Abstract
Previously, we isolated a novel gene, drs, which was downregulated by retroviral oncogenes such as v-src and v-K-ras, from a cDNA library of primary rat embryo fibroblasts. Experiments using a temperature-sensitive mutant of the v-src gene indicated that downregulation of drs mRNA was dependent on functional expression of v-Src. In addition, expression of drs mRNA was also reduced by serum stimulation of G0-arrested normal rat fibroblast cells. To clarify the function of the drs gene in cell transformation and proliferation, we introduced drs linked to a potent promoter into a normal rat cell line, F2408, and examined the effect of ectopic expression of exogenous drs on the transformation by the v-src gene and growth properties. Cells expressing exogenous drs gene showed significantly decreased efficiency of transformation by v-src irrespective of functional expression of v-Src kinase, while the growth rate and G1/S progression of the cells were not suppressed by expression of exogenous drs gene, indicating that drs has the ability to suppress v-src transformation without disturbing cell proliferation.
Transformation by viral oncogenes induces a variety of cellular changes such as cell rounding, loss of contact inhibition, decrease of serum requirement for cell proliferation, and anchorage-independent growth. The v-src oncogene of Rous sarcoma virus (RSV) has been most intensively investigated (29). The product of v-src is a membrane-associated phosphoprotein, pp60v-src, which has tyrosine-specific protein kinase activity (9, 20, 32). The v-src gene also positively or negatively alters the expression of many cellular genes (1, 6, 12, 14, 16, 28, 33, 40, 44, 45, 49). A few of the genes whose expression is negatively regulated by v-src have been shown to function as tumor suppressor genes (11, 33, 41). We have reported that a suppressive factor(s) for v-src transformation is expressed in primary rat embryo fibroblasts (REF) (25, 51, 52). On searching for such transformation suppressor genes, we recently isolated a novel gene, drs (downregulated by v-src), which was expressed in normal rat fibroblast cells but completely suppressed in the cells transformed by the v-src gene, from a cDNA library of REF (42). The drs gene was also demonstrated to be downregulated by other retroviral oncogenes such as v-fps, v-ras, v-mos, v-sis, and v-abl. The molecularly cloned cDNA of drs was about 1.8 kb in size, containing an open reading frame composed of 464 amino acid residues. This protein had one transmembrane domain in the C terminus and three consensus repeats conserved in various numbers in the extracellular domain among the selectin family of adhesion molecules and complement binding proteins (4, 30). Marked downregulation of drs mRNA in oncogene-transformed cells suggests that the drs gene plays a role in suppression of transformation.
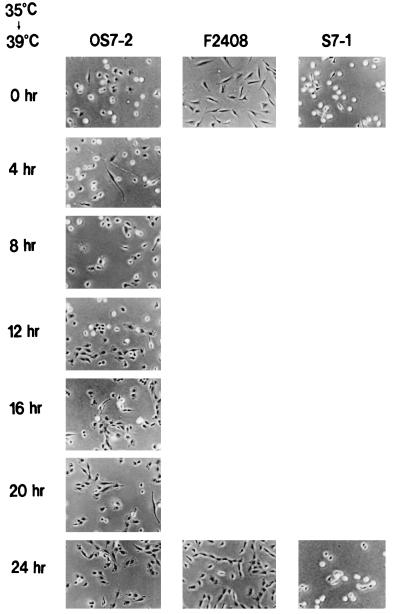
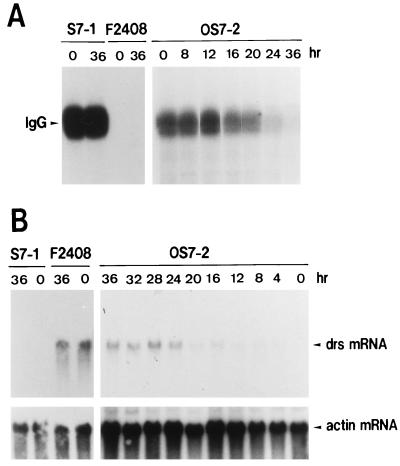
To clarify the function of drs for cell transformation, we initially investigated the correlation between downregulation of drs mRNA and functional expression of the v-src gene. To perform this experiment, we used a rat F2408 cell clone (OS7-2) containing a temperature-sensitive mutant (OS122) of RSV (13, 38, 39, 54). As shown in Fig. 1, OS7-2 displays round refractile morphology typical of transformed cells when incubated at 35°C. When the temperature was shifted to 39°C from 35°C, the morphology of OS7-2 was gradually converted to a flat phenotype within 24 h. Tyrosine kinase activity of v-Src by in vitro kinase assay with anti-Src serum was also reduced by temperature shift from 35 to 39°C (Fig. 2A). The change of tyrosine kinase activity roughly paralleled the morphological change in OS7-2 cells. The level of drs mRNA gradually increased after the temperature increase to 39°C in parallel with the decrease of the kinase activity in OS7-2 cells (Fig. 2B). In the F2408 cell clone S7-1 (23), containing wild-type RSV, the transformed morphology, tyrosine kinase activity, and level of drs mRNA were not changed by temperature shift (Fig. 1 and 2). The expression of drs mRNA in untransformed F2408 cells was also not affected by temperature shift (Fig. 2B). These results indicate that downregulation of drs mRNA depends on functional expression of v-Src tyrosine kinase and parallels the expression of transformed morphology.
FIG. 1.
Morphological change of OS7-2 cells by temperature shifting. F2408 is a rat fibroblast cell line established from REF of Fisher rat (13). S7-1 is an F2408 cell line transformed by the SR-D strain of RSV (23). OS7-2 is an F2408 cell line containing a temperature-sensitive mutant (OS122) of RSV (38, 39, 54). The cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 5% FCS (growth medium). OS7-2, S7-1, and F2408 cells were inoculated into plastic dishes containing growth medium and incubated at 35°C (permissive temperature). After incubation for 24 h, the culture temperature was increased to 39°C. At 0, 4, 8, 12, 16, 20, and 24 h after temperature shifting (0 and 24 h for S7-1 and F2408), cell morphologies were observed with a phase-contrast microscope and photographed.
FIG. 2.
Alterations of protein kinase activity of v-Src (A) and expression of drs mRNA (B) by temperature shifting in OS7-2 cells. (A) In vitro protein kinase assay. Temperature shifting was performed as described in the legend to Fig. 1. For examination of protein kinase activity of v-Src, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 20 mM Tris-HCl [pH 7.4], 150 mM sodium chloride, 5 mM EDTA, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 20 μg of aprotinin/ml) and centrifuged at 13,000 × g for 30 min at 4°C. The resulting supernatant was used for kinase assay of v-Src as described by Inoue et al. (22). Cell extracts containing 100 μg of protein were immunoprecipitated with 5 μl of anti-Src serum for 1 h at 4°C. The immunocomplexes were bound to protein A-Sepharose, washed three times with RIPA buffer, and suspended in 20 μl of kinase buffer (20 mM Tris-HCl [pH 7.4], 10 mM MnCl2) containing 5 μCi of [γ-32P]ATP (3,000 Ci/mmol; Amersham). After incubation for 10 min at 20°C, the reaction was stopped by addition of 15 μl of 4× Laemmli-SDS buffer (0.25 M Tris-Cl [pH 6.8], 40% glycerol, 8% SDS, 20% 2-mercaptoethanol, 0.01% bromophenol blue) and boiled for 2 min. The reaction mixtures were centrifuged at 10,000 × g for 2 min, and samples of the supernatant were analyzed by SDS-polyacrylamide gel electrophoresis. The position of the phosphorylated immunoglobulin G (IgG) heavy chain is indicated. (B) Northern blot analysis. Cellular RNA was isolated from cells by the guanidium isothiocyanate-cesium chloride method (8). Samples (20 μg) of total RNA from the cells were subjected to electrophoresis on a 1% agarose gel containing 2.2 M formaldehyde and transferred to a nylon filter. The filter was hybridized at 42°C overnight in a solution containing 50% formamide, 0.6 M sodium chloride, 60 mM sodium citrate, 0.2% SDS, 0.1% bovine serum albumin, 0.1% Ficoll, 0.1% polyvinylpyrrolidone, and 50 μg of herring sperm DNA/ml with a labeled DNA probe. The hybridized filter was washed with 15 mM sodium chloride–1.5 mM sodium citrate–0.1% SDS at 50°C and autoradiographed. Probes used in this experiment were a drs DNA fragment and a human β-actin DNA fragment (37).
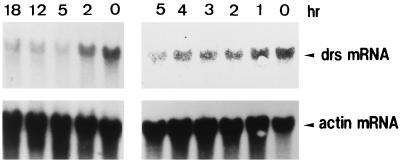
To examine whether drs mRNA expression varies with cell cycle progression, F2408 cells arrested in G0 phase by serum starvation were stimulated by serum (10% fetal calf serum [FCS]) and the change in the amount of drs mRNA was examined (Fig. 3). Under these conditions, G0-arrested F2408 cells synchronously progressed to S phase 12 h after serum stimulation (26). Expression of drs mRNA was gradually reduced until 5 h after serum stimulation (Fig. 3). The level of drs mRNA was unchanged from 5 to 18 h (the peak of S phase) (Fig. 3). These results suggest that expression of the drs gene is also regulated during the cell cycle.
FIG. 3.
Expression of drs mRNA during cell cycle progression of F2408 cells. Confluent cultures of F2408 cells were serum starved for 24 h and then supplemented with 10% FCS. At 0, 1, 2, 3, 4, 5, 12, and 18 h after serum stimulation, cellular RNA was isolated. Isolation of cellular RNA and Northern blot hybridization were carried out as described in the legend to Fig. 2B.
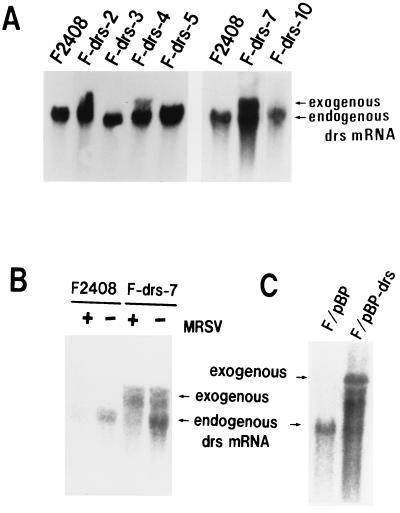
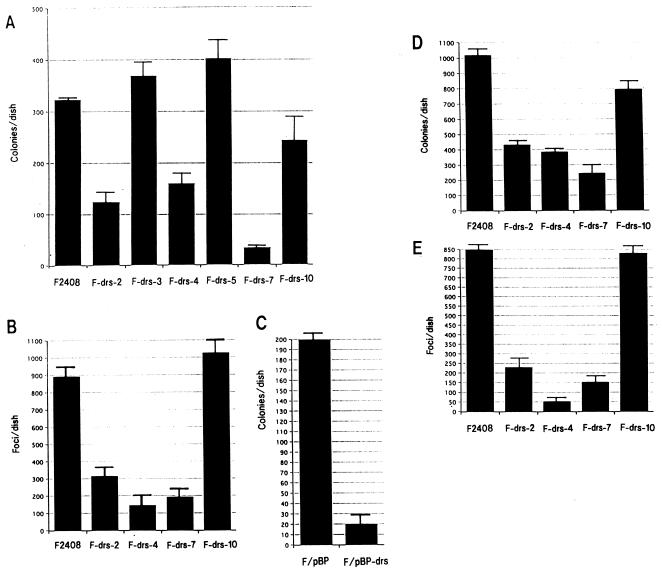
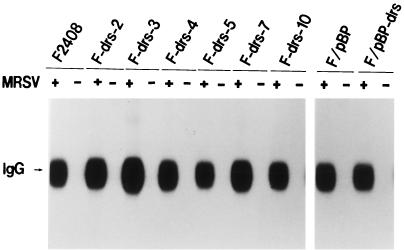
To assess the function of the drs gene in v-src transformation and cell proliferation, we constructed a recombinant plasmid, pSRαNeo/drs, that expresses the drs gene under the potent promoter-enhancer SRα (50), and transfected this plasmid into an F2408 cell line. After G418 selection, six independent G418-resistant clones (F-drs-2, -3, -4, -5, -7, and -10) were isolated, and expression of exogenous drs gene in these clones was examined by Northern blot hybridization. As shown in Fig. 4A, three clones (F-drs-2, -4, and -7) expressed high levels of exogenous drs mRNA (upper band) in addition to expressing endogenous drs mRNA (lower band), while another three clones (F-drs-3, -5, and -10) expressed only endogenous drs mRNA. The cell morphologies of F2408 and the six G418-resistant clones were similar (data not shown). To clarify whether the drs gene has the activity to suppress v-src transformation, F2408 and these clones were infected with a high titer of a recombinant murine retrovirus (MRSV) containing the v-src gene (2), and the colony-forming abilities in soft agar and the focus-forming abilities in liquid culture of these cells were investigated. As shown in Fig. 5A, the colony-forming efficiencies of the clones expressing exogenous drs (F-drs-2, -4, and -7) were significantly lower than those of F2408 and the clones expressing only endogenous drs. Focus-forming efficiencies of F-drs-2, F-drs-4, and F-drs-7 by MRSV were also markedly decreased compared with those of F2408 and F-drs-10 (Fig. 5B). These results suggest that the drs gene acts to suppress v-src transformation. To exclude the possibility that expression of functional v-Src protein was inhibited in the clones expressing exogenous drs gene, the tyrosine kinase activity of v-Src protein in MRSV-infected cells was examined by in vitro protein kinase assay with anti-Src serum. Figure 6 shows the results. v-Src kinase activities of F-drs-2, -4, and -7 infected with MRSV were not reduced compared with those of MRSV-infected F2408, F-drs-3, -5, and -10, indicating that suppression of v-src transformation in the clones expressing exogenous drs gene is not due to the reduced expression of v-Src tyrosine kinase. We also examined the expression of exogenous and endogenous drs mRNA in mock- and MRSV-infected F-drs-7 cells. As shown in Fig. 4B, the level of endogenous drs mRNA was reduced by MRSV infection, whereas the expression of exogenous drs mRNA driven from the SRα promoter was not affected by MRSV, confirming that v-src certainly functions to downregulate endogenous drs in MRSV-infected F2408 cells expressing exogenous drs. However, the drs gene driven from the exogenous promoter was not downregulated by v-src. This result further supports our conclusion that an ectopically expressed exogenous drs acts to suppress transformation by v-src.
FIG. 4.
Expression of endogenous and exogenous drs mRNA in F2408 and F-drs cells (A and C) and the effect of MRSV infection on drs mRNA expression (B). (A) The expression vector in this experiment was pSRαNeo, which contains the SRα promoter (50) for efficient expression of inserted cDNA and the neomycin-resistant gene as a selective marker. A 1.8-kb cDNA fragment (BamHI) containing the open reading frame of the drs gene was inserted into the BamHI-cleaved pSRαNeo vector. The recombinant plasmid, pSRαNeo/drs, in which a drs gene was inserted in the sense orientation, was used for DNA transfection experiments. pSRαNeo/drs plasmid DNA was introduced into F2408 by the calcium-phosphate transfection method (17), and G418-resistant clones were isolated. Isolation of cellular RNA and Northern blot hybridization were carried out as described in the legend to Fig. 2B. (B) Cellular RNA was isolated 3 days after MRSV infection. The upper and lower bands indicate exogenous and endogenous drs transcripts, respectively. (C) A 1.8-kb drs cDNA fragment was inserted into BamHI-cleaved pBabePuro retrovirus vector (36) in the sense orientation. The recombinant plasmid, pBabePuro-drs, was introduced into a packaging cell line, ψ2 (34), by DNA transfection, and puromycin-resistant clones containing the drs gene were isolated. F2408 cells were infected with culture medium of cells containing pBabePuro-drs or vector (pBabePuro) virus, and puromycin-resistant cells were pooled (F/pBP-drs and F/pBP).
FIG. 5.
Transformation of F2408 and F-drs cells by MRSV and Ki-MSV. F2408 and F-drs clones transfected with pSRαNeo/drs plasmid were infected with MRSV (A and B) or Ki-MSV (D and E). F/pBP and F/pBP-drs cells were infected with MRSV (C). For virus infection, inocula of 2 × 105 cells were plated in 60-mm-diameter plastic dishes with growth medium and incubated overnight at 37°C. After treatment of the cultures with Polybrene (2 μg/ml) for 30 min at 37°C, the medium was removed and 0.3 ml of virus preparation was added to each culture. After adsorption for 1 h at 37°C, the cultures were covered with growth medium and incubated at 37°C. For soft agar assays (A, C, and D), 3 days after virus infection, the cells were trypsinized and portions of 104 cells were inoculated into 0.4% soft agar. Colonies were scored after incubation for 2 weeks. For focus assays (B and E), transformed foci were scored 10 days after virus infection.
FIG. 6.
v-Src kinase activities in MRSV-infected F2408 and F-drs cells. Three days after MRSV infection, the mock- and MRSV-infected cells were lysed in RIPA buffer. The cell lysates containing 100 μg of protein were used for protein kinase assay of v-Src as described in the legend to Fig. 2A.
To further confirm the correlation between expression of exogenous drs and suppression of transformation by v-src, we constructed a recombinant retrovirus containing the drs gene and the puromycin-resistant gene as a selective marker (pBabePuro-drs) and infected F2408 cells with the virus. After incubation in selection medium containing 1 μg of puromycin/ml, puromycin-resistant cells were pooled, infected with MRSV, and inoculated into soft agar. F2408 cells infected with pBabePuro-drs virus expressed a considerable amount of viral mRNA hybridized with drs cDNA (Fig. 4C). As shown in Fig. 5C, the colony-forming efficiency of F2408 cells containing pBabePuro-drs virus (F/pBP-drs) by MRSV was markedly decreased compared with that of F2408 cells containing vector virus (F/pBP). v-Src kinase activities in MRSV-infected F/pBP and F/pBP-drs cells were also almost similar (Fig. 6), confirming the suppression function of drs for v-src transformation. In addition, we also examined colony formation in soft agar and focus formation of F2408, F-drs-2, F-drs-4, F-drs-7, and F-drs-10 cells by a murine retrovirus containing v-K-ras (Ki-MSV). As shown in Fig. 5D (soft agar assay) and E (focus assay), transformation efficiencies of F-drs-2, F-drs-4 and F-drs-7 were also significantly decreased compared with those of F2408 and F-drs-10. These results, together with those of v-src transformation, indicate that ectopic expression of the drs gene suppresses transformation by viral oncogenes such as v-src and v-K-ras.
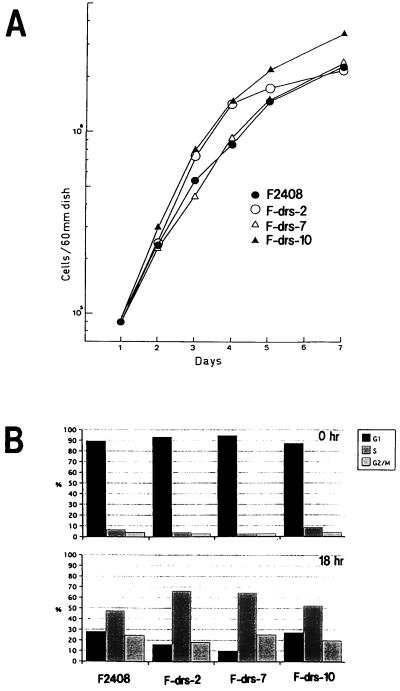
To investigate the effect of ectopic expression of the drs gene on growth properties of F2408 cells, we examined the growth rates of F2408, F-drs-2, F-drs-7, and F-drs-10 cells. As shown in Fig. 7A, expression of exogenous drs gene did not affect the growth rate of the cells. The growth rates of F/pBP and F/pBP-drs cells were also similar (data not shown). To examine whether overexpression of exogenous drs gene suppresses G1/S progression of the cell cycle, F2408, F-drs-2, F-drs-7, and F-drs-10 cells were arrested in G0 phase by serum starvation and stimulated with serum. Progression of the cell cycle of these cells was examined by flow cytometry-activated cell sorting (Fig. 7B). The entry into S phase at 18 h after serum stimulation was not reduced by expression of exogenous drs gene in F-drs-2 and F-drs-7 cells compared with that of F2408 and F-drs-10 cells, indicating that the exogenous drs gene does not suppress G1/S progression of the cell cycle. From these results, we concluded that the drs gene acts to suppress transformation induced by v-src and v-ras without affecting cell proliferation in F2408 cells.
FIG. 7.
Growth curves (A) and cell cycle analyses (B) of F2408 and F-drs clones. Cell cycle analysis was carried out by measuring the cellular DNA content by flow cytometry-activated cell sorting as previously described (26). To isolate and stain the cell nuclei, the Cycle TEST PLUS DNA Reagent Kit (Becton Dickinson) was used. Cells were washed with phosphate-buffered saline, suspended in sodium citrate buffer, quickly frozen in a bath of dry ice-methanol, and stored at −80°C until use. The cells were thawed, and their DNA was stained with Cycle TEST reagent by the procedures recommended by the manufacturer. The fluorescence of the cells was measured with a FACScan system (Becton Dickinson), and the percentages of the cells in G1, S, and G2/M phases were determined by the CellFit program (Becton Dickinson).
Previously, we showed that drs mRNA was markedly reduced in rat cell lines transformed by v-src, v-fps, v-ras, v-mos, v-sis, v-abl, or middle T antigen of polyomavirus but not in cell lines transformed by large T antigen of simian virus 40 or the E6 and E7 genes of human papillomavirus type 16 (24, 42). As the oncogenes that reduced drs mRNA are considered to act upstream of the p42/p44 mitogen-activated protein (MAP) kinase pathway (19, 43), we speculated that expression of the drs gene is negatively regulated by mitogenic signals from growth factors downstream of the MAP kinase pathway but upstream of the cyclin/CDK-Rb pathway. In fact, as shown in Fig. 3, the level of drs mRNA was considerably reduced by serum stimulation of G0-arrested cells although the expression was not completely suppressed. This result suggested that downregulation of drs mRNA by mitogenic signals plays a role in the progression of the cell cycle. However, overexpression of the exogenous drs gene by the SRα promoter did not affect cell proliferation (Fig. 7). Although mitogenic factors in the serum act to modestly regulate the expression of drs gene, the downregulation might not be critical for G1/S progression of the cell cycle. However, we cannot completely rule out the possibility that the level of exogenous drs mRNA is not sufficient to affect cell proliferation in this cell line.
Figure 4B shows that introduction of the v-src gene reduced the level of endogenous drs mRNA but did not affect that of exogenous drs mRNA driven by the SRα promoter. This result also implies that downregulation of the drs gene by v-src is caused by transcriptional repression in the 5′ regulatory region of the drs gene. Activated MAP kinase moves into the nucleus and acts to regulate gene transcription (19, 43). Viral oncogenes such as v-src and mitogenic factors in serum may repress transcription of the drs gene through the MAP kinase pathway. The mechanism of downregulation of the drs gene by oncogenes and mitogens still remains to be worked out.
Introduction of the v-src gene into normal cells results in multiple cellular events including morphological change, activation of the mitogenic pathway, and anchorage-independent growth. Overexpression of the drs gene with the SRα promoter significantly suppressed anchorage-independent growth and focus formation by v-src without disturbing usual cell proliferation. Expression of v-Src tyrosine kinase by MRSV infection was not affected by ectopic expression of the drs gene (Fig. 6), indicating that drs acts to suppress v-src transformation after function of v-Src kinase. The most prominent biochemical change induced by v-Src is an extensive tyrosine phosphorylation of cellular proteins (29). Most of these target proteins of v-Src kinase are localized in the focal adhesions linked to the plasma membrane. Deregulated phosphorylation of these focal adhesion proteins, such as paxillin, focal adhesion kinase, talin, and tensin, is considered to be the cause of rounding and disordered proliferation of the cells. The gene structure of drs implies the membrane-associated localization of Drs protein. It seems possible that the product of the drs gene interacts with these focal adhesion proteins at the membrane and interferes with transformation by v-Src. The drs gene also contains repeated motifs conserved in the extracellular domain among selectin family adhesion molecules (4, 30). Three selectins (L-selectin, E-selectin, and P-selectin) are included in this family. They share a similar molecular structure, consisting of an amino-terminal C-type lectin domain, an epidermal growth factor-like domain, from two to nine short complement regulatory repeats, a transmembrane domain, and a short cytoplasmic tail (5, 27, 31, 48, 53). The complement regulatory repeats of the selectin family had homology with three consensus repeats of the drs gene (42). The selectin family is considered to be crucial for the initial step of leukocyte-endothelial interaction in response to inflamatory stimuli such as injury and infection (4, 30). Recently, in addition to adhesion function, E-selectin was shown to associate with actin-associated proteins such as α-actinin, vinculin, filamin, paxillin, and focal adhesion kinase (57). L-selectin has also been reported to act as a signaling molecule which activates MAP kinase and Ras pathways through tyrosine phosphorylation (7, 56). In this regard, selectins resemble another family of adhesion molecules, integrins. The integrin-mediated signaling pathway is thought to play an important role in adhesion-dependent cell cycle progression as well as regulation of the cytoskeleton (3, 21, 46, 47). Accumulating evidence indicates that some of the adhesion molecules localized in the plasma membrane are able to act as tumor suppressor genes (15, 18, 55). The drs gene may also function as a receptor for adhesion signaling and be involved in the anchorage-dependent pathway. Further investigation of the drs gene is necessary to clarify the mechanism of suppression of transformation. Recently, we found that the drs gene is highly homologous (80% in nucleotide sequence) to a human gene which is deleted in patients with X-linked retinitis pigmentosa (10, 35), suggesting that drs has significant physiological functions in a variety of cell types.
Acknowledgments
This work was supported by a Grant-in-Aid for Special Project Research, Cancer-Bioscience, from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Adams S L, Alwine J A, de Crombrugghe B, Pastan I. Use of recombinant plasmids to characterize collagen RNAs in normal and transformed chicken embryo fibroblasts. J Biol Chem. 1979;254:4935–4938. [PubMed] [Google Scholar]
- 2.Anderson S M, Scolnick E M. Construction and isolation of a transforming murine retrovirus containing the src gene of Rous sarcoma virus. J Virol. 1983;46:594–605. doi: 10.1128/jvi.46.2.594-605.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assoian R K. Anchorage-dependent cell cycle progression. J Cell Biol. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevilacqua M P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 5.Bevilacqua M P, Stenselin S, Gimbrone M A, Jr, Reed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 6.Birdenall-Roberts M C, Ruscetti F W, Kasper J, Lee H-D, Friedman R, Geiser A, Sporn M B, Roberts A B, Kim S-J. Transcriptional regulation of the transforming growth factor β1 promoter by v-src gene products is mediated through the AP-1 complex. Mol Cell Biol. 1990;10:4978–4983. doi: 10.1128/mcb.10.9.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner B, Gulbins E, Schlottmann K, Koppenhoefer U, Busch G L, Walzog B, Steinhausen H, Coggeshall K M, Linderkamp O, Lang F. L-selectin activates the ras pathway via the tyrosine kinase p56lck. Proc Natl Acad Sci USA. 1996;93:15376–15381. doi: 10.1073/pnas.93.26.15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 9.Collett M, Purchio A F, Erikson R L. Avian sarcoma virus transforming protein, pp60src, shows protein kinase activity specific for tyrosine. Nature. 1980;285:167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- 10.Dry K L, Alfred M A, Edgar A J, Brown J, Manson F O, Keuren M-F, Kurnit D M, Bird A C, Jay M, Monaco A P, Wright A F. Identification of a novel gene, ETX1, for Xp21.1, a candidate gene for X-linked retinitis pigmentosa (RP3) Hum Mol Genet. 1995;4:2347–2355. doi: 10.1093/hmg/4.12.2347. [DOI] [PubMed] [Google Scholar]
- 11.Enomoto H, Ozaki T, Takahashi E, Ohnuma N, Tanaka M, Iwai I, Yoshida H, Matsunaga T, Sakiyama S. Identification of human DAN gene, mapping to the putative neuroblastoma tumor suppressor locus. Oncogene. 1994;9:2785–2791. [PubMed] [Google Scholar]
- 12.Fagan J B, Sobel M E, Yamada K M, de Crombrogghe B, Pastan I. Effects of transformation on fibronectin cDNA. J Biol Chem. 1981;256:520–525. [PubMed] [Google Scholar]
- 13.Freeman A E, Igel H J, Price P J. In vitro transformation of rat embryo cells: correlations with the known tumorigenic activities of chemicals in rodents. Carcinog In Vitro. 1975;11:107–119. doi: 10.1007/BF02624083. [DOI] [PubMed] [Google Scholar]
- 14.Fujii M, Shalloway D, Verma I M. Gene regulation by tyrosine kinases: src protein activates various promoters, including c-fos. Mol Cell Biol. 1989;9:2493–2499. doi: 10.1128/mcb.9.6.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giancotti F G, Ruoslahti E. Elevated levels of the α5β1 fibronectin receptor suppress the transformed phenotype of chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 16.Glen S, Martinerie C, Perbal B, Hanafusa H. Transcriptional down regulation of the nov proto-oncogene in fibroblasts transformed by p60v-src. Mol Cell Biol. 1996;16:481–486. doi: 10.1128/mcb.16.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 18.Hedrick L, Cho K R, Vogelstein B. Cell adhesion molecules as tumor suppressor. Trends Cell Biol. 1993;3:36–39. doi: 10.1016/0962-8924(93)90148-t. [DOI] [PubMed] [Google Scholar]
- 19.Hunter T. Oncoprotein networks. Cell. 1997;88:333–346. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- 20.Hunter T, Sefton B M. Transforming product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci USA. 1980;77:1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hynes R O. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 22.Inoue H, Isaka M, Takeda S, Hakura A. Simple system for isolation of cellular and viral mutants for transformation by retroviruses. J Med Virol. 1991;35:246–249. doi: 10.1002/jmv.1890350407. [DOI] [PubMed] [Google Scholar]
- 23.Inoue H, Owada M K, Yutsodo M, Hakura A. A rat mutant cell clone showing temperature-dependent transformed phenotypes with functional expression of the src gene product. Virology. 1989;168:57–66. doi: 10.1016/0042-6822(89)90403-0. [DOI] [PubMed] [Google Scholar]
- 24.Inoue, H., J. Pan, and A. Hakura. Unpublished data.
- 25.Inoue H, Tavoloni N, Hanafusa H. Suppression of v-Src transformation in primary rat embryo fibroblasts. Oncogene. 1995;11:231–238. [PubMed] [Google Scholar]
- 26.Inoue H, Yamashita A, Hakura A. Adhesion-dependency of serum-induced p42/p44 MAP kinase activation is released by retroviral oncogenes. Virology. 1996;225:223–226. doi: 10.1006/viro.1996.0591. [DOI] [PubMed] [Google Scholar]
- 27.Johnston G I, Cook R G, McEver R P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989;56:1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- 28.Joseph C K, Qureshi S A, Wallace D J, Foster D A. MARCKS protein is transcriptionally down-regulated in v-src-transformed BALB/c 3T3 cells. J Biol Chem. 1992;267:1327–1330. [PubMed] [Google Scholar]
- 29.Jove R, Hanafusa H. Cell transformation by the viral src gene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- 30.Kansas G S. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 31.Larsen E, Celi A, Gilbert G E, Furie B C, Erban J K, Bonfanti R, Wagner D D, Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 32.Levinson A D, Opperman H, Varmu H E, Bishop J M. The purified product of the transforming gene of avian sarcoma virus phosphorylate tyrosine. J Biol Chem. 1980;255:11973–11980. [PubMed] [Google Scholar]
- 33.Lin X, Nelson P J, Frankfort B, Tombler E, Johnson R, Gelman I H. Isolation and characterization of a novel mitogenic regulating gene, 322, which is transcriptionally suppressed in cells transformed by src and ras. Mol Cell Biol. 1995;15:2754–2762. doi: 10.1128/mcb.15.5.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann R, Mulligan R C, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983;33:153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- 35.Meindl A, Carvolho M R S, Herrmann K, Lorenz B, Achatz H, Lorenz B, Apfelstedt-Sylla E, Wittwer B, Ross M, Meitinger T. A gene (SRPX) encoding a sushi-repeat-containing protein deleted in patients with X-linked retinitis pigmentosa. Hum Mol Genet. 1995;4:2339–2346. doi: 10.1093/hmg/4.12.2339. [DOI] [PubMed] [Google Scholar]
- 36.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vector with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakajima-Iijima S, Hamada H, Reddy P, Kakunaga T. Molecular structure of the human cytoplasmic β-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci USA. 1985;82:6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama T, Irikura M, Setoguchi Y, Matsuo H, Inoue H, Yutsudo M, Hakura A. Purification and properties of a non-histone chromatin protein with a molecular weight of 38,000 daltons specific for transformed rat cells. Biochem Biophys Res Commun. 1987;149:1156–1164. doi: 10.1016/0006-291x(87)90529-8. [DOI] [PubMed] [Google Scholar]
- 39.Owada M, Moelling K. Temperature-sensitive kinase activity associated with various mutants of avian sarcoma viruses which are temperature sensitive for transformation. Virology. 1980;101:157–168. doi: 10.1016/0042-6822(80)90492-4. [DOI] [PubMed] [Google Scholar]
- 40.Ozaki T, Sakiyama S. Molecular cloning and characterization of a cDNA showing negative regulation in v-src-transformed 3Y1 rat fibroblasts. Proc Natl Acad Sci USA. 1993;90:2593–2597. doi: 10.1073/pnas.90.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozaki T, Sakiyama S. Tumor-suppressive activity of N03 gene product in v-src-transformed rat 3Y1 fibroblasts. Cancer Res. 1994;54:646–648. [PubMed] [Google Scholar]
- 42.Pan J, Nakanishi K, Yutsudo M, Inoue H, Li Q, Oka K, Yoshioka N, Hakura A. Isolation of a novel gene down-regulated by v-src. FEBS Lett. 1996;383:21–25. doi: 10.1016/0014-5793(96)00210-4. [DOI] [PubMed] [Google Scholar]
- 43.Pelech T G, Sarshern J S. Mitogen-activated protein kinases: versatile transducer for cell signaling. Trends Biochem Sci. 1992;17:235–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- 44.Pierani A, Pouponnot C, Calothy G. Transcriptional down regulation of the retina-specific QR1 gene by pp60v-src and identification of a novel v-src-responsive unit. Mol Cell Biol. 1993;13:3401–3414. doi: 10.1128/mcb.13.6.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qureshi S A, Rim M H, Alexandropoulos K, Berg K, Sukhatme V P, Foster D A. Sustained induction of egr-1 by v-src correlates with a lack of Fos-mediated repression of the egr-1 promoter. Oncogene. 1992;7:121–125. [PubMed] [Google Scholar]
- 46.Ruoslahti E, Reed J C. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz M A, Schaller M D, Ginsberg M H. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 48.Siegelman M H, Van de Rijin M, Weissman I L. Mouse lymph node homing receptor cDNA clone encodes a glycoprotein revealing tandem interaction domains. Science. 1989;243:1165–1172. doi: 10.1126/science.2646713. [DOI] [PubMed] [Google Scholar]
- 49.Sugano S, Stoeckle M Y, Hanafusa H. Transcription by Rous sarcoma virus induces a novel gene with homology to a mitogenic platelet protein. Cell. 1995;49:321–328. doi: 10.1016/0092-8674(87)90284-4. [DOI] [PubMed] [Google Scholar]
- 50.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SRα promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tavoloni N, Inoue H. Cellular aging is a critical determinant of primary cell resistance to v-src transformation. J Virol. 1997;71:237–247. doi: 10.1128/jvi.71.1.237-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tavoloni N, Inoue H, Sabe H, Hanafusa H. v-src transformation of rat embryo fibroblasts. Inefficient conversion to anchorage-independent growth involves heterogeneity of primary cultures. J Cell Biol. 1994;126:475–483. doi: 10.1083/jcb.126.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tedder T E, Isaacs C M, Ernst T J, Dometri G D, Adler D A, Disteche C M. Isolation and chromosomal localization of cDNAs encoding a novel human lymphocyte cell surface molecule, LAM-1. J Exp Med. 1989;170:123–133. doi: 10.1084/jem.170.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toyoshima K, Owada M, Kozai Y. Tumor producing capacity of temperature sensitive mutants of avian sarcoma viruses in chicks. Biken J. 1973;16:103–110. [PubMed] [Google Scholar]
- 55.Tsukita S, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Submembranous junctional plaque proteins include potential tumor suppressor molecules. J Cell Biol. 1994;123:1049–1053. doi: 10.1083/jcb.123.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woddell T K, Fialkow L, Chan C K, Kishimoto T K, Downey G P. Signaling functions of L-selectin. Enhancement of tyrosine phosphorylation and activation of MAP kinase. J Biol Chem. 1995;270:15403–15411. doi: 10.1074/jbc.270.25.15403. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida M, Westlin W F, Wang N, Ingber D E, Rosenzweig A, Resnick N, Gimbrone M A., Jr Leukocyte adhesion to vascular endothelium induces E-selectin linkage to the actin cytoskeleton. J Cell Biol. 1996;133:445–455. doi: 10.1083/jcb.133.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]