Abstract
Introduction
Clinical guidelines can contribute to medication errors but there is no overall understanding of how and where these occur.
Objectives
We aimed to identify guideline-related medication errors reported via a national incident reporting system, and describe types of error, stages of medication use, guidelines, drugs, specialties and clinical locations most commonly associated with such errors.
Methods
Retrospective analysis of reports to the National Reporting and Learning System for England and Wales. A hierarchical task analysis (HTA) was developed, describing expected practice when using guidelines. A free-text search was conducted of medication incident reports (2016–2021) using search terms related to common guidelines. All identified reports linked to moderate-severe harm or death, and a random sample of 5100 no/low-harm reports were coded to describe deviations from the HTA. A random sample of 500 cases were independently double-coded.
Results
In total, 28,217 reports were identified, with 608 relating to moderate-severe harm or death. Fleiss’ kappa for interrater reliability was 0.46. Of the 5708 reports coded, 642 described an HTA step discrepancy (including four linked to a death), suggesting over 3200 discrepancies in the entire dataset of 28,217 reports. Discrepancies related to finding guidelines (n = 300 reports), finding information within guidelines (n = 166) and using information (n = 176). Discrepancies were most frequently identified for guidelines produced by a local organisation (n = 405), and most occurred during prescribing (n = 277) or medication administration (n = 241).
Conclusion
Difficulties finding and using information from clinical guidelines contribute to thousands of prescribing and medication administration incidents, some of which are associated with substantial patient harm.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-024-01396-7.
Key Points
| Difficulties finding and using information from clinical guidelines (especially those produced by local healthcare organisations) contribute to thousands of prescribing and medication administration incidents. |
| Some of these incidents are associated with substantial patient harm. |
| Further development and implementation of techniques that may be effective in preventing such incidents (such as iterative user testing) is required. |
Introduction
Medication errors are common, with an estimated 237 million occurring each year in England alone, costing the National Health Service (NHS) £98 million and causing or contributing to an estimated 1708 deaths [1]. Many factors have been identified that can contribute to medication errors [2], one of which is the plethora and complexity of medication-related guidelines.
Guidelines can play a key role in ensuring healthcare professionals provide safe and consistent patient care, and can also themselves contribute to both prescribing [3–9] and medication administration errors [10–13]. For example, Jones et al. [14] previously analysed video recordings of medication preparation and administration in a simulated paediatric emergency setting to find which steps of using the NHS Injectable Medicines Guide were susceptible to misinterpretation and caused clinically significant medication errors. The study suggests that 70% of discrepancies in the guideline use process arose from steps involved in finding the correct guideline (with using the incorrect guideline for the patient’s age a significant issue), contributing to 14 medication errors. As a result of evidence such as this, written guidance that is contradictory, incomprehensible or otherwise of poor quality [15], as well as problems with instructions about procedures [16], have been included in two frameworks describing factors that contribute to patient safety incidents. Additionally, the clarity of writing and formatting of guidelines have been recognised as two of six key domains influencing their uptake [17].
Given the challenges of interpreting and using guidelines, several studies have explored iterative ‘user testing’ and subsequent modification of documents as a structured approach to improving the usability and utility of health-related guidelines. While much of this work has studied information aimed at patients and the public [18], more recent work has tested this approach with clinical guidelines aimed at healthcare staff [19–21]. For example, Jones et al. [21] found participants understood significantly more information when interpreting medication administration guidelines that had been modified following iterative user testing, and that nurses using the revised guidelines made fewer errors in an in situ simulation [22]. A cost-effectiveness analysis concluded that user testing and modifying this guideline had a 99% chance of being cost effective [23].
However, no published study has examined guideline-related errors across a whole health system or even a whole organisation. Previous studies have focused on specific scenarios requiring a limited number of guidelines. We therefore lack an overall picture of how and where mistakes are made with the use of guidelines, what medication errors occur as a result, and which guidelines are most often affected. An understanding of this would allow the identification and prioritisation of areas in which to apply user testing or other approaches to the improvement of guidelines to support patient safety.
We therefore aimed to address this gap in the literature. Our objectives were to identify guideline-related medication errors reported via a national incident reporting database; to describe the types of error, stages of medication use, types of guideline, drugs, specialties and clinical locations most commonly associated with guideline-related medication errors; and to make recommendations for both practice and research in this area.
Methods
We carried out a retrospective analysis of medication errors relating to clinical guidelines that had been reported to the National Reporting and Learning System (NRLS) for England and Wales.
Database and Searches
The NRLS collects data on patient safety incident reports that are voluntarily and anonymously reported by staff working in the NHS and other healthcare organisations in England and Wales. These reports include incidents and near misses. The report template allows the input of categorical data (e.g. incident type and location) as well as free-text fields that allow staff to describe the incident, its perceived causes and the actions taken. Incidents within the database are classified by the reporter as having been linked to death, severe harm, moderate harm, low harm or no harm.
To identify incident reports relating to guideline use, a free-text field search was conducted by the national NRLS team using the following search terms related to medicines guidelines commonly used in England and Wales: BNF, British National Formulary, Guideline, NICE, SPC, SmPC, Summary of Product Characteristics, CKS, Clinical Knowledge Summary/ies, Medusa, Injectable Medicines Guide and IMG. The search was restricted to incidents reported to the NRLS between 1 January 2016 and 31 December 2021 (inclusive) that had been classified by the reporter as medication incidents. The identified incident reports, including both categorical and free-text data fields, were then supplied to the research team.
Hierarchical Task Analysis (HTA)
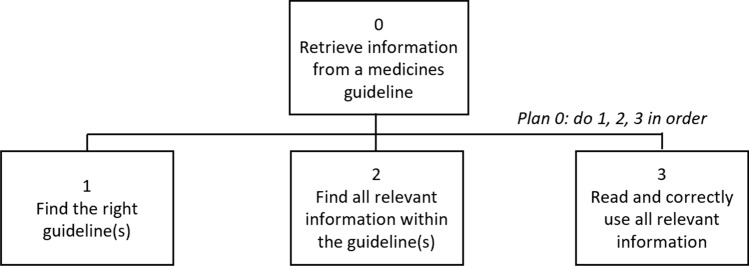
Hierarchical task analysis (HTA), a method that describes the workflow of a task or activity [24], was used as the basis of this study. To develop this HTA, data from the identified incidents reported to have resulted in moderate harm, severe harm or death were first inductively coded to identify the stage of the process of guideline use to which the incident related. These inductive codes were then used to inform the adaptation of an HTA from a previous study that was specific to one guideline [14] to consider the use of guidelines in general. The adapted HTA describing the process of retrieving information from any medicines guideline is shown in Fig. 1.
Fig. 1.
The hierarchical task analysis describing the process of retrieving information from a medicines guideline
Coding of Medication Incident Reports
A list of all medication incidents that were linked by the reporter to moderate or severe harm or death (608 reports), plus a random sample of 5100 linked to no harm and low harm, was generated using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). This sample size was based on pragmatic considerations and resources available. Each report was then coded by one of a team of five researchers (all final-year Master of Pharmacy students), based on the free-text descriptions of the incident, its causes and preventive actions. Any deviations from expected practice (as described by the HTA) when retrieving information from a medicines guideline were defined as ‘step discrepancies’. Each step discrepancy was then further coded using a subset of error modes (Box 1) adapted from those used in a previous study [14] that were drawn from a generic human error taxonomy [25]. To focus on the initial cause of difficulties using guidelines and to enable clear presentation of results without ‘double counting’, only the first discrepancy of a series of step discrepancies was coded. When a step discrepancy was identified, the guideline(s) to which it related was also coded. Separate codes were assigned to reports that did not describe a step discrepancy or those for which it was not clear whether or not a step discrepancy had occurred. Coding was supported by operational definitions agreed by the research team before coding commenced (electronic supplementary material [ESM] Tables S1 and S2).
Box 1.
Error modes used to code step discrepancies from expected practice when retrieving information from a medicines guideline, as described in the hierarchical task analysis
| Error mode code | Error mode description |
|---|---|
| A1 | Operation took too long |
| R1 | Information not obtained |
| R2 | Wrong information obtained |
| R3 | Incomplete information obtained |
| R4 | Information not sought |
| R5 | Information not available |
Error modes coded ‘A’ are ‘action errors’ and those coded ‘R’ are ‘retrieval errors’ [25]
Prior to coding the data, the team of five researchers each read relevant articles from the research and practice literature and attended a series of meetings with experienced medication safety pharmacist-researchers (BDF, SG and MDJ) to discuss how the operational definitions should be applied. To evaluate interrater reliability of coding among the researchers, the team of five researchers, plus a sixth researcher with specific expertise in this area, first independently coded the same 100 randomly selected reports as a pilot. These six researchers then discussed cases where there was disagreement and agreed some amendments to the operational definitions (ESM Table S2). This revised framework was subsequently used to code the remaining reports.
Following this, the team of five researchers each coded 1000 no-harm and low-harm cases and approximately 120 of the moderate, severe and death cases. After coding was completed for all the cases, each reviewer double-coded a further randomly selected 100 cases to further establish the level of interrater reliability.
Data Analysis Processes
The coded data were analysed with descriptive statistics using SPSS (version 27; IBM Corporation, Armonk, NY, USA). The interrater reliability for the coding of error modes was calculated using Fleiss’ kappa. The frequencies of different types of step discrepancy, error mode, guideline and medicine were calculated. The total numbers of no-harm and low-harm discrepancies in the entire NRLS data extract were estimated using Eq. 1 (rounded to the nearest integer):
| 1 |
Eh,s = estimated total number of discrepancies from HTA step ‘s’ in the entire NRLS data extract linked to harm level ‘h’
Dh,s = number of incidents coded as discrepancies from HTA step ‘s’ linked to harm level ‘h’
Th = total number of incidents in the entire NRLS data extract linked to harm level ‘h’
Ch = number of incidents coded linked to harm level ‘h’
h = level of harm linked to an incident, either ‘no harm’ or ‘low harm’
s = HTA step number
This was not necessary for higher levels of harm, as all the reports extracted from the NRLS were coded. Cross tabulations of step discrepancies were made against the guideline involved, stage in the medicines use process, and the care setting.
Ethics
We used exclusively anonymous data and therefore after review through the University of Bath Ethical Implications of Research Activity process, no Ethics Committee review was required. Data-sharing agreements were in place between NHS England and each data analysis site. Identifiable data were encrypted and were only accessible from secured university systems.
Results
National Reporting and Learning System (NRLS) Database Search
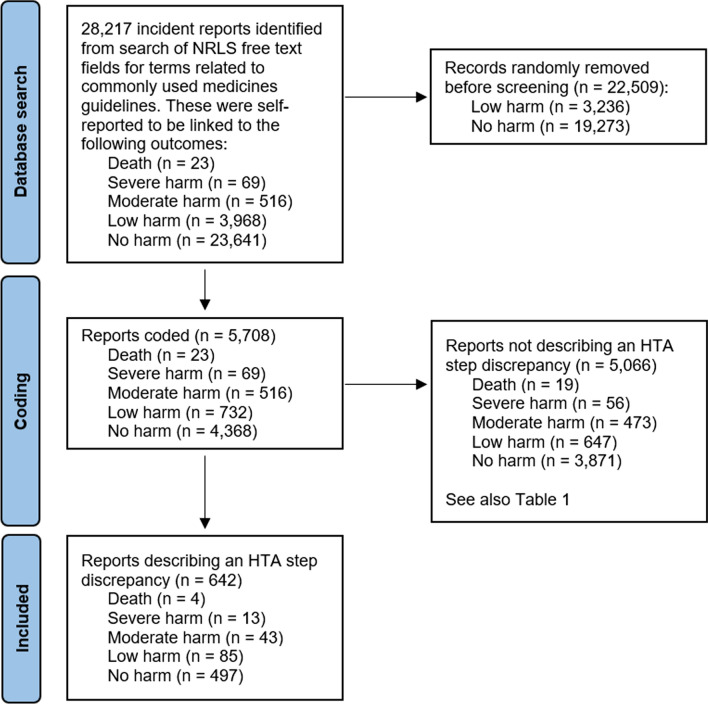
Our search strategy identified 28,217 incident reports of a total of 1,238,649 medication errors reported to the NRLS over the 6-year study period [26]. Of these 28,217, 23 were reported to be linked to death, 69 to severe harm, 516 to moderate harm, 3968 to low harm, and 23,641 to no harm. All reports linked to death and severe and moderate harm were coded, along with the random sample of 5100 low- and no-harm reports, giving a total of 5708 reports coded (Fig. 2).
Fig. 2.
Summary of the incident report search and coding process followed during this study. HTA hierarchical task analysis, NRLS National Reporting and Leaning System
Interrater Reliability
Fleiss’ kappa for interrater reliability during the pilot coding (100 reports each coded by six researchers) was 0.43. During the main coding, this was 0.46 (random sample of 500 reports, each coded by two researchers).
HTA Step Discrepancies
A total of 642 reports of the 5708 coded described an HTA step discrepancy leading to a medication incident (Table 1 and Fig. 2). Finding the right guideline was the HTA step most often associated with a step discrepancy. The remaining 5066 reports did not describe an HTA step discrepancy, although in many cases this largely reflected lack of information in the incident report. Scaling up using Eq. 1 produced an estimate of approximately 3200 HTA step discrepancies described in all 28,217 incident reports (Table 2).
Table 1.
Number of incident reports coded as describing or not describing an HTA step discrepancy relating to use of guidelines
| Number of reports coded | |||||||
|---|---|---|---|---|---|---|---|
| No harm | Low harm | Moderate harm | Severe harm | Death | All levels of harm | ||
| Reports describing an HTA step discrepancy | Step 1 – find the right guideline(s) | 238 | 36 | 17 | 6 | 3 | 300 |
| Step 2 – find all relevant information within the guideline(s) | 129 | 26 | 7 | 4 | 0 | 166 | |
| Step 3 – read and correctly use all relevant information | 130 | 23 | 19 | 3 | 1 | 176 | |
| Subtotal | 497 | 85 | 43 | 13 | 4 | 642 | |
| Reports not describing an HTA step discrepancy | Guideline not followed for reason unrelated to the process of retrieving information from a guideline | 356 | 52 | 31 | 8 | 3 | 450 |
| Guideline not followed; reasons unknown | 1556 | 269 | 180 | 23 | 12 | 2040 | |
| Guideline content incorrect or out-of-date | 62 | 13 | 9 | 3 | 1 | 88 | |
| Report not related to guideline use | 1728 | 292 | 205 | 15 | 3 | 2243 | |
| Insufficient information to determine if guideline followed | 168 | 20 | 48 | 7 | 0 | 243 | |
| Does not relate to a medication incident | 1 | 1 | 0 | 0 | 0 | 2 | |
HTA hierarchical task analysis
Table 2.
Estimated number of incident reports describing or not describing an HTA step discrepancy relating to use of guidelines in the total NRLS data extract
| Estimated total number of incidentsa | Actual number of reports codedb | Estimated totalc | |||||
|---|---|---|---|---|---|---|---|
| No harm | Low harm | Moderate harm | Severe harm | Death | |||
| Reports describing an HTA step discrepancy | Step 1 – find the right guideline(s)d | 1288 (85.4%) | 195 (12.9%) | 17 (1.1%) | 6 (0.4%) | 3 (0.2%) | 1509 (100.0%) |
| Step 2 – find all relevant information within the guideline(s)d | 698 (82.1%) | 141 (16.6%) | 7 (0.8%) | 4 (0.5%) | 0 (0.0%) | 850 (100.0%) | |
| Step 3 – read and correctly use all relevant informationd | 704 (82.6%) | 125 (14.7%) | 19 (2.2%) | 3 (0.4%) | 1 (0.1%) | 852 (100.0%) | |
| Subtotald | 2690 (83.8%) | 461 (14.4%) | 43 (1.3%) | 13 (0.4%) | 4 (0.1%) | 3211 (100.0%) | |
| Reports not describing an HTA step discrepancy | Guideline not followed; reason unrelated to HTA | 1927 | 282 | 31 | 8 | 3 | 2251 |
| Guideline not followed; reasons unknown | 8422 | 1458 | 180 | 23 | 12 | 10,095 | |
| Guideline content incorrect or out-of-date | 336 | 70 | 9 | 3 | 1 | 419 | |
| Report not related to guideline use | 9352 | 1583 | 205 | 15 | 3 | 11,158 | |
| Insufficient information to determine if guideline followed | 909 | 108 | 48 | 7 | 0 | 1072 | |
| Does not relate to a medication incident | 5 | 5 | 0 | 0 | 0 | 10 | |
a4368 of a total of 23,641 no-harm incidents and 732 of a total of 3968 low-harm incidents were coded. The total number of no-harm and low-harm discrepancies in the NRLS data extract was estimated using Eq. 1. This was not necessary for higher levels of harm as all the reports extracted from the NRLS were coded
bExact counts (rather than estimates) are provided for incidents associated with moderate harm, severe harm and death as all of these incidents were coded
cThe estimated total number of incidents of any level of harm in the NRLS data extract was calculated by summing the estimated total number of no- and low-harm incidents with the number of reports coded at the moderate, severe and death levels of harm
dPercentages are within each HTA step
HTA hierarchical task analysis, NRLS National Reporting and Learning System
All six error modes (Box 1) were involved in HTA step discrepancies (Table 3). The most frequent type of discrepancy was misunderstanding the correct guideline (HTA step 3, error mode R2). Other common types of discrepancy included mistakenly using the wrong guideline (HTA step 1, error mode R2), relying on memory rather than checking a guideline (HTA step 1, error mode R4), an organisation not providing an appropriate guideline (HTA step 1, error mode R5), using information from the wrong section of a guideline (HTA step 2, error mode R2) and a guideline not including the required information (HTA step 2, error mode R5). These were also the types of discrepancy associated with reports of death and severe harm (Table 3), along with an appropriate guideline being provided by the organisation but not found by staff (HTA step 1, error mode R1).
Table 3.
Number of HTA step discrepancies coded with each error mode, and an example of each
| HTA step | Error modea | Number of reports coded | Summary of example incident report (reported degree of harm) | |
|---|---|---|---|---|
| All levels of harm | Death and severe harm | |||
| Step 1 – find the right guideline(s) | A1 | 5 | 0 | NHS Injectable Medicines Guide website offline, therefore administration of intravenous metoprolol delayed (No harm) |
| R1 | 30 | 1 | Local unfractionated heparin intravenous infusion guideline not located on hospital intranet, and ward’s printed copy lost. Infusion rate should have been adjusted at 09:30 h, but was not reviewed until 19:00 h (No harm) | |
| R2 | 79 | 3 | Elderly patient with infective exacerbation of bronchiectasis prescribed intravenous tobramycin 10 mg/kg/day instead of 3 mg/kg/day, as the cystic fibrosis guideline was inappropriately used. Patient developed acute kidney injury and died (Death) | |
| R3 | 41 | 0 | Intravenous omeprazole infusion administered at 0.6 mg/h instead of 8 mg/h. Local guideline should have been used to clarify the infusion rate in addition to the NHS Injectable Medicines Guide (No harm) | |
| R4 | 73 | 3 | Paediatric insulin infusion prescribed without checking local guidelines. Prescribed infusion rate was 10 times too high and was administered for 1 h, leading to hypoglycaemia (Severe) | |
| R5 | 72 | 2 | Patient not prescribed venous thromboembolism prophylaxis on discharge for 28 days following cancer-related surgery, as no local guideline for specific type of surgery. Patient readmitted with deep vein thrombosis after 7 days (Moderate) | |
| Step 2 – find all relevant information within the guideline(s)b | R1 | 1 | 0 | Information on contraindication of morphine in severe headache not found in the Joint Royal Colleges Ambulance Liaison Committee guideline, therefore inappropriately administered (No harm) |
| R2 | 68 | 1 | Prescribed nitrofurantoin to a first-trimester pregnant patient (contraindicated), as followed ‘non-pregnant’ section of the urinary tract infection guidelines (No harm) | |
| R3 | 20 | 0 | Intravenous phenytoin infusion administered without an inline filter as this advice was not noticed in the NHS Injectable Medicines Guide (No harm) | |
| R5 | 77 | 3 | Patient with poor renal function prescribed gentamicin for sepsis. Local guidelines advised obtaining advice from a microbiologist in this situation, but this advice was unavailable at the time (03:00 h). Subsequent gentamicin plasma levels were high (Low) | |
| Step 3 – read and correctly use all relevant informationc | A1 | 1 | 0 | Amiodarone infusion administered 2 h late due to discussion between nursing staff around the meaning of information provided in the NHS Injectable Medicines Guide (Low) |
| R1 | 33 | 0 | Labetalol intravenous infusion administered several hours late as nursing staff could not understand NHS Injectable Medicines Guide advice. Eventually administered in accordance with colleagues’ advice (No harm) | |
| R2 | 135 | 4 | Child prescribed 150 mg of oral cefalexin instead of 75 mg, due to misreading of the British National Formulary (No harm) | |
| R3 | 3 | 0 | Psychiatrist recommended increasing a patient’s zuclopenthixol decanoate depot injection from 600 mg to 1 g every 2 weeks. Pharmacist used British National Formulary and Summary of Product Characteristics to confirm this was within the maximum monthly dose, but did not notice that the maximum single dose should be 600 mg. Error detected after patient discharge, leading to community review of treatment options (Low) | |
| R4 | 4 | 0 | Morphine and codeine prescribed for a palliative care patient with severe renal impairment. Online palliative care guideline had been used but prescriber did not seek relevant information on prescribing these drugs in renal impairment (No harm) | |
aErrors modes: A1 = operation took too long; R1 = information not obtained; R2 = wrong information obtained; R3 = incomplete information obtained; R4 = information not sought; R5 = information not available
bNo HTA step 2 discrepancies were coded with error mode R4
cError mode R5 was not applicable to HTA step 3
HTA hierarchical task analysis, NHS National Health Service
HTA step discrepancies were associated with five main types of guideline (Table 4), of which the most frequently identified were guidelines produced by a specific local organisation or group of organisations (‘local guidelines’).
Table 4.
Number of HTA step discrepancies associated with specific types of guidelines
| HTA step | BNF | Local guideline | NHS Injectable Medicines Guide | NICE guideline | SPC | Other guidelinea | Unknown guidelineb |
|---|---|---|---|---|---|---|---|
| Step 1 – find the right guideline(s) | 36 | 193 | 34 | 6 | 8 | 36 | 12 |
| Step 2 – find all relevant information within the guideline(s) | 11 | 104 | 18 | 2 | 7 | 19 | 13 |
| Step 3 – read and correctly use all relevant information | 25 | 108 | 30 | 1 | 6 | 19 | 6 |
| Totalc | 72 | 405 | 82 | 9 | 21 | 74 | 31 |
aOther guidelines were typically nationally published guidelines on specialist topics, such paediatrics, mental health or the administration of medicines to patients with swallowing difficulties
bThe type of guideline was unknown when insufficient information was given in the incident report
cSome discrepancies were associated with more than one guideline, therefore the table sums to more than the 642 identified HTA step discrepancies
BNF British National Formulary, HTA hierarchical task analysis, NHS National Health Service, NICE National Institute for Health and Care Excellence, SPC Summary of Product Characteristics
Most reported HTA step discrepancies occurred during one of two stages of the medicines use process, i.e. prescribing or administration (Table 5). Similarly, the most common types of medication error associated with HTA step discrepancies were wrong or unclear doses, frequencies or quantities, and the omission of medicines (ESM Table S3).
Table 5.
Number of HTA step discrepancies associated with each stage of the medicines use process and the associated types of guideline
| Prescribinga | Preparation or dispensinga | Supply of OTC medicinea | Administrationa | Monitoringa | Advicea | Other processa | |
|---|---|---|---|---|---|---|---|
| Total number of HTA step discrepancies associated with each medicines use process | 277 (100.0%) | 22 (100.0%) | 3 (100.0%) | 241 (100.0%) | 35 (100.0%) | 23 (100.0%) | 41 (100.0%) |
| Number of times specific types of guideline were associated with an HTA step discrepancy at each stage of the medicines use processb | |||||||
| BNF | 50 (18.1%) | 3 (13.6%) | 1 (33.3%) | 13 (5.4%) | 2 (5.7%) | 1 (4.3%) | 2 (4.9%) |
| Local guideline | 185 (66.8%) | 14 (63.6%) | 1 (33.3%) | 134 (55.6%) | 27 (77.1%) | 11 (47.8%) | 33 (80.5%) |
| NHS Injectable Medicines Guide | 13 (4.7%) | 5 (22.7%) | 0 (0.0%) | 56 (23.2%) | 0 (0.0%) | 6 (26.1%) | 2 (4.9%) |
| NICE guideline | 3 (1.1%) | 0 (0.0%) | 0 (0.0%) | 4 (1.7%) | 0 (0.0%) | 1 (4.3%) | 1 (2.4%) |
| SPC | 8 (2.9%) | 0 (0.0%) | 1 (33.3%) | 8 (3.3%) | 0 (0.0%) | 3 (13.0%) | 1 (2.4%) |
| Other guidelinec | 28 (10.1%) | 0 (0.0%) | 1 (33.3%) | 38 (15.8%) | 3 (8.6%) | 2 (8.7%) | 2 (4.9%) |
| Unknown guidelined | 19 (6.9%) | 2 (9.1%) | 0 (0.0%) | 7 (2.9%) | 2 (5.7%) | 0 (0.0%) | 1 (2.4%) |
aPercentages are within each medicines use stage
bSome discrepancies were associated with more than one guideline and other discrepancies involved failure to consult any guideline (HTA step 1, error mode R4), therefore column totals for both counts and percentages differ to the total number of HTA step discrepancies associated with each medicines use process
cOther guidelines were typically nationally published guidelines on specialist topics, such as paediatrics, mental health or the administration of medicines to patients with swallowing difficulties
dThe type of guideline was unknown when insufficient information was given in the incident report
BNF British National Formulary, HTA hierarchical task analysis, NHS National Health Service, NICE National Institute for Health and Care Excellence, OTC over the counter, SPC Summary of Product Characteristics
Fourteen medicines were mentioned in at least 10 incidents that described an HTA step discrepancy (Table 6 ESM Table S4), including four antibiotics and three anticoagulants.
Table 6.
Medicines mentioned in at least 10 incidents that described an HTA step discrepancy (for a list of all medicines, see ESM Table S4)
| Medicine | Number of associated HTA step discrepancies |
|---|---|
| Gentamicin | 38 |
| Insulins | 32 |
| Heparin | 19 |
| Vancomycin | 19 |
| Paracetamol | 18 |
| Dalteparin | 17 |
| Enoxaparin | 16 |
| Ceftriaxone | 14 |
| Amiodarone | 13 |
| Morphine | 13 |
| Parenteral nutrition | 12 |
| Potassium chloride | 11 |
| Teicoplanin | 11 |
| Glucose | 10 |
| Total for this table | 233 |
HTA hierarchical task analysis
The vast majority of reported HTA step discrepancies occurred in acute hospitals (590 reports). Other settings associated with one or more discrepancies were ambulance services (19 reports), community services (19 reports), mental health services (9 reports), community pharmacies (3 reports), general practice (1 report) and learning disability services (1 report). Medical specialties were most frequently associated with HTA step discrepancies occurring in acute hospitals (Table 7).
Table 7.
Number of HTA step discrepancies associated with various acute hospital specialties
| HTA step | Accident and emergency | Anaesthesia, pain and critical care | Children's specialties | Dentistry | Diagnostic services | Medical specialties | Obstetrics and gynaecology | Surgical specialties | Other |
|---|---|---|---|---|---|---|---|---|---|
| Step 1 – find the right guideline(s) | 32 | 17 | 10 | 1 | 3 | 80 | 25 | 30 | 73 |
| Step 2 – find all relevant information within the guideline(s) | 13 | 6 | 5 | 1 | 3 | 52 | 18 | 23 | 34 |
| Step 3 – read and correctly use all relevant information | 24 | 7 | 12 | 0 | 2 | 44 | 12 | 21 | 42 |
| Total | 69 | 30 | 27 | 2 | 8 | 176 | 55 | 74 | 149 |
HTA hierarchical task analysis
Discussion
A wide variety of difficulties occur in finding and using information in clinical guidelines, some of which contribute to patient harm. Difficulty finding the correct guideline was the most common type of guideline-related incident, and several deaths were reported in incident reports following guideline-related medication errors. Difficulties finding and using information were most frequently associated with guidelines produced by specific local organisations (particularly acute hospitals) in relation to either the prescribing or administration of typical ‘high risk’ parenteral drugs, particularly inappropriately omitted medicines and incorrect drug doses or frequencies. Approximately 3200 guideline-related incidents are a small proportion of the more than 1.2 million medication errors reported to the NRLS during the study period, let alone the 237 million medication errors estimated to occur in England each year [1]. However, this figure is likely to be a significant underestimate, as 40% of reports contained insufficient information to determine whether they were linked to difficulties finding and using information in clinical guidelines. In addition, it is likely that the initial search of the NRLS database did not recover all reports of guideline-related incidents, especially as awareness of the contribution to medication errors of difficulties finding and using information is likely to be low among incident reporters.
Comparison with Previous Literature
The present study makes an important contribution to the literature by providing the first detailed examination of how difficulties in the use of guidelines contribute to medication errors in clinical practice. Previous studies of medication errors have identified that difficulties using guidelines can contribute to both prescribing [3–9] and administration errors [10–13]. However, these have not considered the guidelines use process in sufficient detail to identify that problems with both finding and using information can contribute to medication errors. The exception is a study of simulated rather than actual practice, which identified 21 guidelines-use discrepancies contributing to a medication error, of which 14 discrepancies related to finding the guideline and seven discrepancies related to reading it [14]. Similarly, previous user testing studies have identified problems finding and understanding information in guidelines, but were not designed to investigate a subsequent contribution to medication errors [19, 20, 27, 28].
The present study is also the first to quantify the reported level of harm associated with guideline-related medication incidents in practice, although a previous in situ simulation study that compared the use of ‘standard’ and ‘modified following iterative user testing’ intravenous administration guidelines rated the potential clinical significance of 280 guideline-related errors [22]. Six were likely to result in severe consequences, 214 in moderate consequences and 60 in minor consequences. As this previous study considered only the high-risk route of intravenous drug administration, it is unsurprising that the present study (which includes all routes of drug administration) observed a lower proportion of harmful incidents.
The contribution of guidelines produced by local organisations to medication errors has not been investigated by previous studies, although this has been highlighted in relation to national guidelines such as the British National Formulary (particularly dose and frequency errors) [4, 29] and the NHS Injectable Medicines Guide [14, 22]. The present study is also the first to investigate all types of guideline-related medication incident and has thus demonstrated an approximately equal contribution to both prescribing and administration errors, whereas previous studies were not able to draw comparisons between types of medication error.
Implications for Practice
The results suggest that actions to reduce the contribution of difficulties finding and using information in clinical guidelines to medication errors may reduce medication-related harm. This especially applies to local guidelines relating to both the prescribing and administration of high-risk drugs in acute settings. Such work should focus on how easily accessible guidelines are, as well as the guidelines themselves. Both simplicity of the message given within guidelines and the formatting of guidelines may be important to address [17], and iterative user testing and subsequent modification of guidelines may be an effective technique to address these concerns [19, 20, 22, 27]. While local hospital guidelines were the most commonly identified as contributing to errors, and focusing on these is therefore important, the same principles should also be applied to all guidelines. In addition, those reporting, monitoring and investigating medication-related incidents should be aware of guidelines as a potential contributing factor and ensure details are reported and appropriate actions taken.
Implications for Future Research
The process of iterative user testing and modification of intravenous drug administration guidelines has been shown to reduce the frequency of medication administration errors and is a cost-effective approach [22, 23], but this has not been investigated for the prescribing errors linked to local guidelines that were prevalent in the findings of the present study. This question should therefore be addressed by future research, as user testing and subsequent modification has the potential to improve local prescribing guideline design and thus prevent medication errors. Such research should also focus on the development of user testing processes that are feasible given the resources available within individual healthcare providers. While iterative user testing and subsequent modification has been shown to make it easier for health professionals to find and understand information within one specific guideline [19, 20, 27], it does not address the process of finding the correct guideline to use, which contributed to many of incidents reported in the present study. Future research should therefore investigate whether techniques such as iterative usability testing of wider guideline information systems [28] are able to prevent further medication errors. Given the limitations of research based on voluntary reporting of medication incidents, future studies utilising prospective qualitative or quantitative data collection from staff involved in incidents would give greater certainty about the characteristics and prevalence of guideline-related medication errors. This is especially important in sectors such as primary care, which are underrepresented in the voluntary incident report data used in the present study. In addition, all future research into the causes of medication errors should consider the potential contribution of difficulties finding and using information in guidelines.
Strengths and Limitations
A retrospective analysis of a national database has the advantage of giving a broad pool of evidence across different locations and settings within England and Wales. However, incident reports have a number of limitations. The most significant of these is underreporting, with as few as 0.12% of patient safety incidents reported [30–32], leading to underestimation of the number of medication errors. In particular, rates of reporting are influenced by local organisational culture and awareness, and are much higher from secondary care. In addition, it is only mandatory for NHS organisations to report incidents leading to death or severe harm to the national NRLS system. Reports from patients and carers are generally not included. Incident reports are often submitted before completion of a local investigation and hence there may be missing data, especially on contributory factors. Therefore, many reports did not contain sufficient information to determine whether or how guidelines contributed to errors. Furthermore, the level of harm resulting from the incident may not have been clear at the time of report, hence NRLS database reports may relate to actual or potential harm based only on the judgement of the reporter.
It is likely that the initial database search missed some relevant reports. An inductive approach to this free-text field search, with additional search terms identified from the results of initial searches, might have increased the sensitivity of this stage. The fact that so many errors were related to guidelines, despite these limitations, strengthens our finding that guidelines are an important area to address in medication safety.
Another limitation was the relatively low interrater reliability for coding to the stages of the HTA process. This may also have been due to the limited level of information in the reports. As a result of this, findings need to be interpreted with some caution. As only the first of a series of step discrepancies occurring in one incident was coded, any chains of discrepancies were not recorded. Finally, the database relates to England and Wales only, therefore it is uncertain as to how far the findings are generalisable to other countries.
Conclusion
Difficulties finding and using information in clinical guidelines (especially those produced by local healthcare organisations) could contribute to thousands of prescribing and medication administration incidents, some of which are associated with substantial patient harm. Further development and implementation of techniques that may be effective in preventing such incidents (such as iterative user testing and subsequent guideline modification) is required.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the NRLS Data Requests team (NHS England) for providing the data and advice on the development of this study.
Declarations
Funding
No specific funding was received to support this study. BDF is part funded by the National Institute for Health and Care Research (NIHR) North West London Patient Safety Research Collaboration (PSRC). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Conflicts of Interest
Bryony Dean Franklin previously supervised a PhD student who was part funded by Cerner, an electronic health record systems vendor. Matthew D. Jones, Shaojun Liu, Freyja Powell, Asma Samsor, Felicity Ting Chao Ru, Nikolaos Veliotis, Yin Mei Wong, and Sara Garfield have declared no conflicts of interest.
Ethics Approval
This study used exclusively anonymous data and therefore after review through the University of Bath Ethical Implications of Research Activity process, no Ethics Committee review was required.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The coded anonymised dataset generated and analysed during the current study is available in the University of Bath Research Data Archive (https://researchdata.bath.ac.uk/id/eprint/1307). The raw data can be sought by application to NHS England.
Code Availability
Not applicable.
Author Contributions
Conceptualisation: BDF, MJ. Methodology: BDF, SG, MJ. Formal analysis and investigation: BDF, MDJ, SL FP, AS, FCRT, NV, YMW. Writing – original draft preparation: BDF, SG, MJ. Writing – review and editing: BDF, SG, MDJ, SL, FP, AS, FCRT, NV, YMW. Supervision: SG, MJ.
References
- 1.Elliott RA, Camacho E, Jankovic D, Sculpher MJ, Faria R. Economic analysis of the prevalence and clinical and economic burden of medication error in England. BMJ Qual Saf. 2021;30(2):96–105. doi: 10.1136/bmjqs-2019-010206. [DOI] [PubMed] [Google Scholar]
- 2.Naseralallah L, Stewart D, Azfar Ali R, Paudyal V. An umbrella review of systematic reviews on contributory factors to medication errors in health-care settings. Expert Opin Drug Saf. 2022;21(11):1379–1399. doi: 10.1080/14740338.2022.2147921. [DOI] [PubMed] [Google Scholar]
- 3.Coombes ID, Stowasser DA, Coombes JA, Mitchell C. Why do interns make prescribing errors? A qualitative study. Med J Aust. 2008;188(2):89–94. doi: 10.5694/j.1326-5377.2008.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 4.Dornan T, Ashcroft D, Heathfield H, Lewis P, Miles J, Taylor D, et al. An in depth investigation into causes of prescribing errors by foundation trainees in relation to their medical education. EQUIP study. General Medical Council; 2009.
- 5.Lederman RM, Parkes C. Systems failure in hospitals - using Reason's model to predict problems in a prescribing information system. J Med Syst. 2005;29(1):33–43. doi: 10.1007/s10916-005-1102-2. [DOI] [PubMed] [Google Scholar]
- 6.Franklin BD, Reynolds M, Shebl NA, Burnett S, Jacklin A. Prescribing errors in hospital inpatients: a three-centre study of their prevalence, types and causes. Postgrad Med J. 2011;87(1033):739–745. doi: 10.1136/pgmj.2011.117879. [DOI] [PubMed] [Google Scholar]
- 7.Mahomedradja RF, Schinkel M, Sigaloff KCE, Reumerman MO, Otten RHJ, Tichelaar J, et al. Factors influencing in-hospital prescribing errors: a systematic review. Br J Clin Pharmacol. 2023;89(6):1724–1735. doi: 10.1111/bcp.15694. [DOI] [PubMed] [Google Scholar]
- 8.Tully MP, Ashcroft DM, Dornan T, Lewis PJ, Taylor D, Wass V. The causes of and factors associated with prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(10):819–836. doi: 10.2165/11316560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Nichols P, Copeland TS, Craib IA, Hopkins P, Bruce DG. Learning from error: identifying contributory causes of medication errors in an Australian hospital. Med J Aust. 2008;188(5):276–279. doi: 10.5694/j.1326-5377.2008.tb01619.x. [DOI] [PubMed] [Google Scholar]
- 10.Keers RN, Williams SD, Cooke J, Ashcroft DM. Understanding the causes of intravenous medication administration errors in hospitals: a qualitative critical incident study. BMJ Open. 2015;5(3):e005948. doi: 10.1136/bmjopen-2014-005948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taxis K, Barber N. Causes of intravenous medication errors: an ethnographic study. Qual Saf Health Care. 2003;12(5):343–348. doi: 10.1136/qhc.12.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cousins DH, Sabatier B, Begue D, Schmitt C, Hoppe-Tichy T. Medication errors in intravenous drug preparation and administration: a multicentre audit in the UK, Germany and France. Qual Saf Health Care. 2005;14(3):190–195. doi: 10.1136/qshc.2003.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appelbaum N, Clarke J, Feather C, Franklin B, Sinha R, Pratt P, et al. Medication errors during simulated paediatric resuscitations: a prospective, observational human reliability analysis. BMJ Open. 2019;9(11):e032686. doi: 10.1136/bmjopen-2019-032686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones MD, Clarke J, Feather C, Franklin BD, Sinha R, Maconochie I, et al. Use of pediatric injectable medicines guidelines and associated medication administration errors: a human reliability analysis. Ann Pharmacother. 2021;55(11):1333–1340. doi: 10.1177/1060028021999647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawton R, McEachan RR, Giles SJ, Sirriyeh R, Watt IS, Wright J. Development of an evidence-based framework of factors contributing to patient safety incidents in hospital settings: a systematic review. BMJ Qual Saf. 2012;21(5):369–380. doi: 10.1136/bmjqs-2011-000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang A, Schyve PM, Croteau RJ, O'Leary DS, Loeb JM. The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events. Int J Qual Health Care. 2005;17(2):95–105. doi: 10.1093/intqhc/mzi021. [DOI] [PubMed] [Google Scholar]
- 17.Kastner M, Bhattacharyya O, Hayden L, Makarski J, Estey E, Durocher L, et al. Guideline uptake is influenced by six implementability domains for creating and communicating guidelines: a realist review. J Clin Epidemiol. 2015;68(5):498–509. doi: 10.1016/j.jclinepi.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Raynor DK, Knapp P, Silcock J, Parkinson B, Feeney K. "User-testing" as a method for testing the fitness-for-purpose of written medicine information. Patient Educ Couns. 2011;83(3):404–410. doi: 10.1016/j.pec.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Raynor DK, Veene PD, Bryant D. The effectiveness of the Summary of Product Characteristics (SmPC) and recommendations for improvement. Ther Innov Regul Sci. 2013;48(2):255–265. doi: 10.1177/2168479013501311. [DOI] [PubMed] [Google Scholar]
- 20.Verhoeven F, Steehouder MF, Hendrix RMG, Van Gemert-Pijnen JEWC. From expert-driven to user-oriented communication of infection control guidelines. Int J Hum-Comput St. 2010;68(6):328–343. doi: 10.1016/j.ijhcs.2009.07.003. [DOI] [Google Scholar]
- 21.Jones MD, Franklin BD, Watson MC, Raynor DK. User testing to improve retrieval and comprehension of information in guidelines to improve medicines safety. J Patient Saf. 2022;18(1):e172–e179. doi: 10.1097/PTS.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones MD, McGrogan A, Raynor DKT, Watson MW, Franklin BD. User-testing guidelines to improve the safety of intravenous medicines administration: a randomised in-situ simulation study. BMJ Qual Saf. 2020;30:17–26. doi: 10.1136/bmjqs-2020-010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones MD, Franklin BD, Raynor DK, Thom H, Watson MC, Kandiyali R. Costs and cost-effectiveness of user-testing of health professionals’ guidelines to reduce the frequency of intravenous medicines administration errors by nurses in the United Kingdom: a probabilistic model based on voriconazole administration. Appl Health Econ. 2022;20(1):91–104. doi: 10.1007/s40258-021-00675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Privitera MB, editor. Applied human factors in medical device design. Academic Press; 2019. [Google Scholar]
- 25.Lane R, Stanton NA, Harrison D. Applying hierarchical task analysis to medication administration errors. Appl Ergon. 2006;37(5):669–679. doi: 10.1016/j.apergo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 26.NHS England. National patient safety incident reports. 2022. Available at: https://www.england.nhs.uk/patient-safety/national-patient-safety-incident-reports/
- 27.Jones MD, Franklin BD, Watson MC, Raynor DK. User testing to improve retrieval and comprehension of information in guidelines to improve medicines safety. J Patient Saf. 2020;18(1):e172–e179. doi: 10.1097/PTS.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Choi D, Yu CH. Effective web-based clinical practice guidelines resources: recommendations from a mixed methods usability study. BMC Prim Care. 2023;24(1):29. doi: 10.1186/s12875-023-01974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong E, Taylor Z, Thompson J, Tuthill D. A simplified gentamicin dosing chart is quicker and more accurate for nurse verification than the BNFc. Arch Dis Child. 2009;94(7):542–545. doi: 10.1136/adc.2007.137026. [DOI] [PubMed] [Google Scholar]
- 30.Franklin BD, Birch S, Savage I, Wong I, Woloshynowych M, Jacklin A, et al. Methodological variability in detecting prescribing errors and consequences for the evaluation of interventions. Pharmacoepidemiol Drug Saf. 2009;18(11):992–999. doi: 10.1002/pds.1811. [DOI] [PubMed] [Google Scholar]
- 31.Westbrook JI, Li L, Lehnbom EC, Baysari MT, Braithwaite J, Burke R, et al. What are incident reports telling us? A comparative study at two Australian hospitals of medication errors identified at audit, detected by staff and reported to an incident system. Int J Qual Health Care. 2015;27(1):1–9. doi: 10.1093/intqhc/mzu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sari AB, Sheldon TA, Cracknell A, Turnbull A. Sensitivity of routine system for reporting patient safety incidents in an NHS hospital: retrospective patient case note review. BMJ. 2007;334(7584):79. doi: 10.1136/bmj.39031.507153.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.