Abstract
Human milk oligosaccharides (HMOs) impact neonate immunity and health outcomes. However, the environmental factors influencing HMO composition remain understudied. This study examined the associations between ambient air pollutant (AAP) exposure and HMOs at 1-month postpartum. Human milk samples were collected at 1-month postpartum (n = 185). AAP (PM2.5, PM10, NO2) exposure included the 9-month pregnancy period through 1-month postpartum. Associations between AAP with (1) HMO diversity, (2) the sum of sialylated and fucosylated HMOs, (3) 6 a priori HMOs linked with infant health, and (4) all HMOs were examined using multivariable linear regression and principal component analysis (PCA). Exposure to AAP was associated with lower HMO diversity. PM2.5 and PM10 exposure was positively associated with the HMO 3-fucosyllactose (3FL); PM2.5 exposure was positively associated with the sum of total HMOs, sum of fucosylated HMOs, and the HMO 2′-fucosyllactose (2′FL). PCA indicated the PM2.5, PM10, and NO2 exposures were associated with HMO profiles. Individual models indicated that AAP exposure was associated with five additional HMOs (LNFP I, LNFP II, DFLNT, LNH). This is the first study to demonstrate associations between AAP and breast milk HMOs. Future longitudinal studies will help determine the long-term impact of AAP on human milk composition.
Keywords: Air pollution, Human milk oligosaccharides, Early life, Infant development, Environmental exposure
Subject terms: Physiology, Environmental impact
Introduction
Human milk plays an important role in infant nutrition and overall well-being, offering protection against numerous infections and diseases such as colitis, infantile diarrhea, diabetes, and obesity1–3. It is enriched with specific bioactive compounds that are instrumental in infant growth and developmental processes4–6. Notably, human milk oligosaccharides (HMOs), which rank as the third most abundant component in human milk, comprise over 150 distinct oligosaccharides7,8.
HMOs, which are synthesized from five core monosaccharides9–11, serve as prebiotics for the developing gut microbiome12, protect against infections13, stimulate neurological development14, and act as anti-inflammatory and immune modulators15,16. Moreover, a reduced HMO diversity has been linked to adverse infant health outcomes, such as necrotizing enterocolitis (NEC)17,18. Experimental investigations have highlighted specific HMOs, such as DSLNT, for their protective attributes against NEC18. One study19 highlighted the potential of 2’FL and 3FL in modulating immunity, as they bind to adhesion molecules pivotal for dendritic cell activation and emulate cell surface receptors, offering protection against infection16. Additionally, research has shown that LNT and 6’SL can activate GPCR35, a mediator in alleviating pain and colitis symptoms20. Collectively, these findings underscore the integral role of various HMOs in promoting infant health.
The composition and concentration of HMOs varies greatly among women21 and across the time course of lactation22 Variations in HMO concentrations have been linked with genetic secretor status9,23, geographic location9,10,24, socioeconomic status25, pre-pregnancy body mass index (BMI)26, maternal age26, ethnicity9, obesity27, and lactation period9,22. Beyond these factors, exposure to air pollutants may also impact human milk composition. Exposure to air pollution varies by geographic location28 and airborne pollutant concentrations have been mitigated by vegetation and green space29. Accordingly, geography has been linked to differences in HMO profiles9,30, as have residential green environments31. Air pollution exposure has also been found to impact human milk composition, including lipid biosynthesis32. Given the genetic underpinnings of HMO biosynthesis, exposure to air pollution might modulate epigenetic pathways33,34, subsequently influencing HMO production within mammary glands.
The primary aim of this study was to therefore determine whether maternal AAP exposure was associated with summary measures of HMOs (i.e., diversity, sum of sialylated and fucosylated) as well as concentrations of six HMOs that have been previously linked with infant health (i.e., 2’FL, 3FL, 6’SL, LNnT, LNT, DSLNT). Based on existing literature, it was hypothesized that higher exposure to AAP would be associated with lower HMO diversity and the concentrations of six HMOs that have previously been found to impact infant health12,16,19,20,35–37. As a secondary aim, the associations between AAP and HMOs were examined using principal component analysis (PCA) as a data reduction approach. Lastly, as an exploratory and hypothesis generating analysis, we examined the associations between AAP with all 19 HMOs our analytical platform can quantify with confidence. Furthermore, this study follows a cohort of Latino and Hispanic women. Hispanic and Latino individuals have a greater risk of developing type 2 diabetes38 and obesity39, but are underrepresented in biomedical research40. Results from this study have the potential to uncover potentially important associations between AAP and human milk composition in an unstudied population of Latino and Hispanic postpartum women.
Results
Study population characteristics
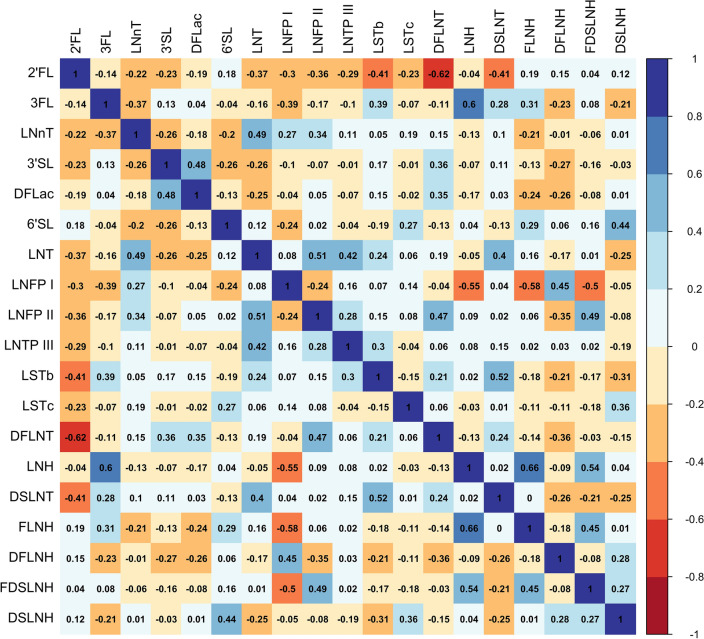
Table 1 displays the mean physical, social, and environmental characteristics of the 185 mothers included in the analysis. On average, mothers were 29 years of age (range: 18–45 years), and overweight before pregnancy (BMI: 28.68 ± 6.03 kg/m2). Additionally, 28.6% of mothers were healthy weight, 32.4% were overweight, and 38.9% were obese. All mothers in the sample were in the mature milk stage where, on average, breastmilk samples were collected 32.5 days after delivery. The Hollingshead Index was used to measure the participant’s socioeconomic status, where 51.9% had low or very low SES (Hollingshead score below 26.5). Maternal exposure to PM2.5, PM10, and NO2 were moderately to highly correlated. As shown in Table 2, NO2 was moderately correlated with PM10 (r = 0.61, p < 2.2e-16) and PM2.5 (r = 0.65, p < 2.2e-16). Additionally, PM10 and PM2.5 were highly correlated (r = 0.85, p < 2.2e-16). When examining HMOs, we observed that the strength of the Pearson correlation coefficients among the HMOs varied and was contingent upon the specific HMOs being examined (Fig. 1). Lastly, we examined correlations between maternal factors and HMOs, finding that maternal age and BMI were only weakly correlated with HMO concentrations (Supplemental Fig. 1).
Table 1.
Descriptive statistics.
| Variable | Mean ± SD |
|---|---|
| Maternal characteristics | |
| Pre-pregnancy BMI (kg/) | 28.68 ± 6.03 |
| Age (years) | 28.99 ± 6.22 |
| Breast milk stage (days after delivery) | 32.47 ± 4.11 |
| SES status (hollingshead index) | 26.24 ± 11.78 |
| Maternal AAP exposure* | |
| PM2.5 (µg/) | 11.89 ± 1.02 |
| PM10 (µg/) | 29.97 ± 3.65 |
| NO2 (ppb) | 17.99 ± 2.23 |
Table shows the mean ± standard deviation (SD) for 185 mothers included in this analysis. Units for all values are indicated. * Maternal AAP exposure is shown. Briefly, air pollution exposures were calculated using a weighted average of exposure during the pregnancy period (281 days) through the postpartum period defined as .
Table 2.
Correlations among maternal ambient air pollutant exposures.
| NO2 | PM10 | PM2.5 | |
|---|---|---|---|
| NO2 | – | 0.61* | 0.65* |
| PM10 | 0.61* | – | 0.85* |
| PM2.5 | 0.65* | 0.85* | – |
Correlation matrix among maternal ambient air pollutant exposures using Pearson’s correlation coefficient (r). All correlations were statistically significant with *p < 2.2e-16.
Figure 1.
Correlations among HMOs examined in the Mother’s Milk Study. Correlation matrix among HMOs (nmol/mL) using Pearson correlation coefficients. The color of each square, either red (negative) or blue (positive), denotes the direction of the correlation.
Ambient air pollution exposure was associated with HMO composition
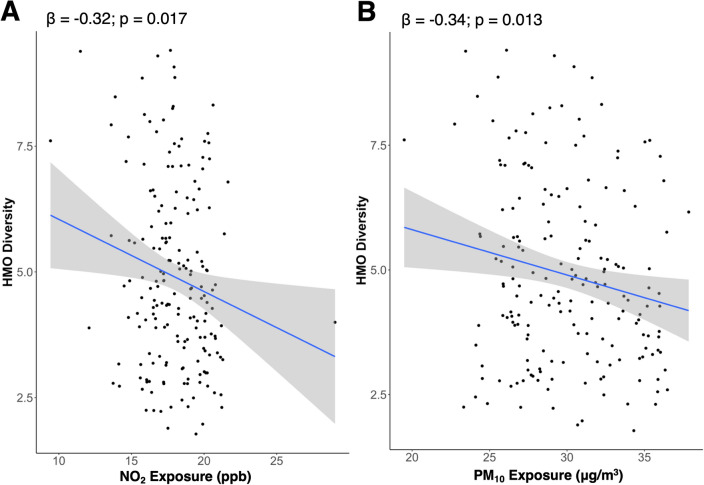
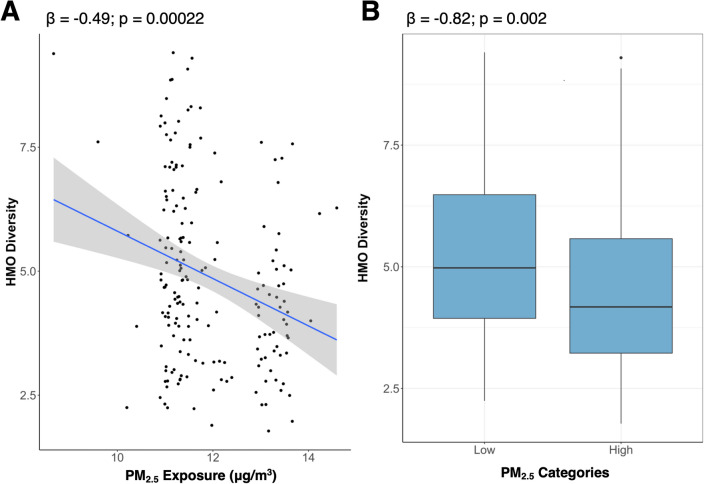
As shown in Table 3, breastmilk HMOs at 1-month post-partum were associated with maternal exposure to AAP. For example, a 1-SD higher NO2, PM10, and PM2.5 was associated with a 0.32 (p = 0.02), 0.34 (p = 0.01) and 0.49 lower HMO diversity (p < 0.001), respectively (Table 3 and Fig. 2). Further, NO2 was negatively associated with the sum of sialylated HMOs (β = − 114.53, p = 0.04). PM10 exposure was positively associated with 3FL, where each 1-SD increase in PM10 exposure was associated with a 239.38 nmol/mL higher 3FL (p = 0.02). Additionally, a 1-SD higher PM2.5 exposure was positively associated with the total sum of all HMOs (β = 398.96, p = 0.002) and the sum of fucosylated HMOs (β = 287.84, p = 0.04), as well as human breast milk concentrations of 2’FL (β = 558.66, p = 0.01) and 3FL (β = 336.68, p < 0.001). In the current study, PM2.5 had a bimodal distribution. Therefore, PM2.5 was also examined as a categorical variable based on a median split (high/low) where results remained largely consistent with those observed when examining PM2.5 as a continuous exposure (Table 4). For example, increased exposure to PM2.5 was associated with lower HMO diversity when treated as a continuous (p < 0.001) and as a categorial exposure (p = 0.002) (Fig. 3A,B). However, the sum of fucosylated HMOs was no longer significantly associated with PM2.5 exposure when examined as a dichotomous variable (Table 4).
Table 3.
Ambient air pollution exposure was associated with a priori HMOs.
| NO2 | PM10 | PM2.5 | ||||
|---|---|---|---|---|---|---|
| β | P Value | β | P Value | β | P Value | |
| HMO summary measures | ||||||
| Diversity | − 0.32 | 0.02 | − 0.34 | 0.01 | − 0.49 | 0.0002 |
| Sum (nmol/mL) | 71.98 | 0.58 | 177.46 | 0.17 | 398.96 | 0.002 |
| HMO-bound sialic acid (nmol/mL) | − 114.53 | 0.04 | − 61.79 | 0.28 | − 60.40 | 0.28 |
| HMO-bound fucose (nmol/mL) | 132.87 | 0.34 | 115.37 | 0.41 | 287.84 | 0.04 |
| HMO concentrations | ||||||
| 2’FL (nmol/mL) | 250.83 | 0.27 | 251.06 | 0.27 | 558.66 | 0.01 |
| 6’SL (nmol/mL) | 1.18 | 0.96 | 38.53 | 0.11 | 27.64 | 0.25 |
| DSLNT (nmol/mL) | − 19.31 | 0.18 | − 12.45 | 0.39 | − 12.72 | 0.37 |
| 3FL (nmol/mL) | 35.84 | 0.72 | 239.38 | 0.02 | 336.68 | 0.0008 |
| LNT (nmol/mL) | − 75.40 | 0.09 | − 41.95 | 0.36 | − 41.18 | 0.36 |
| LNnT (nmol/mL) | 2.56 | 0.91 | − 12.66 | 0.58 | − 12.78 | 0.57 |
Effect estimates (β) and unadjusted p values from the multivariate linear regression models are shown. Models adjusted for maternal age and socioeconomic status. All effect estimates (β) are reported for a one standard deviation increase in NO2 (SD: 2.23 ppb), PM10 (SD: 3.65 µg/), and PM2.5 exposure (SD: 1.02 µg/). Unadjusted P-values less than 0.05 are denoted in bold text.
Figure 2.
Higher exposure to NO2 and PM10 was significantly associated with lower and HMO diversity. Figures show unadjusted data with the effect estimates (β) and unadjusted p values from the multivariate linear regression models for NO2 (A) and PM10 (B). Multivariable models adjusted for maternal age and socioeconomic status. Effect estimates (β) are reported for a one standard deviation increase in NO2 (SD: 2.23 ppb) and PM10 (SD: 3.65 µg/) exposure.
Table 4.
High PM2.5 exposure was associated with a priori HMOs.
| β | P Value | |
|---|---|---|
| HMO summary measures | ||
| Diversity | − 0.82 | 0.002 |
| Sum (nmol/mL) | 788.70 | 0.002 |
| HMO-bound sialic acid (nmol/mL) | 24.82 | 0.83 |
| HMO-bound fucose (nmol/mL) | 485.25 | 0.08 |
| HMO concentrations | ||
| 2’FL (nmol/mL) | 899.63 | 0.05 |
| 6’SL (nmol/mL) | 61.71 | 0.20 |
| DSLNT (nmol/mL) | 3.75 | 0.89 |
| 3FL (nmol/mL) | 763.23 | 0.0001 |
| LNT (nmol/mL) | − 48.53 | 0.59 |
| LNnT (nmol/mL) | − 53.24 | 0.24 |
Effect estimates (β) and unadjusted p values from the multivariate linear regression models are shown where PM2.5 was treated as a categorical variable (high/low). Multivariable models adjusted for maternal age and socioeconomic status. Unadjusted P-values less than 0.05 are denoted in bold text.
Figure 3.
Higher exposure PM2.5 was associated with lower HMO diversity. (A) Figure shows the associations between PM2.5 exposure and HMO diversity. (A) Effect estimates (β) and unadjusted p values from the multivariate linear regression models are shown on the unadjusted plot. The effect estimate (β) is reported for a one standard deviation increase in PM2.5 exposure (SD: 1.02 µg/). (B) Boxplots for HMO diversity in the high and lower PM2.5 exposure groups. Effect estimates (β) and unadjusted p values from the multivariate linear regression models are shown on the unadjusted boxplot. All models adjusted for maternal age and socioeconomic status.
Ambient air pollution exposure was associated HMO PCs
A principal components (PC) analysis was performed on all 19 HMOs as a data reduction technique to further examine the associations between AAP exposures and HMOs. The eigenvalues for the top six PCs, which explain ~ 72% of the total variance in the data, are shown in Table 5. Eigenvalues were further explored if they met a threshold ≥ 1. The loadings of each HMO against PC1 (x-axis) and PC2 (y-axis), where 2’FL, DFLNT, LSTb, DSLNT, LNT, and FLNH contributed to PC1 and LNFPI, LNH, FLNH, 3FL, FDSLNH, and DFLNH contributed to PC2 (Supplemental Fig. 2). PCs 1 to 6 were analyzed with respect to AAP after adjusting for maternal age and socioeconomic status (Table 6). Briefly, PM2.5 was negatively associated with PC1 (p = 0.009), while NO2 was positively associated with PC2 (p = 0.05), and PM10 (p = 0.01) and PM2.5 (p = 0.005) were positively associated with PC4. Given these findings, PC1, PC2, and PC4 were then further analyzed to determine which HMOs contributed most to their profiles. This was done by ranking the magnitude of the loading scores of the HMOs in the profiles (Table 7). The HMOs with the highest contribution to PC1 were 2’FL, DFLNT, LSTb, DSLNT, LNT, and FLNH. The HMOs with the highest contribution to PC2 were LNFPI, LNH, FLNH, 3FL, FDSLNH, and DFLNH. The HMOs with the highest contribution to PC4 were DSLNH, DFLac, DFLNT, LSTc, LNFP II, and 3’SL. Of these HMOs, four (i.e., 2’FL, DSLNT, 3FL, and LNT) overlapped with those that we explored as part of our a priori analysis.
Table 5.
Eigenvalues and aggregate proportion of variance explained by principal components.
| Principal component | Eigenvalue | Aggregate proportion of variance explained (%) |
|---|---|---|
| PC1 | 1.88 | 18.66 |
| PC2 | 1.79 | 35.46 |
| PC3 | 1.58 | 48.65 |
| PC4 | 1.39 | 58.83 |
| PC5 | 1.21 | 66.50 |
| PC6 | 1.07 | 72.53 |
Eigenvalues of principal components (PCs) 1–6 that were all above a value of one, indicating strength of the PC. Aggregate proportion of variance explained by including each additional PC is also shown.
Table 6.
Exposure to ambient air pollution was associated with HMOs based on a principal component analysis.
| NO2 | PM10 | PM2.5 | ||||
|---|---|---|---|---|---|---|
| β | P Value | β | P Value | β | P Value | |
| PC1 | − 0.17 | 0.23 | − 0.26 | 0.06 | − 0.36 | 0.009 |
| PC2 | 0.26 | 0.05 | − 0.05 | 0.73 | − 0.18 | 0.19 |
| PC3 | − 0.13 | 0.27 | − 0.08 | 0.49 | − 0.13 | 0.26 |
| PC4 | 0.18 | 0.09 | 0.26 | 0.01 | 0.29 | 0.005 |
| PC5 | − 0.03 | 0.75 | 0.11 | 0.25 | 0.05 | 0.59 |
| PC6 | 0.04 | 0.62 | 0.04 | 0.65 | 0.13 | 0.10 |
Shows the results from the multivariate linear regression analysis with ambient air pollutants as the independent variables and PC1 through PC6 as the dependent variables. Models adjusted for maternal age and socioeconomic status. All effect estimates (β) are reported for a one standard deviation increase in NO2 (SD: 2.23 ppb), PM10 (SD: 3.65 µg/), and PM2.5 exposure (SD: 1.02 µg/). Unadjusted P-values less than 0.05 are denoted in bold text.
Table 7.
HMO loading scores for PC1, PC2, and PC4.
| Loading Scores | |||||
|---|---|---|---|---|---|
| PC1 | PC2 | PC4 | |||
| 2’FL (nmol/mL) | − 0.39 | LNFP I (nmol/mL) | 0.42 | DSLNH (nmol/mL) | − 0.37 |
| DFLNT (nmol/mL) | 0.34 | LNH (nmol/mL) | − 0.42 | DFLac (nmol/mL) | − 0.36 |
| LSTb (nmol/mL) | 0.32 | FLNH (nmol/mL) | − 0.38 | DFLNT (nmol/mL) | − 0.35 |
| DSLNT (nmol/mL) | 0.29 | 3FL (nmol/mL) | − 0.33 | LSTc (nmol/mL) | − 0.29 |
| LNT (nmol/mL) | 0.27 | FDSLNH (nmol/mL) | − 0.33 | LNFP II (nmol/mL) | − 0.27 |
| FLNH (nmol/mL) | − 0.23 | DFLNH (nmol/mL) | 0.31 | 3’SL (nmol/mL) | − 0.26 |
Shows the loading scores for the HMOs that contributed most to PC1, PC2, and PC4. A higher magnitude indicates a greater contribution to the PC.
Exploratory analysis identified that ambient air pollution exposure was associated with five additional HMOs
As an exploratory analysis, the relationship between AAP exposure with HMOs was examined (Table 8). Again, a multivariate regression analysis was performed that adjusted for maternal age and socioeconomic status. While none of these associations survived correction for multiple testing (PFDR > 0.05), adjusted p-values indicated that AAP exposure was associated with several HMOs. Specifically, AAP was associated with five HMOs, including a negative association with LNFP I, LNFP II, and DFLNT, and a positive association with LNH and FLNH (unadjusted p < 0.05).
Table 8.
Ambient air pollution exposure was associated with breastmilk HMOs.
| NO2 | PM10 | PM2.5 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | P Value | PFDR | β | P Value | PFDR | β | P Value | PFDR | |
| LNFP I (nmol/mL) | 105.47 | 0.23 | 0.43 | − 36.10 | 0.68 | 0.74 | − 178.41 | 0.041 | 0.22 |
| LNFP II (nmol/mL) | − 83.34 | 0.0045 | 0.17 | − 68.33 | 0.021 | 0.17 | − 70.27 | 0.017 | 0.17 |
| DFLNT (nmol/mL) | − 74.56 | 0.18 | 0.38 | − 119.02 | 0.032 | 0.21 | − 145.13 | 0.008 | 0.17 |
| LNH (nmol/mL) | − 2.42 | 0.61 | 0.69 | 6.43 | 0.18 | 0.38 | 9.38 | 0.047 | 0.22 |
| FLNH (nmol/mL) | − 8.28 | 0.33 | 0.52 | 14.58 | 0.09 | 0.24 | 19.47 | 0.021 | 0.17 |
Multivariable models adjusted for maternal age and socioeconomic status. Unadjusted P-values less than 0.05 are denoted in bold text. The table also includes the adjusted (PFDR) p-values from the exploratory analysis. All effect estimates (β) are reported for a one standard deviation increase in NO2 (SD: 2.23 ppb), PM10 (SD: 3.65 µg/), and PM2.5 exposure (SD: 1.02 µg/).
Discussion
To our knowledge, this is the first study to examine the associations between AAP and HMO composition. Increasing evidence suggests that HMOs may impact infant health, including establishing the infant gut microbiome, modulating host-epithelial immune responses, and stimulating neurological development15,41. Previous research also suggests that HMO composition varies significantly across mothers due to factors that are not fully understood but do appear to include genetics23 and geography9,10,24. Therefore, the current study sought to determine if exposure to AAP was associated with breastmilk HMOs among 185 Latino mothers at 1-month post-partum. Using three analytical approaches, we found that exposure to AAP was associated with lower HMO diversity as well as an increased sum of HMOs, sum of sialylated HMOs, and abundance of concentrations of 2′FL and 3FL. These results provide the first evidence that exposure to air pollutants may impact the composition of human breast milk.
Results from this study indicate that higher exposure to AAP was associated with lower HMO diversity, which has been linked to adverse health outcomes in infants, including poor immunity and increased susceptibility to infections25. Additionally, higher HMO diversity has been associated with lower fat mass in infants42. A previous study found that HMO diversity differed among women from different geographic locations10, suggesting that exposure to air pollutants may partially explain these geographic differences. Another study31 found that increased green space was correlated with higher HMO diversity. As green space can mitigate air pollutant concentrations29, our findings of an inverse relationship between HMO diversity and air pollution exposure are consistent with this study. In addition to examining summary measures of HMOs, we also explored six a priori HMOs that have been linked with infant growth and development. Among these HMOs, PM10 exposure was positively associated with 3FL, and PM2.5 was positively associated with both 2′FL and 3FL. 2′FL and 3FL have been linked to infant immunity, with 2’FL being protective against necrotising enterocolitis by inhibiting TLR-437 and 3FL binding adhesion molecules to impact immunity19. However, the protective effects of specific HMOs may vary based on the overall composition of breastmilk, the infant gut microbiota, maternal and infant genetics, or the health status of the infant. Further, previous studies have found that breastfeeding–possibly due to HMO composition–has a protective effect against some of the adverse health effects of air pollution on the respiratory and immune systems43. Therefore, it is also possible that this effect is partly due to HMO mitigation, where women exposed to higher levels of air pollution may produce more of these HMOs to provide infants with enhanced immunity.
In addition to examining six a priori HMOs, principal component (PC) analysis was performed as a data reduction method. Based on this approach, each PC can be interpreted as a unique HMO “profile”, in which different HMOs have varying levels of importance for a specific profile based on their loading score. AAP exposure was associated with the profiles contained with PC1, PC2, and PC4. For example, PM2.5 exposure was associated with PC1 and PC4, PM10 exposure was also associated with PC4, and NO2 exposure was associated with PC2. Based on the loading scores, we observed that PC1 was largely defined by 2′FL, DFLNT, LSTb, DSLNT, LNT, and FLNH, PC2 was largely defined by LNFPI, LNH, FLNH, 3FL, FDSLNH, and DFLNH, and PC4 was largely defined by DSLNH, DFLac, DFLNT, LSTc, LNFP II, and 3′SL. Of these HMOs, four (i.e., 2′FL, 3FL, DSLNT, LNT) overlapped with our six a priori HMOs.
Lastly, we took an agnostic approach and examined the individual associations between each air pollutant and the HMOs we hadn’t included in our a priori HMO analysis. Using this approach, we identified an additional five HMOs beyond 2’FL and 3FL that were associated with one or more air pollutant. For example, PM2.5 exposure was associated with a lower LNFP I concentration and higher LNH concentration. Both LNFP I and LNH have been linked with the composition of the infant gut microbiota44, including an higher abundance of Staphylococcus44. Because air pollution exposure may also impact the gut microbiome45–47, additional research into these associations would help elucidate the relationship between air pollution, HMOs, and the gut microbiome. Furthermore, exposure to NO2, PM10, and PM2.5 was inversely associated LNFP II. This is important since higher concentrations of LNFP II have been associated with fewer respiratory problems in infants48. Hence, mothers exposed to higher levels of air pollution may also produce lower levels of LNFP II, which could place their infants at an increased risk for respiratory issues due to their existing higher air pollution exposures and lower LNFP II intake. Lastly, we found that FLNH was positively associated with PM2.5 and DFLNT was inversely associated with both PM10 and PM2.5 exposures, although the health implications of these HMOs are largely unknown.
Previous work suggests that exposure to air pollution impacts breastmilk composition. For example, an experimental study demonstrated that rabbits exposed to diesel particulate matter during pregnancy led to the development of fat globules in the mammary glands as well as increased expression of genes for enzymes involved in lipid biosynthesis32. Due to the importance of genetic factors in synthesizing HMOs, there is biological plausibility that exposure to air pollution may impact HMO production and composition. Emerging data suggests that air pollution exposure modulates DNA methylation throughout the lifespan33. Additionally, during pregnancy there are DNA methylation changes that occur in mammary epithelial cells49 where studies suggest that epigenetics may play an role in mammary gland development and function50. Additionally, exposure to air pollution has been linked with circulating miRNAs51,52, which may alter gene expression and HMO composition via impacts on the mammary gland53,54. Given that there is a genetic component of HMO biosynthesis55, and HMOs are solely made in the mammary gland, evaluating the effect of air pollution on HMO composition warrants further research.
While this is the first study to examine the relationship between AAP and HMO composition, there are several limitations which should be considered. This study was cross-sectional, making it difficult to establish a causal relationship between air pollution and HMO composition. Additionally, air pollution estimates were generated using individual residential address histories, so misclassification of exposures may have occurred due to time spent away from the provided addresses, or sources of indoor air pollution56. The inverse distance-squared weighting (IDW2) exposure assessment method used is also limited by spatial resolution compared to other exposure assessment methods such as land-use-regression or hybrid models57. However, prior work has shown that the IDW2 method in California has little bias on average (0.7 ppb for NO2, 0.4 µg/m3 for PM2.5, and 0.5 µg/m3 for PM10)57,58. This study was also limited to one geographical region and only included Latino/Hispanic participants, which may limit generalizability. However, our focus on Latino/Hispanic women in Southern California is warranted given that this is an understudied population which experiences greater exposure to air pollution than non-Hispanic White individuals59. Future studies should adopt a longitudinal approach and include participants of various backgrounds from different geographic locations.
Overall, this study found that higher exposure to ambient air pollution was associated with lower HMO diversity, as well as the concentration of several HMOs. These findings hold potential importance as HMO composition is known to impact infant immunity and growth. Hence, interventions and public health policies aimed at reducing air pollution exposure may have the potential to improve infant health and development through important impacts on human breastmilk composition.
Methods
Participants
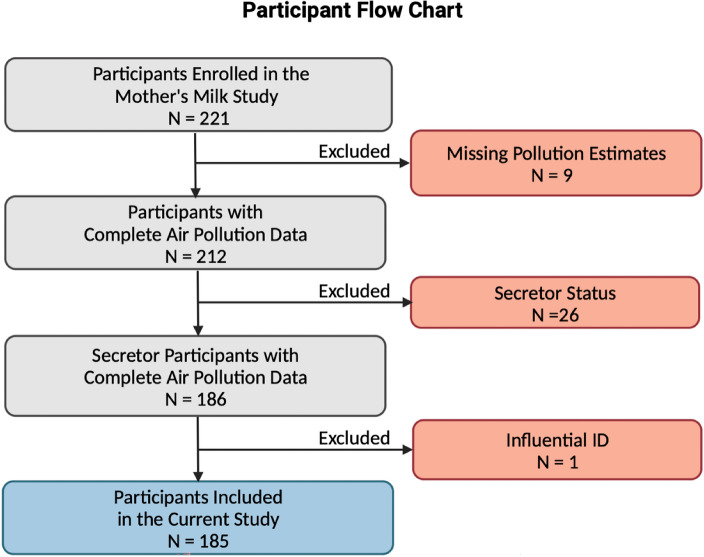
The participants of this study were recruited for the Southern California Mother’s Milk Study, an ongoing longitudinal study examining human breast milk factors and the gut microbiome in a cohort of mother-infant dyads57. Maternal inclusion criteria include ≥ 18 years of age at delivery; healthy, term, singleton birth; enrollment by one month postpartum; self-identified Latino/Hispanic ethnicity; intention to breastfeed for at least three months postpartum; and ability to read at the 5th grade level in either English or Spanish in order to understand study procedures60. Mothers were excluded on the basis of medical conditions or medications which impact health, nutritional status, or metabolism, as well as use of tobacco or recreational drugs, pre-term or low birth weight, and clinically diagnosed fetal abnormalities57. In the current study, 185 of the 221 Mother’s Milk Study participants were included. Briefly, 9 were excluded due to missing air pollution exposure data and 23 were removed since they were classified as HMO “non-secretors” (produced under 500 nmol/mL of the HMO 2’FL). Following this, we observed one participant with intermediate 2′FL levels (559 nmol/mL, which is considered a “non-secretor" by some definitions), marking them as a potential non-secretor. This same individual had an HMO diversity > 2 SD from the mean, further suggesting they were a likely “non-secretor”. Therefore, we opted to remove this individual to maintain data integrity (Fig. 4). Additionally, we examined extreme air pollutant exposure values, which we defined as > 3 SD above the mean. Since the high exposure levels observed were plausible, we conducted thorough model diagnostics to confirm that retaining these cases did not unduly influence the analysis. The Institutional Review Boards of the University of Southern California, Children’s Hospital of Los Angeles, and the University of Colorado Boulder approved study procedures, and all research was performed in accordance with relevant guidelines and regulations. Written informed consent was obtained from participants prior to enrollment and all analyses in this study.
Figure 4.
Participants included in the current analysis from the Southern California Mother’s Milk Study. Nine participants were removed due to missing air pollution estimates. Twenty-six participants were removed for having the “non-secretor” phenotype (produce little/no 2’FL). One participant was removed as they had an intermediate 2’FL value (see methods description).
Clinical measures
The clinical measures used for this study were taken at the baseline visit for participants (1-month postpartum). Maternal weight (kg) was measured using an electronic scale and standing height was measured using a stadiometer (m) to calculate maternal body mass index (BMI, kg/m2). Maternal pre-pregnancy weight status was categorized as having underweight (≤ 18.5 kg/m2), healthy weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obesity (≥ 30 kg/m2). Maternal pre-pregnancy weight, age at delivery, delivery mode, and infant sex were collected at the first clinical visit. Non-consecutive 24-h dietary recalls were performed to represent average maternal dietary intake, as well as to assess dietary information pertaining to nutrient consumption of macronutrients61. Socioeconomic status was measured using a modified version of the Hollingshead index57,62.
Ambient air pollution exposure
Residential exposure estimates to ambient air pollutants (PM2.5, PM10, NO2, and O3) during the pregnancy period (281 days) through 1-month postpartum (30 days) were modeled for all mother-infant pairs. PM2.5 and PM10 were measured in micrograms per cubic meter (µg/m3) while NO2 was assessed in parts per billion (ppb). Residential address histories were obtained from a questionnaire during the study visit and included the prenatal period. Addresses were geocoded with the Texas A&M Geocoder. Monthly averages of pollutant exposure were then estimated using the Environmental Protection Agency (EPA) Air Quality System, which uses monitors to record hourly air quality data. Spatial interpolation of up to four monitoring stations within 50 km of participant’s homes was performed via an inverse distance-squared weighting (IDW2) algorithm. Final air pollution exposures were calculated using a weighted average of exposure during pregnancy (281 days) and 1 month postpartum (30 days).
Human milk oligosaccharides (HMO)
Human milk was collected at 1-month postpartum (mean days after delivery: 32.47), and mothers were instructed to refrain from eating for 1 h and feeding or pumping human milk for 1.5 h before collection. Mothers were instructed to use an electric breast pump and were asked to pump the entire contents of a single breast expression to ensure collection of fore, mid, and hind milk. 20–50 mL of milk was collected, and aliquots were stored at -80 ºC until HMO analysis. HMOs were analyzed using high performance liquid chromatography after fluorescent derivatization allowing for quantification of the 19 most abundant HMOs, which represent more than 90% of the total HMO concentration, and include all structural features found in HMOs as a whole (Berger et al.60). HMO concentrations are reported in nmol/mL, and the sum of all HMOs in a sample was calculated as the sum of all HMOs detected in each sample. HMO-bound fucose and HMO-bound sialic acid were calculated as the sum of all sialic acid and all fucose molecules bound to HMOs in a sample, respectively (e.g., each molecule of 2′FL contains 1 molecule of fucose, and each molecule of DFLNT contains 2 molecules of fucose). HMO diversity was calculated using Simpson’s diversity, which is the reciprocal sum of the square of relative abundance for each measured HMO42. Additionally, as secretor status is known to impact the concentration of HMOs, only secretors (as defined by the presence of > 500 nmol/mL 2′FL HMO) were included in the analysis. Overall, 19 HMOs were identified and quantified: 2′-fucosyllactose (2′FL), 3-fucosyllactose (3FL), 3′-sialyllactose (3′SL), 6′-sialyllactose (6’SL), difucosyllactose (DFLac), difucosyllacto-N-hexaose (DFLNH), difucosyllacto-N-tetrose (DFLNT), disialyllacto-N-hexaose (DSLNH), disialyllacto-N-tetraose (DSLNT), fucodisialyllacto-N-hexaose (FDSLNH), fucosyllacto-N-hexaose (FLNH), lacto-N-fucopentaose (LNFP) I, LNFP II, LNFP III, lacto-N-hexaose (LNH), lacto-N-neotetraose (LNnT), lacto-N-tetrose (LNT), sialyl-lacto-N-tetraose b (LSTb), and sialyl-lacto-N-tetraose c (LSTc).
Statistical analysis
Descriptive statistics were calculated using the mean and standard deviation (SD) for continuous variables and frequencies for categorical variables. Multivariable linear regression was performed to explore the relationship between exposure to air pollution and six a priori breastmilk HMOs. Since PM2.5 had a bimodal distribution, we also examined average differences in HMOs levels based on high and low PM2.5 exposure, which was based on a median split (median: 11.45 µg/m3, 49.19% above and 50.81% below the SD). A directed acyclic graph (DAG) was constructed based on a review of the literature (Fig. 5) to identify potentially important adjustment factors, which included socioeconomic status and maternal age. A principal component analysis (PCA) was also performed as a data reduction method. Given the directed nature of these analyses, we report unadjusted p values. Lastly, multivariable linear regression was used to examine the relationship among AAP and all individual HMOs. Since this analysis was exploratory, we report the raw p values and adjusted p values using the Benjamini–Hochberg procedure with an 5% false discovery rate (FDR). All effect estimates (betas) are reported for a one standard deviation increase in NO2 (SD: 2.23 ppb), PM10 (SD: 3.65 µg/), and PM2.5 exposure (SD: 1.02 µg/). All statistical analyses for this study were performed in R (Version 4.0.3), and a significance threshold of p < 0.05 was used.
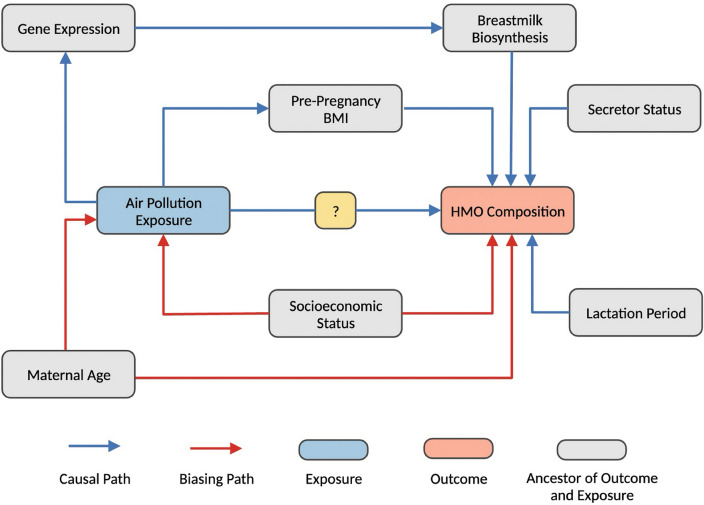
Figure 5.
Directed acyclic graph (DAG). Factors associated with air pollution exposure and HMOs are indicated in the DAG, which was used to identify adjustment variables. Based on this DAG, both maternal age and socioeconomic status were identified as confounding variables and included in our statistical models.
Supplementary Information
Acknowledgements
Research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly funded by the United States Environmental Protection Agency (EPA) (Assistance Award No. CR 83998101) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or motor vehicle and engine manufacturers. Research in this analysis was also supported by the NIH (NIH R01 DK110793). Research reported in this publication was additionally supported by the National Institute of Minority Health and Health Disparities (NIMHD) of the NIH (P50 MD17344).
Author contributions
N.C.N., E.A.H., B.N.C., M.M.C., and T.L.A. had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. N.C.N., E.A.H., B.N.C., and M.M.C. analyzed the data. F.L. generated the air pollution estimates, L.B. generated the H.M.O. data, F.L., M.I.G., and L.B. assisted with manuscript preparation and interpretation of results. All authors provided feedback and approved the final manuscript. M.I.G. conceived of the original Mother’s Milk Study and M.I.G. and T.L.A. conceived of the study and designed the experiments.
Data availability
Data cannot be shared publicly because they include potentially identifying information on human subjects. The data that support the findings of this study are available upon reasonable request from the corresponding author, TLA.
Competing interests
MIG receives book royalties and is a scientific advisor for Yumi. LB is the UC San Diego Chair of Collaborative Human Milk Research endowed by the Family Larsson-Rosenquist Foundation in Switzerland. All other authors declare no additional competing interests to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-57158-z.
References
- 1.Alotiby AA. The role of breastfeeding as a protective factor against the development of the immune-mediated diseases: A systematic review. Front. Pediatr. 2023;11:1086. doi: 10.3389/fped.2023.1086999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JW, et al. The protective effect of exclusive breastfeeding on overweight/obesity in children with high birth weight. J. Korean Med. Sci. 2019;34:e85. doi: 10.3346/jkms.2019.34.e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health. 2014;14:1267. doi: 10.1186/1471-2458-14-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida CC, et al. Bioactive compounds in infant formula and their effects on infant nutrition and health: A systematic literature review. Int. J. Food Sci. 2021;2021:e8850080. doi: 10.1155/2021/8850080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard O, Morrow AL. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. North Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagström H, et al. Associations between human milk oligosaccharides and growth in infancy and early childhood. Am. J. Clin. Nutr. 2020;111:769–778. doi: 10.1093/ajcn/nqaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiciński M, Sawicka E, Gębalski J, Kubiak K, Malinowski B. Human milk oligosaccharides: Health benefits, potential applications in infant formulas, and pharmacology. Nutrients. 2020;12:266. doi: 10.3390/nu12010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X. Human milk oligosaccharides (HMOS): Structure, function, and enzyme-catalyzed synthesis. Adv. Carbohydr. Chem. Biochem. 2015;72:113–190. doi: 10.1016/bs.accb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azad MB, et al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J. Nutr. 2018;148:1733–1742. doi: 10.1093/jn/nxy175. [DOI] [PubMed] [Google Scholar]
- 10.McGuire MK, et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically12. Am. J. Clin. Nutr. 2017;105:1086–1100. doi: 10.3945/ajcn.116.139980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seferovic MD, et al. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 2020;10:22092. doi: 10.1038/s41598-020-79022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandenplas, Y. Human Milk Oligosaccharides: 2’-Fucosyllactose (2’-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. https://www.nestlenutrition-institute.org/resources/publication-series/publications/article/human-milk-oligosaccharides-2-fucosyllactose-2-fl-and-lacto-n-neotetraose-lnnt-infant-formula. [DOI] [PMC free article] [PubMed]
- 13.Moore RE, Xu LL, Townsend SD. Prospecting human milk oligosaccharides as a defense against viral infections. ACS Infect. Dis. 2021;7:254–263. doi: 10.1021/acsinfecdis.0c00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Khafaji AH, Jepsen SD, Christensen KR, Vigsnæs LK. The potential of human milk oligosaccharides to impact the microbiota-gut-brain axis through modulation of the gut microbiota. J. Funct. Foods. 2020;74:104176. doi: 10.1016/j.jff.2020.104176. [DOI] [Google Scholar]
- 15.Ray C, et al. Human milk oligosaccharides: The journey ahead. Int. J. Pediatr. 2019;2019:e2390240. doi: 10.1155/2019/2390240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triantis V, Bode L, van Neerven RJJ. Immunological effects of human milk oligosaccharides. Front. Pediatr. 2018;6:190. doi: 10.3389/fped.2018.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Autran CA, et al. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut. 2018;67:1064–1070. doi: 10.1136/gutjnl-2016-312819. [DOI] [PubMed] [Google Scholar]
- 18.Masi AC, et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut. 2021;70:2273–2282. doi: 10.1136/gutjnl-2020-322771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noll AJ, et al. Human DC-SIGN binds specific human milk glycans. Biochem. J. 2016;473:1343–1353. doi: 10.1042/BCJ20160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foata F, Sprenger N, Rochat F, Damak S. Activation of the G-protein coupled receptor GPR35 by human milk oligosaccharides through different pathways. Sci. Rep. 2020;10:16117. doi: 10.1038/s41598-020-73008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bode L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015;91:619–622. doi: 10.1016/j.earlhumdev.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Plows JF, et al. Longitudinal changes in human milk oligosaccharides (HMOs) over the course of 24 months of lactation. J. Nutr. 2021;151:876–882. doi: 10.1093/jn/nxaa427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellman BP, et al. Elucidating human milk oligosaccharide biosynthetic genes through network-based multi-omics integration. Nat. Commun. 2022;13:2455. doi: 10.1038/s41467-022-29867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, et al. Lactational and geographical variation in the concentration of six oligosaccharides in Chinese breast milk: a multicenter study over 13 months postpartum. Front. Nutr. 2023;10:1267287. doi: 10.3389/fnut.2023.1267287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayechu-Muruzabal V, et al. Diversity of human milk oligosaccharides and effects on early life immune development. Front. Pediatr. 2018;6:239. doi: 10.3389/fped.2018.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzel P, et al. Concentrations of oligosaccharides in human milk and child growth. BMC Pediatr. 2021;21:481. doi: 10.1186/s12887-021-02953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saben JL, Sims CR, Abraham A, Bode L, Andres A. Human milk oligosaccharide concentrations and infant intakes are associated with maternal overweight and obesity and predict infant growth. Nutrients. 2021;13:446. doi: 10.3390/nu13020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eum Y, Song I, Kim H-C, Leem J-H, Kim S-Y. Computation of geographic variables for air pollution prediction models in South Korea. Environ. Health Toxicol. 2015;30:e2015010. doi: 10.5620/eht.e2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diener A, Mudu P. How can vegetation protect us from air pollution? A critical review on green spaces’ mitigation abilities for air-borne particles from a public health perspective–with implications for urban planning. Sci. Total Environ. 2021;796:148605. doi: 10.1016/j.scitotenv.2021.148605. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez C, Fente C, Regal P, Lamas A, Lorenzo MP. Human milk oligosaccharides (HMOs) and infant microbiota: A scoping review. Foods. 2021;10:1429. doi: 10.3390/foods10061429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahdenperä M, et al. Residential green environments are associated with human milk oligosaccharide diversity and composition. Sci. Rep. 2023;13:216. doi: 10.1038/s41598-022-27317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hue-Beauvais C, et al. Impact of exposure to diesel exhaust during pregnancy on mammary gland development and milk composition in the rabbit. PLOS ONE. 2019;14:e0212132. doi: 10.1371/journal.pone.0212132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rider CF, Carlsten C. Air pollution and DNA methylation: effects of exposure in humans. Clin. Epigenetics. 2019;11:131. doi: 10.1186/s13148-019-0713-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shukla A, et al. Air pollution associated epigenetic modifications: Transgenerational inheritance and underlying molecular mechanisms. Sci. Total Environ. 2019;656:760–777. doi: 10.1016/j.scitotenv.2018.11.381. [DOI] [PubMed] [Google Scholar]
- 35.Bode L. Human milk oligosaccharides in the prevention of necrotizing enterocolitis: A journey from in vitro and in vivo models to mother-infant cohort studies. Front. Pediatr. 2018;6:385. doi: 10.3389/fped.2018.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jantscher-Krenn E, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. 2012;61:1417–1425. doi: 10.1136/gutjnl-2011-301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sodhi CP, et al. The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 2021;89:91–101. doi: 10.1038/s41390-020-0852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CDC. Hispanic/Latino Americans and Type 2 Diabetes. Centers for Disease Control and Preventionhttps://www.cdc.gov/diabetes/library/features/hispanic-diabetes.html (2022).
- 39.Obesity and Hispanic Americans | Office of Minority Health. https://minorityhealth.hhs.gov/obesity-and-hispanic-americans.
- 40.Ceballos R, et al. Latino beliefs about biomedical research participation: A qualitative study on the US-Mexico border. J. Empir. Res. Hum. Res. Ethics JERHRE. 2014;9:10–21. doi: 10.1177/1556264614544454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh C, Lane JA, van Sinderen D, Hickey RM. Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. J. Funct. Foods. 2020;72:104074. doi: 10.1016/j.jff.2020.104074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alderete TL, et al. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015;102:1381–1388. doi: 10.3945/ajcn.115.115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zielinska MA, Hamulka J. Protective effect of breastfeeding on the adverse health effects induced by air pollution: Current evidence and possible mechanisms. Int. J. Environ. Res. Public. Health. 2019;16:4181. doi: 10.3390/ijerph16214181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borewicz K, et al. The association between breastmilk oligosaccharides and faecal microbiota in healthy breastfed infants at two, six, and twelve weeks of age. Sci. Rep. 2020;10:4270. doi: 10.1038/s41598-020-61024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alderete TL, et al. Exposure to traffic-related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environ. Res. 2018;161:472–478. doi: 10.1016/j.envres.2017.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey MJ, Naik NN, Wild LE, Patterson WB, Alderete TL. Exposure to air pollutants and the gut microbiota: A potential link between exposure, obesity, and type 2 diabetes. Gut Microbes. 2020;11:1188–1202. doi: 10.1080/19490976.2020.1749754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fouladi F, et al. Air pollution exposure is associated with the gut microbiome as revealed by shotgun metagenomic sequencing. Environ. Int. 2020;138:105604. doi: 10.1016/j.envint.2020.105604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stepans MBF, et al. Early consumption of human milk oligosaccharides is inversely related to subsequent risk of respiratory and enteric disease in infants. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2006;1:207–215. doi: 10.1089/bfm.2006.1.207. [DOI] [PubMed] [Google Scholar]
- 49.Huh SJ, et al. Age- and pregnancy-associated DNA methylation changes in mammary epithelial cells. Stem Cell Rep. 2015;4:297–311. doi: 10.1016/j.stemcr.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rijnkels M, et al. The epigenetic landscape of mammary gland development and functional differentiation. J. Mammary Gland Biol. Neoplasia. 2010;15:85–100. doi: 10.1007/s10911-010-9170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krauskopf J, et al. Short-term exposure to traffic-related air pollution reveals a compound-specific circulating miRNA profile indicating multiple disease risks. Environ. Int. 2019;128:193–200. doi: 10.1016/j.envint.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 52.Li X, et al. Exposure to fine particulate matter air pollution alters mRNA and miRNA expression in bone marrow-derived endothelial progenitor cells from mice. Genes. 2021;12:1058. doi: 10.3390/genes12071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alsaweed M, Hartmann PE, Geddes DT, Kakulas F. MicroRNAs in breastmilk and the lactating breast: Potential immunoprotectors and developmental regulators for the infant and the mother. Int. J. Environ. Res. Public. Health. 2015;12:13981–14020. doi: 10.3390/ijerph121113981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xuan R, et al. Characterization of microRNA profiles in the mammary gland tissue of dairy goats at the late lactation, dry period and late gestation stages. PLOS ONE. 2020;15:e0234427. doi: 10.1371/journal.pone.0234427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams JE, et al. Key genetic variants associated with variation of milk oligosaccharides from diverse human populations. Genomics. 2021;113:1867–1875. doi: 10.1016/j.ygeno.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Crouse DL, et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: A Canadian national-level cohort study. Environ. Health Perspect. 2012;120:708–714. doi: 10.1289/ehp.1104049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patterson WB, et al. Prenatal exposure to ambient air pollutants and early infant growth and adiposity in the Southern California Mother’s milk study. Environ. Health. 2021;20:67. doi: 10.1186/s12940-021-00753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eckel SP, et al. Air pollution affects lung cancer survival. Thorax. 2016;71:891–898. doi: 10.1136/thoraxjnl-2015-207927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tessum CW, et al. Inequity in consumption of goods and services adds to racial–ethnic disparities in air pollution exposure. Proc. Natl. Acad. Sci. 2019;116:6001–6006. doi: 10.1073/pnas.1818859116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berger PK, et al. Human milk oligosaccharides and Hispanic infant weight gain in the first 6 months. Obes. Silver Spring Md. 2020;28:1519–1525. doi: 10.1002/oby.22884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wild LE, et al. Specific amino acids but not total protein attenuate postpartum weight gain among Hispanic women from Southern California. Food Sci. Nutr. 2021;9:1842–1850. doi: 10.1002/fsn3.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juhn YJ, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J. Urban Health Bull N. Y. Acad. Med. 2011;88:933–944. doi: 10.1007/s11524-011-9572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared publicly because they include potentially identifying information on human subjects. The data that support the findings of this study are available upon reasonable request from the corresponding author, TLA.