Abstract
To combat the ongoing opioid epidemic, our laboratory has developed and evaluated an approach to detect fentanyl analogs in urine and plasma by screening LC-QTOF MS/MS spectra for ions that are diagnostic of the core fentanyl structure. MS/MS data from a training set of 142 fentanyl analogs were used to determine the four product ions and six neutral losses that together provided the most complete coverage (97.2%) of the training set compounds. Furthermore, using the diagnostic ion screen against a set of 49 fentanyl analogs not in the training set resulted in 95.9% coverage of those compounds. With this approach, lower reportable limits for fentanyl and a subset of fentanyl-related compounds range from 0.25 ng/mL to 2.5 ng/mL in urine and 0.5 ng/mL to 5.0 ng/mL in plasma. This innovative processing method was applied to evaluate simulated exposure samples of remifentanil and carfentanil in water, and their metabolites remifentanil acid and norcarfentanil in urine. This flexible approach enables a way to search for emerging fentanyl analogs in clinical samples.
Introduction
In the United States, synthetic opioids, primarily fentanyl, have contributed to a 38.4 percent increase in drug overdoses when comparing the 12 months prior to June 2019 and the 12 months prior to May 2020[1]. Forensic cases of fentanyl and fentanyl analogs increased 7500% from 2013 to 2017, as deaths attributed to fentanyl analogs saw a 434% increase from 2014 to 2016[2]. Therefore, exposure detection has become of greater concern. In 2019, the use of 4 new fentanyl analogs were reported in the US for the first time[3]. The growing number of novel fentanyl analogs demonstrates the need for flexible analytical methods to detect these emerging health threats.
Fentanyl was first synthesized by Jansson pharmaceutical for commercial use in the 1960s, however modifications to alter potency or symptom onset have created a large array of analogs in the fentanyl family of compounds[4]. Because of the high potential for modifications to the fentanyl backbone, in 2018 the US Drug Enforcement Administration (DEA) made a ruling to schedule all fentanyl related compounds in an effort to reduce overdose deaths[5].This emphasized the need for flexible detection capabilities. Targeted GC-MS and LC-MS/MS are limited to the analytes predetermined in each method and typically report around 20 fentanyl analogs per method[6–8]. High resolution mass spectrometry is commonly used as a screening technique for detection of up to 160 fentanyl analogs in clinical and forensic samples[9–11]. At the time of writing, commercially available forensic libraries offered by major instrument vendors contain up to 75 fentanyl analogs[12]. To improve the availability of library spectra for fentanyl analogs, CDC has published a free, high resolution mass spectral library in NIST and other vendor formats containing 213 synthetic opioid-related compounds, 192 of which are fentanyl analogs[13]. Creation of a library often requires the use of reference standards, although in some cases predicted products ions have been used[9]. Because reference standards or library spectra may not be available for novel fentanyl analogs, a flexible method is needed to presumptively detect fentanyl analogs.
Detection of unknown compounds in complex biological matrices and at low concentrations can be challenging. By using class-specific information, product ions and neutral losses can be used to identify related compounds[14, 15], This approach has been used for screening designer drugs in urine[16] and bioactive compounds in botanical material[17]. However, the LC-MS/MS instrumentation used required multiple acquisitions to collect data for product ions and neutral losses. LC-HRMS has also been used to monitor product ions and neutral losses, with ready identification of related compounds from traditional Chinese medicines[18]. Given that fentanyl analogs contain common moieties, precursor ions and neutral losses are conserved between multiple analogs[19] and can be used to rapidly recognize emerging analogs in complex matrix backgrounds without additional data acquisition.
In this study, multiple precursor ions and neutral losses were identified using the LC-HRMS data collected from a training set of 142 fentanyl analogs. From this data, a minimum number were selected to ensure broad coverage of known fentanyl analogs. The selected diagnostic ions were screened against more than 100 reference range samples and applied to 49 new analogs, as well as fortified urine and plasma samples to confirm detection capabilities of emerging fentanyl analogs.
Experimental Section
Materials.
High-pressure liquid chromatography (HPLC) grade methanol (Fisher, Hampton, NH), acetonitrile (The Lab Depot, Dawsonville, GA), and dichloromethane (DCM) (The Lab Depot, Dawsonville, GA) were used for all experiments. Deionized (DI) water was prepared with an on-site water purification system (Aqua Solutions Inc., Jasper, GA). Ammonium formate and formic acid (99%) were acquired from Sigma Aldrich (Pittsburg, PA). Three separate lots of each pooled urine and pooled plasma along with fifty each of individual urine and plasma reference samples were purchased from Tennessee Blood Services (Memphis, TN). This study does not meet the definition of human subjects as specified in 45 CFR 46.102 (f) as all urine and plasma samples were acquired from commercial sources with appropriate institutional review board approvals.
Reference Materials and Working Solutions.
The training set was comprised of fentanyl analogs from the Fentanyl Analog Screenig (FAS) Kit and FAS Kit Emergent Panel Version 1 (V1), manufactured by Cayman Chemical (Ann Arbor, MI) and carfentanil from the Opioid CRM Kit, manufactured by Cerilliant (Round Rock, TX). A list of all synthetic opioids and related compounds in the FAS Kit and Emergent Panels can be found on the vendor’s website[20]. FAS kit and Opioid CRM Kit are part of the Traceable Opioid Material® Kits and its development is explored in greater depth by Mojica et. al. [2] Two additional expansion packs, FAS Kit Emergent Panels Version 2 and 3, (FAS Kit V2, and V3) containing 32 and 30 compounds, respectively were used to challenge the training set. Fentanyl 2H5 labeled standard was purchased from Cerilliant (Round Rock, TX).
FAS Kit and FAS V1, V2, and V3 individual stock solutions were prepared at 10 μg/mL in DI water with trace amounts of methanol (1.5 – 2.5%), with individual working solutions generated by diluting to 100 ng/mL in DI water. A 25 ng/mL internal standard working solution was created by diluting a isotopically labeled (2H5) fentanyl standard. Additionally, pooled urine samples were spiked with remifentanil acid and norcarfentanil at 10 ng/mL and water samples were spiked with remifentanil and carfentanil at 1 ng/mL to simulate a documented exposure.
Sample Preparation, Chromatography, and Mass Spectrometry.
Prior to sample extraction, 25 μL of a 25 ng/mL fentanyl internal standard (2H5) solution was mixed with 200 μL of sample and 175 μL of diluent. Sample extraction was carried out using a Biotage ISOLUTE SLE+ 400μL solid-liquid extraction plate followed by reverse phase chromatography using a Phenomenex Kinetex biphenyl column (100 × 3.0 mm, 2.6 μM). Mass spectrometry was performed using positive mode electrospray ionization on an Agilent 6545 QTOF using data dependent acquisition in the AutoMSMS mode of data collection. The in-house spectral library was curated to correct fragment m/z values and remove noise below 1% of the base peak in each spectra. The details associated with this method have been described in a previous publication[11].
Creation of Diagnostic Ion Chromatograms.
Diagnostic ion chromatograms were created from the conserved diagnostic product ions and the conserved neutral losses, which were selected by analysis of the fentanyl analogs in the in-house library. These MS/MS level chromatograms created for each run consisted of a summed extraction ion chromatogram (EIC) trace for the product ions or a summed precursor neutral loss chromatogram (pNLC) trace for the neutral losses. Each of these chromatograms were created with a mass window of ±15 ppm to account for any mass error using MassHunter Qualitative Analysis 10.0. A Gaussian peak smoothing function in the software was applied to each EIC and pNLC with a function width set at 100 points and a gaussian width set at 10 points to create peaks that mimic the width of the chromatographic peak at the MS level. Smoothed peaks with height greater than 2000 counts were investigated further using the software’s FindByFormula function to detect and identify any matches to the in-house personal compound database/library (PCDL). The chromatogram from any compounds detected using FindByFormula were overlaid with the diagnostic ion chromatograms (EIC and pNLC) to associate the identity of each peak in the diagnostic ion chromatograms. Any remaining, unidentified peaks were converted to compounds in the software by first determining the best molecular formula for the precursor ion, then adding the molecular formula and retention time to the in-house PCDL, labeling the compound as an unknown compound. This necessary step allows for a peak in a diagnostic ion chromatogram to be treated as a compound within the software, allowing for library searching of the MS/MS spectra. All unknown compound MS/MS spectra were then searched against the NIST 17 Tandem MS library for identification. If identified, the compound name was updated in the PCDL. In some cases, there were no matches in the NIST library for compounds that were present in most of the blank individual matrix samples. Twelve commonly observed background compounds from urine and plasma samples were added to and denoted in the PCDL as a matrix background ion for ease of future detection.
Results and Discussion
Overview.
The general approach to this work is to mine a large training set of MS/MS data from fentanyl analogs to determine a list of diagnostic product ions and neutral losses that may be used for the detection of emerging fentanyl analogs. The detection method was then tested against urine and plasma samples spiked with fentanyl analogs from the training set and against non-matrix samples spiked with fentanyl analogs and other synthetic opioids not in the training set.
Determination of Neutral Loss and Product Ions.
To determine appropriate product ion and neutral loss masses that would identify a wide array of fentanyl analogs, tandem mass spectrometry data was collected and curated for all compounds present in the FAS and FAS V1 kit. Of the 150 compounds in the FAS and FAS V1 kit, 142 compounds contained fully-substituted 4-aminopiperidines, a core structural component of fentanyl analogs. 2-fluoro MT-45, 4-ANPP, AH 7921, isopropyl U-47700, MT-45, U-47700, U-48800, and U-49900 were excluded as despite their common association with fentanyl and fentanyl analogs, they do not possess a tertiary 4-aminopiperidine. For the 142 compounds not excluded in the FAS Kit, the mass-corrected precursor and product ions collected at collision energies of 20 and 40 eV were compiled. A neutral loss value relative to the precursor ion was determined for each product ion in the curated data. A matrix was created with each possible product ion or neutral loss value on one axis and each compound per collision energy value on the other axis. The matrix was filled in with Boolean values describing whether the product ion or neutral loss value was observed in each spectrum. The count of true Boolean values for each product ion or neutral loss values was calculated and a list of most prevalent product ions and neutral loss values was generated by sorting from high to low count. For the purposes of determining coverage, overlapping product ion or neutral loss values were discarded, as coverage, in this work, is defined as having at least one product ion or neutral loss in at least one of the two spectra collected at different collision energy levels.
One example of overlapping product ions was found with the comparison of product ions m/z 105.0699 and m/z 188.1434.
Both product ions are well known fragments of fentanyl and many fentanyl analogs. From the training set, 111 compounds had the product ion m/z 105.0699 in the spectra for at least one collision energy. Similarly, 103 compounds had the product ion m/z 188.1434 in the spectra for at least one collision energy. Interestingly, all compounds that demonstrated product ion m/z 188.1434 also demonstrated m/z 105.0699, thus all 111 of these compounds could be covered using the product ion at m/z 105.0699, therefore m/z 188.1434 was not included for the detection of fentanyl analogs. Best coverage was empirically determined by assessing various combinations of product ions until the maximum coverage for each product ion and neutral loss screen was reached. Sensitivity may be improved by the inclusion of product ions that were determined to be redundant in terms of coverage, but care must be taken to ensure there is no significant loss in specificity.
Six neutral loss values were selected as diagnostic for fentanyl analogs: m/z 121.0891 was found in 90 compounds (63.3% coverage), m/z 205.1467 was found in 45 compounds (31.7% coverage), m/z 149.0841 was found in 25 compounds (19.7% coverage), m/z 308.1889 was found in 25 compounds (17.6% coverage), m/z 163.0997 was found in 13 compounds (9.2% coverage), and m/z 135.0684 was found in 6 compounds (4.2% coverage) for a combined coverage of 125 compounds (88.0% coverage). Four product ions were also selected as diagnostic for fentanyl analogs: m/z 146.0964 was found in 119 compounds (83.8% coverage), m/z 105.0699 was found in 111 compounds (78.2% coverage), m/z 134.0964 was found in 108 compounds (76.1% coverage), and m/z 160.1121 was found in 32 compounds (22.5% coverage) for a combined coverage of 128 compounds (90.1% coverage). When combined, the neutral loss approach and the product ion approach cover 138 compounds and provide 97.2% coverage on the FAS and FAS V1 kits (Table 1). Only four fentanyl analogs in the training set (2’-fluoro ortho fluoro fentanyl, benzyl acryl fentanyl, N-benzyl furanyl norfentanyl, and 4’-fluoro, para-fluoro (±)-trans-3-methyl fentanyl) are not identified by the selected diagnostic ions. These fentanyl analogs did not have common diagnostic ions, so inclusion would require the addition of a diagnostic ion for each.
Table 1.
Product ions and neutral loss fragments used for semi-targeted analysis of fentanyl analogs along with the percent coverage of the library compounds represented.
| Fragment #1 | Fragment #2 | Fragment #3 | Fragment #4 | Fragment #5 | Fragment #6 | # of Library Compounds Represented | % of Library Compounds Represented | |
|---|---|---|---|---|---|---|---|---|
| Neutral Loss | 121.0891 | 135.0684 | 149.0841 | 163.0997 | 205.1467 | 308.1889 | 125 | 88.0% |
| Product Ion | 105.0699 | 134.0964 | 146.0964 | 160.1121 | 128 | 90.1% | ||
| TOTAL | 138 of 142 | 97.2% |
Structural Importance of Product Ion.
The product ions observed in this work can be divided into two groups as delineated in Figure 1 by color. The orange portions of the structure pertain to product ions in both groups. The product ion at m/z 160.1121, represented in green, contains the aniline group and a portion of the piperidine ring within the core structure of fentanyl. The three product ions in purple (m/z 105.0699, m/z 134.0964, and m/z 146.0964) correspond to the aryl group that is separated by two methylene bridges from the amine of the piperidine ring. For most fentanyl analogs, the conservation of the core structure during MS/MS is not surprising because functional groups are commonly cleaved. The structural significance of the neutral loss values is more difficult to attribute to structure due to the likelihood of more than one leaving group; however, all of the proposed neutral loss values for this approach are supported by the detailed fragmentation patterns reported in the Q. Nan et. al. study[21].
Figure 1.
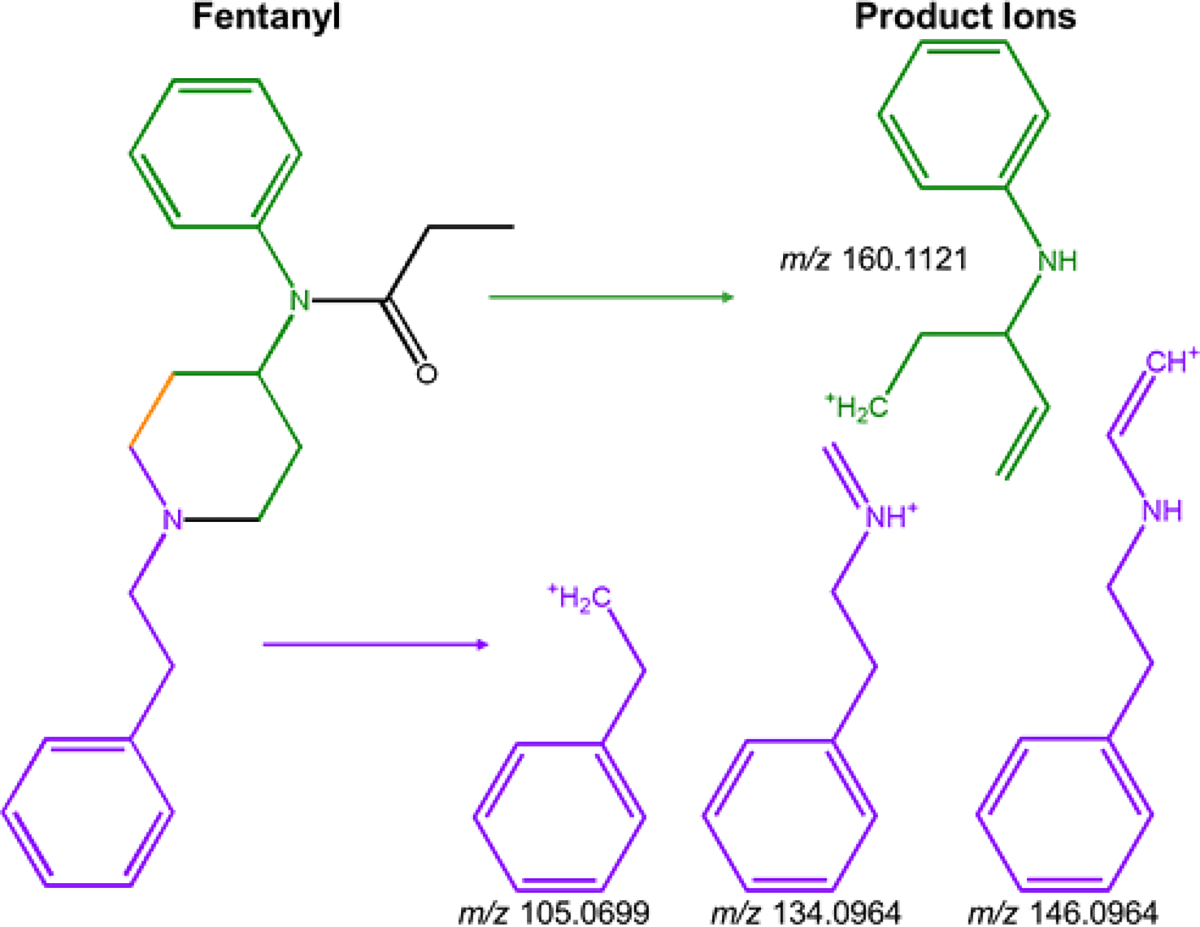
Structures of product ions relative to fentanyl
Demonstration of Diagnostic Ions in Matrix Samples.
Our diagnostic ion technique was applied to 50 individual blank urine and 50 individual blank plasma samples along with aliquots of pooled urine and pooled plasma to determine the specificity against a matrix background. Fentanyl-2H5 was spiked into each sample to monitor the quality of the extraction and sample injection into the instrument. From this analysis, a detection cutoff of 2000 counts was deemed suitable to distinguish between the presence of analyte and noise. Figure 2 demonstrates a representative product ion EIC (blue) and neutral loss pNCL (red) for both blank pooled plasma (A) and blank pooled urine (B). All peaks with a height greater than 2000 counts are identified by name or with an asterisk. For both pooled plasma and pooled urine, two large peaks are detected in addition to the fentanyl-2H5 in the product ion EIC. With neutral loss analysis, one additional peak is detected in pooled plasma and four additional peaks are detected in pooled urine. The observed peaks are consistently detected in analysis of the individual blank urine and plasma samples. The neutral loss peak at 4.2 minutes in plasma was successfully identified as hydrocortisone using the NIST 17 HRMS Tandem Mass Spectral Library; however, the remaining background ions could not be identified using the NIST library. The remaining compounds are likely product ions and neutral losses of endogenous matrix compounds and are not indicative fentanyl analogs. All observed peaks were determined to be consistent background ions or were confirmed using NIST 17 library searching using MassHunter Qualitative Analysis 10.0, thus no false positives were detected. Because this approach is only an adjustment in data processing from the previously published library screening method, Figure 3 demonstrates the ability to apply retroactive screening for diagnostic ions to a previously analyzed quality control plasma sample spiked with ten fentanyl-related compounds along with fentanyl-2H5, with the representative product ion EIC (blue trace in Figure 3A) and neutral loss pNCL (red trace in Figure 3A) displayed. The peaks in panels A and B often appear as doublets, with triplet and quadruplet peaks also visible. This is an effect of the data sampling rate and smoothing on the EIC. The unsmoothed trace of each product ion and neutral loss consists of single-point peaks as the instrument is constantly selecting different precursor ions for MS/MS as part of the data-dependent acquisition. Smoothing is applied to minimize the number of zero abundance points in the middle of a true chromatographic peak. However, the acquisition method also employs active exclusion to optimize the number of compounds selected for MS/MS by reducing redundancy in the data. A doublet peak is likely a single, highly abundant compound with an active exclusion period at the center of the peak. A triplet and quadruplet peak is likely two or more coeluting compounds with one or more active exclusion periods. It can easily be confirmed that a doublet peak belongs to one compound by investigating the accurate mass of the precursor compound for each peak in the doublet. Likewise, the number of compounds present in a triplet or quadruplet peak may be determined using the accurate masses of each precursor present. Further optimization of the data sampling rate and active exclusion timeframe to ensure that quality MS/MS spectra for a compound is only collected once could eliminate this issue.
Figure 2.
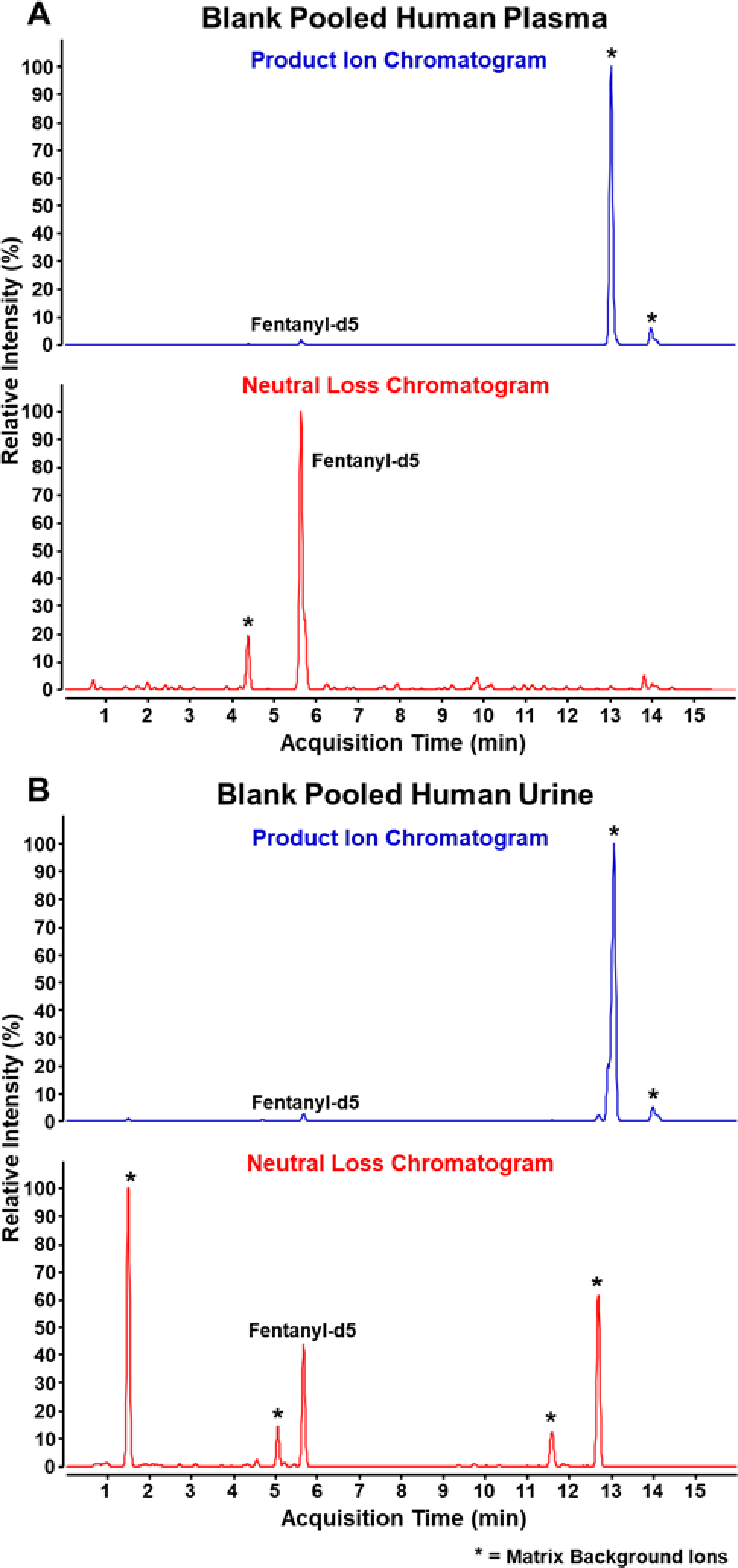
Chromatograms of PI EIC and NL EIC of blank pooled human plasma (A) and blank pooled human urine (B).
Figure 3.
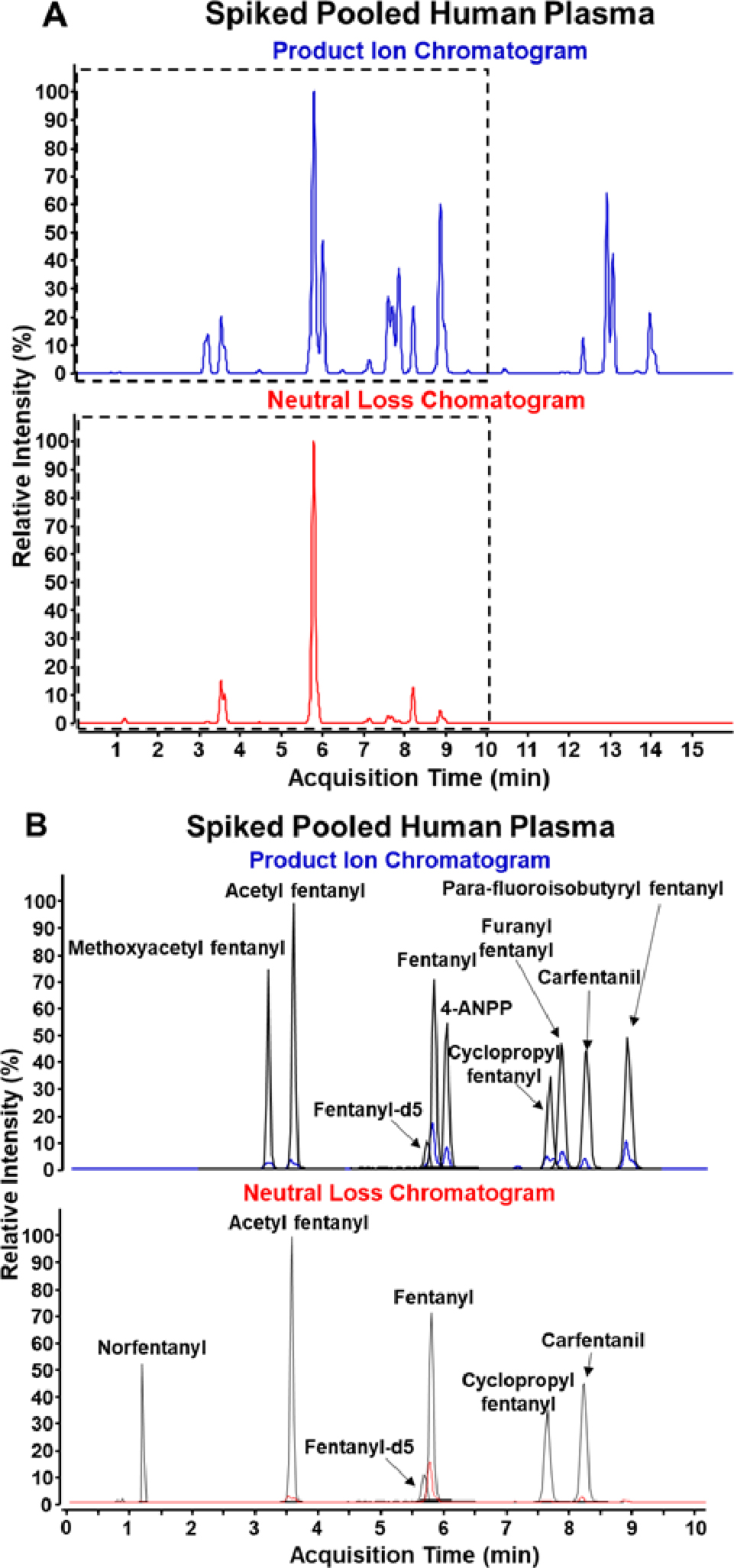
Chromatograms of PI EIC and NL EIC of pooled human plasma spiked at 25 ng/mL without (A) and with annotation (B).
Detection of Compounds not in Training Set.
To further test this detection technique, FAS V2 and V3 compounds, not part of the original training set, were investigated using our diagnostic ion technique (Table 2). As the two expansion kits include both fentanyl analogs and U-series compounds with structures differing greatly from fentanyl they are an ideal test for this technique. With this investigation no U-series compounds, which compose 21.0% of the FAS V2 and V3 compounds, are detected along with no false positives. Among the 49 fentanyl analogs in the FAS V2 and V3 kits, 95.9% (79.0% of the total number of compounds within the kits) are detected with this method. Only two compounds were not found in either the product ion or neutral loss screen: N-DOET fentanyl and despropionyl 2’-fluoro ortho fluoro fentanyl. Overall, this demonstrates the viability of using the selected product ion and neutral losses to screen and detect for new fentanyl analogs while minimizing the chances of false positives from unrelated analytes.
Table 2.
Number of fentanyl analogs from the FAS V2 and V3 compounds that were detected by neutral loss (NL) or product ion (PI) screens and number of false positives seen from U-Series compounds.
| # of Fentanyl Analogs Detected | % of Fentanyl Analogs Detected | # of False Positives from U-Series compounds | |
|---|---|---|---|
| NL | 40 | 81.6% | 0 |
| PI | 37 | 75.5% | 0 |
| TOTAL | 47 of 49 | 95.9% | 0 |
Lower Reportable Limit.
To determine the lower reportable limit (LRL100) of the seven most common fentanyl analogs [20], one blank pooled and three blank individual matrix samples were spiked with analytes at concentration levels ranging from 0.075 to 5 ng/mL. The lowest concentration level in which the compound was positively identified across all 4 replicates is reported as the LRL100 for the matrix and can be found in Table 3. The selected approach to determine LRL results in a simplified estimate to best describe method performance across time and variable conditions.
Table 3.
LRL100 for select compounds in ng/mL for plasma and urine.
| Compound Name | Plasma (ng/mL) | Urine (ng/mL) |
|---|---|---|
| Acetyl fentanyl | 1.0 | 2.5 |
| Carfentanil | 0.75 | 1.0 |
| Cyclopropyl fentanyl | 2.5 | 0.75 |
| Fentanyl | 0.50 | 0.25 |
| Furanyl fentanyl | 2.5 | 2.5 |
| Methoxyacetyl fentanyl | 5.0 | 0.75 |
| Para-fluoro isobutyryl fentanyl | 0.75 | 0.75 |
The LRL100 values range from 0.25 ng/mL to 5 ng/mL, with no discernable trend between the LRL100 values in urine and plasma. Reported concentrations of fentanyl and fentanyl analogs following exposure have varied greatly, with reported fentanyl, carfentanil, acetyl fentanyl, and furanyl fentanyl concentrations ranging from 0.0102 ng/mL to 827 ng/mL in hu man matrices[22–28]. Although sensitivity may preclude the detection of all exposures due to delayed sample collection or opioid toxicity, the LRL values of the eight chosen fentanyl analogues suggest that this method has the capability to identify fentanyl related compounds in true exposure samples. Ultimately, however, the absence of any analyte cannot be definitively reported without characterization of the individual compound’s LRL100.
Synthetic Clinical Sample.
True exposure samples were not available for use, so spiked solvent and urine samples were created to mimic exposure to a mixture of incapacitating agents, remifentanil and carfentanil[29]. Remifentanil and norcarfentanil, a common metabolite for carfentanil, are part of the FAS Kit and were used in the training set for this method. Carfentanil and remifentanil acid, a common metabolite for remifentanil, are not included in the FAS Kit or FAS V1 Kit, making them good candidates for testing the method to determine the presence of unknown analytes.
Remifentanil and carfentanil were spiked into water at 1 ng/mL to simulate an extraction where the native fentanyl analogs would likely be present. Because the sample contained no clinical matrix material, the SLE extraction was not performed on these samples. After analysis, both compounds were detected above the intensity cutoff in at least one of the product ion EIC or neutral loss EIC as seen in Figure 4A. A peak at 2.3 minutes in the neutral loss EIC corresponds to a precursor ion at m/z 377.2064 which was confirmed by a library match to be remifentanil. Additionally, a peak at 7.8 minutes in the product ion EIC corresponds to a precursor ion at m/z 395.2324 which was confirmed by a library match to be carfentanil. No significant matrix background ions or chemical noise are observed. For this reason, the authors suggest the 2000 count detection cutoff may be lowered for samples that are not in a complex matrix like urine or plasma. This improved sensitivity could prove the conserved diagnostic ions workflow even more useful in a matrix such as water.
Figure 4.
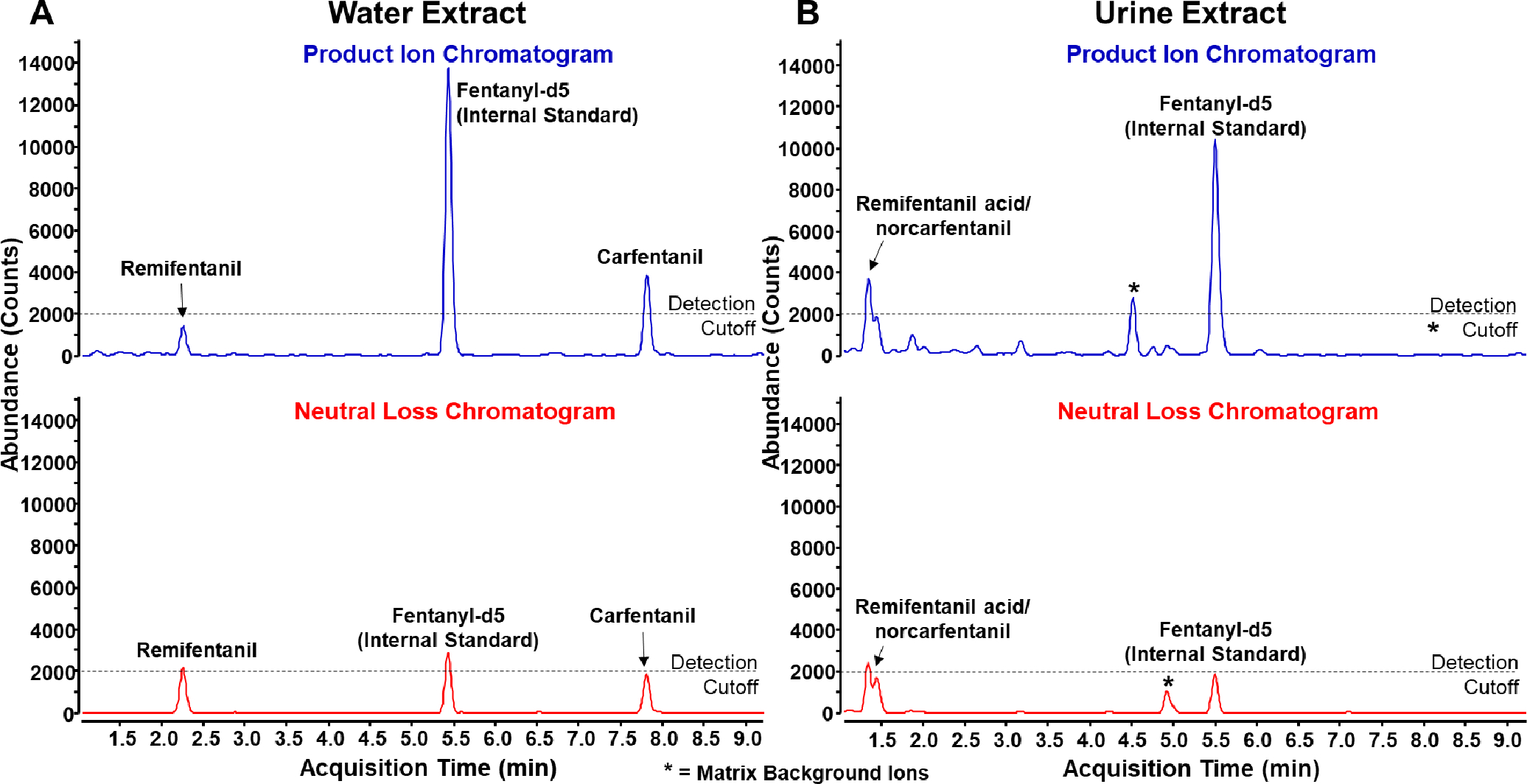
Product ion (blue) and neutral loss (red) chromatograms of a simulated extract in water (A) and simulated human exposure with urine extract (B)
As urine is more likely to contain compound metabolites, remifentanil acid and norcarfentanil were spiked into urine at 10 ng/mL to simulate a human exposure sample, extracted, and analyzed. In Figure 4B, a doublet is observed at 1.4 minutes. The precursor mass of the first peak in the doublet (m/z 363.1912) does not match the precursor mass of the second peak (m/z 291.1708). The precursor masses match those of remifentanil acid and norcarfentanil respectively, an indication that the compounds are coeluting. Nevertheless, because the product ion and neutral losses can be linked with precursor masses due to our data acquisition approach, we are still able to confirm the presence of both compounds in the sample. In this sample two previously determined matrix background ions were observed similar to Figure 2, although only one was above the detection cutoff. Due to their presence in blank urine samples, we do not believe they have any relation to the analytes spiked into the sample. Sensitivity is generally lower in the urine sample compared to the water sample, as seen by the height of fentanyl-2H5, which was spiked at the same concentration in both samples. These can be attributed to analyte loss from sample extraction and ion suppression from matrix analytes. In addition, extraction, chromatography, and data collection were optimized for fentanyl analogs rather than metabolites, which may result in poorer metabolite ion detection relative to fentanyl analogs, as metabolites tend to be poorly retained.
Conclusions
The developed semi-targeted data processing approach described may be used to support overdose surveillance involving synthetic opioids by enabling the detection of fentanyl analogs that may not be present in available databases/libraries. Fentanyl-specific product ions and neutral losses used to detect fentanyl-like compounds were determined by mining in-house library data containing 142 fentanyl analogs. This approach was then applied to compounds not in the original training set and was able to detect 95.9% of those fentanyl analogs. Additionally, this approach was applied to a simulated exposure scenario and detected fentanyl analogs and metabolites in both urine and solvent extract. This method utilizes a data dependent acquisition approach where the diagnostic ions are used to screen the data during data processing. Additional diagnostic ions beyond those presented here may slightly improve coverage and sensitivity but may reduce selectivity as additional background interferences may be observed. Although we found during analysis of the training set that there was little benefit to the use of additional diagnostic ions for the detection of fentanyl analogs, the flexibility inherent in this process allows the efficient addition and evaluation of new diagnostic ions through retrospective data analysis, which could be used to screen for additional compound classes.
ACKNOWLEDGMENT
The authors would like to thank Craig Seymour for providing thoughtful discussion and technical expertise regarding the structural elucidation of fentanyl analogs. In addition, the authors would like to acknowledge Melissa Carter for her work in the design and development of the FAS Kit and related Emergent Panels as part of the TOM Kits® product line.
Laboratory findings were made possible, in part, by the Centers for Disease Control and Prevention’s design and support of Traceable Opioid Material® Kits. This work was funded through CDC’s National Center for Injury Prevention and Control. The authors would like to especially thank the National Center for Injury Prevention and Control and the many CDC offices that provided support in the areas of contracting, policy, communications, ethics, technology transfer, general counsel, and administrative services.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.O’Donnell J, et al. , Vital Signs: Characteristics of Drug Overdoes Deaths Involving Opioids and Stimulants - 24 States and the District of Columbia January-June 2019. Morbidity and Mortality Weekly Report, 2020. 69(35): p. 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mojica MA, et al. , Designing traceable opioid material( section sign) kits to improve laboratory testing during the U.S. opioid overdose crisis. Toxicol Lett, 2019. 317: p. 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DEA, 2019 Emerging Threat Report. 2019. [Google Scholar]
- 4.Vardanyan RS and Hruby VJ, Fentanyl-related compounds and derivatives: current status and future prospects for pharmaceutical applications. Future Med Chem, 2014. 6(4): p. 385–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DEA, Schedules of Controlled Substances: Temporary Placement of Fentanyl-Related Substances in Schedule I, Justice D.o., Editor. 2018. [Google Scholar]
- 6.Fogarty MF, Papsun DM, and Logan BK, Analysis of Fentanyl and 18 Novel Fentanyl Analogs and Metabolites by LC-MS-MS, and report of Fatalities Associated with Methoxyacetylfentanyl and Cyclopropylfentanyl. J Anal Toxicol, 2018. 42(9): p. 592–604. [DOI] [PubMed] [Google Scholar]
- 7.Busardo FP, et al. , Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry Assay for Quantifying Fentanyl and 22 Analogs and Metabolites in Whole Blood, Urine, and Hair. Front Chem, 2019. 7: p. 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strayer KE, et al. , LC-MS/MS-Based Method for the Multiplex Detection of 24 Fentanyl Analogues and Metabolites in Whole Blood at Sub ng mL(−1) Concentrations. ACS Omega, 2018. 3(1): p. 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noble C, et al. , Application of a screening method for fentanyl and its analogues using UHPLC-QTOF-MS with data-independent acquisition (DIA) in MS(E) mode and retrospective analysis of authentic forensic blood samples. Drug Test Anal, 2018. 10(4): p. 651–662. [DOI] [PubMed] [Google Scholar]
- 10.Palmquist KB and Swortwood MJ, Data-independent screening method for 14 fentanyl analogs in whole blood and oral fluid using LC-QTOF-MS. Forensic Sci Int, 2019. 297: p. 189–197. [DOI] [PubMed] [Google Scholar]
- 11.Krajewski LC, et al. , Application of the fentanyl analog screening kit toward the identification of emerging synthetic opioids in human plasma and urine by LC-QTOF. Toxicol Lett, 2020. 320: p. 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.m/z Cloud Advanced Mass Spectral Database. June/3/2021]; Available from: https://www.mzcloud.org/.
- 13.High-Resolution Mass Spectral Libraries for Opioid Analysis. February/24/2021]; Available from: https://www.cdc.gov/nceh/dls/erb_hrms_libraries.html.
- 14.Grabenauer M, et al. , Analysis of synthetic cannabinoids using high-resolution mass spectrometry and mass defect filtering: implications for nontargeted screening of designer drugs. Anal Chem, 2012. 84(13): p. 5574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasin D, et al. , Current applications of high-resolution mass spectrometry for the analysis of new psychoactive substances: a critical review. Anal Bioanal Chem, 2017. 409(25): p. 5821–5836. [DOI] [PubMed] [Google Scholar]
- 16.Montesano C, et al. , Screening of methylenedioxyamphetamine- and piperazine-derived designer drugs in urine by LC-MS/MS using neutral loss and precursor ion scan. Journal of Mass Spectrometry, 2013. 48(1): p. 49–59. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, et al. , Neutral Loss Scan - Based Strategy for Integrated Identification of Amorfrutin Derivatives, New Peroxisome Proliferator-Activated Receptor Gamma Agonists, from Amorpha Fruticosa by UPLC-QqQ-MS/MS and UPLC-Q-TOF-MS. J Am Soc Mass Spectrom, 2018. 29(4): p. 685–693. [DOI] [PubMed] [Google Scholar]
- 18.Ren D, et al. , Integrated strategy for identifying minor components in complex samples combining mass defect, diagnostic ions and neutral loss information based on ultra-performance liquid chromatography-high resolution mass spectrometry platform: Folium Artemisiae Argyi as a case study. J Chromatogr A, 2018. 1550: p. 35–44. [DOI] [PubMed] [Google Scholar]
- 19.Klingberg J, et al. , Collision-Induced Dissociation Studies of Synthetic Opioids for Non-targeted Analysis. Front Chem, 2019. 7: p. 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fentanyl Analog Screening (FAS) Kit. 2-25-21]; Available from: https://www.caymanchem.com/forensics/faskit/.
- 21.Nan Q, et al. , Investigation of Fragmentation Pathways of Fentanyl Analogues and Novel Synthetic Opioids by Electron Ionization High-Resolution Mass Spectrometry and Electrospray Ionization High-Resolution Tandem Mass Spectrometry. Journal of the American Society for Mass Spectrometry, 2020. 31(2): p. 277–291. [DOI] [PubMed] [Google Scholar]
- 22.Butler DC, et al. , Three Cases of Fatal Acrylfentanyl Toxicity in the United States and a Review of Literature. J Anal Toxicol, 2018. 42(1): p. e6–e11. [DOI] [PubMed] [Google Scholar]
- 23.Henderson GL, Fentanyl-Related Deaths Demographics, Circumstances, and Toxicology of 112 Cases. Journal of Forensic Sciences, 1991. 36: p. 422–433. [PubMed] [Google Scholar]
- 24.Martucci HFH, et al. , Distribution of furanyl fentanyl and 4-ANPP in an accidental acute death: A case report. Forensic Sci Int, 2018. 283: p. e13–e17. [DOI] [PubMed] [Google Scholar]
- 25.Mochizuki A, et al. , Postmortem distribution of mepirapim and acetyl fentanyl in biological fluid and solid tissue specimens measured by the standard addition method. Forensic Toxicol, 2019. 37(1): p. 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanks KG and Behonick GS, Detection of Carfentanil by LC–MS-MS and Reports of Associated Fatalities in the USA. Journal of Analytical Toxicology, 2017. 41(6): p. 466–472. [DOI] [PubMed] [Google Scholar]
- 27.Sofalvi S, et al. , An LC-MS-MS Method for the Analysis of Carfentanil, 3-Methylfentanyl, 2-Furanyl Fentanyl, Acetyl Fentanyl, Fentanyl and Norfentanyl in Postmortem and Impaired-Driving Cases. J Anal Toxicol, 2017. 41(6): p. 473–483. [DOI] [PubMed] [Google Scholar]
- 28.Swanson DM, et al. , Fatalities Involving Carfentanil and Furanyl Fentanyl: Two Case Reports. J Anal Toxicol, 2017. 41(6): p. 498–502. [DOI] [PubMed] [Google Scholar]
- 29.Riches JR, et al. , Analysis of clothing and urine from Moscow theatre siege casualties reveals carfentanil and remifentanil use. J Anal Toxicol, 2012. 36(9): p. 647–56. [DOI] [PubMed] [Google Scholar]


