Summary
Drought is a deleterious abiotic stress factor that constrains crop growth and development. Post‐translational modification of proteins mediated by the ubiquitin–proteasome system is an effective strategy for directing plant responses to stress, but the regulatory mechanisms in wheat remain unclear. In this study, we showed that TaSDIR1‐4A is a positive modulator of the drought response. Overexpression of TaSDIR1‐4A increased the hypersensitivity of stomata, root length and endogenous abscisic acid (ABA) content under drought conditions. TaSDIR1‐4A encodes a C3H2C3‐type RING finger protein with E3 ligase activity. Amino acid mutation in its conserved domain led to loss of activity and altered the subcellular localization. The membrane‐bound transcription factor TaWRKY29 was identified by yeast two‐hybrid screening, and it was confirmed as interacting with TaSDIR1‐4A both in vivo and in vitro. TaSDIR1‐4A mediated the polyubiquitination and proteolysis of the C‐terminal amino acid of TaWRKY29, and its translocation from the plasma membrane to the nucleus. Activated TaWRKY29 bound to the TaABI5 promoter to stimulate its expression, thereby positively regulating the ABA signalling pathway and drought response. Our findings demonstrate the positive role of TaSDIR1‐4A in drought tolerance and provide new insights into the involvement of UPS in the wheat stress response.
Keywords: wheat, drought resistance, E3 ubiquitin ligase, membrane‐bound WRKY transcription factor, TaSDIR1‐4A
Introduction
Wheat (Triticum aestivum L.) is one of the major food crops grown throughout the world, and its yield is closely related to global food security (Mao et al., 2022; Zhu, 2016). Wheat grows mainly in arid and semi‐arid areas, and thus drought is the main abiotic factor that limits wheat production (Lesk et al., 2016; Ma et al., 2023; Zhu, 2016). Therefore, elucidating the mechanism associated with the drought stress response is highly significant for improving drought tolerance in wheat and ensuring global food security.
The resistance of plants to abiotic stresses depends greatly on proteomic plasticity (Xu and Xue, 2019). The post‐translational modification of proteins mediated by the ubiquitin–proteasome system (UPS) is a rapid and effective strategy for sensing and responding to environmental stresses (Santner and Estelle, 2010; Smalle and Vierstra, 2004). The UPS is responsible for the selective breakdown of most intracellular proteins (Gagne et al., 2004). Single or multiple ubiquitin molecules are covalently attached to the target protein and the tagged substrate protein is then recognized and degraded by the 26S proteasome (Gagne et al., 2004; Qin et al., 2008; Stone et al., 2005). This process involves catalysis by three enzymes comprising E1 (ubiquitin‐activating enzyme), E2 (ubiquitin‐conjugating enzyme) and E3 (ubiquitin ligase) (Moon et al., 2004; Qin et al., 2008; Smalle and Vierstra, 2004). The diversity of E3 ubiquitin ligase confers specificity to the substrate protein selected, which plays a key role in the ubiquitination process (He et al., 2023; Stone et al., 2005; Xu and Xue, 2019; Yu et al., 2013). AtPUB1 acts as an E3 ubiquitin ligase and degrades the receptor‐like protein kinases LRR1 and KIN7, thereby negatively regulating the response of Arabidopsis thaliana to drought stress (Chen et al., 2021). OsRINGzf1 encodes E3 ubiquitin ligase and promotes the degradation of aquaporin OsPIP2; 1 in a UPS‐dependent manner to reduce the loss of water and positively regulate drought resistance in rice (Chen et al., 2022). E3 ubiquitin ligase PRL1 promotes the degradation of MYB43 to weaken the inhibition of CBF gene expression and the antagonism of ICE1 by MYB43, thereby actively regulating the tolerance of cold in Arabidopsis (Zheng et al., 2023).
E3 ligases are classified into the following four types according to the mechanism of action and subunit: U‐box, Really Interesting New Gene (RING), Cullin (Cul)‐RING ligases and Homologous to the E6‐AP Carboxyl Terminus (HECT) (Vierstra, 2009). Many studies have shown that RING E3 ligases play important roles in responses to abiotic stress environments via different mechanisms, such as signal transduction, hormone sensing and transcriptional factors (Chapagain et al., 2018; Cho et al., 2011; Liu and Stone, 2010; Lyzenga and Stone, 2012; Shu and Yang, 2017). SDIR1 is a salt and drought‐induced gene that encodes a C3H2C3‐type RING finger protein with E3 ligase activity (Zhang et al., 2007). AtSDIR1 negatively regulates the stability of SDIRIP1 through the 26S proteasome pathway, thereby regulating abscisic acid (ABA) signalling and the salt stress response (Zhang et al., 2015). Overexpression of TaSDIR1 reduces the accumulation of reactive oxygen species and restricts the inward flow of Na+ to maintain a high K+/Na+ ratio in vivo, which positively regulates the tolerance of salt in wheat (Chen et al., 2023). TaSDIR1‐4A is a negative regulator of grain size (Wang et al., 2020). However, the mechanism that allows TaSDIR1 to regulate the response to drought stress in wheat has not been reported.
Membrane‐bound transcription factors (MTFs) are a class of transcription factors located on membranes in a dormant state (Hoppe et al., 2001; Kim et al., 2007a). MTFs are proteolytically processed and then transported to the nucleus, where they perform transcriptional regulatory functions, thereby adapting to adverse environmental changes (Hoppe et al., 2001; Kim et al., 2007b; Liu et al., 2018; Seo et al., 2008). AtbZIP17 is a MTF that is proteolytically processed under salt stress dependent on AtS1P, and the cleaved N‐terminal component translocates into the nucleus. Activated AtbZIP17 induces the expression of salt stress response genes and enhances the tolerance of salt by Arabidopsis seedlings (Liu et al., 2007, 2008). Under heat stress and endoplasmic reticulum stress, OsNTL3 protein migrates from the plasma membrane to the nucleus to regulate the expression of OsbZIP74 and other genes involved in the unfolded protein response (Liu et al., 2020). However, the molecular mechanisms associated with the translocation and activation of WRKY family MTFs in plant stress responses have not been reported.
In the present study, we showed that RING E3 ubiquitin ligase TaSDIR1‐4A specifically mediates the activation and translocation of membrane‐bound WRKY transcription factor TaWRKY29 via the 26S proteasome degradation pathway. The activated TaWRKY29 protein binds to the TaABI5 promoter to regulate its transcription, thereby enhancing drought resistance in wheat.
Results
Characterization of TaSDIR1‐4A and expression patterns
In this study, we obtained the gene sequence of TaSDIR1 from hexaploid Chinese spring wheat by homologous cloning using the Arabidopsis thaliana sequence for AtSDIR1. Comparison of the cDNA and genome alignment of the amplified gene showed that TaSDIR1 had eight exons and seven introns (Figure S1a). In addition, comparisons with the wheat database showed that TaSDIR1 was located on chromosome 4A. We utilized genomic DNA from Chinese Spring nullisomic 4A‐tetrasomic 4B (N4A‐T4B), nullisomic 4B‐tetrasomic 4D (N4B‐T4D) and nullisomic 4D‐tetrasomic 4B (N4D‐T4B) lines to locate TaSDIR1 on chromosome 4A (Figure S1b). Evolutionary analysis indicated that TaSDIR1‐4A has a close relationship with the homologous genes in rice (Figure S1c). Tissue‐specific expression analysis indicated that the highest expression levels of TaSDIR‐4A were found in the spike, followed by leaf, stem and root tissues (Figure 1a). qRT‐PCR assays indicated that the TaSDIR1‐4A expression levels were significantly higher under treatments with NaCl, PEG6000 and ABA (Figure 1b–d). However, the expression levels of TaSDIR1‐4A were reduced under cold treatment and heat treatment (Figure S2a,b).
Figure 1.
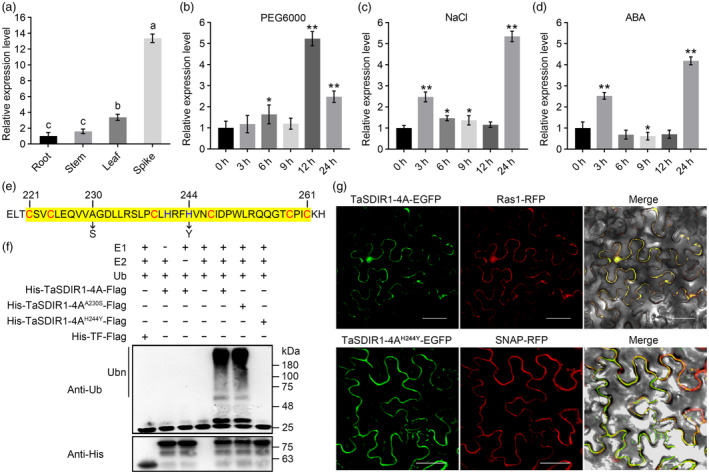
Expression patterns and ubiquitin ligase activity of TaSDIR1‐4A. (a) Expression patterns of TaSDIR1‐4A in different tissues. Data represent the mean ± SD based on three independent replicates. Values with nonidentical letters are significantly different (P < 0.05; Student's t‐test). (b–d) Expression patterns of the TaSDIR1‐4A when exposed to 15% PEG6000, 200 mm NaCl and 0.2 μm ABA, respectively. Leaves were collected at different time points, as indicated. The expression levels were normalized to 0 h for TaSDIR1‐4A. Actin was used as the internal control. Data represent the mean ± SD based on three independent replicates. Significant differences were determined using the Student's t‐test: **P < 0.01; *P < 0.05. (e) Schematic diagram showing the mutation sites in the TaSDIR1‐4A C3H2C3‐type RING domain. The H244Y mutation affected the biological function whereas the A230S mutation did not affect the biological function. (f) E3 ubiquitin ligase activity assays for TaSDIR1‐4A and TaSDIR1‐4AH244Y. Immunological detection of the E3 ubiquitin ligase activity was confirmed using anti‐ubiquitin and anti‐His antibodies. (g) Subcellular localization of TaSDIR1‐4A and TaSDIR1‐4AH244Y in tobacco epidermis cells. Ras1‐RFP was used as a marker of nuclear and plasma membrane localization. SNAP‐RFP was used as a marker of plasma membrane localization. Bar = 50 μm.
TaSDIR1‐4A has an E3 ubiquitin ligase function and the conserved RING domain is essential for its activity
Previous studies have shown that TaSDIR1‐4A encodes a C3H2C3 RING protein with E3 ligase activity (Wang et al., 2020). We also found that TaSDIR1‐4AH244Y and the control had no E3 ligase activity, whereas TaSDIR1‐4AA230S and TaSDIR1‐4A had E3 ligase activities (Figure 1e,f). Interestingly, we also found that TaSDIR1‐4A and TaSDIR1‐4AH244Y had distinct subcellular localizations. TaSDIR1‐4A was localized in the plasma membrane and nucleus, whereas TaSDIR1‐4AH244Y was only localized on the plasma membrane (Figure 1g). These results indicate that TaSDIR1‐4A has an E3 ligase activity, and the conserved domain is essential for its activity and localization.
TaSDIR1‐4A confers drought tolerance in wheat
We overexpressed TaSDIR1‐4A driven by an ubiquitin promoter in the wheat cultivar Fielder to investigate drought tolerance. The three T3 homozygous transgenic lines that we obtained were used for subsequent functional verification (Figure S3). Under normal growth conditions, there were no significant differences between the transgenic lines and WT plants. However, under drought stress treatment, the transgenic lines continued to grow whereas the WT was relatively wilted (Figure 2a). The survival rates of the transgenic lines after rewatering were higher than those of the WT plants (Figure 2b), thereby demonstrating that TaSDIR1‐4A had a positive regulatory effect on drought resistance in wheat. Our results also indicated that the stomatal aperture was smaller in the transgenic TaSDIR1‐4A wheat lines than the WT plants after drought treatment (Figure 2c,d), and thus the water loss rate was lower in the transgenic lines than the WT plants (Figure 2e). Moreover, the ABA contents were higher in the transgenic lines than the WT plants under drought treatment (Figure 2f). We also treated the seedlings of TaSDIR1‐4A overexpressing transgenic plants with 15% PEG6000 to induce drought stress. Compared with the WT plants, the root length was longer in the transgenic TaSDIR1‐4A lines under PEG6000 treatment, but there were no significant differences under normal conditions (Figure 2g,h).
Figure 2.

TaSDIR1‐4A enhanced drought resistance in wheat. (a) Phenotypes of wild‐type (WT) and overexpression lines before and after drought treatment. (b) Survival rates of plants after rewatering. Representative stomatal images (c) and stomatal aperture (d) for WT and overexpression lines after drought treatment. Bar = 50 μm. Data represent the mean ± SD based on three independent replicates. 10 stomata were observed per replicate. Significant differences were determined using the Student's t‐test: **P < 0.01; *P < 0.05. (e) Water loss rate from leaves detached from WT and overexpression lines at different times. (f) Endogenous ABA contents of leaves after drought treatment. (g) Seed root length phenotypes in WT and overexpression lines under normal conditions and after treatment with 15% PEG6000 for 7 days. Bar = 2 cm. (h) Root lengths calculated with and without 15% PEG6000 treatment. Data represent the mean ± SD based on three independent replicates. 30 seeds were tested per replicate. Significant differences were determined using Student's t‐test: **P < 0.01; *P < 0.05. (i) Phenotypes of TaSDIR1‐4A knockdown plants and empty vector control after drought treatment for about 15 days. (j) Wheat survival rates after rewatering. Data represent the mean ± SD based on three independent replicates. 20 plants were tested per replicate. Significant differences were determined using Student's t‐test: **P < 0.01; *P < 0.05.
VIGS was used to examine the role of TaSDIR1‐4A in the wheat cultivar Fielder in the seedling stage. 14 days after inoculation with BSMV‐mediated VIGS, green algae mottled mosaic symptoms appeared on the newly emerged leaves (Figure S4a). The leaf tissues were harvested from the silenced BSMV:TaSDIR1‐4A lines and qRT‐PCR analysis showed that TaSDIR1‐4A expression was significantly reduced by 60%–70% compared with the BSMV:00 lines (Figure S4b). Under drought conditions, the silenced plants had severely wilted leaves and a lower survival rate compared with the WT plants (Figure 2i,j). These results demonstrate that TaSDIR1‐4A had a positive regulatory role in drought resistance in wheat.
TaSDIR1‐4A regulates the expression of ABA signalling‐related genes under drought treatment
We identified three ABA‐responsive transcription factor (ABF) genes regulated by TaSDIR1‐4A after comparing Arabidopsis ABF genes with wheat genome databases. Further evolutionary analysis showed that TaABF1 and TaABF2 were closely related to Arabidopsis AtABF1/AtABF2, whereas TaABI5 was closely related to AtABI5 (Figure 3a). We selected these ABA signalling‐related genes for expression analysis. Without drought treatment, the expression levels of these genes were not significantly different in the TaSDIR1‐4A overexpressing lines and WT plants. However, the expression levels of these genes were higher in the transgenic plants than WT plants under drought treatment (Figure 3b–d). These results indicate that TaSDIR1‐4A enhanced drought resistance in plants by regulating ABA signalling‐related genes.
Figure 3.

Expression of ABA signalling pathway‐related genes in wild‐type (WT) and overexpression lines. (a) Evolutionary analysis of ABA signalling pathway‐related genes in wheat and ABF family genes in Arabidopsis thaliana. AtABF1 (AT1G49720.2), AtABF2 (AT1G45249.3), AtABF3 (AT4G34000.4), AtABF4 (AT3G19290.3), AtABI5 (AT2G36270.2), TaABF1 (TraesCS5A02G237200.2), TaABF2 (TraesCS7A02G170600.1) and TaABI5 (TraesCS6D02G312800.1). The cladogram was constructed using MEGA 6.0 software. (b–d) Expression levels of TaABF1, TaABF2 and TaABI5 in WT and overexpression lines under normal and drought conditions. Data represent the mean ± SD based on three independent replicates. Significant differences were determined using Student's t‐test: **P < 0.01; *P < 0.05.
TaSDIR1‐4A interacts with TaWRKY29 in vitro and in vivo
To identify the protein that interacted with TaSDIR1‐4A, we obtained a WRKY transcription factor by screening the yeast library, which was designated as TaWRKY29 (GenBank: OK065946.1) based on its evolutionary relationship with the Arabidopsis WRKY family (Figure S5). We found that TaSDRI1‐4A could interact with TaWRKY29 in Y2H assays (Figure 4a,b). Pull‐down experiments further proved that His‐TaSDIR1‐4A‐Flag, but not His‐TF‐Flag, interacted with TaWRKY29 in vitro (Figure 4c).
Figure 4.

TaSDIR1‐4A interacts with TaWRKY29. (a) Amino acid structures of TaSDIR1‐4A and TaSDIR1‐4A‐∆TM (without the membrane domain containing an 82‐amino acid deletion in the N‐terminus). TM, transmembrane domain. (b) TaSDIR1‐4A‐∆TM interacts with TaWRKY29 in yeast. BD and AD indicate the PGBKT7 bait vector and PGADT7 prey vector, respectively. BD/TaSDIR1‐4A‐∆TM, TaSDIR1‐4A‐∆TM fused to PGBKT7 as the bait. AD/TaWRKY29, TaWRKY29 fused to PGADT7 as the prey. SD/−L‐T: synthetic dropout medium lacking leucine and tryptophan. SD/−L‐T‐H‐A: synthetic dropout medium lacking leucine, tryptophan, histidine and adenine. (c) In vitro pull‐down detection of TaSDIR1‐4A and TaWRKY29. Purified His‐TsSDIR1‐4A‐Flag protein was incubated with TaWRKY29‐Myc. His‐TF‐Flag protein was used as a negative control. The samples were subsequently detected with anti‐His and anti‐Myc antibodies. (d) Bimolecular fluorescence complementation assay of the interaction between TaSDIR1‐4A and TaWRKY29 in tobacco leaves. Bar = 50 μm. (e) Co‐immunoprecipitation assay conducted to detect the interaction between TaSDIR1‐4A and TaWRKY29 in vivo. Flag‐TaSDIR1‐4A and HA‐TaWRKY29‐V5 were simultaneously injected into tobacco leaves with P19, before analysis using anti‐HA and anti‐Flag antibodies. 1302‐6 × Flag was used as the negative control. The horizontal line represents the full‐length TaWRKY29 form, and the asterisk represents the cleaved TaWRKY29 form.
In addition, we used tobacco leaves in bimolecular fluorescence complementation experiments to show that TaWRKY29 could interact with TaSDIR1‐4A and TaSDIR1‐4AH244Y on the cell membrane (Figure 4d). In co‐immunoprecipitation assays, HA‐TaWRKY29‐V5 was specifically detected but the control combination could not be detected by using anti‐HA antibody (Figure 4e). These results demonstrate that TaSDIR1‐4A and TaWRKY29 could interact in vivo and in vitro.
TaWRKY29 is a membrane‐bound transcription factor that can be specifically activated to localize to the nucleus by TaSDIR1‐4A
Subcellular localization analysis showed that TaWRKY29 localized to the plasma membrane (Figure 5b). Analysis of the protein structure of TaWRKY29 showed that TaWRKY29 had strong hydrophobic domains at its N‐terminus and C‐terminus, so we constructed different gene fragments to determine the location of the membrane signal (Figure 5a). Interestingly, we observed that the fragments of TaWRKY29 with truncated N‐terminal hydrophobic domain (TaWRKY29‐ΔN1 and TaWRKY29‐ΔN2), were also expressed on the plasma membrane. However, when the C‐terminal hydrophobic domain of TaWRKY29 was truncated (TaWRKY29‐ΔC1 and TaWRKY29‐ΔC2), the fluorescence signal was transferred from the plasma membrane to the nucleus. Therefore, it can be inferred that lacking of the C‐terminal hydrophobic domain of TaWRKY29 lead to translocation from the plasma membrane to the nucleus (Figure 5b). In addition, injection of ABA strongly promoted TaWRKY29 to enter the nucleus (Figure 5c), thereby demonstrating that TaWRKY29 protein can respond to ABA treatment.
Figure 5.

TaSDIR1‐4A mediates the nuclear translocation of membrane‐bound transcription factor TaWRKY29. (a) Schematic illustration of structures of the full‐length TaWRKY29 and its different truncated fragments. WRKY, conserved WRKY domain. HD, hydrophobic domain. (b) Subcellular localizations of full‐length TaWRKY29 and its different truncated fragments in N. benthamiana leaves, Bar = 50 μm. (c) N. benthamiana leaves expressing EGFP‐TaWRKY29 fusion protein were treated with 150 μm ABA for 6 h. The EGFP signal pattern was detected by confocal imaging at 0 and 6 h after ABA treatment. SNAP‐RFP and HY5‐RFP were used as plasma membrane‐localized and nucleus‐localized markers, respectively. Bar = 50 μm. (d) Co‐localization of EGFP‐TaWRKY29 and RFP, and TaSDIR1‐4A‐RFP and TaSDIR1‐4AH244Y‐RFP. Bar = 50 μm.
When 35S:EGFP‐TaWRKY29 and 35S:TaSDIR1‐4A‐RFP were co‐injected into tobacco, the EGFP‐TaWRKY29 fluorescent signal was observed in the nucleus, but the combination with 35S:RFP and 35S:TaSDIR1‐4AH244Y‐RFP only produced a fluorescent signal on the plasma membrane (Figure 5d). These results suggest that TaSDIR1‐4A specifically mediates the cleavage and nuclear translocation of TaWRKY29.
TaSDIR1‐4A degrades TaWRKY29 to obtain the cleaved nuclear fraction via the 26S proteasome pathway
We conducted in vitro ubiquitination assays in order to show that TaWRKY29 may be one of the target proteins of TaSDIR1‐4A. The results showed that TaWRKY29 could be specifically ubiquitinated in the presence of E1, E2, ubiquitin and TaSDIR1‐4A, whereas TaWRKY29‐ΔC2 could not be ubiquitinated (Figure 6a).
Figure 6.

TaSDIR1‐4A degrades TaWRKY29 to obtain the cleaved nuclear form via the 26S proteasome pathway. (a) TaSDIR1‐4A ubiquitinated TaWRKY29 in vitro. The ubiquitination of TaWRKY29 was detected with anti‐Myc antibody. Ubiquitinated proteins were immunoblotted with anti‐ubiquitin antibody. (b) TaSDIR1‐4A promoted TaWRKY29 degradation to form the cleaved fraction. Flag‐TaSDIR1‐4A and HA‐TaWRKY29‐V5 were injected into tobacco leaves with P19, before the mixed proteins in equal proportions was detected with or without the protease inhibitor MG132 by using anti‐HA, anti‐Flag and anti‐V5 antibodies. Actin was used as a control of ensure consistent sample loading. The asterisk represents the cleaved TaWRKY29 form. (c) TaSDIR1‐4A induces TaWRKY29 to enter the nucleus to form the cleaved fraction. Cellular fractionation and immunoblot analysis of co‐injection of Flag‐TaSDIR1‐4A and HA‐TaWRKY29‐V5 Agrobacterium into N. benthamiana leaves. The total and membrane proteins and nuclear proteins were separated and determined by immunoblot analysis. T, total protein; M, membrane protein; N, nuclear protein. H+‐ATPase, Histone H3 and cFBPase were used as plasma membrane, nuclear and cytosolic fraction markers, respectively. The horizontal line represents the full‐length TaWRKY29 form and the asterisk represents the cleaved TaWRKY29 form.
To investigate whether TaSDIR1‐4A mediates the degradation of TaWRKY29, we mixed TaWKRY29 protein with TaSDIR1‐4A expressed in tobacco leaves. The results showed that the abundance of the TaWKRY29 protein gradually decreased and a lower molecular weight protein fragment was produced. Moreover, protein degradation was effectively blocked by the 26S proteasome inhibitor MG132, thereby indicating that TaWKRY29 is degraded via the 26S proteasome pathway (Figure 6b). In cell separation experiments, we extracted nuclear proteins for immunoblotting using anti‐HA antibody and a smaller nuclear fragment was detected (Figure 6c). These results suggest that TaSDIR1‐4A degrades TaWRKY29 via the 26S proteasome pathway to obtain the cleaved nuclear fraction.
Activated TaWRKY29 interacts directly with the promoter of TaABI5
WRKY transcription factors regulate their target genes by binding to the W‐box in the promoter (Rushton et al., 2010). Thus, we investigated the relationship between TaABI5 and TaWRKY29. We found that the activated TaWRKY29 could bind to the promoter region of TaABI5 according to Y1H assays (Figure 7a). In addition, in vitro EMSA showed that the activated TaWRKY29 could specifically bind to the W‐box region of TaABI5 (Figure 7b).
Figure 7.

TaWRKY29 promotes the expression of TaABI5 by TaSDIR1‐4A. (a) Prey and bait vectors used for the yeast one‐hybrid assay. SD/−Ura/−Leu, synthetically defined medium (SD) lacking uracil (Ura) and leucine (Leu). AbA, aureobasidin A. (b) EMSA using purified His‐TaWRKY29‐ΔC2 protein incubated with biotin‐labelled probes for TaABI5 promoter fragment. ‘−’, absence; ‘+’, presence. ‘50×’ and ‘100×’ indicate that the molarity of the unlabelled probe was 50‐fold and 100‐fold higher than that of the biotin probe. Cold‐Probe indicates the unlabelled probe. Cold‐mProbe indicates the unlabelled mutant probe. All probe sequences used for EMSA are listed in Table S1. (c) Schematic structures of the effector (p1302‐WRKY29), reporter vector (mini35S‐GUS and W‐box‐mini35S‐GUS) and accelerator vector (p1302‐TaSDIR1‐4A) used for transient expression analysis. (d) GUS staining and activity analysis in Nicotiana benthamiana leaves. Empty represents the pCAMBIA1302 vector. (e) Transient expression assay of the promoter activity using N. benthamiana leaves co‐transformed with the effector (35S:TaWRKY29), reporter (35S:REN‐promoter:LUC), and accelerator (35S:TaSDIR1‐4A). Data represent the mean ± SD based on three independent replicates. Values with nonidentical letters are significantly different (P < 0.05; one‐way ANOVA).
GUS staining experiments were conducted to demonstrate that TaWRKY29 could regulate the expression of TaABI5 under the action of TaSDIR1‐4A. The results showed that the darkest blue GUS staining occurred with the combination of TaWRKY29 and TaSDIR‐4A compared with the other combinations and their respective individual controls (Figure 7c,d). At the same time, The GUS activities were highest in Nicotiana benthamiana leaves that co‐expressed the TaSDIR1‐4A and TaWRKY29 (Figure 7d). Furthermore, D‐luciferase assays showed that the combination of TaWRKY29 and TaSDIR‐4A obtained the highest relative luciferase activities (Figure 7e). These findings indicate that TaSDIR1‐4A can promote the effect of activated TaWRKY29 on upregulating TaABI5 gene expression.
Activated TaWRKY29 positively regulates plant drought resistance
To further explore the biological function of TaWRKY29 in response to drought stress, we produced TaWRKY29 and TaWRKY29‐ΔC2 overexpression lines in Arabidopsis (Figure S6a). Under normal growth conditions, there were no significant differences between the transgenic lines and WT plants. Under drought conditions, the biological phenotypes of the TaWRKY29 transgenic overexpression plants in Arabidopsis were similar to those of WT plants. However, the TaWRKY29‐ΔC2 overexpression plants were a significantly stronger green colour and had higher survival rates compared with the WT plants (Figure S6b,c). These results indicate that activated TaWRKY29 has a positive regulatory role in plant drought resistance.
Discussion
It is well known that gene expression regulation and post‐translational modification play key roles in the evolutionary adaptation of multicellular organisms to environmental changes. The UPS is a highly conserved regulatory mechanism for intracellular protein degradation, and E3 is a key component in the ubiquitination pathway (Lyzenga and Stone, 2012). RING‐type E3 ubiquitin ligase is characterized by a cysteine‐ and histidine‐rich domain that can form two symplectic domains. RING‐HC (C3HC4 type) and RING‐H2 (C3H2C3 type) are two relatively common RING domain proteins. Previous studies have demonstrated that RING‐type E3 is involved in the regulation of plant responses to abiotic stresses (Baek et al., 2021; Joo et al., 2020; Li et al., 2020; Yu et al., 2021). AtATL78 encoded RING‐H2 type E3 ubiquitin ligase. The expression of AtATL78 was upregulated by cold stress and down‐regulated by drought stress, thus playing opposite functions in cold and drought stress response (Kim and Kim, 2013). AtSDIR1 was upregulated by drought and salt stress, but not by ABA, and positively regulates stress‐responsive ABA signalling in Arabidopsis (Zhang et al., 2007). OsSDIR1 actively responded to and participated in drought regulation, but its expression was inhibited by cold stress (Gao et al., 2011). In this study, the expression of TaSDIR1‐4A was significantly upregulated by PEG6000, NaCl and ABA stress (Figure 1b–d). However, the inhibition of its expression under cold and heat stress may be regulated by other stress‐related factors (Figure S2). We determined that TaSDIR1‐4A had a RING‐H2 (C3H2C3 type) domain (Figure 1e). We also showed that TaSDIR1‐4A had an E3 ubiquitin ligase activity and the conserved RING domain was necessary for its activity and localization (Figure 1f,g).
Plants have developed many defence strategies to cope with adverse environments, including regulating plant hormones and stomatal movement, and inducing drought‐responsive genes (Lee and Luan, 2012; Lim et al., 2007; Zhu, 2002). At the cellular level, the plant hormone ABA is the most important factor for the regulation of stomatal movement (Kollist et al., 2014; Murata et al., 2015; Osakabe et al., 2014; Raghavendra et al., 2010). A previous study showed that AtMYB44‐overexpressing transgenic plants exhibited faster ABA‐induced stomatal closure and slower water loss rates than WT and atmyb44 knockout lines, with enhanced drought resistance in the former (Jung et al., 2008). In addition, the overexpression of HRF1 in rice and PbWRKY53 in tobacco increased the ABA content and promoted stomatal closure to significantly enhance the water retention capacity and drought tolerance (Liu et al., 2019; Zhang et al., 2011). In addition, plants can regulate stress‐related genes to cope with external adverse factors. Genes related to the ABA signalling pathway are usually strongly associated with drought resistance (Kilian et al., 2007; Li et al., 2017; Zhao et al., 2016). Similarly, we found that transgenic TaSDIR1‐4A wheat lines had higher ABA contents, smaller stomatal apertures and slower water loss rates than WT plants under drought conditions, thereby enhancing the water retention capacity of the former (Figure 2a–e). Under drought treatment, qRT‐PCR analysis showed that TaSDIR1‐4A‐overexpressing lines exhibited significantly upregulated expression of genes involved in ABA signalling metabolic pathways, including TaABF1, TaABF2 and TaABI5 (Figure 3a–d). These findings demonstrate that TaSDIR1‐4A had a positive role in mediating drought resistance by regulating the ABA signalling pathway.
Previous studies have shown that most MTFs translocate from the membrane to the nucleus under external or internal stimulation (Liang et al., 2015; Liu et al., 2018). The release of MTFs from membranes is critical for their transcriptional regulatory functions (Che et al., 2010; Kim et al., 2010; Yang et al., 2014). In rice, the nuclear import of MTFs ONAC054 and OsMADS18 requires cleavage of the C‐terminal transmembrane domain (Sakuraba et al., 2020; Yin et al., 2019). AtbZIP60 localizes to the endoplasmic reticulum membrane and undergoes cleavage in response to endoplasmic reticulum stress, and the N‐terminal fragment containing the bZIP structural domain is subsequently translocated to the nucleus (Iwata et al., 2008; Iwata and Koizumi, 2005). Overexpression of the full‐length NTL8 will not produce the expected phenotype whereas plants that overexpress truncated forms without the transmembrane domain exhibit the expected phenotype (Kim et al., 2007a). Overexpression of the truncated form of OsNTL3 without the transmembrane domain increases heat tolerance in rice seedlings (Liu et al., 2020). In the present study, the membrane localization signal of TaWRKY29 was detected in the C‐terminus (Figure 5b). TaWRKY29 produced a nuclear localization signal after ABA treatment (Figure 5c). The E3 ubiquitin ligase TaSDIR1‐4A degraded TaWRKY29 via the 26S proteasome pathway, thereby facilitating the entry of cleaved TaWRKY29 into the nucleus (Figure 6). In addition, the physiological phenotypes of full‐length TaWRKY29 overexpressing plants were not significantly different from WT plants, whereas truncated TaWRKY29 overexpressing Arabidopsis plants exhibited significantly increased drought tolerance (Figure S6).
The activation of MTFs involves regulating the expression of downstream genes (Duan et al., 2017; Liu and Howell, 2010). The WRKY transcription factors regulate gene expression by binding to the W‐box (TTGACC/T) region in the promoters of target genes to participate in different stress responses (Eulgem et al., 2000; Jiang et al., 2012; Ren et al., 2010). TaWRKY2 and TaWRKY19 can directly bind to the RD29B promoter to activate its expression or indirectly induce the expression of downstream stress‐related genes to positively regulate stress tolerance (Niu et al., 2012). AtWRKY18, AtWRKY60 and AtWRKY40 interact with the W‐box in the promoters of the ABI4 and ABI5 genes to together repress their expression, with a negative role in ABA signalling (Liu et al., 2012b). In the present study, we demonstrated the direct and specific interaction between cleaved TaWRKY29 and the promoter of TaABI5 based on Y1H assays and EMSA (Figure 7a,b). In addition, transient expression assays showed that TaWRKY29 could activate and promote the expression of TaABI5 under the action of TaSDIR1‐4A (Figure 7c–e). We also found that activated TaWRKY29 could enhance drought resistance, which may have been mediated by regulating the expression of TaABI5.
In summary, we propose a working model for TaSDIR1‐4A‐mediated drought resistance (Figure 8). We identified TaSDIR1‐4A as a RING‐type E3 ubiquitin ligase. Under drought stress, TaSDIR1‐4A responds rapidly and its expression mediates proteolysis of the MTF TaWRKY29 via the 26S proteasome degradation pathway, which translocates from the plasma membrane to the nucleus. Activated TaWRKY29 regulates the expression of TaABI5 to positively regulate plant drought resistance by regulating ABA‐related signalling pathways.
Figure 8.

Proposed working model for TaSDIR1‐4A mediated drought resistance. Under drought stress, TaSDIR1‐4A responds rapidly and its expression mediates the proteolysis of membrane‐bound transcription factor TaWRKY29 via the 26S proteasome degradation pathway, which translocates from the plasma membrane to the nucleus. Activated TaWRKY29 regulates the expression of TaABI5 by binding to the W‐box region. Finally, TaSDIR1‐4A enhances drought resistance in plants by regulating ABA‐related signalling pathways.
Experimental procedures
Plant materials, growth conditions, and stress treatments
The common hexaploid wheat variety Chinese Spring was grown in Petri dishes at 25 °C under a 16 h:8 h, light: dark cycle. 2‐week‐old seedlings were stressed with 0.2 mm ABA, NaCl (200 mm), 15% PEG6000, cold (4 °C) and heat (42 °C). Leaf tissues were sampled after 0, 3, 6, 9, 12 and 24 h under the different stress treatments. Tissues comprising the root, stem, leaf and spikes were collected at the heading date. Chinese Spring nullisomic 4A‐tetrasomic 4B (N4A‐T4B), nullisomic 4B‐tetrasomic 4D (N4B‐T4D) and nullisomic 4D‐tetrasomic 4B (N4D‐T4B) were used for chromosomal localization.
Arabidopsis thaliana plants were grown under long‐day conditions at 23 °C, 60% relative humidity under a 16 h:8 h, light: dark cycle. Nicotiana benthamiana plants grown under the same conditions as Arabidopsis thaliana were used for agroinfiltration experiments. Wheat cultivar Fielder seedlings were grown in a growth chamber at 25 °C under a 14 h:10 h, light: dark cycle.
Sequence analysis and chromosomal localization of TaSDIR1
The AtSDIR1 protein (At3G55530.1) was used to screen the Triticum aestivum database with TBLASTN in URGI (https://urgi.versailles.inra.fr/blast/). The predicted open reading frame (ORF) was spliced with FGENESH (http://www.softberry.com/). According to the splicing results, the primers TaSDIR‐F and TaSDIR‐R were designed to amplify the ORF fragment of TaSDIR1 from cDNA in leaf samples from Chinese Spring seedlings. Sequence analyses were conducted using DNAMAN. Primary structural analysis was performed using SMART. A phylogenetic tree was constructed with MEGA5.
The chromosome localization of TaSDIR1 was determined using a PCR strategy based on a previously described method (Yang et al., 2012). The TaSDIR1‐specific primer pair was employed to amplify genomic DNA from Chinese Spring nullisomic‐tetrasomic lines.
Quantitative real‐time PCR (qRT‐PCR) analysis
Total RNA was isolated from the samples using TRizol reagent according to the manufacturer's instructions (TaKaRa, Dalian, China). After confirming that the concentration and quality of the RNA samples satisfied the requirements, total RNA was reverse transcribed to synthesize cDNA with SuperScript™ III Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The cDNA templates were used for PCR amplification. qRT‐PCR was conducted with a Light Cycler® 96 instrument (Roche, Switzerland) and FastStart Essential DNA Green Master (Roche, Germany) according to the manufacturer's instructions. The wheat actin gene was used as an internal control. Relative expression levels of detected genes were determined by using the relative 2−ΔΔCt method. The primers employed are listed in Table S1.
Transgenic vector construction and plant transformation
Homologous recombination was employed for vector construction. The ORF regions of TaWRKY29 and TaWRKY29‐ΔC2 were constructed in the pCAMBIA1302‐EGFP vector. The constructs were introduced separately into Agrobacterium GV3101. Arabidopsis transformation was performed using the floral dip method. The constructed 1302‐Ubi‐TaSDIR1‐4A‐EGFP vector was transformed into Agrobacterium EHA105 and then used to transform wheat (variety Fielder) plants with the Agrobacterium tumefaciens‐mediated method.
Phenotypic characteristics of transgenic plants
The transgenic wheat plants and Arabidopsis plants were grown in a pot containing soil with water for 3 weeks, and drought treatment was then applied. The plants were watered again 3 days after the treatment, and the survival rate was determined.
Leaf materials were obtained from the transgenic TaSDIR1‐4A wheat and wild‐type (WT) plants, which were placed on filter paper to measure the moisture lost from the leaves every 1 h while incubating at a constant temperature of 25 °C. Fielder seeds and transgenic TaSDIR1‐4A seeds were treated with 5% NaClO for about 30 min, and then treated overnight with 2% H2O2 at 4 °C. Sterilized seeds were grown in Petri dishes containing filter paper. Root lengths were measured, and photographs were taken after treatment for 7 days with water and 15% PEG6000. 30 seeds were counted in each group and three independent replicates were conducted.
ABA content and stomatal aperture measurement
Three‐week‐old transgenic TaSDIR1‐4A and Fielder wheat seedlings were cultivated in a light incubator and watering was stopped for 1 week. The leaves were then collected to measure the ABA content and stomatal size. Endogenous ABA was extracted from the wheat leaves after drought treatment according to a previously described method (Liu et al., 2012a). Leaf guard cells were observed using a confocal microscope (IX83‐FV1200, Olympus Inc, Tokyo, Japan) with objective lens (UPLSAPO20× NA:0.75). Photographs were taken and the ratio of the stomatal width relative to length was calculated as the stomatal aperture.
Barley stripe mosaic virus‐induced gene silencing (BSMV‐VIGS)
We constructed recombinant vectors by selecting specific target regions in the 3′‐terminal sequence and 3′‐untranslated region of TaSDIR1‐4A and inserting into the BSMV‐γ vector according to a previously described method (Wang et al., 2020). The BSMV‐β genome was linearized with SpeI. Genomes of the BSMV‐α, BSMV‐γ and recombinant BSMV‐γ vectors were linearized using MluI. Vectors were linearized in vitro by reverse transcription using an mMessage mMachine™ T7 transcription kit (AM1344, Invitrogen) according to the manufacturer's instructions. The BSMV transcripts were mixed at a ratio of 1 : 1 : 1, before mixing with 1× FES inoculation buffer and inoculating into wheat stems at the two‐leaf stage. BSMV:PDS was used as a positive control and BSMV:γ as a negative control. At 14 days after viral infection, the leaves were collected for quantitative pattern analysis and photography. Photographs were taken, and survival statistics analysed 10 days after the plants were subjected to drought treatment.
Yeast two‐hybrid (Y2H) assay
We constructed a cDNA library with the prey plasmid PGADT7/AD using tissues from the root, stem, leaves and spikes and different seedling development stages. Deletion of two transmembrane domains of TaSDIR1‐4A fused to the bait PGBKT7/BD was conducted used to generate BD‐TaSDIR1‐4A‐ΔTM for transcription activity and toxicity assays. Yeast libraries were screened according to the manufacturer's instructions (Clontech). The NCBI database was used to sequence and validate proteins that interacted positively with TaSDIR1‐4A. The full‐length coding region of each interacting protein was cloned into AD plasmid and co‐transformed with BD‐TaSDIR1‐4A‐ΔTM into Y2H yeast for further experimental verification on SD/−Trp/−Leu and SD/−Trp/−Leu/‐His/−Ade plates.
E3 ubiquitin ligase activity assay
The ORF of TaSDIR1‐4A was introduced into the pCold‐TF‐QM‐Flag vector and expressed in Escherichia coli BL21(DE3). The expressed fusion protein His‐TaSDIR1‐4A‐Flag was purified according to the manufacturer's instructions (Sigma‐Aldrich). The conserved RING finger domain was mutated using a MutanBEST Kit (TaKaRa) according to the protocol provided by the manufacturer. The mutated primers used for Ala 230 to Ser 230 and for His 244 to Tyr 244 are shown in Table S1. The ubiquitination assays were performed as described previously (Park et al., 2018). The reaction system (30 μL final volume) comprised 1.5 μL 20× reaction buffer, 200 ng human E1 (UBE1), 200 ng human E2 (UBCh5b), 8 μg ubiquitin and 1 μg purified E3 (His‐TaSDIR1‐4A‐Flag). The reaction mixture was incubated at 30 °C for 2 h. Purified His‐TF‐Flag was used as a negative control. The products were separated by 8% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and subjected to Western blot analysis using anti‐ubiquitin (R&D) and anti‐His (Sigma‐Aldrich) antibodies.
Subcellular localization
We inserted the ORFs of TaSDIR1‐4A and TaSDIR1‐4A H244Y into the N‐terminus of EGFP to construct 35S:TaSDIR1‐4A‐EGFP and 35S:TaSDIR1‐4AH244Y‐EGFP, respectively. In addition, we constructed TaWRKY29 and used different truncated fragments from the C‐terminus of EGFP to determine the membrane localization region of TaWRKY29. These constructed vectors were transformed into Agrobacterium GV3101 and injected into the leaves of 4‐week‐old N. benthamiana plants. The fluorescence signal was observed after incubating at 23 °C for 40 h by confocal laser scanning microscopy (LSCM X83‐FV1200, Olympus).
Pull‐down assay
The ORF of TaWRKY29 was constructed in the pCold‐TF‐Myc vector to obtain a translational fusion protein with the Myc tag and then expressed in BL21(DE3). The Myc‐tagged recombinant fusion proteins were purified from bacterial lysates by using His beaded magnetic agarose (Sigma‐Aldrich) to obtain TaWRKY29‐Myc protein. Similarly, the coding region of TaSDIR1‐4A was connected to the pCold‐TF‐QM‐Flag vector to express the His‐tagged recombinant fusion proteins. The expressed His‐TF‐Flag was used as a negative control. The TaWRKY29‐Myc and His‐TaSDIR1‐Flag fusion proteins were co‐incubated at 4 °C for 3 h in pull‐down buffer (40 mm HEPES, 10 mm KCl, 3 mm MgCl2, 0.4 m sucrose, 1 mm EDTA, 1 mm dithiothreitol and 0.2% Triton X‐100) including His beaded magnetic agarose. Separation was conducted with a magnetic frame, before washing eight times with 1× PBS, and the protein obtained was finally boiled. The boiled proteins were separated by 8% SDS‐PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The blot was analysed with Myc antibody and Flag antibody (MBL).
Bimolecular fluorescence complementary assay
The coding regions of TaSDIR1‐4A, TaSDIR1‐4AH244Y and TaWRKY29 were cloned into the pCAMBIA1302‐cEYFP and pCAMBIA1302‐nEYFP vectors to generate TaSDIR1‐4A‐cEYFP, TaSDIR1‐4AH244Y‐cEYFP and TaWRKY29‐nEYFP, respectively. The constructed vector plasmids were transformed into Agrobacterium GV3101 and co‐injected into N. benthamiana leaves as described previously (Xu et al., 2018). The YFP fluorescence signal was observed using laser scanning confocal microscopy (IX83‐FV1200, Olympus).
Co‐immunoprecipitation assay
The coding regions of TaSDIR1‐4A and TaWRKY29 were fused to pCAMBIA1302‐6 × Flag and pCAMBIA1302‐6 × HA‐6 × V5 to generate the fusion vectors 35S:TaSDIR1‐4A‐6 × Flag and 35S:6 × HA‐TaWRKY29‐6 × V5, respectively. The constructed vectors were then transformed into Agrobacterium GV3101 and co‐injected into N. benthamiana leaves. pCAMBIA1302‐6 × Flag was used as a negative control. In order to prevent the degradation of the interacting protein, 100 μM of MG132 protease inhibitor was injected 12 h before sampling. Next, 100 μL of Anti‐HA magnetic agarose (Sigma‐Aldrich) was used to capture the protein complex from the total protein extracted from the treated tobacco leaves. Each prepared protein sample was washed six times with 1 mL of phosphate‐buffered saline and the immune reaction products were then detected by immunoblot analyses using anti‐HA or anti‐Flag antibodies (MBL).
In vitro substrate ubiquitination and degradation assays
The ORFs of TaWRKY29 and TaWRKY29‐ΔC2 were constructed in the pCold‐TF‐Myc vector and expressed in Escherichia coli BL21(DE3). The His‐TaWRKY29‐Myc and His‐TaWRKY29‐ΔC2‐Myc recombinant fusion proteins were purified from bacterial lysates by using His beaded magnetic agarose (Sigma‐Aldrich). The reaction system (final volume of 30 μL) contained 1.5 μL 20× reaction buffer, 200 ng human E1 (UBE1), 200 ng human E2 (UBCh5b), 4 μg ubiquitin, 1 μg purified E3 (His‐TaSDIR1‐4A‐Flag) and 2 μg purified His‐TaWRKY29‐Myc. His‐TF‐Myc and His‐TaWRKY29‐ΔC2‐Myc were used as the negative controls. The reaction mixture was incubated at 30 °C for 3 h. The products were separated by 8% SDS‐PAGE and subjected to Western blot analysis using anti‐ubiquitin and anti‐Myc antibodies.
Flag‐TaSDIR1‐4A and HA‐TaWRKY29‐V5 fused were injected separately into tobacco leaves and the total proteins were then extracted. Equal amounts of the two differently tagged proteins were mixed with the 20× buffer (1 m Tris, pH 7.5, 80 mm MgCl2, 140 mm ATP, 40 mm dithiothreitol) and incubated at 30 °C for 40 min in the presence or absence of 100 μm MG132 (Xiong et al., 2019). The protein samples were separated by 8% SDS‐PAGE and detected by immunoblotting using anti‐Flag, anti‐V5 and anti‐HA antibodies (MBL).
Cell fragment separation experiment
The 35S:TaSDIR1‐4A‐6 × Flag (Flag‐TaSDIR1‐4A) and 35S:6 × HA‐TaWRKY29‐6 × V5 (HA‐TaWRKY29‐V5) constructed vectors were then transformed into Agrobacterium GV3101 and co‐injected into N. benthamiana leaves. Total protein, membrane protein and nuclear protein were extracted separately from leaves at 2 days after injection according to the manufacturer's instructions (BestBio). The extracted proteins were separated by SDS‐PAGE and transferred to PVDF membranes for western blot detection by using anti‐HA, anti‐Flag and anti‐V5 antibodies (MBL). In addition, H+‐ATPase, Histone H3 and cFBPase were used as plasma membrane, nuclear and cytosolic fraction internal reference antibodies (AmyJet Scientific) to detect total protein, membrane protein and nuclear protein extracts, respectively.
Yeast one‐hybrid (Y1H) assays
We constructed W‐box elements in the pAbAi vector, before transformation into yeast Y1H competent cells after digestion. The minimal concentration of AbA was determined and the AD‐TaWRKY29‐ΔC2 recombinant plasmid was translated into Y1H competent cells containing the plasmid described above. The empty AD plasmid was used as a negative control, and the yeast solution was serially diluted and spotted on SD/‐Ura/−Leu plates with or without 400 ng/mL AbA.
Electrophoretic mobility shift assay (EMSA)
The TaWRKY29‐ΔC2 fragment was connected to pCold‐TF‐Myc to obtain a translational fusion protein with a Myc tag. The His‐TF‐Myc and His‐TaWRKY29‐ΔC2‐Myc recombinant fusion proteins were purified from bacterial lysates by using His beaded magnetic agarose (Sigma‐Aldrich). The W‐box core region of TaABI5 was used as a probe for subsequent experiments. All sequences of the probes and mutant probes used in the present study are listed in Table S1. The single‐stranded complementary fragments of the probe were used for synthesis and labelled with biotin at the 5′‐ends (Sangon Biotech). The double‐stranded DNA probe was obtained by PCR amplification and collected as a prepared probe. Gel‐shift assays were conducted using a LightShift Chemiluminescent EMSA Kit (Thermo Fisher Scientific, Waltham, MA). Binding reactions were conducted for 30 min at room temperature and the reaction products were transferred to nylon membranes after electrophoresis. The membranes were subjected to cross‐linking treatment with ultraviolet light. The biotinylated DNA was detected by chemiluminescence.
β‐Glucuronidase (GUS) staining and promoter‐luciferase assay
The PCR product of W‐box‐minimal‐100 CaMV 35S (m35S) was obtained from PBI121 using the adapter primer. The product was constructed with the PBI121 vector where the 35S promoter had been removed. The recombinant plasmid vector was transformed into Agrobacterium GV3101 for use as the reporter gene. The TaWRKY29 overexpression construct was used as the effector gene. In addition, we used the TaSDIR1‐4A overexpression construct as an accelerator to explore the binding effect of TaWRKY29 and the promoter of TaABI5. A. tumefaciens‐mediated transformation of tobacco leaves was conducted using the effector, reporter gene and accelerator gene in GV3101 at a ratio of 1 : 1 : 1, whereas infection with only m35S‐GUS, the effector gene, and accelerator gene was performed for the control. After incubating at 23 °C for 36 h, leaf tissues were collected. GUS Staining Solution (Coolaber, Beijing), a GUS Activity Detection Kit (Coolaber, Beijing), were used for GUS staining, GUS activity assays, respectively.
The ORF of TaWRKY29 was connected to pGreenII 62‐SK to use as the effector plasmid vector. A 250‐bp promoter fragment of TaABI5 was constructed in pGreen II 0800‐LUC to act as the reporter plasmid vector. The TaSDIR1‐4A overexpression construct was used as the accelerator. The constructed vectors were transformed into A. tumefaciens GV3101 cells. A. tumefaciens‐mediated transformation of tobacco leaves was conducted using the effector, reporter and accelerator genes in GV3101 at a ratio of 1 : 1 : 1. After incubating for 2 days at 23 °C, leaf tissues were collected for analysis with firefly luciferase (LUC) and Renilla luciferase (REN) using the Dual‐Luciferase® Reporter Assay System (Promega) with an Infinite 200 Proreader (Tecan, Männedorf, Switzerland). The promoter activity was analysed based on the relative LUC activity (LUC/LUC) when the LUC/REN ratio was set as 1.
Statistical analysis
Differences between data were tested by analysis of variance using SPSS software. Data were expressed as the mean ± standard deviation based on three independent replicates. Significant differences were determined using Student's t‐test: **P < 0.01; *P < 0.05. All figures were prepared using GraphPad Prism 7 software.
Conflicts of interest
None declared.
Author contributions
XL, QL and YM designed the research. QL, YM and LC performed the experiments. QL, YM, BW, LC and WY analysed the data. YM and QL wrote the manuscript. LL, BX, WY, YL, YX and XL revised the article. YM and QL contributed equally to this work.
Supporting information
Figure S1 Genomic structure, chromosomal localization and phylogenetic relationships for TaSDIR1‐4A.
Figure S2 Expression patterns of TaSDIR1‐4A in stress.
Figure S3 Translational levels in transgenic TaSDIR1‐4A wheat plants.
Figure S4 Green algae mottled mosaic symptoms of BSMV‐infected plants and specificity of knock down.
Figure S5 Phylogenetic relationships of TaWRKY29 and WRKY family genes in Arabidopsis.
Figure S6 Phenotypes of transgenic TaWRKY29 and TaWRKY29‐ΔC2 overexpressing Arabidopsis plants.
Table S1 Details of primers used in the experiments
Acknowledgements
This study was supported by the Key Program of Shaanxi Agricultural Cooperative Innovation and Promotion Alliance (No. LMZD202104), National Major Agricultural Science and Technology Project (NK2022060503), Program of Introducing Talents of Innovative Discipline to Universities (Project 111) from the State Administration of Foreign Experts Affairs (#B18042) ‘Crop breeding for disease resistance and genetic improvement’ and Chinese Universities Scientific Fund (2452022112).
Data availability statement
All relevant data generated or analyzed are included in the manuscript and the supporting materials.
References
- Baek, W. , Lim, C.W. and Lee, S.C. (2021) Pepper E3 ligase CaAIRE1 promotes ABA sensitivity and drought tolerance by degradation of protein phosphatase CaAITP1. J. Exp. Bot. 72, 4520–4534. [DOI] [PubMed] [Google Scholar]
- Chapagain, S. , Park, Y.C. , Kim, J.H. and Jang, C.S. (2018) Oryza sativa salt‐induced RING E3 ligase 2 (OsSIRP2) acts as a positive regulator of transketolase in plant response to salinity and osmotic stress. Planta, 247, 925–939. [DOI] [PubMed] [Google Scholar]
- Che, P. , Bussell, J.D. , Zhou, W. , Estavillo, G.M. , Pogson, B.J. and Smith, S.M. (2010) Signaling from the endoplasmic reticulum activates brassinosteroid signaling and promotes acclimation to stress in Arabidopsis . Sci. Signal. 3, ra69. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Wang, T. , Rehman, A.U. , Wang, Y. , Qi, J. , Li, Z. , Song, C. et al. (2021) Arabidopsis U‐box E3 ubiquitin ligase PUB11 negatively regulates drought tolerance by degrading the receptor‐like protein kinases LRR1 and KIN7. J. Integr. Plant Biol. 63, 494–509. [DOI] [PubMed] [Google Scholar]
- Chen, S. , Xu, K. , Kong, D. , Wu, L. , Chen, Q. , Ma, X. , Ma, S. et al. (2022) Ubiquitin ligase OsRINGzf1 regulates drought resistance by controlling the turnover of OsPIP2;1. Plant Biotechnol. J. 20, 1743–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Meng, Y. , Yang, W. , Lv, Q. , Zhou, L. , Liu, S. , Tang, C. et al. (2023) Genome‐wide analysis and identification of TaRING‐H2 gene family and TaSDIR1 positively regulates salt stress tolerance in wheat. Int. J. Biol. Macromol. 242, 125162. [DOI] [PubMed] [Google Scholar]
- Cho, S.K. , Ryu, M.Y. , Seo, D.H. , Kang, B.G. and Kim, W.T. (2011) The Arabidopsis RING E3 ubiquitin ligase AtAIRP2 plays combinatory roles with AtAIRP1 in abscisic acid‐mediated drought stress responses. Plant Physiol. 157, 2240–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, M. , Zhang, R. , Zhu, F. , Zhang, Z. , Gou, L. , Wen, J. , Dong, J. et al. (2017) A lipid‐anchored NAC transcription factor is translocated into the nucleus and activates glyoxalase I expression during drought stress. Plant Cell, 29, 1748–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem, T. , Rushton, P.J. , Robatzek, S. and Somssich, I.E. (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5, 199–206. [DOI] [PubMed] [Google Scholar]
- Gagne, J.M. , Smalle, J. , Gingerich, D.J. , Walker, J.M. , Yoo, S.D. , Yanagisawa, S. and Vierstra, R.D. (2004) Arabidopsis EIN3‐binding F‐box 1 and 2 form ubiquitin‐protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc. Natl Acad. Sci. U.S.A. 101, 6803–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, T. , Wu, Y. , Zhang, Y. , Liu, L. , Ning, Y. , Wang, D. , Tong, H. et al. (2011) OsSDIR1 overexpression greatly improves drought tolerance in transgenic rice. Plant Mol. Biol. 76, 145–156. [DOI] [PubMed] [Google Scholar]
- He, W. , Wang, R. , Zhang, Q. , Fan, M. , Lyu, Y. , Chen, S. , Chen, D. et al. (2023) E3 ligase ATL5 positively regulates seed longevity by mediating the degradation of ABT1 in Arabidopsis . New Phytol. 239, 1754–1770. [DOI] [PubMed] [Google Scholar]
- Hoppe, T. , Rape, M. and Jentsch, S. (2001) Membrane‐bound transcription factors: regulated release by RIP or RUP. Curr. Opin. Cell Biol. 13, 344–348. [DOI] [PubMed] [Google Scholar]
- Iwata, Y. and Koizumi, N. (2005) An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc. Natl Acad. Sci. U.S.A. 102, 5280–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata, Y. , Fedoroff, N.V. and Koizumi, N. (2008) Arabidopsis bZIP60 is a proteolysis‐activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell, 20, 3107–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. , Liang, G. and Yu, D. (2012) Activated expression of WRKY57 confers drought tolerance in Arabidopsis . Mol. Plant, 5, 1375–1388. [DOI] [PubMed] [Google Scholar]
- Joo, H. , Lim, C.W. and Lee, S.C. (2020) The pepper RING‐type E3 ligase, CaATIR1, positively regulates abscisic acid signalling and drought response by modulating the stability of CaATBZ1. Plant Cell Environ. 43, 1911–1924. [DOI] [PubMed] [Google Scholar]
- Jung, C. , Seo, J.S. , Han, S.W. , Koo, Y.J. , Kim, C.H. , Song, S.I. , Nahm, B.H. et al. (2008) Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 146, 623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian, J. , Whitehead, D. , Horak, J. , Wanke, D. , Weinl, S. , Batistic, O. , D'Angelo, C. et al. (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV‐B light, drought and cold stress responses. Plant J. 50, 347–363. [DOI] [PubMed] [Google Scholar]
- Kim, S.J. and Kim, W.T. (2013) Suppression of Arabidopsis RING E3 ubiquitin ligase AtATL78 increases tolerance to cold stress and decreases tolerance to drought stress. FEBS Lett. 587, 2584–2590. [DOI] [PubMed] [Google Scholar]
- Kim, S.G. , Kim, S.Y. and Park, C.M. (2007a) A membrane‐associated NAC transcription factor regulates salt‐responsive flowering via FLOWERING LOCUS T in Arabidopsis . Planta, 226, 647–654. [DOI] [PubMed] [Google Scholar]
- Kim, S.Y. , Kim, S.G. , Kim, Y.S. , Seo, P.J. , Bae, M. , Yoon, H.K. and Park, C.M. (2007b) Exploring membrane‐associated NAC transcription factors in Arabidopsis: implications for membrane biology in genome regulation. Nucleic Acids Res. 35, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.G. , Lee, S. , Seo, P.J. , Kim, S.K. , Kim, J.K. and Park, C.M. (2010) Genome‐scale screening and molecular characterization of membrane‐bound transcription factors in Arabidopsis and rice. Genomics, 95, 56–65. [DOI] [PubMed] [Google Scholar]
- Kollist, H. , Nuhkat, M. and Roelfsema, M.R.G. (2014) Closing gaps: linking elements that control stomatal movement. New Phytol. 203, 44–62. [DOI] [PubMed] [Google Scholar]
- Lee, S.C. and Luan, S. (2012) ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 35, 53–60. [DOI] [PubMed] [Google Scholar]
- Lesk, C. , Rowhani, P. and Ramankutty, N. (2016) Influence of extreme weather disasters on global crop production. Nature, 529, 84–87. [DOI] [PubMed] [Google Scholar]
- Li, W. , Nguyen, K.H. , Chu, H.D. , Ha, C.V. , Watanabe, Y. , Osakabe, Y. , Leyva‐Gonzalez, M.A. et al. (2017) The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana . PLoS Genet. 13, e1007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Serio, R.J. , Schofield, A. , Liu, H. , Rasmussen, S.R. , Hofius, D. and Stone, S.L. (2020) Arabidopsis RING‐type E3 ubiquitin ligase XBAT35.2 promotes proteasome‐dependent degradation of ACD11 to attenuate abiotic stress tolerance. Plant J. 104, 1712–1723. [DOI] [PubMed] [Google Scholar]
- Liang, M. , Li, H. , Zhou, F. , Li, H. , Liu, J. , Hao, Y. , Wang, Y. et al. (2015) Subcellular distribution of NTL transcription factors in Arabidopsis thaliana . Traffic 16, 1062–1074. [DOI] [PubMed] [Google Scholar]
- Lim, P.O. , Kim, H.J. and Nam, H.G. (2007) Leaf senescence. Annu. Rev. Plant Biol. 58, 115–136. [DOI] [PubMed] [Google Scholar]
- Liu, J.X. and Howell, S.H. (2010) bZIP28 and NF‐Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis . Plant Cell, 22, 782–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H.X. and Stone, S.L. (2010) Abscisic acid increases Arabidopsis ABI5 transcription factor levels by promoting KEG E3 ligase self‐ubiquitination and proteasomal degradation. Plant Cell, 22, 2630–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J.X. , Srivastava, R. , Che, P. and Howell, S.H. (2007) Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J. 51, 897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J.X. , Srivastava, R. and Howell, S.H. (2008) Stress‐induced expression of an activated form of AtbZIP17 provides protection from salt stress in Arabidopsis . Plant Cell Environ. 31, 1735–1743. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Li, X. , Xiao, J. and Wang, S. (2012a) A convenient method for simultaneous quantification of multiple phytohormones and metabolites: application in study of rice‐bacterium interaction. Plant Methods 8, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z.Q. , Yan, L. , Wu, Z. , Mei, C. , Lu, K. , Yu, Y.T. , Liang, S. et al. (2012b) Cooperation of three WRKY‐domain transcription factors WRKY18, WRKY40, and WRKY60 in repressing two ABA‐responsive genes ABI4 and ABI5 in Arabidopsis . J. Exp. Bot. 63, 6371–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Li, P.Y. , Fan, L. and Wu, M.H. (2018) The nuclear transportation routes of membrane‐bound transcription factors. Cell Commun. Signal. 16, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Yang, T. , Lin, Z. , Gu, B. , Xing, C. , Zhao, L. , Dong, H. et al. (2019) A WRKY transcription factor PbrWRKY53 from Pyrus betulaefolia is involved in drought tolerance and AsA accumulation. Plant Biotechnol. J. 9, 1770–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X.H. , Lyu, Y.S. , Yang, W. , Yang, Z.T. , Lu, S.J. and Liu, J.X. (2020) A membrane‐associated NAC transcription factor OsNTL3 is involved in thermotolerance in rice. Plant Biotechnol. J. 18, 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyzenga, W.J. and Stone, S.L. (2012) Abiotic stress tolerance mediated by protein ubiquitination. J. Exp. Bot. 63, 599–616. [DOI] [PubMed] [Google Scholar]
- Ma, J. , Geng, Y. , Liu, H. , Zhang, M. , Liu, S. , Hao, C. , Hou, J. et al. (2023) TaTIP41 and TaTAP46 positively regulate drought tolerance in wheat by inhibiting PP2A activity. J. Integr. Plant Biol. 65, 2056–2070. [DOI] [PubMed] [Google Scholar]
- Mao, H.D. , Li, S.M. , Chen, B. , Jian, C. , Mei, F.M. , Zhang, Y.F. , Li, F.F. et al. (2022) Variation in cis‐regulation of a NAC transcription factor contributes to tolerance in wheat. Mol. Plant, 15, 276–292. [DOI] [PubMed] [Google Scholar]
- Moon, J. , Parry, G. and Estelle, M. (2004) The ubiquitin‐proteasome pathway and plant development. Plant Cell, 16, 3181–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata, Y. , Mori, I.C. and Munemasa, S. (2015) Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 66, 369–392. [DOI] [PubMed] [Google Scholar]
- Niu, C.F. , Wei, W. , Zhou, Q.Y. , Tian, A.G. , Hao, Y.J. , Zhang, W.K. , Ma, B.A. et al. (2012) Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ. 35, 1156–1170. [DOI] [PubMed] [Google Scholar]
- Osakabe, Y. , Osakabe, K. , Shinozaki, K. and Tran, L.S. (2014) Response of plants to water stress. Front. Plant Sci. 5, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.H. , Kang, C.H. , Nawkar, G.M. , Lee, E.S. , Paeng, S.K. , Chae, H.B. , Chi, Y.H. et al. (2018) EMR, a cytosolic‐abundant ring finger E3 ligase, mediates ER‐associated protein degradation in Arabidopsis. New Phytol. 220, 163–177. [DOI] [PubMed] [Google Scholar]
- Qin, F. , Sakuma, Y. , Tran, L.‐S.P. , Maruyama, K. , Kidokoro, S. , Fujita, Y. , Fujita, M. et al. (2008) Arabidopsis DREB2A‐interacting proteins function as RING E3 ligases and negatively regulate plant drought stress–responsive gene expression. Plant Cell, 20, 1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra, A.S. , Gonugunta, V.K. , Christmann, A. and Grill, E. (2010) ABA perception and signalling. Trends Plant Sci. 15, 395–401. [DOI] [PubMed] [Google Scholar]
- Ren, X. , Chen, Z. , Liu, Y. , Zhang, H. , Zhang, M. , Liu, Q. , Hong, X. et al. (2010) ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J. 63, 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton, P.J. , Somssich, I.E. , Ringler, P. and Shen, Q.J. (2010) WRKY transcription factors. Trends Plant Sci. 15, 247–258. [DOI] [PubMed] [Google Scholar]
- Sakuraba, Y. , Kim, D. , Han, S.H. , Kim, S.H. , Piao, W. , Yanagisawa, S. , An, G. et al. (2020) Multilayered regulation of membrane‐bound ONAC054 is essential for abscisic acid‐induced leaf senescence in rice. Plant Cell, 32, 630–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santner, A. and Estelle, M. (2010) The ubiquitin‐proteasome system regulates plant hormone signaling. Plant J. 61, 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, P.J. , Kim, S.G. and Park, C.M. (2008) Membrane‐bound transcription factors in plants. Trends Plant Sci. 13, 550–556. [DOI] [PubMed] [Google Scholar]
- Shu, K. and Yang, W. (2017) E3 Ubiquitin ligases: ubiquitous actors in plant development and abiotic stress responses. Plant Cell Physiol. 58, 1461–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle, J. and Vierstra, R.D. (2004) The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 55, 555–590. [DOI] [PubMed] [Google Scholar]
- Stone, S.L. , Hauksdottir, H. , Troy, A. , Herschleb, J. , Kraft, E. and Callis, J. (2005) Functional analysis of the RING‐type ubiquitin ligase family of Arabidopsis. Plant Physiol. 137, 13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra, R.D. (2009) The ubiquitin‐26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 10, 385–397. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wang, R. , Mao, X. , Zhang, J. , Liu, Y. , Xie, Q. , Yang, X. et al. (2020) RING finger ubiquitin E3 ligase gene TaSDIR1‐4A contributes to determination of grain size in common wheat. J. Exp. Bot. 71, 5377–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, C. , Luo, D. , Lin, A. , Zhang, C. , Shan, L. , He, P. , Li, B. et al. (2019) A tomato B‐box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome‐mediated degradation. New Phytol. 221, 279–294. [DOI] [PubMed] [Google Scholar]
- Xu, F.Q. and Xue, H.W. (2019) The ubiquitin‐proteasome system in plant responses to environments. Plant Cell Environ. 42, 2931–2944. [DOI] [PubMed] [Google Scholar]
- Xu, R. , Duan, P. , Yu, H. , Zhou, Z. , Zhang, B. , Wang, R. , Li, J. et al. (2018) Control of grain size and weight by the OsMKKK10‐OsMKK4‐OsMAPK6 signaling pathway in rice. Mol. Plant 11, 860–873. [DOI] [PubMed] [Google Scholar]
- Yang, Z. , Bai, Z. , Li, X. , Wang, P. , Wu, Q. , Yang, L. , Li, L. et al. (2012) SNP identification and allelic‐specific PCR markers development for TaGW2, a gene linked to wheat kernel weight. Theor. Appl. Genet. 125, 1057–1068. [DOI] [PubMed] [Google Scholar]
- Yang, Z.T. , Wang, M.J. , Sun, L. , Lu, S.J. , Bi, D.L. , Sun, L. , Song, Z.T. et al. (2014) The membrane‐associated transcription factor NAC089 controls ER‐stress‐induced programmed cell death in plants. PLoS Genet. 10, e1004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X. , Liu, X. , Xu, B. , Lu, P. , Dong, T. , Yang, D. , Ye, T. et al. (2019) OsMADS18, a membrane‐bound MADS‐box transcription factor, modulates plant architecture and the abscisic acid response in rice. J. Exp. Bot. 70, 3895–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. , Xu, W. , Wang, J. , Wang, L. , Yao, W. , Yang, Y. , Xu, Y. et al. (2013) The Chinese wild grapevine (Vitis pseudoreticulata) E3 ubiquitin ligase Erysiphe necator‐induced RING finger protein 1 (EIRP1) activates plant defense responses by inducing proteolysis of the VpWRKY11 transcription factor. New Phytol. 200, 834–846. [DOI] [PubMed] [Google Scholar]
- Yu, J. , Kang, L. , Li, Y. , Wu, C. , Zheng, C. , Liu, P. and Huang, J. (2021) RING finger protein RGLG1 and RGLG2 negatively modulate MAPKKK18 mediated drought stress tolerance in Arabidopsis . J. Integr. Plant Biol. 63, 484–493. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Yang, C. , Li, Y. , Zheng, N. , Chen, H. , Zhao, Q. , Gao, T. et al. (2007) SDIR1 is a RING finger E3 ligase that positively regulates stress‐responsive abscisic acid signaling in Arabidopsis . Plant Cell, 19, 1912–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Xiao, S. , Li, W. , Feng, W. , Li, J. , Wu, Z. , Gao, X. et al. (2011) Overexpression of a Harpin‐encoding gene hrf1 in rice enhances drought tolerance. J. Exp. Bot. 62, 4229–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Cui, F. , Wu, Y. , Lou, L. , Liu, L. , Tian, M. , Ning, Y. et al. (2015) The RING finger ubiquitin E3 ligase SDIR1 targets SDIR1‐INTERACTING PROTEIN1 for degradation to modulate the salt stress response and ABA signaling in Arabidopsis . Plant Cell, 27, 214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Chan, Z. , Gao, J. , Xing, L. , Cao, M. , Yu, C. , Hu, Y. et al. (2016) ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl Acad. Sci. U.S.A. 113, 1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, P. , Cao, L. , Zhang, C. , Fang, X. , Wang, L. , Miao, M. , Tang, X. et al. (2023) The transcription factor MYB43 antagonizes with ICE1 to regulate freezing tolerance in Arabidopsis . New Phytol. 238, 2440–2459. [DOI] [PubMed] [Google Scholar]
- Zhu, J.K. (2002) Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J.K. (2016) Abiotic stress signaling and responses in plants. Cell, 167, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Genomic structure, chromosomal localization and phylogenetic relationships for TaSDIR1‐4A.
Figure S2 Expression patterns of TaSDIR1‐4A in stress.
Figure S3 Translational levels in transgenic TaSDIR1‐4A wheat plants.
Figure S4 Green algae mottled mosaic symptoms of BSMV‐infected plants and specificity of knock down.
Figure S5 Phylogenetic relationships of TaWRKY29 and WRKY family genes in Arabidopsis.
Figure S6 Phenotypes of transgenic TaWRKY29 and TaWRKY29‐ΔC2 overexpressing Arabidopsis plants.
Table S1 Details of primers used in the experiments
Data Availability Statement
All relevant data generated or analyzed are included in the manuscript and the supporting materials.


