Abstract
Leishmaniasis, which is caused by a parasitic protozoan of the genus Leishmania, is still a major threat to global health, impacting millions of individuals worldwide in endemic areas. Chemotherapy has been the principal method for managing leishmaniasis; nevertheless, the evolution of drug resistance offers a significant obstacle to therapeutic success. Drug-resistant behavior in these parasites is a complex phenomenon including both innate and acquired mechanisms. Resistance is frequently related to changes in drug transportation, drug target alterations, and enhanced efflux of the drug from the pathogen. This review has revealed specific genetic mutations in Leishmania parasites that are associated with resistance to commonly used antileishmanial drugs such as pentavalent antimonials, miltefosine, amphotericin B, and paromomycin, resulting in changes in gene expression along with the functioning of various proteins involved in drug uptake, metabolism, and efflux. Understanding the genetic changes linked to drug resistance in Leishmania parasites is essential for creating approaches for tackling and avoiding the spread of drug-resistant variants. Based on which specific treatments focus on mutations and pathways could potentially improve treatment efficacy and help long-term leishmaniasis control. More study is needed to uncover the complete range of genetic changes generating medication resistance and to develop new therapies based on available information.
1. Introduction
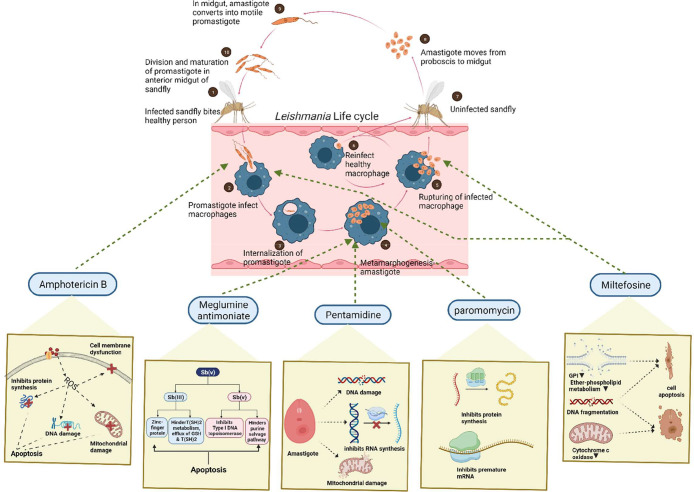
Leishmania is a parasitic eukaryotic protozoan that belongs to the class Trypansomatidae (order Kinetoplastida). These species are distinguished by the presence of a prominent Feulgen stain-positive kinetoplast. All of the species of this class are pathogenic from invertebrates to vertebrates, and their morphology changes as they progress through their life cycle. Over fourteen Leishmania species are harmful to mammals, nine of which are identified as human parasites.1,2 Based on the morphological characterization, Leishmania exists in two different forms: a promastigote stage that penetrates inside the host’s phagocytic cell and later transforms into an obligatory intracellular amastigote stage. This parasite performs a digenetic life cycle; each time the parasite shuttles between the host and the carrier, it undergoes morphological differentiation.3,4 The female sandfly (Old World: genus Phlebotomus and New World: Lutzomyia and Psychodopygus) becomes infected after consuming blood from a diseased host. Once the parasite is inside the sandfly, it goes through the first stage of differentiation and becomes a procyclic promastigote. Promastigotes are flagellated and migratory parasites with thin bodies that range in length from 15 to 20 μm and 0.5–3.5 μm in width. The flagellar size is about 5–14 μm, which aids the parasite in adhering to the sandfly’s gut.5 The procyclic forms divide in the sandfly’s abdominal midgut, culminating in the formation of indivisible nectomonad promastigotes. These nectomonad promastigotes pass through the midgut of the abdomen to the anterior midgut, where they change into leptomonad promastigotes. The leptomonad promastigotes then mature into metacyclic promastigotes and travel to the insect’s proboscis, where they can be transferred to mammalian hosts. The following phase in the lifecycle is known as metacyclogenesis, and the promastigotes are introduced into the host species via a sandfly bite. Through the proboscis by biting, metacyclic promastigotes transmit to the host organism, where they initiate a phagocytic procedure by attaching to the cell membrane. In this way, the promastigotes infiltrate the phagocytes and attack the parasitophorous vacuole. Here, promastigotes convert into oval-shaped amastigotes two and four micrometers in width. It is crucial to highlight how the parasitic organism survives both the macrophages’ acidic circumstances and the stomach’s acidic environment. The amastigotes grow and multiply within the parasitophorous vacuole until the macrophage breaks off, secreting all matured amastigotes. These released amastigotes set off a chain reaction that finally develop into leishmaniasis as shown in Figure 1.3,4
Figure 1.
Life cycle of the Leishmania parasite along with its mode of action.
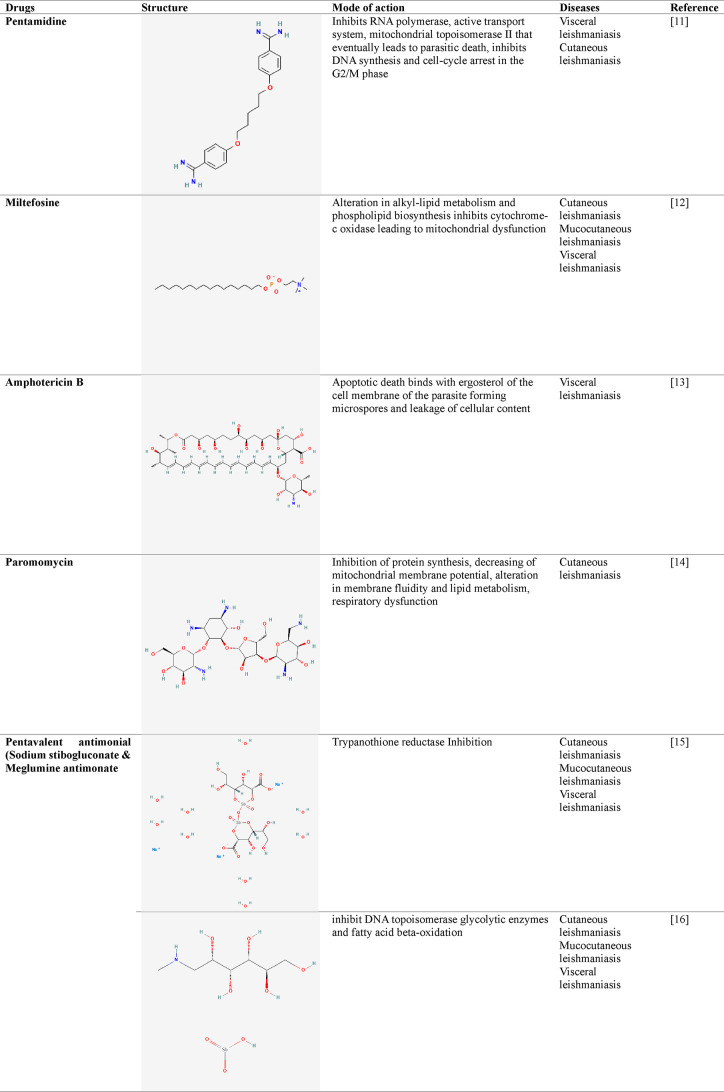
According to the World Health Organization (WHO), leishmaniasis is a parasitic infection that causes tropical and subtropical illnesses that are spread in over 89 countries including Africa, Asia, America, and the Mediterranean, thereby creating a global health crisis. About 10 to 15 million individuals worldwide are affected, with an annual incidence of new infections exceeding 0.7–1 million.6 This prevailing epidemiology shows a wide range of clinical symptoms ranging from minor skin lesions to life-threatening systemic infections, in the case of cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL) respectively.7 There are medications available for such illnesses, including antimonial, pentamidine, amphotericin B, miltefosine, paromomycin, and others, as shown in Figure 1. The drug structure (PubChem) and its mode of action is described in Table 1.8 Despite continued attempts to manage and treat leishmaniasis, the emergence of drug-resistant strains presents a serious problem, weakening the effectiveness of current treatment strategies. Drug resistance in leishmaniasis is a complex problem driven by genetic, environmental, and clinical factors that could be considered innate and acquired mechanisms. The innate mechanism in clinically relevant Leishmania species shows that the presence of different molecular and biochemical components generates variances in in vitro drug susceptibility. Although pharmacokinetics and host immune responses play a role in the infected host, these species-specific changes often result in distinct in vivo medication efficacy. On the contrary, due to the defensive acquired mechanisms in the Leishmania parasite, it exhibits amazing genomic plasticity; genetic alterations can rapidly occur, allowing it to survive under pharmacological strain. The development of such resistance mechanisms can be linked to changes in drug uptake, drug efflux or sequestration, enzymatic drug inactivation, improved cellular responses to deal with drug-induced stress or cell damage, and/or changes in the expression, abundance, or drug binding affinity of the primary therapeutic target.9,10 The convoluted life cycle of Leishmania, which involves both mammalian hosts and sandfly vectors, adds to the complication of drug resistance development. Furthermore, socioeconomic factors, inadequate healthcare infrastructure, and restricted access to effective treatment choices have led to the proliferation of drug-resistant parasites. The goal of this study is to provide a complete overview of the mechanisms causing drug resistance in leishmaniasis, the factors that contribute to its emergence, and the strategies used to tackle this challenge. This review focuses on investigating the genetic basis of drug resistance and looking at how changes in critical genes can lead to resistance to antileishmanial medicines. In addition, it also focuses on the influence of treatment practices, such as monotherapy and incomplete treatment sessions, on the selection of drug-resistant strains. To address this critical issue, researchers and healthcare practitioners are implementing a variety of strategies to reduce the impact of drug resistance. Combination therapy, which involves the administration of numerous medications with diverse mechanisms of action, has the potential to postpone or overcome resistance. Understanding the complicated host-parasite interactions in leishmaniasis reveals new targets for innovative drug development. This review aims to shed light on the complex interplay between drug resistance and leishmaniasis by an examination of current research and clinical experiences, emphasizing the significance of a multidisciplinary approach to address this public health concern. We can pave the road for more tailored therapies and better treatment results in leishmaniasis by identifying the mechanisms causing drug resistance and evaluating the effectiveness of various strategies.10
Table 1. Antileishmanial Drugs along with Their Mode of Action−.
2. The Genome of Leishmania
Leishmania species have a wide range of chromosomes and gene sets. A recent genome assembly of L. major reported 32.8 Megabase genomic with 11,238 genes scattered among thirty-six chromosomes.23 Initially, Leishmania species were assumed to possess atypically diploid genomes; however, mosaic aneuploidy has since been determined to be the norm in these parasites’ genomes, with aneuploidy varied depending on strain or species.24 Almost all trypanosomatid protein-coding genes lack introns and organize themselves in unidirectional polycistronic transcriptional zones that lack functional gene associations. Polycistronic transcription yields pre-mRNAs, which are later processed to form mature mRNAs. Every gene is continuously produced, mainly by RNA polymerase II, but the timing of the initial stages of the gene transcriptional process is unknown due to canonical promoter sites not yet being identified in these parasitic organisms.25 Epigenetic processes that influence DNA accessibility seem to have an essential function in Leishmania transcription initiation. Having the base J results in transcriptional completion at the final point of each polycistronic transcriptional region.26 However, due to the lack of transcriptional regulation, it is hypothesized in the literature that protein production in these species is modulated via post-transcriptional mechanisms such as ribonucleic acid deterioration, translational management, and protein breakdown. Numerous investigations have discovered a link between the chromosome copy number and transcription levels, lending support to the theory that expression regulation occurs after transcription. However, transcript and protein levels are not always related.27
3. Genome Variation and Plasticity
Mutations within the parasite gene have been connected to geographic location and illness symptoms, which may impact leishmaniasis treatment. A surprisingly extensive study incorporating an enormous number of isolates from many different regions indicates that genetic variation is considerably greater than earlier stated.28 Single-cell sequencing recently showed the existence of multiple distinct karyotypes inside the same Leishmanial clone, implying that multigenotype infections can occur within the same host’s cells as well as in tissue.29,30
Aside from the function of mutations in parasite variety, these species’ genomes are exceptionally flexible and constantly reorganize, which leads to changes in gene copy values, sets of DNAs, as well as the entire chromosome makeup. As a result, mosaic aneuploidy is not common within several species, but it also serves as a key adaptation process, allowing a certain genomic pattern to be rapidly selected during times of difficulty.31,32 Ploidy alterations are not entirely random but appear to follow an identical sequence in specimens exposed to a variety of stressors, and each strain often follows the same pattern, indicating the presence of selecting processes.33 In this ploidy, a gene’s copy number could either be altered by inserting or removing genes in tandem, as well as through making extrachromosomal replicas of genetic material, that are either circular or linear.34,38 These genes are generally detected under wild-type conditions but are expressed through alterations in Leishmania populations under stress conditions. In Leishmania, the genome contains sets of recurring regions enclosing several genes, and double-stranded DNA breakages close to or inside the repetitive regions can promote homologous recombination, and this is linked to an increase in gene reorganizations.35–37
3.1. TXNPx
TXNPx is a member of the 2-cysteine peroxiredoxin group and is classified according to as it is found in the cytosol or the mitochondria.39 Enzymes such as these have been extensively conserved and found in a variety of Leishmania species. Most parasitic organisms, involving Leishmania spp., tend to be more vulnerable to reactive oxygen compounds than their hosts. Organisms have evolved several antioxidant defense systems to counteract cell damage caused by reactive oxygen species (ROS).40 Compared to other eukaryotes that utilize glutathione and catalase, trypanosomatid parasites possess trypanothione [N1, N8-bis(glutathionyl)spermidine] (TS2) that acts as the main detoxifying agent against oxidative damage. Trypanothione synthetase (TryS) plays a role in this dithiol synthesis which is later reduced to T(SH)2 by the trypanothione reductase (TR). The tryparedoxin/tryparedoxin peroxidase I (TXN/TXNPx) complex uses this T(SH)2 to reduce the negative effect produced by macrophages through hydrogen peroxide neutralization. By catalyzing the reduction of H2O2, and small-chain organic hydroperoxides to alcohol and water, respectively, these antioxidant enzymes protect against chemical and oxidative stress.41 The cooperative function of trypanothione reductase, tryparedoxin, and tryparedoxin peroxidase is thus critical for maintaining a low hydrogen peroxide (H2O2) content.42 This enzyme is essential for Leishmania’s survival during oxidative stress caused by macrophages and medications.43
3.2. ABC Transporters
ABC transporters make up a well-known family of proteins that perform important physiological tasks. These molecules observed in organisms such as prokaryotes as well as in humans utilize ATP hydrolysis for the elimination of numerous kinds of substances throughout the cell membranes.44 ABC proteins have an important activity in resistance toward drugs via 2 modes of action. The initial phase is when ABC-carrying genes are amplified or expressed more, increasing carriers at the cell surface membrane and have been associated with a large amount of drug efflux through cells.45 The subsequent phase is a mutation in the gene, which alters the biochemical characteristics of ABC transporters and, thus, their carrier capacity. ABC protein is a multidrug-resistant protein (MRP) in Leishmania that contributes to metal resistance via thiol metabolic processes and drug elimination pathways. And, the P-glycoprotein A (PGPA) is a member of the MRP ABC transporter family that has been linked to arsenite and antimoniate resistance. The Leishmanial parasite genome has forty-two ABC genes, designated ABCA to ABCH. In L. major infection, some investigations suggest that there is a link between nonresponding to glucantime and alleles such as ABCC7, ABCC3, ABCG2, and ABCI4.46,47 The ABCI4 transporter, which is present in parasite mitochondria and cell membranes, is involved in heavy metal transfer. The ABCI4 gene is found on chromosome no. 33.48 The ABCG2 transporter is in intracellular vesicles and the plasma membrane and is expressed with the help of the ABCG2 gene on the sixth chromosome. It transports the conjugated thiol-antimony complex outside the amastigote cell. The ABCC7 (PRP1) transporter is also in intracellular vesicles and is expressed by ABCC7 on chromosome 31.49 Similarly, the ABCC3 transporter (MRPA), encoded by the ABCC3 gene on chromosome 23, is present in vesicles across the nuclei and the flagellar pocket of the cell. As these are related to tubulin vesicles, ABCC7 and MRPA proteins bind in both endocytosis and exocytosis processes. These protein carriers are important in drug response.50
3.3. Glycoprotein
The zinc-dependent metalloprotease glycoprotein 63 (GP63) or leishmanolysin, which was discovered on the surface of the Leishmania parasite in the 1980s, has been identified genetically as well as biochemically as a crucial surface antigen presented on Leishmania promastigotes from different species and to have an array of materials including casein, gelatin, albumin, hemoglobin, and fibrinogen.51,52 This protease is included in the metzincin class, which includes the HExxHxxGxxH sequence motif and the N-terminus pro-peptide that suppresses the pro-enzyme during translation and is eliminated during maturation and activation.53,54 GP63 is prevalent in promastigotes, but not in amastigotes. As it is found in both phases, it is expected to serve various functions based on the parasitic stage. GP63 was discovered to cleave C3b into iC3b in promastigotes of L. amazonensis and L. major, hence assisting the parasite to evade complement-mediated lysis. Alongside, iC3b production can operate as an opsonin, allowing the parasite to engage with macrophages via complement receptors 1 and 3, thereby promoting parasite internalization. GP63 has also been shown to have interactions with the fibronectin receptor (FR), which may aid parasite adhesion to macrophages.55 Another significant discovery was that when L. mexicana promastigotes encounter the outermost layer of subcutaneous tissue, GP63 could break down extracellular components, allowing for faster migration over matrix gel in vitro. According to these findings, GP63 shows a drastic impact on macrophage processes that favor Leishmania survival by cleaving and/or degrading different proteins.56
3.4. Aquaglyceroporins
The AQPs are a superfamily of aquaporins as well as aquaglyceroporins, which are channels in animals ranging from prokaryotes to eukaryotes that aid in the submissive pathway of water and tiny neutral molecules through the plasma membranes. Despite these channels not being found in many microorganisms, most eukaryotic genomes encode a minimum of one channel; plants have more than 30, some vertebrates have more than ten, and Plasmodium spp. and other Apicomplexa generally have one.57 There are three types such as AQP1, AQP2, and AQP3 out of which AQP1 is a flagellar membrane protein, and the AQP3 is a cell membrane protein that carries water, glycerol, urea, dihydroxyacetone, and ammonia. Along with in vitro analysis, it was found that the deletion of these genes leads to resistance to antimonial drugs.58
3.5. l-Asparaginase
l-Asparagine synthase is essential for Leishmania survival and was recently recognized as a potential therapeutic candidate. A whole proteome BLAST revealed that among numerous protozoan diseases, only Leishmania, Giardia, and Trichomonasspp. have a unique genomic region coding for suspected l-asparaginase.59l-Asparaginases are amidohydrolase enzymes that have been employed as efficient antileukemic medicines. Also, some studies suggest that this l-asparagine (substrate for l-asparaginase) has an inhibitory influence on the Leishmanial autophagosomal pathway. According to a recent study, Mycobacterium tuberculosisl-asparaginase (MyAnsA) provides survival advantages to the pathogen by decreasing the acidic conditions inside the host cell. Each M. tuberculosis and Leishmania donovani uses a phagolysosome fusion process to establish infection. Transaminases and synthases may collaborate in a particular subcellular region to restore important chemical byproducts, as it was hypothesized that LdAI might not be the only one contributing to the metabolism of nitrogen. The STRING database added to the evidence by anticipating a functional relationship between LdAI and important enzymes that govern the metabolism of parasite aspartate, arginine, and purine metabolism. Some of the major enzymes, arginosuccinate synthase engaged in arginine biosynthesis and adenylate synthetase involved in purine biosynthesis salvage pathway, were predicted to interact strongly with LdAI.60,61 These two enzymes were demonstrated to produce arginosuccinate (ATP dependent) and adenylate (GTP dependent) using ASP as a source. It has been demonstrated that functionally inactivating these enzymes causes deficiencies in parasite development and contagiousness. The knowledge that could be combined to successfully target the N2 metabolic processes of parasites does, therefore, indicate the synergistic interdependence of these pathways. Fortunately, utilizing an overexpression system, this undesirable potential was eliminated; by overexpression of LdAI in parasites, it was found that longevity increased by 20 percent, by having the ability to withstand detrimental impact at all doses of both inhibiting agents. The findings conclusively show l-asparaginase of L. donovani is one of the essential enzymes involved in metabolism for early cautious reaction to Amphotericin B used to treat visceral leishmaniasis.62
3.6. Cysteine Synthase
Cysteine is a sulfur-containing amino acid that plays a crucial role in cellular redox homeostasis; cysteine synthase plays an important role in the synthesis of cysteine, which is a precursor for the antioxidant molecules glutathione, and trypanothione, which is crucial for maintaining cellular redox balance and protecting against oxidative stress.63 An upregulation of cysteine synthase could contribute to increased levels of cysteine and, subsequently, glutathione, enhancing the pathogen’s antioxidant defense mechanisms and aiding in the detoxification of drugs.64 The de novo or assimilatory and reverse transsulfuration (RTS) processes are 2 pathways for cysteine production. RTS has been established in fungi and mammals, and it comprises the entire process leading to cysteine from methionine via cystathionine synthesis. Cystathionine β-synthase (CS), which synthesizes cystathionine from homocysteine and serine, and cystathionine γ-lyase (CGL), which produces cysteine from cystathionine, catalyze these processes. The de novo process is likewise a two-step catalytic reaction that begins with serine acetyltransferase (SAT) to make O-acetyl serine (OAS) from l-serine and acetyl coenzyme A, followed by an alanyl transfer reaction driven by cysteine synthase (CS). This de novo pathway for cysteine biosynthesis is found in plants, bacteria, and several protozoan parasites such as Entamoeba histolytica, Entamoeba dispar, Leishmania major, and Leishmania donovani.65,66
3.7. Ascorbate Peroxidase
Ascorbate peroxidase (APx) is an enzyme that is essential for the glutathione-ascorbate cycle. Glutathione, which keeps cells in a reducing environment, is likely to be responsible for the reduction of many cellular components.67 A single copy of the Leishmania major ascorbate peroxidase gene has been shown to possess a crucial role in the H2O2 detoxification, which is produced by both endogenous and exogenous processes such as an oxidative rupture of diseased host macrophages and Leishmania parasite medication mechanism.68 Aminotriazole or sodium azide, which blocks heme-containing enzymes like catalase and peroxidase, dramatically slowed the elimination of H2O2 from amastigotes. Overexpressing ascorbate peroxidase in L. major promastigotes enhanced adaptability toward oxidative stress-induced apoptosis. Overexpression of APx (LmAPx) in L. major mitochondria protects cells from the harmful effects of oxidative damage, such as mitochondrial dysfunction and apoptosis.69
3.8. Silent Information Regulator 2
The silent information regulator 2 (SIR2) includes a family of NAD+-dependent protein deacetylases and is conserved in microbial to eukaryotic organisms. These genes regulate a wide range of functions in eukaryotic cells, including transcriptional repression, recombination, the division of cells, cellular responses to DNA-damaging substances, and spindle organization.70 Sirtuins are currently studied in parasitic protozoa such as Plasmodium and trypanosomes as they are required for proper cellular functioning and proliferation. Recently it was discovered to have both conserved and unique functions that regulate a wide range of biological processes; parasitic sirtuins are promising therapeutic options for treatment. SIR2RP1, SIR2RP2, and SIR2RP3 are SIR2 homologues found in the Leishmania genome.71 The cytosolic sirtuin SIR2RP1 is known to be critical for parasite infectivity and survival, making it an interesting therapeutic target for antileishmanial treatment. The role of SIR2RP2 in L. donovani was explored. LdSIR2RP2 is linked to the human orthologue HsSIRT4, which corresponds to Sirtuin class II, according to phylogenetic and sequencing analyses. Some studies show that LdSIR2RP2, like HsSIRT4, exclusively exhibits NAD+-dependent ADP-ribosyltransferase activity. This protein, like HsSIRT4, was discovered to be localized in the mitochondria of the parasite. LdSIR2RP2 was not proven to be needed for parasite survival, but null variants showed delayed growth and lower infectivity. A G2/M block was discovered in the null mutant protozoa cell cycle, which could explain a mutant line growth issue. Thus, in Leishmania, deletion of the mitochondrial sirtuin LdSIR2RP2 affects mitochondrial activity, leading to lower ATP content and thus slower growth kinetics.72
3.9. Sterols
Lanosterol 14-demethylase (CYP51) is a cytochrome P450 (CYP) monooxygenase that stimulates the elimination of the 14-methyl group from various sterol substrates. This elimination process includes an essential stage within the cholesterol synthesis process, which produces cholesterol in mammals and ergosterol and ergosterol-like sterols in plants, fungi, and protozoa. As a result, CYP51 is being identified as an option for drugs since human cells possess sterol in their outermost layer, whereas parasitic organisms need ergosterol. Leishmania CYP51 is a prime example of a naturally occurring plant-like sterol 14-demethylase that can be addressed specifically to produce potent antileishmanial medications.73,74 Cell surface sterols are crucial biological elements that help to produce functional cell membranes. Sterol 14-demethylase inhibition prevents sterol production, which is fatal in the affected organism. Sterol methyltransferase (SMT) catalyzes the conversion of a methyl group from adenosine to methionine into the sterol end chain of the C24 position. This enzyme is necessary for the protection of mitochondrial membrane potential, ROS generation, and parasite pathogenicity in Leishmania major.75
4. Drug Resistance Linked to Mutation
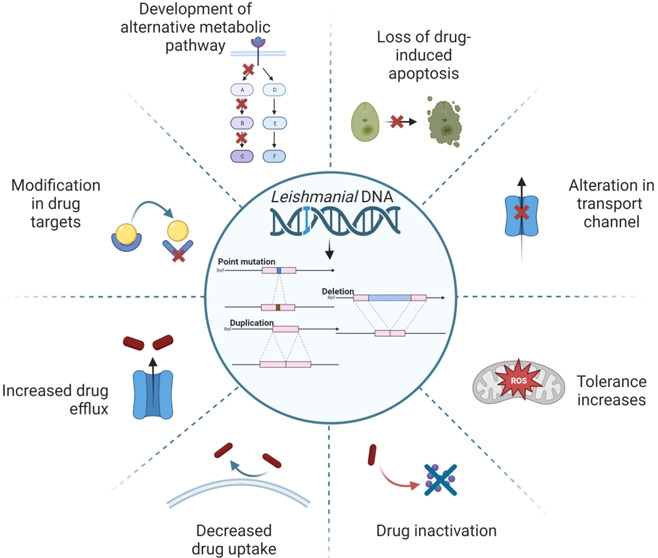
As drug resistance is frequent in these parasites, gene variation is postulated as the primary factor in the evolution of various drug-resistant phenotypes. There is no single indicator for evaluating tolerance in clinical specimens since a variety of alterations may cause resistance to existing medicines. It is critical to understand the genetic basis of medication resistance to create successful treatment techniques.76 By studying the specific genetic variations that contribute to drug resistance, researchers can identify potential targets for new drugs or therapies. Additionally, investigating the correlation between genetic variation and drug susceptibility in clinical specimens and in vitro tests can provide valuable insights into the mechanisms underlying drug resistance and guide future research efforts. Some of the examples of drugs associated with the mutation leading to its resistance are given in Table 2.
Table 2. Gene Mutation along with Its Associated Drug.
| associated drug | gene name | protein | function | type of mutation | mutation (a.a.) | clinical relevance | references |
|---|---|---|---|---|---|---|---|
| Pentamidine | MRPA | Multidrug resistance protein | Role in detoxification | Amplification, overexpression | F360S, gene duplication, increased expression | Increased drug efflux | (77) |
| AQP1 | Aquaglyceroporin 1 | Maintains osmoregulation, drug uptake | Deletion | Protein truncation, G126E | Decreased drug uptake | (78) | |
| Altered channel role | |||||||
| TDR1 | Thiol-dependent reductase I | Redox regulation | Point mutation | G146S | Reduced drug accumulation | (79) | |
| PTR1 | Pteridine reductase 1 | Increases resistance against oxidative stress | Point mutation | R30L | Altered drug metabolism | (80) | |
| DHFR-TS | Dihydrofolate reductase, thymidylate synthase | DNA synthesis | Overexpression | S91F, F185Y, I164L | Reduced drug binding | (81) | |
| Deletion | Gene deletion | Impaired drug-target interaction | |||||
| Miltefosine | LdMT | Miltefosine transporter | ATP-dependent transport of NBD-labeled phosphatidylethanolamine (PE) and phosphatidylcholine | Deletion point mutation | Gene deletion T245I, L832F, T420N, L856P | Impaired drug uptake | (82) |
| LdRos3 | Miltefosine transporter beta subunit | Maintains phospholipid symmetry | Point mutation | L262P | Reduced drug influx | (83) | |
| Paromomycin | PMP3, PMP24 | Phospholipid-Methyltransferase 3 and 24 | Lipid metabolism | Point mutation | L42S, L55P | Altered drug susceptibility | (84) |
| Gene deletion | L62P | ||||||
| HSP23 | Heat shock protein 23 | Maintains cellular integrity during stress condition | Deletion | Gene deletion | Reduced drug uptake | (85) | |
| Amphotericin-B | ERG1 | Ergosterol biosynthesis 1 | Sterol biosynthesis | Point Mutation | G487S, S405A | Altered ergosterol biosynthesis | (86) |
| LmABCB3 | ABC transporter protein | Transportation | Deletion | Gene deletion | Impaired drug transport | (87) | |
| ERG11 | Ergosterol Biosynthesis 11 | Encodes the enzyme lanosterol 14-alpha demethylase | Point Mutation | Y306F | Reduced drug binding | (88) | |
| G117V | |||||||
| Antimony | AQP1 | Aquaglyceroporin 1 | Transports water, other small molecules across the membrane, maintains osmotic balance & metabolic process | Point mutation | SL75L, N146D, G245A | Altered water channel function | (89) |
| TDR1 | Tryparedoxin peroxidase 1 | Protecting cells against oxidative stress | Amplification | Gene duplication | Increased drug efflux | (90) | |
| MRPA (ABCG2-like) | Multi-drug resistance protein A1 | Role in drug efflux | Amplification | Gene duplication | Increased drug efflux | (91, 92) | |
| PGPA (ABCB1-like) | P-Glycoprotein A | Role in drug efflux | Amplification | Gene duplication | Increased drug efflux | (93) |
4.1. Pentamidine
Pentamidine, an aromatic diamidine, was initially used for treating insomnia in 1937. The first use of this drug in the treatment of VL in antimony-resistant patients in India was in 1949. PMD is utilized to treat L. guyanensis and L. panamensis-caused systemic CL. However, growing resistance to PMD and unexpected consequences like low blood sugar, low blood pressure, a cold, myocarditis, and renal toxicity were the main reasons for the drug’s discontinuance throughout India in the nineties. Although the precise mechanism of PMD activity in Leishmania is unknown, a few studies suggest that it alters the parasite organism’s mitochondrial internal membrane potential.94 Pentamidine buildup in mitochondria may cause death by apoptosis in L. donovani by blocking respiratory cycle complexes I to III, producing reactive oxygen species, and elevating cytosolic Ca2+. It can also act on DNA topoisomerases (TOPs), an enzyme that is required for the topology of DNA modulation during transcription, replication, recombination, and reparation. TOPI and TOPII of the parasite Leishmania differ significantly in structure and biochemistry by comparing with the human enzymes, as they play an important part within the parasite’s kinetoplast DNA network organization.95 PMD has been demonstrated as an arginine transport competitor in L. donovani as well as a noncompetitive antagonist of spermidine along with putrescine transportation in L. infantum, L. donovani, as well as in L. mexicana. The medication can enter both forms, promastigote and amastigote of L. mexicana, through a transporter-mediated process by identifying the medicine. Amastigotes, on the other hand, have a far higher absorption rate than promastigotes.96
Mutations in several transporters have been associated with PMD tolerance in the Leishmania parasites. ABC transporters were recently discovered in a variety of Leishmania species, and several of these species have been widely investigated and linked to treatment resistance.97 The ABC transporters superfamily (ABCC7) comprises the P-glycoprotein (PGP), which includes the pentamidine resistance protein 1 (PRP1). Aquaglyceroporin 2 (AQP2) is a transporter in trypanosomes that regulates the resistance to pentamidine and melarsoprol. AQP2 is a surface channel protein that aids in the passive movement of liquids and tiny noncharged substances through the plasma membrane. More research is required, but an AQP2 mutation may be the cause of pentamidine resistance in Leishmania parasites.98,99 Verapamil, a Ca2+ channel and P-glycoprotein inhibitor, has been demonstrated to restrict its efflux, resulting in PMD buildup in resistant parasites.100 Flavonoid dimers were synthesized to avoid pentamidine resistance in Leishmania parasites, and they demonstrated much stronger reversal activity to pentamidine tolerance in L. enriettii, because of increased drug accumulation in mitochondria. These synthesized flavonoids work as reversal inhibitors in parasite Leishmania to overcome PMD resistance. The identical dimers functioned in tandem with quinacrine also used to reverse PMD resistance in the Leishmania parasite.101
4.2. Antimonial
Sodium stibogluconate (SSG), as well as meglumine antimoniate (MA), is currently the only viable therapy for all types of leishmaniasis with positive clinical and scientific outcomes. Because there are no oral medicines available, these therapies have serious side effects such as pancreatitis and renal and cardiac toxicity and can only be administered via an injection. Both SSG and MA have been shown to inhibit trypanothione reductase (TR), which is thought to be important for the survival of parasites in the host organism. TR degrades trypanothione, which is employed for neutralizing ROS generated by macrophages during infection.102 In contrast to glutathione (GSH), which is the principal redox defense molecule in mammals, trypanothione is the primary detoxifying pathway for oxidative damage in Trypanosomatid parasites. Pentavalent antimonials are considered prodrugs because their in vivo transformation results in the generation of active as well as toxic trivalent antimonials Sb(III), which causes Leishmania apoptotic death. Acidic pH values and high temperatures aid in Sb(V) reduction.103 Both macrophages and parasites can experience a decrease in antimonial levels. The ability of Leishmania to convert Sb(V) to Sb(III) changes with each stage. Because amastigotes can transform Sb(V) to Sb(III), they are more sensitive to Sb(V), while promastigotes are unable to. Many patients’ incorrect antimonial therapy causes pharmacological stress on the parasite organisms, resulting in adaptation and, finally, persistence against Sb(V).103,104
The gradual evolution of antimony resistance raises the notion that numerous mutations need to occur to produce a resistant phenotype. Several in vitro processes may clarify the reported antimonial resistance, albeit it should be noted that the in vitro response is not always translated into clinical resistance. The reduction in the concentration of drugs in the parasitic protozoan may be due to reduced absorption or by higher outflow of medicine; drug activity suppression; deactivation of the active drug; and gene amplification, all possible explanations for resistance evolution.105 Sb(III) resistance relates to TXNPx overexpression and greater intracellular thiol levels. In vivo, antimonial resistance was demonstrated by inhibiting Sb(V) activity and decreasing amastigotes’ absorption of active Sb(III) in thiol production. The gene encoding aquaglyceroporin 1, gamma-glutamylcysteine synthetase, and ornithine decarboxylase, which are involved in Sb(III) uptake and glutathione and trypanothione digestion, was lowered throughout the procedure.106,107 The overexpression of membrane ABC transporters on the surface parasites is also the reason for the resistance. This transport route influences drug efflux and intracellular accumulation, which contribute to resistance development. Elimination of the drug as a metal–thiol in combination with ABC carriers such as ABCI4 and ABCG2 may improve antimony resistance. Sb-resistant L. donovani parasites also boost host cell production of the MRP, and the P-gp, reducing antimony influx and hence suppressing drug accumulation inside the cell. For the binding of parasites, the cholesterol membrane of the host is required as well to invade phagocytes.108 Cholesterol is an important lipid membrane component in eukaryotic organisms, where it aids in the structure, dynamics, and activity of its constituents. Statins, such as lovastatin, work by inhibiting HMG-CoA reductase, which is the rate-limiting enzyme in the cholesterol production pathway. Lovastatin inhibits the proteins MRP1 and P- glycoprotein in L. donovani, enabling antimony to accumulate and decreasing Leishmanial cell growth, macrophage infection, and causing its death.109 As a result, the statin class mitigates the Sb resistance. Flavonoids naturally inhibit P-glycoprotein and its related ABC transporters in Leishmania. Synthetic flavonoid dimers were employed to overcome antimony drug resistance in L. donovani, enhancing intracellular drug accumulation.110,111 Heat shock proteins (HSP70) were found to play a function in antimony tolerance by employing functional cloning to extract drug-resistance genes. Antimony and Sb(III)-resistant variant cells were found to have high levels of HSP70 proteins.
Transfected Leishmania species developed antimony resistance, most likely due to improved cell tolerance to metals; this enabled the cell to become a more specialized and more efficient defense activity. HSP90 has been related to the development of resistance in Leishmania in recent research. In summary, antimonial resistance is a difficult issue. Several antimonial resistance mechanisms in experimental Leishmania isolates have been identified.112,113
4.3. Miltefosine
An alkyl phospholipid called miltefosine (also known as hexadecyl phosphocholine, or MT) was first created as an anticancer medication. As MT received approval as an initial oral medication for VL in India in 2002. MT is now used for treating VL as well as CL diseases, as it is the first-line treatment for CL. It is easier to acquire and has lesser toxicities when compared to antimonials.114 Hepatotoxicity and nephrotoxicity are two of the medication’s side effects. The main disadvantages of MT involve its teratogenicity, the possibility of resistance resulting from its extended half-life (7 days), the occurrence of subtherapeutic dosages over time, and the high price.115 Phosphatidylcholine formation is inhibited by MT, changing the phospholipid biosynthesis. The proposed mechanism of action begins with adhesion to the plasma membrane and then proceeds to internalization via 2 protein membranes: the miltefosine transporter (LdMT), as Ld stands for the species L. donovani; this transporter is a member of the P4-ATPase subfamily, as well as possessing LdRos3, a potential noncatalytic component of LdMT. These 2 proteins reside mostly in the cell membrane of Leishmania and present as an important factor for the quick intracellular absorption of alkyl phosphocholine medications. LdMT and LdRos3 combined to form a stable protein assembly, allowing phospholipids to migrate across the cell membrane from the exoplasmic to the cytoplasmic sections.116,117 It is also shown that MT inhibits mitochondrial cytochrome c oxidase, resulting in a decrease in the L. donovani oxygen utilization rate and ATP levels. In addition to other immunologic and inflammatory effects on macrophages, MT was discovered to cause the death of cells during the promastigote phase of L. donovani through programmed cell death. Considering the most recent emergence of miltefosine use, therapeutically resistant parasites were observed in Nepal in the occurrence of VL. In the lab, mutants were generated to forecast the development of MT resistance and define the mutants that resulted.118
In the Indian subcontinent, miltefosine-resistant parasites have been discovered. In the laboratory, 2 strains of visceral leishmaniasis with evolved miltefosine resistance (L. donovani) were morphologically as well as genotypically characterized.119 Even though there are just a few MT-resistant clinical strains, their genetic and biological characteristics are strikingly similar to those of laboratory-selected strains. Although the precise mechanism of MT resistance is unknown, every miltefosine-resistant Leishmania strain examined demonstrated a reduction in drug accumulation. This could be because of decreased drug absorption, increased efflux, faster metabolic rate, and altered cell membrane permeability. A single locus variation within the LdMT gene is two different alleles rendering the transporter protein LdMT inactive. The LdMT mutations in genes L832F, T420N, or L856P increased susceptibility (includes in vitro and in vivo), as well as decreased absorption, greater emission, quicker metabolic processes, and alterations in the lipid makeup of parasitic cell membranes. Other LdMT gene variants such as V176D, W210, the lately found Y354F, and F1078Y, M1 mutation of LdRos3.120 Overexpression of ABC carriers has been identified as another avenue for MT resistance. The first molecule associated with experimental MT resistance was LMDR1/ABCB4, a P-glycoprotein-like transporter found among ABC transporters. Leishmania is more resistant to MT when the ABC transporters such as ABCB4 (MDR1), ABCG4, and ABCG6 are overexpressed, resulting in a decrease in their accumulation inside the cell because of increased drug efflux across a cell membrane.121,122 Changes in MT-resistant promastigotes’ membrane lipid content sterol production may also alter drug-membrane interactions. Recently, it was revealed that the use of cosmid-based functional cloning in conjunction with next-generation sequencing genes implicated in ergosterol production and translocation of phospholipid could result in L. infantum resistance.120
To fight MT resistance, several substances have been produced. Sesquiterpenes have been found to overcome multidrug resistance with the help of ABC transporters and by boosting intracellular drug concentration. An additional flavonoid analogue is effective in reversing LMDR1 overexpression at modest dosages. Sitamaquine, an 8-aminoquinoline, reverses LMDR1-mediated MT resistance by elevating intracellular drug accumulation, resulting in an effective LMDR1-mediated miltefosine resistance reversal agent in this parasitic organism.123
4.4. Amphotericin B
As the second-line therapy for VL, amphotericin B (AMB) is a polyene medication produced by the Streptomyces nodosus filamentous bacterium. It is the most effective drug for pentavalent antimonial resistance.124 Although it has substantial adverse reactions, including severe renal toxicity, which necessitated inpatient treatment and 4 weeks of patient monitoring. Another significant downside is the expensive price of AMB. To overcome these limitations, liposomal AMB (LAMB), a lipid-associated composition with less harmful effects and a prolonged plasma lifespan that allows for a single infusion, was developed. To treat leishmaniasis, oral formulations of AMB are being developed. AMB’s mechanism of activity could include interacting with surface sterols, resulting in membrane disarray and increased accessibility of protons and monovalent cations.125 AMB may influence cells due to oxidation along with the following creation of reactive oxygen species. Cell damage produced by AMB may be linked to ion mobility, oxidative effects, and the formation of ROS. Because of the advent of drug resistance to past therapies, amphotericin B has become a more important therapy for leishmaniasis. Because there are few options, the emergence of AMB resistance is a real possibility. As a result, figuring out how AMB resistance develops is a primary priority, which led to the development of laboratory-based amphotericin B, species that are resistant to it.126 A recurrence incidence of about 3.7% following therapy with liposomal amphotericin demonstrates that resistance to LAMB can also emerge. Regardless of this minimal risk, recurrence is important in transmission dynamics because it increases the worldwide population of parasitic organisms in the host that are set to be transmitted to the vector; in persons with HIV who are not receiving antiretroviral medication, VL recurrence raises the possibility of spreading by suppressing the immune system, raising the parasite burden, along with a lack of responsiveness to treatment, and there is a chance that parasites may become resistant to antileishmanial drugs.127
To predict the emergence of resistance, several experimental approaches have been developed. Many Leishmania species are resistant to AMB. Promastigotes were selected and studied through elevated drug pressure. Many alterations were detected when the biological properties of these resistant varieties were examined in the wild-type parent strain. Variation within sterol biosynthesis, enzyme lanosterol 14-demethylase (CYP51) was shown to have a function in L. mexicana. Several AMB-resistant Leishmania strains exhibited genetic changes in other sterol biosynthesis enzymes, including sterol C24-methyltransferase (SMT), which adds the C24-methyl group to the ergosterol side chain, and sterol C5-desaturase (SC5D), which is required for sterol 5(6)-7(8) double- bond pairing.128 Overall, resistance is associated with decreased AMB adherence to a cell membrane due to sterol modifications (declination of SMT gene expression). MDR1 effluxes AMB from the membrane, whereas the rest of the intracellular AMB auto-oxidizes and generates ROS. The thiol metabolic pathway’s tryparedoxin cascade may be able to counteract the adverse effects of this ROS. The cumulative effects of transformed membrane characteristics, including MDR1 and the tryparedoxin cascade, might be the cause of AMB-resistance.129
4.5. Paromomycin (PMM)
PMM is an aminoglycoside medication that was approved for the therapy of VL in 2006. PMM tends to be tolerated effectively, can be given orally or intramuscularly, and has few side effects.130 PMM inhibits the production of proteins by interfering with the ribosomal subunits and enhances the ribosomal subunit interaction in bacterial infections. Escherichia coli was discovered to connect to the major cleft at the A-site of the 16S rRNA sequence and to activate mRNA misreading. However, the method through which it works in Leishmania is uncertain. PMM may inhibit protein production in Leishmania parasites by binding to the 16S ribosomal unit and creating a specific structural alteration in the 16S rRNA’s A site. Alteration at the N1 sites of A1492 and A1493 on the minor groove regions of the A-site RNA showed modal activity during the translational process. Changes in membrane fluidity and lipid metabolism, a decrease in mitochondrial membrane potential, and respiratory failure have all been proposed as possible mechanisms of action.131,132 Endocytosis increases PMM intake by binding PMM to parasitic membrane proteins like PFR 1D and 2D, inhibiting, as well as a P-type H+ ATPase, the main purpose of which is to induce endocytosis thus allowing the drug within vacuoles.133 PMM provides several benefits, including affordable prices, quick administration, good protection characteristics, and convenience of use. Paromomycin’s physicochemical nature, on the other hand, prevents appropriate accumulation at the location of infection. The application of solid nanoparticles of lipids as a PMM transport vehicle increased drug absorption into macrophages, raised immunological response, and hence improved PMM efficacy.134 To target Leishmania parasites in monocytes, a combination of albumin microspheres infused with PMM was created for VL therapy. This formulation has the advantage of preferentially targeting macrophages, which means less toxicity and an easier procedure over an intramuscular injection.135 PMM is additionally combined with stearyl amine (SA)-containing phosphatidylcholine (PC) liposomes. PMM-SA-PM revealed enhanced immune protection, antileishmanial activity, and no toxicity.136
5. Conclusions
Owing to the variation in the parasitic class, it is extremely difficult to develop drugs that can be useful in curing diseases triggered by distinct species or subspecies. Certain-omics-based studies (metabolomics, genomics, proteomics, and transcriptomics) have also focused on how the resistivity is enhanced and demonstrated in the Leishmania genus due to the synthesis of specific proteins that provide such traits. Furthermore, because these interactions are crucial for the success of treatments, it is necessary to explore the host-parasite interactions. Gene editing in parasites is an intriguing method for learning more about proteins that aid in drug metabolism. There are now various techniques for genetically modifying Leishmania, including deletion via allelic substitution, overexpression, and heterologous expression. In addition, parasite genome sequencing can work as a novel and crucial method, since the parasitic genes could be a potential therapeutic target for drug discovery, and the data collected could act as a beneficiary repository for TritrypDB-like databases focused on Leishmania.
Despite all of the tools available, studying the resistant effect exhibited by parasites needs in vitro and artificial laboratory-based genetic manipulation like the ones that are exhibited by the parasite inside the host organism owing to their genetic plasticity and vulnerable environment tolerance capacity. Because parasites can undergo various modifications in vitro to survive, it was proposed that genetic changes be undertaken directly in clinical specimens to avoid experimental artifacts. Similarly, gene deletion can lead to the selection of parasitic organisms with aneuploidies and altered phenotypes that are not found in nature. Unfortunately, inducible strategies for modulating gene expression in Leishmania between active and inactive states are currently being researched, and little is known about their use. The diversity of the Leishmania genus and the factors that determine diversity are the subject of this review. We emphasize the need to apply genetic manipulation methods to learn more about leishmaniasis drug resistance mechanisms and chemotherapeutic targets. Single-cell sequencing will allow the discovery of numerous new parasite species that were previously unknown. An integrated study that combines data from these many approaches and aspects of host-parasite linkages would improve understanding of the intricacies of medication, resistance mechanisms, therapeutic failure, and the sly parasites’ incredible durability. This understanding will pave the path for the development of more effective treatment choices as well as the discovery of new therapeutic targets. It will also help to create preventive techniques to stop the spread of drug-resistant strains, lowering the burden of leishmaniasis on affected people.
Acknowledgments
The authors are thankful to the Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research Group Project under grant no. R.G.P.2/557/44. We are also thankful to Department of Life Sciences, Parul Institute of Applied Sciences and Research & Development Cell, Parul University, Vadodara, Gujarat, India 391760.
Author Contributions
K.B. and R.S.K. conceived and designed the study. K.B. and R.S.K. conducted the literature search and data collection. R.S.K. and T.K.U. performed data analysis and compilation. K.B. and R.S.K. wrote the initial draft of the manuscript. R.S.K. and T.K.U. provided critical revisions and intellectual input. All authors have read and approved the final manuscript.
This research was funded by the Deanship of Scientific Research at King Khalid University for funding this work through the large Research Group Project under grant number RGP.02/317/44.
The authors declare no competing financial interest.
References
- Stuart K.; Brun R.; Croft S.; Fairlamb A.; Gurtler R. E.; McKerrow J.; Reed S.; Tarleton R. Kinetoplastids: related protozoan pathogens, different diseases. J. Clin. Invest. 2008, 118 (4), 1301–1310. 10.1172/JCI33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S. Leishmania. Advances in parasitology 1964, 2, 35–96. 10.1016/S0065-308X(08)60586-2. [DOI] [PubMed] [Google Scholar]
- Lazar L. T. Y.; Abass K. S. Morphology, life cycle, pathogenesis and virulence factors of genus Leishmania: a review. Plant Archives 2020, 20 (2), 4057–4060. [Google Scholar]
- Sasidharan S.; Saudagar P. Leishmaniasis: where are we and where are we heading?. Parasitology research 2021, 120, 1541–1554. 10.1007/s00436-021-07139-2. [DOI] [PubMed] [Google Scholar]
- Cecílio P.; Cordeiro-da-Silva A.; Oliveira F. Sand flies Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Communications Biology 2022, 5 (1), 305. 10.1038/s42003-022-03240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M.; Welburn S. C. Leishmaniasis beyond east Africa. Frontiers in Veterinary Science 2021, 8, 618766 10.3389/fvets.2021.618766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S.; Frasca K.; Scherrer S.; Henao-Martínez A. F.; Newman S.; Ramanan P.; Suarez J. A. A review of leishmaniasis: current knowledge and future directions. Current tropical medicine reports 2021, 8, 121–132. 10.1007/s40475-021-00232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar B.; Shelper T. B.; Avery V. M. Leishmaniasis drug discovery: recent progress and challenges in assay development. Drug discovery today 2017, 22 (10), 1516–1531. 10.1016/j.drudis.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Wijnant G. J.; Dumetz F.; Dirkx L.; Bulté D.; Cuypers B.; Van Bocxlaer K.; Hendrickx S. Tackling drug resistance and other causes of treatment failure in leishmaniasis. Frontiers in Tropical Diseases 2022, 3, 837460–22. 10.3389/fitd.2022.837460. [DOI] [Google Scholar]
- Santi A. M. M.; Murta S. M. F. Impact of genetic diversity and genome plasticity of Leishmania spp. in treatment and the search for novel chemotherapeutic targets. Frontiers in Cellular and Infection Microbiology 2022, 12, 9. 10.3389/fcimb.2022.826287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.; Madhu M.; Murti K. An overview on leishmaniasis. Viral, Parasitic, Bacterial, and Fungal Infections 2023, 389–406. 10.1016/B978-0-323-85730-7.00055-2. [DOI] [Google Scholar]
- Palić S.; Beijnen J. H.; Dorlo T. P. An update on the clinical pharmacology of miltefosine in the treatment of leishmaniasis. Int. J. Antimicrob. Agents 2022, 59 (1), 106459 10.1016/j.ijantimicag.2021.106459. [DOI] [PubMed] [Google Scholar]
- Kumari S.; Kumar V.; Tiwari R. K.; Ravidas V.; Pandey K.; Kumar A. Amphotericin B: A drug of choice for Visceral Leishmaniasis. Acta Tropica 2022, 235, 106661 10.1016/j.actatropica.2022.106661. [DOI] [PubMed] [Google Scholar]
- Matos A. P. S.; Viçosa A. L.; Ré M. I.; Ricci-Júnior E.; Holandino C. A review of current treatments strategies based on paromomycin for leishmaniasis. Journal of Drug Delivery Science and Technology 2020, 57, 101664 10.1016/j.jddst.2020.101664. [DOI] [Google Scholar]
- van Griensven J.; Dorlo T. P.; Diro E.; Costa C.; Burza S. The status of combination therapy for visceral leishmaniasis: an updated review. The Lancet Infectious Diseases 2023, 24, E36–E46. 10.1016/S1473-3099(23)00353-5. [DOI] [PubMed] [Google Scholar]
- Demicheli C.; Vallejos V. M. R.; Lanza J. S.; Ramos G. S.; Do Prado B. R.; Pomel S.; Loiseau P. M.; Frezard F. Supramolecular assemblies from antimony (V) complexes for the treatment of leishmaniasis. Biophysical Reviews 2023, 15, 751. 10.1007/s12551-023-01073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos S. S.; de Araújo R. V.; Giarolla J.; El Seoud O.; Ferreira E. I. Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis: a review. International journal of antimicrobial agents 2020, 55 (4), 105906 10.1016/j.ijantimicag.2020.105906. [DOI] [PubMed] [Google Scholar]
- Momeni A. Z.; Jalayer T.; Emamjomeh M.; Bashardost N.; Ghassemi R. L.; Meghdadi M.; Aminjavaheri M. Treatment of cutaneous leishmaniasis with itraconazole: randomized double-blind study. Arch. Dermatol. 1996, 132 (7), 784–786. [PubMed] [Google Scholar]
- Martinez S.; Gonzalez M.; Vernaza M. E. Treatment of cutaneous leishmaniasis with allopurinol and stibogluconate. Clinical Infectious Diseases 1997, 24 (2), 165–169. 10.1093/clinids/24.2.165. [DOI] [PubMed] [Google Scholar]
- Nico D.; Conde L.; Palatnik de Sousa C. B.. Classical and Modern Drug Treatments for Leishmaniasis; Antiprotozoal Drug Development and Delivery, 2021; pp 1–21. [Google Scholar]
- Loiseau P. M.; Cojean S.; Schrével J. Sitamaquine as a putative antileishmanial drug candidate: from the mechanism of action to the risk of drug resistance. Parasite: journal de la Société Française de Parasitologie 2011, 18 (2), 115. 10.1051/parasite/2011182115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Nava G.; Tirado-Sánchez A.; Fernández-Figueroa E. A.; Becker I.; Bonifaz A.. Efficacy of imiquimod 5% cream as first-line management in cutaneous leishmaniasis caused by Leishmania mexicana. Revista da Sociedade Brasileira de Medicina Tropical 2021, 54, 10.1590/0037-8682-0305-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho E.; González-de la Fuente S.; Solana J. C.; Rastrojo A.; Carrasco-Ramiro F.; Requena J. M.; Aguado B. Gene annotation and transcriptome delineation on a de novo genome assembly for the reference Leishmania major Friedlin strain. Genes 2021, 12 (9), 1359. 10.3390/genes12091359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterkers Y.; Crobu L.; Lachaud L.; Pagès M.; Bastien P. Parasexuality and mosaic aneuploidy in Leishmania: alternative genetics. Trends in parasitology 2014, 30 (9), 429–435. 10.1016/j.pt.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Iantorno S. A.; Durrant C.; Khan A.; Sanders M. J.; Beverley S. M.; Warren W. C.; Berriman M.; Sacks D. L.; Cotton J. A.; Grigg M. E. Gene expression in Leishmania is regulated predominantly by gene dosage. MBio 2017, 8 (5), 10–1128. 10.1128/mBio.01393-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeu D. C.; Teixeira S. M. R.; Cruz A. K. Genomics and functional genomics in Leishmania and Trypanosoma cruzi: statuses, challenges and perspectives. Memórias do Instituto Oswaldo Cruz 2021, 116, e200634 10.1590/0074-02760200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivens A. C.; Peacock C. S.; Worthey E. A.; Murphy L.; Aggarwal G.; Berriman M.; Myler P. J. The genome of the kinetoplastid parasite Leishmania major. Science 2005, 309 (5733), 436–442. 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackay A.; Cotton J. A.; Sanders M.; Hailu A.; Nasereddin A.; Warburg A.; Jaffe C. L. Genome wide comparison of Ethiopian Leishmania donovani strains reveals differences potentially related to parasite survival. PLoS genetics 2018, 14 (1), e1007133 10.1371/journal.pgen.1007133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura H.; Monsieurs P.; Jara M.; Sanders M.; Maes I.; Vanaerschot M.; Berriman M.; Cotton J. A.; Dujardin J.-C.; Domagalska M. A. Evaluation of whole genome amplification and bioinformatic methods for the characterization of Leishmania genomes at a single cell level. Sci. Rep. 2020, 10 (1), 15043 10.1038/s41598-020-71882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto Leite C. M.; Mariano-Neto E.; Rocha P. L. B. D. Biodiversity thresholds in invertebrate communities: the responses of dung beetle subgroups to forest loss. PLoS One 2018, 13 (8), e0201368 10.1371/journal.pone.0201368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska M. A.; Imamura H.; Sanders M.; Van den Broeck F.; Bhattarai N. R.; Vanaerschot M.; Maes I.; D’Haenens E.; Rai K.; Rijal S.; Berriman M.; Cotton J. A.; Dujardin J.-C. Genomes of Leishmania parasites directly sequenced from patients with visceral leishmaniasis in the Indian subcontinent. PLoS Neglected Tropical Diseases 2019, 13 (12), e0007900 10.1371/journal.pntd.0007900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douanne N.; Dong G.; Amin A.; Bernardo L.; Blanchette M.; Langlais D.; Olivier M.; Fernandez-Prada C. Leishmania parasites exchange drug-resistance genes through extracellular vesicles. Cell reports 2022, 40 (3), 111121. 10.1016/j.celrep.2022.111121. [DOI] [PubMed] [Google Scholar]
- Laffitte M. C. N.; Leprohon P.; Papadopoulou B.; Ouellette M. Plasticity of the Leishmania genome leading to gene copy number variations and drug resistance. F1000Research 2016, 5, 2350. 10.12688/f1000research.9218.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprohon P.; Legare D.; Raymond F.; Madore E.; Hardiman G.; Corbeil J.; Ouellette M. Gene expression modulation is associated with gene amplification, supernumerary chromosomes and chromosome loss in antimony-resistant Leishmania infantum. Nucleic acids research 2009, 37 (5), 1387–1399. 10.1093/nar/gkn1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnielli J. B.T.; Crouch K.; Forrester S.; Silva V. C.; Carvalho S. F.G.; Damasceno J. D.; Brown E.; Dickens N. J.; Costa D. L.; Costa C. H.N.; Dietze R.; Jeffares D. C.; Mottram J. C. A Leishmania infantum genetic marker associated with miltefosine treatment failure for visceral leishmaniasis. EBioMedicine 2018, 36, 83–91. 10.1016/j.ebiom.2018.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva M. S. DNA double-strand breaks a double-edged sword for trypanosomatids. Frontiers in Cell and Developmental Biology 2021, 9, 945. 10.3389/fcell.2021.669041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasceno J. D.; Marques C. A; Beraldi D.; Crouch K.; Lapsley C.; Obonaga R.; Tosi L. R.; McCulloch R. Genome duplication in Leishmania major relies on persistent subtelomeric DNA replication. eLife 2020, 9, e58030 10.7554/eLife.58030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H.; Cáceres A. G.; Gomez E. A.; Tabbabi A.; Mizushima D.; Yamamoto D. S.; Hashiguchi Y. Prevalence of genetically complex Leishmania strains with hybrid and mito-nuclear discordance. Frontiers in Cellular and Infection Microbiology 2021, 11, 625001 10.3389/fcimb.2021.625001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro H.; Tomás A. M. Peroxidases of trypanosomatids. Antioxidants & redox signaling. Antioxidants Redox Signaling 2008, 10 (9), 1593–1606. 10.1089/ars.2008.2050. [DOI] [PubMed] [Google Scholar]
- Iyer J. P.; Kaprakkaden A.; Choudhary M. L.; Shaha C. Crucial role of cytosolic tryparedoxin peroxidase in Leishmania donovani survival, drug response and virulence. Molecular microbiology 2008, 68 (2), 372–391. 10.1111/j.1365-2958.2008.06154.x. [DOI] [PubMed] [Google Scholar]
- Day B. J. Catalase and glutathione peroxidase mimics. Biochemical pharmacology 2009, 77 (3), 285–296. 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo A.; Colotti G.; Boffi A.; Baiocco P.; Ilari A. The crystal structures of the tryparedoxin-tryparedoxin peroxidase couple unveil the structural determinants of Leishmania detoxification pathway 2012, 6, e1781. 10.1371/journal.pntd.0001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade J. M.; Murta S. M. Functional analysis of cytosolic tryparedoxin peroxidase in antimony-resistant and–susceptible Leishmania braziliensis and Leishmania infantum lines. Parasites vectors 2014, 7, 406. 10.1186/1756-3305-7-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M.; Légaré D.; Haimeur A.; Grondin K.; Roy G.; Brochu C.; Papadopoulou B. ABC transporters in Leishmania and their role in drug resistance. Drug Resistance Updates 1998, 1 (1), 43–48. 10.1016/S1368-7646(98)80213-6. [DOI] [PubMed] [Google Scholar]
- Croft S. L.; Sundar S.; Fairlamb A. H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19 (1), 111–126. 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik P. K.; Alam M. N.; Roy Chowdhury D.; Chakraborti T. Drug resistance in protozoan parasites: an incessant wrestle for survival. Journal of Global Antimicrobial Resistance 2019, 18, 1–11. 10.1016/j.jgar.2019.01.023. [DOI] [PubMed] [Google Scholar]
- Leprohon P.; Fernandez-Prada C.; Gazanion É.; Monte-Neto R.; Ouellette M. Drug resistance analysis by next generation sequencing in Leishmania. International Journal for Parasitology: Drugs and Drug Resistance 2015, 5 (1), 26–35. 10.1016/j.ijpddr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boozhmehrani M. J.; Eslami G.; Khamesipour A.; Jafari A. A.; Vakili M.; Hosseini S. S.; Askari V. The role of ATP-binding cassette transporter genes expression in treatment failure cutaneous leishmaniasis. AMB Express 2022, 12 (1), 78. 10.1186/s13568-022-01419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastrojo A.; Garcia-Hernandez R.; Vargas P.; Camacho E.; Corvo L.; Imamura H.; Dujardin J.-C.; Castanys S.; Aguado B.; Gamarro F.; Requena J. M. Genomic and transcriptomic alterations in Leishmania donovani lines experimentally resistant to antileishmanial drugs. International Journal for Parasitology: Drugs and Drug Resistance 2018, 8 (2), 246–264. 10.1016/j.ijpddr.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprohon P.; Légaré D.; Ouellette M. ABC transporters involved in drug resistance in human parasites. Essays in biochemistry 2011, 50, 121–144. 10.1042/bse0500121. [DOI] [PubMed] [Google Scholar]
- Yao C.; Donelson J. E.; Wilson M. E. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Molecular and biochemical parasitology 2003, 132 (1), 1–16. 10.1016/S0166-6851(03)00211-1. [DOI] [PubMed] [Google Scholar]
- Passalacqua T. G.; Torres F. A.E.; Nogueira C. T.; de Almeida L.; Del Cistia M. L.; dos Santos M. B.; Regasini L. O.; Graminha M. A.S.; Marchetto R.; Zottis A. The 2′, 4′-dihydroxychalcone could be explored to develop new inhibitors against the glycerol-3- phosphate dehydrogenase from Leishmania species. Bioorg. Med. Chem. Lett. 2015, 25 (17), 3564–3568. 10.1016/j.bmcl.2015.06.085. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf E.; Etges R.; Metcalf P. The crystal structure of the Leishmania major surface proteinase leishmanolysin (gp63). Structure 1998, 6 (8), 1035–1046. 10.1016/S0969-2126(98)00104-X. [DOI] [PubMed] [Google Scholar]
- Mercado-Camargo J.; Cervantes-Ceballos L.; Vivas-Reyes R.; Pedretti A.; Serrano-García M. L.; Gómez-Estrada H. Homology modeling of leishmanolysin (gp63) from Leishmania panamensis and molecular docking of flavonoids. ACS omega 2020, 5 (24), 14741–14749. 10.1021/acsomega.0c01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C.; Donelson J. E.; Wilson M. E. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Molecular and biochemical parasitology 2003, 132 (1), 1–16. 10.1016/S0166-6851(03)00211-1. [DOI] [PubMed] [Google Scholar]
- Olivier M.; Atayde V. D.; Isnard A.; Hassani K.; Shio M. T. Leishmania virulence factors: focus on the metalloprotease GP63. Microbes and infection 2012, 14 (15), 1377–1389. 10.1016/j.micinf.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R.; Bhattacharjee H.; Rosen B. P. Aquaglyceroporins: generalized metalloid channels. Biochimica et Biophysica Acta (BBA)-General Subjects 2014, 1840 (5), 1583–1591. 10.1016/j.bbagen.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto R. L. D. M.; Laffitte M. C. N.; Leprohon P.; Reis P.; Frézard F.; Ouellette M. Intrachromosomal Amplification, Locus Deletion and Point Mutation in the Aquaglyceroporin AQP1 Gene in Antimony Resistant Leishmania (Viannia) guyanensis 2015, 9, e0003476. 10.1371/journal.pntd.0003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhas R.; Tripathi P.; Khan S.; Sethu Lakshmi B.; Lal S. K.; Gowri V. S.; Sharma A.; Madhubala R. Identification and functional characterization of a novel bacterial type asparagine synthetase A: a tRNA synthetase paralog from Leishmania donovani. J. Biol. Chem. 2014, 289 (17), 12096–12108. 10.1074/jbc.M114.554642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. George S.; Bishop J. V.; Titus R. G.; Selitrennikoff C. P. Novel compounds active against Leishmania major. Antimicrobial agents and chemotherapy 2006, 50 (2), 474–479. 10.1128/AAC.50.2.474-479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzy A.; Larrouy-Maumus G.; Bottai D.; Levillain F.; Dumas A.; Wallach J. B.; Neyrolles O. Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathogens 2014, 10 (2), e1003928 10.1371/journal.ppat.1003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J.; Srivastava A.; Jha P.; Sinha K. K.; Kundu B. L-Asparaginase as a new molecular target against leishmaniasis: insights into the mechanism of action and structure-based inhibitor design. Molecular biosystems 2015, 11 (7), 1887–1896. 10.1039/C5MB00251F. [DOI] [PubMed] [Google Scholar]
- Romero I.; Téllez J.; Romanha A. J.; Steindel M.; Grisard E. C. Upregulation of cysteine synthase and cystathionine β-synthase contributes to Leishmania braziliensis survival under oxidative stress. Antimicrob. Agents Chemother. 2015, 59 (8), 4770–4781. 10.1128/AAC.04880-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. A.; Westrop G. D.; Coombs G. H. Two pathways for cysteine biosynthesis in Leishmania major. Biochemical Journal. Biochemical J. 2009, 420 (3), 451–462. 10.1042/BJ20082441. [DOI] [PubMed] [Google Scholar]
- Saxena V. K.; Vedamurthy G. V.; Swarnkar C. P.; Kadam V.; Onteru S. K.; Ahmad H.; Singh R. De novo pathway is an active metabolic pathway of cysteine synthesis in Haemonchus contortus. Biochimie 2021, 187, 110–120. 10.1016/j.biochi.2021.05.014. [DOI] [PubMed] [Google Scholar]
- Sowerby K.; Freitag-Pohl S.; Murillo A. M.; Silber A. M.; Pohl E. Cysteine synthase: multiple structures of a key enzyme in cysteine synthesis and a potential drug target for Chagas disease and leishmaniasis. Acta Crystallographica Section D: Structural Biology 2023, 79 (6), 518. 10.1107/S2059798323003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L.; Laranjeira-Silva M. F.; Maeda F. Y.; Hauzel J.; Andrews N. W.; Mittra B. Ascorbate-Dependent peroxidase (APX) from Leishmania amazonensis is a reactive oxygen species-induced essential enzyme that regulates virulence. Infect. Immun. 2019, 87 (12), 10–1128. 10.1128/IAI.00193-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. K.; Gorain B.; Choudhury H.; Singh S. K.; Whadwa P.; Sahu S.; Kesharwani P. Macrophage targeted amphotericin B nanodelivery systems against visceral leishmaniasis. Materials Science and Engineering: B 2020, 258, 114571 10.1016/j.mseb.2020.114571. [DOI] [Google Scholar]
- Kashif M.; Paladhi A.; Singh R.; Bhattacharyya S.; Hira S. K.; Manna P. P.. Leishmanicidal activity of an in silico-screened novel inhibitor against ascorbate peroxidase of Leishmania donovani. Antimicrobial Agents and Chemotherapy 2020, 64( (7), ), 10.1128/AAC.01766-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar L.; Coron R. P.; KongThoo Lin P.; Costa D. M.; Perez-Cabezas B.; Tavares J.; Cordeiro-da-Silva A. Inhibitors of Trypanosoma cruzi Sir2 related protein 1 as potential drugs against Chagas disease. PLoS neglected tropical diseases 2018, 12 (1), e0006180 10.1371/journal.pntd.0006180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronin C.; Costa D. M.; Tavares J.; Faria J.; Ciesielski F.; Ciapetti P.; Smith T. K.; MacDougall J.; Cordeiro-da-Silva A.; Pemberton I. K. The crystal structure of the Leishmania infantum Silent Information Regulator 2 related protein 1: Implications to protein function and drug design. PLoS One 2018, 13 (3), e0193602 10.1371/journal.pone.0193602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal N.; Muthuswami R.; Madhubala R. The mitochondrial SIR2 related protein 2 (SIR2RP2) impacts Leishmania donovani growth and infectivity. PLoS Neglected Tropical Diseases 2017, 11 (5), e0005590 10.1371/journal.pntd.0005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva G. I.; Waterman M. R. Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis. Current topics in medicinal chemistry 2011, 11 (16), 2060–2071. 10.2174/156802611796575902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza W.; Rodrigues J. C. F. Sterol Biosynthesis Pathway As Target for Anti-Trypanosomatid Drugs. Interdiscip Perspect Infect Dis. 2009, 2009, 642502. 10.1155/2009/642502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. T. Jr; Gaylor J. L. Isolation and purification of an S-adenosylmethionine: Δ24-sterol methyltransferase from yeast. J. Biol. Chem. 1969, 244 (23), 6334–6340. 10.1016/S0021-9258(18)63470-2. [DOI] [PubMed] [Google Scholar]
- Laffitte M. C. N.; Leprohon P.; Papadopoulou B.; Ouellette M. Plasticity of the Leishmania genome leading to gene copy number variations and drug resistance. F1000Research 2016, 5, 2350. 10.12688/f1000research.9218.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha M.; Sarkar A. Review on multiple facets of drug resistance: a rising challenge in the 21st century. Journal of xenobiotics 2021, 11 (4), 197–214. 10.3390/jox11040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Kerkhof M.; Sterckx Y. G. J.; Leprohon P.; Maes L.; Caljon G. Experimental strategies to explore drug action and resistance in kinetoplastid parasites. Microorganisms 2020, 8 (6), 950. 10.3390/microorganisms8060950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana J. F.; Field M. C. Evolution, function and roles in drug sensitivity of trypanosome aquaglyceroporins. Parasitology 2021, 148 (10), 1137–1142. 10.1017/S0031182021000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopu B.; Kour P.; Pandian R.; Singh K. Insights into the drug screening approaches in leishmaniasis. International Immunopharmacology 2023, 114, 109591 10.1016/j.intimp.2022.109591. [DOI] [PubMed] [Google Scholar]
- Shtaiwi A. Thiadiazine-thiones as inhibitors of leishmania pteridine reductase (PTR1) target: Investigations and in silico approach. J. Biomol. Struct. Dyn. 2023, 1–10. 10.1080/07391102.2023.2246589. [DOI] [PubMed] [Google Scholar]
- Herrera-Acevedo C.; de Menezes R. P. B.; de Sousa N. F.; Scotti L.; Scotti M. T.; Coy-Barrera E. Kaurane-Type Diterpenoids as Potential Inhibitors of Dihydrofolate Reductase-Thymidylate Synthase in New World Leishmania Species. Antibiotics 2023, 12 (4), 663. 10.3390/antibiotics12040663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx S.; Van Bockstal L.; Bulte D.; Mondelaers A.; Aslan H.; Rivas L.; Maes L.; Caljon G. Phenotypic adaptations of Leishmania donovani to recurrent miltefosine exposure and impact on sand fly infection. Parasites & vectors 2020, 13, 96. 10.1186/s13071-020-3972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S.; Ali V.; Singh Y.; Kanojia S.; Goyal N. Leishmania donovani chaperonin TCP1γ subunit protects miltefosine induced oxidative damage. Int. J. Biol. Macromol. 2020, 165, 2607–2620. 10.1016/j.ijbiomac.2020.10.134. [DOI] [PubMed] [Google Scholar]
- Pradhan S.; Schwartz R. A.; Patil A.; Grabbe S.; Goldust M. Treatment options for leishmaniasis. Clinical and experimental dermatology 2022, 47 (3), 516–521. 10.1111/ced.14919. [DOI] [PubMed] [Google Scholar]
- Kröber-Boncardo C.New Insights into Leishmania Stress Tolerance: The Impact of the Small Heat Shock Protein 23 and Casein Kinase 1.2, Doctoral dissertation, Staats-und Universitätsbibliothek Hamburg Carl von Ossietzky, 2020. [Google Scholar]
- Carolus H.; Pierson S.; Lagrou K.; Van Dijck P. Amphotericin B and other polyenes—Discovery, clinical use, mode of action, and drug resistance. Journal of Fungi 2020, 6 (4), 321. 10.3390/jof6040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P.; Saha S.; BoseDasgupta S. The ultimate fate determinants of drug induced cell-death mechanisms in Trypanosomatids. International Journal for Parasitology: Drugs and Drug Resistance 2021, 15, 81–91. 10.1016/j.ijpddr.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORAES D. C. Recent developments on the anti-Candida effect of amphotericin B combined with a second drug-a mini-review. Anais da Academia Brasileira de Ciências 2023, 95, e20220033 10.1590/0001-3765202320220033. [DOI] [PubMed] [Google Scholar]
- Potvin J. E.; Leprohon P.; Queffeulou M.; Sundar S.; Ouellette M. Mutations in an aquaglyceroporin as a proven marker of antimony clinical resistance in the parasite Leishmania donovani. Clinical Infectious Diseases 2021, 72 (10), e526–e532. 10.1093/cid/ciaa1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirkx L.Characterizing the bone marrow as a parasitological niche responsible for antileishmanial treatment failure, Doctoral dissertation, University of Antwerp, 2023. [Google Scholar]
- Douanne N.; Wagner V.; Roy G.; Leprohon P.; Ouellette M.; Fernandez-Prada C. MRPA-independent mechanisms of antimony resistance in Leishmania infantum. International Journal for Parasitology: Drugs and Drug Resistance 2020, 13, 28–37. 10.1016/j.ijpddr.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugani J. N.; Gontijo C. M. F.; Frézard F.; Soares R. P.; Monte-Neto R. L. D.. Antimony resistance in Leishmania (Viannia) braziliensis clinical isolates from atypical lesions associates with increased ARM56/ARM58 transcripts and reduced drug uptake; Memórias do Instituto Oswaldo Cruz, 2019; p 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejazi S. H.; Saberi S.; Arjmand R.; Soleimanifard S. The study of P-glycoprotein A, G- glutamylcysteine synthetase 1, and aquaglyceroporin 1 genes expression in non-healing zoonotic cutaneous leishmaniasis cases. Journal of Shahrekord University of Medical Sciences 2021, 23 (4), 162–167. 10.34172/jsums.2021.27. [DOI] [Google Scholar]
- Piccica M.; Lagi F.; Bartoloni A.; Zammarchi L. Efficacy and safety of pentamidine isethionate for tegumentary and visceral human leishmaniasis: a systematic review. Journal of Travel Medicine 2021, 28 (6), taab065. 10.1093/jtm/taab065. [DOI] [PubMed] [Google Scholar]
- Abirami M.; Karan Kumar B.; Faheem; Dey S.; Johri S.; Reguera R. M.; Balana-Fouce R.; Gowri Chandra Sekhar K. V.; Sankaranarayanan M. Molecular-level strategic goals and repressors in Leishmaniasis–Integrated data to accelerate target-based heterocyclic scaffolds. Eur. J. Med. Chem. 2023, 257, 115471 10.1016/j.ejmech.2023.115471. [DOI] [PubMed] [Google Scholar]
- Sundar S.; Chakravarty J.; Meena L. P. Leishmaniasis: treatment, drug resistance, and emerging therapies. Expert Opinion on Orphan Drugs 2019, 7 (1), 1–10. 10.1080/21678707.2019.1552853. [DOI] [Google Scholar]
- Pramanik P. K.; Alam M. N.; Roy Chowdhury D.; Chakraborti T. Drug resistance in protozoan parasites: an incessant wrestle for survival. Journal of Global Antimicrobial Resistance 2019, 18, 1–11. 10.1016/j.jgar.2019.01.023. [DOI] [PubMed] [Google Scholar]
- Roy A. DNA Topoisomerase as Drug Target in unicellular protozoan parasite Leishmania donovani. Indian Journal of Physiology and Allied Sciences 2022, 74 (02), 28–35. 10.55184/ijpas.v74i02.55. [DOI] [Google Scholar]
- Saha S.; Chowdhury S. R.; Majumder H. K. DNA topoisomerases of kinetoplastid parasites: brief overview and recent perspectives. Current Issues in Molecular Biology 2019, 31 (1), 45–62. 10.21775/cimb.031.045. [DOI] [PubMed] [Google Scholar]
- Jeacock L.; Baker N.; Wiedemar N.; Mäser P.; Horn D. Aquaglyceroporin-null trypanosomes display glycerol transport defects and respiratory-inhibitor sensitivity. PLoS pathogens 2017, 13 (3), e1006307 10.1371/journal.ppat.1006307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong I. L.; Chan K. F.; Zhao Y.; Chan T. H.; Chow L. M. Quinacrine and a novel apigenin dimer can synergistically increase the pentamidine susceptibility of the protozoan parasite Leishmania. Journal of antimicrobial chemotherapy 2009, 63 (6), 1179–1190. 10.1093/jac/dkp130. [DOI] [PubMed] [Google Scholar]
- Kwofie S. K.; Broni E.; Dankwa B.; Enninful K. S.; Kwarko G. B.; Darko L.; Durvasula R.; Kempaiah P.; Rathi B.; Miller III W. A.; Yaya A.; Wilson M. D. Outwitting an old neglected nemesis: a review on leveraging integrated data-driven approaches to aid in unraveling of leishmanicides of therapeutic potential. Current Topics in Medicinal Chemistry 2020, 20 (5), 349–366. 10.2174/1568026620666200128160454. [DOI] [PubMed] [Google Scholar]
- Salari S.; Bamorovat M.; Sharifi I.; Almani P. G. N. Global distribution of treatment resistance gene markers for leishmaniasis. Journal of Clinical Laboratory Analysis 2022, 36 (8), e24599 10.1002/jcla.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee B.; Mukherjee K.; Nanda P.; Mukhopadhayay R.; Ravichandiran V.; Bhattacharyya S. N.; Roy S. Probing the molecular mechanism of aggressive infection by antimony resistant Leishmania donovani. Cytokine. Cytokine 2021, 145, 155245 10.1016/j.cyto.2020.155245. [DOI] [PubMed] [Google Scholar]
- Moody R. Genome plasticity and its role in leishmania adaptation and drug resistance. Journal of Medical Research and Innovation 2021, 5 (2), 15–22. 10.25259/JMRI_3_2021. [DOI] [Google Scholar]
- Colotti G.; Baiocco P.; Fiorillo A.; Boffi A.; Poser E.; Chiaro F. D.; Ilari A. Structural insights into the enzymes of the trypanothione pathway: targets for antileishmaniasis drugs. Future medicinal chemistry 2013, 5 (15), 1861–1875. 10.4155/fmc.13.146. [DOI] [PubMed] [Google Scholar]
- Ibarra-Meneses A. V.; Corbeil A.; Wagner V.; Beaudry F.; do Monte-Neto R. L.; Fernandez-Prada C. Exploring direct and indirect targets of current antileishmanial drugs using a novel thermal proteomics profiling approach. Frontiers in Cellular and Infection Microbiology 2022, 12, 954144 10.3389/fcimb.2022.954144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klokouzas A.; Shahi S.; Hladky S. B.; Barrand M. A.; van Veen H. W. ABC transporters and drug resistance in parasitic protozoa. International journal of antimicrobial agents 2003, 22 (3), 301–317. 10.1016/S0924-8579(03)00210-3. [DOI] [PubMed] [Google Scholar]
- Sakyi P. O.; Amewu R. K.; Devine R. N.; Bienibuor A. K.; Miller W. A. III; Kwofie S. K. Unravelling the myth surrounding sterol biosynthesis as plausible target for drug design against leishmaniasis. Journal of Parasitic Diseases 2021, 45 (4), 1152–1171. 10.1007/s12639-021-01390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S.; Singh R. P. Nyctanthes arbor-tristis L.: Perspective of phytochemical-based inhibition of fatty acid biosynthesis in Mycobacterium tuberculosis. International Journal of Plant-Based Pharmaceuticals. International Journal of Plant Based Pharmaceuticals 2022, 2 (2), 166–175. 10.55484/ijpbp.1049943. [DOI] [Google Scholar]
- Kaur P.; Garg M.; Hombach-Barrigah A.; Clos J.; Goyal N. MAPK1 of Leishmania donovani interacts and phosphorylates HSP70 and HSP90 subunits of foldosome complex. Sci. Rep. 2017, 7 (1), 10202 10.1038/s41598-017-09725-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach-Barrigah A.; Bartsch K.; Smirlis D.; Rosenqvist H.; MacDonald A.; Dingli F.; Loew D.; Spath G. F.; Rachidi N.; Wiese M.; Clos J. Leishmania donovani 90 kD heat shock protein–impact of phosphosites on parasite fitness, infectivity and casein kinase affinity. Sci. Rep. 2019, 9 (1), 5074. 10.1038/s41598-019-41640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhjazi G.; Gabrielli A. F.; Ruiz-Postigo J. A.; Atta H.; Osman M.; Bashour H.; Al Tawil A.; Husseiny H.; Allahham R.; Allan R. Cutaneous leishmaniasis in Syria: A review of available data during the war years: 2011–2018. PLoS neglected tropical diseases 2019, 13 (12), e0007827 10.1371/journal.pntd.0007827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle A. S.; Khare S.; Kumar A. B.; Supek F.; Buchynskyy A.; Mathison C. J. N.; Chennamaneni N. K.; Pendem N.; Buckner F. S.; Gelb M. H.; Molteni V. Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Chem. Rev. 2014, 114 (22), 11305–11347. 10.1021/cr500365f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. Drug resistance in leishmaniasis: Newer developments. Tropical parasitology 2014, 4 (1), 4. 10.4103/2229-5070.129142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Pachioni J.; Magalhães J. G.; Lima E. J. C.; de Moura Bueno L.; Barbosa J. F.; de Sá M. M.; Rangel-Yagui C. O. Alkylphospholipids–a promising class of chemotherapeutic agents with a broad pharmacological spectrum. Journal of Pharmacy & Pharmaceutical Sciences 2013, 16 (5), 742–759. 10.18433/J3CW23. [DOI] [PubMed] [Google Scholar]
- Pinto-Martinez A. K.; Rodriguez-Durán J.; Serrano-Martin X.; Hernandez-Rodriguez V.; Benaim G. Mechanism of action of miltefosine on Leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane Ca2+ channel. Antimicrob. Agents Chemother. 2018, 62 (1), e01614-17. 10.1128/AAC.01614-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner K. G.; Vacchina P.; Robles-Murguia M.; Wadsworth M.; McDowell M. A.; Morales M. A. Fitness and phenotypic characterization of miltefosine-resistant Leishmania major. PLoS neglected tropical diseases. PLoS Negl Trop Dis 2015, 9 (7), e0003948 10.1371/journal.pntd.0003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage E. G.; Alqaisi A. Q. I.; Godzien J.; Pena I.; Mbekeani A. J.; Alonso-Herranz V.; Lopez-Gonzalvez A.; Martin J.; Gabarro R.; Denny P. W.; Barrett M. P.; Barbas C. Complex interplay between sphingolipid and sterol metabolism revealed by perturbations to the Leishmania metabolome caused by miltefosine. Antimicrob. Agents Chemother. 2018, 62 (5), 10–1128. 10.1128/AAC.02095-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Victoria J. M.; Bavchvarov B. I.; Torrecillas I. R.; Martinez-Garcia M.; Lopez-Martin C.; Campillo M.; Castanys S.; Gamarro F. Sitamaquine overcomes ABC-mediated resistance to miltefosine and antimony in Leishmania. Antimicrobial agents and chemotherapy. Antimicrob Agents Chemother 2011, 55 (8), 3838–3844. 10.1128/AAC.00065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa K. M.; Valente R. C.; Salustiano E. J.; Gentile L. B.; Freire-de-Lima L.; Mendonça-Previato L.; Previato J. O. Functional characterization of ABCC proteins from Trypanosoma cruzi and their involvement with thiol transport. Frontiers in microbiology 2018, 9, 205. 10.3389/fmicb.2018.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt E.; Bulte D.; Van Bockstal L.; Van den Kerkhof M.; Cos P.; Delputte P.; Hendrickx S.; Maes L.; Caljon G. Miltefosine enhances the fitness of a non-virulent drug-resistant Leishmania infantum strain. J. Antimicrob. Chemother. 2019, 74 (2), 395–406. 10.1093/jac/dky450. [DOI] [PubMed] [Google Scholar]
- Götte M., Berghuis A., Matlashewski G., Wainberg M. A., Sheppard D., Eds. Handbook of Antimicrobial Resistance; Springer: New York, 2017; 606 p. [Google Scholar]
- Ghorbani M.; Farhoudi R. Leishmaniasis in humans: drug or vaccine therapy?. Drug design, development and therapy 2018, 12, 25–40. 10.2147/DDDT.S146521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkait B.; Kumar A.; Nandi N.; Sardar A. H.; Das S.; Kumar S.; Pandey K.; Ravidas V.; Kumar M.; De T.; Singh D.; Das P. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrobial agents and chemotherapy. Antimicrob Agents Chemother 2012, 56 (2), 1031–1041. 10.1128/AAC.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pountain A. W.; Weidt S. K.; Regnault C.; Bates P. A.; Donachie A. M.; Dickens N. J.; Barrett M. P. Genomic instability at the locus of sterol C24-methyltransferase promotes amphotericin B resistance in Leishmania parasites. PLoS neglected tropical diseases. PLoS Negl Trop Dis 2019, 13 (2), e0007052 10.1371/journal.pntd.0007052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirve S.; Boelaert M.; Matlashewski G.; Mondal D.; Arana B.; Kroeger A.; Olliaro P. Transmission dynamics of visceral leishmaniasis in the Indian subcontinent–a systematic literature review. PLoS neglected tropical diseases 2016, 10 (8), e0004896 10.1371/journal.pntd.0004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinc R. New developments in the treatment of cutaneous leishmaniasis. Asian Pacific Journal of Tropical Medicine. Asian Pac J Trop Med. 2022, 15 (5), 196–205. 10.4103/1995-7645.345944. [DOI] [Google Scholar]
- Wiwanitkit V. Interest in paromomycin for the treatment of visceral leishmaniasis (kala-azar). Therapeutics and clinical risk management 2012, 323–328. 10.2147/TCRM.S30139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela R.; Moreira R.; Lopes F. An overview of drug resistance in protozoal diseases. International journal of molecular sciences 2019, 20 (22), 5748. 10.3390/ijms20225748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari-Kharaji M.; Taheri T.; Doroud D.; Habibzadeh S.; Badirzadeh A.; Rafati S. Enhanced paromomycin efficacy by solid lipid nanoparticle formulation against Leishmania in mice model. Parasite immunology 2016, 38 (10), 599–608. 10.1111/pim.12340. [DOI] [PubMed] [Google Scholar]
- Naderer T.; McConville M. J. Intracellular growth and pathogenesis of Leishmania parasites. Essays in biochemistry 2011, 51, 81–95. 10.1042/bse0510081. [DOI] [PubMed] [Google Scholar]
- Matos A. P. S.; Viçosa A. L.; Ré M. I.; Ricci-Júnior E.; Holandino C. A review of current treatments strategies based on paromomycin for leishmaniasis. Journal of Drug Delivery Science and Technology 2020, 57, 101664 10.1016/j.jddst.2020.101664. [DOI] [Google Scholar]
- Bhandari V.; Sundar S.; Dujardin J. C.; Salotra P. Elucidation of cellular mechanisms involved in experimental paromomycin resistance in Leishmania donovani. Antimicrobial agents and chemotherapy. Antimicrob Agents Chemother. 2014, 58 (5), 2580–2585. 10.1128/AAC.01574-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaza S. Recent advances in identification of potential drug targets and development of novel drugs in parasitic diseases. Part II: Parasite targets. Parasitologists United Journal 2022, 15 (1), 22–38. 10.21608/PUJ.2022.129311.1160. [DOI] [Google Scholar]