Abstract
The aim of this study was to determine the levels of superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and malondialdehyde (MDA) in patients with refractory epilepsy. Serum superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and malondialdehyde (MDA) levels were determined using the spectrophotometer method. Refractory epilepsy patients’ serum superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and malondialdehyde (MDA) levels were statistically significant compared to the healthy control group (p < 0.05). In conclusion, superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and malondialdehyde (MDA) levels may play an important role in the etiopathogenesis of refractory epilepsy. This study was the first to investigate some parameters in refractory epilepsy disease.
Keywords: Catalase, Malondialdehyde, Refractory epilepsy, Superoxide dismutase
Subject terms: Development of the nervous system, Neuroimmunology
Introduction
Epilepsy is known as a serious disease of the nervous system. Loss of consciousness usually occurs in epilepsy patients1–4.Epilepsy is a very commonly observed disease globally. This disease causes physical, psychosocial and economic problems in society5. Recently, studies of epilepsy have intensified. Despite the effective doses of antiepileptics used in the treatment of epilepsy, seizure control cannot be achieved, because the disease has become very resistant to drugs. In epilepsy, seizures can occur continuously6,7. Refractory epilepsy is an important disease in which seizure control cannot be achieved. Different drugs and doses are used to fight against this disease. However, seizure recurrence continues in epileptic patients8,9. The main method for diagnosis, treatment and follow-up of epilepsy is age, gender, seizure types, genomic studies, technological advances, electroencephalography (EEG) and imaging10. Drugs used in the treatment of epilepsy provide seizure control, but do not have an effect on epileptogenesis11.
Free radicals are unpaired electron pairs. The main free radicals are compounds such as hydroxy (OH−), peroxy (ROO.), and superoxide (O2−) radical. When free radicals are formed, they become stable. Also, these radicals enter the structure of cells and damage them. As a result of this damage, various diseases may occur. It has been reported that free oxygen radicals play an important role in the etiopathogenesis of various diseases. It has been determined that free oxygen radical damage occurs in diseases such as bladder disease, prostate cancer, sepsis, myocardial infarction, stroke, perinatal hypoxic brain injury, glomerulonephritis, uveitis, various cancers and arthritis in laboratory, clinical and experiments12–18. However, antioxidants protect the cell against these radicals17–20.
Malondialdehyde (MDA) is the end product of lipid peroxidation. MDA occurs in the formation of free oxygen radicals or when arachidonic acid occurs. MDA is known as an indicator of oxidative stress. When free radicals increase, MDA level rise as well. In the literature, it has been found that MDA, a marker of oxidative stress, has a higher level in patients with epilepsy21. Another study with similar results reported that MDA levels are higher in epilepsy patients compared to healthy individuals22.
Catalase (CAT) is an enzyme in the oxidoreductase group. It has four heme groups. It is in the structure of CAT hemoprotein. CAT converts hydrogen peroxide (H2O2) into water and oxygen. It is one of the most powerful antioxidants23. It has been reported that CAT activity in patients with adult epilepsy have decreased activity compared to healthy individuals in the patient group24. Literature studies have determined that CAT activity in epilepsy patients have decreased activity compared to healthy individuals22.
Serum superoxide dismutase (SOD) is a very resistant antioxidant in the cell. It is also a free radical scavenger. It converts the superoxide radical into hydrogen peroxide and oxygen25. In a study on epilepsy, it has been found that SOD activity increased in the patient group26. Conversely, in another study, SOD activity has been found to be lower in epilepsy patients than in healthy individuals22.
Reduced glutathione (GSH) removes reactive oxygen molecules from the cell. It is synthesized in the liver. At the same time, it has 3 tripeptides in its structure. There are two types of GSH, endogenous and exogenous27. In a study, it has been reported that the GSH level in epileptic patients did not change compared to healthy individuals28. In another study, it was determined that the GSH level increased in epileptic patients29.
In this study, we provide a brief discussion on the role of oxidative stress and some antioxidant activities in the pathophysiology of refractory epilepsy. The role of neuroprotectants in the therapeutic strategy to prevent or treat epilepsy is also discussed. In this study, our first aim was to investigate the relationship between oxidative stress and in refractory epilepsy. This study was the first to investigate some parameters in refractory epilepsy disease. Additionally, the second aim of this study is to determine the levels of superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and malondialdehyde (MDA) in resistant epilepsy patients who have not been studied before.
Materıals and methods
Patients who experience more than 1 seizure a month and show a tolerance to medication were accepted as refractory epileptic30.
In this study, blood samples were taken from 35 healthy individuals and 35 patients diagnosed with refractory epilepsy. 2 ml of blood was taken from the antecubital venous vein and added to a biochemistry tube.
Method
A total of 70 people, 35 of whom were diagnosed with refractory epilepsy and 35 healthy controls, who applied to the Neurology Department of Van Yüzüncü Yıl University, were included in the study. The age range of the patient group was 33.09 ± 13.4. The age range of the control group was 34 ± 13. Most of the subjects included in this study were patients whose seizures could not be fully controlled despite using effective doses of 1st generation and 2nd generation antiepileptic drugs31. All of the patients were examined in detail and EEG and IMR of the patients were taken. In addition, demographic characteristics of the patients, frequency of seizures, seizure type, duration of disease and medications they used were recorded (Table 1). Patients with hypertension, diabetes, rheumatoid arthritis, chronic neurological disease, a history of smoking, alcohol and those using any other non-prescription drugs were not included in the study. Patients with refractory epilepsy who had inflammatory conditions and those who took antioxidants as supplements or used medication were also excluded from the study. We assured that all methods were carried out in accordance with Scientific Report’s relevant guidelines and regulations and also informed consent was obtained from all subjects and/or their legal guardians.
Table 1.
Demographic data of the patient and control groups.
| Patient | Control | |
|---|---|---|
| n | 35 | 35 |
| Age (mean ± S.D) | 33.09 ± 13.4 | 34 ± 13 (p = 0.142) |
| Gender | ||
| Male (n,%) | 14, 40% | 24, 68.6% |
| Female (n,%) | 21, 60% | 11, 31.4% |
| Patients | ||
| EEG None (n, %) | 21, 60% | |
| Pathological (n, %) | 14, 40% | |
| MR None (n, %) | 23, 65.7% | |
| Pathological (n, %) | 12, 34.3% | |
| Seizurefrequency (n, %) | 1 times 3 months: 2, 5.7% | |
| 1 times month: 1, 2.8% | ||
| 2 times month: 24, 68.6% | ||
| 2–3 times month:1, 2.9% | ||
| 3 times month: 3, 8.6% | ||
| 3–4 times month: 3, 8.6% | ||
| 3 times month: 1, 2.8% | ||
| Seizure type (n, %) | Focal: 9, 25.8% | |
| Focal-jk: 18, 51.4% | ||
| JK: 8, 22.8% | ||
| Drugs (n, %) | Lev: 7, 20% | |
| Other: 28, 80% | ||
In the study, biochemical parameters were determined with serum samples. Local ethics committee approval was obtained from Van Yüzüncü Yıl University Medical Faculty Hospital before blood samples were taken (decision number:03 and date:10.02.2021). Laboratory and imaging results of the patients were recorded. For this study, 2 ml of blood was taken from the antecubital venous vein and added to a biochemistry tube. Afterwards, the blood samples were centrifuged at 5.000 rpm for 10 min and the serums were separated from the plasma. From the serum samples, superoxide dismutase (SOD) activity, catalase (CAT) activity, reduced glutathione (GSH) levels and malondialdehyde (MDA) levels were determined.
Biochemical investigations
Determination of superoxide dismutase (SOD) activity
SOD activity was measured in serum samples in both the patient and healthy control groups. SOD activity was determined in accordance with the method proposed by Gunduz et al18. The SOD analysis was measured as thus: Two tubes were taken, one was serum and the other tube was blank. 1.425 µl of reagent was added to the blank and sample tubes. 50 µl of serum was added to the sample tube. In the blank tube, no serum was added. Then, while 100 µl of bidistilled water was added to the blank tube, it was not added to the sample tube. 25 µl of xanthine oxidase was added to both the blank and the sample tube. This mixture was incubated at 25 °C for 20 min. Finally, the blank and sample tubes were scanned vis a vis bidistilled water at 560 nm.
% Inhibition = [(blank OD–Sample OD)/blank OD] × 100
Determination of reduced glutathione (GSH) level
GSH level was measured in serum samples in both refractory epilepsy and healthy control groups. The GSH level was determined in accordance with the Tietz method32. 800 µl of phosphate buffer was added to 200 µl of serum. Initial absorbance (OD1) at 412 nm was measured. 100 µl of Ellman’s reagent was added to the same tube and then the second absorbance (OD2) was measured.
Calculation
Glutathione concentration was calculated in mmol/g protein unit.
C/1000 = (OD2 − OD1) / 13,600 × E1 × 5/2 × ½
13,600: Molar extinction coefficient of yellow color formed during the interaction of GSH and 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB).
E1: If a band with a width greater than 6 nm is used, a derivative extrusion coefficient is used for correcting both light path and bandwidth differences. The width of the tape we used was 2 nm. It was taken as E1 = 1 in the calculations.
1000: conversion coefficient to mmol.
C: mmol/glutathione (mg/dl).
Determination of catalase (CAT) activity
CAT activity was measured in serum samples in both refractory epilepsy and healthy control groups. In this study, CAT activity was determined in accordance with the Aeibi method. Firstly, 1.4 ml of 30 mM H2O2 was placed in the blank tube and 0.1 ml of phosphate buffer was added to it. 1.4 ml of 30 mM H2O2 was placed in the sample tube and 0.1 ml of sample was added to it and the tubes were vortexed. The absorbance values were then measured twice at 240 nm at 30 s intervals using the spectrophotometric method33.
Activity account:
Activity = (2.3/ΔX) × [(log A1/log A2)].
ΔX: 30 s.
2.3: 1 mmol optical density of H2O2 in 1 cm light path.
Determination of malondialdehyde (MDA) level
MDA level was measured according to the Bird and Draper method34. 200 ml of serum was added to the sample tube, then 800 ml of phosphate buffer, 25 ml of butylated hydroxy toluene (BHT) solution and 500 ml of 30% tricarboxylic acid (TCA) were added to it. The tubes were mixed by vortexing and kept on ice for 2 h. It was then centrifuged at 2000 rpm for 15 min. 1 ml of the obtained supernatant was taken and transferred to another tube. Then 75 ml of ethylenediamine tetraacetic acid (EDTA) and 250 ml of thiobarbutiric acid (TBA) were added to this mixture. The tubes were mixed by vortex again and kept in a hot water bath for 15 min. The tubes were then brought to room temperature. Absorbance values were read in the spectrophotometer at 532 nm.
Calculation of malondialdehyde level:
C = F × 6.41 × A.
C: Concentration, F: Dilution factor, A: Absorbance.
Statistical analysis
The descriptive statistics for the features mentioned were expressed as mean, standard deviation, median, min and max for continuous variables, numbers and percentages for categorical variables. The population of the study consists of a total of 200 refractory epilepsy patients who applied to Van Yüzüncü Yıl University Dursun Odabaşı Medical Center, Department of Neurology in the last 6 months. The number of samples was determined by the Universal Population Volume Known Sampling Calculation Formula (n = Nz2pq/d2(N − 1) + z2pq). According to the literature review; It was determined that the "effect size" value was 0.15. According to this; Taking the Primary Type Error as 5% (Z = 1.96), the Power of the Test as 80% and the effect size as 0.15 units; the minimum sample size was calculated as n = 35.2. Thus, considering the study process, the study was planned with a total of 70 volunteers, including 35 patients diagnosed with resistant epilepsy and 35 healthy controls. The T-Test was used in the comparison of binary groups providing the condition of normal distribution. The statistics of the Mann–Whitney U-test was used when the condition of normal distribution was not provided. The statistical significance level was taken as p < 0.05 and the SPSS statistical software pack version 19.0 (SPSS Inc, Chicago, III, USA) was used for analyses.
Ethical approval
The authors confirm that they have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Results
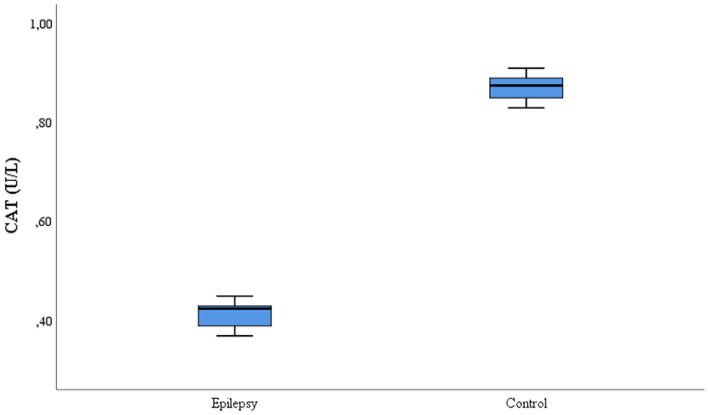
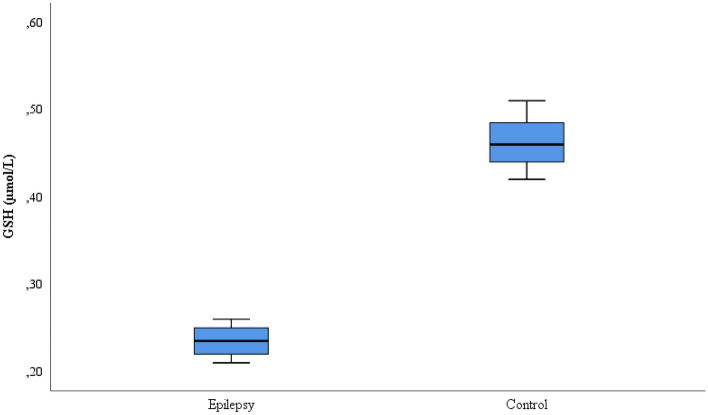
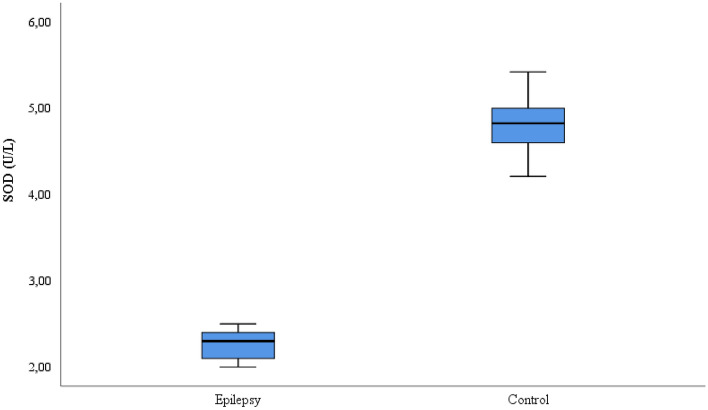
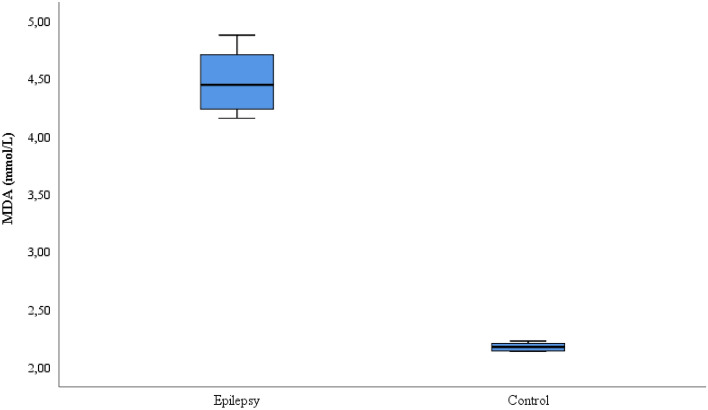
This study included 35 patients with refractory epilepsy and 35 control subjects. The age, gender, seizure frequency, seizure type, EEG, IMR and medication information of the patient and control groups is given in Table 1. The CAT, SOD, GSH, and MDA values comparison for the patient and control groups is shown on Table 2. While the CAT, GSH and SOD values were lower in the refractory epilepsy group compared to the control group, the MDA values were higher in the refractory epilepsy group (Figs. 1, 2, 3 and 4).
Table 2.
Biochemical parameters between refractory epilepsy and healthy control group.
| Refractory epilepsy | Control | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.D | Median | 25% | 75% | Mean | S.D | Median | 25% | 75% | ||
| CAT (U/L) | 0.42 | ± 0.03 | 0.43 | 0.39 | 0.43 | 0.87 | ± 0.02 | 0.88 | 0.85 | 0.89 | < 0.001 |
| GSH (µmol/L) | 0.24 | ± 0.02 | 0.24 | 0.22 | 0.25 | 0.46 | ± 0.03 | 0.46 | 0.44 | 0.49 | < 0.001 |
| SOD (U/L) | 2.28 | ± 0.17 | 2.30 | 2.1 | 2.4 | 4.84 | ± 0.33 | 4.82 | 4.6 | 5.0 | < 0.001 |
| MDA(mmol/L) | 4.48 | ± 0.25 | 4.45 | 4.24 | 4.71 | 2.15 | ± 0.08 | 2.18 | 2.15 | 2.21 | < 0.001 |
Figure 1.
CAT activities between the refractory epilepsy and the healthy control group.
Figure 2.
GSH levels between the refractory epilepsy and the healthy control group.
Figure 3.
SOD activities between the refractory epilepsy and the healthy control group.
Figure 4.
MDA levels between the refractory epilepsy and the healthy control group.
Discussion
Epilepsy is a neurological disorder. It is a very common disease. Refractory epilepsy is a much more serious disease. In these patients, seizure control is never achieved8,9. Epilepsy patients face very serious risks in daily life. The quality of life of these patients declines significantly. Likewise, these patients have serious psychological problems35. Epilepsy is more common in men than in women in general. Seizures in this disease are often more common in the elderly people and younger children. Controversy continues about the causes of this disease. The research of the etiopathonenesis of this disease are still ongoing36. Surgery has recently become important in the treatment of refractory epilepsy8.
Free radicals cause malondialdehyde levels to rise which causes oxidative stress. Free radicals are unpaired atoms. It is known that free radicals are responsible for diseases such as atherosclerosis, diabetes, epilepsy, inflammatory diseases and cancer. Oxidative stress occurs when the balance between oxidant and antioxidants is damaged37,38. Oxidative stress leads to metabolic problems in the membrane lipids, DNA, RNA, and the increased risk of seizures and recurrent seizures39. There is a relationship between the brain and oxidative stress. Thus, when the input of the oxygen to the metabolism is intense, the brain signals. As a result of increased oxidative stress, cerebral diseases such as brain damage or epilepsy occur39,40.
It has been determined that diseases such as Alzheimer's, Parkinson’s, and multiple sclerosis (MS) are neurodegenerative diseases and that there is an increase in the level of oxidative stress in patients with these diseases. Free oxygen radicals or oxidative stress cause structural changes in the cell. Studies have reported that oxidative stress is effective in depression and different psychological stress diseases41–43.
MDA is a marker reflecting oxidative stress. MDA also shows lipid peroxidation. The autocatalytic chain reaction involved in lipid peroxidation is very dangerous to the cell and initiates cellular damage. In a study, it was found that the MDA level increased in epilepsy patients compared to the healthy control group21. In another study, the MDA levels were found to be higher in individuals with normal epilepsy44. In this study, MDA levels were found to be significantly higher in patients with refractory epilepsy when compared to healthy individuals (p < 0.05). (Fig. 4). According to these results, high MDA levels in refractory epilepsy patients may be another indicator of increased oxidative stress. The results show us that brain damage has occurred. Because of the high amount of lipids in the brain, intense oxygen consumption and increased oxidative metabolism show us that the brain is sensitive to oxidative stress. This may be the beginning of refractory epilepsy disease. In addition, MDA may cause structural differences in the platelet mechanism. Thus, prostaglandin release and cerebral blood flow decrease in the metabolism. In conclusion, increased oxidative stress may play an important role in the pathogenesis of refractory epilepsy. MDA was investigated for the first time in refractory epilepsy. In addition, this study will contribute to the literature.
Antioxidants fight free radicals and also reduce the effects of free radicals. SOD, CAT, GST, GR (glutathione reductase), GPx (glutathione peroxidase) and GSH are the main antioxidants. Antioxidant enzymes are protective type enzymes that increase their activity under oxidative stress conditions17–20. Antioxidants are required as compensatory mechanisms of oxidative stress. Otherwise, oxidative stress may cause cellular damage. Strong antioxidants are needed to conteract this. Thanks to the antioxidants found in the body, it is possible to protect from cellular damage.
SOD is a very powerful antioxidant enzyme. SOD ensures the excretion of many toxic and harmful compounds from the body. It also removes free radical products from the cell. In a study, it was reported that the SOD level decreased significantly in patients with migraine17. In another study, it was reported that SOD activity decreased in bladder cancer patients compared to healthy control groups45. In a presented study, it was reported that the SOD activity decreased in epilepsy patients26. In the literature, it has been determined that SOD activity is decreased in epilepsy patients compared to healthy individuals.22 In this study, it was observed that SOD enzyme activity decreased significantly in refractory epilepsy patients compared to healthy individuals (p < 0.05) (Fig. 3). The cause of this is likely the high accumulation of lipid compounds in the brain in patients with refractory epilepsy. Oxidative stress rises and can also trigger refractory epilepsy. Thus, antioxidant agents, especially SOD activity, begin to decrease in the body. SOD activity in refractory epilepsy was studied for the first time. The role of antioxidants in the treatment of refractory epilepsy should be researched in more detail. This study will greatly contribute to the the current literature in these respects.
CAT is one of the most important antioxidant agents. It is a leading compound in the removal of hydrogen peroxide, which is toxic in the cell. It converts hydrogen peroxide into water and molecular oxygen in metabolism. At the same time, CAT prevents cellular damage18. . It has been reported that CAT activity is decreased in young epilepsy patients compared to healthy individuals24. In another study, CAT activity has been found to be lower in epilepsy patients22. In this study, the serum CAT activity of refractory epilepsy patients was statistically significant compared to the healthy control group (p < 0.05), and it was also found to be low in patients. (Fig. 1). This study is the first to reflect CAT activity in refractory epilepsy. It is certain that this study will contribute to the literature.
GSH level protects the cell against reactive oxygen molecules. However, it is a very powerful antioxidant as well. Because GSH protects the cell from all kinds of threats against oxidative stress, it has been reported that cellular damage may occur in GSH deficient patients. The GSH level is higher in patients with prostate cancer and bladder disease16,17,46. In experimental rat models, it has been determined that the seizures in animals did not recur for a long time with the administration of some antioxidant agents in animals with epilepsy47. In a study on patients with epilepsy, it has been reported that there was no significant difference in the GSH level when compared to healthy individuals28. In another study in the literature, it was reported that the GSH level increased in epilepsy patients29. In this study, the GSH level decreased significantly in the patient group when compared to the healthy control group (p < 0.05) (Fig. 2). This is likely due to an increase in reactive oxygen radicals or oxidative stress in the body. In such a case, damage to the brain may occur which may accelerate the epilepsy process. This study is the first to investigate the level of GSH in refractory epilepsy. This study will contribute to the literature.
In general, low SOD and CAT and high MDA levels were found in epilepsy patients in the literature. But, in some studies, preoperative SOD activity in epileptic patients did not change compared to post-operative patients. Also, in the same study, CAT enzyme activity before and after surgery was not found to be significant compared to the healthy control group48. A study by Dalton et al49. is the first to determine that severe brain damage in a rat model of epilepsy is in part due to oxidative stress. In a reported study, further research on the possible role of oxidative stress in epilepsy and refractory epilepsy was recommended50. This study differs from other studies in the literature, as it is the first study in which parameters such as SOD, CAT, GSH and MDA are measured in the refractory epilepsy disease.
There is evidence that neuronal overstimulation and oxidative damage caused by excessive free radical production may play a role in the onset and progression of epilepsy. Understanding the role of oxidative stress in epileptogenesis is important for determining appropriate treatment strategies. Neuroprotectant or antioxidant compounds may exert positive effects when associated with antiepileptic drugs (AEDs). The role of neuroprotectants in the therapeutic strategy to prevent or treat epilepsy is also discussed. Although some mysteries need to be clarified in the future, antioxidant compounds are useful neuroprotective agents in ameliorating brain damage in patients with refractory epilepsy. As a result, it is important that some antioxidant substances should be included in the treatment protocol for refractory epilepsy. Increased SOD, CAT and GSH levels can be used as a good antioxidant agent against refractory epilepsy disease. Also, MDA may be a precursor marker in identifying the refractory epilepsy disease.
The risk of seizures may increase in refractory epilepsy as a result of the reduction of the antioxidant defense system with the formation of active oxygen metabolites. In such a situation, oxidative stress may play an important role in the mechanism of neuronal death due to epilepsy. Ultimately, the occurrence of epileptic seizures may be the cause and consequence of reactive oxygen radicals. Thus, oxidative phosphorylation in mitochondria can generate large numbers of oxygen radicals in the nervous system. In such cases, it is necessary to reduce the excess oxygen radicals in the nervous system. For this, high antioxidant systems are needed to keep the body immunity strong.
As a result, it is important that some antioxidant substances should be included in the treatment protocol for refractory epilepsy. Increased SOD, CAT and GSH levels can be used as a good antioxidant agent against refractory epilepsy disease. Also, MDA may be a precursor marker in identifying the refractory epilepsy disease. This study was the first to investigate some parameters in refractory epilepsy disease
Author contributions
Abdullah Yilgor. Data Collection or Processing, Data Collection or Processing Analysis or Interpretation:, Literature Search, Writing. Canan Demir Processing Analysis or Interpretation.
Data availability
The datasets used and/or analyzed during the current study can be provided by the corresponding author upon reasonable request, either by providing it in a supplementary file or by depositing it in a public repository and providing the details on how to access it.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ozer IJ. Images of epilepsy in literatüre. Epilepsia. 1991;32:798–809. doi: 10.1111/j.1528-1157.1991.tb05536.x. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson KJ, Koivikko MJ. Prevalence, classification, and severity of epilepsy and epileptic syndromes in children. Epilepsia. 1997;38:1275–1282. doi: 10.1111/j.1528-1157.1997.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 3.Cerić I, Mehić-Basara N. Ibn Sina–psychology and psychological disorders. Med. Arh. 1997;51:21–23. [PubMed] [Google Scholar]
- 4.Naderi S, Acar F, Mertol T, Arda MN. Functional anatomy of the spine by Avicenna in his eleventh century treatise, Al-Qanun fial-Tibb. Canons Med. Neurosurg. 2003;52:1449–1453. doi: 10.1227/01.NEU.0000064811.30933.7F. [DOI] [PubMed] [Google Scholar]
- 5.Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 6.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 7.Olowe R, Sandouka S, Saadi A, Shekh-Ahmad T. Approaches for reactive oxygen species and oxidative stress quantification in epilepsy. Antioxidants. 2020;9(10):990. doi: 10.3390/antiox9100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyllie E, Comair YG, Kotagal P, Bulacio J, Bingaman W, Ruggieri P. Seizure outcome after epilepsy surgery in children and adolescents. Ann. Neurol. 1998;44(5):740–748. doi: 10.1002/ana.410440507. [DOI] [PubMed] [Google Scholar]
- 9.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 10.Janz D. When should antiepileptic drug treatment be terminated In:Wolf P, Dam M, Janz D. And Dreifuss F eds., Advances in Epileptology, Proc. The Xth Epilepsy İnternational Symposium, New York: Raven Press. 365–372 (1987).
- 11.Pitkänen A, Lukasiuk K, Dudek FE, Staley KJ. Epileptogenesis. Cold Spring Harb. Perspect. Med. 2015;5(10):1–17. doi: 10.1101/cshperspect.a022822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneeberger H, Illner WD, Abendroth D, Bulkley G, Rutili F, Williams M, et al. First elinical experiences with superoxide dismutase in kidney transplantation. Results of a double blind randomized study. Transpl. Proc. 1989;21(1):1245–1246. [PubMed] [Google Scholar]
- 13.Palmer C, Vannucci RC, Towfighi J. Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Ped. Resc. 1990;27(4):332–336. doi: 10.1203/00006450-199004000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman JJ. Therapeutic application of oxygen radical scavengers. Chest. 1990;100(3):189–192. doi: 10.1378/chest.100.3_Supplement.189S. [DOI] [PubMed] [Google Scholar]
- 15.El Kossi MM, Zakhary MM. Oxidative stress in the context of acute cerebrovascular stroke. Stroke. 2000;31(8):1889–1892. doi: 10.1161/01.STR.31.8.1889. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed Amar SA, Eryilmaz R, Demir H, Aykan S, Demir C. Determination of oxidative stress levels and some antioxidant enzyme activities in prostate cancer. Aging Male. 2019;22(3):198–206. doi: 10.1080/13685538.2018.1488955. [DOI] [PubMed] [Google Scholar]
- 17.Günes M, Eryilmaz R, Aslan R, Taken K, Demir H, Demir C. Oxidant-antioxidant levels in patients with bladder tumours. Aging Male. 2020;23(5):1176–1181. doi: 10.1080/13685538.2020.1718636. [DOI] [PubMed] [Google Scholar]
- 18.Gündüz AM, Demir H, Toprak N, Akdeniz H, Demir C, Arslan A, Göya C. The effect of computed tomography on oxidative stress level and some antioxidant parameters. Acta Radiol. 2021;62(2):260–265. doi: 10.1177/0284185120922135. [DOI] [PubMed] [Google Scholar]
- 19.Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Leonart ME. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013;12:376–390. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Sayır F, Şehitoğulları A, Demir H, Aslan M, Çobanoğlu U, Bilgin C. Serum prolidase activity, total oxidant/antioxidant, and nitric oxide levels in patients with esophageal squamous cell carcinoma. Turk. J. Thorac. Cardiovasc. Surg. 2019;27(2):206–211. doi: 10.5606/tgkdc.dergisi.2019.16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin. Chim Acta. 2001;303(1–2):19–24. doi: 10.1016/S0009-8981(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 22.Liao KH, Mei QY, Zhou YC. Determination of antioxidants in plasma and erythrocyte in patients with epilepsy. J. Central South Univ. Med. Sci. 2004;29(1):72–74. [PubMed] [Google Scholar]
- 23.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc. Diabetol. 2005;4(5):4–11. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedin AI, Starykh EV, Baranova OA, Chekanov AV, Torshin DV, Mikhailova EV. Oxidative stress in focal symptomatic and cryptogenic epilepsy in young patients. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova. 2020;120(7):17–22. doi: 10.17116/jnevro202012007117. [DOI] [PubMed] [Google Scholar]
- 25.Kohen R, Nyska A. Oxidation of biological systems: Oxidative stres phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002;30(6):620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, Zhang X, Jia W, Zhang C, Boczek T, Harding M, Liu Y, Li M, Zhang S, Lei S, Zhang D, Guo F. Circulating glutathione peroxidase and superoxide dismutase levels in patients with epilepsy: A meta-analysis. Seizure. 2021;91:278–286. doi: 10.1016/j.seizure.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Schulz BJ, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 28.Menon B, Ramalingam K, Kumar RV. Low plasma antioxidant status in patients with epilepsy and the role of antiepileptic drugs on oxidative stress. Ann. Indian Acad. Neurol. 2014;17(4):398–404. doi: 10.4103/0972-2327.144008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller SG, Trabesinger AH, Boesiger P, Wieser HG. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology. 2001;57(8):1422–1427. doi: 10.1212/WNL.57.8.1422. [DOI] [PubMed] [Google Scholar]
- 30.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE Official report: A practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 31.Langeh U, Chawla P, Gupta GD, Singh S. A novel approach to refractory epilepsy by targeting Pgp peripherally and centrally: Therapeutic targets and future perspectives. CNS Neurol. Disord. Drug Targets. 2020;19(10):741–749. doi: 10.2174/1871527319999200819093109. [DOI] [PubMed] [Google Scholar]
- 32.Tietz F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 33.Aebi H. Methods of Enzymatic Analysis. Academic Press; 1974. Catalase in Vitro; pp. 673–680. [Google Scholar]
- 34.Bird RP, Draper HH. Comparative studies on different methods of malonaldehyde determination. Methods Enzymol. 1984;105:299–305. doi: 10.1016/S0076-6879(84)05038-2. [DOI] [PubMed] [Google Scholar]
- 35.Asia P, Sharma A, Kumar Ahirwar A, Garg S, Elgiva John J, Gopal N. The study of ischemia modified albumin as an early biomarker of epilepsy in adolescent population: A cross sectional study. Horm. Mol. Biol. Clin. Investig. 2021;42(2):183–187. doi: 10.1515/hmbci-2020-0060. [DOI] [PubMed] [Google Scholar]
- 36.Khaled S, Hammad E, Hassan FA, Badry R. Trace element, oxidant, and antioxidant enzyme values in blood of children with refractory epilepsy. Int. J. Neurosci. 2014;124(3):181–186. doi: 10.3109/00207454.2013.831851. [DOI] [PubMed] [Google Scholar]
- 37.Sittsworth JD, Jr, Lanthorn TH. Lactate mimics only some effects of D-glucose on epileptic depolarization and long-term synaptic failure. Brain Res. 1993;630:21–27. doi: 10.1016/0006-8993(93)90637-3. [DOI] [PubMed] [Google Scholar]
- 38.Zarnowski T, Kleinrok Z, Turski WA. 2,3-dihydroxy-6-nitro-7 sulfamoylbenzo(F)quinoxaline enhances the protective activity of common antiepileptic drugs against maximal electroshock-induced seizures in mice. Neuropharmacology. 1993;32:895–900. doi: 10.1016/0028-3908(93)90145-S. [DOI] [PubMed] [Google Scholar]
- 39.Menon B, Ramalingam K, Kumar RV. Oxidative stress inpatients with epilepsy is independent of antiepileptic drugs. Seizure. 2012;21(10):780–784. doi: 10.1016/j.seizure.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Gokce C, Aytac B, Durak ZE, Yoldas T. Serum oxidant and antioxidant status of patients with chronic tension-type headache: possible effects of medical treatment. Neurol. Sci. 2015;36(10):1171–1175. doi: 10.1007/s10072-015-2240-z. [DOI] [PubMed] [Google Scholar]
- 41.Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J. Biol. Chem. 2013;37:26489–26496. doi: 10.1074/jbc.R113.462929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katı C, Karadas S, Aslan M, Gonullu H, Duran L, Demir H. Serum paraoxonase and arylesterase activities and oxidative stress levels in patients with SSRI intoxication. J. Membr. Biol. 2014;247:17–21. doi: 10.1007/s00232-013-9606-z. [DOI] [PubMed] [Google Scholar]
- 43.Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014;47:326–332. doi: 10.1016/j.clinbiochem.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Çevik MU, Varol S, Yücel Y, Akıl E, Çelepkolu T, Arıkanoğlu A, Yüksel H, Ufuk UM. Serum paraoxonase-1 activities and malondialdehyde levels in patients with epilepsy. Dicle Med. J. 2012;39(4):557–560. doi: 10.5798/diclemedj.0921.2012.04.0200. [DOI] [Google Scholar]
- 45.Shimomura T, Kowa H, Nakamo T, Kitano A, Marukawa H, Urakami K, Takahashi K. Platelet superoxide dismutase in migraine and tension-type headache. Cephalalgia. 1994;14:215–218. doi: 10.1046/j.1468-2982.1994.014003215.x. [DOI] [PubMed] [Google Scholar]
- 46.Cotgreave IA, Johansson U, Moldeus P, Brattsand R. The effect of acute cigarette smoke inhalation on pulmonary and systemic cysteine and glutathione redox states in the rat. Toxicology. 1987;45:203–212. doi: 10.1016/0300-483X(87)90106-5. [DOI] [PubMed] [Google Scholar]
- 47.Hassanzadeh P, Arbabi E, Rostami F. The ameliorative effects of sesamol against seizures, cognitive impairment and oxidative stressin the experimental model of epilepsy. Iran. J. Basic Med. Sci. 2014;17(2):100–107. [PMC free article] [PubMed] [Google Scholar]
- 48.López J, González ME, Lorigados L, Morales L, Riverón G, Bauzá JY. Oxidative stress markers in surgically treated patients with refractory epilepsy. Clin Biochem. 2007;5(40):292–298. doi: 10.1016/j.clinbiochem.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Dalton T, Pazdernik TL, Wagner J, Samson F, Andrews GK. Temporal spatial patterns of expression of metallothionein-I and -III and other stress related genes in rat brain after kainic acid-induced seizures. Neurochem. Int. 1995;27:59–71. doi: 10.1016/0197-0186(94)00168-T. [DOI] [PubMed] [Google Scholar]
- 50.Cardenas-Rodriguez N, Huerta-Gertrudis B, Rivera-Espinosa L, Montesinos-Correa H, Bandala C, Carmona-Aparicio L, Coballase-Urrutia E. Role of oxidative stress in refractory epilepsy: Evidence in patients and experimental models. Int. J. Mol. Sci. 2013;14(1):1455–1476. doi: 10.3390/ijms14011455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study can be provided by the corresponding author upon reasonable request, either by providing it in a supplementary file or by depositing it in a public repository and providing the details on how to access it.