This cross-sectional study evaluates the rate of detection of cutaneous phosphorylated α-synuclein by skin biopsy in individuals with and without synucleinopathy.
Key Points
Question
Can skin biopsy detect phosphorylated α-synuclein in individuals with synucleinopathies such as Parkinson disease (PD), multiple system atrophy (MSA), dementia with Lewy bodies (DLB), and pure autonomic failure (PAF)?
Finding
In this cross-sectional study of 428 participants, the proportions of individuals with cutaneous phosphorylated α-synuclein detected by skin biopsy were 92.7% with PD, 98.2% with MSA, 96.0% with DLB, 100% with PAF, and 3.3% with no history of synucleinopathy (controls).
Meaning
A high proportion of individuals meeting clinical consensus criteria for PD, DLB, MSA, and PAF had a skin biopsy positive for phosphorylated α-synuclein in this study, although further research is needed to validate the findings in unselected clinical populations.
Abstract
Importance
Finding a reliable diagnostic biomarker for the disorders collectively known as synucleinopathies (Parkinson disease [PD], dementia with Lewy bodies [DLB], multiple system atrophy [MSA], and pure autonomic failure [PAF]) is an urgent unmet need. Immunohistochemical detection of cutaneous phosphorylated α-synuclein may be a sensitive and specific clinical test for the diagnosis of synucleinopathies.
Objective
To evaluate the positivity rate of cutaneous α-synuclein deposition in patients with PD, DLB, MSA, and PAF.
Design, Setting, and Participants
This blinded, 30-site, cross-sectional study of academic and community-based neurology practices conducted from February 2021 through March 2023 included patients aged 40 to 99 years with a clinical diagnosis of PD, DLB, MSA, or PAF based on clinical consensus criteria and confirmed by an expert review panel and control participants aged 40 to 99 years with no history of examination findings or symptoms suggestive of a synucleinopathy or neurodegenerative disease. All participants completed detailed neurologic examinations and disease-specific questionnaires and underwent skin biopsy for detection of phosphorylated α-synuclein. An expert review panel blinded to pathologic data determined the final participant diagnosis.
Exposure
Skin biopsy for detection of phosphorylated α-synuclein.
Main Outcomes
Rates of detection of cutaneous α-synuclein in patients with PD, MSA, DLB, and PAF and controls without synucleinopathy.
Results
Of 428 enrolled participants, 343 were included in the primary analysis (mean [SD] age, 69.5 [9.1] years; 175 [51.0%] male); 223 met the consensus criteria for a synucleinopathy and 120 met criteria as controls after expert panel review. The proportions of individuals with cutaneous phosphorylated α-synuclein detected by skin biopsy were 92.7% (89 of 96) with PD, 98.2% (54 of 55) with MSA, 96.0% (48 of 50) with DLB, and 100% (22 of 22) with PAF; 3.3% (4 of 120) of controls had cutaneous phosphorylated α-synuclein detected.
Conclusions and Relevance
In this cross-sectional study, a high proportion of individuals meeting clinical consensus criteria for PD, DLB, MSA, and PAF had phosphorylated α-synuclein detected by skin biopsy. Further research is needed in unselected clinical populations to externally validate the findings and fully characterize the potential role of skin biopsy detection of phosphorylated α-synuclein in clinical care.
Introduction
A group of neurodegenerative disorders collectively known as synucleinopathies are characterized by the deposition of phosphorylated α-synuclein (P-SYN) within the central and peripheral nervous systems. These include the Lewy body diseases (Parkinson disease [PD], dementia with Lewy bodies [DLB]), multiple system atrophy (MSA), and pure autonomic failure (PAF). All 4 synucleinopathies have overlapping clinical features and are characterized by progressive neurodegeneration and disability.
Finding an easily accessible and reliable diagnostic biomarker for these diseases is an urgent unmet priority. Based on data collected from 1991 to 2010, it is estimated that 2.5 million people in the US have a diagnosis of a synucleinopathy and approximately 180 000 new patients are diagnosed each year.1,2,3,4 No disease-modifying therapy exists for these diseases,5,6 and many patients with synucleinopathy experience diagnostic delay or an initial misdiagnosis even when evaluated at movement disorder centers.7,8 We therefore sought to evaluate the frequency of detection of cutaneous α-synuclein deposition as a biomarker in clinically confirmed cases of PD, DLB, MSA, and PAF in a multicenter study that included university- and community-based neurologists.
Methods
Study Design
The study design and methods have been previously published.9 In brief, this was a blinded, multicenter, cross-sectional study conducted from February 2021 through March 2023 to evaluate the detection of P-SYN by skin biopsy in individuals with PD, DLB, MSA, and PAF and control individuals without synucleinopathy. The study was approved by a central institutional review board (Sterling: 8385). Written informed consent was obtained from all participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Inclusion Criteria
The study included adults aged 40 to 99 years with a clinical diagnosis of PD, DLB, MSA, or PAF based on consensus criteria as previously published.9,10,11,12 Control participants were adults aged 40 to 99 years with no history of clinical examination findings or symptoms suggestive of synucleinopathy (including hyposmia, constipation, rapid-eye-movement [REM] sleep behavior disorder, mild cognitive impairment, or dementia) or other neurodegenerative diseases. Controls were recruited through all study sites via local advertisements and at routine visits with local primary care physicians. Exclusion criteria for all participants were published previously9 and include risks associated with performing a biopsy and diseases that could mimic symptoms of a synucleinopathy.
Examination and Clinical Testing
Examination for all participants included the Movement Disorders Society Unified Parkinson Disease Rating Scale (MDS-UPDRS),13 the Hoehn and Yahr scale,14 orthostatic vital signs, and the Montreal Cognitive Assessment.15 All participants completed the following questionnaires: the European Quality of Life Instrument (EQ-5D and EQ-VAS),16 the 39-item Parkinson Disease Questionnaire,17 the Multiple System Atrophy Quality of Life Questionnaire,18 the Orthostatic Hypotension Questionnaire,19 and the REM sleep behavior disorder screening questionnaire.20 Vital signs were measured in the supine resting position and after 3 minutes of standing. Historical data on disease and symptom duration were extracted from the medical records.
Standardization of Data and Tissue Collection
All participants had 3-mm punch skin biopsy samples taken from the distal leg 10 cm above the lateral malleolus, the distal thigh 10 cm above the lateral knee, and the posterior cervical region 3 cm lateral to the C-7 spinous process. All study-related data were acquired using electronic data capture.
Data Blinding, Data Review, and Tissue Processing
Skin Biopsies
Skin biopsy specimens were received at the central processing site. Biopsy specimens were separated from any clinical data using random number assignment for data blinding. All skin biopsy specimens were sectioned, with tissue specimens processed at CND Life Sciences and half of the specimens also processed at Beth Israel Deaconess Medical Center. A complete summary of the skin biopsy processing methods is provided in the eMethods in Supplement 1.
P-SYN Quantitation
Quantitation of P-SYN deposition for all tissue sections followed previously reported standards21 using strict blinding measures for all readers and both laboratories, including slide randomization, random number generation, and blinding between reviewers and study staff.22 A quantitative analysis of P-SYN was performed for each biopsy sample with a sum total for each participant as previously described21 (results expressed as synuclein units and reported in detail in eMethods in Supplement 1). Intraepidermal nerve fiber density for each biopsy specimen was calculated using standard methods, with results reported as nerve fibers per millimeter.23
Expert Consensus Panel for Diagnosis of Synucleinopathy
The referring physician who examined the participant indicated their clinical diagnosis (PD, MSA, DLB, PAF, or no synucleinopathy). The participant history, examination scores, medical records, and ancillary test data were then sent for central review by 2 disease experts (C.A., R.F.) to confirm the diagnosis of the specific synucleinopathy based on defined consensus and inclusion and exclusion criteria for the diagnoses or controls. The expert review panel was blinded to skin biopsy results.
Study participants reclassified by the expert panel to another specific synucleinopathy were included in the primary outcome analysis. Patients classified as having undifferentiated synucleinopathy (defined by symptoms and signs that precluded expert panel agreement on a specific synucleinopathy) or an unknown diagnosis (did not meet prespecified diagnostic criteria for a synucleinopathy) were included in the secondary outcome analysis. Similarly, controls who did not fulfill prespecified criteria for inclusion as controls were included in the secondary outcome analysis.
Outcomes
The primary outcome of this study was to define the positivity rate of skin biopsies (using a binary outcome of P-SYN present or absent) to detect P-SYN deposition in clinically confirmed cases of PD, MSA, DLB, and PAF (including those reclassified to a different synucleinopathy by the expert panel). Prespecified secondary outcomes included quantitation of P-SYN in study participants who did not meet the primary outcome criteria (eg, those categorized as having an undifferentiated synucleinopathy or an unknown diagnosis, excluded controls), number of adverse events, and correlations between P-SYN deposition and P-SYN location, disease type, disease stage, time since diagnosis, duration of symptoms, intraepidermal nerve fiber density, and clinical features.
Sample Size Justification
The sample size was calculated as the number of participants needed to differentiate synucleinopathy subgroups (PD, MSA, DLB, and PAF) from the group without synucleinopathy by cutaneous deposition of P-SYN (binary present or absent). The true-positive fraction was estimated at 0.90 and the false-positive fraction at 0.05.24 With a 2-sided α of .01 and a power of 0.95, we estimated that a total of 150 participants were required to complete the primary objective. To differentiate P-SYN deposition among the synucleinopathies,21 the cohort with synucleinopathy was increased to 300, with 200 controls.
Statistical Analysis
Statistical analysis was performed using SPSS, version 29.0 (IBM Corp). Data are presented as means and SDs. The frequency with which skin biopsy–detected P-SYN was determined for each subgroup, with the expert panel clinical diagnosis as the gold standard. Differences between groups were analyzed by analysis of variance with the Tamhane T2 post hoc test when normally distributed (Shapiro-Wilk test). If data were not normally distributed, a Kruskal-Wallis test with the Dunn method for post hoc analysis was used. Test reproducibility between laboratories and examiners was completed using the intraclass correlation coefficient. Associations between α-synuclein deposition, examination scores, and questionnaire scores were expressed with Pearson correlations. A post hoc exploratory analysis of the primary results using a subgroup of controls to ensure age-matched data was completed. For all results, P < .05 was considered significant (with Bonferroni corrections for multiple calculations) and statistical analysis was 2-sided unless otherwise specified. Analysis of missing data was performed to determine frequency and randomness with investigation into patterns of potential bias (eg, site specific or disease specific). For any cases with primary missing data (diagnosis, skin biopsy), the participant was not included in the analysis. For all other missing data, if less than 1% of total data were missing, pairwise deletion was performed. If missing data were 1% or more of total data, maximum likelihood analysis was performed.
Results
Study Population
From February 2021 through March 2023, 428 participants (151 controls and 277 patients with synucleinopathy) were enrolled in the study by 11 academic and 19 community-based neurology practices. The flow of participants is shown in the Figure. The protocol-predefined primary analysis included 343 participants (mean [SD] age, 69.5 [9.1] years; 168 [49.0%] female; 175 [51.0%] male), of whom 223 met the consensus criteria for a synucleinopathy (96 [28.0%] with PD, 50 [14.6%] with DLB, 55 [16.0%] with MSA, and 22 [6.4%] with PAF) and 120 were controls without synucleinopathy. Eighty-five individuals (54 patients and 31 controls) did not meet predefined study criteria as assessed by the expert review panel and were included in the secondary analysis. The number of participants with reclassified diagnoses was similar between academic and community-based sites, with similar rates noted (9 of 11 academic sites [81.8%] and 13 of 19 community-based sites [68.4%] had at least 1 patient with a reclassified diagnosis). The characteristics of the included participants by diagnostic subgroup are reported in Table 1. A detailed description of the reclassified patients and controls is shown in the Figure and reported in the eResults and eTables 2 to 3 in Supplement 1.
Figure. Participant Flow Diagram.
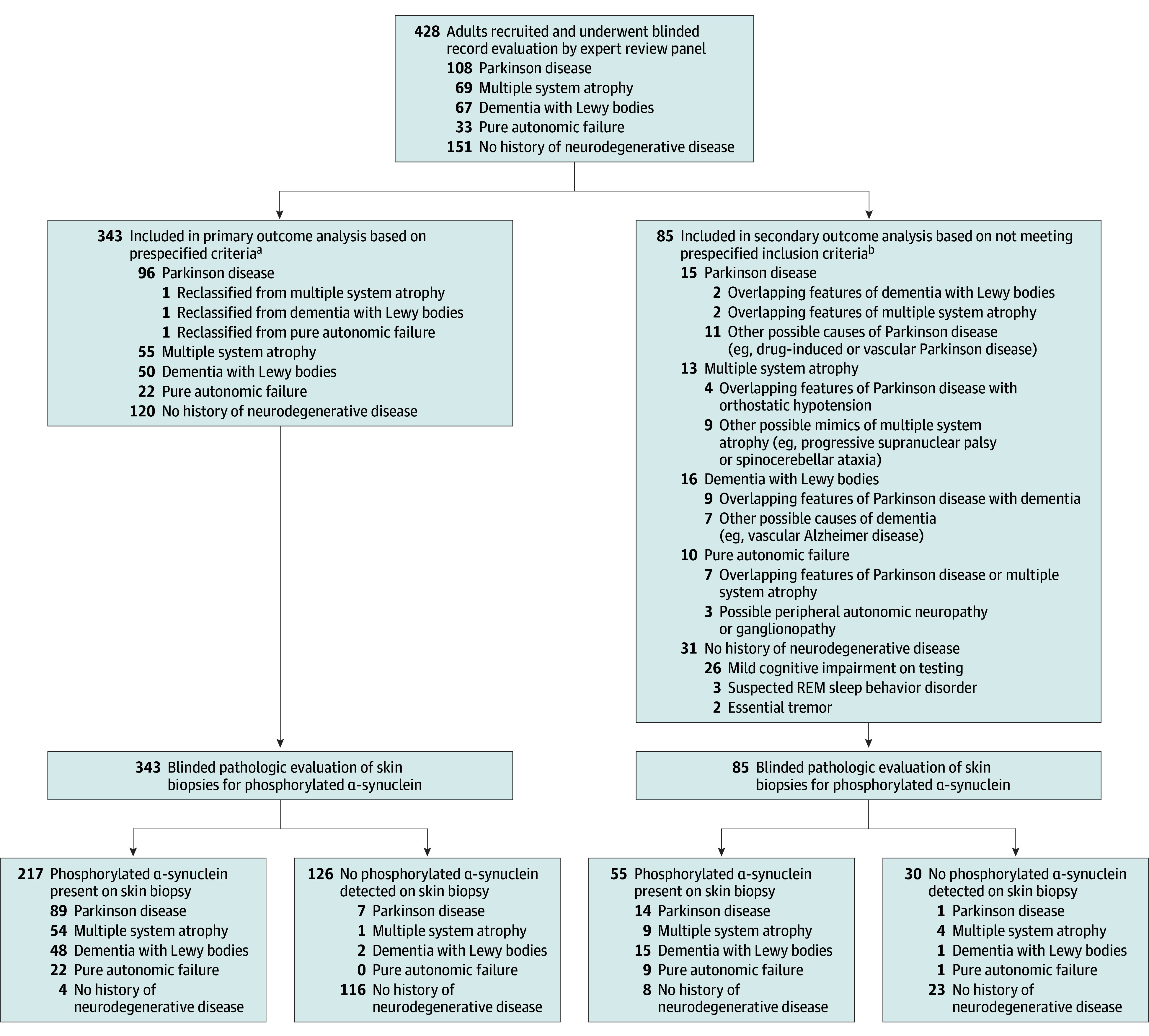
REM indicates rapid-eye-movement.
aUpdated diagnoses based on expert review are shown.
bOriginal diagnoses and reasons for exclusion are shown.
Table 1. Demographic Information and Clinical Test Data.
| Characteristic | Participantsa | ||||
|---|---|---|---|---|---|
| Parkinson disease (n = 96) | Multiple system atrophy (n = 55) | Dementia with Lewy bodies (n = 50) | Pure autonomic failure (n = 22) | Controls (n = 120) | |
| Age, median (IQR), y | 70 (63-76) | 67 (60-74) | 75 (71-80) | 72 (66-77) | 59 (41-70) |
| Sex | |||||
| Female | 40 (41.7) | 28 (50.9) | 10 (20.0) | 8 (36.4) | 82 (68.3) |
| Male | 56 (58.3) | 27 (49.1) | 40 (80.0) | 14 (63.6) | 38 (31.7) |
| Time since diagnosis, mean (SD), y | 6.0 (4.9) | 1.9 (1.2) | 1.6 (2.6) | 2.9 (2.7) | NA |
| Symptom duration, mean (SD), y | 7.5 (5.0) | 4.0 (1.6) | 4.1 (5.9) | 6.1 (3.7) | NA |
| Comorbidities | |||||
| Hyperlipidemia | 40 (41.6) | 21 (38.2) | 29 (58.0) | 14 (63.6) | 34 (28.3) |
| Hypertension | 37 (38.5) | 15 (27.3) | 23 (46.0) | 5 (22.7) | 34 (28.3) |
| Depression | 27 (28.1) | 15 (27.2) | 27 (54.0) | 7 (31.8) | 12 (10.0) |
| Diabetes or prediabetes | 24 (25.0) | 2 (3.6) | 8 (16.0) | 2 (9.1) | 8 (6.7) |
| Anxiety | 20 (20.8) | 13 (23.6) | 14 (28.0) | 6 (27.2) | 12 (10.0) |
| REM sleep behavior disorder | 19 (19.8) | 26 (47.3) | 24 (48.0) | 9 (40.9) | 0 |
| Medications | |||||
| Levodopa and dopamine agonists | 91 (94.7) | 17 (30.9) | 12 (24.0) | 0 | 0 |
| Sedatives | 55 (57.2) | 27 (49.1) | 41 (82.0) | 18 (81.8) | 6 (5.0) |
| Antihypertensives | 42 (43.8) | 6 (10.9) | 14 (28.0) | 3 (13.6) | 26 (21.6) |
| Antidepressants | 22 (22.9) | 14 (25.4) | 26 (52.0) | 8 (36.4) | 12 (10) |
| Antiorthostatic hypotension medications | 12 (12.5) | 33 (60.0) | 16 (32.0) | 17 (77.3) | 0 |
| Anxiolytics | 12 (12.5) | 11 (19.6) | 10 (20.0) | 9 (40.9) | 4 (3.3) |
| Diabetes medications | 9 (9.4) | 2 (3.6) | 3 (6.0) | 0 | 2 (1.7) |
| MDS-UPDRS, mean (SD), scoreb | |||||
| Part II | 8.5 (7.5) | 23.5 (12.2) | 15.7 (10.6) | 5.8 (6.6) | 0.2 (0.8) |
| Part III | 28.6 (13.7) | 44.2 (13.4) | 33.6 (16.6) | 7.5 (14) | 0.5 (1.2) |
| Total | 49.5 (24.4) | 83.1 (32.1) | 68.3 (28.4) | 25.7 (21.7) | 1.9 (3.1) |
| Hoehn and Yahr, mean (SD), scorec | 2.1 (0.6) | 3.4 (1.4) | 2.4 (1.0) | 0.5 (1.1) | 0 |
| Questionnaires, mean (SD), score | |||||
| MOCAd | 25.2 (3.7) | 24.8 (3.6) | 16.1 (5.7) | 26.4 (2.6) | 28.1 (1.6) |
| PDQ-39e | 26.5 (22.9) | 62.3 (31.3) | 51.9 (33.6) | 27.5 (21.1) | 1.3 (3.2) |
| EQ-5Df | 6.6 (1.7) | 9.0 (2.3) | 8.1 (2.1) | 7.3 (1.5) | 5.2 (0.7) |
| OHQg | 9.6 (12.9) | 20.6 (15.2) | 16.3 (14.4) | 22.3 (14.1) | 0.9 (2.6) |
| OHDASh | 5.4 (8.7) | 16.7 (14.5) | 9.7 (10.9) | 15.3 (11.9) | 0.4 (1.5) |
| RBDSQi | 4.9 (3.1) | 5.7 (2.8) | 6.3 (2.6) | 6.7 (2.5) | 0.8 (1.3) |
| Orthostatic hypotension, SBP change, mean (SD), mm Hgj | −6.9 (14.5) | −30.0 (36.3) | −15.4 (17.8) | −37.3 (26) | 0.8 (12.8) |
| P-SYN, mean (SD), SUsk | |||||
| Total | 4.63 (4.2) | 8.6 (4.9) | 8.3 (6.0) | 11.4 (5.7) | 0.1 (0.8) |
| Posterior cervical | 2.1 (2.0) | 2.6 (2.4) | 3.3 (2.0) | 4.3 (2.7) | 0 (0.3) |
| Distal thigh | 1.6 (2.1) | 3.2 (2.6) | 2.7 (2.6) | 3.9 (3.3) | 0 (0.2) |
| Distal leg | 0.9 (1.5) | 2.8 (2.7) | 2.3 (2.2) | 3.1 (2.3) | 0 (0.4) |
| P-SYN distribution coefficient, mean (SD) | 0.45 (0.52) | 0.06 (0.54) | 0.10 (0.45) | 0.12 (0.43) | 0.20 (1.00) |
| P-SYN in the subepidermal plexus | 3 (3.1) | 27 (49.1) | 5 (10.0) | 2 (9.1) | 0 |
| IENFD, mean (SD), fibers per mm | |||||
| Posterior cervical | 28.1 (7.0) | 30.0 (6.9) | 28.5 (7.1) | 30.2 (6.1) | 29.9 (6.6) |
| Distal thigh | 10.0 (3.5) | 11.3 (3.3) | 9.5 (3.6) | 9.9 (4.2) | 12.7 (3.3) |
| Distal leg | 4.6 (3.0) | 6.1 (3.0) | 3.9 (2.6) | 4.2 (3.0) | 7.8 (2.7) |
Abbreviations: EQ-5D, European Quality of Life Instrument; IENFD, intraepidermal nerve fiber density; MDS-UPDRS, Movement Disorders Society Unified Parkinson Disease Rating Scale; MOCA, Montreal Cognitive Assessment; NA, not applicable; OHDAS, Orthostatic Hypotension Daily Activity Scale; OHQ, Orthostatic Hypotension Questionnaire; PDQ-39, 39-item Parkinson Disease Questionnaire; P-SYN, phosphorylated α-synuclein; RBDSQ, REM sleep behavior disorder screening questionnaire; REM, rapid-eye-movement; SBP, systolic blood pressure; SUs, synuclein units.
Data are presented as number (percentage) of participants unless otherwise indicated.
MDS-UPDRS score ranges from 0 (low score with no symptoms or signs of parkinsonism) to 200 (maximal disability) in 4 parts: part I (nonmotor experiences of daily living: 0-52 points), part II (motor experiences of daily living: 0-52 points), part III (motor examination: 0-68 points), and part IV (motor complications: 0-24 points).
Hoehn and Yarh score ranges from 0 (asymptomatic) to 5 (wheelchair or bedbound).
MOCA score ranges from 0 to 30, with 26 or higher indicating no cognitive impairment.
PDQ-39 score ranges from 0 (asymptomatic) to 195 (maximal symptoms).
EQ-5D score ranges from 0 (asymptomatic) to 20 (maximal symptoms).
OHQ score ranges from 0 (asymptomatic) to 60 (maximal symptoms).
OHDAS score ranges from 0 (asymptomatic) to 40 (maximal symptoms).
RBDSQ score ranges from 0 (asymptomatic) to 13 (maximal symptoms), with scores greater than 5 suggestive of REM sleep behavior disorder.
Defined as the difference in SBP from the supine to standing position within 3 minutes.
Each biopsy has a range of 0 to 12, and the total score range is 0 to 36.
Missing Data
All 428 participants had complete primary data (biopsy data and clinical diagnoses). Missing data from examinations, questionnaires, or other clinical information were less than 0.05% of total data and were missing at random.
Biopsy Outcomes
The proportion of individuals with cutaneous P-SYN detected by skin biopsy was 95.5% (213 of 223) of those with an expert panel–confirmed diagnosis of synucleinopathy. Of these individuals, 92.7% (89 of 96) were P-SYN positive with PD, 98.2% (54 of 55) were P-SYN positive with MSA, 96% (48 of 50) were P-SYN positive with DLB, 100% (22 of 22) were P-SYN positive with PAF, and 3.3% (4 of 120) were P-SYN–positive controls (Table 2).
Table 2. Primary Outcomes.
| Diagnosis | Participants, No. | Proportion of participants positive for P-SYN, % (95% CI) | |
|---|---|---|---|
| P-SYN positivea | P-SYN negativeb | ||
| Synucleinopathy | 213 | 10 | 95.5 (91.9-97.8) |
| Parkinson disease | 89 | 7 | 92.7 (85.6-97.0) |
| Multiple system atrophy | 54 | 1 | 98.2 (91.7-99.9) |
| Dementia with Lewy bodies | 48 | 2 | 96.0 (86.3-99.5) |
| Pure autonomic failure | 22 | 0 | 100 (84.6-100) |
| No synucleinopathy | 4 | 116 | 3.3 (1.3-8.0) |
Abbreviation: P-SYN, phosphorylated α-synuclein.
Positive for P-SYN in at least 1 biopsy site.
Negative for P-SYN in all biopsy sites.
Secondary Analysis
Two subgroups of participants were excluded from the primary analysis. The first subgroup included 27 individuals with undifferentiated synucleinopathy, defined by overlapping diagnostic features that did not fulfill expert panel diagnostic criteria for a specific synucleinopathy. The proportion of individuals with undifferentiated synucleinopathy with P-SYN detected by skin biopsy was 85.2% (23 of 27). The second subgroup included 58 individuals with an unknown diagnosis that included 30 individuals originally diagnosed with synucleinopathy and 28 controls. The proportion of individuals classified as having an unknown diagnosis with P-SYN detected by skin biopsy was 55.2% (32 of 58). Full details of the P-SYN–positive controls and the reclassified participants are provided in the eResults and eTables 1 to 3 in Supplement 1.
Quantitative analysis of P-SYN revealed differences by synucleinopathy subtype and biopsy location as noted in Table 1. Among individuals with PD, there was no difference in P-SYN positivity between those with a time since diagnosis of less than 5 years (43 of 46 [93.5%] positive; 3 were P-SYN negative) and those with a time since diagnosis of 5 years or more (46 of 50 [92.0%] positive; 4 were P-SYN negative).
The deposition of P-SYN within the subepidermal plexus differed among synucleinopathy subtypes and was more common in patients with MSA (27 [49.1%]) than in those with PD (3 [3.1%]), DLB (5 [10.0%]), or PAF (2 [9.1%]) (χ2 test, P = .001). Length-dependent small fiber neuropathy differed among synucleinopathy subtypes. Neuropathy was most common in patients with DLB (39 [78.0%]) followed by those with PD (60 [62.5%]), PAF (10 [45.5]%), and MSA (12 [21.8%]). Detailed data are reported in Table 1.
Correlations Between α-Synuclein Deposition and Time Since Diagnosis, Symptom Duration, Neurologic Examination Findings, and Symptom Scores
The total P-SYN for each participant correlated with examination scores as measured by the MDS-UPDRS total score (R = 0.48; P < .001), the Hoehn and Yahr score (R = 0.44; P < .001), the Montreal Cognitive Assessment score (R = −0.32; P < .001), and the orthostatic change in systolic blood pressure (R = −0.44; P < .001). The total P-SYN for each participant correlated with the 39-item Parkinson Disease Questionnaire score (R = 0.42; P < .001), the European Quality of Life EQ-5D score (R = 0.37; P < .001), the Orthostatic Hypotension Questionnaire score (R = 0.44; P < .001), and the REM sleep behavior disorder screening questionnaire score (R = 0.49; P < .001). The total P-SYN for each participant correlated with the time since diagnosis of PD (R = 0.25; P = .009), MSA (R = 0.34; P = .004), and PAF (R = 0.41; P = .04).
Adverse Events and Quality Control and Concordance
In 428 participants, 1284 skin biopsies were completed (3 per participant). Two participants (0.5% of cases) had bleeding at a single biopsy site that required treatment with an additional bandage but did not require additional medical care. No infections or other serious complications were noted. A full summary of the interrater and interlaboratory reproducibility testing is reported in the eMethods and eFigure in Supplement 1.
Discussion
This prospective, blinded investigation of the detection of cutaneous P-SYN in all 4 synucleinopathies found that (1) cutaneous P-SYN was detected in greater than 92% of patients with MSA, PD, DLB, and PAF; (2) quantitative measures of P-SYN deposition correlated with disease severity; and (3) skin biopsies were well tolerated, with few adverse effects. There is a need for an office-based, accurate, and reproducible diagnostic test for the synucleinopathies. Clinical diagnosis based on a combination of signs and symptoms is still the in vivo standard, and the results of autopsy are the postmortem gold standard. However, autopsy studies of patients with clinical diagnoses of PD and MSA have drawn attention to the high misdiagnosis rate.7,25 In the present study, the expert review panel reclassified 20.5% of diagnoses in study participants, suggesting a similar rate of misdiagnosis. Accurate diagnosis is of vital importance for patient and family counseling, initiation of appropriate symptomatic therapies, and enrollment into clinical trials of potential disease-modifying therapies. The accuracy and overall test reproducibility reported in this study will be an important contributor to address inaccurate patient diagnosis. Expert review panels offer a complementary path to improve diagnostic accuracy, although this approach may be suitable only for clinical trials.
The results in the present study are consistent with prior work by some of us21 in a different patient population showing that both patients with MSA and those with PD exhibited P-SYN deposition. The prior findings were extended in this study with a larger number of patients and the inclusion of patients with DLB and PAF. Furthermore, the investigator sites were broadened to include community-based neurologists, which enhanced the generalizability of the results.
The Lewy body diseases PD and DLB are characterized by α-synuclein aggregates in neurons, Lewy bodies, and Lewy neurites, whereas the pathologic hallmark of MSA is glial cytoplasmic inclusions in oligodendrocytes.25 In PD, neuronal loss is predominantly in the brainstem and subcortical regions; in DLB, the pathology is more extensive in the neocortex; and in MSA, the pathology is widespread throughout the central nervous system.25,26 The theory that different α-synuclein strains have molecular, morphologic, and functional properties that underlie the central pathologic signature and clinical phenotype has gained credence in recent years.27 It is likely that the distribution, topographic location, and amount of synuclein deposition in the periphery has a similar basis. This theory is supported by data in a recent study28 that detected P-SYN deposition within cutaneous Schwann cells of patients with MSA but not in patients with PD or DLB. The mechanism whereby different synuclein strains might result in specific central and peripheral pathologic signatures has not been elucidated to date.
Skin biopsy detection of P-SYN was lower in patients with PD than in those with other synucleinopathies. One explanation for the lower P-SYN detection in patients with PD is the greater disease heterogeneity (eg, the body-first vs brain-first subtypes).29 Parkinson disease and DLB have overlapping clinical and pathologic features, but the amount of P-SYN was typically greater and P-SYN was more widespread in patients with DLB than in those with PD, similar to the pattern seen in the central nervous system. Finally, individuals with some genetic forms of PD have lower or even absent P-SYN deposition. Many of the study participants with undifferentiated synucleinopathy or an unknown diagnosis (particularly those with suspected DLB) were also positive for P-SYN. In most cases, the treating physicians strongly suspected a synucleinopathy but the patient did not meet the prespecified diagnostic criteria. These observations will require further investigation.
These results should be viewed within the context of a recently reported multicenter study30 of a spinal fluid assay in which small amounts of P-SYN were amplified to augment detection using prion-protein amplification techniques (seed assay amplification). Unlike that study, the cohort in our study was not enriched by including patients with positive dopamine transporter scan findings that indicated basal ganglia dopamine depletion in PD. Despite study differences, the accuracy of skin immunohistochemical analysis for α-synuclein was similar to the spinal fluid seed amplification assay results in patients with PD. Both studies showed sensitivities and specificities similar to those in a prior autopsy-based study of skin seed assay amplification testing in patients with PD.31 To our knowledge, only 1 study32 has directly compared spinal fluid P-SYN seed amplification assays with skin biopsy immunofluorescent staining in patients with synucleinopathies. In a study of PD, MSA, DLB, and PAF,32 skin immunofluorescence was 90% sensitive and 100% specific whereas spinal fluid amplification was 78% sensitive and 100% specific. In a similar comparison study33 of patients with isolated REM sleep behavior disorder, skin immunofluorescent staining was 78% sensitive and 100% specific and spinal fluid amplification was 67% sensitive and 72% specific. In contrast, a skin and spinal fluid amplification study34 of patients with isolated REM sleep behavior disorder showed that spinal fluid amplification was 75% sensitive and 98% specific and skin amplification was 77% sensitive and 98% specific.
In the present study, skin biopsy was introduced as a new procedure for many movement disorder and cognitive neurology specialists. The procedure was well tolerated by all participants, with a rate of minor complications of 0.5%. Taken together, the results of this study make an important contribution to clinical care and may accelerate drug development for the synucleinopathies by improving patient homogeneity in clinical trials.
Limitations
This study has several limitations. First, the gold standard for diagnosis was clinical consensus criteria supported by a review by an independent expert review panel without video review or autopsy confirmation. We used the Parkinson UK Brain Bank diagnostic criteria,10 which differ from the most recent International Parkinson and Movement Disorder Society criteria. Second, the sensitivity of detection for early or prodromal disease was not investigated in this study. Third, other disease mimics, such as essential tremor, progressive supranuclear palsy, corticobasal degeneration, and Alzheimer disease, were not included as disease controls. Fourth, other biomarkers, specifically neuroimaging and biofluid biomarkers, were not a requirement for inclusion in the study. Fifth, we did not perform genetic testing on patients. Some genetic forms of PD do not have α-synuclein deposition, and exclusion of such patients could have increased the proportion of individuals in whom P-SYN detection was observed. Sixth, the control participants were younger than participants in the other groups. This was a consequence of our per protocol approach to ensure adequate control data by decile across the full spectrum of age of neurodegenerative disease. Seventh, only 3 biopsy sites were analyzed; a greater number of biopsy sites could have increased test sensitivity.
Conclusions
In this cross-sectional study, a high proportion of individuals meeting clinical consensus criteria for PD, DLB, MSA, and PAF had a skin biopsy positive for P-SYN. Further research is needed in unselected clinical populations to externally validate the findings and fully characterize the potential role of skin biopsy detection of P-SYN in clinical care.
eMethods.
eResults.
eFigure. Inter-laboratory synuclein and nerve density testing
eTable 1. P-SYN positive control data
eTable 2. Reclassified to undifferentiated synucleinopathy
eTable 3. Reclassified to unknown
Data Sharing Statement
References
- 1.Marras C, Beck JC, Bower JH, et al. ; Parkinson’s Foundation P4 Group . Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21. doi: 10.1038/s41531-018-0058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savica R, Grossardt BR, Bower JH, et al. Survival and causes of death among people with clinically diagnosed synucleinopathies with parkinsonism: a population-based study. JAMA Neurol. 2017;74(7):839-846. doi: 10.1001/jamaneurol.2017.0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013;70(11):1396-1402. doi: 10.1001/jamaneurol.2013.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol. 2013;70(7):859-866. doi: 10.1001/jamaneurol.2013.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann H, Norcliffe-Kaufmann L, Palma JA, et al. ; Autonomic Disorders Consortium . Natural history of pure autonomic failure: a United States prospective cohort. Ann Neurol. 2017;81(2):287-297. doi: 10.1002/ana.24877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbons CH, Freeman R. Clinical implications of delayed orthostatic hypotension: a 10-year follow-up study. Neurology. 2015;85(16):1362-1367. doi: 10.1212/WNL.0000000000002030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler CH, Beach TG, Zhang N, et al. Clinical diagnostic accuracy of early/advanced Parkinson disease: updated clinicopathologic study. Neurol Clin Pract. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beach TG, Adler CH. Importance of low diagnostic accuracy for early Parkinson’s disease. Mov Disord. 2018;33(10):1551-1554. doi: 10.1002/mds.27485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbons CH, Freeman R, Bellaire B, Adler CH, Moore D, Levine T. Synuclein-One study: skin biopsy detection of phosphorylated α-synuclein for diagnosis of synucleinopathies. Biomark Med. 2022;16(7):499-509. doi: 10.2217/bmm-2021-0646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42(6):1142-1146. doi: 10.1212/WNL.42.6.1142 [DOI] [PubMed] [Google Scholar]
- 11.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670-676. doi: 10.1212/01.wnl.0000324625.00404.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. doi: 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetz CG. Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): a new scale for the evaluation of Parkinson’s disease. Article in French. Rev Neurol (Paris). 2010;166(1):1-4. doi: 10.1016/j.neurol.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 14.Goetz CG, Poewe W, Rascol O, et al. ; Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease . Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020-1028. doi: 10.1002/mds.20213 [DOI] [PubMed] [Google Scholar]
- 15.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal Cognitive Assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord. 2008;23(7):1043-1046. doi: 10.1002/mds.22017 [DOI] [PubMed] [Google Scholar]
- 16.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343. doi: 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing. 1997;26(5):353-357. doi: 10.1093/ageing/26.5.353 [DOI] [PubMed] [Google Scholar]
- 18.Schrag A, Selai C, Mathias C, et al. Measuring health-related quality of life in MSA: the MSA-QoL. Mov Disord. 2007;22(16):2332-2338. doi: 10.1002/mds.21649 [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22(2):79-90. doi: 10.1007/s10286-011-0146-2 [DOI] [PubMed] [Google Scholar]
- 20.Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov Disord. 2007;22(16):2386-2393. doi: 10.1002/mds.21740 [DOI] [PubMed] [Google Scholar]
- 21.Gibbons C, Wang N, Rajan S, et al. Cutaneous α-synuclein signatures in patients with multiple system atrophy and Parkinson disease. Neurology. 2023;100(15):e1529-e1539. doi: 10.1212/WNL.0000000000206772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Garcia J, Freeman R, Gibbons CH. Phosphorylated alpha-synuclein within cutaneous autonomic nerves of patients with Parkinson’s disease: the implications of sample thickness on results. J Histochem Cytochem. 2020;68(10):669-678. doi: 10.1369/0022155420960250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provitera V, Gibbons CH, Wendelschafer-Crabb G, et al. A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur J Neurol. 2016;23(2):333-338. doi: 10.1111/ene.12842 [DOI] [PubMed] [Google Scholar]
- 24.Pepe M. The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford University Press; 2003. [Google Scholar]
- 25.Koga S, Aoki N, Uitti RJ, et al. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology. 2015;85(5):404-412. doi: 10.1212/WNL.0000000000001807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickson DW. Neuropathology of Parkinson disease. Parkinsonism Relat Disord. 2018;46(Suppl 1)(suppl 1):S30-S33. doi: 10.1016/j.parkreldis.2017.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayers JI, Lee J, Monteiro O, et al. Different α-synuclein prion strains cause dementia with Lewy bodies and multiple system atrophy. Proc Natl Acad Sci U S A. 2022;119(6):e2113489119. doi: 10.1073/pnas.2113489119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donadio V, Incensi A, Rizzo G, et al. Phosphorylated alpha-synuclein in skin Schwann cells: a new biomarker for multiple system atrophy. Brain. 2023;146(3):1065-1074. doi: 10.1093/brain/awac124 [DOI] [PubMed] [Google Scholar]
- 29.Borghammer P, Horsager J, Andersen K, et al. Neuropathological evidence of body-first vs. brain-first Lewy body disease. Neurobiol Dis. 2021;161:105557. doi: 10.1016/j.nbd.2021.105557 [DOI] [PubMed] [Google Scholar]
- 30.Siderowf A, Concha-Marambio L, Lafontant DE, et al. ; Parkinson’s Progression Markers Initiative . Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023;22(5):407-417. doi: 10.1016/S1474-4422(23)00109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Becker K, Donadio V, et al. Skin α-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol. 2020;78(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donadio V, Wang Z, Incensi A, et al. In vivo diagnosis of synucleinopathies: a comparative study of skin biopsy and RT-QuIC. Neurology. 2021;96(20):e2513-e2524. doi: 10.1212/WNL.0000000000011935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liguori R, Donadio V, Wang Z, et al. A comparative blind study between skin biopsy and seed amplification assay to disclose pathological α-synuclein in RBD. NPJ Parkinsons Dis. 2023;9(1):34. doi: 10.1038/s41531-023-00473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iranzo A, Mammana A, Muñoz-Lopetegi A, et al. Misfolded α-synuclein assessment in the skin and CSF by RT-QuIC in isolated REM sleep behavior disorder. Neurology. 2023;100(18):e1944-e1954. doi: 10.1212/WNL.0000000000207147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eResults.
eFigure. Inter-laboratory synuclein and nerve density testing
eTable 1. P-SYN positive control data
eTable 2. Reclassified to undifferentiated synucleinopathy
eTable 3. Reclassified to unknown
Data Sharing Statement


