodes two proteins and is required for cleavage of genomic viral DNA. J Virol. 1994;68:8118–8124. doi: 10.1128/jvi.68.12.8118-8124.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bataille D, Epstein A. Herpes simplex virus replicative concatemers contain L components in inverted orientation. Virology. 1994;203:384–388. doi: 10.1006/viro.1994.1498. [DOI] [PubMed] [Google Scholar]
- 8.Bazinet C, King J. The DNA translocating vertex of dsDNA bacteriophage. Annu Rev Microbiol. 1985;39:109–129. doi: 10.1146/annurev.mi.39.100185.000545. [DOI] [PubMed] [Google Scholar]
- 9.Black L W. DNA packaging in ds DNA bacteriophages. Annu Rev Microbiol. 1989;43:267–292. doi: 10.1146/annurev.mi.43.100189.001411. [DOI] [PubMed] [Google Scholar]
- 10.Bush M, Yager D R, Gao M, Weisshart K, Marcy A I, Coen D M, Knipe D M. Correct intranuclear localization of herpes simplex virus DNA polymerase requires the viral ICP8 DNA-binding protein. J Virol. 1991;65:1082–1089. doi: 10.1128/jvi.65.3.1082-1089.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casjens S, Hendrix R. Control mechanism in dsDNA bacteriophage assembly. In: Calendar R, editor. The bacteriophages. Vol. 1. New York, N.Y: Plenum Press; 1988. pp. 15–91. [Google Scholar]
- 12.Catalano C E, Tomka M A. Role of gpFI protein in DNA packaging by bacteriophage lambda. Biochemistry. 1995;34:10036–10042. doi: 10.1021/bi00031a027. [DOI] [PubMed] [Google Scholar]
- 13.Chang Y E, Poon A P, Roizman B. Properties of the protein encoded by the UL32 open reading frame of herpes simplex virus type 1. J Virol. 1996;70:3938–3946. doi: 10.1128/jvi.70.6.3938-3946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coen D M, Aschman D P, Gelep P T, Retondo M J, Weller S K, Schaffer P A. Fine mapping and molecular cloning of mutations in the herpes simplex virus DNA polymerase locus. J Virol. 1984;49:236–247. doi: 10.1128/jvi.49.1.236-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davison A J. Channel catfish virus: a new type of herpesvirus. Virology. 1992;186:9–14. doi: 10.1016/0042-6822(92)90056-u. [DOI] [PubMed] [Google Scholar]
- 16.de Bruyn Kops A, Knipe D M. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988;55:857–868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]
- 17.Desai P, Ramakrishnan R, Lin Z W, Osak B, Glorioso J C, Levine M. The RR1 gene of herpes simplex virus type 1 is uniquely trans activated by ICP0 during infection. J Virol. 1993;67:6125–6135. doi: 10.1128/jvi.67.10.6125-6135.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dube P, Tavares P, Lurz R, van Heel M. The portal protein of bacteriophage Spp1: a DNA pump with 13-fold symmetry. EMBO J. 1993;12:1303–1309. doi: 10.1002/j.1460-2075.1993.tb05775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 20.Gibson W, Roizman B. Proteins specified by herpes simplex virus. VIII. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972;10:1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein D J, Weller S K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein D J, Weller S K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein D J, Weller S K. An ICP6::lacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J Virol. 1988;62:2970–2977. doi: 10.1128/jvi.62.8.2970-2977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodrich L D, Schaffer P A, Dorsky D I, Crumpacker C S, Parris D S. Localization of the herpes simplex virus type 1 65-kilodalton DNA-binding protein and DNA polymerase in the presence and absence of viral DNA synthesis. J Virol. 1990;64:5738–5749. doi: 10.1128/jvi.64.12.5738-5749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 26.Knipe D M, Senechek D, Rice S A, Smith J L. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J Virol. 1987;61:276–284. doi: 10.1128/jvi.61.2.276-284.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamberti C, Weller S K. The herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology. 1996;226:403–407. doi: 10.1006/viro.1996.0668. [DOI] [PubMed] [Google Scholar]
- 28.Lamberti, C., and S. K. Weller. Unpublished results.
- 29.Liptak L M, Uprichard S L, Knipe D M. Functional order of assembly of herpes simplex virus DNA replication proteins into prereplicative structures. J Virol. 1996;70:1759–1767. doi: 10.1128/jvi.70.3.1759-1767.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukonis C J, Weller S K. Characterization of nuclear structures in cells infected with herpes simplex virus type 1 in the absence of viral DNA replication. J Virol. 1996;70:1751–1758. doi: 10.1128/jvi.70.3.1751-1758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukonis C J, Weller S K. Formation of herpes simplex virus 1 replication compartments by transfection: requirements and localization to nuclear domain 10. J Virol. 1997;71:2390–2399. doi: 10.1128/jvi.71.3.2390-2399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukonis C J, Weller S K. The herpes simplex virus type 1 transactivator ICP0 mediates aberrant intracellular localization of the viral helicase/primase complex subunits. Virology. 1996;220:495–501. doi: 10.1006/viro.1996.0338. [DOI] [PubMed] [Google Scholar]
- 33.Malik A K, Shao L, Shanley J D, Weller S K. Intracellular localization of the herpes simplex virus type-1 origin binding protein, UL9. Virology. 1996;224:380–389. doi: 10.1006/viro.1996.0545. [DOI] [PubMed] [Google Scholar]
- 34.Martinez R, Sarisky R T, Weber P C, Weller S K. Herpes simplex virus type 1 alkaline nuclease is required for efficient processing of viral DNA replication intermediates. J Virol. 1996;70:2075–2085. doi: 10.1128/jvi.70.4.2075-2085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez R M, Shao L, Weller S K. The conserved helicase motifs of the herpes simplex virus type 1 origin-binding protein UL9 are important for function. J Virol. 1992;66:6735–6746. doi: 10.1128/jvi.66.11.6735-6746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matusick-Kumar L, McCann III P J, Robertson B J, Newcomb W W, Brown J C, Gao M. Release of the catalytic domain No from the herpes simplex virus type 1 protease is required for viral growth. J Virol. 1995;69:7113–7121. doi: 10.1128/jvi.69.11.7113-7121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNab A R, Desai P, Person S, Roof L L, Thomsen D R, Newcomb W W, Brown J C, Homa F L. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J Virol. 1998;72:1060–1070. doi: 10.1128/jvi.72.2.1060-1070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McVoy M A, Adler S P. Human cytomegalovirus DNA replicates after early circularization by concatemer formation, and inversion occurs within the concatemer. J Virol. 1994;68:1040–1051. doi: 10.1128/jvi.68.2.1040-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosig G, Ghosal D, Bock S. Interactions between the maturation protein gp17 and the single-stranded DNA binding protein gp32 initiate DNA packaging and compete with initiation of secondary DNA replication forks in phage T4. Prog Clin Biol Res. 1981;64:139–150. [PubMed] [Google Scholar]
- 40.Murialdo H, Tzamtzis D. Mutations of the coat protein gene of bacteriophage lambda that overcome the necessity for the FI gene; the EFi domain. Mol Microbiol. 1997;24:341–353. doi: 10.1046/j.1365-2958.1997.3321698.x. [DOI] [PubMed] [Google Scholar]
- 40a.Newcomb, W. W., and J. C. Brown. Personal communication.
- 41.Newcomb W W, Homa F L, Thomsen D R, Booy F P, Trus B L, Steven A C, Spencer J V, Brown J C. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid formation. J Mol Biol. 1996;263:432–446. doi: 10.1006/jmbi.1996.0587. [DOI] [PubMed] [Google Scholar]
- 42.Newcomb W W, Homa F L, Thomsen D R, Ye Z, Brown J C. Cell-free assembly of the herpes simplex virus capsid. J Virol. 1994;68:6059–6063. doi: 10.1128/jvi.68.9.6059-6063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olivo P D, Nelson N J, Challberg M D. Herpes simplex virus type 1 gene products required for DNA replication: identification and overexpression. J Virol. 1989;63:196–204. doi: 10.1128/jvi.63.1.196-204.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel A H, Maclean J B. The product of UL6 gene of herpes simplex virus type 1 is associated with virus capsids. Virology. 1995;206:465–478. doi: 10.1016/s0042-6822(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 45.Patel A H, Rixon F J, Cunningham C, Davison A J. Isolation and characterization of herpes simplex virus type 1 mutants defective in the UL6. Virology. 1996;217:111–123. doi: 10.1006/viro.1996.0098. [DOI] [PubMed] [Google Scholar]
- 46.Perdue M L, Cohen J C, Randall C C, O’Callaghan D J. Biochemical studies of the maturation of herpesvirus nucleocapsid species. Virology. 1976;74:194–208. [PubMed] [Google Scholar]
- 47.Phelan A, Dunlop J, Patel A H, Stow N D, Clements J B. Nuclear sites of herpes simplex virus type 1 DNA replication and transcription colocalize at early times postinfection and are largely distinct from RNA processing factors. J Virol. 1997;71:1124–1132. doi: 10.1128/jvi.71.2.1124-1132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poon A P W, Roizman B. Characterization of a temperature-sensitive mutant of the UL15 open reading frame of herpes simplex virus 1. J Virol. 1993;67:4497–4503. doi: 10.1128/jvi.67.8.4497-4503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinlan M P, Chen L B, Knipe D M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984;36:857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- 50.Randall R E. Intranuclear localization of herpes simplex virus immediate-early and delayed-early proteins: evidence that ICP4 is associated with progeny virus DNA. J Gen Virol. 1986;67:2163–2177. doi: 10.1099/0022-1317-67-10-2163. [DOI] [PubMed] [Google Scholar]
- 51.Schaffer P A, Aron G M, Biswal N, Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973;52:57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- 52.Severini A, Morgan A R, Tovell D R, Tyrrel L J. Study of the structure of replicative intermediates of HSV-1 DNA by pulsed-field gel electrophoresis. Virology. 1994;200:428–435. doi: 10.1006/viro.1994.1206. [DOI] [PubMed] [Google Scholar]
- 53.Shelton L S G, Albright A G, Ruyechan W T, Jenkins F J. Retention of the herpes simplex virus type 1 (HSV-1) UL37 protein on single-stranded DNA columns requires the HSV-1 ICP8. J Virol. 1994;68:521–525. doi: 10.1128/jvi.68.1.521-525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherman G, Bachenheimer S. DNA processing in temperature-sensitive morphogenic mutants of HSV-1. Virology. 1987;158:427–430. doi: 10.1016/0042-6822(87)90214-5. [DOI] [PubMed] [Google Scholar]
- 55.Sherman G, Bachenheimer S L. Characterization of intranuclear capsids made by ts morphogenic mutants of HSV-1. Virology. 1988;163:471–480. doi: 10.1016/0042-6822(88)90288-7. [DOI] [PubMed] [Google Scholar]
- 56.Steven A C, Spear P G. Herpesvirus capsid assembly and envelopment. In: Burnet R, Chiu W, Garcea R, editors. Structural biology of viruses. New York, N.Y: Oxford University Press; 1996. [Google Scholar]
- 57.Sze P, Herman R C. The herpes simplex virus type 1 ICP6 gene is regulated by a leaky early promoter. Virus Res. 1992;26:141–152. doi: 10.1016/0168-1702(92)90153-z. [DOI] [PubMed] [Google Scholar]
- 58.Szilagyi J F, Cunningham C. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J Gen Virol. 1991;72:661–668. doi: 10.1099/0022-1317-72-3-661. [DOI] [PubMed] [Google Scholar]
- 59.Tengelsen L A, Pederson N E, Shaver P R, Wathen M W, Homa F L. Herpes simplex virus type 1 DNA cleavage and encapsidation require the product of the UL28 gene: isolation and characterization of two UL28 deletion mutants. J Virol. 1993;67:3470–3480. doi: 10.1128/jvi.67.6.3470-3480.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomsen D R, Roof L L, Homa F L. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J Virol. 1994;68:2442–2457. doi: 10.1128/jvi.68.4.2442-2457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Towbin H, Staehlein T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trus B L, Booy F P, Newcomb W W, Brown J C, Homa F L, Thomsen D R, Steven A C. The herpes simplex virus capsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly. J Mol Biol. 1996;263:447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 63.Trus B L, Newcomb W W, Booy F P, Brown J C, Steven A C. Distinct monoclonal antibodies separately label the hexons or the pentons of herpes simplex virus capsid. Proc Natl Acad Sci USA. 1992;89:11508–11512. doi: 10.1073/pnas.89.23.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ward P L, Ogle W O, Roizman B. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J Virol. 1996;70:4623–4631. doi: 10.1128/jvi.70.7.4623-4631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weller S K, Aschman D P, Sacks W R, Coen D M, Schaffer P A. Genetic analysis of temperature-sensitive mutants of HSV-1: the combined use of complementation and physical mapping for cistron assignment. Virology. 1983;130:290–305. doi: 10.1016/0042-6822(83)90084-3. [DOI] [PubMed] [Google Scholar]
- 66.Weller S K, Carmichael E P, Aschman D P, Goldstein D J, Schaffer P A. Genetic and phenotypic characterization of mutants in four essential genes that map to the left half of HSV-1 UL DNA. Virology. 1987;161:198–210. doi: 10.1016/0042-6822(87)90186-3. [DOI] [PubMed] [Google Scholar]
- 67.Weller S K, Spadaro A, Schaffer J E, Murray A W, Maxam A M, Schaffer P A. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol Cell Biol. 1985;5:930–942. doi: 10.1128/mcb.5.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu D, Sheaffer A K, Tenney D J, Weller S K. Characterization of ICP6::lacZ insertion mutants of the UL15 gene of herpes simplex virus type 1 reveals the translation of two proteins. J Virol. 1997;71:2656–2665. doi: 10.1128/jvi.71.4.2656-2665.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu, D., and S. K. Weller. Unpublished results.
- 70.Zhang X, Efstathiou S, Simmons A. Identification of novel herpes simplex virus replicative intermediates by field inversion gel electrophoresis: implications for viral DNA amplification strategies. Virology. 1994;202:530–539. doi: 10.1006/viro.1994.1375. [DOI] [PubMed] [Google Scholar]
- 71.Zhu L, Weller S K. The UL5 gene of herpes simplex virus type 1: isolation of a lacZ insertion mutant and association of the UL5 gene product with other members of the helicase-primase complex. J Virol. 1992;66:458–468. doi: 10.1128/jvi.66.1.458-468.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu L, Weller S K. UL5, a protein required for HSV DNA synthesis: genetic analysis, overexpression in Escherichia coli, and generation of polyclonal antibodies. Virology. 1988;166:366–378. doi: 10.1016/0042-6822(88)90507-7. [DOI] [PubMed] [Google Scholar]