Abstract
Background
Little is known about the etiology, clinical presentation, management, and outcome of central nervous system (CNS) infections in Indonesia, a country with a high burden of infectious diseases and a rising prevalence of HIV.
Methods
We included adult patients with suspected CNS infections at two referral hospitals in a prospective cohort between April 2019 and December 2021. Clinical, laboratory, and radiological assessments were standardized. We recorded initial and final diagnoses, treatments, and outcomes during 6 months of follow-up.
Results
Of 1051 patients screened, 793 were diagnosed with a CNS infection. Patients (median age 33 years, 62% male, 38% HIV-infected) presented a median of 14 days (IQR 7–30) after symptom onset, often with altered consciousness (63%), motor deficits (73%), and seizures (21%). Among HIV-uninfected patients, CNS tuberculosis (TB) was most common (60%), while viral (8%) and bacterial (4%) disease were uncommon. Among HIV-infected patients, cerebral toxoplasmosis (41%) was most common, followed by CNS TB (19%), neurosyphilis (15%), and cryptococcal meningitis (10%). A microbiologically confirmed diagnosis was achieved in 25% of cases, and initial diagnoses were revised in 46% of cases. In-hospital mortality was 30%, and at six months, 45% of patients had died, and 12% suffered from severe disability. Six-month mortality was associated with older age, HIV, and severe clinical, radiological and CSF markers at presentation.
Conclusion
CNS infections in Indonesia are characterized by late presentation, severe disease, frequent HIV coinfection, low microbiological confirmation and high mortality. These findings highlight the need for earlier disease recognition, faster and more accurate diagnosis, and optimized treatment, coupled with wider efforts to improve the uptake of HIV services.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-023-02170-0.
Keywords: CNS infection, Indonesia, Adult, Diagnosis, Management, Outcome
Introduction
Central nervous system (CNS) infections cause significant morbidity and mortality, especially in low-resource settings. The Global Burden of Disease study reported an estimated 2.82 million cases of meningitis globally with 318,400 deaths in 2016 [1]. CNS infections can present in several clinical syndromes, most frequently meningitis, followed by encephalitis, brain abscesses and myelitis [2, 3].
There are many barriers in the diagnosis and treatment of CNS infections: initial signs and symptoms are often nonspecific, leading to patient and doctor delays; physicians may be reluctant to perform diagnostic lumbar punctures (LPs); cerebral imaging, CSF analysis and specific microbiological tests are often unavailable, especially in resource-limited settings; and even with appropriate diagnostic modalities and appropriate treatment, morbidity and mortality remain high. Further context-specific research is needed to understand these barriers and take targeted actions to improve diagnosis, treatment and patient outcomes [4].
Indonesia, a lower-middle-income country with the world’s fourth largest population (275 million), continues to have a high burden of infectious diseases. Little is known regarding the etiology, management and outcomes of CNS infections and their association with the rising number of HIV cases in the country. Previous studies have been limited due to the relatively small number of participants being from a single center and/or focused on one specific pathogen [2, 5–9].
To address these knowledge gaps, we conducted a prospective cohort study in two large referral hospitals to examine the clinical presentation, etiology, treatment and outcome of patients with suspected CNS infections. As diagnosing CNS infections is difficult and timely treatment is vital, we also examined how clinical diagnosis evolved over time in these patients.
Methods
Study design and setting
We included adult patients with suspected CNS infections. Patients who refused to give consent were excluded. This prospective cohort study was conducted at two tertiary referral hospitals, Dr. Cipto Mangunkusumo Hospital in Jakarta and Hasan Sadikin Hospital in Bandung, West Java. Both hospitals serve patients in the two most populated provinces in Indonesia, with approximately 2000–3000 emergency department visits and 40,000–60,000 outpatient visits each month per hospital [10]. Patients are either referred from lower-level hospitals or primary health centers, and approximately two-thirds are self-referrals [11]. Both hospitals have neurologists with specific expertise on CNS infections and routine and microbiological CSF tests available, and both hospitals offer HIV services. Brain CT and MRI are available, although access may be constrained due to costs or limited capacity. Nearly all patients attending the hospitals are covered by the National Health Insurance scheme. Ethical approval was obtained from the institutional review board of Universitas Indonesia (No. 1365/UN.2.F1/ETIK/2018). Patients or their proxy provided written informed study consent, which included sample storage for future studies.
Study procedures
We recruited consecutive patients ≥ 18 years of age who presented between April 2019 and December 2021 with suspected CNS infections, as judged by the attending physicians. Participants were recruited from the emergency room, neurology and HIV outpatient clinic, and medical and neurology wards. All participants underwent a standardized diagnostic work-up with systematic recording of signs and symptoms; neurological examination, including functional status using the modified Rankin scale [12]; and blood examination, including complete blood count (CBC), serum glucose, serum electrolytes, liver functions, creatinine, and HIV testing (followed by CD4 + T-cell count for those who tested positive). Routine CSF examination (cells, protein, glucose) was performed, except if LP was deemed contraindicated [13]. Clinical and ancillary data were documented in an electronic study database. We stored baseline CSF, blood samples, urine samples, and any isolated microbial pathogens for future study.
Microbiological testing
A minimum of 10 mL cerebrospinal fluid (CSF) was collected unless contraindicated. Three milliliters was used for routine CSF analysis (leukocytes, protein, glucose), India ink, and Gram staining; bacterial culture was performed at the physician’s request. The remaining CSF (minimum 6 mL) was used for TB diagnostics, for which CSF was concentrated by centrifugation (3000 ×g for 15 min), and CSF sediment was used for microscopy, GeneXpert MTB/Rif, and mycobacteria growth indicator tube (MGIT or MODS) followed by Lowenstein-Jensen or Ogawa subculture. Cryptococcal antigen (CrAg) was measured in CSF from HIV-infected patients using a lateral flow assay (LFA).
In individual cases, attending physicians might decide on additional testing, including CSF real-time PCR for viral infection (herpes simplex virus, varicella-zoster virus, cytomegalovirus); fungal or bacterial culture; quantitative serum toxoplasma IgG for CNS toxoplasmosis; Rapid Plasma Reagin (RPR) and Treponema Pallidum Hemagglutination Assay (TPHA) for neurosyphilis; anti-NMDA receptor in suspected autoimmune encephalitis; and cytology assessment for CNS lymphoma.
Radiological evaluation
Brain CT with contrast using a dual-source 128-slice (1.3 mm) scanner was part of routine procedures unless contraindicated. Brain MRI (1.5 T) with contrast was performed on clinical indication only. Neuroimaging assessment was performed systematically by the attending radiologists recording the presence or absence of abnormalities, including meningeal enhancement, hydrocephalus, tuberculoma, encephalitis or cerebritis, abscess, infarction, and herniation. Meningeal enhancement was defined as any pathological contrast enhancement of the meninges with linear or gyri form appearance [14]; hydrocephalus was defined as the distention of lateral cerebral ventricles with Evans ratio > 30% and/or the size of one or both temporal horns > 2 mm [15]; tuberculoma was defined as isohyperdense solitary or multiple lesions with ring, nodular, or irregular nonhomogeneous contrast enhancement [16]. Encephalitis or cerebritis refers to poorly marginated cortical or subcortical hypodensity with variable patterns of contrast enhancement [17]; abscesses as round, oval, or multiloculated space-occupying lesions with ring enhancement appearance [17]; infarctions as hypodense lesions on CT scan or variable intensity based on the age of infarction on MRI in a vascular distribution [15]; and herniation as parenchymal displacement to the adjacent structures due to the mass effect [18]. All participants underwent routine chest X-rays, with evaluation for signs of pulmonary TB [19].
Evaluation of diagnostic process and treatment
Diagnoses were evaluated and reviewed by the study team at three time points. First, an ‘initial diagnosis’ was made < 24 h after initial evaluation by the neurologist and used to guide initial empirical treatment and further investigations. (2) A ‘discharge diagnosis’ was made at the time of hospital discharge or in-hospital death, updated by the study team according to further testing or clinical judgment. Possible treatment adjustments during hospitalization were recorded, and the discharge diagnosis was used to guide treatment and follow-up after discharge. (3) Finally, a ‘final diagnosis’ was made retrospectively by the clinical investigators taking into account all information available (e.g., including retrospective CSF testing). Throughout the process, as much as possible, standardized case definitions were followed (Table 1).
Table 1.
Case definitions
| Final Diagnosis | Definition | Refs. | |
|---|---|---|---|
| CNS tuberculosis | TBM Definite | Positive CSF microscopy for acid-fast bacilli, Mycobacterium tuberculosis culture, or PCR (Xpert TB) | [20] |
| TBM Probable | TBM research case definition score ≥ 10 (without available CT imaging) or ≥ 12 (with CT), and exclusion of alternative diagnoses | [20] | |
| TBM Possible | TBM research case definition score of 6–9 points (without available CT imaging) or 6–11 points (with CT), and exclusion of an alternative diagnosis | [20] | |
| Tuberculoma | Iso-hyperdense solitary or multiple lesions with ring, nodular, or irregular nonhomogenous contrast enhancement compatible with tuberculous etiology | [16] | |
| Cerebral toxoplasmosis (presumptive) | HIV infection and presence of 1 or more cerebral mass lesions on CT or MRI or IgG antibody to Toxoplasma, and exclusion of an alternative diagnosis | [22] | |
| Cryptococcal meningitis | Positive CSF India ink examination (budding encapsulated yeasts), or Cryptococcus neoformans culture, or cryptococcal antigen lateral flow antigen test | [23] | |
| Viral encephalitis (CMV, HSV, VZV, or other viruses) | Definite | Positive CSF PCR for HSV, CMV, VZV, or other viruses with the present of clinical syndrome | [21] |
| Probable |
Clinical syndrome: Altered mental status plus 2 or more of the following: fever ≥ 38 °C; seizures; new onset of focal neurologic findings; CSF WBC count ≥ 5/mm3; abnormality of brain parenchyma on neuroimaging suggestive of encephalitis; abnormal EEG |
||
| Bacterial meningitis | Definite | Meningitis with detection of bacteria in CSF by microscopy or culture | [40] |
| Probable | Compatible clinical syndrome, positive blood culture, plus 1 of the following CSF changes: > 5 leukocytes/mm3; glucose < 40 mg/dL or CSF/blood glucose ratio < 0.5; or protein > 100 mg/dL | [40] | |
| Bacterial brain abscess | Definite | Evidence of brain abscess seen during surgery or histopathological examination | [41] |
| Radiologically confirmed | Brain CT and/or MRI with supporting evidence of brain abscess; and compatible clinical syndrome (headache, fever, localized neurological signs and/or impaired consciousness | ||
| Neurosyphilis | Positive blood VDRL or TPHA and reactive CSF VDRL and/or elevated CSF cell count or elevated CSF protein or focal neurological signs plus exclusion of alternative diagnoses | [42] | |
| CNS infection of unknown etiology | Suspected CNS infection based on combination of compatible clinical signs, CSF findings, and brain CT scan, and no other diagnosis established | Con-sensus | |
TBM berculous meningitis, CSF Cerebrospinal fluid, PCR Polymerase chain reaction, MTB Mycobacterium tuberculosis, RIF Rifampicin, CT Computed tomography, MRI Magnetic resonance imaging, HSV Herpes simplex virus, VZV Varicella-zoster virus, CMV Cytomegalovirus, WBC White blood cells, EEG Electroencephalography, RPR Rapid plasma regain, VDRL venereal disease research laboratory, CNS Central nervous system
Initial treatment, based on clinical judgment and available baseline test results, was given in accordance with national/hospital guidelines (Supplement 1). Anti-retroviral (ART) initiation was performed according to the national guidelines 4–8 weeks after the start of therapy for TB meningitis, 2 weeks after the start of therapy for toxoplasma encephalitis, and 4–6 weeks after the start of therapy for cryptococcal meningitis. All patients with TB meningitis received adjunctive corticosteroids.
Follow-up
We documented in-hospital and 6 month survival based on medical records and phone calls to patients and relatives. For patients who were alive at discharge or at 6 months, we recorded the Glasgow Coma Scale (GCS) and modified Rankin scale (mRS), a 6-point disability scale that measures dependence in performing daily activities [12]. Patients discharged from the hospital will receive monthly phone calls until the 6 month. Three unanswered calls will be considered lost to follow-up, and a social worker will conduct home visits to reach the patients. In the event of a patient's death during the follow-up period, the cause of death will be investigated using the WHO verbal autopsy method or by interviewing the family during the home visit.
Statistical analysis
We made comparisons between groups according to HIV status using Chi-square/Fisher exact (categorical data) or parametric/nonparametric tests (numerical data). Survival analysis was presented using Kaplan‒Meier curves with log-rank tests, and univariate and multivariate Cox regression analyses were used to identify factors associated with death. All statistical analyses were performed using SPSS 23 for Windows (SPSS Inc., Chicago, IL). All figures were created with RStudio in R 4.4.2.
Results
Clinical characteristics and patient mortality
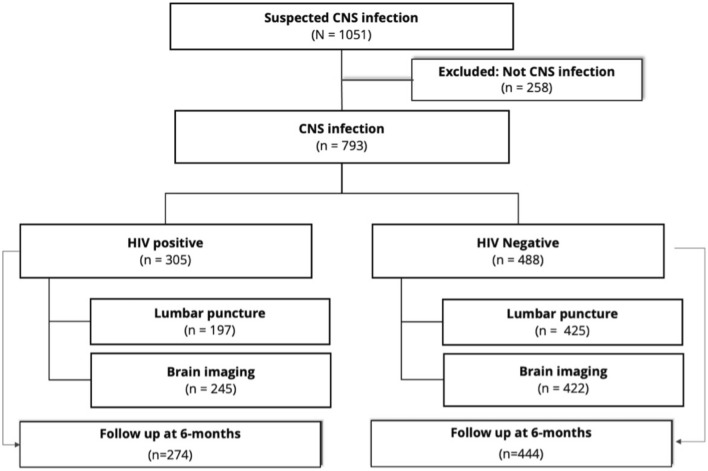
We screened 1051 patients with clinically suspected CNS infection for the study. We excluded 258 patients due to alternative diagnoses, such as primary headache, autoimmune-related neurological disorders, secondary syphilis, psychiatric disorders, or stroke. A total of 793 patients were enrolled, of whom 78% underwent lumbar puncture, and 84% had brain imaging (Fig. 1). The median age was 33 (IQR 26–44) years, and the majority were male (62%) and self-referred (59%). The median time between the onset of neurological symptoms and hospital presentation was 14 days (IQR 7–30). The most frequent symptoms were fever, headache, seizures, lowered consciousness, motor abnormalities, and cranial nerve palsies (Table 2). HIV infection was present in 305 patients (38%), mostly at an advanced stage, with median CD4 T-cell counts of 22 (IQR 13–48) cells/mL among those newly diagnosed with HIV and 36 (IQR 12–120.5) cells/mL among those already known to have HIV. Brain imaging and lumbar puncture were performed a median of 24 and 26 h after admission, respectively. CSF examination and neuroimaging results are summarized in Table 2.
Fig. 1.
Patient flowchart
Table 2.
Baseline characteristics (stratified by HIV status)
| Variables | Data completeness | HIV positive (n = 305) | HIV negative (n = 488) | p |
|---|---|---|---|---|
| Age (years), median (IQR) | 100% | 36 (29.5–42) | 32 (24–46) | 0.02 |
| Male, n (%) | 100% | 238 (78) | 251 (51) | < 0.001 |
| HIV diagnosis and treatment | ||||
| New HIV diagnosis, n (%) | 100% | 43 (14) | - | |
| Known HIV, not on ART | 95.4% | 146 (59) | - | |
| Known HIV, history of recent ART | 95.4% | 102 (41) | - | |
| TB treatment, n(%) | ||||
| On admission | 100% | 42 (14) | 83 (17) | 0.1 |
| History of previous TB treatment | 100% | 61 (20) | 69 (14) | 0.1 |
| Symptoms | ||||
| Time since first neurological symptom (days), median (IQR) | 98.1% | 21 (7–60) | 14 (6–30) | < 0.001 |
| Fever, n (%) | 100% | 151 (50) | 339 (70) | < 0.001 |
| Headache, n (%) | 100% | 199 (65) | 330 (68) | 0.5 |
| Loss of consciousness, n (%) | 99.7% | 159 (52) | 344 (71) | < 0.001 |
| Seizures, n (%) | 100% | 55 (18) | 113 (23) | 0.09 |
| Behavioral change, n (%) | 97% | 56 (19) | 121 (26) | 0.02 |
| Vomiting, n (%) | 100% | 74 (23) | 126 (25) | 0.7 |
| Chronic cough, n (%) | 100% | 72 (24) | 144 (30) | 0.1 |
| Signs | ||||
| Body temperature > 38 (oC) | 99.6% | 43 (14) | 78 (16) | 0.5 |
| GCS, median (IQR) | 100% | 14 (12–15) | 13 (11–15) | < 0.001 |
| Neck stiffness, n (%) | 98.6% | 116 (38) | 294 (61) | < 0.001 |
| Cranial nerve palsy, n (%) | 99.7% | 168 (55) | 312 (64) | 0.01 |
| Motor abnormality, n (%) | 100% | 164 (54) | 290 (59) | 0.1 |
| Lumbar puncture & CSF analysis | ||||
| Time to lumbar puncture (hours), median (IQR) | 100% | 29 (14–68) | 24 (14–68) | 0.5 |
| Opening pressure (cmH2O), median (IQR) | 31% | 14 (8–20) | 12 (10–17) | 0.8 |
| Leukocytes (cells/uL), median (IQR) | 99.8% | 7 (2–24) | 32 (4–130) | < 0.001 |
| Neutrophils (%), median (IQR) | 99.7% | 18.5 (0–50) | 24 (5–53.8) | 0.1 |
| Protein (mg/dL), median (IQR) | 92% | 56 (40–122.3) | 125 (58–278) | < 0.001 |
| CSF glucose ratio, median (IQR) | 93% | 0.5 (0.4–0.6) | 0.4 (0.2–0.6) | < 0.001 |
| Blood | ||||
| Moderate/severe anemiaa, n (%) | 99.2% | 76 (25.4) | 90 (18.4) | 0.02 |
| Leukocytes (× 109/L), median (IQR) | 99.1% | 6.4 (4.5–9.4) | 10.9 (7.7–14.6) | < 0.001 |
| Thrombocytes (× 109/L), median (IQR) | 99.1% | 253 (184–318) | 300 (214–386) | < 0.001 |
| Moderate/severe hyponatremiab, n (%) | 96.7% | 103 (36) | 198 (41) | 0.1 |
| CD4 count (cells/mL), median (IQR) | 62.9% | 32 (12.3–98.8) | - | |
| Brain imaging findings | ||||
| Meningeal enhancement, n (%) | 100% | 59 (19.3) | 190 (38.9) | < 0.001 |
| Infarct, n (%) | 100% | 41 (13.4) | 76(16) | 0.4 |
| Hydrocephalus, n (%) | 100% | 34 (11) | 115 (24) | < 0.001 |
| Tuberculoma, n (%) | 100% | 8 (2.6) | 49 (10) | 0.001 |
| Herniation, n (%) | 100% | 26 (9) | 8 (2) | < 0.001 |
| Brain abscess, n (%) | 100% | 30 (9.8) | 26 (5.3) | 0.02 |
| Encephalitis, n (%) | 100% | 70 (23) | 24 (4.9) | < 0.001 |
| Mortality | ||||
| At discharge | 100% | 82 (27) | 152 (31) | 0.2 |
| At 6 months | 90.5% | 131 (48) | 226 (51) | 0.6 |
ART antiretroviral therapy, IQR interquartile range, GCS Glasgow coma scale, CSF cerebrospinal fluid
aModerate/severe anemia defined as hemoglobin level < 10 mg/dL
bModerate/severe hyponatremia defined as serum sodium level < 130 mEq/L
Final diagnosis
Among HIV-uninfected patients (n = 488), CNS tuberculosis was the most common etiology (60%), while viral (8%) and bacterial disease (4%) were uncommon. Among HIV-infected patients (n = 305), cerebral toxoplasmosis was the most common diagnosis (41%), followed by CNS tuberculosis (19%), neurosyphilis (15%) and cryptococcal meningitis (10%; Table 3). Acute bacterial meningitis, bacterial abscess and viral encephalitis were uncommon in both groups. A microbiologically confirmed diagnosis was established in 198 of 793 patients with a suspected CNS infection (25%); a final diagnosis was reached by consensus or international guidelines in 58% [20–23]; and no clear etiology was established in 17% (Table 3). Among 325 patients diagnosed with TB meningitis, microbiological confirmation was reached in 154 (47%) patients by Xpert MTB/RIF (n = 58), culture (n = 45) and/or microscopy (n = 15).
Table 3.
CNS infections, final diagnosis
| No | Diagnosis | HIV status | Total (n = 793) | |
|---|---|---|---|---|
| HIV positive (n = 305) | HIV negative (n = 488) | |||
| 1 | Tuberculosis | 58 (19) | 291(60) | 349 (44) |
| Definite TBM | 15 | 139 | 154 | |
| Probable TBM | 40 | 131 | 171 | |
| Tuberculoma | 3 | 21 | 24 | |
| 2 | Cerebral toxoplasmosis | 127 (41) | 0 (0) | 127 (16) |
| 3 | Neurosyphilis | 46 (15) | 3 (1) | 49 (6) |
| 4 | Viral encephalitis | 3 (1) | 38 (8) | 41 (5) |
| Definite | 0 | 5 | 5 | |
| Clinically suspected | 3 | 34 | 37 | |
| 5 | Cryptococcal meningitis | 29 (10) | 1 (0) | 30 (4) |
| 6 | Brain abscess | 2 (1) | 23 (5) | 25 (3) |
| Definite | 0 | 4 | 4 | |
| Radiologically suspected | 2 | 19 | 21 | |
| 7 | Bacterial meningitis | 0 (0) | 20 (4) | 20 (3) |
| Definite | 0 | 5 | 5 | |
| Clinically suspected | 0 | 15 | 15 | |
| 8 | Other CNS infectiona | 2 (1) | 13 (3) | 15 (2) |
| 9 | Suspected CNS infection, unknown etiology | 38 (12) | 99 (20) | 137 (17) |
aOther infections included infectious myelitis, progressive multifocal leukoencephalopathy, cerebral aspergillosis, and subdural empyema
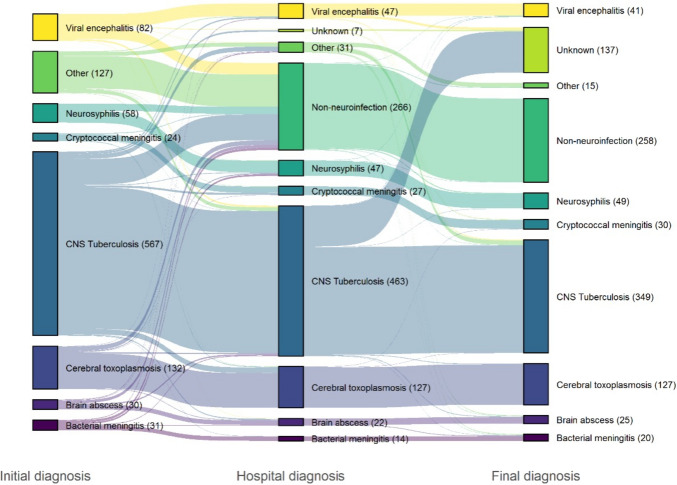
The most common initial diagnosis was CNS tuberculosis (54%; Fig. 2). Of those who were initially diagnosed with CNS tuberculosis (n = 567), CNS infection was deemed unlikely at discharge in 79 (14%), and the final diagnosis was another CNS infection in 52 (9%). Conversely, in 18 patients (3%), CNS tuberculosis was not considered on admission but was diagnosed later during hospitalization with further test results and/or after exclusion of other diagnoses (Fig. 2). A correct initial diagnosis of cerebral toxoplasmosis, cryptococcal meningitis, and neurosyphilis was more likely based on cerebral imaging, serum IgG anti-toxoplasma, CSF cryptococcal antigen (CrAg) immunoassay test and VDRL, respectively. The median time between hospital presentation and appropriate treatment based on the initial diagnosis was 1 day (excluding those who were already on treatment for pulmonary TB when entering the cohort).
Fig. 2.
Patient diagnoses over time The initial diagnosis was recorded within 24 h of admission, the hospital diagnosis was determined after hospitalization, and the final diagnosis was determined after all test results were gathered. Each bar is connected by the same color ribbon to illustrate changes in diagnosis over time. The height of the bars represents the number of patients for each diagnosis.
Treatment outcome and prognostic factors
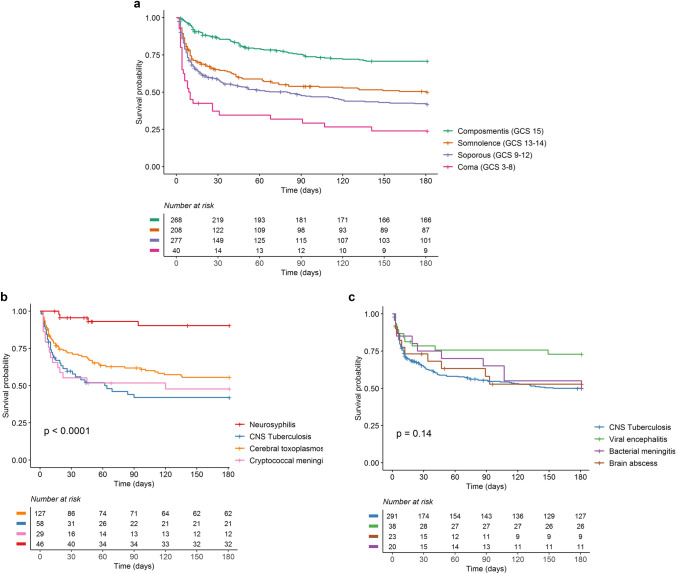
The overall in-hospital mortality was 30% (234/793) and was highest for cryptococcal meningitis (47%, 14/30), followed by bacterial meningitis (35%, 7/20) and CNS TB (32%, 112/349). Among 721 (91%) participants for whom 6 month outcome data were available, 357 (50%) died, a median of 10 days after hospital admission. Mortality was higher and occurred earlier for those presenting with more severe neurological disease (Fig. 3a). Mortality appeared highest for those with unknown etiology, followed by TB meningitis in both groups (Fig. 3b, c). Among 137 patients with suspected CNS infection of unknown etiology, in-hospital and 6-month mortality was 35% and 52%, respectively. After exclusion of patients with neurosyphilis (all of whom survived), the risk of death was associated with older age, HIV infection, decreased consciousness at the time of admission, and other clinical (fever, headache), radiological (hydrocephalus) and CSF markers (pleocytosis and lower CSF glucose ratio) indicating severe disease (Table 4).
Fig. 3a.
Patient mortality according to Glasgow coma scale at time of diagnosis b. Top 4 etiology in HIV positive. c. Top 4 etiology in HIV negative
Table 4.
Factors associated with 6 months mortality (n = 744)
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Clinical | ||||
| Age (per 10 year) increase | 1.2 (1.1–1.3) | < 0.001 | 1.1 (1–1.3) | 0.01 |
| Male | 1.0 (0.8–1.2) | 0.7 | 0.7 (0.5–0.9) | 0.04 |
| HIV positive | 1.1 (0.8–1.3) | 0.5 | 1.8 (1.3–2.6) | 0.002 |
| Symptoms | ||||
| Time since first neurological symptoms (per 1 day increase) | 1.0 (0.9–1.0) | 0.8 | 1.0 (0.9–1.0) | 0.4 |
| Fever | 1.5 (1.2–1.9) | < 0.001 | 1.5 (1.1–2.1) | 0.02 |
| Headache | 0.9 (0.7–1.1) | 0.4 | 0.9 (0.7–1.3) | 0.6 |
| Loss of consciousness | 2.2 (1.7–2.9) | < 0.001 | 2.6 (1.7–3.9) | < 0.001 |
| Seizure | 0.9 (0.7–1.1) | 0.3 | 0.9 (0.7–1.4) | 0.8 |
| Behavioral changes | 0.9 (0.7–1.2) | 0.6 | 1.0 (0.7–1.4) | 0.9 |
| Vomiting | 0.9 (0.7–1.1) | 0.4 | 0.8 (0.6–1.2) | 0.3 |
| Chronic cough | 1.2 (0.9–1.5) | 0.2 | 0.9 (0.6–1.2) | 0.4 |
| Signs | ||||
| Body temperature > 38 (oC) | 1.4 (1.1–1.9) | 0.01 | 1.2 (0.9–1.8) | 0.3 |
| GCS (per 1-point increase) | 0.9 (0.9–0.9) | < 0.001 | 1.0 (1.0–1.1) | 0.6 |
| Neck stiffness | 1.6 (1.3–2.0) | < 0.001 | 0.9 (0.6–1.3) | 0.6 |
| Cranial nerve palsy, n (%) | 1.4 (1.1–1.7) | < 0.001 | 1.1 (0.8–1.6) | 0.5 |
| Motor abnormality(s), n (%) | 1.4 (1.1–1.8) | < 0.001 | 1.3 (0.9–1.7) | 0.04 |
| Lumbar puncture & CSF analysis | ||||
| Opening pressure (per 1-point cmH2O increase) | 1.0 (0.9–1.0) | 0.3 | NA | NA |
| Leukocytes (per 1 cells/µL increase) | 1.0 (0.9–1.0) | 0.2 | 1.0 (0.9–1.0) | 0.04 |
| Neutrophils (per 10% increase) | 1.0 (0.9–1.0) | 0.1 | 1.0 (0.9–1.0) | 0.2 |
| Protein (per 10 mg/dL increase) | 1.0 (0.9–1.0) | 0.3 | 1.0 (0.9–1.0) | 0.9 |
| CSF glucose ratio (per 0.10 increase) | 0.4 (0.2–0.7) | < 0.001 | 0.3 (0.1–0.6) | 0.001 |
| Blood | ||||
| Moderate/severe anemia | 1.5 (1.2–1.9) | < 0.001 | 1.1 (0.8–1.5) | 0.7 |
| Leukocytes (per 109/L increase) | 1.0 (0.9–1.0) | 0.4 | 1.1 (1–1.02) | 0.1 |
| Thrombocytes (per 109/L increase) | 1.0 (0.9–1.0) | 0.3 | 1.0 (0.9–1.0) | 0.8 |
| CD4 (per 10 cells/mL increase) | 1.0 (0.9–1.0) | 0.1 | NA | NA |
| Brain imaging findings | ||||
| Meningeal enhancement | 1.1 (0.9–1.3) | 0.5 | 1 (0.7–1.3) | 0.8 |
| Infarct | 1.1 (0.8–1.4) | 0.7 | 1.1 (0.7–1.5) | 0.8 |
| Hydrocephalus | 1.5 (1.2–1.9) | < 0.001 | 1.5 (1.1–2.1) | 0.01 |
| Tuberculoma | 0.8 (0.5–1.2) | 0.3 | 0.9 (0.5–1.6) | 0.7 |
| Herniation | 0.8 (0.5–1.4) | 0.5 | 1.2 (0.3–4.7) | 0.8 |
| Brain abscess | 0.5 (0.3–0.9) | 0.01 | 0.5 (0.2–1.5) | 0.2 |
| Encephalitis | 0.8 (0.6 -1.2) | 0.3 | 0.9 (0.6–1.5) | 0.8 |
GCS glasgow coma scale, CSF cerebrospinal fluid, NA Not applicable
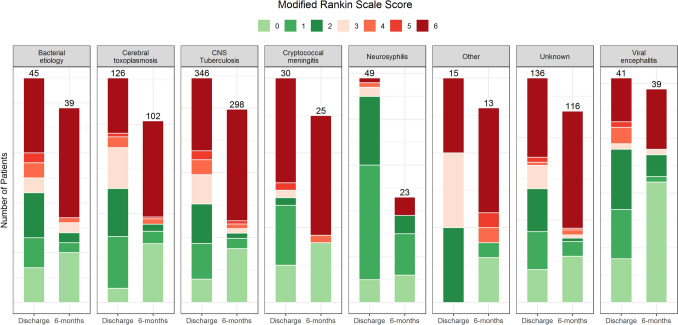
Many patients who were alive after 6 months of follow-up suffered from long-term disabilities (Fig. 4). For instance, of 126 CNS tuberculosis patients (median age 29) with available MRS data at 6 months, 19 (15%) had moderate or severe disabilities and required support from others for their daily activities.
Fig. 4.
Functional outcome using the modified Rankin scale Functional outcome for those with available modified Rankin Scale data at discharge (99.4%) and after 6 months (82.6%), stratified by final diagnosis. MRS 0 = no symptoms; 1 = no significant disability; 2 = slight disability, 3 = moderate disability, 4 = moderately severe disability, 5 = severe disability, 6 = dead. The numbers above the bar refer to the total number of patients with available data at each time point.
Discussion
We prospectively evaluated all patients who presented with a suspected CNS infection over a 32-month period in two referral hospitals in Indonesia. Patients were mostly young and severely ill on presentation, and one-third suffered from advanced HIV infection. CNS tuberculosis was the most common final diagnosis overall, cerebral toxoplasmosis was the most common among HIV-infected patients, and viral and bacterial meningitis were uncommon. There were substantial diagnostic challenges, with only 25% of patients receiving a definite diagnosis. Establishing a timely diagnosis proved challenging, often leading to delays in the initiation of appropriate treatment. The overall in-hospital mortality was 30%, the six-month mortality was 45%, and significant disabilities were common among the patients who survived.
Patients in this cohort presented late and with very severe disease, similar to previous smaller studies from this setting [11], and often more severe than in studies from other countries. For instance, in neuro-infection cohorts in Vietnam [24] and England [3], 38% and 55% of patients (versus 66% in our cohort) were unconscious, and 4% and 36% had focal neurological deficits (versus 58% in our cohort). This difference might be attributed to challenges in timely access or referral to appropriate care. Despite being a tertiary-referral hospital, most patients were self-referred, and our group has established that patients with TB meningitis often visit many formal and informal health providers before receiving an appropriate diagnosis [25]. This underlines a need for interventions to increase early diagnosis and treatment, for instance, through enhancing health literacy among patients and health professionals in the community or facilitating timely referral to higher-level care.
Tuberculosis was the most common cause of CNS infection, similar to previous studies from Indonesia [11] and India [26], where tuberculosis accounted for 92/274 (34%) and 205/401 (51%) of cases, respectively. Obviously, this rate is much higher than that found in countries with a low tuberculosis burden, such as Australia (1.8% of 725 CNS infections) [2], Singapore (20% of 110 cases) [27], and Vietnam (14% of 617 cases) [28]. In those studies, viral and bacterial meningitis were more common, similar to studies from Africa, Europe, and the United States [29]. We hypothesize that many patients with acute CNS infections, such as those caused by S. pneumoniae or herpes simplex virus, may die at home and that those with self-resolving disease (such as those caused by entero- or arboviruses) might not reach our facilities either.
Establishing the etiology of CNS infections is difficult. Despite a thorough microbiological evaluation, microbiological confirmation was only made in 25% of cases. This is not unique for lower-resource settings. For instance, in a large CNS infection cohort from Australia and a meningitis cohort from the UK, 29% and 42% were of unknown etiology [2, 30]. Higher microbiological confirmation was reported from Singapore [27], Vietnam [28], and Thailand [31]; at least one pathogen was found in 60%, 52%, and 48% of cases, respectively. It is challenging to identify and diagnose patients with a rare but potentially life-threatening CNS infection in a timely manner amidst the multitude of patients presenting with nonspecific symptoms. Neuroimaging and CSF analysis may appear normal in early disease, and CSF testing is not sufficiently sensitive for many pathogens [32, 33]. In contrast to the difficulty of finding pathogens in the CNS, a report from a large cohort of acute febrile illnesses in Indonesia showed a positive laboratory finding in 67.5% of participants, mostly from blood samples [34]. Metagenomic next-generation sequencing (NGS) is now introduced as a promising tool to increase the diagnostic yield for CNS infections [35, 36].
Mortality in this study was high, and many survivors suffered from significant disabilities. Consistent with previous studies [37], HIV infection was strongly associated with poor outcome. This is likely due to the advanced stage of HIV in our cohort, with very low CD4 counts, both among those newly diagnosed with HIV and those already known with HIV (but either having dropped out of care or infected with resistant HIV viruses). This highlights the importance of earlier HIV testing and efforts to improve retention, adherence and viral load testing for patients on ART.
The strengths of the study are that it is a large cohort comprising all patients with suspected CNS infection (rather than with one particular infection, such as tuberculous or cryptococcal meningitis) recruited in two reference centers. Another strength is its comprehensive, largely standardized diagnostic workup and use of clear diagnostic classification. The study has several limitations. First, we were not able to apply MRI and the latest molecular tools to establish disease etiology, but this reflects the often-restricted diagnostic capabilities in hospitals in high-burden countries. Second, our findings may not be representative of other, smaller hospitals in Indonesia with different patient populations and etiologies. Both our hospitals have been engaged in clinical research related to TB meningitis and other CNS infections for years, and this has increased awareness and quality of patient management [23, 38, 39]. HIV testing, lumbar puncture rates, CSF analysis and treatment are even more challenging in smaller hospitals, and mortality might even be higher. Further research is therefore needed to help improve triage and referral, early diagnosis, appropriate treatment and better supportive care for patients with CNS infections in high-burden countries such as Indonesia.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 16 KB) Patients with suspected or confirmed TBM received an intensive anti-TB fixed drug combination (FDC) containing rifampicin 150 mg/isoniazid 75 mg/pyrazinamide 400 mg/ethambutol 275 mg based on body weight and an additional dose of 300 mg rifampicin for 2 months, followed by a 10 month maintenance phase of rifampicin and isoniazid at the same dose. We followed the TB National Guideline to define the daily dose according to the patients’ body weight. The Bandung site also implemented a routine of high-dose rifampicin (20 mg/kg) for the initial 30 days of intensive treatment. Tapered-dose dexamethasone for 6–8 weeks was also given as the adjunctive treatment (reference). Suspected bacterial meningitis was treated with ceftriaxone IV 4 g/d for 2 weeks and dexamethasone 20 mg/d for 4 days. Acute viral encephalitis was given aciclovir 10 mg/kg/d for 2 weeks in suspected HSV as etiology or ganciclovir 5 mg/kg/12 h for 2 weeks in confirmed CMV. Probable cerebral toxoplasmosis was initiated with pyrimethamine 200 mg/d followed by 50–75 mg/d and clindamycin 2400 mg/d for 6 weeks. Definite cryptococcal meningitis received amphotericin-B IV 0.7-1 mg/kg/d and fluconazole 800 mg/d for 2 weeks. Fluconazole IV 1200 mg/d for 2 weeks was given as an alternative if amphotericin-B was not available. The neurosurgeon was consulted in case of a suspected bacterial brain abscess for abscess drainage, and this was empirically treated with Ceftriaxone IV 4 g/d and Metronidazole 1500 mg/d for 4 weeks (8 weeks if not surgically evacuated). In a suspected case of neurosyphilis, ceftriaxone IV 2 g/d for 2 weeks was administered. Treatment was then adjusted based on the definite diagnosis.
Acknowledgements
We thank Rubiah Adawiyah, Gina Nadia Mokogita, Rani Trisnawati, and Fanny Despitawanda, for data collection and extending follow-up after discharge; neurology residents, for monitoring patients and the director of Hasan Sadikin General Hospital, for accommodating the research.
Author contribution
KM, SD, EA and PWA performed statistical analyses. FK and DP were the field physician for most of the research period, under the supervision of SD, RE, DI, KM, and AR GLC, A R, AK, RA, DS performed the microbiology and parasitology assays. EY supervised the HIV clinics, REY. was helping in neuroradiology reading, LW supervised the sample processing and archiving, JvI was a consultant on mycobacteriology laboratory. KM, and SD performed the literature search and wrote the first complete draft of the report. RLH, and BA contributed to the idea of the research and to the manuscript. RvC led the research group, and DI and SD, were the Indonesian principal investigator of the cohort. All authors have read and approved the final version of the article.
Funding
This work was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health (Grant Number R01AI145781) and Medical Research Council UK (Grant Number MR/S004963/1). This study was carried out as part of the routine work.
Data and materials availability
Data will be made available upon personal contact to authors.
Declarations
Ethical approval
Ethical approval was obtained from the institutional review board of Universitas Indonesia (No. 1365/UN.2. F1/ETIK/2018).
Consent to participate
Patients or their proxy provided written informed study consent, which included sample storage for future studies. All data analyzed during this study was anonymized prior to analysis.
Consent for publication
Not applicable.
Conflict of interests
The authors: No reported conflicts of interest.
Footnotes
Kartika Maharani and Sofiati Dian contributed equally to this manuscript and are co-first authors.
References
- 1.Collaborators GM. Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(12):1061–1082. doi: 10.1016/S1474-4422(18)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gora H, et al. The epidemiology and outcomes of central nervous system infections in Far North Queensland, tropical Australia; 2000–2019. PLoS ONE. 2022;17(3):e0265410. doi: 10.1371/journal.pone.0265410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granerod J, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 4.Seddon JA, et al. Knowledge gaps and research priorities in tuberculous meningitis. Wellcome Open Res. 2019;4:188. doi: 10.12688/wellcomeopenres.15573.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dian S, Ganiem AR, van Laarhoven A. Central nervous system tuberculosis. Curr Opin Neurol. 2021;34(3):396–402. doi: 10.1097/WCO.0000000000000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mawuntu AHP, et al. Detection of central nervous system viral infections in adults in Manado, North Sulawesi, Indonesia. PLoS ONE. 2018;13(11):e0207440. doi: 10.1371/journal.pone.0207440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susilawathi NM, et al. Streptococcus suis-Associated Meningitis, Bali, Indonesia, 2014–2017. Emerg Infect Dis. 2019;25(12):2235–2242. doi: 10.3201/eid2512.181709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logan C, et al. Presentations and outcomes of central nervous system TB in a UK cohort: the high burden of neurological morbidity. J Infect. 2021;82(1):90–97. doi: 10.1016/j.jinf.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, et al. Epidemiology of patients with central nervous system infections, mainly neurosurgical patients: a retrospective study from 2012 to 2019 in a teaching hospital in China. BMC Infect Dis. 2021;21(1):826. doi: 10.1186/s12879-021-06561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liastuti LD, Sadad AR, et al. (2021) Laporan Akuntabilitas Kinerja Instansi Pemerintah RSUP Nasional Dr. Cipto Mangunkusumo.
- 11.Imran D, et al. Presentation, etiology, and outcome of brain infections in an Indonesian hospital: a cohort study. Neurol Clin Pract. 2018;8(5):379–388. doi: 10.1212/CPJ.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19(12):1497–1500. doi: 10.1161/01.STR.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 13.Engelborghs S, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement. 2017;8:111–126. doi: 10.1016/j.dadm.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smirniotopoulos JG, et al. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27(2):525–551. doi: 10.1148/rg.272065155. [DOI] [PubMed] [Google Scholar]
- 15.Garg RK, Malhotra HS, Jain A. Neuroimaging in tuberculous meningitis. Neurol India. 2016;64(2):219–227. doi: 10.4103/0028-3886.177608. [DOI] [PubMed] [Google Scholar]
- 16.Sanei Taheri M, et al. Central nervous system tuberculosis: an imaging-focused review of a reemerging disease. Radiol Res Pract. 2015;2015:202806. doi: 10.1155/2015/202806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rath TJ, et al. Imaging of cerebritis, encephalitis, and brain abscess. Neuroimaging Clin N Am. 2012;22(4):585–607. doi: 10.1016/j.nic.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Riveros Gilardi B, et al. Types of cerebral herniation and their imaging features. Radiographics. 2019;39(6):1598–1610. doi: 10.1148/rg.2019190018. [DOI] [PubMed] [Google Scholar]
- 19.Bhalla AS, et al. Chest tuberculosis: radiological review and imaging recommendations. Indian J Radiol Imaging. 2015;25(3):213–225. doi: 10.4103/0971-3026.161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marais B, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 21.Venkatesan A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portegies P, et al. Guidelines for the diagnosis and management of neurological complications of HIV infection. Eur J Neurol. 2004;11(5):297–304. doi: 10.1111/j.1468-1331.2004.00856.x. [DOI] [PubMed] [Google Scholar]
- 23.Beardsley J, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374(6):542–554. doi: 10.1056/NEJMoa1509024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabor JJ, et al. Aetiologies and clinical presentation of central nervous system infections in Vietnamese patients: a prospective study. Sci Rep. 2022;12(1):18065. doi: 10.1038/s41598-022-23007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nijman G, et al. Tuberculous meningitis patient pathways and delays to diagnosis in Indonesia: a retrospective cohort study. BMJ Public Health. 2023 doi: 10.1136/bmjph-2023-000052. [DOI] [Google Scholar]
- 26.Kumar D, et al. The epidemiology and clinical spectrum of infections of the central nervous system in adults in north India. Trop Doct. 2021;51(1):48–57. doi: 10.1177/0049475520959905. [DOI] [PubMed] [Google Scholar]
- 27.Zellweger RM, et al. Disentangling etiologies of CNS infections in Singapore using multiple correspondence analysis and random forest. Sci Rep. 2020;10(1):18219. doi: 10.1038/s41598-020-75088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trung HD, et al. Aetiologies of central nervous system infection in Viet Nam: a prospective provincial hospital-based descriptive surveillance study. PLoS One. 2021 doi: 10.1371/journal.pone.0037825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson FC, et al. Epidemiology of central nervous system infectious diseases: a meta-analysis and systematic review with implications for neurosurgeons worldwide. J Neurosurg. 2018;130(4):1107. doi: 10.3171/2017.10.JNS17359. [DOI] [PubMed] [Google Scholar]
- 30.McGill F, et al. Incidence, aetiology, and sequelae of viral meningitis in UK adults: a multicentre prospective observational cohort study. Lancet Infect Dis. 2018;18(9):992–1003. doi: 10.1016/S1473-3099(18)30245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saraya A, et al. Autoimmune causes of encephalitis syndrome in Thailand: prospective study of 103 patients. BMC Neurol. 2013;13:150. doi: 10.1186/1471-2377-13-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purohit M, Mustafa T. Laboratory diagnosis of extra-pulmonary tuberculosis (EPTB) in resource-constrained setting: state of the art, challenges and the need. J Clin Diagn Res. 2015 doi: 10.7860/JCDR/2015/12422.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta PK, et al. Diagnosis of extrapulmonary tuberculosis by PCR. FEMS Immunol Med Microbiol. 2012;66(1):20–36. doi: 10.1111/j.1574-695X.2012.00987.x. [DOI] [PubMed] [Google Scholar]
- 34.Gasem MH, et al. An observational prospective cohort study of the epidemiology of hospitalized patients with acute febrile illness in Indonesia. PLoS Negl Trop Dis. 2020;14(1):e0007927. doi: 10.1371/journal.pntd.0007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Y, et al. Trends and developments in the detection of pathogens in central nervous system infections: a bibliometric study. Front Cell Infect Microbiol. 2022;12:856845. doi: 10.3389/fcimb.2022.856845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bahr NC, et al. Recurrence of symptoms following cryptococcal meningitis—characterizing a diagnostic conundrum with multiple etiologies. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Laarhoven A, et al. Clinical parameters, routine inflammatory markers, and LTA4H genotype as predictors of mortality among 608 patients with tuberculous meningitis in indonesia. J Infect Dis. 2017;215(7):1029–1039. doi: 10.1093/infdis/jix051. [DOI] [PubMed] [Google Scholar]
- 38.Ruslami R, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13(1):27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 39.Dian S, et al. Double-blind, randomized, placebo-controlled phase ii dose-finding study to evaluate high-dose rifampin for tuberculous meningitis. Antimicrob Agents Chemother. 2018 doi: 10.1128/AAC.01014-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tunkel AR, et al. The management of encephalitis: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis. 2008;47(3):303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 41.Lu CH, et al. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. QJM. 2002;95(8):501–509. doi: 10.1093/qjmed/95.8.501. [DOI] [PubMed] [Google Scholar]
- 42.Workowski KA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (DOCX 16 KB) Patients with suspected or confirmed TBM received an intensive anti-TB fixed drug combination (FDC) containing rifampicin 150 mg/isoniazid 75 mg/pyrazinamide 400 mg/ethambutol 275 mg based on body weight and an additional dose of 300 mg rifampicin for 2 months, followed by a 10 month maintenance phase of rifampicin and isoniazid at the same dose. We followed the TB National Guideline to define the daily dose according to the patients’ body weight. The Bandung site also implemented a routine of high-dose rifampicin (20 mg/kg) for the initial 30 days of intensive treatment. Tapered-dose dexamethasone for 6–8 weeks was also given as the adjunctive treatment (reference). Suspected bacterial meningitis was treated with ceftriaxone IV 4 g/d for 2 weeks and dexamethasone 20 mg/d for 4 days. Acute viral encephalitis was given aciclovir 10 mg/kg/d for 2 weeks in suspected HSV as etiology or ganciclovir 5 mg/kg/12 h for 2 weeks in confirmed CMV. Probable cerebral toxoplasmosis was initiated with pyrimethamine 200 mg/d followed by 50–75 mg/d and clindamycin 2400 mg/d for 6 weeks. Definite cryptococcal meningitis received amphotericin-B IV 0.7-1 mg/kg/d and fluconazole 800 mg/d for 2 weeks. Fluconazole IV 1200 mg/d for 2 weeks was given as an alternative if amphotericin-B was not available. The neurosurgeon was consulted in case of a suspected bacterial brain abscess for abscess drainage, and this was empirically treated with Ceftriaxone IV 4 g/d and Metronidazole 1500 mg/d for 4 weeks (8 weeks if not surgically evacuated). In a suspected case of neurosyphilis, ceftriaxone IV 2 g/d for 2 weeks was administered. Treatment was then adjusted based on the definite diagnosis.
Data Availability Statement
Data will be made available upon personal contact to authors.