Abstract
Background
Transcranial Direct Current Stimulation (tDCS) is a non-invasive brain stimulation technique. Constant electric current is passed through the patient's scalp with the aim of modulating cortical excitability. Stroke is a cerebrovascular disease characterized by hemorrhage or cerebral ischemia. This systematic review and meta-analysis are aimed at comparing the efficacy of motor cortex stimulation with that of cerebellar stimulation by using transcranial direct current stimulation.
Method
Google Scholar, PubMed, EMBASE, Cochrane CENTRAL, and Physiotherapy Evidence Database (Pedro) databases were searched for studies. The extracted qualitative data was synthesized systematically. Cochrane RevMan software was used to conduct a meta-analysis of quantitative data. The fixed effects mean difference of the collected data was calculated at a 95% confidence interval (CI) for the changes in balance and side effects.
Results
This research included 10 articles with seven studies assessing changes in balance (outcome measured in CoP and FMA scores) and side effects (tingling and itching were the most prevalent). There was no significant difference between the efficacy levels of m1-tDCS versus ctDCS (P = 0.18), m1-tDCS versus sham (P = 0.92), and ctDCS versus sham (P = 0.19). Itching and tingling sensation were the most common and were significantly prevalent in sham interventions (P < 0.00001).
Conclusion
We found that motor cortex and cerebellar stimulations are both effective in improving motor function in stroke patients. There are no adverse effects to using the interventions besides mild itching and tingling experienced during the stimulation.
Keywords: Transcranial direct current stimulation, Cerebellar transcranial direct current stimulation (tDCS) motor cortex cerebellar-transcranial direct current stimulation
What is known?
The literature shows that transcranial direct current stimulation is likely effective in improving motor function in both cerebral motor cortex stimulation and cerebellar stimulation in stroke patients. Still, the evidence is greatly lacking when we compare the two stimulation sites.
What is new?
Current studies aim to investigate the efficacy of motor cortex stimulation compared to cerebellar stimulation using transcranial direct current stimulation. Although a previous study stated cerebellar stimulation to be more effective, we found no statistical difference between the effectiveness of the two modalities in improving motor function.
1. Introduction
Transcranial Direct Current Stimulation (tDCS) involves brain stimulation techniques where a constant electric current is applied to a patient's scalp. It is a non-invasive method that modulates cortical excitability [1]. Stroke is a cerebrovascular disease characterized by hemorrhage or cerebral ischemia. Stroke often leads to various health challenges, including impaired cognitive function, decreased muscle strength, proprioceptive abilities, and cognitive function impairment [2]. These sensorimotor deficits ultimately affect the postural and balance control abilities of stroke patients [3]. Statistically, around 80% of patients who have experienced stroke encounter difficulties in maintaining motor function [4]. Additionally, approximately 38% of them remain in a non-ambulatory state for more than 180 days after the stroke, whether it is chronic or mild [5]. Data from Błaszcz et al. non-ambulatory post-stroke patients revealed that with a proper rehabilitation regimen, physical activity can be restored after 6 weeks [6].
During the past decades, therapies based on modulating plasticity and motor learning have significantly improved and continued to evolve greatly. Transcranial direct current stimulation has been an innovative, favorable, and non-invasive stimulation where a weak direct current of approximately 1–2 mA is applied using electrodes over a patient's scalp. The impact of tDCS is specifically related to the polarity of the electrodes; cathodal stimulation reduces motor cortex excitability, while anodal stimulation can increase excitability [7]. Recent investigations by Takano et al. provide a clearer and updated understanding of the clinical application of tDCS by monitoring changes in corticospinal excitability and motor control during stimulation in healthy individuals [8]. Different tDCS montages induce diverse results on a patient's brain networks. Stimulation results directly correlate with the polarity and positioning of the electrodes. The polarity of electrode montages defines the specific implications of the stimulation [7]. This is a result of the effects of the medical procedure on the modulation of cortical excitability, especially when directed at the primary motor cortex [9].
Stroke is one of the most common medical conditions to which brain stimulation can be applied [10]. Considerable research has been conducted over the years to assess the efficacy and safety of tDCS for improving motor function in stroke patients. A number of studies have concluded that tDCS is safe and effective in improving cognitive and motor function in stroke patients [[11], [12], [13]]. Moreover, He et al. and Huang et al. emphasize the scarcity of data to completely inform the safety and efficacy of this modality [12,13]. Similar sentiments of data limitation have been echoed by the latest research on the subject that monitored changes in cognitive function after stimulation of schizophrenic patients [14].
The concept of modulation of the patient's neuronal activities prompted by tDCS has not been fully analyzed. However, according to numerous studies, the electric current generated through the stimulation significantly interferes with the resting membrane potential of neuronal cells, thereby modulating voluntary activities in brain circuits [15]. According to some researchers, tDCS may affect the strength of neuronal synapses, which, in turn, alters the activity of GABA and NMDA receptors. Ultimately, this process may activate the plasticity process, including long-term depression and potentiation [16]. When a stimulus is applied, it creates a time varying electrofield in the brain, which may trigger action potentials in cortical neurons [17]. Chen and Liu provide an overview of neuronal activity and the generated action potentials in neurons, highlighting the potential of tDCS in modulating the electrical potential of neuronal cells, thereby influencing their voluntary activities in brain circuits [18].
The long-term effects of the brain stimulation procedure are also believed to have a strong correlation with changes in gene expression and alterations in protein synthesis [19]. Furthermore, according to a previous neuroimaging study, changes in blood flow occur following stimulation, which may correlate with the effect of brain stimulation on blood flow, leading to an increase in oxygen supply in cortical areas followed by a boost in neuronal excitability [20]. No extreme side effects are documented in any clinical trials; some mild side effects include a burning sensation, low-intensity discomfort, itching, tingling under the electrode, and mild skin irritation. Apart from the aforementioned side effects, tDCS is relatively safe [21]. According to researchers, the procedure has the potential to impact deeper brain structures, and this possibility has supported broader investigations into brain stimulation procedures for different disorders, including stroke patients. Given the prevalence of the procedure in stroke treatment, it is necessary to conduct research to test alternative targets for tDCS to enhance motor recovery in stroke patients.
The cerebellum is an additional subcortical region, besides cortical areas, associated with motor functions. It is vital in numerous aspects of motricity as well as balanced and fine motor functions. Besides the role of the cerebellum in motor functions, researchers suggest that the process may also have an effect on cognition, including motor learning [22]. Considering the role played by the cerebellum in aspects of motion, scientists have perceived the cerebellum as a likely focus of stimulation using tDCS to improve motor recovery after a stroke [23]. Stimulation through the cerebellum can be applied to improve a patient's motor, balance, and lower limb functioning. With the apparent divide between the efficacy of motor cortex and cerebellum tDCS, this study aims to compare the two. The purpose of this systematic review and meta-analysis is to compare the efficacy and safety of motor cortex stimulation with cerebellar stimulation using tDCS.
2. Methodology
2.1. Study design
This is a systematic review and meta-analysis conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.2. Literature search
An online database search was conducted on Google Scholar, PubMed, EMBASE, Cochrane CENTRAL, and Physiotherapy Evidence Database (Pedro) databases until October 2023. The search strategy employed keyword combinations and Boolean operators to prepare a search string. Primary keywords were "transcranial direct current stimulation" OR "cerebellar transcranial direct current stimulation" OR "ctDCS" OR "tDCS" AND "motor cortex cerebellar transcranial direct current stimulation" AND "m1-tDCS". A hand search through reference lists was also used to identify new articles for inclusion.
2.3. Inclusion and exclusion criteria
A limitation was given to scholars working together with articles from peer-reviewed publications from conventional channels; any unpublished randomized controlled trials were excluded. Also, all articles written in languages other than English, authored before 1999, or that were not relevant to the research topic were excluded. A PECOS criterion of study selection was used to formulate the inclusion and exclusion criteria for this systematic review and meta-analysis. Participants (P) for trials had to be stroke patients. The groups in these experiments had to have been exposed (E) to motor cortex tDCS, with the findings observed against cerebellar tDCS as the comparator (C). Sham comparisons were also allowed. To compare the effectiveness of each stimulation location, the trials had to have observed the following outcomes (O): changes in the outcome of balance, movement function, joint range of motion, sensation, and side effects such as tingling and itching on the stimulated area. Study designs (S) were exclusively randomized controlled trials or clinical trials. All reviews, cohort studies, or case reports were excluded from inclusion. All disagreements or variances were settled through concessions or consultations.
2.4. Data extraction
Excel was used to prepare standardized data tables for data extraction. The information recorded from the articles includes the study author(s) and publication's year, sample size, and intervention characteristics (type, frequencies, durations, and intensities). Patient attributes at baseline, such as mean age and randomization, were extracted. Key outcomes of the studies were extracted and sub-grouped in line with comparisons, type of outcome, and nature of stimulation. The author resolved any inconsistencies relating to the extraction of any data item before the data was synthesized.
2.5. Data synthesis
The core of this investigation is a quantitative approach to data analysis, which requires the conduction of a meta-analysis. The Cochrane RevMan program/software was used to conduct the meta-analysis. A fixed-effects model computed the mean difference at a 95% confidence interval (CI). The level of significance of the comparison was reported by the P-value (significant exists at P ≤ 0.05). Heterogeneity was reported by the I2 statistic and judged according to the value, which ranges from 0% (complete consistency), 50% (low consistency), 75% (high inconsistency), to 100% (complete inconsistency). Moreover, a systematic descriptive analysis of the collected data was also done. Results were reported using forest plots, and the publication bias among the analyzed studies was expressed on the funnel plot.
3. Results
3.1. Study selection
All the identified studies were assessed against the predetermined criteria of eligibility. Studies relevant to high-frequency external muscle stimulation for neuropathy were the first ones excluded from the review process. An online search identified 3139 studies (PubMed = 1440, Embase = 391, Cochrane CENTRAL = 668, and Pedro = 640). Following the removal of copies through automated filters, 1996 research/studies were left. The remaining copies were filtered against the eligibility criteria, leaving 953 studies. The abstracts and headlines of the 953 searches/studies were scanned, determining their significance for the present study. The process eliminated 899 studies and left 54 studies for full-text screening. Due to failure on several eligibility requirements, 40 studies were eliminated. Most of these failed the inclusion for not reporting our outcomes of interest. A further hand search through reference lists of the 7 included studies identified three additional studies that were included. Finally, 10 studies were included in the study. Fig. 1 is a PRISMA flow diagram illustrating the selection process.
Fig. 1.
P.R.I.S.M.A Flow-diagram outlining research/studies selecting procedure for present systematic-review and meta-analysis.
3.2. Study characteristics
Ten studies were selected for this meta-analysis, all including 347 participants randomized between m1-tDCS, ctDCS, or sham stimulations. Table 1, Table 2, and Table 3 represent data extracted from the selected studies grouped according to the outcome measures of center of pressure (CoP), Fugl-Meyer Assessment (FMA) scores, and the side effects of itching and tingling sensations.
-
1.
Extracted Data on Changes in CoP Values
-
2.
Extracted Data on Fugl-Meyer Assessment (FMA) Scores
-
3.
Extracted Data on the Side Effects of Stimulation
Table 1.
Study characteristics for the studies reporting the outcome Center of Pressure (CoP).
Table 2.
Study characteristics from the studies reporting Fugl-Meyer Assessment (FMA) scores.
Table 3.
Study characteristics reporting the side effects of stimulation.
3.3. Statistical analysis
Three comparisons were adopted for this meta-analysis: motor cortex tDCS versus cerebellar tDCS, motor cortex tDCS versus sham stimulation, and cerebellar tDCS versus sham stimulation. The first comparison looked into the outcome of balance measured by the center of pressure (CoP); the second comparison investigated the outcome of balance measured by CoP and Fugl-Meyer Assessment (FMA) scores. Additionally, there was a look into the side effects of the stimulation, with data on tingling sensation and itching analyzed. The latter comparison (ctDCS vs. Sham) investigated the same outcomes without a subgroup of FMA scores.
-
1.
Changes in Balance Post-Stimulation
A total of seven studies were assessed for this outcome. In the first comparison (m1-tDCS vs. ctDCS), two studies [24,25] investigating 52 participants (equally randomized) were used to calculate the fixed effects mean difference (MD) of changes in CoP. The MD was 0.73 [−1.66, 3.13] at the 95% confidence interval (CI), and the overall effect was Z = 0.60 (P = 0.55). Fig. 2 below is the forest plot of the meta-analysis, and Fig. 3 is a funnel plot of two studies.
Fig. 2.
Forest plot of the CoP changes comparing m1-tDCS vs. ctDCS.
Fig. 3.
Funnel plot of the CoP changes meta-analysis comparing m1-tDCs vs. ctDCS.
To look at changes in balance between m1-tDCS stimulation and sham stimulation in a subgroup, five studies used CoP data [24,25] and FMA scores [[28], [29], [30]] from their studies. The overall fixed effects MD was 0.15 [−2.80, 3.09] at 95% CI. With a test or overall effect being Z = 0.10 (P = 0.92). Fig. 3 shows the results of this subgroup analysis, and Fig. 5 is a funnel plot of five studies (see Fig. 7) (see Fig. 6).
Fig. 5.
Funnel plot of the CoP changes and FMA scores meta-analysis comparing m1-tDCS vs. sham stimulation.
Fig. 7.
Funnel plot of the CoP changes meta-analysis comparing ctDCS vs. sham stimulation.
Fig. 6.
Forest plot of the CoP changes comparing ctDCS vs. sham stimulation.
The last comparison showing changes in the balance was ctDCS vs. sham stimulation. Four studies [[24], [25], [26], [27]] investigating 122 participants (equally randomized) were included. The fixed effects MD was −1.80 [−4.32, 0.72] at 95% CI with Z = 1.40 (P = 0.16) as the test for overall effect. The results of this meta-analysis are shown in Fig. 4 below.
Fig. 4.
Forest plot of the CoP and FMA scores changes comparing m1-tDCS vs. sham stimulation.
The p-values of each of the three comparisons were P = 0.55, P = 0.92, and P = 0.16. This signifies the lack of a significant difference between balance changes after m1-tDCS, ctDCS, and sham stimulations.
-
2.
Side Effects of the Stimulation Intervention
Two common side effects were identified from 3 studies and sub-grouped with respect to the current placement. The outcomes of tingling sensation and itching after tDCS were only reported in three studies [[31], [32], [33]], which reported different results when stimulation was cathodal or anodal.
3.3.1. Tingling sensation
The meta-analysis results report an overall fixed effects MD of 0.62 [0.55, 0.68] at 95% CI (P < 0.00001) when comparing m1-tDCS vs. sham stimulation. On the other hand, a fixed effects MD of 0.40 [0.36, 0.45] at 95% CI (P < 0.00001) when comparing ctDCS vs. sham stimulation. Fig. 8, Fig. 10 (forest plots) show a clear and significant difference in edged towards sham stimulation (see Fig. 11) (see Fig. 9).
Fig. 8.
Forest plot of the tingling sensation side effect comparing m1-tDCS vs. sham stimulation.
Fig. 10.
Forest plot of the tingling sensation side effect comparing ctDCS vs. sham stimulation.
Fig. 11.
Funnel plot of the side effects meta-analysis comparing tingling sensation in ctDCS vs. sham stimulation.
Fig. 9.
Funnel plot of the side effects meta-analysis comparing tingling sensation in m1-tDCS vs. sham stimulation.
3.3.2. Itching
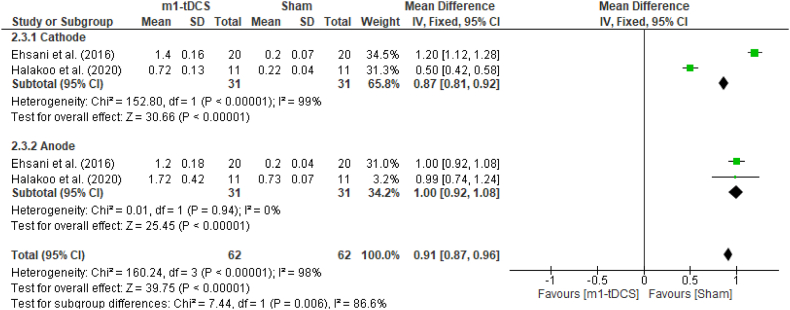
The meta-analysis results report a fixed effects MD of 0.91 [0.87, 0.96] at 95% CI (P < 0.00001) when comparing m1-tDCS with sham stimulation. In the case of ctDCS vs. sham stimulation, the fixed effects MF for itching were 0.96 [0.92, 1.00] at 95% CI (P < 0.0001). Fig. 12, Fig. 14 (forest plots) show a clear significant difference in edged towards sham stimulation (see Fig. 15) (see Fig. 13).
Fig. 12.
Forest plot of the itching sensation side effect comparing m1-tDCS vs. sham stimulation.
Fig. 14.
Forest plot of itching side effect comparing ctDCS vs. sham stimulation.
Fig. 15.
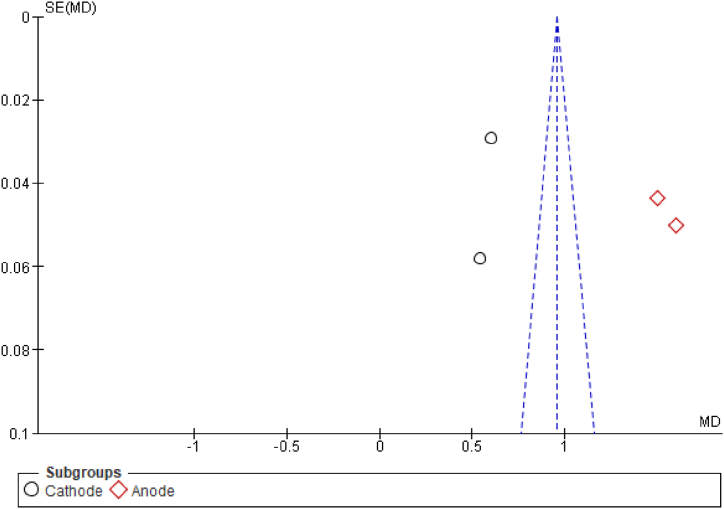
Funnel plot of the side effects meta-analysis comparing itching in ctDCS vs. sham stimulation.
Fig. 13.
Funnel plot of the side effects meta-analysis comparing itching in m1-tDCS vs. sham stimulation.
3.4. Risks of Biases
We used the Cochrane Risk of bias tool (RoB2) to appraise the quality of the selected studies. The tool assesses seven elements bias as per the Cochrane-handbook of Systematic-reviews for intervention (5.4). There was a moderately low bias, as indicated in the risk of bias graph (Fig. 16). Bias was lowest in selection, performance, and detection, while it remained moderately high in attrition and reporting. Fig. 16, Fig. 17 are the RoB graph and RoB summary, which represent the results of the assessment.
Fig. 16.
Risk of bias graph from the quality appraisal in RoB2.
Fig. 17.
Risk of bias summary of the included studies from the quality appraisal in RoB2.
4. Discussion
Current stimulation has been used clinically to treat stroke patients. The idea is to improve motor function as well as other secondary effects of stroke. Electrical stimulations provide input to the neural pathways in the stroke-damaged portion of the brain. Such stimulations engage brain neuroplasticity, a process of self-rewiring which the brain uses to heal stroke injuries [34]. As we shall see in this discussion, stroke improves mobility, sensation, cognition, and several other outcomes. Every study included in this meta-analysis treated patients with at least two non-invasive brain stimulation techniques: tDCS and bi-hemispheric tDCS. Transcranial direct current is applied bilaterally through the scalp over the primary motor cortex or cerebellar region to modulate cortical excitability [35,36]. The effectiveness of tDCS can be improved by secondary therapies such as moto imagery and upper limb functional training. Bi-hemispheric tDCS is a more specialized form of tDCS that targets a single hemisphere of the brain rather than bilateral [35,36].
The majority of the current literature that conducts statistical pooling on the efficacy of tDCS on the after-effects of stroke focuses primarily on stimulating motor networks to improve other impaired functions. The present meta-analysis seems to be the first pooling of statistics to assess improvements in motor function after stimulation. tDCS has demonstrated positive results in aphasia recovery [37,38]. Ehsani et al. provide a more generalized perspective on the improvement in activities of daily living (ADLs) after tDCS treatments [31]. The majority of the evidence presented points to significant improvement in ADL performance, which includes function, muscle strength, cognitive abilities, and spatial neglect [31]. This study opens up the possible applications of tDCS, with more research focusing on specific outcomes. More recently, tDCS has been found to improve a lot of physiologic functions in stroke patients. Our focus sought to look at the efficacy of tDCS in improving motor functions in stroke patients. However, besides stroke patients, tDCS has been found to significantly improvecortical functions in patients with various neurological disorders. A 13-study meta-analysis found that anodal tDCS resulted in improved general cognitive performance in stroke patients [39]. Patients with cognitive impairment after stroke are more likely to show a significant change in general cognition after stimulation. Other impairments reported to improve significantly after stimulation include attention and concentration, figural memory, logical reasoning, reaction behavior, and functional independence measure (FIM) [40].
4.1. Efficacy of motor cortex and cerebellar tDCS
In our study, seven of the 10 studies included in the meta-analysis report on the changes in balance were observed through changes in CoP and FMA scores. Statistical analysis shows that there is no significant difference (0.73 [95% CI (−1.66-3.13)] (P = 0.18)) in the improvement of motor function when comparing m1-tDCS versus ctDCS. Similar findings have been replicated in the next two comparisons: m1-tDCS versus sham stimulation (0.15 [95% CI (−2.80-3.09)] (P = 0.92)) and ctDCS versus sham stimulation (−1.80 [95% CI (−4.32-0.72)] (P = 0.19)). While looking at static balance, Baharlouei et al. concluded that the effects exerted by each form of stimulation on the participants' postural balance were relatively the same [24]. These results do not necessarily provide evidence of which intervention gives superior results. Our meta-analysis findings indicate that, despite a lack of significant difference, the direction of the mean difference leans towards ctDCS and m1-tDCS when ether modalities are compared to a sham stimulation. Still, this point remains heavily suggestive rather than conclusive. This outcome is perfectly summed up by Galea et al., who state that the cerebellar and motor cortex have distinct functional roles. While anodal cerebellar tDCS enhances acquisition or motor function, anodal motor cortex tDCS influences an increase in retention of the visuomotor transformation. These findings have been reiterated in other studies that suggest that multi-session cerebellar and motor cortex tDCS positively impacts various aspects of motor function, especially static and dynamic postural [31]. Direct comparisons to sham stimulations have not demonstrated any conclusive results to indicate the superiority of each intervention over another.
On the contrary, findings by Poortvliet et al. and Jackson et al. have all positively concluded the utility of cerebellar tDCS in the short-term improvement of motor skills compared to sham stimulation [26,27]. Poortvliet et al. state that active tDCS significantly improved postural steadiness during vibration and reduced forward displacement and variability in COP derivatives during recovery [26]. Such immediate improvement in postural steadiness indicates rapid acquisition of adaptive motor skills. Similarly, ctDCS applied over three days significantly improves the force of accuracy during a visuo-motor isometric pinch grip task [27]. Other studies have reported a similar outcome, indicating significant improvements in motor function. When comparing bi-hemispheric tDCS with sham stimulation, Lindenberg et al. observed that effects persist after the intervention for at least a week [28]. Functional changes in the activation of the motor cortex were present along with these effects.
Same results where m1-tDCS and ctDCS induce significantly better outcomes have been reported by Ang et al. and Rocha et al. [29,30]. This consistency is an intriguing element when attempting to understand the underlying reasons why intervention stimulations are significantly better than sham stimulations. Applying weak direct current through the scalp has been observed to modulate excitability in the motor cortex. Cathodal direct current stimulations upregulate contra-lesional cortical excitability. On the other hand, anodal direct current stimulation may upregulate ipsi-lesional cortical excitability. Lindenberg et al. make the same point, demonstrating improvements in activation of the ipsi-lesional motor cortex following bi-hemispheric ctDCS [28]. Other findings suggest that interventional stimulations may have a pronounced influence on the activity of intracortical inhibitory neurons [41]. Therefore, such influence could exert a neuromodulatory effect on the ipsi-lesional motor cortex through transcallosal pathways.
Variations of current stimulation have been employed clinically to test for subtle differences in results when treating neurodegenerative disorders. Transcranial alternating current stimulation (tACS) has been compared to tDCS for memory enhancement in Alzheimer's patients [42]. Patients receiving tACS recalled significantly fewer words and made more memory errors compared to tDCS. Other variations, such as delayed current stimulation, have received almost no attention to compare the differences in recovery outcomes. The bulk of the research primarily explores the motor cortex and cerebellar stimulations. The choice of stimulation has largely been based on specific motor deficits and the stage of stroke. On one hand, anodal cerebellar is compared to anodal cerebral tDCS to assess improved gait, balance, and risk of fall in stroke patients [43]. On the other hand, cerebellar tDCS is used as an effective and safe treatment to promote recovery of upper limb motor function [44] or functional balance in chronic stroke patients [45]. Optimal stimulation parameters remain a point of contention to determine the long-term effects of tDCS on motor function. A general consensus from the literature is that either m1-tDCS or ctDCS should be applied when improving functional recovery in stroke patients. Different contexts have described functional recovery from a motor perspective or a psychological or cortical perspective.
4.2. Safety and side effects of tDCS
The general impression of both intervention modalities is that they are safe, with mild and meagerly reported adverse effects and various side effects. In our assessment, there is a significant difference in the amount of tingling sensation caused by sham stimulation compared to m1-tDCS or ctDCS. Comparison with m1-tDCS has a mean difference of 0.91 [95% CI (0.87–0.96)] (P < 0.00001) and there is a fixed effect MD of 0.96 [95% CI (0.92–1.00)] (P < 0.00001) in the ctDCS comparison. Similarly, patients experienced more itching with sham stimulation than m1-tDCS (MD 0.62 [95% CI (0.55–0.68)] (P < 0.00001)) and ctDCS (MD 0.40 [95% CI (0.36–0.45) (P < 0.00001)). Ehsani et al. report itching and tingling sensation as a general discomfort experienced over the area of stimulation [31]. The tolerance of participants was high in both study groups, with no adverse effects reports recorded. According to Samaei et al., itching was the most common side effect recorded during the study [32]. In this case, there were no side effects identified at the end of the study. Without any burning sensation or pain under the electrodes, our assessment concludes that using tDCS on the motor cortex or cerebellar area is a safe intervention with minimum side effects for the stroke patients [33]. Beyond slight irritation and tingling during the current application, there seem to be no adverse effects during treatment.
Electric stimulation, like any other element of science, is under constant development. Medical advancements will constantly morph it into different mechanisms, optimally maximizing efficacy and minimizing side effects. Some studies have suggested that a single session of tDCS, either cerebellar or motor cortex stimulation, may fail to impact motor function in stroke patients [46]. Therefore, a multi-session cerebellar or motor cortex tDCS may have some accumulative impacts [47]. Numerous sections of the cerebellar, principally the vermis, have major impacts on postural control by processing and receiving inputs from the auditory, vestibular, visual, and somatosensory systems and by having control over the muscles that have been affected [48]. We have discussed other potential mechanisms of motor cortex and cerebellar tDCS in improving motor function, such as enhancing regional cerebral blood flow (rCBF). Others include modulating neuronal excitability and plasticity, activating the ipsi-lesional motor cortex, facilitating neuroplasticity and brain reorganization, and enhancing the levels of cerebellar activity [35,44].
5. Limitations
The primary limitation of this study is the scarcity of statistical data on individual outcomes of interest. There is a high probability of bias because there were varied intrinsic differences between particular trials focusing on diverse aspects. In many cases, studies did not provide sufficient data on within, and between-group testing. The included trials also failed to state the blinding procedures for the therapists, participants, and assessors, further contributing to selection bias. Subgroup analyses were not possible in every outcome analysis owing to the few studies that met the eligibility criteria for inclusion.
6. Conclusion
In this systematic and meta-analysis, we can conclusively state that tDCS is safe without any adverse effects reported. Expectedly, mild discomfort might be experienced during the stimulation. Itching and tingling sensation were the most common side effects and were significantly prevalent in sham interventions (P < 0.00001). Moreover, this study could not find any significant difference between the efficacy levels of m1-tDCS versus ctDCS (P = 0.18), m1-tDCS versus sham (P = 0.92), and ctDCS versus sham (P = 0.19). With the limited data pooled from these analysis groups, it was not possible to offer conclusive results on the superiority of the intervention. However, the descriptive review uncovers that m1-tDCS and ctDCS offer almost similar levels of efficacy, affecting various physiological mechanisms to improve motor function.
Data availability statement
Data associated with the study has not been deposited into a publicly available repository. However, it can be available on request to the corresponding author.
Funding
This study was supported by the National Natural Science Foundation of China (U1913216, 31972907).
The research work was supported by researchers supporting project number (RSP2024R110) at King Saud University.
CRediT authorship contribution statement
Qurat ul-ain: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Saad Ilyas: Software, Methodology, Investigation, Formal analysis, Data curation. Hamid Ali: Writing – original draft, Formal analysis. Ijaz Ali: Software, Investigation, Formal analysis. Riaz Ullah: Project administration, Methodology, Investigation. Hafsah Arshad: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Sana Khalid: Writing – review & editing, Data curation, Conceptualization. Muhammad Ehab Azim: Validation, Methodology, Investigation, Conceptualization. Tian Liu: Conceptualization, Data curation, Funding acquisition, Supervision. Jue Wang: Supervision, Resources, Project administration, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Jue Wang reports financial support was provided by National Natural Science Foundation of China.
Acknowledgements
The authors would like to thank Researchers Supporting Project number RSP2024R110 at King Saud University, Riyadh Saudia Arabia for financial Support.
References
- 1.Yosephi M.H., et al. Multi-session anodal tDCS enhances the effects of postural training on balance and postural stability in older adults with high fall risk: primary motor cortex versus cerebellar stimulation. Brain stimulation. 2018;11(6):1239–1250. doi: 10.1016/j.brs.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaban B., Tok F. Gait Disturbances in patients with stroke. PM&R. 2014;6(7):635–642. doi: 10.1016/j.pmrj.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Tyson S.F., et al. Balance disability after stroke. Phys. Ther. 2006;86(1):30–38. doi: 10.1093/ptj/86.1.30. [DOI] [PubMed] [Google Scholar]
- 5.Kollen B., Kwakkel G., Lindeman E. Longitudinal robustness of variables predicting independent gait following severe middle cerebral artery stroke: a prospective cohort study. Clin. Rehabil. 2006;20(3):262–268. doi: 10.1191/0269215506cr910oa. [DOI] [PubMed] [Google Scholar]
- 6.Błaszcz M., et al. Physical activity, psychological and functional outcomes in non-ambulatory stroke patients during rehabilitation—a pilot study. J. Clin. Med. 2022;11(24):7260. doi: 10.3390/jcm11247260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitsche M.A., Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 8.Takano K., et al. Changes in corticospinal excitability and motor control during cerebellar transcranial direct current stimulation in healthy individuals. Cerebellum. 2023;22(5):905–914. doi: 10.1007/s12311-022-01469-2. [DOI] [PubMed] [Google Scholar]
- 9.Stagg C.J., Nitsche M.A. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 10.Boggio P.S., et al. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor. Neurol. Neurosci. 2007;25(2):123–129. [PubMed] [Google Scholar]
- 11.Salehinejad M.A., et al. Cognitive functions and underlying parameters of human brain physiology are associated with chronotype. Nat. Commun. 2021;12(1):4672. doi: 10.1038/s41467-021-24885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y., et al. Effects of transcranial direct current stimulation over the left primary motor cortex on verbal intelligence. Front. Hum. Neurosci. 2022;16 doi: 10.3389/fnhum.2022.888590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He W., et al. Safety and effects of transcranial direct current stimulation on hand function in preschool children with hemiplegic cerebral palsy: a pilot study. Frontiers in Behavioral Neuroscience. 2022;16 doi: 10.3389/fnbeh.2022.925122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y., et al. 2023. Efficacy and Safety of Transcranial Direct Current Stimulation (tDCS) on Cognitive Function in Chronic Schizophrenia with Tardive Dyskinesia (TD): a Randomized, Double-Blind, Sham-Controlled, Clinical Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebetanz D., et al. Pharmacological approach to the mechanisms of transcranial DC‐stimulation‐induced after‐effects of human motor cortex excitability. Brain. 2002;125(10):2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 16.Polanía R., et al. Introducing graph theory to track for neuroplastic alterations in the resting human brain: a transcranial direct current stimulation study. Neuroimage. 2011;54(3):2287–2296. doi: 10.1016/j.neuroimage.2010.09.085. [DOI] [PubMed] [Google Scholar]
- 17.Siebner H.R., et al. Transcranial magnetic stimulation of the brain: what is stimulated?–a consensus and critical position paper. Clin. Neurophysiol. 2022;140:59–97. doi: 10.1016/j.clinph.2022.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen I., Lui F. 2019. Neuroanatomy, Neuron Action Potential. [PubMed] [Google Scholar]
- 19.Hattori Y., Moriwaki A., Hori Y. Biphasic effects of polarizing current on adenosine-sensitive generation of cyclic AMP in rat cerebral cortex. Neurosci. Lett. 1990;116(3):320–324. doi: 10.1016/0304-3940(90)90094-p. [DOI] [PubMed] [Google Scholar]
- 20.Zheng X., Alsop D.C., Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58(1):26–33. doi: 10.1016/j.neuroimage.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poreisz C., et al. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007;72(4–6):208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Bastian A.J. Moving, sensing and learning with cerebellar damage. Curr. Opin. Neurobiol. 2011;21(4):596–601. doi: 10.1016/j.conb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wessel M.J., Hummel F.C. Non-invasive cerebellar stimulation: a promising approach for stroke recovery? Cerebellum. 2018;17(3):359–371. doi: 10.1007/s12311-017-0906-1. [DOI] [PubMed] [Google Scholar]
- 24.Baharlouei H., et al. Comparison of transcranial direct current stimulation of the primary motor cortex and cerebellum on static balance in older adults. Iran. Red Crescent Med. J. 2020;22(3) [Google Scholar]
- 25.Galea J.M., et al. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cerebr. Cortex. 2011;21(8):1761–1770. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poortvliet P., et al. Cerebellar transcranial direct current stimulation improves adaptive postural control. Clin. Neurophysiol. 2018;129(1):33–41. doi: 10.1016/j.clinph.2017.09.118. [DOI] [PubMed] [Google Scholar]
- 27.Jackson A.K., et al. Cerebellar transcranial direct current stimulation enhances motor learning in a complex overhand throwing task. Cerebellum. 2019;18:813–816. doi: 10.1007/s12311-019-01040-6. [DOI] [PubMed] [Google Scholar]
- 28.Lindenberg R., et al. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ang K.K., et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Archives of physical medicine and rehabilitation. 2015;96(3):S79–S87. doi: 10.1016/j.apmr.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Rocha S., et al. The impact of transcranial direct current stimulation (tDCS) combined with modified constraint-induced movement therapy (mCIMT) on upper limb function in chronic stroke: a double-blind randomized controlled trial. Disabil. Rehabil. 2016;38(7):653–660. doi: 10.3109/09638288.2015.1055382. [DOI] [PubMed] [Google Scholar]
- 31.Ehsani F., et al. Differential effects of primary motor cortex and cerebellar transcranial direct current stimulation on motor learning in healthy individuals: a randomized double-blind sham-controlled study. Neurosci. Res. 2016;112:10–19. doi: 10.1016/j.neures.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Samaei A., et al. Online and offline effects of cerebellar transcranial direct current stimulation on motor learning in healthy older adults: a randomized double-blind sham-controlled study. Eur. J. Neurosci. 2017;45(9):1177–1185. doi: 10.1111/ejn.13559. [DOI] [PubMed] [Google Scholar]
- 33.Halakoo S., et al. Does anodal trans-cranial direct current stimulation of the damaged primary motor cortex affects wrist flexor muscle spasticity and also activity of the wrist flexor and extensor muscles in patients with stroke?: a Randomized Clinical Trial. Neurol. Sci. 2021;42(7):2763–2773. doi: 10.1007/s10072-020-04858-9. [DOI] [PubMed] [Google Scholar]
- 34.Yukihiro H. Rehabilitation with functional electrical stimulation in stroke patients. International Journal of Physical Medicine & Rehabilitation. 2013;1(6) [Google Scholar]
- 35.Gomez Palacio Schjetnan A., et al. Transcranial direct current stimulation in stroke rehabilitation: a review of recent advancements. Stroke Res. Treat. 2013;2013 doi: 10.1155/2013/170256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefebvre S., et al. Neural substrates underlying stimulation-enhanced motor skill learning after stroke. Brain. 2015;138(Pt 1):149–163. doi: 10.1093/brain/awu336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosso C., et al. Repetitive sessions of tDCS to improve naming in post-stroke aphasia: Insights from an individual patient data (IPD) meta-analysis. Restor. Neurol. Neurosci. 2018;36(1):107–116. doi: 10.3233/RNN-170783. [DOI] [PubMed] [Google Scholar]
- 38.Biou E., et al. Transcranial direct current stimulation in post-stroke aphasia rehabilitation: a systematic review. Ann Phys Rehabil Med. 2019;62(2):104–121. doi: 10.1016/j.rehab.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Yan R.B., et al. Effect of transcranial direct-current stimulation on cognitive function in stroke patients: a systematic review and meta-analysis. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaker H.A., et al. Effect of transcranial direct current stimulation on cognitive function in stroke patients. Egypt J Neurol Psychiatr Neurosurg. 2018;54(1):32. doi: 10.1186/s41983-018-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodwill A.M., et al. Bihemispheric-tDCS and upper limb rehabilitation improves retention of motor function in chronic stroke: a pilot study. Front. Hum. Neurosci. 2016;10:258. doi: 10.3389/fnhum.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray N.W.G., et al. Theta tACS impairs episodic memory more than tDCS. Sci. Rep. 2023;13(1):716. doi: 10.1038/s41598-022-27190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qurat Ul A., et al. Short term effects of anodal cerebellar vs. anodal cerebral transcranial direct current stimulation in stroke patients, a randomized control trial. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.1035558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong Q., et al. Effects of cerebellar transcranial direct current stimulation on rehabilitation of upper limb motor function after stroke. Front. Neurol. 2023;14 doi: 10.3389/fneur.2023.1044333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohammadi R., Mahmoudi Z., Mahmoodian N. Effects of cerebellar transcranial direct current stimulation (tDCS) on timed up and Go test with Foot Placement in chronic stroke patients. Middle East J Rehabil Health Stud. 2021;8(1) [Google Scholar]
- 46.Steiner K.M., et al. Cerebellar tDCS Does not improve learning in a complex Whole Body dynamic balance task in Young healthy subjects. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0163598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duarte Nde A., et al. Effect of transcranial direct-current stimulation combined with treadmill training on balance and functional performance in children with cerebral palsy: a double-blind randomized controlled trial. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0105777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacLullich A.M., et al. Size of the neocerebellar vermis is associated with cognition in healthy elderly men. Brain Cogn. 2004;56(3):344–348. doi: 10.1016/j.bandc.2004.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with the study has not been deposited into a publicly available repository. However, it can be available on request to the corresponding author.