Abstract
Background
In single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) studies, attenuation artifacts frequently cause false positives, which can be partially overcome by computed tomography attenuation correction (CT-AC) or gated acquisition [gated myocardial perfusion imaging (GMPI)]. The purpose of this study is to evaluate their relative diagnostic performances for coronary artery disease (CAD).
Patients and methods
We enrolled 181 patients who underwent gated SPECT with CT-AC in this study. Two observers who were blinded to the clinical data interpreted the GMPI and CT-AC images. Coronary angiography was considered as the reference standard. The diagnostic efficacy was evaluated based on sex, BMI, and individual coronary arteries.
Results
The diagnostic accuracy of GMPI was higher than that of nonattenuation correction overall, as well as for men, overweight individuals, and right CAD (P < 0.05). Compared with CT-AC, GMPI overall had a higher specificity (96.3 vs. 86.9%, P = 0.014) but the same sensitivity, achieving an increased accuracy and area under the curve (AUC, P > 0.05). For diagnosing right CAD, GMPI had a higher diagnostic efficacy (AUC: 0.733 vs. 0.596, P < 0.001) because of its higher sensitivity (52.0 vs. 26.0%, P = 0.008); for men, the diagnostic efficacy of GMPI was significantly higher than that of CT-AC (AUC: 0.754 vs. 0.681, P = 0.038).
Conclusion
Both CT-AC and GMPI led to an increased diagnostic efficacy compared with nonattenuation correction in differentiating attenuation artifacts from fixed perfusion defects. These improvements were, however, more obvious for GMPI than for CT-AC, especially in men and right CAD.
Keywords: computed tomography attenuation correction, coronary artery disease, gated myocardial perfusion imaging, single-photon emission computed tomography
Introduction
Radionuclide myocardial perfusion imaging (MPI) plays an important role in the diagnosis of coronary artery disease (CAD). However, myocardial perfusion single-photon emission computed tomography (SPECT) images are susceptible to soft-tissue attenuation artifacts (breast attenuation in women and diaphragmatic attenuation in men). If the patient’s stress–rest position remains constant [1], soft-tissue attenuation artifacts usually manifest as fixed perfusion defects, which are indistinguishable from a myocardial infarction (MI). Left ventricular (LV) function parameters gained from gated MPI (GMPI), such as wall motion (WM) and wall thickening, help obtain a better differentiation between MI and attenuation artifacts. Alternatively, attenuation correction (AC) using computed tomography (CT) or a line source is a mathematic algorithm that compensates for the effects of soft-tissue attenuation [2], which makes the count distribution more homogeneous in LV myocardium. The clinical values for identifying the attenuation artifacts by AC and gating have been shown in a number of studies [3–7]. As the two technologies are not mutually exclusive, it is important to understand their relative roles in differentiating MI from attenuation artifacts that are due to fixed defects. Currently, differences in the diagnostic accuracies of CT-AC and GMPI are assessed mainly by the experience of the laboratory, the availability of SPECT/CT, and the variety of accuracies among different hospitals [8]. Relative accuracy evaluations between the two methods in patients with suspected CAD or subgroup analyses based on sex, BMI, and individual coronary arteries have rarely been reported. The aim of this study was to evaluate the relative diagnostic efficacy of GMPI and CT-AC for the detection of CAD using SPECT.
Patients and methods
Patient enrollment
One hundred eighty-one consecutive patients with suspected CAD who underwent gated technetium-99m sestamibi (99mTc-MIBI) SPECT/CT scans at the Third Affiliated Hospital of Soochow University from March 2012 to September 2016 were retrospectively analyzed in our study. Patients with a BMI of at least 24kg/m2 were considered to be overweight according to Chinese guidelines [9]. A coronary angiography (CAG) was considered as the reference standard, and a single-vessel or multivessel stenosis of more than 50% was defined as CAD. The exclusion criteria were as follows: (i) prior percutaneous coronary intervention or a coronary artery bypass graft; (ii) patients with left bundle branch block, a pacemaker, severe arrhythmia, sick sinus syndrome, atrial fibrillation, or paroxysmal tachycardia; (iii) hypotension (blood pressure <90/60 mmHg; 1 mmHg=0.133 kPa); (iv) age below 18 years; or (v) pregnancy. An informed consent was signed by all enrolled patients, and the clinical study was approved by the ethics committee of the Third Affiliated Hospital of Soochow University.
Image acquisition
According to a 2-day standard imaging protocol, a gated stress–rest 99mTc-MIBI (radiochemical purity >95%) MPI with a CT-AC was performed for each patient. Images were acquired 60–90 min after injection of 99mTc-MIBI at a dose of 740–925 MBq for both the stress and rest studies. The exercise stress test was conducted on a bicycle according to the modified Bruce protocol, and the pharmacologic stress test was conducted with a slow intravenous administration of 140 μg/kg/min of adenosine over 6 min [10].
Each patient was placed on the examination bed in a supine position with their arms over their heads. SPECT images were acquired using a 90°-angled dual-head camera (Symbia T16; Siemens Medical Systems, Erlangen, Germany) equipped with a low-energy and high-resolution parallel hole collimator and a 180° orbit from the 45° right anterior oblique to the 45° left anterior oblique positions. The stress–rest GMPI was performed using eight frames per cardiac cycle with an acquisition matrix of 64×64, a magnification of 1.45, and a 20% window centered on the 140 keV peak energy.
Tomographic image acquisition for MPI was performed first, and a low-dose CT chest scan for AC was subsequently performed (voltage: 130 kV; tube current: 100 mAs; thickness: 3 mm; scanning range: ~20 cm; and pitch: 1.0).
Image processing and interpretation
Planar SPECT images were reconstructed using ordered subset expectation maximization (OSEM) with 16 iterations and two subsets, reoriented into short axis, horizontal long axis, and vertical long axis images. The SPECT and CT images would be coregistered manually if there was a misregistration. The aforementioned operation and processing were completed by experienced nuclear imaging technologists.
According to guidelines [10], the nonattenuation correction (NAC) and CT-AC images were interpreted independently by two experienced nuclear physicians based on qualitative visual interpretation. They were blinded to the clinical data (including the CAG results) of the patients. A third expert was called in when there was a disagreement. If there were any fixed perfusion abnormalities when interpreting the NAC images, the QGS software package (QGS 2009; Cedars-Sinai Medical Center, Los Angeles, California, USA) was used to obtain the summed motion score and summed thickening score. WM and thickening were scored using a LV 17-segment model according to the American Heart Association. A scale of 0–5 was used to grade the WM for each segment (0 = normal, 1 = mildly hypokinetic, 2 = moderately hypokinetic, 3 = severely hypokinetic, 4 = akinetic, and 5 = dyskinetic), and a scale of 0–3 to grade the thickening for each segment (0 = normal, 1 = mildly reduced, 2 = moderately to severely reduced, and 3 = no thickening). The criterion for abnormality in the motion and thickening was both defined as a score of at least 2 [11]. The assignment of individual myocardial segments to the coronary arterial territories was determined according to a previous study [12].
Statistical analysis
All statistical analyses in this study were performed using IBM SPSS (version 21.0; SPSS Inc., Chicago, Illinois, USA). Continuous data are expressed as the mean ± SD, and categorical data are expressed as a number and percentage and compared using a χ2-test. Either independent tests or Wilcoxon’s nonparametric tests were used to compare the differences in the quantitative paired data. The receiver operating characteristic curve was drawn by MedCalc (version 15.2.2; MedCalc Software, Ostend, Belgium), and the area under the curve (AUC) was calculated to measure the diagnostic performance. P values less than 0.05 were considered to be statistically significant.
Results
Table 1 lists the clinical characteristics and the CAG results of the studied population (123 men and 58 women). The age was 61.5 ± 8.7 years, with a BMI of 25.1 ± 3.0kg/m2. The pretest probability of CAD was ~50.2 ± 23.2% [13]. In our study, the prevalence of CAD is the only statistically significant difference between men and women. Significant differences in hypertension, diabetes, and dyslipidemia between overweight and normal-weight participants were observed.
Table 1.
Baseline characteristics of patients included in this study (n=181)
| Patient characteristics | Value |
|---|---|
|
| |
| Age (years) | 61.5 ± 8.7 |
| Sex (male/female) | 123/58 |
| BMI (kg/m2) | 25.1 ± 3.0 |
| Pretest probability of CAD (%) | 50.2 ± 23.2 |
| Chest pain [n (%)] | 125 (69.1) |
| Hypertension [n (%)] | 129 (71.3) |
| Diabetes [n (%)] | 40 (22.1) |
| Dyslipidemia [n (%)] | 68 (37.6) |
| Patients with CAD [n (%)] | 74 (40.9) |
| Single-vessel disease [n (%)] | 20 (27) |
| Double-vessel disease [n (%)] | 16 (21.6) |
| Multivessel disease [n (%)] | 38 (51.4) |
| LAD lesions [n (%)] | 67 (90.5) |
| LCX lesions [n (%)] | 39 (52.7) |
| RCA lesions [n (%)] | 50 (67.6) |
CAD, coronary artery disease; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery.
Diagnostic efficacy of nonattenuation correction, computed tomography attenuation correction, and gated myocardial perfusion imaging for overall coronary artery disease
GMPI had a higher specificity (96.3 vs. 55.1%, P < 0.001), accuracy (77.9 vs. 63.5%, P = 0.003), and AUC (0.738 vs. 0.654, P=0.034) but a lower sensitivity (51.4 vs. 75.7%, P = 0.002) than NAC did for CAD diagnosis. However, compared with CT-AC, GMPI increased the specificity (96.3 vs. 86.9%, P=0.014) while preserving a similar sensitivity (51.4 vs. 52.7%, P=0.869). The AUC of GMPI was larger than that of CT-AC (0.738 vs. 0.698, P=0.233), but the difference was not statistically significant, as shown in Table 2 and Figs 1 and 2.
Table 2.
Sensitivity, specificity, and accuracy of nonattenuation correction, CT attenuation correction, and gated myocardial perfusion imaging in single-photon emission computed tomography studies for overall and right coronary artery disease
| NAC | CT-AC | GMPI | P value# | P value& | |
|---|---|---|---|---|---|
|
| |||||
| Overall (n = 181) | |||||
| Sensitivity | 75.7 | 52.7 | 51.4 | 0.002# | 0.869 |
| Specificity | 55.1 | 86.9 | 96.3 | < 0.001# | 0.014& |
| Accuracy | 63.5 | 72.9 | 77.9 | 0.003# | 0.272 |
| RCA (n = 181) | |||||
| Sensitivity | 60.0 | 26.0 | 52.0 | 0.420 | 0.008& |
| Specificity | 64.9 | 93.1 | 94.7 | < 0.001# | 0.606 |
| Accuracy | 63.5 | 74.6 | 82.9 | < 0.001# | 0.054 |
CAD, coronary artery disease; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery.
CT-AC, CT attenuation correction; GMPI, gated myocardial perfusion imaging; NAC, nonattenuation correction; RCA, right coronary artery.
P value refers to test accuracy.
P<0.05 for NAC and GMPI.
P<0.05 for CT-AC and GMPI.
Fig. 1.

Analysis of area under the curve (AUC). (a) Overall (AUC for GMPI, CT-AC, and NAC: 0.738 vs. 0.698 vs. 0.654#). (b) Right CAD (AUC for GMPI, CT-AC, and NAC: 0.733 vs. 0.596 vs. 0.624#,&). (c) Men (AUC for GMPI, CT-AC, and NAC: 0.754 vs. 0.681 vs. 0.563*,#,&). (d) Overweight individuals (AUC for GMPI, CT-AC, and NAC: 0.730 vs. 0.686 vs. 0.600#). *P<0.05 for NAC and CT-AC, #P<0.05 for NAC and GMPI, and &P<0.05 for CT-AC and GMPI. CAD, coronary artery disease; CT-AC, CT attenuation correction; GMPI, gated myocardial perfusion imaging; NAC, nonattenuation correction.
Fig. 2.
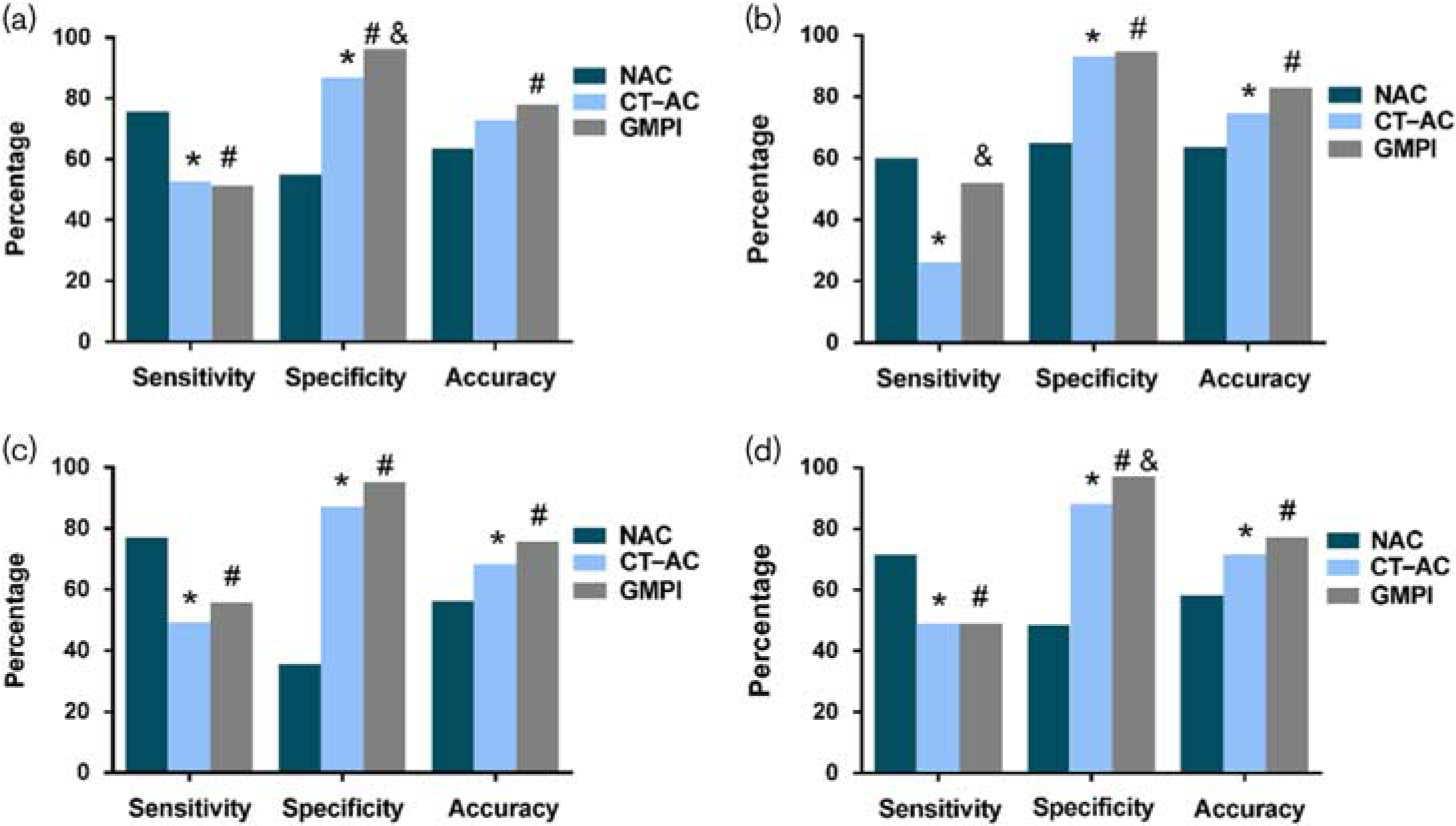
Sensitivity, specificity, and accuracy of NAC, CT-AC, and GSPECT (a) Overall (b) Right CAD (c) Men (d) Overweight individuals.*P<0.05 for NAC and CT-AC, #P<0.05 for NAC and GMPI, and &P<0.05 for CT-AC and GMPI. CAD, coronary artery disease; CT-AC, CT attenuation correction; GMPI, gated myocardial perfusion imaging; GSPECT, gated single-photon emission computed tomography; NAC, nonattenuation correction.
Diagnostic efficacy of nonattenuation correction, computed tomography attenuation correction, and gated myocardial perfusion imaging for individual coronary arteries
For diagnosing right CAD, GMPI had a higher specificity (94.7 vs. 64.9%, P < 0.001), a higher accuracy (82.9 vs. 63.5%, P < 0.001), and a larger AUC (0.733 vs. 0.624, P=0.02) than NAC, but their sensitivities were identical. Compared with CT-AC, GMPI had a similar specificity (94.7 vs. 93.1%, P = 0.606), a significantly higher sensitivity (52.0 vs. 26.0%, P=0.008), a higher AUC (0.733 vs. 0.596, P < 0.001), and an increased accuracy (82.9 vs. 74.6%, P = 0.054). Moreover, of the 131 patients with a normal right coronary artery (RCA), there were 46 false positives when using NAC [46/131 (35.1%)] and nine false positives when using CT-AC [9/131 (6.9%), 46 false positives from NAC included nine from CT-AC], suggesting that 37 cases were corrected by CT-AC. There were only four false positives [4/131 (3.1%)] when GMPI was used. In addition, the images of 17 patients with RCA lesions were overcorrected by CT-AC, which was not found in GMPI, as shown in Figs 3 and 4.
Fig. 3.
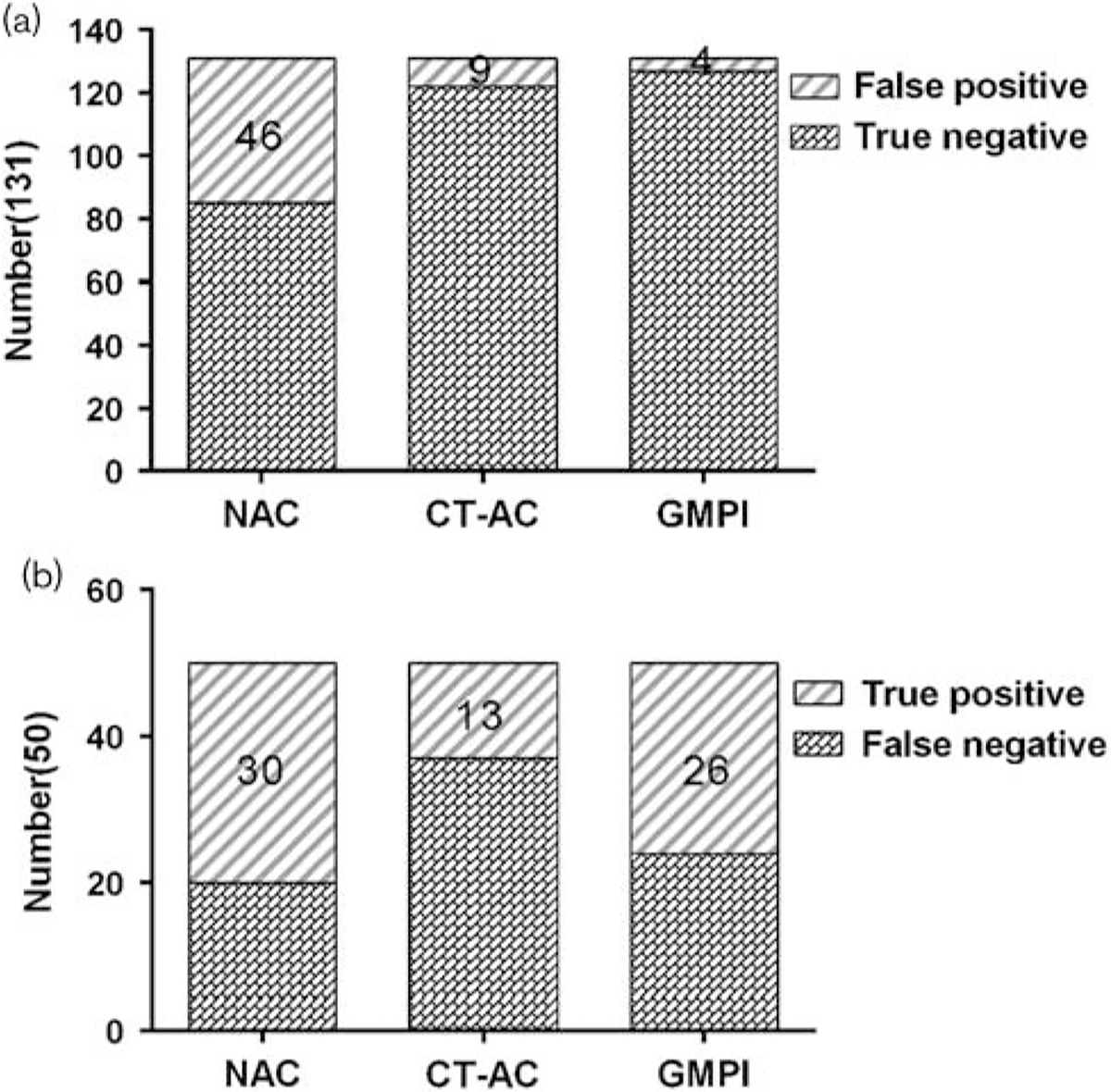
(a) False positive findings using NAC, CT-AC, and GMPI in normal RCA. (b) True positive findings using NAC, CT-AC, and GMPI for diagnosing RCA lesion. CAD, coronary artery disease; CT-AC, CT attenuation correction; GMPI, gated myocardial perfusion imaging; NAC, nonattenuation correction; RCA, right coronary artery.
Fig. 4.
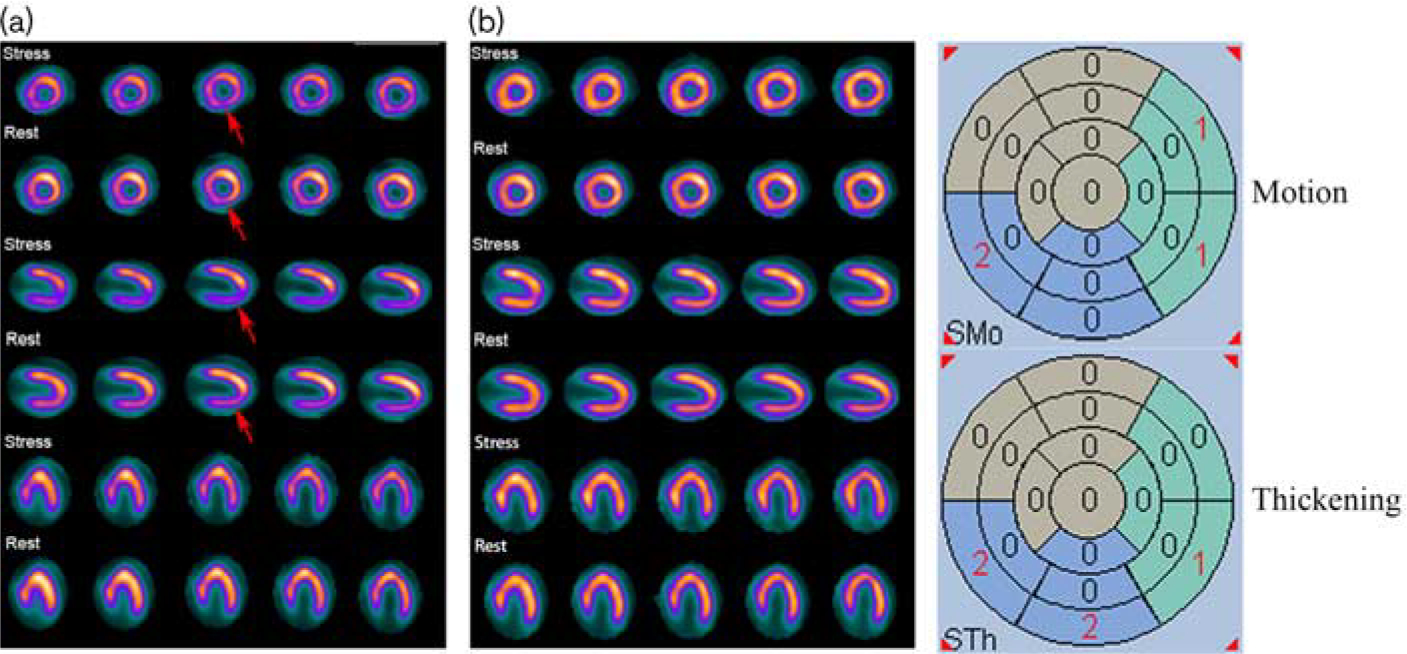
A 72-year-old man with a BMI of 30.5. He noted chest tightness for 1 year and aggravation for 1 month, which was not associated with exertion. The NAC stress–rest SPECT images (a) show a mixed radioactivity count reduction involving the partial inferior and inferoseptal wall (red arrow). The gated images show abnormal inferior and inferoseptal wall motion and thickening by automatic quantitative analysis. However, the CT-AC stress–rest SPECT images (b) show normal perfusion. A coronary angiography showed 90% RCA stenosis. This figure indicates that there was an overcorrection in the inferior and inferoseptal wall when using CT-AC. CT-AC, CT attenuation correction; NAC, nonattenuation correction; RCA, right coronary artery; SPECT, single-photon emission computed tomography.
There was no significant difference between GMPI and CT-AC in the efficacy of diagnosing CAD with left anterior descending or left circumflex coronary artery lesions.
Diagnostic efficacy of nonattenuation correction, computed tomography attenuation correction, and gated myocardial perfusion imaging for sex and BMI
As shown in Table 3 and Figs 1 and 2, in the diagnosis of suspected CAD for men, compared with NAC, GMPI showed a higher specificity (95.2 vs. 35.5%, P < 0.001), a higher accuracy (75.6 vs. 56.1%, P = 0.001), and a lower sensitivity (55.7 vs. 77.0%, P=0.013). In contrast, the specificity, accuracy, and sensitivity of GMPI were higher than those of CT-AC, although the differences were not statistically significant. Finally, the AUC of GMPI was larger than that of CT-AC and NAC (0.754 vs. 0.681 vs. 0.563, P < 0.05).
Table 3.
Sensitivity, specificity, and accuracy of nonattenuation correction, CT attenuation correction, and gated myocardial perfusion imaging in single-photon emission computed tomography studies for men and overweight individuals
| NAC | CT-AC | GMPI | P value# | P value& | |
|---|---|---|---|---|---|
|
| |||||
| Men (n = 123) | |||||
| Sensitivity | 77.0 | 49.2 | 55.7 | 0.013# | 0.468 |
| Specificity | 35.5 | 87.1 | 95.2 | < 0.001# | 0.114 |
| Accuracy | 56.1 | 68.3 | 75.6 | 0.001# | 0.201 |
| Overweight individuals (n = 117) | |||||
| Sensitivity | 71.4 | 49.0 | 49.0 | 0.023# | 1 |
| Specificity | 48.5 | 88.2 | 97.1 | < 0.001# | 0.049& |
| Accuracy | 58.1 | 71.8 | 77.0 | 0.002# | 0.369 |
CT-AC, CT attenuation correction; GMPI, gated myocardial perfusion imaging; NAC, nonattenuation correction.
P value refers to test accuracy.
P<0.05 for NAC and GMPI.
P<0.05 for CT-AC and GMPI.
In the diagnosis of suspected CAD for overweight individuals, compared with NAC, GMPI had a higher specificity (97.1 vs. 48.5%, P<0.001), a higher accuracy (77.0 vs. 58.1%, P = 0.002), and a reduced sensitivity (49.0 vs. 71.4%, P=0.023). Compared with CT-AC, GMPI had a greater specificity (97.1 vs. 88.2%, P=0.049) for CAD diagnosis, while preserving a similar sensitivity and accuracy. The AUC of GMPI was larger than that of NAC (0.730 vs. 0.600, P=0.006), but there was no significant difference between GMPI and CT-AC.
In the diagnosis of suspected CAD for women and normal-weight patients, the diagnostic efficacy showed no significant difference between GMPI and CT-AC.
Discussion
Myocardial perfusion SPECT imaging has become an important noninvasive examination for the diagnosis, risk stratification, and treatment of CAD [14]. Attenuated artifacts, which are usually manifested as fixed perfusion abnormalities that are difficult to identify from MI, are one of the common limitations of SPECT MPI. A relatively higher incidence of false positives caused by attenuation artifacts may lead to unnecessary further testing and potentially increase the medical costs. GMPI observes myocardial systolic function in segments with fixed perfusion defects, and AC utilizes an external radioactive line source or CT images for the acquisition of transmission maps. Both of these are important techniques that allow the readers to discern attenuation artifacts from fixed perfusion abnormalities.
Application of attenuation correction for diagnosing coronary artery disease
In a large meta-analysis involving 13 different clinical studies (including 1327 patients), Garcia [15] found that the specificity (77 vs. 57%) and normalcy rate (92 vs. 72%) for diagnosing CAD increased when using AC, but there was no significant difference in the sensitivity (86 vs. 86%). The clinical value of AC in identifying attenuation artifacts has been confirmed by other studies [3–5]. However, Apostolopoulos and Savvopoulos [16] studied 120 patients who underwent Tl-201 myocardial perfusion SPECT imaging and found that the overall sensitivity of CT-AC in diagnosing CAD was lower than that of NAC (70 vs. 87%, P=0.04), and the specificity was slightly improved (62 vs. 54%, P>0.05), but the difference in accuracy was not significant (66 vs. 69%, P>0.05). In addition, many studies [15,17,18] have noted that AC may overcorrect the inferior wall in some patients with excessive bowel intake. Therefore, the routine clinical application of AC is still controversial because of the inconsistent results [4,18,19]. In fact, there is still no clear requirement for the routine use of AC in the guidelines, and nearly half of SPECT cameras in China are not equipped with spiral CT for AC [20].
Application of gated myocardial perfusion imaging for diagnosing coronary artery disease
However, with the popularity of SPECT in China, GMPI is increasingly being used as an important adjunct to SPECT. Additional information provided by GMPI not only helps identify soft-tissue attenuation artifacts but also improves the prognosis [6,7,21,22]. Iskandrian and Gracia [23] argued that if there was no history of MI, there were no Q waves on the ECG, and there was normal inferior WM on GMPI, the fixed perfusion defects reduction in the inferior wall could be attributed to diaphragmatic attenuation instead of MI. The reason may be that the fixed defects caused by MI cause a decrease in WM and thickening, whereas the fixed defects caused by artifacts are generally normal [6]. In other words, combined with clinical data and the ECG results, GMPI together with perfusion information will improve reader confidence and artifact recognition, thereby improving the efficacy of clinical decision-making. Fleischman et al. [7] showed that for patients without a history of MI, 90% of the patients with normal systolic function of fixed perfusion defects occurred in the inferior wall for men (87%) and the anterior wall for women (3%). The application of additional GMPI reduced the number of patients with unexplained fixed perfusion defects (without clinical MI) from 29 to 10% by reinterpreting these cases, and these perfusion defects were most likely caused by attenuation artifacts. Therefore, GMPI has a considerable value in identifying the fixed perfusion defects and may potentially improve diagnostic accuracy. DePuey and Rozanski [6] also suggested that incorporating the LV function into MPI interpretation could reduce the false positive rate from 14 to 3%. Smanio et al. [24] reported that the number of ‘borderline normal’ and ‘borderline abnormal’ interpretations were significantly decreased if the stress–rest perfusion images were interpreted in combination with information such as WM and thickening provided by GMPI. Moreover, Lima et al. [25] demonstrated that abnormal WM/thickening was more sensitive in detecting severe multivessel CAD than perfusion alone.
Evaluation of computed tomography attenuation correction and gated myocardial perfusion imaging for diagnosing coronary artery disease
Our previous research [26] showed that CT-AC helped in detecting false positives, which might be more pronounced in the subgroups of men, overweight individuals, and right CAD. Combined with CAD pretest, CT-AC images together with NAC images could improve the diagnostic efficacy for CAD. However, only a few studies [1,8,27] have focused on the combined effects of AC and gating in myocardial SPECT imaging interpretation. In this study, compared with CT-AC, the addition of GMPI led to an improvement in specificity (96.3 vs. 86.9%) and accuracy (77.9 vs. 72.9%) with no change in sensitivity. Benkiran et al. [27] found that compared with NAC, both AC and GMPI led to an increased specificity (98 vs. 81 vs. 60%) and accuracy (93 vs. 79 vs. 63%) without a significant reduction in sensitivity. These improvements were, however, undoubtedly more obvious for GMPI than for AC. Their results are more likely to support the conclusion that gating may be more effective than AC in detecting false positives caused by attenuation artifacts. Thus, the method of combining gating with perfusion data to identify artifacts from fixed perfusion defects may be more feasible and effective, which is consistent with our findings.
Subgroup analysis of computed tomography attenuation correction and gated myocardial perfusion imaging for diagnosing coronary artery disease
A subgroup analysis of patients with suspected CAD revealed that GMPI had a higher diagnostic performance than NAC for men, overweight individuals, and right CAD because of the improvement in specificity. The sensitivity reduction from CT-AC was more obvious than that from GMPI in the diagnosis of right CAD. This is most likely owing to an overcorrection of the perfusion defects in RCA when using CT-AC, as shown in Fig. 4. The diagnostic performance of GMPI was higher for men than that of CT-AC. Singh et al. [2] demonstrated that the left diaphragm in men tended to cause attenuation of the inferior wall, leading to potential perfusion artifacts in right CAD, which was estimated to account for 25% in myocardial perfusion studies. However, the incidences of inferior wall attenuation artifacts in our study were 28.5% in men and 6.9% in women. The higher incidence of inferior attenuation artifacts in men was likely because of a higher diaphragmatic position during acquisition in a supine position. This might in part relate to different respiration patterns between male and female patients; male patients favor diaphragmatic as opposed to intercostal respiratory patterns. Genovesi [8] evaluated the LV function by visual interpretation and showed that in overweight men, the specificity in the diagnosis of RCA disease was 66.7% using GMPI and 100% using CT-AC, but they had the same sensitivity; the AUC of CT-AC was significantly higher than that of GMPI (0.90 vs 0.72, P < 0.05). No significant differences were found for the evaluation of the left anterior descending or left circumflex coronary artery territory between the two methods. A methodological inconsistency may be one of the reasons for the inconsistent results. The automated quantitative analysis used in our study is not only reproducible but can also avoid any biases possibly caused by a visual analysis, which has been validated in large sample size populations [28]. In a recent study by Sharir [22], the author used an automatic quantitative analysis to assess stress–rest myocardial WM and thickening, which obtained a remarkable increase in diagnostic performance over perfusion alone. Another reason may be the somatotype differences of the selected population.
Radiation exposure
The effective radiation dose for a standard 99mTc-MIBI imaging is ~10 mSv [29], whereas for cardiac imaging, the dose from a CT scan for AC is typically ~0.90 mSv in the default setting (2.5 mA) and 0.36 mSv for a 1.0mA tube current [30]. Evidence [31] indicates that the ionizing radiation exposure of CT-AC is still a concern despite the relatively low dose because additional CT scans increase the risk of breast malignancy, especially for younger women. In contrast, there is no extra ionizing radiation exposure for GMPI.
Limitation
This is a single-center study, and the number of patients is relatively small. CAD may manifest as a coronary microvascular disease without significant epicardial coronary artery lesions. The presence of an underlying microvascular disease may not be adequately ruled out, which may produce false positive results. A scatter correction with compensation resolution was not used in our study. Correcting for attenuation but not for scatter may cause a difference in both the sensitivity and diagnostic accuracy. The aforementioned points still need to be determined through a multicenter prospective study with a large sample size.
Conclusion
Both CT-AC and GMPI led to a higher diagnostic efficacy than NAC did in differentiating between MI or attenuation artifacts for fixed defects. These improvements were more obvious by GMPI than by CT-AC, especially when dealing with a population of men and right CAD. In analyzing patients in the entire group and other subgroups, the diagnostic performance of GMPI is similar or slightly improved over that of CT-AC.
Acknowledgements
This research was supported in part by grants from the Natural Science Foundation of China (81471690 and 81871381, PI: Yuetao Wang); the Key Development Foundation of Jiangsu Province (BE2015635, PI: Yuetao Wang); the Youth Science Fund Project of National Natural Science Foundation of China (81701734, PI: Xiaoliang Shao); the Youth Science Fund Project of National Natural Science Foundation of China (81701737, PI: Jianfeng Wang); the Social Development Foundation of Changzhou Science and Technology Bureau, Jiangsu Province, China (CE20175029, PI: Jianfeng Wang); and the American Heart Association (17AIREA33700016, PI: Weihua Zhou).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Links JM, DePuey EG, Taillefer R, Becker LC. Attenuation correction and gating synergistically improve the diagnostic accuracy of myocardial perfusion SPECT. J Nucl Cardiol 2002; 9:183–187. [DOI] [PubMed] [Google Scholar]
- 2.Singh B, Bateman TM, Case JA, Heller G. Attenuation artifact, attenuation correction, and the future of myocardial perfusion SPECT. J Nucl Cardiol 2007; 14:153–164. [DOI] [PubMed] [Google Scholar]
- 3.Masood Y, Liu YH, Depuey G, Taillefer R, Araujo LI, Allen S, et al. Clinical validation of SPECT attenuation correction using x-ray computed tomography-derived attenuation maps: multicenter clinical trial with angiographic correlation. J Nucl Cardiol 2005; 12:676–686. [DOI] [PubMed] [Google Scholar]
- 4.Huang JY, Huang CK, Yen RF, Wu HY, Tu YK, Cheng MF, et al. Diagnostic performance of attenuation-corrected myocardial perfusion imaging for coronary artery disease: a systematic review and meta-analysis. J Nucl Med 2016; 57:1893–1898. [DOI] [PubMed] [Google Scholar]
- 5.Huang R, Li F, Zhao Z, Liu B, Ou X, Tian R, et al. Hybrid SPECT/CT for attenuation correction of stress myocardial perfusion imaging. Clin Nucl Med 2011; 36:344–349. [DOI] [PubMed] [Google Scholar]
- 6.DePuey EG, Rozanski A. Using gated technetium-99m-sestamibi SPECT to characterize fixed myocardial defects as infarct or artifact. J Nucl Med 1995; 36:952–955. [PubMed] [Google Scholar]
- 7.Fleischmann S, Koepfli P, Namdar M, Wyss CA, Jenni R, Kaufmann PA. Gated (99m)Tc-tetrofosmin SPECT for discriminating infarct from artifact in fixed myocardial perfusion defects. J Nucl Med 2004; 45:754–759. [PubMed] [Google Scholar]
- 8.Genovesi D, Giorgetti A, Gimelli A, Kusch A, D’Aragona Tagliavia I, Casagranda M, et al. Impact of attenuation correction and gated acquisition in SPECT myocardial perfusion imaging: results of the multicentre SPAG (SPECT Attenuation Correction vs Gated) study. Eur J Nucl Med Mol Imaging 2011; 38:1890–1898. [DOI] [PubMed] [Google Scholar]
- 9.Chen CM. Chinese adult overweight and obesity prevention and control guidelines. Beijing: People’s Health Press; 2006. [Google Scholar]
- 10.Hesse B, Tagil K, Cuocolo A, Anagnostopoulos C, Bardies M, Bax J, et al. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur J Nucl Med Mol Imaging 2005; 32:855–897. [DOI] [PubMed] [Google Scholar]
- 11.Sharir T, Berman DS, Waechter PB, Areeda J, Kavanagh PB, Gerlach J, et al. Quantitative analysis of regional motion and thickening by gated myocardial perfusion SPECT: normal heterogeneity and criteria for abnormality. J Nucl Med 2001; 42:1630–1638. [PubMed] [Google Scholar]
- 12.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol 2002; 9:240–245. [DOI] [PubMed] [Google Scholar]
- 13.Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013; 34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 14.Zafrir N, Shafir G, Kovalski G, Mats I, Bouhnik JP, Battler A, et al. Yield of a novel ultra-low-dose computed tomography device mounted on a dedicated cardiac SPECT system in improving the accuracy of myocardial perfusion imaging and the detection of chest abnormalities. J Nucl Cardiol 2012; 19:303–310. [DOI] [PubMed] [Google Scholar]
- 15.Garcia EV. SPECT attenuation correction: an essential tool to realize nuclear cardiology’s manifest destiny. J Nucl Cardiol 2007; 14:16–24. [DOI] [PubMed] [Google Scholar]
- 16.Apostolopoulos DJ, Savvopoulos C. What is the benefit of CT-based attenuation correction in myocardial perfusion SPET? Hell J Nucl Med 2016; 19:89–92. [DOI] [PubMed] [Google Scholar]
- 17.Malkerneker D, Brenner R, Martin WH, Sampson UK, Feurer ID, Kronenberg MW, et al. CT-based attenuation correction versus prone imaging to decrease equivocal interpretations of rest/stress Tc-99m tetrofosmin SPECT MPI. J Nucl Cardiol 2007; 14:314–323. [DOI] [PubMed] [Google Scholar]
- 18.Sharma P, Patel CD, Karunanithi S, Maharjan S, Malhotra A. Comparative accuracy of CT attenuation-corrected and non-attenuationcorrected SPECT myocardial perfusion imaging. Clin Nucl Med 2012; 37:332–338. [DOI] [PubMed] [Google Scholar]
- 19.Hendel RC, Berman DS, Cullom SJ, Follansbee W, Heller GV, Kiat H, et al. Multicenter clinical trial to evaluate the efficacy of correction for photon attenuation and scatter in SPECT myocardial perfusion imaging. Circulation 1999; 99:2742–2749. [DOI] [PubMed] [Google Scholar]
- 20.Chinese Society of Nuclear Medicine. A brief report on the results of the national survey of nuclear medicine in 2016. Chin J Nucl Med Mol Imaging 2016; 36:479–480. [Google Scholar]
- 21.Sciagrà R The expanding role of left ventricular functional assessment using gated myocardial perfusion SPECT: the supporting actor is stealing the scene. Eur J Nucl Med Mol Imaging 2007; 34:1107–1122. [DOI] [PubMed] [Google Scholar]
- 22.Sharir T What is the value of motion and thickening in gated myocardial perfusion SPECT? J Nucl Cardiol 2018; 25:754–757. [DOI] [PubMed] [Google Scholar]
- 23.Iskandrian AE, Garcia EV. Atlas of nuclear cardiology: imaging companion to Braunwald’s heart disease E-book. Elsevier Health Sciences; 2011. [Google Scholar]
- 24.Smanio PE, Watson DD, Segalla DL, Vinson EL, Smith WH, Beller GA. Value of gating of technetium-99m sestamibi single-photon emission computed tomographic imaging. J Am Coll Cardiol 1997; 30:1687–1692. [DOI] [PubMed] [Google Scholar]
- 25.Lima RS, Watson DD, Goode AR, Siadaty MS, Ragosta M, Beller GA, et al. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol 2003; 42:64–70. [DOI] [PubMed] [Google Scholar]
- 26.Xin WC, Shao XL, Wang YT, Wang JF, Wang XS, Yang L, et al. Is there an incremental value to use myocardial perfusion imaging with or without CT attenuation for the diagnosis of coronary artery disease? A study in Chinese patients. Hell J Nucl Med 2018; 21:48–54. [DOI] [PubMed] [Google Scholar]
- 27.Benkiran M, Mariano-Goulart D, Bourdon A, Sibille L, Bouallègue FB. Is computed tomography attenuation correction more efficient than gated single photon emission computed tomography analysis in improving the diagnostic performance of myocardial perfusion imaging in patients with low prevalence of ischemic heart disease? Nucl Med Commun 2015; 36:69–77. [DOI] [PubMed] [Google Scholar]
- 28.Slomka PJ, Berman DS, Xu Y, Kavanagh P, Hayes SW, Dorbala S, et al. Fully automated wall motion and thickening scoring system for myocardial perfusion SPECT: method development and validation in large population. J Nucl Cardiol 2012; 19:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007; 116:1290–1305. [DOI] [PubMed] [Google Scholar]
- 30.Preuss R, Weise R, Lindner O, Fricke E, Fricke H, Burchert W. Optimisation of protocol for low dose CT-derived attenuation correction in myocardial perfusion SPECT imaging. Eur J Nucl Med Mol Imaging 2008; 35:1133–1141. [DOI] [PubMed] [Google Scholar]


