Abstract
Alterations of nitric oxide (NO) homeostasis have been described in mood disorders. However, the analytical challenges associated with the direct measurement of NO have prompted the search for alternative biomarkers of NO synthesis. We investigated the published evidence of the association between these alternative biomarkers and mood disorders (depressive disorder or bipolar disorder). Electronic databases were searched from inception to the June 30, 2023. In 20 studies, there was a trend towards significantly higher asymmetric dimethylarginine (ADMA) in mood disorders vs. controls (p = 0.072), and non-significant differences in arginine (p = 0.29), citrulline (p = 0.35), symmetric dimethylarginine (SDMA; p = 0.23), and ornithine (p = 0.42). In subgroup analyses, the SMD for ADMA was significant in bipolar disorder (p < 0.001) and European studies (p = 0.02), the SMDs for SDMA (p = 0.001) and citrulline (p = 0.038) in European studies, and the SMD for ornithine in bipolar disorder (p = 0.007), Asian (p = 0.001) and American studies (p = 0.005), and patients treated with antidepressants (p = 0.029). The abnormal concentrations of ADMA, SDMA, citrulline, and ornithine in subgroups of mood disorders, particularly bipolar disorder, warrant further research to unravel their pathophysiological role and identify novel treatments in this group (The protocol was registered in PROSPERO: CRD42023445962)
Keywords: Arginine, NO, ADMA, SDMA, Citrulline, Ornithine, Mood disorder, Bipolar disorder
1. Introduction
Despite significant advances, largely based on the monoamine hypothesis which was formulated more than 50 years ago [1], the treatment failure in patients suffering from the two main subtypes of mood disorder, depressive disorder and bipolar disorder, remains unacceptably high, with negative implications for the community and healthcare systems [[2], [3], [4], [5]]. This vexing issue is likely the result of the incomplete knowledge regarding the pathophysiological mechanisms involved in mood disorders, which prevents the discovery of new disease biomarkers, druggable targets, and more effective therapies [[6], [7], [8], [9]].
In the ongoing search for breakthrough molecular mechanisms, studies have sought to determine the pathophysiological role of nitric oxide (NO), synthetised by three NO synthase (NOS) isoforms (Fig. 1) [10,11]. All NOS isoforms modulate cell signalling in the brain, particularly the neuronal isoform, nNOS, which co-locates with key receptors [12,13], and is also influenced by muscarinic and purinergic receptors and by serotoninergic pathways [[14], [15], [16]]. NO, itself can nitrosylate several proteins, influencing their activity and regulating neuronal function and local blood flow [[17], [18], [19]]. Despite the various homeostatic effects exerted by NO experimental and human studies have provided conflicting results regarding whether mood disorders are associated with an increased or a decreased synthesis and availability of NO [10,11,20,21]. This issue is particularly relevant given that alterations in NO concentrations can either exert beneficial effects on cellular homeostatic mechanisms or toxicity [22,23]. However, the high reactivity of NO has traditionally represented a major barrier to its measurement in biological samples [24,25]. The measurement of alternative metabolites, e.g., nitrite and nitrate, is also affected by a number of factors that have curtailed its routine use in research and clinical studies [26,27].
Fig. 1.
Arginine metabolic pathways. PRMTs, protein arginine methyltransferases; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; DDAH1, isoform 1 of dimethylarginine dimethylaminohydrolase; eNOS, endothelial nitric oxide synthase; nNOS, neuronal nitric oxide synthase; iNOS, inducible nitric oxide synthase; NO, nitric oxide; OAT, ornithine aminotransferase; ODC, ornithine decarboxylase; OTC, ornithine transcarbamylase.
To address this issue, a number of metabolites within the arginine biochemical pathways have been investigated in health and disease states given their direct or indirect effects in modulating NO synthesis (Fig. 1) [[28], [29], [30], [31], [32], [33]]. Their assessment has also been proposed to further investigate the role of NO and arginine pathways in mood disorders [29,34].
Therefore, we critically appraised the available evidence of the association between these arginine metabolites, mood disorders, and specific clinical and demographic characteristics, including the type of mood disorder (depressive vs. bipolar) [35], and the use of antidepressant treatment. Our initial hypothesis was that mood disorders were associated with abnormalities in one or more arginine metabolites when compared to healthy controls.
2. Methods
2.1. Literature search
Two independent investigators searched the electronic databases until the June 30, 2023 using the search strategy described in Supplementary Tab. 1 and the following inclusion criteria: the measurement of arginine, asymmetric (ADMA) and symmetric (SDMA) dimethylarginine, citrulline, or ornithine in adult patients with mood disorder and healthy subjects in fully available articles written in English. Individual articles’ references were hand searched for further articles.
First author, publication year, study country, sample size, age, male to female ratio, use of antidepressant medications, and analytical method used for arginine metabolomics evaluation were extracted from each article.
The risk of bias and the certainty of evidence were assessed using standard methods [36,37], the PRISMA 2020 statement was adhered to (Supplementary Tab. 1) [38], and the review was registered (PROSPERO registration number: CRD42023445962).
2.2. Statistical analysis
Forest plots were generated to investigate differences between patients suffering from mood disorder and controls using standard mean differences (SMDs) [39,40]. Heterogeneity was assessed using the Q statistic [41,42]. Sensitivity analysis assessed whether the pooled SMD values were stable [43], and standard methods were used to assess publication bias [[44], [45], [46]]. We further investigated associations between the effect size and pre-defined patient and study characteristics. Statistical analyses were conducted using Stata 14 (Stata Corp., College Station, TX, USA).
3. Results
3.1. Screening process
The study selection is described in Fig. 2. Of the 4168 articles initially identified, 4145 were excluded. Of the remaining 23 articles, a further three were excluded, leaving 20 studies [[47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66]] (Table 1). Nineteen studies had a low risk of bias [[47], [48], [49],[51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66]], and the remaining one had a moderate risk of bias [50] (Supplementary Tab. 2). The cross-sectional study design reduced the initial certainty of evidence to low.
Fig. 2.
PRISMA 2020 flow diagram.
Table 1.
Study characteristics.
|
Study |
Healthy controls |
Patients with depressive disorder |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Age (Years) | M/F |
Arginine ADMA SDMA (Mean ± SD) |
Citrulline Ornithine (Mean ± SD) |
n | Age (Years) | M/F |
Arginine ADMA SDMA (Mean ± SD) |
Citrulline Ornithine (Mean ± SD) |
|
| Abou-Saleh MT et al., 1998, UAE [47] | 38 | 36 | 0/38 | 65.4 ± 24.13 NR NR |
NR 90.2 ± 33.2 |
30 | 35 | 0/30 | 89.1 ± 30.5 NR NR |
NR 67.4 ± 23.3 |
| Maes M et al., 1998, Belgium [48] | 15 | 48 | 5/10 | 137 ± 28 NR NR |
NR NR |
35 | 51 | 18/17 | 124 ± 22 NR NR |
NR NR |
| Mauri MC et al., 1998, Italy [49] | 28 | 42 | 16/12 | 87.6 ± 30.9 NR NR |
NR NR |
29 | 47 | 15/14 | 83.5 ± 100.6 NR NR |
NR NR |
| Mitani H et al., 2006, Japan [50] | 31 | 41 | 17/14 | 93.2 ± 42.8 NR NR |
NR NR |
23 | 38 | 11/12 | 116.8 ± 54.8 NR NR |
NR NR |
| Pinto VL et al., 2012, Brazil [51] | 5 | NR | NR | 130 ± 19 NR NR |
NR 62 ± 27 |
5 | NR | NR | 104 ± 9 NR NR |
NR 97 ± 5 |
| Canpolat S et al., 2014, Turkey [52] | 44 | 27 | 14/30 | NR 6.8 ± 4.0 NR |
NR NR |
41 | 26 | 13/28 | NR 7.3 ± 5.3 NR |
NR NR |
| Baranyi A et al., 2015, UK [53] | 48 | 40 | 31/17 | 96.1 ± 13.8 0.61 ± 0.08 0.71 ± 0.15 |
30.6 ± 7.6 90 ± 23 |
71 | 50 | 48/23 | 100.2 ± 19.8 0.64 ± 0.09 0.64 ± 0.11 |
29.9 ± 6.2 96.3 ± 11.2 |
| Woo HI et al. (a), 2015, Korea [54] | 22 | 68 | 4/18 | 57.7 ± 26.7 NR NR |
30.9 ± 8.1 110 ± 32 |
68 | 65 | 16/22 | 70.9 ± 33.4 NR NR |
34.9 ± 15.1 99.2 ± 43 |
| Woo HI et al. (b), 2015, Korea [54] | 22 | 68 | 4/18 | 57.7 ± 26.7 NR NR |
30.9 ± 8.1 110 ± 32 |
68 | 65 | 16/22 | 77.1 ± 33.1 NR NR |
36.1 ± 19.3 99.8 ± 50.7 |
| Yoshimi N et al., 2016, Japan [55] | 39 | 36 | 39/0 | 122 ± 19 NR NR |
37.0 ± 6.4 79 ± 19 |
54 | 41 | 54/0 | 131 ± 23 NR NR |
38.0 ± 7.1 71 ± 16 |
| Hess S et al., 2017, Canada [56] | 36 | 26 | 20/16 | 84.9 ± 25.2 NR NR |
35.2 ± 6.8 NR |
35 | 27 | 20/15 | 73.5 ± 21.5 NR NR |
31.6 ± 6.0 NR |
| Kageyama Y et al. (a), 2017, Japan [57] | 19 | 36 | 10/9 | 102 ± 22 NR NR |
31.9 ± 10.9 56 ± 11.6 |
9 | 39 | 3/6 | 85 ± 18 NR NR |
28.9 ± 12.4 47.6 ± 16.3 |
| Kageyama Y et al. (b), 2017, Japan [57] | 19 | 36 | 10/9 | 102 ± 22 NR NR |
31.9 ± 10.9 56 ± 11.6 |
6 | 42 | 1/5 | 79 ± 19 NR NR |
21.4 ± 5.1 46.2 ± 10.7 |
| Ali-Sisto T et al., 2018, Finland [58] | 253 | 55 | 124/129 | 118 ± 35 0.79 ± 0.34 1.43 ± 0.61 |
28.2 ± 8.7 90.6 ± 34.4 |
99 | 39 | 43/56 | 99 ± 24 0.77 ± 0.29 1.27 ± 0.50 |
27.4 ± 10 89.6 ± 37.9 |
| Moaddel R et al., 2018, USA [59] | 25 | NR | NR | NR NR NR |
23.8 ± 7.22 NR |
29 | NR | NR | NR NR NR |
19.25 ± 5.17 NR |
| Ogawa S et al. (a), 2018, Japan [60] | 217 | 41 | 100/117 | 79.2 ± 25.7 NR NR |
35.3 ± 8.6 87.1 ± 28.6 |
147 | 42 | 74/73 | 77.9 ± 21.9 NR NR |
36.1 ± 8.6 74.7 ± 27.4 |
| Ogawa S et al. (b), 2018, Japan [60] | 65 | 43 | 20/45 | 90.6 ± 28.3 NR NR |
32.2 ± 10.3 65.3 ± 17.6 |
51 | 44 | 27/24 | 83.7 ± 24.1 NR NR |
31.9 ± 11.6 67.9 ± 18.0 |
| Yilmaz E et al., 2019, Turkey [61] | 30 | NR | 13/17 | 0.34 ± 0.23* NR NR |
NR NR |
30 | NR | 13/17 | 0.63 ± 0.41* NR NR |
NR NR |
| Ozden A et al., 2020, USA [62] | 27 | 38 | 7/20 | 1.4 ± 1.3 0.16 ± 0.08 0.01 ± 0.01 |
4.4 ± 1.0 4.5 ± 1.9 |
77 | 41 | 28/49 | 6.6 ± 7.2 0.1 ± 0.03 0.02 ± 0.01 |
5.2 ± 1.8 7.83 ± 4.33 |
| Ustundag MF et al., 2020, Turkey [63] | 30 | 35 | 12/18 | 74.7 ± 9.2 NR 3.67 ± 0.98 |
NR NR |
30 | 35 | 13/17 | 91.6 ± 18.8 NR 4.52 ± 0.85 |
NR NR |
| Bilbao AV et al., 2021, USA [64] | 30 | NR | NR | NR 0.42 ± 0.07° 0.36 ± 0.05° |
NR NR |
30 | NR | NR | NR 0.42 ± 0.09° 0.41 ± 0.05° |
NR NR |
| Braun D et al. (a), 2021, Germany [65] | 16 | 31 | 8/8 | NR 82 ± 12.6* NR |
NR NR |
14 | 52 | 9/5 | NR 151 ± 36.9* NR |
NR NR |
| Braun D et al. (b), 2021, Germany [65] | 16 | 31 | 8/8 | NR 82 ± 12.6* |
NR NR |
44 | 50 | 27/17 | NR 138 ± 39* NR |
NR NR |
| Loeb E et al., 2022, France [66] | 895 | 40 | 457/438 | 81.8 ± 19.5 NR NR |
30.1 ± 8.1 NR |
460 | 46 | 145/315 | 90.9 ± 22.8 NR NR |
27.2 ± 8.0 NR |
Legend: ADMA, asymmetric dimethylarginine; F, female; M, male; NR, not reported; SDMA, symmetric dimethylarginine.
The concentration is expressed in μmol/L except where otherwise indicated. *, ng/mL; °, pg/mL.
3.2. Arginine
Sixteen studies including 19 group comparators reported arginine in 1327 patients with mood disorders and 1839 controls (Table 1) [[47], [48], [49], [50], [51],[53], [54], [55], [56], [57], [58],[60], [61], [62], [63],66]. Fifteen study groups included patients with depressive disorder and four with bipolar disorder. Eight study groups included patients receiving antidepressants, ten untreated patients, whereas relevant information was not available in one. Fifteen studies had a low risk of bias and one moderate (Supplementary Tab. 2).
The forest plot showed that the serum concentrations of arginine in mood disorders were similar to controls (Fig. 3). Sensitivity analysis confirmed the stability of the results (Supplementary F. 1). No significant publication bias was observed (Supplementary F. 2).
Fig. 3.
Forest plot of arginine concentrations in patients with mood disorders and healthy controls.
No significant associations were observed between the SMD and age (t = 0.17, p = 0.87), sex distribution (t = −0.91, p = 0.38), study size (t = 0.37, p = 0.72), or publication year (t = 0.22, p = 0.83). The pooled SMD were similar (p = 0.30) in studies in depressive disorder and bipolar disorder (Supplementary F. 3), between Asian, European, and American studies (p = 0.25; Supplementary F. 4), and between studies using liquid chromatography and other methods (p = 0.67) (Supplementary F. 5). Among liquid chromatography studies, the pooled SMD was similar (p = 0.58) in studies using fluorimetric detection and mass spectrometry (Supplementary F. 6). Finally, the effect size was similar (p = 0.22) in studies in treated and untreated patients (Supplementary F. 7).
The high and unexplainable heterogeneity led to the downgrading of the level of certainty to very low.
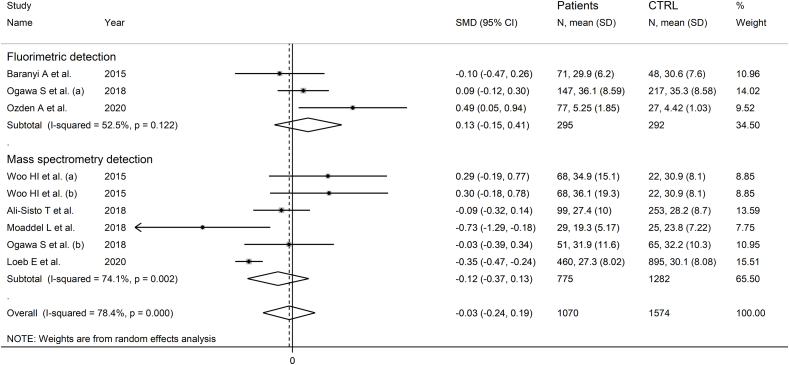
3.3. Asymmetric dimethylarginine
Seven studies including eight group comparators reported ADMA in 406 patients with mood disorders and 464 controls (Table 1) [52,53,58,[62], [63], [64], [65]]. Five studies included participants with depressive disorder and two with bipolar disorder. Three study groups included patients receiving antidepressants, three untreated patients, whereas no information was available in two. All studies had a low risk of bias (Supplementary Tab. 2).
The forest plot showed that patients with mood disorders had a trend towards statistically higher ADMA concentrations compared to controls (Fig. 4). The direction of the pooled SMD remained consistent in sensitivity analysis (Supplementary F. 8).
Fig. 4.
Forest plot of ADMA concentrations in patients with mood disorders and healthy controls.
Publication bias and meta-regression could not be evaluated because of the insufficient number of studies for these types of analyses. There was a significant difference (p = 0.037) in the pooled SMD between studies in depressive disorder and bipolar disorder (Fig. 5) with the bipolar subgroup exhibiting a lower heterogeneity. In addition, the pooled SMD was statistically significant in European, but not in Asian or American studies (Supplementary F. 9), whereas there were no significant differences between studies of treated and untreated patients (Supplementary F. 10).
Fig. 5.
Forest plot of ADMA concentrations in patients with mood disorders and healthy controls according to disease type (depressive disorder vs. mood disorder).
The lack of evaluation of publication bias led to the downgrading of the level of certainty to very low.
3.4. Symmetric dimethylarginine
Five studies investigated SDMA in 307 patients with mood disorders and 388 controls (Table 1) [53,58,[62], [63], [64]]. Patients with depressive disorder were investigated in four studies and with bipolar disorder in the remaining one. In one study group patients were receiving antidepressants, in two patients were untreated, whereas in the remaining two no information regarding treatment was provided. All studies had low risk of bias (Supplementary Tab. 2).
The forest plot showed that SDMA was similar between patients with mood disorders and controls (Fig. 6). The corresponding pooled SMD was stable (Supplementary F. 11).
Fig. 6.
Forest plot of SDMA concentrations in patients with mood disorders and healthy controls.
We could not assess publication bias or perform meta-regression analysis because of the insufficient number of selected studies. There was a significant difference (p = 0.02) in effect size between European and Asian studies (Supplementary F. 12), with a substantial reduction in heterogeneity in both subgroups.
Similar to ADMA, the lack of evaluation of publication bias led to the downgrading of the level of certainty to very low.
3.5. Citrulline
Ten studies including 13 group comparators reported citrulline in 1174 patients with mood disorders and 1687 healthy controls (Table 1) [[53], [54], [55], [56], [57], [58], [59], [60],62,66]. Eleven study groups included patients with depressive disorder and the remaining two bipolar disorder. Eight study groups included untreated patients and the remaining five patients receiving antidepressants. All studies had low risk of bias (Supplementary Tab. 2).
The forest plot showed that citrulline was similar between the two groups (Fig. 7), with stable pooled SMD values (Supplementary F. 13). There was no publication bias. The addition of one missing study with the “trim-and-fill” method did not substantially change the effect size (Supplementary F. 14).
Fig. 7.
Forest plot of citrulline concentrations in patients with mood disorders and healthy controls.
No associations were observed between the effect size and age of participants (t = −0.63, p = 0.59), year of publication (t = −0.54, p = 0.60), and sample size (t = −0.68, p = 0.51). However, a significant association was observed with sex distribution (Supplementary F. 15A and 15B).
The pooled SMD was similar (p = 0.74) in studies in depressive disorder and bipolar disorder (Supplementary F. 16). The pooled SMD was significant in European, but not in Asian or American studies (Supplementary F. 17), with a lower heterogeneity in the Asian subgroup. The pooled SMD was similar (p = 0.64) in studies using liquid chromatography and capillary electrophoresis (Supplementary F. 18). In studies using liquid chromatography, the pooled SMD was similar (p = 0.31) in studies with fluorimetric detection and mass spectrometry detection (Supplementary F. 19). The pooled SMD was also similar (p = 0.19) between studies in patients receiving antidepressants and untreated patients (Supplementary F. 20).
The final level of certainty was unchanged (low).
3.6. Ornithine
Nine studies including 12 group comparators investigated ornithine in 718 patients with mood disorders and 806 healthy controls (Table 1) [47,51,[53], [54], [55],57,58,60,62]. Ten study groups included patients with depressive disorder and the remaining two with bipolar disorder. Six study groups included untreated patients and the remaining six patients receiving antidepressants. All studies had low risk of bias (Supplementary Tab. 2).
The forest plot showed that ornithine was similar in patients with mood disorders and controls (Fig. 8). No significant deviations in pooled SMD were observed in sensitivity analysis (Supplementary F. 21) and no publication bias was detected (Supplementary F. 22).
Fig. 8.
Forest plot of studies examining ornithine concentrations in patients with mood disorders and healthy controls.
No significant associations were observed between the SMD and participant age (t = 0.19, p = 0.85), sex distribution (t = 0.60, p = 0.56), publication year (t = 1.26, p = 0.24), or participant number (t = −0.32, p = 0.76). The pooled SMD was significant in studies of bipolar disorder but not depressive disorder (Fig. 9), with a reduced heterogeneity in the bipolar subgroup. Moreover, the pooled SMD was significantly different in Asian and American, but not European studies (Supplementary F. 23), with a lower heterogeneity in the American subgroup. The SMD was also statistically significant using capillary electrophoresis but not liquid chromatography (Supplementary F. 24), with a lower heterogeneity in the capillary electrophoresis subgroup. In liquid chromatography studies, there was no significant difference (p = 0.43) in pooled SMD between fluorimetric detection and mass spectrometry detection (Supplementary F. 25), with absent between-study variance in the mass spectrometry subgroup. Finally, the pooled SMD was statistically significant in treated, but not untreated patients (Supplementary F. 26).
Fig. 9.
Forest plot of ornithine concentrations in patients with mood disorders and healthy controls according to disease type (depressive disorder vs. bipolar disorder).
The final level of certainty remained unchanged (low).
4. Discussion
This study has reported non-significant differences in key metabolites of the arginine pathways, arginine, ornithine, citrulline, and SDMA, between individuals suffering from mood disorders and healthy controls, and a trend towards higher ADMA concentrations in patients with mood disorders. However, in subgroup analysis ADMA was significantly higher in subjects with bipolar disorder vs. controls and in European studies. Opposite, significant effects were observed for SDMA in European vs. Asian studies, and significantly lower concentrations were observed with citrulline only in European studies. Furthermore, ornithine concentrations were significantly lower specifically in bipolar disorder and in subjects with mood disorders receiving antidepressants, whereas opposite, significant effects were observed in Asian vs. American studies. Additional subgroup differences for ornithine were observed according to the type of analytical method used. The overall absence of significant associations between the effect size of other metabolites and analytical method suggests the potential validity of several methodological approaches for the routine assessment of arginine metabolomics in research and clinical studies [26,67]. No further associations were observed barring a significant correlation between the SMD of citrulline and sex distribution.
ADMA and SDMA are formed following methylation of arginine contained in proteins (Fig. 1) [68,69]. After protein degradation, ADMA is either transformed to citrulline and dimethylamine by dimethylarginine dimethylaminohydrolase 1 (DDAH1) or transported into the extracellular compartment for remote DDAH1 metabolism or renal elimination [30,[70], [71], [72], [73]]. Differently from ADMA, SDMA is not metabolised by DDAH1 and undergoes renal elimination [28,71,74]. Both ADMA and SDMA have been shown to downregulate NO synthesis. However, whilst ADMA is a potent reversible inhibitor of all NOS isoforms (Fig. 1) [75,76], the interaction between SDMA and NOS is more controversial, with studies reporting that this methylated arginine can favour NOS uncoupling and inhibit arginine transport, reducing the amount of arginine as a NOS substrate [71,77,78]. Our subgroup analyses suggest a selective increase in circulating ADMA concentrations, with consequent reduction in NO synthesis, in bipolar depression. Although the relatively small number of studies captured in search warrants caution with data interpretation, this observation suggests that NO may have a different pathophysiological role in specific subtypes of mood disorder. Furthermore, the reported associations between the SMD of ADMA and geographical location and treatment with antidepressants suggest new avenues for research investigating the interplay between arginine metabolomics, NO, and mood disorders. Previous studies investigating the association between ADMA concentrations and specific ethnic groups have provided conflicting results, with some reports suggesting a link [[79], [80], [81], [82], [83]], while others reporting negative findings [84,85]. Regarding SDMA, the reported link between its circulating concentrations and ethnicity reported in other studies should also prompt further research to investigate the pathophysiological role of this indirect modulator of NO synthesis in mood disorders [83,84].
The role of citrulline in arginine metabolism is complex as this metabolite is a product of reactions catalysed by NOS, DDAH1, and ornithine transcarbamylase, as well as an argininosuccinate synthetase substrate in reactions leading to the synthesis of arginine (Fig. 1) [31]. Whilst no overall significant associations were observed between citrulline concentrations and mood disorders, the lower concentrations observed in European studies suggests the need for additional research, also given the results of recent studies in non-psychiatric populations reporting significant ethnic-related differences in citrulline concentrations [79,86]. Ornithine also plays a complex role within arginine metabolism, serving as both a substrate for ornithine aminotransferase for the synthesis of l-glutamate 5-semialdehyde and either glutamate or proline, for ornithine decarboxylase as the first step in the synthesis of polyamines, and for ornithine transcarbamylase which is involved in the synthesis of citrulline, and a product of arginase which uses arginine as substrate [31]. The significantly lower ornithine concentrations in bipolar, but not depressive disorder, suggest, similarly to ADMA, the presence of disease subtype-specific differences in the pathophysiological role of arginine metabolism. Such alterations could be secondary to reduced arginase activity and/or increased ornithine aminotransferase, ornithine decarboxylase, or ornithine transcarbamylase activity. A reduced arginase activity has been previously reported in bipolar disorder. In one study, the plasma arginase activity in 43 patients with bipolar disorder (male to female ratio: 33/10) was significantly lower compared to that measured in 31 age- and sex-matched control subjects (male to female ratio: 22/9; F = 18.79, p < 0.001) [87]. However, another study failed to report significant differences in arginase expression in platelets in subjects with bipolar disorder and controls [88]. Whilst the expression/activity of ornithine aminotransferase and ornithine transcarbamylase have not been specifically investigated in experimental and human studies of depressive or bipolar disorder, a recent study has reported that individuals with bipolar disorder had significantly higher plasma concentrations of spermine, a member of the polyamine family that is the end product of a series of enzymatic reactions that include ornithine decarboxylase, compared to healthy controls (879 ± 1021 vs. 174 ± 83 mmol/mL, p = 0.015) [89]. The hypothesis that alterations in ornithine concentrations in bipolar disorder reflect changes in the expression/activity of arginase and/or ornithine decarboxylase requires confirmation in further studies that should also investigate the activity of specific antidepressants on these enzymes. In this context, a study investigating the effects of antidepressant agents failed to demonstrate significant effects on arginase [90], whereas another reported a five-fold increase in ornithine decarboxylase activity with the tricyclic antidepressant imipramine [91]. Finally, the associations between ornithine and study continent are in line with previously reported differences in the concentrations of this metabolite associated with ethnicity [79].
A previously published systematic review and meta-analysis has investigated arginine, ornithine, and citrulline in major depressive disorder and reported no significant alterations of the three metabolites nor associations with pharmacological treatment [92]. Our meta-analysis captured a larger number of studies (20 vs. 13), which also included patients with bipolar disorders and the assessment of ADMA and SDMA, allowing a comprehensive investigation of the interplay between arginine metabolites with direct or indirect effects on NO synthesis and subtypes of mood disorder (depressive vs. bipolar).
Strengths of our work are the comprehensive assessment of arginine metabolomics in mood disorders (overall and by subtype), the investigation of potential correlations between the SMD and various parameters, and the robust assessment of the certainty of evidence and risk of bias. Limitations include the small number of articles, particularly studies investigating ADMA and SDMA. A further limitation is the high between-study heterogeneity observed. However, in subgroup analysis we identified potential sources of heterogeneity for ADMA (type of mood disorder), SDMA (study continent), citrulline (study continent), and ornithine (type of mood disorder, study continent, analytical method, and detection method).
In conclusion, our study has identified significant abnormalities in arginine metabolites and biomarkers of NO synthesis, i.e., ADMA, SDMA, citrulline, and ornithine, in subgroups of patients with mood disorders. These findings indicate a dysregulation of NO, particularly in bipolar disorder, and are hypothesis-generating for further research to unravel the pathophysiological role of arginine metabolism and NO and identify novel druggable targets in these patients.
Data availability statement
The data that support the findings of this systematic review and meta-analysis are available from Angelo Zinellu upon reasonable request.
Ethics declaration statement
Review and/or approval by an ethics committee was not needed as this is a systematic review and meta-analysis of published studies.
CRediT authorship contribution statement
Angelo Zinellu: Writing – review & editing, Methodology, Formal analysis, Data curation, Conceptualization. Sara Tommasi: Writing – review & editing, Methodology, Conceptualization. Stefania Sedda: Writing – review & editing, Methodology. Arduino A. Mangoni: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Stefania Sedda is funded by the project e.INS - Ecosystem of Innovation for Next Generation Sardinia (cod. ECS 00000038) by the Italian Ministry for Research and Education (MUR) under the National Recovery and Resilience Plan (NRRP) - MISSION 4 COMPONENT 2, “From research to business” INVESTMENT 1.5, “Creation and strengthening of Ecosystems of innovation” and construction of “Territorial R&D Leaders”.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27292.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
figs9.
figs10.
figs11.
figs12.
figs13.
figs14.
figs15.
figs16.
figs17.
figs18.
figs19.
figs20.
figs21.
figs22.
figs23.
figs24.
figs25.
figs26.
References
- 1.Schildkraut J.J. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am. J. Psychiatr. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn T.P. Depressive disorders: treatment failures and poor prognosis over the last 50 years. Pharmacol Res Perspect. 2019;7 doi: 10.1002/prp2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhdanava M., Pilon D., Ghelerter I., Chow W., Joshi K., Lefebvre P., Sheehan J.J. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J. Clin. Psychiatry. 2021:82. doi: 10.4088/JCP.20m13699. [DOI] [PubMed] [Google Scholar]
- 4.Baldessarini R.J., Vazquez G.H., Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8:1. doi: 10.1186/s40345-019-0160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui Poon S., Sim K., Baldessarini R.J. Pharmacological approaches for treatment-resistant bipolar disorder. Curr. Neuropharmacol. 2015;13:592–604. doi: 10.2174/1570159x13666150630171954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatr. 2010;9:155–161. doi: 10.1002/j.2051-5545.2010.tb00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu B., Liu J., Wang M., Zhang Y., Li L. From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front. Cell. Neurosci. 2017;11:305. doi: 10.3389/fncel.2017.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips M.L., Kupfer D.J. Bipolar disorder diagnosis: challenges and future directions. Lancet. 2013;381:1663–1671. doi: 10.1016/S0140-6736(13)60989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieta E., Berk M., Schulze T.G., Carvalho A.F., Suppes T., Calabrese J.R., Gao K., Miskowiak K.W., Grande I. Bipolar disorders. Nat. Rev. Dis. Prim. 2018;4 doi: 10.1038/nrdp.2018.8. [DOI] [PubMed] [Google Scholar]
- 10.Dhir A., Kulkarni S.K. Nitric oxide and major depression. Nitric Oxide. 2011;24:125–131. doi: 10.1016/j.niox.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Joca S.R.L., Sartim A.G., Roncalho A.L., Diniz C.F.A., Wegener G. Nitric oxide signalling and antidepressant action revisited. Cell Tissue Res. 2019;377:45–58. doi: 10.1007/s00441-018-02987-4. [DOI] [PubMed] [Google Scholar]
- 12.Fan X., Jin W.Y., Wang Y.T. The NMDA receptor complex: a multifunctional machine at the glutamatergic synapse. Front. Cell. Neurosci. 2014;8:160. doi: 10.3389/fncel.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L.H., Talman W.T. Coexistence of NMDA and AMPA receptor subunits with nNOS in the nucleus tractus solitarii of rat. J. Chem. Neuroanat. 2002;24:287–296. doi: 10.1016/s0891-0618(02)00069-8. [DOI] [PubMed] [Google Scholar]
- 14.Huang C.C., Hsu K.S. Activation of muscarinic acetylcholine receptors induces a nitric oxide-dependent long-term depression in rat medial prefrontal cortex. Cerebr. Cortex. 2010;20:982–996. doi: 10.1093/cercor/bhp161. [DOI] [PubMed] [Google Scholar]
- 15.Granjeiro E.M., Pajolla G.P., Accorsi-Mendonca D., Machado B.H. Interaction of purinergic and nitrergic mechanisms in the caudal nucleus tractus solitarii of rats. Auton. Neurosci. 2009;151:117–126. doi: 10.1016/j.autneu.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Garthwaite J. Neuronal nitric oxide synthase and the serotonin transporter get harmonious. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7739–7740. doi: 10.1073/pnas.0702508104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prast H., Philippu A. Nitric oxide as modulator of neuronal function. Prog. Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Stella A.M. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 20.McNeill R.V., Kehrwald C., Brum M., Knopf K., Brunkhorst-Kanaan N., Etyemez S., Koreny C., Bittner R.A., Freudenberg F., Herterich S., Reif A., Kittel-Schneider S. Uncovering associations between mental illness diagnosis, nitric oxide synthase gene variation, and peripheral nitric oxide concentration. Brain Behav. Immun. 2022;101:275–283. doi: 10.1016/j.bbi.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Amini-Khoei H., Nasiri Boroujeni S., Maghsoudi F., Rahimi-Madiseh M., Bijad E., Moradi M., Lorigooini Z. Possible involvement of L-arginine-nitric oxide pathway in the antidepressant activity of Auraptene in mice. Behav. Brain Funct. 2022;18:4. doi: 10.1186/s12993-022-00189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuste J.E., Tarragon E., Campuzano C.M., Ros-Bernal F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015;9:322. doi: 10.3389/fncel.2015.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dzoljic E., Grbatinic I., Kostic V. Why is nitric oxide important for our brain? Funct. Neurol. 2015;30:159–163. doi: 10.11138/fneur/2015.30.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goshi E., Zhou G., He Q. Nitric oxide detection methods in vitro and in vivo. Med. Gas Res. 2019;9:192–207. doi: 10.4103/2045-9912.273957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moller M.N., Rios N., Trujillo M., Radi R., Denicola A., Alvarez B. Detection and quantification of nitric oxide-derived oxidants in biological systems. J. Biol. Chem. 2019;294:14776–14802. doi: 10.1074/jbc.REV119.006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic. Res. 2005;39:797–815. doi: 10.1080/10715760500053651. [DOI] [PubMed] [Google Scholar]
- 27.Viinikka L. Nitric oxide as a challenge for the clinical chemistry laboratory. Scand. J. Clin. Lab. Invest. 1996;56:577–581. doi: 10.3109/00365519609090591. [DOI] [PubMed] [Google Scholar]
- 28.Mangoni A.A. The emerging role of symmetric dimethylarginine in vascular disease. Adv. Clin. Chem. 2009;48:73–94. doi: 10.1016/s0065-2423(09)48003-x. [DOI] [PubMed] [Google Scholar]
- 29.Mangoni A.A., Rodionov R.N., McEvoy M., Zinellu A., Carru C., Sotgia S. New horizons in arginine metabolism, ageing and chronic disease states. Age Ageing. 2019;48:776–782. doi: 10.1093/ageing/afz083. [DOI] [PubMed] [Google Scholar]
- 30.Jarzebska N., Mangoni A.A., Martens-Lobenhoffer J., Bode-Boger S.M., Rodionov R.N. The second life of methylarginines as cardiovascular targets. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20184592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris S.M., Jr. Arginine metabolism revisited. J. Nutr. 2016;146:2579S. doi: 10.3945/jn.115.226621. 86S. [DOI] [PubMed] [Google Scholar]
- 32.Sotgia S., Zinellu A., Paliogiannis P., Pinna G.A., Mangoni A.A., Milanesi L., Carru C. A diethylpyrocarbonate-based derivatization method for the LC-MS/MS measurement of plasma arginine and its chemically related metabolites and analogs. Clin. Chim. Acta. 2019;492:29–36. doi: 10.1016/j.cca.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 33.van Dyk M., Mangoni A.A., McEvoy M., Attia J.R., Sorich M.J., Rowland A. Targeted arginine metabolomics: a rapid, simple UPLC-QToF-MS(E) based approach for assessing the involvement of arginine metabolism in human disease. Clin. Chim. Acta. 2015;447:59–65. doi: 10.1016/j.cca.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 34.McEvoy M.A., Schofield P., Smith W., Agho K., Mangoni A.A., Soiza R.L., Peel R., Hancock S., Kelly B., Inder K., Carru C., Zinellu A., Attia J. Serum methylarginines and incident depression in a cohort of older adults. J. Affect. Disord. 2013;151:493–499. doi: 10.1016/j.jad.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Fawcett J. An overview of mood disorders in the DSM-5. Curr. Psychiatr. Rep. 2010;12:531–538. doi: 10.1007/s11920-010-0154-2. [DOI] [PubMed] [Google Scholar]
- 36.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., Currie M., Qureshi R., Mattis P., Lisy K., Mu P.-F. Joanna Briggs Institute Reviewer's Manual, edsAromataris E, Munn Z. Johanna Briggs Institute; Adelaide, Australia: 2017. Systematic reviews of etiology and risk. [Google Scholar]
- 37.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., Jaeschke R., Rind D., Meerpohl J., Dahm P., Schunemann H.J. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hrobjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 42.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Technical Bulletin. 1999;47:15–17. [Google Scholar]
- 44.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 45.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 46.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 47.Abou-Saleh M.T., Karim L., Krymsky M. The biology of depression in Arab culture. Nord. J. Psychiatr. 1998;52:177–182. doi: 10.1080/08039489850139067. [DOI] [Google Scholar]
- 48.Maes M., Verkerk R., Vandoolaeghe E., Lin A., Scharpe S. Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatr. Scand. 1998;97:302–308. doi: 10.1111/j.1600-0447.1998.tb10004.x. [DOI] [PubMed] [Google Scholar]
- 49.Mauri M.C., Ferrara A., Boscati L., Bravin S., Zamberlan F., Alecci M., Invernizzi G. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. 1998;37:124–129. doi: 10.1159/000026491. [DOI] [PubMed] [Google Scholar]
- 50.Mitani H., Shirayama Y., Yamada T., Maeda K., Ashby C.R., Jr., Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2006;30:1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 51.Pinto V.L., de Souza P.F., Brunini T.M., Oliveira M.B., Moss M.B., Siqueira M.A., Ferraz M.R., Mendes-Ribeiro A.C. Low plasma levels of L-arginine, impaired intraplatelet nitric oxide and platelet hyperaggregability: implications for cardiovascular disease in depressive patients. J. Affect. Disord. 2012;140:187–192. doi: 10.1016/j.jad.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Canpolat S., Kirpinar I., Deveci E., Aksoy H., Bayraktutan Z., Eren I., Demir R., Selek S., Aydin N. Relationship of asymmetrical dimethylarginine, nitric oxide, and sustained attention during attack in patients with major depressive disorder. Sci. World J. 2014;2014 doi: 10.1155/2014/624395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baranyi A., Amouzadeh-Ghadikolai O., Rothenhausler H.B., Theokas S., Robier C., Baranyi M., Koppitz M., Reicht G., Hlade P., Meinitzer A. Nitric oxide-related biological pathways in patients with major depression. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woo H.I., Chun M.R., Yang J.S., Lim S.W., Kim M.J., Kim S.W., Myung W.J., Kim D.K., Lee S.Y. Plasma amino acid profiling in major depressive disorder treated with selective serotonin reuptake inhibitors. CNS Neurosci. Ther. 2015;21:417–424. doi: 10.1111/cns.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimi N., Futamura T., Kakumoto K., Salehi A.M., Sellgren C.M., Holmen-Larsson J., Jakobsson J., Palsson E., Landen M., Hashimoto K. Blood metabolomics analysis identifies abnormalities in the citric acid cycle, urea cycle, and amino acid metabolism in bipolar disorder. BBA Clin. 2016;5:151–158. doi: 10.1016/j.bbacli.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hess S., Baker G., Gyenes G., Tsuyuki R., Newman S., Le Melledo J.M. Decreased serum L-arginine and L-citrulline levels in major depression. Psychopharmacology (Berl) 2017;234:3241–3247. doi: 10.1007/s00213-017-4712-8. [DOI] [PubMed] [Google Scholar]
- 57.Kageyama Y., Kasahara T., Morishita H., Mataga N., Deguchi Y., Tani M., Kuroda K., Hattori K., Yoshida S., Inoue K., Kato T. Search for plasma biomarkers in drug-free patients with bipolar disorder and schizophrenia using metabolome analysis. Psychiatr. Clin. Neurosci. 2017;71:115–123. doi: 10.1111/pcn.12461. [DOI] [PubMed] [Google Scholar]
- 58.Ali-Sisto T., Tolmunen T., Viinamaki H., Mantyselka P., Valkonen-Korhonen M., Koivumaa-Honkanen H., Honkalampi K., Ruusunen A., Nandania J., Velagapudi V., Lehto S.M. Global arginine bioavailability ratio is decreased in patients with major depressive disorder. J. Affect. Disord. 2018;229:145–151. doi: 10.1016/j.jad.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 59.Moaddel R., Shardell M., Khadeer M., Lovett J., Kadriu B., Ravichandran S., Morris P.J., Yuan P., Thomas C.J., Gould T.D., Ferrucci L., Zarate C.A. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology (Berl) 2018;235:3017–3030. doi: 10.1007/s00213-018-4992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogawa S., Koga N., Hattori K., Matsuo J., Ota M., Hori H., Sasayama D., Teraishi T., Ishida I., Yoshida F., Yoshida S., Noda T., Higuchi T., Kunugi H. Plasma amino acid profile in major depressive disorder: analyses in two independent case-control sample sets. J. Psychiatr. Res. 2018;96:23–32. doi: 10.1016/j.jpsychires.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Yilmaz E., Sekeroglu M.R., Yilmaz E., Cokluk E. Evaluation of plasma agmatine level and its metabolic pathway in patients with bipolar disorder during manic episode and remission period. Int. J. Psychiatr. Clin. Pract. 2019;23:128–133. doi: 10.1080/13651501.2019.1569237. [DOI] [PubMed] [Google Scholar]
- 62.Ozden A., Angelos H., Feyza A., Elizabeth W., John P. Altered plasma levels of arginine metabolites in depression. J. Psychiatr. Res. 2020;120:21–28. doi: 10.1016/j.jpsychires.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Ustundag M.F., Ozcan H., Gencer A.G., Yilmaz E.D., Ugur K., Oral E., Bilici M. Nitric oxide, asymmetric dimethylarginine, symmetric dimethylarginine and L-arginine levels in psychotic exacerbation of schizophrenia and bipolar disorder manic episode. Saudi Med. J. 2020;41:38–45. doi: 10.15537/smj.2020.1.24817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bilbao A.V., Goldschmied J., Jang A., Ehrmann D., Kaplish N., Pitt B., Arnedt T., Sen S., Dalack G., Deldin P.J. A preliminary study on the relationship between sleep, depression and cardiovascular dysfunction in a 4 sample population. Int J Cardiol Heart Vasc. 2021;35 doi: 10.1016/j.ijcha.2021.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braun D., Schlossmann J., Haen E. Asymmetric dimethylarginine in psychiatric disorders. Psychiatr. Res. 2021;300 doi: 10.1016/j.psychres.2021.113901. [DOI] [PubMed] [Google Scholar]
- 66.Loeb E., El Asmar K., Trabado S., Gressier F., Colle R., Rigal A., Martin S., Verstuyft C., Feve B., Chanson P., Becquemont L., Corruble E. Nitric Oxide Synthase activity in major depressive episodes before and after antidepressant treatment: results of a large case-control treatment study. Psychol. Med. 2022;52:80–89. doi: 10.1017/S0033291720001749. [DOI] [PubMed] [Google Scholar]
- 67.Desiderio C., Iavarone F., Rossetti D.V., Messana I., Castagnola M. Capillary electrophoresis-mass spectrometry for the analysis of amino acids. J. Separ. Sci. 2010;33:2385–2393. doi: 10.1002/jssc.201000171. [DOI] [PubMed] [Google Scholar]
- 68.Ghosh S.K., Paik W.K., Kim S. Purification and molecular identification of two protein methylases I from calf brain. Myelin basic protein- and histone-specific enzyme. J. Biol. Chem. 1988;263:19024–19033. [PubMed] [Google Scholar]
- 69.Rawal N., Rajpurohit R., Paik W.K., Kim S. Purification and characterization of S-adenosylmethionine-protein-arginine N-methyltransferase from rat liver. Biochem. J. 1994;300(Pt 2):483–489. doi: 10.1042/bj3000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wadham C., Mangoni A.A. Dimethylarginine dimethylaminohydrolase regulation: a novel therapeutic target in cardiovascular disease. Expet Opin. Drug Metabol. Toxicol. 2009;5:303–319. doi: 10.1517/17425250902785172. [DOI] [PubMed] [Google Scholar]
- 71.Closs E.I., Basha F.Z., Habermeier A., Forstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 72.Strobel J., Muller F., Zolk O., Endress B., Konig J., Fromm M.F., Maas R. Transport of asymmetric dimethylarginine (ADMA) by cationic amino acid transporter 2 (CAT2), organic cation transporter 2 (OCT2) and multidrug and toxin extrusion protein 1 (MATE1) Amino Acids. 2013;45:989–1002. doi: 10.1007/s00726-013-1556-3. [DOI] [PubMed] [Google Scholar]
- 73.Ragavan V.N., Nair P.C., Jarzebska N., Angom R.S., Ruta L., Bianconi E., Grottelli S., Tararova N.D., Ryazanskiy D., Lentz S.R., Tommasi S., Martens-Lobenhoffer J., Suzuki-Yamamoto T., Kimoto M., Rubets E., Chau S., Chen Y., Hu X., Bernhardt N., Spieth P.M., Weiss N., Bornstein S.R., Mukhopadhyay D., Bode-Boger S.M., Maas R., Wang Y., Macchiarulo A., Mangoni A.A., Cellini B., Rodionov R.N. A multicentric consortium study demonstrates that dimethylarginine dimethylaminohydrolase 2 is not a dimethylarginine dimethylaminohydrolase. Nat. Commun. 2023;14:3392. doi: 10.1038/s41467-023-38467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tain Y.L., Hsu C.N. Toxic dimethylarginines: asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) Toxins. 2017;9 doi: 10.3390/toxins9030092. [DOI] [Google Scholar]
- 75.Rees D.D., Palmer R.M., Schulz R., Hodson H.F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mangoni A.A., Tommasi S., Sotgia S., Zinellu A., Paliogiannis P., Piga M., Cauli A., Pintus G., Carru C., Erre G.L. Asymmetric dimethylarginine: a key player in the pathophysiology of endothelial dysfunction, vascular inflammation and atherosclerosis in rheumatoid arthritis? Curr. Pharmaceut. Des. 2021;27:2131–2140. doi: 10.2174/1381612827666210106144247. [DOI] [PubMed] [Google Scholar]
- 77.Strobel J., Mieth M., Endress B., Auge D., Konig J., Fromm M.F., Maas R. Interaction of the cardiovascular risk marker asymmetric dimethylarginine (ADMA) with the human cationic amino acid transporter 1 (CAT1) J. Mol. Cell. Cardiol. 2012;53:392–400. doi: 10.1016/j.yjmcc.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Feliers D., Lee D.Y., Gorin Y., Kasinath B.S. Symmetric dimethylarginine alters endothelial nitric oxide activity in glomerular endothelial cells. Cell. Signal. 2015;27:1–5. doi: 10.1016/j.cellsig.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 79.Althoff M.D., Peterson R., McGrath M., Jin Y., Grasemann H., Sharma S., Federman A., Wisnivesky J.P., Holguin F. Phenotypic characteristics of asthma and morbidity are associated with distinct longitudinal changes in L-arginine metabolism. BMJ Open Respir Res. 2023:10. doi: 10.1136/bmjresp-2023-001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sydow K., Fortmann S.P., Fair J.M., Varady A., Hlatky M.A., Go A.S., Iribarren C., Tsao P.S., Investigators A. Distribution of asymmetric dimethylarginine among 980 healthy, older adults of different ethnicities. Clin. Chem. 2010;56:111–120. doi: 10.1373/clinchem.2009.136200. [DOI] [PubMed] [Google Scholar]
- 81.Sandrim V.C., Palei A.C., Metzger I.F., Cavalli R.C., Duarte G., Tanus-Santos J.E. Interethnic differences in ADMA concentrations and negative association with nitric oxide formation in preeclampsia. Clin. Chim. Acta. 2010;411:1457–1460. doi: 10.1016/j.cca.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 82.Drew D.A., Tighiouart H., Scott T., Kantor A., Fan L., Artusi C., Plebani M., Weiner D.E., Sarnak M.J. Asymmetric dimethylarginine, race, and mortality in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014;9:1426–1433. doi: 10.2215/CJN.00770114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mels C.M., Huisman H.W., Smith W., Schutte R., Schwedhelm E., Atzler D., Boger R.H., Ware L.J., Schutte A.E. The relationship of nitric oxide synthesis capacity, oxidative stress, and albumin-to-creatinine ratio in black and white men: the SABPA study. Age (Dordr) 2016;38:9. doi: 10.1007/s11357-016-9873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bollenbach A., Schutte A.E., Kruger R., Tsikas D. An ethnic comparison of arginine dimethylation and cardiometabolic factors in healthy black and white youth: the ASOS and african-PREDICT studies. J. Clin. Med. 2020;9 doi: 10.3390/jcm9030844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Glyn M.C., Anderssohn M., Luneburg N., Van Rooyen J.M., Schutte R., Huisman H.W., Fourie C.M., Smith W., Malan L., Malan N.T., Mels C.M., Boger R.H., Schutte A.E. Ethnicity-specific differences in L-arginine status in South African men. J. Hum. Hypertens. 2012;26:737–743. doi: 10.1038/jhh.2011.103. [DOI] [PubMed] [Google Scholar]
- 86.Mels C.M.C., Loots I., Schwedhelm E., Atzler D., Boger R.H., Schutte A.E. Nitric oxide synthesis capacity, ambulatory blood pressure and end organ damage in a black and white population: the SABPA study. Amino Acids. 2016;48:801–810. doi: 10.1007/s00726-015-2128-5. [DOI] [PubMed] [Google Scholar]
- 87.Yanik M., Vural H., Tutkun H., Zoroglu S.S., Savas H.A., Herken H., Kocyigit A., Keles H., Akyol O. The role of the arginine-nitric oxide pathway in the pathogenesis of bipolar affective disorder. Eur. Arch. Psychiatr. Clin. Neurosci. 2004;254:43–47. doi: 10.1007/s00406-004-0453-x. [DOI] [PubMed] [Google Scholar]
- 88.Fontoura P.C., Pinto V.L., Matsuura C., Resende Ade C., de Bem G.F., Ferraz M.R., Cheniaux E., Brunini T.M., Mendes-Ribeiro A.C. Defective nitric oxide-cyclic guanosine monophosphate signaling in patients with bipolar disorder: a potential role for platelet dysfunction. Psychosom. Med. 2012;74:873–877. doi: 10.1097/PSY.0b013e3182689460. [DOI] [PubMed] [Google Scholar]
- 89.Baytunca M.B., Ongur D. Plasma spermine levels in patients with schizophrenia and bipolar disorder: a preliminary study. Schizophr. Res. 2020;216:534–535. doi: 10.1016/j.schres.2019.11.040. [DOI] [PubMed] [Google Scholar]
- 90.Roman A., Rogoz Z., Kubera M., Nawrat D., Nalepa I. Concomitant administration of fluoxetine and amantadine modulates the activity of peritoneal macrophages of rats subjected to a forced swimming test. Pharmacol. Rep. 2009;61:1069–1077. doi: 10.1016/s1734-1140(09)70169-0. [DOI] [PubMed] [Google Scholar]
- 91.Hietala O.A., Laitinen S.I., Laitinen P.H., Lapinjoki S.P., Pajunen A.E. The inverse changes of mouse brain ornithine and S-adenosylmethionine decarboxylase activities by chlorpromazine and imipramine. Dependence of ornithine decarboxylase induction on beta-adrenoceptors. Biochem. Pharmacol. 1983;32:1581–1585. doi: 10.1016/0006-2952(83)90331-3. [DOI] [PubMed] [Google Scholar]
- 92.Fan M., Gao X., Li L., Ren Z., Lui L.M.W., McIntyre R.S., Teopiz K.M., Deng P., Cao B. The association between concentrations of arginine, ornithine, citrulline and major depressive disorder: a meta-analysis. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.686973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this systematic review and meta-analysis are available from Angelo Zinellu upon reasonable request.