Abstract
A chicken gene conferring susceptibility to subgroup A avian sarcoma and leukosis viruses (ASLV-A) was recently identified by a gene transfer strategy. Classical genetic approaches had previously identified a locus, TVA, that controls susceptibility to ASLV-A. Using restriction fragment length polymorphism (RFLP) mapping in inbred susceptible (TVA*S) and resistant (TVA*R) chicken lines, we demonstrate that in 93 F2 progeny an RFLP for the cloned receptor gene segregates with TVA. From these analyses we calculate that the cloned receptor gene lies within 5 centimorgans of TVA, making it highly probable that the cloned gene is the previously identified locus TVA. The polymorphism that distinguishes the two alleles of TVA in these inbred lines affects the encoded amino acid sequence of the region of Tva that encompasses the viral binding domain. However, analysis of the genomic sequence encoding this region of Tva in randomly bred chickens suggests that the altered virus binding domain is not the basis for genetic resistance in the chicken lines analyzed.
Avian sarcoma and leukosis viruses (ASLV) may be classified into several subgroups based on properties of the viral envelope proteins. Five major subgroups of ASLV (A to E) have been defined based on immunological reactivity of the viral envelope proteins, host range, and infection interference patterns (reviewed in reference 18). The subgroup specificity of the virus maps to the env gene, and distinct hypervariable regions within env have been shown to determine the viral subgroup (2, 3, 7, 8).
Patterns of susceptibility of inbred chicken lines to infection with the various ASLV subgroups suggest that three loci control viral entry for the five major ASLV subgroups (11, 12, 15, 17). Dominant alleles at the TVA and TVC (TVA*S and TVC*S) loci confer susceptibility to subgroups A and C viruses, respectively. The TVB locus is more complex, with various alleles determining infection by subgroup B, D, and E viruses. TVA, TVB, and TVC appear to act at the level of viral entry into the cell; therefore, it has been assumed that the TV loci encode or control expression of the cellular receptors for ASLV (5, 13).
Recently, a gene that confers susceptibility to subgroup A ASLV (ASLV-A) was cloned by a gene transfer strategy (1, 19). Molecular clones containing coding sequences for this gene relieve the block to viral entry when expressed in a number of mammalian cell lines (1, 19). The gene encodes a surface glycoprotein that has been shown to bind directly and specifically to the ASLV-A envelope protein (4, 9). In addition, binding of the receptor protein to ASLV-A envelope protein induces conformational changes in the viral glycoproteins that appear to be associated with viral entry (10). Finally, an antibody to the receptor protein specifically blocks infection of chicken cells by subgroup A viruses, suggesting that this gene encodes the normal ASLV-A receptor on chicken cells (1). Taken together, these results demonstrate that the cloned gene encodes a subgroup A virus receptor.
Since it is clear that the cloned gene encodes an ASLV-A receptor, the protein supposed to be encoded by the classical TVA locus, we asked whether the identified receptor gene mapped to the TVA locus. In this paper we show that a restriction fragment length polymorphism (RFLP) of DNA from inbred chickens can be used to demonstrate that the cloned ASLV-A receptor gene maps to within 5 centimorgans (cM) of the TVA locus as defined by susceptibility to ASLV-A, making it highly probable that the cloned gene is TVA. In addition, analysis of the genomic sequences encoding the viral interaction domain of the receptor in both inbred and randomly bred chickens demonstrates that alterations in this region of the receptor are not responsible for the resistance phenotype.
TVA segregation analysis.
Inbred chicken lines homozygous for TVA alleles encoding either sensitivity (TVA*S) or resistance (TVA*R) were used to generate birds in which the TVA segregation pattern could be studied. Regional Poultry Research Lab lines 63 (TVA*S/*S TVB*S/*S) and 72 (TVA*R/*R TVB*R/*R) (6) were crossed, and the resulting heterozygous F1 progeny were mated to generate 93 F2 chicks. The susceptibility phenotype of the F2 chicks was determined on the basis of tumor formation following subcutaneous injection of 500 focus-forming units (FFU) of subgroup A Bryan high-titer Rous sarcoma virus (RSV) (RAV-1) and 500 FFU of subgroup B Bryan high-titer RSV (RAV-2) into the right and left wing webs, respectively, of 4-week-old chicks. At 2, 3, and 4 weeks postinjection the wings were palpated to check for tumor development. Chicks were scored as positive for susceptibility (e.g., TVA*S/*S or TVA*S/*R) if a tumor of any size formed in the appropriate wing web.
Table 1 summarizes the distribution of susceptibility to subgroups A and B RSV in the F2 chicks. Segregation of TVB yielded roughly the predicted frequency of susceptible (67 observed versus 70 expected) and resistant (26 observed versus 23 expected) progeny for a dominant locus (P > 0.05). In contrast, the distribution of subgroup A-susceptible and -resistant birds deviated significantly from the expected 3:1 ratio, with an excessive number of subgroup A-resistant chicks observed and a ratio of 2:1 (χ2 = 3.4) (Table 1). Previous analysis of the segregation of TVA using the same chicken lines did not reveal an altered distribution of sensitive and resistant birds (6), suggesting that the bias seen here may be a chance deviation from the expected ratio. Segregation of two endogenous virus loci, ALVE2 (previously designated ev2) (carried by line 72) and ALVE3 (previously designated ev3) (carried by line 63), also gave roughly the expected 3:1 ratio in these F2 chicks (data not shown). When the segregation bias in the ASLV-A-susceptible birds was accounted for, then susceptibility to subgroup A and B RSV segregated independently, as was expected since the TVA and TVB genes have been reported to be unlinked (6). Recent mapping studies also confirmed that TVA and TVB are found on different linkage groups (3a).
TABLE 1.
Segregation of resistance and susceptibility to subgroup A and B viruses in F2 progeny obtained by crossing lines 63 and 72
| Subgroup B susceptibility phenotypea | No. of progeny with subgroup A susceptibility phenotypeb
|
Total | |
|---|---|---|---|
| Susceptible | Resistant | ||
| Susceptible | 45 | 22 | 67 |
| Resistant | 17 | 9 | 26 |
| Total | 62 | 31 | |
Susceptibility to subgroup B viruses was determined by injection of 500 FFU of Bryan high-titer RSV (RAV-2) into the wing webs of 4-week-old chicks. Tumors were scored on the basis of palpation of the wing web at 2, 3, and 4 weeks postinfection.
Susceptibility to subgroup A viruses was determined as described for subgroup B except that Bryan high-titer RSV (RAV-1) was used.
Identification of an RFLP in the ASLV-A receptor.
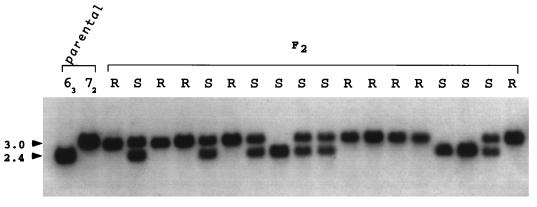
In an attempt to define an RFLP that differentiated alleles of the cloned ASLV-A receptor gene in line 72 and 63 birds, genomic DNA from the parental lines was digested with a battery of 18 different restriction enzymes and then Southern blotted. Four unique-sequence DNA probes from the cloned chicken ASLV-A receptor gene corresponding to regions in exons 2, 3, and 4 and intron 1 were prepared by random oligonucleotide priming of purified DNA fragments and hybridized to these blots. From these experiments, a single TaqI polymorphism that distinguishes alleles of the receptor gene in lines 72 and 63 was found (Fig. 1). The polymorphism was seen with a probe encompassing exon 3 of the receptor gene and generated a 3.0-kb fragment in DNA from line 72 (TVA*R/*R) and a 2.4-kb band in DNA from line 63 (TVA*S/*S).
FIG. 1.
RFLP mapping of the cloned receptor gene. Genomic DNA from the parental lines, 72 and 63, and 18 F2 progeny was digested with TaqI and then analyzed by Southern blotting with a random-primer-labeled 375-bp probe specific for the cloned ASLV-A receptor gene. The approximate sizes (in kilobases) of the two hybridizing fragments are indicated. The susceptibility of the F2 progeny to ASLV-A infection is indicated. R, resistant; S, sensitive.
Analysis of the Taq RFLP segregation in the F2 progeny.
The TaqI polymorphism was used to correlate segregation of the ASLV-A receptor in the F2 chicks with the TVA phenotype as determined by tumor induction. Genomic DNA prepared from blood of the F2 progeny was digested with TaqI, Southern blotted, and hybridized with the exon 3 probe. Figure 1 shows the distribution of the TaqI polymorphism in 18 of the F2 progeny. There is an absolute correlation of the 2.4-kb fragment diagnostic for the receptor allele from line 63 (TVA*S/*S), with sensitivity to ASLV-A. As expected for a dominant gene, both homozygotes and heterozygotes carrying this allele were susceptible to subgroup A virus. Similarly, all the F2 progeny that were homozygous for the 3.0-kb TaqI fragment inherited from line 72 were resistant to infection, as would be expected for birds carrying the recessive TVA*R allele. Analysis of segregation of the TaqI polymorphism in these birds plus the remaining 75 F2 birds demonstrated that the 2.4-kb allele was present in all 62 of the F2 progeny susceptible to ASLV-A (Table 2). Furthermore, all 31 birds homozygous for the 3.0-kb TaqI fragment inherited from the line 72 parent were resistant to ASLV-A. Thus, in all the examined progeny from the 63 × 72 cross, the TaqI polymorphism that distinguishes the alleles of the cloned receptor gene cosegregated with alleles of TVA.
TABLE 2.
Cosegregation of the TaqI RFLP and TVA phenotype
| TVA phenotypea | Size(s) (kb) of TaqI fragment(s) observedb | No. of F2 progeny |
|---|---|---|
| Susceptible | 2.4 | 16 |
| Susceptible | 2.4, 3.0 | 46 |
| Resistant | 3.0 | 31 |
The TVA phenotype was scored by wing web injection of Bryan high-titer RSV (RAV-1) as described in TABLE 1, footnote a.
Sizes of fragments observed after hybridization with the exon 3 probe from the cloned ASLV-A receptor gene.
The perfect correlation in the segregation pattern of the TaqI polymorphism that distinguishes alleles of the cloned receptor gene with the ASLV-A susceptibility phenotype conferred by TVA strongly suggests that the cloned gene is equivalent to the TVA locus. Calculation of the expected recombination frequency supports this conclusion. If the genetic distance between the RFLP and TVA were 5 cM, then there would be a 99% probability that in 93 progeny we would have observed at least one recombinant in which the RFLP and TVA phenotype segregated independently. Since no such recombinants were observed, the receptor gene is at least within 5 cM of TVA. Given that the cloned receptor gene encodes a protein that binds specifically to the subgroup A envelope and confers ASLV-A susceptibility to mammalian cells, and that this gene is within 5 cM of TVA in a genome of more than 3,000 cM, it is highly probable that the cloned gene is TVA.
The TaqI polymorphism alters the receptor viral binding domain.
Within the extracellular domain of the protein encoded by cloned receptor gene is a 40-residue region closely related to the ligand binding domain of the low-density-lipoprotein receptor (LDLR) (1). Since this region is necessary and sufficient for receptor function (14, 20), we sought to determine if the phenotypes of lines 63 and 72 could be accounted for by differences in the sequence of the viral interaction domain. To address this question, genomic DNA from lines 63 and 72 was amplified by PCR, using primers flanking the LDLR motif in the receptor sequence. Genomic DNA was prepared from approximately 200 to 300 mg of bursa tissue by digestion in 5 ml of DNA preparation buffer (100 mM NaCl, 10 mM Tris-Cl [pH 8], 25 mM EDTA [pH 8], 0.5% sodium dodecyl sulfate, and 0.1 mg of proteinase K per ml) at 50°C overnight in a rotating incubator. After purification of the genomic DNA by standard techniques (16), the region of exon 2 in the receptor gene encoding the LDLR-like motif was amplified by 30 cycles of PCR with the primers 5′ AGCAGGCCCGCCCGTACCTGT 3′ and 5′ CAGGTTCTTTGGCGCAGT 3′.
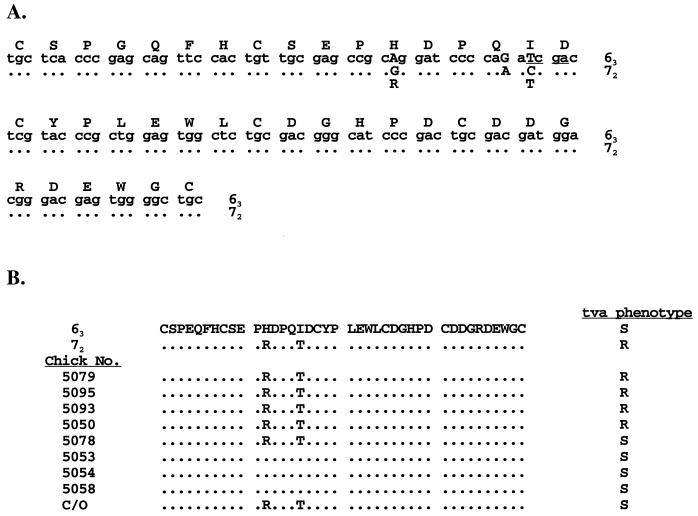
Sequence analysis of the PCR-amplified fragments from the LDLR motif revealed that the polymorphic TaqI site that distinguishes the receptor gene in lines 63 and 72 lies within the region of the receptor gene encoding the viral binding domain (Fig. 2A). Furthermore, the TaqI polymorphism and a linked alteration immediately upstream change the amino acid sequence at two positions in the LDLR motif of lines 63 and 72 (Fig. 2A). The receptor sequence in the ASLV-A-resistant line 72 encodes an arginine and a threonine at positions 12 and 16 of the LDLR-like motif, whereas in the susceptible line 63 the receptor sequence encodes histidine and isoleucine at these positions (Fig. 2A). Thus, the TaqI polymorphism that segregates with TVA lies in exon 2 of the cloned receptor gene and introduces an amino acid variation in the ligand binding domain of the ASLV-A receptor.
FIG. 2.
Sequence analysis of the viral interaction region in the ALSV-A receptor. (A) The viral interaction region from the ASLV-A receptor gene in lines 63 and 72 was amplified by PCR and the DNA sequence was determined. The deduced amino acid sequence and DNA sequence for line 63 are shown in the top lines. Nucleotide differences between the two chicken lines are indicated by capital letters, and dots represent identical sequences. For line 72, only the altered amino acid residues are shown. The polymorphic TaqI site is underlined. (B) The viral interaction region of the receptor gene from genomic DNA of randomly bred broiler-type chickens was amplified by PCR and directly sequenced. The deduced amino acid sequence and susceptibility to ASLV-A infection are shown compared to the line 63 sequence. Dots indicate identical residues. R, resistant to ASLV-A; S, sensitive to ASLV-A. C/O represents the sequence of the functional ASLV-A receptor gene identified by gene transfer (19).
An altered viral binding domain is not the mechanism of genetic resistance to ASLV-A infection.
To address whether the altered residues in the receptor of line 72 are responsible for the resistance phenotype in birds of this line, we examined the sequence of the viral binding domain of the receptor gene in a number of randomly bred broiler-type chickens that had been screened for their susceptibility phenotypes by wing web injection of virus as described above. Genomic DNA was isolated and amplified by PCR with the primers listed above. Comparison of the deduced amino acid sequences of the LDLR-like motif from the randomly bred birds demonstrates that the arginine and threonine residues found in line 72 (TVA*R/*R) do not correlate with resistance to ASLV-A in the randomly bred chickens (Fig. 2B). For example, the receptor gene in chicken 5078 encodes arginine and threonine and does not have the polymorphic TaqI site, yet this chicken is sensitive to ASLV-A infection. In addition, the receptor gene that we first isolated by gene transfer (C/O [Fig. 2B]) encodes arginine and threonine at these positions, yet this gene confers susceptibility to ASLV-A when introduced into mammalian cells (19) or into chicken embryo fibroblasts derived from line 72 embryos (1b). Furthermore, additional data from RFLP mapping of the receptor gene in randomly bred chickens demonstrates that a number of ASLV-A-resistant birds carry the polymorphic TaqI site and thus presumably carry a receptor allele similar to that of the ASLV-A-sensitive line 63 (1a). Therefore, an altered viral binding domain in the receptor is not the basis for the recessive genetic resistance seen in the chicken lines analyzed here. Presumably another change in the receptor alleles in the ASLV-A-resistant line 72 is linked to the polymorphic site, but the mechanism of resistance to ASLV-A conferred by specific alleles of TVA remains to be determined.
Acknowledgments
We thank Leonard Provencher for expert technical assistance in support of this project. In addition, we thank members of the Bates lab for useful discussions during the course of this work.
This work was supported by the USDA ARS base fund to the Avian Disease and Oncology Laboratory (L.B.C.) and grants to P.B. from the American Cancer Society (IRG 135N), NIH (CA63531), and American Heart Association (95015200) and to H.E.V. from the NIH (CA39832).
REFERENCES
- 1.Bates P, Young J A, Varmus H E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 1a.Bates, P., and L. B. Crittenden. Unpublished data.
- 1b.Bates, P., and R. Damico. Unpublished observation.
- 2.Bova C A, Manfredi J P, Swanstrom R. env genes of avian retroviruses: nucleotide sequence and molecular recombinants define host range determinants. Virology. 1986;152:343–354. doi: 10.1016/0042-6822(86)90137-6. [DOI] [PubMed] [Google Scholar]
- 3.Bova C A, Olsen J C, Swanstrom R. The avian retrovirus env gene family: molecular analysis of host range and antigenic variants. J Virol. 1988;62:75–83. doi: 10.1128/jvi.62.1.75-83.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Cheng, H., and L. B. Crittenden. Unpublished results.
- 4.Connolly L, Zingler K, Young J A T. A soluble form of a receptor for subgroup A avian leukosis and sarcoma viruses (ALSV-A) blocks infection and binds directly to ALSV-A. J Virol. 1994;68:2760–2764. doi: 10.1128/jvi.68.4.2760-2764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crittenden L B. Observations on the nature of a genetic cellular resistance to avian tumor viruses. J Natl Cancer Inst. 1968;41:145–153. [PubMed] [Google Scholar]
- 6.Crittenden L B, Stone H A, Reamer R H, Okazaki W. Two loci controlling genetic cellular resistance to avian leukosis-sarcoma viruses. J Virol. 1967;1:898–904. doi: 10.1128/jvi.1.5.898-904.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorner A J, Coffin J M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986;45:365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- 8.Dorner A J, Stoye J P, Coffin J M. Molecular basis of host range variation in avian retroviruses. J Virol. 1985;53:32–39. doi: 10.1128/jvi.53.1.32-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert J M, Bates P, Varmus H E, White J M. The receptor for the subgroup A avian leukosis-sarcoma viruses binds to subgroup A but not to subgroup C envelope glycoprotein. J Virol. 1994;68:5623–5628. doi: 10.1128/jvi.68.9.5623-5628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert J M, Hernandez L D, Balliet J W, Bates P, White J M. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J Virol. 1995;69:7410–7415. doi: 10.1128/jvi.69.12.7410-7415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payne L N, Biggs P M. Genetic basis of cellular susceptibility to the Schmidt-Ruppin and Harris strains of Rous sarcoma virus. Virology. 1966;29:190–198. doi: 10.1016/0042-6822(66)90025-0. [DOI] [PubMed] [Google Scholar]
- 12.Payne L N, Crittenden L B, Okazaki W. Influence of host genotype on responses to four strains of avian leukosis virus. J Natl Cancer Inst. 1968;40:907–916. [PubMed] [Google Scholar]
- 13.Piraino F. The mechanism of genetic resistance of chick embryo cells to infection by Rous sarcoma virus-Bryan strain (BS-RSV) Virology. 1967;32:700–707. doi: 10.1016/0042-6822(67)90046-3. [DOI] [PubMed] [Google Scholar]
- 14.Rong L, Bates P. Analysis of the subgroup A avian sarcoma and leukosis virus receptor: the 40-residue, cysteine-rich, low-density lipoprotein receptor repeat motif of Tva is sufficient to mediate viral entry. J Virol. 1995;69:4847–4853. doi: 10.1128/jvi.69.8.4847-4853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin H. Genetic control of susceptibility to pseudotypes of Rous sarcoma virus. Virology. 1965;26:270–276. doi: 10.1016/0042-6822(65)90274-6. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 17.Vogt P K, Ishizaki R. Reciprocal patterns of genetic resistance to avian tumor viruses in two lines of chickens. Virology. 1965;26:664–672. doi: 10.1016/0042-6822(65)90329-6. [DOI] [PubMed] [Google Scholar]
- 18.Weiss R. Cellular receptors and viral glycoproteins involved in retrovirus entry. In: Levy J, editor. The retroviruses. Vol. 2. New York, N.Y: Plenum Press; 1992. pp. 1–108. [Google Scholar]
- 19.Young J A T, Bates P, Varmus H E. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J Virol. 1993;67:1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zingler K, Bélanger C, Peters R, Agard D, Young J A T. Identification and characterization of the viral interaction determinant of the subgroup A avian leukosis virus receptor. J Virol. 1995;69:4261–4266. doi: 10.1128/jvi.69.7.4261-4266.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]