Summary
Background
Repurposed drugs with host-directed antiviral and immunomodulatory properties have shown promise in the treatment of COVID-19, but few trials have studied combinations of these agents. The aim of this trial was to assess the effectiveness of affordable, widely available, repurposed drugs used in combination for treatment of COVID-19, which may be particularly relevant to low-resource countries.
Methods
We conducted an open-label, randomized, outpatient, controlled trial in Thailand from October 1, 2021, to June 21, 2022, to assess whether early treatment within 48-h of symptoms onset with combinations of fluvoxamine, bromhexine, cyproheptadine, and niclosamide, given to adults with confirmed mild SARS-CoV-2 infection, can prevent 28-day clinical deterioration compared to standard care. Participants were randomly assigned to receive treatment with fluvoxamine alone, fluvoxamine + bromhexine, fluvoxamine + cyproheptadine, niclosamide + bromhexine, or standard care. The primary outcome measured was clinical deterioration within 9, 14, or 28 days using a 6-point ordinal scale. This trial is registered with ClinicalTrials.gov (NCT05087381).
Findings
Among 1900 recruited, a total of 995 participants completed the trial. No participants had clinical deterioration by day 9, 14, or 28 days among those treated with fluvoxamine plus bromhexine (0%), fluvoxamine plus cyproheptadine (0%), or niclosamide plus bromhexine (0%). Nine participants (5.6%) in the fluvoxamine arm had clinical deterioration by day 28, requiring low-flow oxygen. In contrast, most standard care arm participants had clinical deterioration by 9, 14, and 28 days. By day 9, 32.7% (110) of patients in the standard care arm had been hospitalized without requiring supplemental oxygen but needing ongoing medical care. By day 28, this percentage increased to 37.5% (21). Additionally, 20.8% (70) of patients in the standard care arm required low-flow oxygen by day 9, and 12.5% (16) needed non-invasive or mechanical ventilation by day 28. All treated groups significantly differed from the standard care group by days 9, 14, and 28 (p < 0.0001). Also, by day 28, the three 2-drug treatments were significantly better than the fluvoxamine arm (p < 0.0001). No deaths occurred in any study group. Compared to standard care, participants treated with the combination agents had significantly decreased viral loads as early as day 3 of treatment (p < 0.0001), decreased levels of serum cytokines interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β) as early as day 5 of treatment, and interleukin-8 (IL-8) by day 7 of treatment (p < 0.0001) and lower incidence of post-acute sequelae of COVID-19 (PASC) symptoms (p < 0.0001). 23 serious adverse events occurred in the standard care arm, while only 1 serious adverse event was reported in the fluvoxamine arm, and zero serious adverse events occurred in the other arms.
Interpretation
Early treatment with these combinations among outpatients diagnosed with COVID-19 was associated with lower likelihood of clinical deterioration, and with significant and rapid reduction in the viral load and serum cytokines, and with lower burden of PASC symptoms. When started very soon after symptom onset, these repurposed drugs have high potential to prevent clinical deterioration and death in vaccinated and unvaccinated COVID-19 patients.
Funding
Ped Thai Su Phai (Thai Ducks Fighting Danger) social giver group.
Keywords: Early treatment, Fluvoxamine, Bromhexine, Cyproheptadine, Niclosamide, COVID-19 treatment
Research in context.
Evidence before this study
On September 10, 2023, a search was conducted on PubMed using the search criteria ‘(randomized OR trial) AND (fluvoxamine OR bromhexine OR cyproheptadine OR niclosamide OR combination OR antidepressants OR selective serotonin reuptake inhibitors OR SSRIs) AND (COVID∗ OR SARS-CoV-2 OR SARS-CoV)’ without any restrictions on date or language. This search yielded multiple preclinical and clinical studies that suggest a significant connection between the usage of fluvoxamine, bromhexine, and niclosamide, and a reduced risk of intubation or mortality in adult outpatients exhibiting symptomatic COVID-19. Notably, there have been no trials examining the effectiveness of combinations of fluvoxamine, bromhexine, cyproheptadine, and niclosamide in the treatment of SARS-CoV-2 infection.
Added value of this study
We conducted a controlled trial to evaluate the effect of early treatment with fluvoxamine, bromhexine, cyproheptadine, and niclosamide within 48 h of symptom onset in adults with mild SARS-CoV-2 infection. The study aimed to determine if this combination therapy could prevent clinical deterioration in the targeted population. Early treatment with these drug combinations in COVID-19 outpatients reduced the likelihood of clinical deterioration significantly. It also led to rapid reductions in viral load, serum cytokines, and the burden of post-acute sequelae of SARS-CoV-2 (PASC) symptoms.
Implications of all the available evidence
Early treatment with these drug combinations can significantly alter the clinical course of COVID-19, leading to fewer hospitalizations and potentially serving as important outpatient interventions. The findings of this study hold particular significance for regions where vaccination and costly antiviral treatments are not easily accessible.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes coronavirus disease 2019 (COVID-19), which can lead to serious illness, hospitalization, intensive care unit admission, and death.1,2 Vaccination has reduced the risk of severe disease, but waves of infection by new immune-evasive variants still pose challenges for healthcare systems around the world.3, 4, 5, 6 For low-income countries, in particular, the lack of access to mRNA vaccines and the expense associated with procuring novel antivirals have created an urgent need to identify affordable, safe, and widely available treatment options.3,4,6, 7, 8, 9, 10, 11 Clinical deterioration of COVID-19 typically occurs during the second week of illness, with hospitalization most often occurring within 8–10 days of the initial symptoms.2 Progression to severe COVID-19 is characterized by immune dysregulation and excessive production of pro-inflammatory cytokines, especially IL-6, TNF-α, IL-8, and IL-1β. Elevations in these serum cytokines are predictive of disease progression, mortality, and post-acute sequelae of COVID-19 (PASC).1,2,12,13 Curtailing this immune dysregulation at an early stage using safe and affordable therapeutics may reduce illness severity, improve clinical outcomes, and reduce the long-term risk of PASC development.1
Previous clinical studies have shown that fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is associated with benefit in the treatment of acute COVID-1914, 15, 16, 17 possibly by reducing damaging aspects of the inflammatory response to SARS-CoV-2 infection,14,16,18 lowering the risk of hypercoagulable state by reducing platelet serotonin levels,14,16,18 and/or other possible mechanisms.12 The initially hypothesized mechanism leading to clinical trials of fluvoxamine in COVID-19 was its anti-inflammatory action through activation of the sigma-1 (S1R).19 Fluvoxamine may also have antiviral effects through interaction with viral proteins and inhibition of acid sphingomyelinase.12,20, 21, 22 Other commonly available drugs have immune-modulating and antiviral properties and have shown some evidence of effectiveness against SARS-CoV-2: Bromhexine hydrochloride acts as a mucolytic and immune modulator to help clear chest congestion, and as a TMPRSS2 protease blocker may be an effective antiviral against SARS-CoV-223,24 Cyproheptadine acts as an antagonist of 5-hydroxytryptamine (5HT/serotonin) receptor subtype two. It is an immune modulator to prevent potent effects of serotonin on lung vascular tone, respiratory rate, and systemic vascular beds, which may potentially affect COVID-19 clinical outcomes.12,25 The anti-helminthic drug niclosamide may be able to inhibit SARS-CoV-2 viral replication and modulate inflammation by markedly blunting calcium oscillations and membrane conductance in spike-expressing cells by suppressing the activity of TMEM16F, a calcium-activated ion channel, a scramblase responsible for the exposure of phosphatidylserine on the cell surface.26
Though not known to act as direct-acting antiviral agents against SARS-CoV-2, these four agents employ different mechanisms to exert host-directed antiviral and immunomodulatory effects. The mechanism of observed benefit in clinical studies remains unclear and is thought to be multifactorial. Clinically, the effect of combining these agents may bring about faster viral clearance and decreased inflammatory and immunothrombotic responses that are the hallmarks of disease progression in COVID-19. We, therefore, hypothesized that combinations of these agents may act synergistically to potentiate the beneficial effects of each agent alone, and early treatment with the combination of these agents may positively alter the trajectory of SARS-CoV-2 infection and reduce the risk of disease progression in COVID-19. The aim of this trial was to investigate this hypothesis.
Methods
Study design
This was an open-label, multi-arm, randomized controlled trial that compared early treatment with fluvoxamine, fluvoxamine plus bromhexine, fluvoxamine plus cyproheptadine, niclosamide plus bromhexine, versus standard care, in adult outpatients with confirmed SARS-CoV-2 infection. Standard care is not specified or mandated in the protocol but refers to the care that participants received, decided on by the responsible clinicians based on the COVID-19 treatment guidelines in Thailand at the time of the trial enrollment. The trial protocol and statistical analysis plan appear in the Supplement and ClinicalTrials.gov (NCT05087381). The several institutional review boards separately approved the study at Rajavithi Hospital in Bangkok, Thailand (224/2021), Vibhavadi Hospital in Bangkok, Thailand (01/64), Chiang Mai Neurological Hospital, Thailand (009/64), Thanyarak Pattani Hospital, Pattani, Thailand (008/64). This study was conducted in compliance with the Declaration of Helsinki, the Good Clinical Practice guidelines, and local regulatory requirements (ClinicalTrials.gov Identifier: NCT05087381). All participants provided informed consent electronically.
This trial was conducted in the Bangkok, Nonthaburi, Samut Prakan, Pathum Thani, Nakhon Pathom, Samut Sakhon, Chiang Mai, Chiang Rai and Pattani provinces in Thailand. Participants were recruited from October 1, 2021, to June 21, 2022. The 90-day post-randomization follow-up assessment was completed on September 21, 2022. This was a fully remote (contactless) clinical trial. Participants were recruited via a virtual healthcare system for Thailand's SARS-COV-2 care management program. Participants were enrolled without regard to sex, race, ethnicity, or religion. The participants were first examined (physical and respiratory status assessments) by attending physicians at the virtual healthcare system for the SARS-COV-2 care management program and referred to the study team if deemed eligible. Potential participants underwent second screenings by phone and provided informed consent electronically.
Study supplies were delivered to self-quarantined participants at their homes, quarantine centers, quarantine hotels, and isolation centers. The study materials consisted of the study medication, an oxygen saturation monitor, an automated blood pressure monitor, and a thermometer. Participants then self-assessed using the equipment provided and confirmed vital signs within range (systolic blood pressure between 80 mm Hg and 200 mm Hg, diastolic blood pressure between 40 mm Hg and 120 mm Hg, and pulse rate between 50 beats/min and 120 beats/min), and oxygen saturation of 92% or greater. The study staff called participants, informed them of their eligibility, and instructed them to take the study medication. The study medications were targeted to start on the same day that participants were first contacted and screened by the research team, who also informed the team at the virtual healthcare system for the SARS-COV-2 care management program.
All data collection was done by daily surveys from day 0 through day 14, 28, 60 and 90. The surveys were sent to participants via email, SMS with a secure online-based data collection link, together with the phone as a backup to ensure that individuals without internet access were able to participate. On study days 0, 3, 5, 7, 9, and 14, nasal swabs, blood, and fecal samples were collected from consenting participants only via a test drive-thru service to enable the research team to assess possible differences in disease severity and responses to treatment. The surveys recorded oxygen saturation, vital signs, medication adherence, and COVID-19 symptoms. Phone contact was attempted daily during the first 3 days of the trial to address participants' questions, address any medication-related issues, and encourage assessment completion. Additional phone calls were conducted on a case-by-case basis when participants’ survey data indicated values outside trial ranges. For participants who had worsening SARS-COV-2 illness, study staff recommended that the participants seek medical attention via the virtual healthcare system. Study staff assessed clinical status on a 6-point ordinal scale on study days 9, 14, and 28 using SMS, email, or phone contact consisting of the following categories: 1, death; 2, hospitalized, requiring invasive mechanical ventilation or extracorporeal membrane oxygenation; 3, hospitalized, requiring noninvasive ventilation or use of high-flow oxygen devices; 4, hospitalized, requiring low-flow supplemental oxygen; 5, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (related or not to SARS-COV-2 infection); and 6, not hospitalized. A final assessment was conducted on day 90.
Participants
In this fully remote (contactless) trial, individuals were deemed eligible if they were living in the community, aged 18–60 years, with symptomatic SARS-CoV-2 infection as confirmed by a positive polymerase chain reaction (PCR) assay or rapid antigen test kit (ATK), who had less than 48 h of symptoms. Individuals were excluded from participation if currently hospitalized for COVID-19, if deemed by the recruiting clinician to have significant exclusionary comorbidities (e.g., severe renal or hepatic impairment), if completely asymptomatic from COVID-19 at the time of screening, or if they had onset of COVID-19 symptoms more than 48 h before the time of screening. Additional exclusion criteria applied included individuals with allergies to, contraindications to, or significant drug–drug interactions with any of the drugs in the trial treatment arms; individuals receiving other medications for the treatment of COVID-19 outside of standard care in Thailand at the time of randomization; individuals participating in other COVID-19 treatment trials; pregnancy or breastfeeding; psychiatric diseases; inability to perform study self-assessment procedures; and refusal to participate in the study.
Randomisation
After exclusions, participants were randomized 1:1:1:1:1 to the five arms of (1) fluvoxamine alone, (2) fluvoxamine + bromhexine, (3) fluvoxamine + cyproheptadine, (4) niclosamide + bromhexine, and (5) standard care.
This randomisation scheme comprised these five treatment arms rather than a conventional comparison of a standard care group against the experimental medications. This choice was made in light of the prevailing medical context in Thailand, where favipiravir was categorised as the established standard of care. However, it is important to acknowledge that the evidentiary foundation supporting the use of favipiravir was limited, and there existed ongoing controversies regarding the efficacy of alternative treatment modalities.
In this context, based on 80% power and an alpha level of 0.05, the proportions of clinical deterioration were anticipated as follows: 0 for the fluvoxamine-alone group,14 0.01 for the fluvoxamine combined with bromhexine group,23 fluvoxamine combined with cyproheptadine group, the niclosamide combined27 with bromhexine group, and 0.032 for the standard care group.28 Based on the feasibility of the research, the total sample size with an additional 22%29 dropouts would be 297 participants per arm. Therefore, a total of 1485 participants was planned. The interim analysis of the data was undertaken after 426 patients had been recruited, and the decision was taken at that time to continue the trial until completion.
Randomisation schedules were generated that were stratified by age (18–44, 45–54, 55–64, and ≥65 years) and sex. Treatments were randomly allocated in a block size of 5 using a web-based response system, which displayed randomisation assignment to the outcome assessors, investigators, and research staff, who prepared the study materials, including the study drugs. Upon randomisation, participants were provided with the study self-assessment supplies and the study drugs at their place of residence, and further eligibility criteria were assessed before the participant took the study drugs (systolic blood pressure between 80 mm Hg and 200 mm Hg, diastolic blood pressure between 40 mm Hg and 120 mm Hg, pulse rate between 50 beats/min and 120 beats/min, and oxygen saturation of 92% or greater). Participants who could not clearly confirm eligibility at baseline based on the provided self-assessment supplies, who withdrew from the study prior to taking treatment, or who had abnormal vital signs, including oxygen saturation <92% at baseline, were instructed not to take the study drugs, and were withdrawn from the study.
Procedures
Participants in the fluvoxamine-only arm received fluvoxamine (immediate release) 50 mg orally, one tablet in the morning and one tablet at bedtime for the first two days, then escalated to one 50 mg one tablet in the morning and two tablets before bedtime for days 3 through 12, ending with one 50 mg one tablet in the morning and one tablet at bedtime for days 13 and 14 (additional details appear in Supplement 1). This dose taper and range was determined with regard to participant tolerance, safety, and efficacy signals based on prior fluvoxamine trials in the treatment of COVID-19.14, 15, 16, 17,30,31 Pharmacokinetic models predicting the dose needed for occupancy of the S1R were also considered.32,33 For most patients, a dose of 50 mg twice daily should achieve steady occupancy of the sigma1 receptor by day 2, but if a dose is missed, the level may drop too low. A dose of 100 mg twice daily may be ideal since in most cases it is predicted that S1R occupancy can be achieved after the first dose and never drop below the level that should achieve this. Moreover, a dose of 100 mg twice daily also should have good pharmacokinetic forgivability in case a dose is missed.33 However, some individuals may not tolerate 100 mg doses, as evidenced by drop-out rates from trials that used 100 mg 2–3 times daily.17,32 Therefore, in order to balance tolerability and the need to ensure S1R occupancy, we titrate up to 50 mg in the morning and 100 mg at bedtime. Giving a higher dose at bedtime may also help with tolerability in case patients experience nausea or somnolence.
Participants in the fluvoxamine + bromhexine arm received the same fluvoxamine regimen as the fluvoxamine-only arm, in combination with one 8 mg bromhexine tablet, twice daily, for a total of 10 days.17,32, 33, 34, 35, 36 Participants in the fluvoxamine + cyproheptadine arm received the same fluvoxamine regimen as the fluvoxamine-only arm, combined with one 4 mg cyproheptadine tablet, three times a day for 14 days. Participants in the niclosamide + bromhexine arm received a 1000 mg of niclosamide tablet two times daily for a total of 14 days, in combination with one 8 mg tablet of bromhexine twice daily for a total of 10 days.
Outcomes
The primary endpoint was the distribution of clinical status assessed on the 6-point ordinal scale on study days 9, 14, and 28. The primary endpoint was corroborated by phone discussions with participants and a review of their medical records. The secondary endpoints were the proportion of participants with adverse events, the incidence of PASC symptoms as described in a prior study37 (myalgia, cognitive symptoms/headache, pain, anxiety/depression/fatigue, abdominal symptoms, abnormal breathing and chest/throat pain), the change in respiratory viral clearance (by PCR), and the change in inflammatory markers IL-6, TNF-α, IL-8, and IL-1β.
Due to logistical issues at the time of study implementation, virological and inflammatory markers were accessed only for the subset of participants who voluntarily agreed to attend drive-through test centers. For participants who stopped responding to the surveys prior to day 90 or who had met the primary endpoint, medical records and subsequent calls to these participants were used to determine whether they met the primary endpoint. For participants who met the primary endpoint, hospital records were used to confirm specific healthcare use (e.g., supplemental oxygen use, hospital length of stay, and ventilator support). Adverse events and serious adverse events were recorded each day via participant self-reporting system for 14 days after randomisation.
Statistical analysis
All outcomes were summarized across the treatment arms using means and standard deviations for continuous variables and counts and percentages for categorical variables. For inferential analysis, the primary outcomes (1, death; 2, hospitalized, requiring invasive mechanical ventilation or extracorporeal membrane oxygenation; 3, hospitalized, requiring noninvasive ventilation or use of high-flow oxygen devices; 4, hospitalized, requiring low-flow supplemental oxygen; 5, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (related or not to SARS-COV-2 infection); and 6, not hospitalized) all exhibited complete case separation (i.e. zero observed cases in several treatment arms). Consequently, logistic or log-binomial mixed effect regression-based modeling was not possible (as zero or infinite odds or risk ratios would have resulted). Instead, we were restricted to other strategies to compare the primary outcomes across the five treatment arms. We chose two different approaches. The first was to combine the individual outcomes listed above into an ordinal clinical severity score ranging from 1 = not hospitalized to 6 = death. Then, for each measurement period (by 9, 14, and 28 days), we performed a Kruskal Wallis test on this ordinal severity score to test for a difference in location among the various arms. The second approach focused on a difference in the distribution of patients (across the severity classes) among the five treatment groups. For the global test, we used a generalized version of Fisher's exact test for the resulting 6 severity class x 5 treatment group contingency table (for each measurement day). Specifically, we used the algorithm first implemented by Mehal and Patel38 and then later improved by Clarkson, Fan, and Joe.39 The zero cases in many of the groups meant post-hoc comparison among these groups is not only unnecessary (they are clearly not different), but presents considerable statistical challenges. Consequently, we only compared these zero case groups to groups containing cases. For the secondary outcomes (Cytokine levels), we employed linear mixed models to compare levels at particular times and trends between the treatment arms over time. A perusal of the cytokines data revealed a marked decay in variation over time, so the linear mixed model employed allowed unequal variances for the time within-subject effect. For the final set of outcomes, adverse events and PASC symptoms, we used the 2 × g Fisher's exact test for the omnibus test for difference in incidence of a symptom among the five treatment arms, followed by the standard (2 x 2) Fisher's exact test for pairwise comparisons. p-values from these pairwise tests were adjusted using the Holm method40 to control the accumulation of family-wise Type 1 error. All analysis was conducted using the R statistical package41 and mixed effect modeling was performed using the R library, nlme.42 A significance level of 0.05 was used throughout all inferential statistical analysis. For the analysis of viral load and serum cytokine levels, participants with missing data or those who did not volunteer for testing at each time point, the most recent assessment was used for missing values and missing values were mentioned in each figure and table legend.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Participant distribution and characteristics
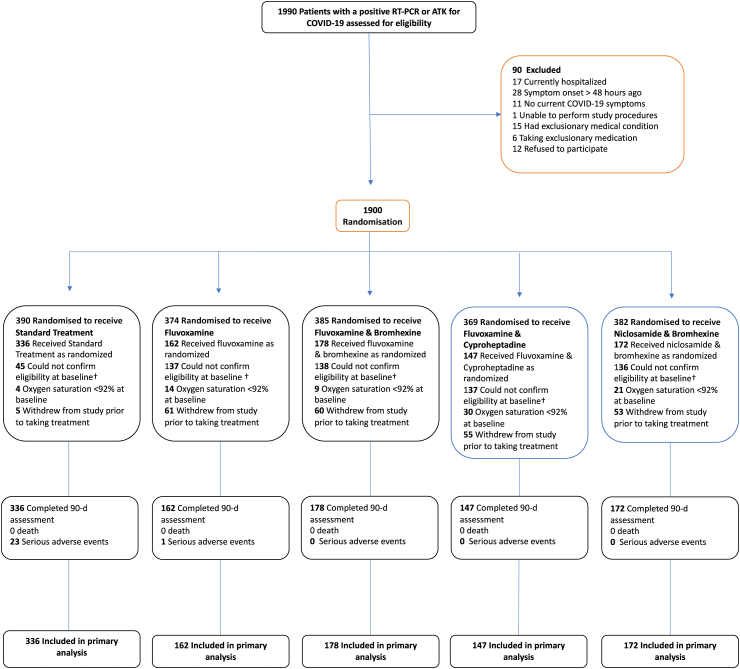
Of the 1990 participants who consented and were assessed for eligibility, 90 were found to be ineligible (Fig. 1). Because of the complexities in performing this trial, of the 1900 who underwent randomization, 905 were subsequently withdrawn for the reasons shown in Table 1. A comparison of the characteristics of those completing the trial and those withdrawn within each treatment allocation group is shown in Table 2. There were no obvious differences, suggesting that the withdrawals may not have caused any major bias despite the withdrawal rate being seen to be far less in the standard care group.
Fig. 1.
Participant flow.
Table 1.
Details of the participants who did not receive treatment after randomisation.
| Reasons for not receiving treatment after randomisation | Standard care (n = 54) | Fluvoxamine (n = 212) | Fluvoxamine + Bromhexine (n = 207) | Fluvoxamine + Cyproheptadine (n = 222) | Niclosamide + Bromhexine (n = 210) |
|---|---|---|---|---|---|
| Experiencing issues related to systolic and diastolic blood pressure | 15 | 40 | 37 | 34 | 30 |
| Refusing to discontinue smoking and alcohol consumption during the trial period | 10 | 25 | 27 | 27 | 20 |
| Taking unknown cosmetic supplements | 5 | 12 | 10 | 12 | 15 |
| Unable to clearly confirm their psychiatric status | 5 | 19 | 17 | 19 | 18 |
| Using illicit drugs or stimulants | 10 | 25 | 33 | 33 | 35 |
| Participants refusing to stop consuming caffeinated beverages | – | 16 | 14 | 17 | 18 |
| Oxygen saturation levels below 92% at baseline | 4 | 14 | 9 | 30 | 21 |
| Withdrew from the study before receiving treatment | 5 | 61 | 60 | 50 | 53 |
Table 2.
Demographics and baseline disease characteristics.
| Characteristic | Standard care |
Fluvoxamine |
Fluvoxamine + Bromhexine |
Fluvoxamine + Cyproheptadine |
Niclosamide + Bromhexine |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Received standard treatment as randomised, N = 336a | Did not receive standard treatment as randomised, N = 54a | Received fluvoxamine as randomised, N = 162a | Did not receive fluvoxamine as randomised, N = 212a | Received fluvoxamine & bromhexine as randomised, N = 178a | Did not receive fluvoxamine & bromhexine as randomised, N = 207a | Received fluvoxamine & cyproheptadine as randomised, N = 147a | Did not receive fluvoxamine & cyproheptadine as randomised, N = 222a | Received niclosamide & bromhexine as randomised, N = 172a | Did not receive niclosamide & bromhexine as randomised, N = 210a | |
| Age years | 36.2 (14.5) | 37.7 (13.2) | 36.1 (15.6) | 35.2 (13.1) | 35.5 (13.1) | 34.5 (13.2) | 35.1 (13.2) | 36.4 (14.6) | 38.1 (14.7) | 37.9 (15.5) |
| Weight kg | 63.6 (20.4) | 61.0 (12.4) | 66.6 (24.3) | 61.8 (19.9) | 64.8 (21.4) | 64.4 (18.6) | 64.8 (21.3) | 67.1 (21.2) | 65.3 (19.9) | 65.5 (23.7) |
| Height cm | 161.8 (9.8) | 161.4 (9.7) | 163.2 (8.8) | 161.5 (11.0) | 162.4 (9.4) | 162.7 (8.8) | 161.4 (9.3) | 163.3 (8.9) | 162.6 (9.4) | 161.4 (8.6) |
| Body mass index (BMI), kg/m2 | 24.2 (7.3) | 23.3 (3.8) | 24.9 (8.7) | 23.6 (7.5) | 24.4 (7.1) | 24.2 (6.2) | 24.9 (8.1) | 25.1 (7.7) | 24.5 (6.6) | 25.1 (8.5) |
| Body temperature celsius | 37.8 (0.7) | 37.7 (0.8) | 37.8 (0.7) | 37.6 (0.6) | 37.7 (0.7) | 37.6 (0.7) | 37.7 (0.7) | 37.9 (0.7) | 37.8 (0.7) | 37.8 (0.7) |
| Systolic blood pressure mmHg | 115.0 (9.8) | 122.0 (13.4) | 115.4 (9.5) | 121.4 (14.3) | 115.9 (9.9) | 120.5 (14.1) | 115.8 (9.6) | 119.6 (14.3) | 115.6 (9.2) | 119.9 (14.5) |
| Diastolic blood pressure mmHg | 74.7 (9.4) | 77.6 (10.8) | 74.6 (9.2) | 79.7 (10.8) | 75.6 (9.0) | 79.7 (11.3) | 75.1 (8.9) | 77.4 (11.9) | 74.6 (9.0) | 77.6 (11.1) |
| Oxygen saturation | 94.4 (1.1) | 94.0 (1.5) | 94.4 (1.1) | 94.1 (1.4) | 94.3 (1.1) | 94.1 (1.2) | 94.2 (1.0) | 93.9 (1.7) | 94.6 (1.1) | 94.2 (1.6) |
| Respiratory rate PR breaths min. | 17.8 (2.3) | 17.7 (1.4) | 18.3 (2.4) | 18.0 (1.6) | 17.8 (2.5) | 18.1 (1.5) | 17.8 (2.1) | 17.9 (3.1) | 18.1 (2.7) | 17.9 (2.7) |
| Heart rate beats min. | 86.6 (19.1) | 87.6 (20.3) | 88.2 (19.4) | 86.4 (17.5) | 88.1 (19.9) | 85.3 (19.5) | 88.1 (19.4) | 89.9 (19.3) | 85.8 (17.4) | 86.1 (18.7) |
| Duration of COVID 19 symptomshrs | ||||||||||
| 0 | 28/336 (8.3%) | 4/54 (7.4%) | 20/162 (12%) | 23/212 (11%) | 22/178 (12%) | 19/207 (9.2%) | 13/147 (8.8%) | 22/222 (9.9%) | 11/172 (6.4%) | 19/210 (9.0%) |
| 12 | 199/336 (59%) | 32/54 (59%) | 103/162 (64%) | 116/212 (55%) | 103/178 (58%) | 110/207 (53%) | 94/147 (64%) | 146/222 (66%) | 104/172 (60%) | 136/210 (65%) |
| 24 | 76/336 (23%) | 15/54 (28%) | 25/162 (15%) | 45/212 (21%) | 33/178 (19%) | 43/207 (21%) | 22/147 (15%) | 41/222 (18%) | 41/172 (24%) | 38/210 (18%) |
| 48 | 33/336 (9.8%) | 3/54 (5.6%) | 14/162 (8.6%) | 28/212 (13%) | 20/178 (11%) | 35/207 (17%) | 18/147 (12%) | 13/222 (5.9%) | 16/172 (9.3%) | 17/210 (8.1%) |
| Ethnicity | ||||||||||
| Thai | 182/336 (54%) | 36/54 (67%) | 91/162 (56%) | 118/212 (56%) | 94/178 (53%) | 111/207 (54%) | 77/147 (52%) | 123/222 (55%) | 102/172 (59%) | 108/210 (51%) |
| Burmese | 65/336 (19%) | 1/54 (1.9%) | 28/162 (17%) | 17/212 (8.0%) | 35/178 (20%) | 11/207 (5.3%) | 27/147 (18%) | 72/222 (32%) | 29/172 (17%) | 53/210 (25%) |
| Lao | 89/336 (26%) | 17/54 (31%) | 43/162 (27%) | 77/212 (36%) | 49/178 (28%) | 85/207 (41%) | 43/147 (29%) | 27/222 (12%) | 41/172 (24%) | 49/210 (23%) |
| Sex | ||||||||||
| Male | 130/336 (39%) | 22/54 (41%) | 70/162 (43%) | 88/212 (42%) | 80/178 (45%) | 82/207 (40%) | 51/147 (35%) | 94/222 (42%) | 75/172 (44%) | 83/210 (40%) |
| Female | 206/336 (61%) | 32/54 (59%) | 92/162 (57%) | 124/212 (58%) | 98/178 (55%) | 125/207 (60%) | 96/147 (65%) | 128/222 (58%) | 97/172 (56%) | 127/210 (60%) |
| Vaccination status | ||||||||||
| Never | 10/336 (3.0%) | 1/54 (1.9%) | 5/162 (3.1%) | 13/212 (6.1%) | 4/178 (2.2%) | 15/207 (7.2%) | 7/147 (4.8%) | 0/222 (0%) | 7/172 (4.1%) | 2/210 (1.0%) |
| RNA (1 shot) | 43/336 (13%) | 1/54 (1.9%) | 19/162 (12%) | 10/212 (4.7%) | 28/178 (16%) | 7/207 (3.4%) | 13/147 (8.8%) | 50/222 (23%) | 27/172 (16%) | 41/210 (20%) |
| Viral vector (1 shot) | 39/336 (12%) | 1/54 (1.9%) | 27/162 (17%) | 4/212 (1.9%) | 25/178 (14%) | 6/207 (2.9%) | 13/147 (8.8%) | 60/222 (27%) | 29/172 (17%) | 40/210 (19%) |
| Inactivated (1 shot) | 53/336 (16%) | 3/54 (5.6%) | 24/162 (15%) | 23/212 (11%) | 29/178 (16%) | 17/207 (8.2%) | 27/147 (18%) | 58/222 (26%) | 26/172 (15%) | 46/210 (22%) |
| RNA (2 shot) | 36/336 (11%) | 3/54 (5.6%) | 11/162 (6.8%) | 20/212 (9.4%) | 18/178 (10%) | 19/207 (9.2%) | 20/147 (14%) | 27/222 (12%) | 21/172 (12%) | 29/210 (14%) |
| Viral vector (2 shot) | 40/336 (12%) | 11/54 (20%) | 19/162 (12%) | 28/212 (13%) | 21/178 (12%) | 33/207 (16%) | 16/147 (11%) | 23/222 (10%) | 25/172 (15%) | 17/210 (8.1%) |
| Inactivated (2 shot) | 5/336 (1.5%) | 2/54 (3.7%) | 2/162 (1.2%) | 4/212 (1.9%) | 2/178 (1.1%) | 7/207 (3.4%) | 4/147 (2.7%) | 0/222 (0%) | 4/172 (2.3%) | 3/210 (1.4%) |
| Viral vector + RNA | 24/336 (7.1%) | 7/54 (13%) | 19/162 (12%) | 28/212 (13%) | 18/178 (10%) | 32/207 (15%) | 10/147 (6.8%) | 3/222 (1.4%) | 13/172 (7.6%) | 12/210 (5.7%) |
| Inactivated + Viral vector | 8/336 (2.4%) | 2/54 (3.7%) | 1/162 (0.6%) | 7/212 (3.3%) | 2/178 (1.1%) | 5/207 (2.4%) | 3/147 (2.0%) | 0/222 (0%) | 0/172 (0%) | 0/210 (0%) |
| Inactivated + RNA | 54/336 (16%) | 12/54 (22%) | 26/162 (16%) | 57/212 (27%) | 18/178 (10%) | 41/207 (20%) | 25/147 (17%) | 1/222 (0.5%) | 13/172 (7.6%) | 16/210 (7.6%) |
| Inactivated + Viral vector + Viral vector | 9/336 (2.7%) | 8/54 (15%) | 7/162 (4.3%) | 9/212 (4.2%) | 5/178 (2.8%) | 9/207 (4.3%) | 1/147 (0.7%) | 0/222 (0%) | 5/172 (2.9%) | 0/210 (0%) |
| Inactivated + RNA + RNA | 4/336 (1.2%) | 1/54 (1.9%) | 0/162 (0%) | 5/212 (2.4%) | 3/178 (1.7%) | 4/207 (1.9%) | 4/147 (2.7%) | 0/222 (0%) | 1/172 (0.6%) | 1/210 (0.5%) |
| Inactivated + Inactivated + Viral vector | 4/336 (1.2%) | 0/54 (0%) | 1/162 (0.6%) | 2/212 (0.9%) | 2/178 (1.1%) | 3/207 (1.4%) | 1/147 (0.7%) | 0/222 (0%) | 0/172 (0%) | 2/210 (1.0%) |
| Inactivated + Inactivated + RNA | 4/336 (1.2%) | 2/54 (3.7%) | 1/162 (0.6%) | 2/212 (0.9%) | 3/178 (1.7%) | 4/207 (1.9%) | 2/147 (1.4%) | 0/222 (0%) | 0/172 (0%) | 1/210 (0.5%) |
| Viral vector + Viral vector + RNA | 2/336 (0.6%) | 0/54 (0%) | 0/162 (0%) | 0/212 (0%) | 0/178 (0%) | 4/207 (1.9%) | 1/147 (0.7%) | 0/222 (0%) | 1/172 (0.6%) | 0/210 (0%) |
| Don't know | 1/336 (0.3%) | 0/54 (0%) | 0/162 (0%) | 0/212 (0%) | 0/178 (0%) | 1/207 (0.5%) | 0/147 (0%) | 0/222 (0%) | 0/172 (0%) | 0/210 (0%) |
| Blood group | ||||||||||
| O− | 6/336 (1.8%) | 1/54 (1.9%) | 2/162 (1.2%) | 4/212 (1.9%) | 2/178 (1.1%) | 7/207 (3.4%) | 2/147 (1.4%) | 0/222 (0%) | 0/172 (0%) | 0/210 (0%) |
| O+ | 73/336 (22%) | 15/54 (28%) | 34/162 (21%) | 33/212 (16%) | 34/178 (19%) | 43/207 (21%) | 31/147 (21%) | 49/222 (22%) | 40/172 (23%) | 48/210 (23%) |
| A− | 63/336 (19%) | 7/54 (13%) | 36/162 (22%) | 30/212 (14%) | 33/178 (19%) | 27/207 (13%) | 23/147 (16%) | 52/222 (23%) | 30/172 (17%) | 47/210 (22%) |
| A+ | 79/336 (24%) | 14/54 (26%) | 33/162 (20%) | 43/212 (20%) | 37/178 (21%) | 24/207 (12%) | 31/147 (21%) | 63/222 (28%) | 35/172 (20%) | 48/210 (23%) |
| B− | 58/336 (17%) | 5/54 (9.3%) | 20/162 (12%) | 44/212 (21%) | 32/178 (18%) | 38/207 (18%) | 32/147 (22%) | 46/222 (21%) | 34/172 (20%) | 34/210 (16%) |
| B+ | 27/336 (8.0%) | 7/54 (13%) | 20/162 (12%) | 28/212 (13%) | 20/178 (11%) | 22/207 (11%) | 13/147 (8.8%) | 12/222 (5.4%) | 22/172 (13%) | 23/210 (11%) |
| AB− | 7/336 (2.1%) | 1/54 (1.9%) | 2/162 (1.2%) | 7/212 (3.3%) | 4/178 (2.2%) | 11/207 (5.3%) | 3/147 (2.0%) | 0/222 (0%) | 5/172 (2.9%) | 2/210 (1.0%) |
| AB+ | 16/336 (4.8%) | 3/54 (5.6%) | 14/162 (8.6%) | 21/212 (9.9%) | 14/178 (7.9%) | 30/207 (14%) | 12/147 (8.2%) | 0/222 (0%) | 6/172 (3.5%) | 6/210 (2.9%) |
| Don't know | 7/336 (2.1%) | 1/54 (1.9%) | 1/162 (0.6%) | 2/212 (0.9%) | 2/178 (1.1%) | 5/207 (2.4%) | 0/147 (0%) | 0/222 (0%) | 0/172 (0%) | 2/210 (1.0%) |
| Smoking level | ||||||||||
| Never | 142/336 (42%) | 26/54 (48%) | 76/162 (47%) | 78/212 (37%) | 80/178 (45%) | 81/207 (39%) | 54/147 (37%) | 117/222 (53%) | 75/172 (44%) | 90/210 (43%) |
| Smoke but not every day | 80/336 (24%) | 11/54 (20%) | 42/162 (26%) | 57/212 (27%) | 37/178 (21%) | 52/207 (25%) | 33/147 (22%) | 47/222 (21%) | 46/172 (27%) | 49/210 (23%) |
| Smoke every day | 66/336 (20%) | 9/54 (17%) | 24/162 (15%) | 53/212 (25%) | 36/178 (20%) | 37/207 (18%) | 39/147 (27%) | 28/222 (13%) | 27/172 (16%) | 43/210 (20%) |
| Smoke but not every day (used to) | 33/336 (9.8%) | 3/54 (5.6%) | 14/162 (8.6%) | 16/212 (7.5%) | 10/178 (5.6%) | 24/207 (12%) | 9/147 (6.1%) | 15/222 (6.8%) | 13/172 (7.6%) | 15/210 (7.1%) |
| Smoke every day (used to) | 15/336 (4.5%) | 5/54 (9.3%) | 6/162 (3.7%) | 8/212 (3.8%) | 15/178 (8.4%) | 13/207 (6.3%) | 12/147 (8.2%) | 15/222 (6.8%) | 11/172 (6.4%) | 13/210 (6.2%) |
| Alcohol drinking level | ||||||||||
| Never | 179/336 (53%) | 13/54 (24%) | 95/162 (59%) | 91/212 (43%) | 92/178 (52%) | 78/207 (38%) | 65/147 (44%) | 157/222 (71%) | 88/172 (51%) | 136/210 (65%) |
| Drink low, 1–2 times a week. | 85/336 (25%) | 20/54 (37%) | 47/162 (29%) | 77/212 (36%) | 47/178 (26%) | 58/207 (28%) | 38/147 (26%) | 40/222 (18%) | 51/172 (30%) | 48/210 (23%) |
| Drink moderately, 3–4 times a week. | 72/336 (21%) | 21/54 (39%) | 20/162 (12%) | 44/212 (21%) | 39/178 (22%) | 71/207 (34%) | 44/147 (30%) | 25/222 (11%) | 33/172 (19%) | 26/210 (12%) |
| Variant | ||||||||||
| Omicron B.1.1.529 | 189/336 (56%) | 32/54 (59%) | 105/162 (65%) | 118/212 (56%) | 107/178 (60%) | 140/207 (68%) | 84/147 (57%) | 126/222 (57%) | 104/172 (60%) | 113/210 (54%) |
| Delta B.1.617.2 | 88/336 (26%) | 12/54 (22%) | 37/162 (23%) | 54/212 (25%) | 48/178 (27%) | 32/207 (15%) | 32/147 (22%) | 62/222 (28%) | 35/172 (20%) | 61/210 (29%) |
| Alpha B.1.1.7 | 59/336 (18%) | 10/54 (19%) | 20/162 (12%) | 40/212 (19%) | 23/178 (13%) | 35/207 (17%) | 31/147 (21%) | 34/222 (15%) | 33/172 (19%) | 36/210 (17%) |
| Symptoms | ||||||||||
| Loss of sense of smell | 204/336 (61%) | 34/54 (63%) | 97/162 (60%) | 112/212 (53%) | 117/178 (66%) | 119/207 (57%) | 93/147 (63%) | 157/222 (71%) | 116/172 (67%) | 146/210 (70%) |
| Fatigue | 181/336 (54%) | 20/54 (37%) | 74/162 (46%) | 112/212 (53%) | 95/178 (53%) | 103/207 (50%) | 71/147 (48%) | 115/222 (52%) | 86/172 (50%) | 110/210 (52%) |
| Body aches | 234/336 (70%) | 43/54 (80%) | 115/162 (71%) | 127/212 (60%) | 113/178 (63%) | 130/207 (63%) | 100/147 (68%) | 168/222 (76%) | 108/172 (63%) | 140/210 (67%) |
| Cough | 169/336 (50%) | 30/54 (56%) | 78/162 (48%) | 105/212 (50%) | 87/178 (49%) | 93/207 (45%) | 68/147 (46%) | 111/222 (50%) | 80/172 (47%) | 96/210 (46%) |
| Subjective fever | 143/336 (43%) | 15/54 (28%) | 62/162 (38%) | 81/212 (38%) | 73/178 (41%) | 77/207 (37%) | 72/147 (49%) | 98/222 (44%) | 79/172 (46%) | 116/210 (55%) |
| Loss of appetite | 194/336 (58%) | 33/54 (61%) | 94/162 (58%) | 107/212 (50%) | 107/178 (60%) | 89/207 (43%) | 82/147 (56%) | 157/222 (71%) | 108/172 (63%) | 140/210 (67%) |
| Chills | 191/336 (57%) | 34/54 (63%) | 96/162 (59%) | 102/212 (48%) | 104/178 (58%) | 95/207 (46%) | 75/147 (51%) | 138/222 (62%) | 90/172 (52%) | 130/210 (62%) |
| Shortness of breath | 230/336 (68%) | 33/54 (61%) | 104/162 (64%) | 125/211 (59%) | 132/177 (75%) | 122/207 (59%) | 103/147 (70%) | 181/222 (82%) | 127/172 (74%) | 169/210 (80%) |
| Loss of taste | 133/336 (40%) | 17/54 (31%) | 71/162 (44%) | 74/211 (35%) | 91/177 (51%) | 90/207 (43%) | 70/147 (48%) | 102/222 (46%) | 76/172 (44%) | 109/210 (52%) |
| Dyspnea | 93/336 (28%) | 9/54 (17%) | 50/162 (31%) | 43/212 (20%) | 64/178 (36%) | 67/207 (32%) | 49/147 (33%) | 79/222 (36%) | 48/172 (28%) | 74/210 (35%) |
| Fatigue | 90/336 (27%) | 5/54 (9.3%) | 43/162 (27%) | 36/212 (17%) | 60/178 (34%) | 39/207 (19%) | 42/147 (29%) | 86/222 (39%) | 39/172 (23%) | 74/210 (35%) |
| Myalgia | 77/336 (23%) | 4/54 (7.4%) | 35/162 (22%) | 22/212 (10%) | 56/178 (31%) | 38/207 (18%) | 34/147 (23%) | 72/222 (32%) | 36/172 (21%) | 70/210 (33%) |
| Dairrhea | 203/336 (60%) | 29/54 (54%) | 89/162 (55%) | 96/212 (45%) | 110/178 (62%) | 107/207 (52%) | 80/147 (54%) | 152/222 (68%) | 101/172 (59%) | 135/210 (64%) |
| Sore throat | 175/336 (52%) | 31/54 (57%) | 82/162 (51%) | 79/212 (37%) | 92/178 (52%) | 100/207 (48%) | 83/147 (56%) | 132/222 (59%) | 90/172 (52%) | 130/210 (62%) |
| Nausea | 240/336 (71%) | 39/54 (72%) | 105/162 (65%) | 133/212 (63%) | 124/178 (70%) | 135/207 (65%) | 106/147 (72%) | 159/222 (72%) | 123/172 (72%) | 164/210 (78%) |
| Concomitant medications, No. (%) | ||||||||||
| Favipiravir | 160/336 (48%) | 21/54 (39%) | – | – | – | – | – | – | – | – |
Mean (SD); n/N (%).
Participants enrolled in the five arms were balanced in demographic and clinical characteristics (Table 2). There were 54.9% (546) were of Thai ethnicity, and the mean age was 36.3 years (SD, 13 years) with a mean BMI (kg/m2) of 24.5 (SD, 7.6). Overall, 57.1% (568) of participants were smokers, and 47.8% (476) of participants consumed alcohol. A minority of participants (3.3%, 10) were not vaccinated for SARS-CoV-2 at screening. A total of 697 (70.1%) patients received treatment within 12 h from symptom onset (Table 2). At initial screening, participants had a mean oxygen saturation of 94.0% (SD, 1.1) while breathing room air, and a mean body temperature of 37.7 °C (SD, 0.7 °C). 59.2% (589) of participants were infected with Omicron B.1.1.529, 24.1% (240) with Delta B.1.617.2 and 16.7% (166) with Alpha B.1.1.7 variant. The presenting COVID-19 symptoms varied, with loss of sense of smell (63% (627)), body aches (67% (670)), shortness of breath (70% (696)) and nausea (70.2% (698)) being the most commonly reported symptoms. The longitudinal data for nasopharyngeal viral load (based on cycle threshold values) and the serum cytokine levels of IL-6, IL-8, TNF-α, IL-1β were available in 57–63 participants from each arm, which corresponded to n = 63 (18.7%) of participants in the standard care arm, n = 62 (38.3%) in fluvoxamine arm, n = 58 (32.6%) in fluvoxamine + bromhexine arm, n = 62 (42.2%) in fluvoxamine + cyproheptadine arm, and n = 57 (33.1%) in niclosamide + bromhexine arm.
Primary endpoint
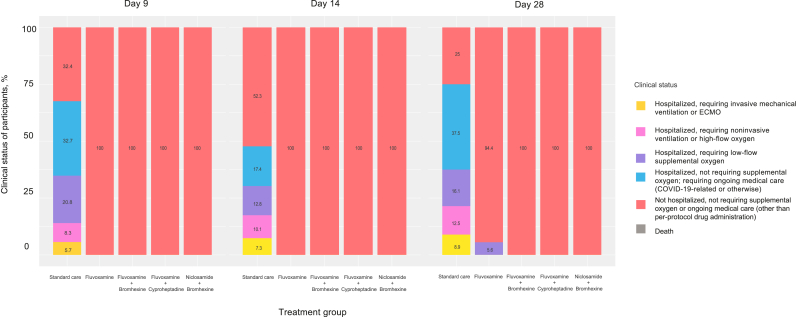
All treated groups (fluvoxamine arm (9 of 163), fluvoxamine plus bromhexine arm (0 of 178), fluvoxamine plus cyproheptadine arm (0 of 147), and niclosamide plus bromhexine arm (0 of 172)) significantly differed from the standard care group (321 of 336) by days 9, 14, and 28 (p < 0.0001) (Fig. 2 and Table 3). Also, by day 28, the three 2-drug treatments (fluvoxamine plus bromhexine arm, fluvoxamine plus cyproheptadine arm, and niclosamide plus bromhexine arm) were significantly better than the fluvoxamine arm (p < 0.0001). Although numerically, a large number of participants in the standard care arm experienced clinical deterioration, the vast majority of these deteriorated cases (53.5% (180) by study day 9, 30.2% (33) by day 14, and 53.6% (30) by day 28 (among the 336) experienced mild symptoms and only required low-flow or no supplemental oxygen. Nevertheless, 14% (47) of participants in the standard care arm experienced clinical deterioration to severe COVID-19 by study day 9, requiring high-flow oxygen, non-invasive or mechanical ventilation. In contrast, none of the participants in the treatment arms of fluvoxamine plus bromhexine (0%), fluvoxamine plus cyproheptadine (0%), or niclosamide plus bromhexine (0%) experienced clinical deterioration through study day 28, while 9 participants (5.6%) in the fluvoxamine arm eventually experienced clinical deterioration requiring low-flow oxygen between study days 14 and 28 (all after completion of the 14-day fluvoxamine course). There were no deaths reported in any study arm. Results of the Kruskal–Wallis tests revealed a difference in severity among at least some of the treatment groups by day 9, 14, and 28 (p < 0.0001). We also tested for differences in the distribution of patients (across the severity classes) among the five groups by day 9, 14, and 28 and found a significant difference (all p < 0.0001). A perusal of Table 3 reveals that the difference is clear between the standard care group and all of the treatment groups. Table 3 also strongly suggests that by 28, the two-drug treatment (fluvoxamine plus bromhexine arm, fluvoxamine plus cyproheptadine arm, and niclosamide plus bromhexine arm) groups were superior to the fluvoxamine arm. We also confirmed this by running a generalized Fisher's exact test involving only the four treated groups (fluvoxamine arm, fluvoxamine plus bromhexine arm, fluvoxamine plus cyproheptadine arm, and niclosamide plus bromhexine arm) (p < 0.0001).
Fig. 2.
Clinical status of participants on a 6-point ordinal scale on study days 9, 14, and 28 by treatment group.
Table 3.
Clinical outcomes on a 6-point ordinal scale by study days 9, 14, and 28. 1 missing value in standard care on day 28.
| Treatment group as number (%) of person in each follow-up day | Outcomes; clinical status on 6-point scale |
||||||
|---|---|---|---|---|---|---|---|
| 1: Death | 2: Hospitalised, requiring invasive mechanical ventilation or extracorporeal membrane oxygenation | 3: Hospitalised, requiring non-invasive ventilation or high-flow oxygen | 4: Hospitalised, requiring low-flow supplemental oxygen | 5: Hospitalised, not requiring supplemental oxygen; requiring ongoing medical care (COVID-19-related or otherwise) | 6: Not hospitalised, not requiring supplemental oxygen or ongoing medical care (other than per-protocol drug administration) | ||
| Day 9 | Standard care | 0 (0.0) | 19 (5.7) | 28 (8.3) | 70 (20.8) | 110 (32.7) | 109 (32.4) |
| Fluvoxamine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 162 (100) | |
| Fluvoxamine + Bromhexine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 178 (100) | |
| Fluvoxamine + Cyproheptadine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 147 (100) | |
| Niclosamide + Bromhexine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 172 (100) | |
| Day 14 | Standard care | 0 (0.0) | 8 (7.3) | 11 (10.1) | 14 (12.8) | 19 (17.4) | 57 (52.3) |
| Fluvoxamine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 162 (100) | |
| Fluvoxamine + Bromhexine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 178 (100) | |
| Fluvoxamine + Cyproheptadine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 147 (100) | |
| Niclosamide + Bromhexine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 172 (100) | |
| Day 28 | Standard care | 0 (0.0) | 5 (8.9) | 7 (12.5) | 9 (16.1) | 21 (37.5) | 14 (25) |
| Fluvoxamine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (5.6) | 0 (0.0) | 153 (94.4) | |
| Fluvoxamine + Bromhexine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 178 (100) | |
| Fluvoxamine + Cyproheptadine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 147 (100) | |
| Niclosamide + Bromhexine | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 172 (100) | |
Adverse events
Adverse events occurred significantly (p < 0.0001) more often among participants in the standard care arm compared with the participants in the four treatment arms (Table 4). Compared with the combination agents, fluvoxamine monotherapy was overall less well-tolerated due to gastrointestinal adverse events such as nausea and vomiting, which occurred most commonly in the fluvoxamine monotherapy arm (24.7% (40)). Other adverse events included headache (most common in the fluvoxamine plus bromhexine arm, 37.1% (66)), and muscle aches (most common in the niclosamide plus bromhexine arm, 27.3% (47)). Among treatment arms, the only serious adverse event occurred in the fluvoxamine monotherapy arm (1 participant, 0.6%). No serious adverse events were reported among participants treated with the combination agents. In contrast, 23 participants (6.8%) in the standard care arm reported serious adverse events.
Table 4.
Adverse event summary.
| Adverse events | No. of participants with event (%) |
||||
|---|---|---|---|---|---|
| Standard care (n = 336) | Fluvoxamine (n = 162) | Fluvoxamine + Bromhexine (n = 178) | Fluvoxamine + Cyproheptadine (n = 147) | Niclosamine + Bromhexine (n = 172) | |
| Event details | |||||
| Gastroenteritis, nausea, or vomiting, n (%) | 27 (8.0) | 40 (24.7) | 1 (0.6) | 3 (2.0) | 2 (1.2) |
| Muscle aches | 87 (25.9) | 32 (19.8) | 2 (1.1) | 17 (11.6) | 47 (27.3) |
| Headache or head pain | 49 (14.6) | 8 (4.9) | 66 (37.1) | 28 (19.0) | 2 (1.2) |
| Loss of coordination with seizures/convulsions | 23 (6.8) | 1 (0.6) | 0 (0) | 0 (0) | 0 (0) |
| Changes in vision | 108 (32.1) | 0 (0) | 0 (0) | 0 (0) | n (0) |
| Changes in hearing | 101 (30.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Teeth chattering | 101 (30.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dehydration | 101 (30.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Chest pain or tightness | 100 (32.1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Difficulties with smelling or tasting | 91 (27.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vasovagal syncope | 91 (27.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Numbness or tingling in the skin | 77 (22.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Low oxygen or hypoxia | 68 (20.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fever | 44 (13.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Bacterial infection | 24 (7.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Acute respiratory failure | 23 (6.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Shortness of breath | 19 (5.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pneumonia | 19 (5.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Seriousness of Event | |||||
| Serious adverse events | 23 (6.8) | 1 (0.6) | 0 (0) | 0 (0) | 0 (0) |
| Other adverse events (people in each group had at least one non-serious AE) | 173 (51.5) | 75 (46.3) | 67 (37.6) | 43 (29.3) | 47 (27.3) |
Dynamics of SARS-CoV-2 viral load
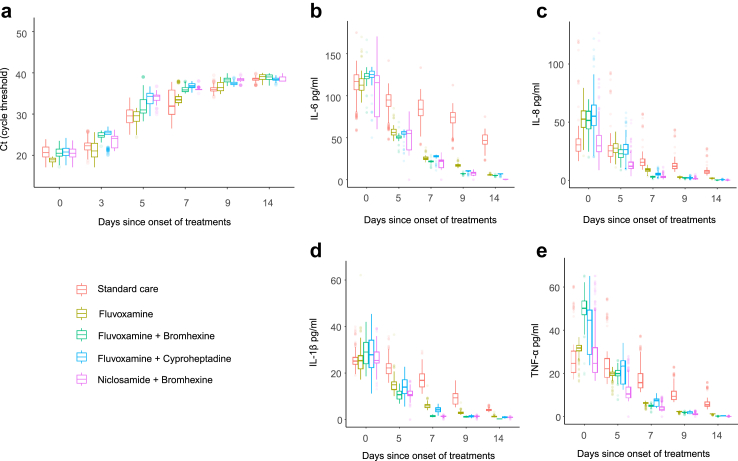
The trend of nasopharyngeal viral load was determined by longitudinal assessment of cycle threshold (Ct) values derived from RT-PCR results of nasopharyngeal samples at the time of randomization (day 0), and on treatment days 3, 5, 7, 9, and 14 (Fig. 3a). Higher Ct values signify lower viral loads in the nasopharynx. However, as early as on day 3 of treatment and throughout days 5, 7 and 9, participants treated with fluvoxamine plus bromhexine (p < 0.0001), fluvoxamine plus cyproheptadine (p < 0.0001), or niclosamide plus bromhexine (p < 0.0001), demonstrated a significantly lower viral load relative to the participants treated with standard care (Supplementary Table S1). However, on day 14, fluvoxamine plus bromhexine (p = 0.0001) demonstrated a significantly lower viral load relative to the participants treated with standard care. The niclosamide plus bromhexine shows a lower viral load compared to fluvoxamine plus bromhexine on days 3 (p < 0.0001), 5 (p < 0.0001), 7 (p < 0.0001), 14 (p < 0.0001), and fluvoxamine plus cyproheptadine on days 3 (p < 0.0001) and 9 (p < 0.0001). The magnitude of the decrease in viral load from baseline was most pronounced between trial arms on day 5 of treatment. An approximate 18–20-fold decrease in viral load (based on Ct values) at day 5, relative to standard care, was observed in participants treated with fluvoxamine plus cyproheptadine or niclosamide plus bromhexine. Monotherapy with fluvoxamine was superior to standard care in decreasing viral load on days 3 (p < 0.0001), 7 (p = 0.02), 9 (p < 0.0001), and 14 (p = 0.0006) but was inferior to treatment with the combination agents on days 3, 5, 7, and 9 (all p < 0.0001). On day 14, fluvoxamine plus cyproheptadine (p = 0.019) and niclosamide plus bromhexine (p = 0.0002) were only superior to treatment with fluvoxamine. Significantly higher viral loads were observed in the fluvoxamine plus cyproheptadine relative to the fluvoxamine plus bromhexine on days 5 (p < 0.0001), 7 (p = 0.023), 9 (p < 0.0001) and 14 (p = 0.008).
Fig. 3.
a) Cycle threshold (Ct) values for different days since onset of treatments, level of systemic pro-inflammatory cytokines b) IL-6, c) IL-8, d) TNF-α, e) IL-1β for different days since treatment initiation. (Standard care (n = 63), Fluvoxamine (n = 62), Fluvoxamine + Bromhexine (n = 58), Fluvoxamine + Cyproheptadine (n = 62), Niclosamide + Bromhexine (n = 57)). 273 participants from the standard care group, 100 participants from the fluvoxamine-only group, 120 participants from the fluvoxamine + bromhexine group, 85 participants from the fluvoxamine + cyproheptadine group, and 115 participants from the niclosamide + bromhexine group declined to provide voluntary nasal and blood samples. In Ct values and cytokine measurements, the most recent assessment was used for analysis as 1 missing value in standard care on day 5, 2 missing values in fluvoxamine on day 9, 2 missing values in fluvoxamine + bromhexine on day 5, 1 missing value in fluvoxamine + cyproheptadine on day 5, and 2 missing values in Niclosamide + Bromhexine on day 14.
Variation of pro-inflammatory cytokines
On days 7, 9, and 14, a reduction in serum levels of IL-6, IL-8, TNF-α, and IL-1β was observed across fluvoxamine (p < 0.0001), fluvoxamine plus bromhexine (p < 0.0001), fluvoxamine plus cyproheptadine (p < 0.0001), and niclosamide plus bromhexine (p < 0.0001) in comparison to standard care (Fig. 3b–e) (Supplementary Tables S2–S5). A comparable reduction persisted on day 5 for TNF-α, IL-6, and IL-1β in all the treatment groups compared to standard care. However, a reduction in IL-8 compared to standard care was observed only in the fluvoxamine plus bromhexine (p < 0.0001) and niclosamide plus bromhexine (p < 0.0001) on day 5.
Notably, TNF-α values were reduced for fluvoxamine plus bromhexine on days 7 (p < 0.0001) and 14 (p < 0.0001), fluvoxamine plus cyproheptadine on days 5 (p = 0.002) and 14 (p = 0.002), and niclosamide plus bromhexine on days 5, 7, 9, and 14 (all p < 0.0001), when compared to fluvoxamine. Furthermore, there was a reduction in IL-8 values for fluvoxamine plus bromhexine on days 5 (p < 0.0001), 7 (p < 0.0001), and 14 (p = 0.0037), fluvoxamine plus cyproheptadine on days 7 (p < 0.0001) and 14 (p = 0.037), and niclosamide plus bromhexine on days 5 (p < 0.0001), 7 (p < 0.0001), 9 (p = 0.0077), and 14 (p = 0.0012), in comparison to fluvoxamine. Moreover, a reduction in IL-6 values was observed for fluvoxamine plus bromhexine on days 5 (p < 0.0001), 7 (p < 0.0001), and 9 (p < 0.0001), fluvoxamine plus cyproheptadine on day 9 (p < 0.0001), and niclosamide plus bromhexine on days 5, 7, 9, and 14 (all p < 0.0001), when compared to fluvoxamine. The reduction in IL-1β values was also noticed for fluvoxamine plus bromhexine on days 5, 7, 9, and 14 (all p < 0.0001), fluvoxamine plus cyproheptadine on day 7 (p < 0.0001) and 9 (p < 0.0001), and niclosamide plus bromhexine on days 5, 7, and 9 (all p < 0.0001), in comparison to fluvoxamine.
Interestingly, niclosamide plus bromhexine demonstrated reduced TNF-α values compared to both fluvoxamine plus bromhexine on days 5 (p < 0.0001), 7 (p < 0.0001), and 9 (p = 0.0022), and fluvoxamine plus cyproheptadine on days 5 (p < 0.0001), 7 (p < 0.0001), and 9 (p = 0.012). Similarly, a reduction was observed for IL-8 in comparison to fluvoxamine plus bromhexine on day 5 (p < 0.0001) and fluvoxamine plus cyproheptadine on days 5 and 7 (all p < 0.0001). Higher IL-6 values were noted for fluvoxamine plus bromhexine on day 14 (p < 0.0001) and fluvoxamine plus cyproheptadine on days 5 (p < 0.0001), 7 (p < 0.0001), 9 (p = 0.0052), and 14 (p < 0.0001) compared to niclosamide plus bromhexine. Additionally, a reduction in IL-1β values was observed for niclosamide plus bromhexine compared to fluvoxamine plus bromhexine on day 14 (p = 0.023) and fluvoxamine plus cyproheptadine on days 5 and 7 (all p < 0.0001). Fluvoxamine plus cyproheptadine demonstrated a reduction in TNF-α on days 5 (p = 0.077) and 7 (p < 0.0001). Similarly, for IL-8, significant reductions were observed on days 5 (p < 0.0001) and 7 (p < 0.0001). Additionally, for IL-6, reductions were noted on days 5 (p = 0.002), 7 (p < 0.0001), and 9 (p = 0.0061). Lastly, for IL-1β, significant reductions were observed on days 5 (p < 0.0001) and 7 (p < 0.0001), with a further reduction on day 14 (p = 0.023), all compared to fluvoxamine plus bromhexine.
Post-acute sequelae of COVID-19 syndrome (PASC)
The presence of PASC symptoms was assessed in the trial participants using a questionnaire 90 days after the end of treatment. The questionnaire used for this assessment was based on the best evidence available on PASC at the time of the trial conduction (authors acknowledge the continually evolving nature of PASC definitions). The percentage of participants reporting any PASC symptoms was significantly (p < 0.0001) higher in the standard care arm relative to those in the treatment arms (Table 5). None of the participants in the treatment arms reported long-term cognitive symptoms on 90-day follow up, and there were substantial reductions in the incidence of other PASC symptoms reported among participants in the treatment arms relative to standard care (Table 5). Compared to fluvoxamine monotherapy, treatment with the combination agents was associated with a lower incidence of all PASC symptoms. We ran 2 x P generalized Fisher's exact test (to examine whether any groups differed) and 2 x 2 Fisher's exact tests to pairwise compare the individual groups (with p-values adjusted for multiple testing). The results of these comparisons can be found in Supplementary Table S7.
Table 5.
PASC symptoms summary.
| Standard care (n = 336) | Fluvoxamine (n = 162) | Fluvoxamine + Bromhexine (n = 178) | Fluvoxamine + Cyproheptadine (n = 147) | Niclosamide + Bromhexine (n = 172) | |
|---|---|---|---|---|---|
| Myalgia (%) | 333 (99.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cognitive symptoms/Headache (%) | 324 (96.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pain (%) | 277 (82.4) | 50 (30.9) | 14 (7.9) | 11 (7.5) | 5 (2.9) |
| Anxiety/Depression/Fatigue (%) | 327 (97.3) | 61 (37.7) | 14 (7.9) | 11 (7.5) | 5 (2.9) |
| Abdominal symptoms (%) | 313 (93.2) | 69 (42.6) | 52 (29.2) | 47 (32.0) | 13 (7.6) |
| Abnormal breathing (%) | 281 (83.6) | 95 (58.6) | 79 (44.4) | 69 (46.9) | 52 (30.2) |
| Chest/Throat pain (%) | 283 (84.2) | 60 (37.0) | 14 (7.9) | 27 (18.4) | 22 (12.8) |
| Anya (%) | 336 (100.0) | 97 (59.9) | 87 (48.9) | 76 (51.7) | 70 (40.7) |
Any of the symptoms in the above rows.
Discussion
In this clinical trial, early treatment with the combination agents of fluvoxamine plus bromhexine, fluvoxamine plus cyproheptadine, or niclosamide plus bromhexine, among outpatients diagnosed with COVID-19 completely eliminated the risk of clinical deterioration within the acute phase (28 days) compared to those who received standard care. Moreover, treatment with the combination agents was superior to fluvoxamine alone, as 9 participants in the fluvoxamine arm eventually experienced clinical deterioration requiring low-flow oxygen between study days 14 and 28 (all after completion of the 14-day fluvoxamine course). This observation supports the hypothesis that the combination agents possess higher efficacy and durability of benefit relative to fluvoxamine alone and to standard care. This benefit was supported by two compelling lines of virologic and biochemical evidence.
Beyond the risk of clinical deterioration in the acute setting, the early use of combination agents in this trial was associated with a lower burden of PASC symptoms relative to standard care in long-term follow-up. PASC is not only a significant source of morbidity and disability for the affected individuals but also a matter of worldwide concern with grave long-term economic and healthcare implications. PASC symptoms are often multisystemic, and can be clustered into three groups of symptoms: primarily cardiorespiratory symptoms, myalgias/autonomic instability, and cognitive impairment.37,43,44 These symptoms can manifest as severe disabling fatigue, cognitive impairment, chest pain, and difficulty breathing. The frequency and severity of PASC symptoms is directly correlated with the severity of the initial COVID-19 infection.44,45 Given the level of disability caused by PASC-associated cognitive impairment,44,46 these findings alone represent an important advancement in knowledge regarding the prevention of this syndrome. It has been suggested that S1R agonists such as fluvoxamine may be associated with lower risk of PASC symptoms.36,47 Furthermore, a recent study linking PASC with reduced levels of serotonin, a neurotransmitter involved in learning, memory, and mood, points toward a new potential mechanism underlying post–COVID-19 conditions,48,49 which might have potential to be mitigated by treatment with SSRIs like fluvoxamine. In addition to vaccination, early treatment with antiviral nirmatrelvir-ritonavir has been associated in a large retrospective analysis with a 26% reduction in the risk of PASC. Similarly, and of more relevance to low-resource settings, SSRI use during acute COVID-19 has also been associated with a 26% reduction in the risk of PASC.12,48 The frequency and severity of PASC symptoms appear to be directly correlated with the severity of the initial COVID-19 infection.50 Specifically, a higher serum IL-6 level upon infection with COVID-19 was associated with a higher risk of PASC later on.51 Additionally, individuals with PASC were found to harbor persistently elevated levels of IL-6, TNF-α, and IL-1β (43, 44), all of which were decreased rapidly and significantly in those treated with the combination agents in this trial. With these factors in mind, it is not unexpected that treatment with the combination agents in this trial lowered the risk of PASC symptoms—particularly the cognitive symptoms—as these agents blunted the elevation in the key cytokine mediators implicated as risk factors for PASC development, suppressed the viral load early on, and reduced the overall severity of the initial disease.
To the best of our knowledge, this is the first large, randomized controlled trial to show significant treatment benefit in a vaccinated population by reducing inflammation and increasing the speed of viral clearance by using repurposed drugs in the early treatment of COVID-19. This open-label, randomized clinical trial also demonstrated the feasibility of a fully remote (contactless) study during the SARS-CoV-2 pandemic. Adult outpatients with mild COVID-19 are often in self-quarantine, but few studies have focused on the care of this vulnerable population.
Given the safety, tolerability, ease of use, low cost, and widespread availability of these medications, our findings might influence national and international guidelines on the clinical management of COVID-19. Our results are consistent with earlier single-therapy fluvoxamine, bromhexine, and niclosamide trials conducted in other countries, and with several observational studies involving a different population of hospitalized SARS-CoV-2 patients.14, 15, 16,23,31,52, 53, 54 However, those studies used different doses of these drugs and included patients with symptoms beginning within 7 days of the screening date. For example, several fluvoxamine trials used 100 mg twice or thrice daily for 10 or 15 days.14, 15, 16,31,36 The doses of fluvoxamine in our trial are lower than in some other studies.14,16 The key difference lies in the early administration within 48 h of symptom onset, which has been shown in our study to reduce viral load and prevent excessive inflammation. Reducing viral load earlier and modulation of immune responses may be the key to prevent the bombardment of inflammation-causing molecules, which spurs the failure of multiple organs and a septic shock-like syndrome.13,55
The underlying mechanisms by which fluvoxamine, bromhexine, cyproheptadine, and niclosamide affect COVID-19 remain outcomes. However, several hypotheses have been suggested, and potential mechanisms are supported by in vitro and in vivo studies as well as numerous clinical studies.14, 15, 16,23,31,52, 53, 54,56 Previous studies have suggested that fluvoxamine, an SSRI, has beneficial effects in the treatment of acute COVID-1915,17,57 and reduces the risk of developing post-acute sequelae of SARS-CoV-2 infection (PASC).15,48 The specific mechanisms underlying these benefits are thought to involve fluvoxamine's activation of the S1R in multiple cell types leading to immunomodulatory effects, and its ability to modulate serotonin in platelets and immune cells.12,48,58 In COVID-19, intense platelet activation occurs due to specific antibodies, which significantly release serotonin from platelets.58, 59, 60 This excessive serotonin release has been implicated in acute lung injury and other pathogenic effects observed in COVID-19.59,61 By depleting platelet serotonin, early use of fluvoxamine may lower the risk of acute lung injury, lung edema, hypoxemia, and other effects caused by platelet serotonin release.62, 63, 64 Additionally, decreased platelet serotonin release may attenuate endothelial injury and immunopathology and reduce the risk of viral persistence.63 Fluvoxamine's benefits may also involve antiviral effects through the functional inhibition of acid sphingomyelinase pathway.63
The 5HT2 receptor plays a crucial role in mediating the pathogenic effects of serotonin.64 Agents with potent 5HT2 receptor antagonism, such as mirtazapine, have shown survival benefits in COVID-19.63,65 In this study, cyproheptadine, a widely available and affordable 5HT2 receptor antagonist,66 was chosen to be used in combination with fluvoxamine to enhance its therapeutic effects and reduce serotonergic side effects. Moreover, bromhexine hydrochloride inhibits TMPRSS2, a key protein involved in SARS-CoV-2 cell entry and replication, potentially reducing viral infectivity and aiding viral clearance.23,52,53,67 Additionally, niclosamide has antiviral effects by blocking spike-induced cell fusion and syncytia formation, inhibiting the procoagulant effect of the virus on platelets, and promoting faster viral clearance and symptom resolution.26,68
Our findings clearly show that the most plausible explanation is that early treatment of COVID-19 using these drug combinations can dampen the immune system's overreaction to SARS-CoV-2 infection. Although these drugs are relatively mild antivirals, our findings show that they can substantially impact viral clearance when given shortly after symptom onset. Given the association between lower viral load in early disease and reduced risk of subsequent hospital admission and mortality in COVID-19, it is plausible that rapid reduction in the viral load as a result of early treatment with the combination agents in this trial may have reduced the risk of clinical deterioration. Furthermore, it is important to note that fluvoxamine has been recognized for its CYP2D6 inhibiting activity. In this trial, the combination of bromhexine and cyproheptadine may elevate the blood levels of fluvoxamine, potentially resulting in beneficial effects. The study population received a mix of vaccination platforms, and due to this mix-match, it may not have produced the expected level of immunity. Our recent study on this matter demonstrated that this mix-match contributed to immune imbalances,6 shedding light on a possible explanation of why individuals in standard care experienced clinical deterioration. Additionally, the type of vaccine received is relevant in the context of evolving evidence on vaccine effectiveness against different variants.3,6,7,9,10,13,69 While all vaccines aim for comparable efficacy, subtle variations in their performance against specific strains might impact outcomes.
Early innate immune evasion strategies used by SARS-CoV2 to circumvent type I interferon signaling to gain a window of opportunity for virus propagation is a key factor in clinical deterioration.1,2 Therefore, an early anti-inflammatory and antiviral response may be most beneficial to prevent severe illness in vulnerable adults if these drugs are taken soon after infection occurs. Curtailing the excessive production of pro-inflammatory cytokines early on with the use of the combination agents in this trial may have further reduced the risk of clinical deterioration and poor outcomes.
Our results also suggest that an early anti-inflammatory and antiviral response lowers the risk of Long COVID symptoms/PASC. Importantly, these drugs can create similar clinical outcomes as currently approved antiviral drugs for SARS-CoV2.30,70, 71, 72, 73, 74, 75 In addition, the absolute number of serious adverse events associated with fluvoxamine-only, fluvoxamine + bromhexine, fluvoxamine + cyproheptadine, and niclosamide + bromhexine was lower than for standard care, potentially reflecting the modulatory effect of these drugs on systemic inflammation in these participants. Other widely available drugs have similar mechanisms of action as the ones used in this trial,76 and we recommend further study into other repurposed, widely available drugs that can prevent clinical deterioration of COVID-19 patients and reduce the threat and burden of SARS-CoV-2 infection.
This study highlights the importance of continued community testing for COVID-19. In addition to identifying when to isolate and thereby helping interrupt chains of transmission,11,77 community testing is crucial for identifying the need for therapeutic interventions that prevent disease progression and clinical deterioration,78 not only for COVID-19 but other infections too.69,79,80 In light of the results of this trial, communities that do not encourage regular testing for COVID-19 may be placed at a disadvantage and face an increased healthcare burden compared to communities that encourage testing only at the point where therapeutic intervention is clearly required.
The main strengths of our trial include the rapid recruitment, enrollment, and treatment of participants within 72 h of symptom onset with drug combinations. Early treatment using these drug combinations appears to drastically change the clinical trajectory resulting in fewer hospitalizations and less PASC symptoms, which suggests these combination therapies could be important interventions for outpatient care. Our study establishes strong evidence for the benefit of fluvoxamine alone, fluvoxamine + bromhexine, fluvoxamine + cyproheptadine, and niclosamide + bromhexine among vaccinated outpatients with early COVID-19.
This study has some limitations. First, this study was conducted as an unblinded, open-label trial due to limited funding as well as the limitations posed by the challenging and costly nature of achieving blinding for a combination of agents that each have distinct dosing regimens. To further confirm the results of this study, authors encourage other trial networks to conduct additional controlled, double-blinded trials of the combination agents that demonstrated efficacy in this trial versus other established COVID-19 treatments and/or placebos. Additionally, an inherent limitation in this study and other outpatient, contactless trials enrolling a population experiencing large and disruptive waves of COVID-19 lies in the large number of participants who may not initiate treatment after randomization for reasons that are not entirely unexpected. In this study, among the 1900 participants who were randomised, 593 participants did not receive treatment after randomisation. This was primarily because they could not clearly confirm their baseline eligibility using the study self-assessment supplies sent to each patient. Additionally, 234 participants voluntarily withdrew from the study before initiating treatment because, during randomisation, some participants provided inaccurate information about their medical history or current lifestyle, including hiding illicit drug use, in an attempt to access COVID-19 care. Furthermore, some participants in the treatment arms withdrew from the study for unknown reasons, possibly influenced by the open-label nature of the study. To overcome potential challenges related to this issue, including conducting the trial in remote settings, we conducted a statistical analysis comparing the demographics and baseline disease characteristics of individuals who did not receive standard treatment as randomised to those who received standard treatment as randomised. No discernible differences were observed, thus ensuring the comparability of arms at baseline. Lastly, some participants in standard care received the anti-viral drug favipiravir and may have experienced some benefits as a result of this treatment. However, it is important to acknowledge that the evidentiary foundation supporting the use of favipiravir was limited, and ongoing controversies existed regarding the efficacy of alternative treatment modalities. Therefore, some participants in the standard care arm chose not take the favipiravir. We did not randomised participants to favipiravir within the standard care and therefore we cannot analyze them as a separate group. However, we believe this will have had minimal effect on the primary outcome as reflected in the results, and if anything would understate the effectiveness of the combination therapies against a ‘no treatment’ arm. The absence of bromhexine-only, cyproheptadine-only, and niclosamide-only arms limits the ability to attribute observed effects specifically to each component. A more comprehensive evaluation, including monotherapy arms, would have provided a clearer understanding of the individual contributions of bromhexine, cyproheptadine and niclosamide.
Early treatment with the combination agents fluvoxamine plus bromhexine, fluvoxamine plus cyproheptadine, or niclosamide plus bromhexine, among outpatients diagnosed with COVID-19 was associated with reduced risk of clinical deterioration, hospitalization, and death in the acute phase, and was associated with significant and rapid reduction in the viral load and in the levels of serum cytokines IL-6, TNF-α, IL-8, and IL-1β. Early treatment with these combination agents was associated with a lower burden of PASC symptoms long-term. Larger, randomized, controlled, double-blinded trials are warranted with these combination agents to confirm the findings of this open-label trial. Given the strong evidence for the benefit of immunomodulators like fluvoxamine and antivirals such as nirmaltravir/ritonavir, it may at this point be most ethical to compare to active single- or combination-agent controls rather than placebos. The current findings may be particularly important in areas of the world where vaccination and expensive new antiviral treatments are not readily available. Our findings strongly suggest that treatment with effective repurposed drugs has high potential to prevent clinical deterioration (including hospitalization and death) in vaccinated and unvaccinated COVID-19 patients.
Contributors
Dhammika Leshan Wannigama supervision, conception, investigation, funding acquisition, data curation, critical review, and editing of the manuscript.
Cameron Hurst data curation, statistical analysis, supervision, critical review, and editing of the manuscript.
Phatthranit Phattharapornjaroen supervision, conception, investigation, funding acquisition, data curation, critical review, and editing of the manuscript.
Parichart Hongsing supervision, conception, investigation, data curation, critical review, and editing of the manuscript.
Natchalaikorn Sirichumroonwit supervision, conception, investigation, funding acquisition, data curation, critical review and editing of the manuscript.
Kanokpoj Chanpiwat supervision, conception, investigation, funding acquisition, data curation, critical review and editing of the manuscript.
Ali Hosseini Rad S. M. supervision, critical review and editing of the manuscript.
Robin James Storer supervision, critical review and editing of the manuscript.
Puey Ounjai supervision, critical review and editing of the manuscript.
Phitsanuruk Kanthawee supervision, critical review and editing of the manuscript.
Natharin Ngamwongsatit supervision, critical review and editing of the manuscript.
Rosalyn Kupwiwat clinical data collection, supervision, critical review and editing of the manuscript.
Chaisit Kupwiwat clinical data collection, supervision, critical review and editing of the manuscript.
James Michael Brimson clinical data collection, supervision, critical review and editing of the manuscript.
Naveen Kumar Devanga Ragupathi supervision, critical review and editing of the manuscript.
Sirirat Luk-in supervision, critical review and editing of the manuscript.
Somrat Charuluxananan supervision, critical review and editing of the manuscript.
Asada Leelahavanichkul supervision, critical review and editing of the manuscript.
Talerngsak Kanjanabuch supervision, critical review and editing of the manuscript.
Paul G. Higgins supervision, critical review and editing of the manuscript.
Vishnu Nayak Badavath supervision, critical review and editing of the manuscript.
Mohan Amarasiri supervision, critical review and editing of the manuscript.
Valerie Verhasselt supervision, critical review and editing of the manuscript.
Anthony Kicic supervision, critical review and editing of the manuscript.
Tanittha Chatsuwan supervision, critical review and editing of the manuscript.
Kashif Pirzada supervision, critical review and editing of the manuscript.
Farid Jalali supervision, conception, investigation, funding acquisition, data curation, critical review and editing of the manuscript.
Angela M. Reiersen supervision, conception, investigation, data curation, critical review and editing of the manuscript.
Shuichi Abe supervision, conception, investigation, funding acquisition, data curation, critical review and editing of the manuscript.
Hitoshi Ishikawa supervision, critical review and editing of the manuscript.
COVID-EarlyMed Trial Team clinical data collection, critical review and editing of the manuscript.
Authors Dhammika Leshan Wannigama, Cameron Hurst, Phatthranit Phattharapornjaroen, and Natchalaikorn Sirichumroonwit have directly accessed and verified the underlying data.
Data sharing statement
This published article and its Supplemental information file includes data generated and analyzed during this study. The additional de-identified participant data will be available upon reasonable request from the corresponding author DLW. Qualified external researchers only can access the data, and types of analysis requests are at the research team's discretion and dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Mechanisms of data availability will be with a signed data access agreement. The requested proposal must include a statistician.
Declaration of interests
Dr. Reiersen is listed as an inventor on a patent application related to methods of treating COVID-19 (including Sigma1 agonists and specifically fluvoxamine), which was filed by Washington University in St. Louis. No other author declares any potential conflict of interest or competing financial or non-financial interest in relation to the manuscript.
Acknowledgements
We thank the trial participants, their families, and all investigators, as well as the clinical and nursing staff at Rajavithi Hospital in Bangkok, Vibhavadi Hospital in Bangkok, Chiang Mai Neurological Hospital Chiang Mai, and Thanyarak Pattani Hospital, Pattani. We also thank the volunteers, the LGBTQIA + community, and marginalized, vulnerable indigenous communities in northern Thailand, who kindly assisted with sample collection, testing, and technical support. We would like to acknowledge the monumental efforts of a departed scholar who went by the Twitter pseudonym @__ice9 for their prolific and early research into the mechanisms of action of the agents used in this trial. We also thank to Dr. Katika Akksilp (Institute of Medical Research and Technology Assessment, Department of Medical Services, Ministry of Public Health, Thailand): Advice regarding protocol, project and funding management, data management, and establishment of randomization schedule. This study was funded by Ped Thai Su Phai (Thai Ducks Fighting Danger) social giver group. Dhammika Leshan Wannigama was supported by Chulalongkorn University (Second Century Fund- C2F Postdoctoral Fellowship), University of Western Australia (Overseas Research Experience Fellowship) and Yamagata Prefectural Central Hospital, Yamagata, Japan (Clinical Residency Fellowship). Anthony Kicic is a Rothwell Family Fellow. The sponsor(s) had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. We, the authors of this paper, embrace inclusive, diverse, and equitable conduct of research. Our team comprises individuals who self-identify as underrepresented ethnic minorities, gender minorities, members of the LGBTQIA + community, and individuals living with disabilities. We actively promote gender balance in our reference list while maintaining scientific relevance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102517.
Contributor Information
Dhammika Leshan Wannigama, Email: Dhammika.L@chula.ac.th.
Phatthranit Phattharapornjaroen, Email: Phatthranit.pha@mahidol.edu.
Kanokpoj Chanpiwat, Email: zoom1630@hotmail.com.
Tanittha Chatsuwan, Email: Tanittha.C@chula.ac.th.
Kashif Pirzada, Email: kashif.pirzada@utoronto.ca.
Farid Jalali, Email: farid.jalali@gmail.com.
Angela M. Reiersen, Email: reiersena@wustl.edu.
Shuichi Abe, Email: abeshu@ypch.gr.jp.
COVID-EarlyMed Trial Team:
Chanikan Tanasatitchai, Supamat Amphol, Ladda Nantawong, Prangrawee Sangchan, Varissara Sinkajarern, Thutpharritchn Phoonakh, Phornnapat Utenpattanun, Aye Mya Sithu Shein, Timporn Vitoonpong, Nichapha Chongthavonsatit, Yahya Mankong, Piyapong Chaichana, Jenjira Yaithet, Dumrongsak Pongprajak, Sukjai Traimuangpak, Gasit Saksirisampant, Phimonsiri Lamloeskittinon, Adam Adam Hamdy, Sinthu Sinthu Kosasih, and Sirirat Sirirat Luk-in
Appendix A. Supplementary data
References
- 1.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F., Zhao Z., Ma C., Nie X., Wu A., Li X. Return to normal pre-COVID-19 life is delayed by inequitable vaccine allocation and SARS-CoV-2 variants. Epidemiol Infect. 2022;150:e46. doi: 10.1017/S0950268822000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wannigama D.L., Amarasiri M., Hongsing P., et al. COVID-19 monitoring with sparse sampling of sewered and non-sewered wastewater in urban and rural communities. iScience. 2023;26 doi: 10.1016/j.isci.2023.107019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wannigama D.L., Amarasiri M., Hurst C., et al. Tracking COVID-19 with wastewater to understand asymptomatic transmission. Int J Infect Dis. 2021;108:296–299. doi: 10.1016/j.ijid.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anupong S., Chantanasaro T., Wilasang C., et al. Modeling vaccination strategies with limited early COVID-19 vaccine access in low- and middle-income countries: a case study of Thailand. Infect Dis Model. 2023;8(4):1177–1189. doi: 10.1016/j.idm.2023.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews N., Stowe J., Kirsebom F., et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(4):831–837. doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stowe J., Andrews N., Kirsebom F., Ramsay M., Bernal J.L. Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation, a test negative case-control study. Nat Commun. 2022;13(1):5736. doi: 10.1038/s41467-022-33378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills E.J., Reis G. Evaluating COVID-19 vaccines in the real world. Lancet. 2022;399(10331):1205–1206. doi: 10.1016/S0140-6736(22)00194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wannigama D.L., Amarasiri M., Phattharapornjaroen P., et al. Tracing the new SARS-CoV-2 variant BA.2.86 in the community through wastewater surveillance in Bangkok, Thailand. Lancet Infect Dis. 2023;23(11):e464–e466. doi: 10.1016/S1473-3099(23)00620-5. [DOI] [PubMed] [Google Scholar]
- 11.Anupong S., Chadsuthi S., Hongsing P., et al. Exploring indoor and outdoor dust as a potential tool for detection and monitoring of COVID-19 transmission. iScience. 2024;27 doi: 10.1016/j.isci.2024.109043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukhatme V.P., Reiersen A.M., Vayttaden S.J., Sukhatme V.V. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rad S.M.A.H., Wannigama D.L., Hirankarn N., McLellan A.D. The impact of non-synonymous mutations on miRNA binding sites within the SARS-CoV-2 NSP3 and NSP4 genes. Sci Rep. 2023;13(1) doi: 10.1038/s41598-023-44219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenze E.J., Mattar C., Zorumski C.F., et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324(22):2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee T.C., Vigod S., Bortolussi-Courval É., et al. Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(4):e226269. doi: 10.1001/jamanetworkopen.2022.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reis G., dos Santos Moreira-Silva E.A., Silva D.C.M., et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10(1):e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sánchez-Rico M., Rezaei K., Lenze E.J., Limosin F., Hoertel N. Efficacy of fluvoxamine in outpatients with COVID-19: understanding conflicting conclusions from 2 recent meta-analyses of the same clinical trials. Ann Pharmacother. 2023 doi: 10.1177/10600280231211304. [DOI] [PubMed] [Google Scholar]
- 18.Schultheiß C., Willscher E., Paschold L., et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022;3(6) doi: 10.1016/j.xcrm.2022.100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicol G.E., Karp J.F., Reiersen A.M., Zorumski C.F., Lenze E.J. "What were you before the war?" Repurposing psychiatry during the COVID-19 pandemic. J Clin Psychiatry. 2020;81(3) doi: 10.4088/JCP.20com13373. [DOI] [PubMed] [Google Scholar]
- 20.Gordon D.E., Hiatt J., Bouhaddou M., et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020;370(6521) doi: 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon D.E., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoertel N., Sánchez-Rico M., Cougoule C., et al. Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID-19: current evidence and potential mechanisms. Mol Psychiatry. 2021;26(12):7098–7099. doi: 10.1038/s41380-021-01254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansarin K., Tolouian R., Ardalan M., et al. Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: a randomized clinical trial. Bioimpacts. 2020;10(4):209–215. doi: 10.34172/bi.2020.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins R., Ferreira I., Jorge D.M.M., et al. In vitro inhibition of SARS-CoV-2 infection by bromhexine hydrochloride. bioRxiv. 2022 doi: 10.1101/2022.12.23.521817. [DOI] [Google Scholar]
- 25.Weinstock L.B., Reiersen A.M., Jain A., Konstantinoff K., James W., Jalali F. Cyproheptadine treatment of COVID-19-induced enteritis: implications for hyperinflammatory phase management. ACG Case Rep J. 2023;10(4) [Google Scholar]
- 26.Weiss A., Touret F., Baronti C., et al. Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2) PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0260958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdulamir A.S., Gorial F.I., Saadi S.J., et al. A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management. Ann Med Surg. 2021;69 doi: 10.1016/j.amsu.2021.102779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai Q., Yang M., Liu D., et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cramer H., Haller H., Dobos G., Lauche R. A systematic review and meta-analysis estimating the expected dropout rates in randomized controlled trials on yoga interventions. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/5859729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen W., Chen C., Tang J., et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis. Ann Med. 2022;54(1):516–523. doi: 10.1080/07853890.2022.2034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seftel D., Boulware D.R. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis. 2021;8(2) doi: 10.1093/ofid/ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcec R., Likic R. Could fluvoxamine dose de-escalation increase treatment compliance without sacrificing efficacy in COVID-19 patients? Clin Pharmacokinet. 2022;61(9):1321–1323. doi: 10.1007/s40262-022-01154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodds M.G., Doyle E.B., Reiersen A.M., Brown F., Rayner C.R. Fluvoxamine for the treatment of COVID-19. Lancet Global Health. 2022;10(3):e332. doi: 10.1016/S2214-109X(22)00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiersen A.M., Mattar C., Bender Ignacio R.A., et al. The STOP COVID 2 study: fluvoxamine vs placebo for outpatients with symptomatic COVID-19, a fully remote randomized controlled trial. Open Forum Infect Dis. 2023;10(8):ofad419. doi: 10.1093/ofid/ofad419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart T.G., Rebolledo P.A., Mourad A., et al. Higher-dose fluvoxamine and time to sustained recovery in outpatients with COVID-19: the ACTIV-6 randomized clinical trial. JAMA. 2023;330(24):2354–2363. doi: 10.1001/jama.2023.23363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashimoto K. Overview of the potential use of fluvoxamine for COVID-19 and long COVID. Discov Ment Health. 2023;3(1):9. doi: 10.1007/s44192-023-00036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis H.E., Assaf G.S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta C.R., Patel N.R. Algorithm 643: fexact: a FORTRAN subroutine for Fisher's exact test on unordered r×c contingency tables. ACM Trans Math Softw. 1986;12(2):154–161. [Google Scholar]
- 39.Clarkson D.B., Fan Y.-A., Joe H. A remark on algorithm 643: fexact: an algorithm for performing Fisher's exact test in r x c contingency tables. ACM Trans Math Softw. 1993;19(4):484–488. [Google Scholar]
- 40.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 41.Team RC . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: a language and environment for statistical computing. [Google Scholar]
- 42.Pinheiro J., Bates D., R Core Team . 2023. Nlme: linear and nonlinear mixed effects models. R package version 31-164. [Google Scholar]
- 43.Xie Y., Bowe B., Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12(1):6571. doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canas L.S., Molteni E., Deng J., et al. Profiling post-COVID syndrome across different variants of SARS-CoV-2. medRxiv. 2022 doi: 10.1101/2022.7.28.22278159. [DOI] [Google Scholar]
- 46.Chasco E.E., Dukes K., Jones D., Comellas A.P., Hoffman R.M., Garg A. Brain fog and fatigue following COVID-19 infection: an exploratory study of patient experiences of long COVID. Int J Environ Res Public Health. 2022;19(23) doi: 10.3390/ijerph192315499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashimoto K. Detrimental effects of COVID-19 in the brain and therapeutic options for long COVID: the role of Epstein-Barr virus and the gut-brain axis. Mol Psychiatry. 2023 doi: 10.1038/s41380-023-02161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidky H., Sahner D.K., Girvin A.T., Hotaling N., Michael S.G., Gersing K. Assessing the effect of selective serotonin reuptake inhibitors in the prevention of post-acute sequelae of COVID-19. medRxiv. 2023 doi: 10.1016/j.csbj.2023.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong A.C., Devason A.S., Umana I.C., et al. Serotonin reduction in post-acute sequelae of viral infection. Cell. 2023;186(22):4851–4867.e20. doi: 10.1016/j.cell.2023.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherif Z.A., Gomez C.R., Connors T.J., Henrich T.J., Reeves W.B. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC) Elife. 2023;12 doi: 10.7554/eLife.86002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin J.-X., Agbana Y.L., Sun Z.-S., et al. Increased interleukin-6 is associated with long COVID-19: a systematic review and meta-analysis. Infect Dis Poverty. 2023;12(1):43. doi: 10.1186/s40249-023-01086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuerdo A.M., Ogbac M.K., Tamayo J.E. Effect of bromhexine among COVID-19 patients - a meta-anaylsis. ERJ Open Res. 2022;8(suppl 8):104. [Google Scholar]
- 53.Mikhaylov E.N., Lyubimtseva T.A., Vakhrushev A.D., et al. Bromhexine hydrochloride prophylaxis of COVID-19 for medical personnel: a randomized open-label study. Interdiscip Perspect Infect Dis. 2022;2022 doi: 10.1155/2022/4693121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunzelmann K. Getting hands on a drug for Covid-19: inhaled and intranasal niclosamide. Lancet Reg Health Eur. 2021;4:100094. doi: 10.1016/j.lanepe.2021.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wannigama D.L., Jacquet A. NOD2-dependent BCG-induced trained immunity: a way to regulate innate responses to SARS-CoV2? Int J Infect Dis. 2020;101:52–55. doi: 10.1016/j.ijid.2020.09.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalali F., Rezaie S., Rola P., Kyle-Sidell C. COVID-19 pathophysiology: are platelets and serotonin hiding in plain sight? SSRN Electron J. 2021 doi: 10.2139/ssrn.3800402. [DOI] [Google Scholar]
- 57.Deng J., Rayner D., Ramaraju H.B., et al. Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: a systematic review and meta-analysis. Clin Microbiol Infect. 2023;29(5):578–586. doi: 10.1016/j.cmi.2023.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaid Y., Guessous F., Puhm F., et al. Platelet reactivity to thrombin differs between patients with COVID-19 and those with ARDS unrelated to COVID-19. Blood Adv. 2021;5(3):635–639. doi: 10.1182/bloodadvances.2020003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El Mdawar M.B., Maître B., Magnenat S., et al. Platelet FcγRIIA-induced serotonin release exacerbates the severity of transfusion-related acute lung injury in mice. Blood Adv. 2021;5(23):4817–4830. doi: 10.1182/bloodadvances.2021004336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zaid Y., Khalki L., Jalali F., et al. Low α-thrombin/GPIbα interaction is a potential contributor to platelet hyper-reactivity in COVID-19 patients. Thromb Haemost. 2023;123(8):804–807. doi: 10.1055/a-2072-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conway E.M., Mackman N., Warren R.Q., et al. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022;22(10):639–649. doi: 10.1038/s41577-022-00762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cloutier N., Allaeys I., Marcoux G., et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc Natl Acad Sci U S A. 2018;115(7):E1550–E1559. doi: 10.1073/pnas.1720553115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoertel N., Sánchez-Rico M., Kornhuber J., et al. Antidepressant use and its association with 28-day mortality in inpatients with SARS-CoV-2: support for the FIASMA model against COVID-19. J Clin Med. 2022;11(19):5882. doi: 10.3390/jcm11195882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Retamal J.S., Grace M.S., Dill L.K., et al. Serotonin-induced vascular permeability is mediated by transient receptor potential vanilloid 4 in the airways and upper gastrointestinal tract of mice. Lab Invest. 2021;101(7):851–864. doi: 10.1038/s41374-021-00593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakhaee H., Zangiabadian M., Bayati R., Rahmanian M., Ghaffari Jolfayi A., Rakhshanderou S. The effect of antidepressants on the severity of COVID-19 in hospitalized patients: a systematic review and meta-analysis. PLoS One. 2022;17(10) doi: 10.1371/journal.pone.0267423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woodrum S.T., Brown C.S. Management of SSRI-induced sexual dysfunction. Ann Pharmacother. 1998;32(11):1209–1215. doi: 10.1345/aph.17428. [DOI] [PubMed] [Google Scholar]
- 67.Maggio R., Corsini G.U. Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection. Pharmacol Res. 2020;157 doi: 10.1016/j.phrs.2020.104837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cairns D.M., Dulko D., Griffiths J.K., et al. Efficacy of niclosamide vs placebo in SARS-CoV-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19: a phase 2 randomized clinical trial. JAMA Netw Open. 2022;5(2) doi: 10.1001/jamanetworkopen.2021.44942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Imsuwansri T., Jongthitinon T., Pojdoung N., et al. Assessment of safety and intranasal neutralizing antibodies of HPMC-based human anti-SARS-CoV-2 IgG1 nasal spray in healthy volunteers. Sci Rep. 2023;13(1) doi: 10.1038/s41598-023-42539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spinner C.D., Gottlieb R.L., Criner G.J., et al. Effect of remdesivir vs standard care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vegivinti C.T.R., Evanson K.W., Lyons H., et al. Efficacy of antiviral therapies for COVID-19: a systematic review of randomized controlled trials. BMC Infect Dis. 2022;22(1):107. doi: 10.1186/s12879-022-07068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bai Y., Shen M., Zhang L. Antiviral efficacy of molnupiravir for COVID-19 treatment. Viruses. 2022;14(4):763. doi: 10.3390/v14040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong C.K.H., Au I.C.H., Lau K.T.K., Lau E.H.Y., Cowling B.J., Leung G.M. Real-world effectiveness of early molnupiravir or nirmatrelvir–ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22(12):1681–1693. doi: 10.1016/S1473-3099(22)00507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Najjar-Debbiny R., Gronich N., Weber G., et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2022;76:ciac443. doi: 10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shah M.M., Joyce B., Plumb I.D., et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19 - United States, April-September 2022. MMWR Morb Mortal Wkly Rep. 2022;71(48):1531–1537. doi: 10.15585/mmwr.mm7148e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vardanyan R.S., Hruby V.J. In: Synthesis of essential drugs. Vardanyan R.S., Hruby V.J., editors. Elsevier; Amsterdam: 2006. 16 - Antihistamine drugs; pp. 219–235. [Google Scholar]
- 77.Manabe Y.C., Sharfstein J.S., Armstrong K. The need for more and better testing for COVID-19. JAMA. 2020;324(21):2153–2154. doi: 10.1001/jama.2020.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wroe E.B., Seung K.J., Baker B.K., Farmer P.E. Test and treat: a missing link in the global fight against COVID-19. Lancet Global Health. 2022;10(2):e181–e182. doi: 10.1016/S2214-109X(21)00568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wannigama D.L., Amarasiri M., Phattharapornjaroen P., et al. Tracing the transmission of mpox through wastewater surveillance in Southeast Asia. J Trav Med. 2023;30(5) doi: 10.1093/jtm/taad096. [DOI] [PubMed] [Google Scholar]
- 80.Wannigama D.L., Amarasiri M., Hongsing P., et al. Multiple traces of monkeypox detected in non-sewered wastewater with sparse sampling from a densely populated metropolitan area in Asia. Sci Total Environ. 2023;858 doi: 10.1016/j.scitotenv.2022.159816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.