Abstract
The role of nitric oxide after poliovirus infection of the human HeLa (carcinoma) and U937 (promonocytic) cell lines has been analyzed. Both types of cells produced detectable levels of nitric oxide after poliovirus infection. However, this production was not sufficient to limit viral productivity. On the other hand, pretreatment with the nitric oxide donor glycerine trinitrate lengthened the course of poliovirus infection.
It has been demonstrated that nitric oxide (NO) plays an important role in defense against a wide spectrum of microbial pathogens (22). Nevertheless, the antiviral activity of NO has not been observed until recently (6, 10). In those first reports, murine macrophages produced NO after activation with gamma interferon and resisted infection with herpes simplex virus type 1 (HSV-1) (6), vaccinia virus, or ectromelia virus (10). Further reports pointed to NO as a first line of defense against infections in murine systems with RNA viruses (e.g., vesicular stomatitis virus [4, 12], Friend leukemia virus [3], encephalomyocarditis virus [8]; Sindbis virus [SV] [25], or Japanese encephalitis virus [15]) and DNA viruses, such as HSV-1 (6) or vaccinia virus (9, 24). Nevertheless, in some cases the effect of the production of NO in cultured cells is difficult to extrapolate to animals systems (14, 23).
As regards human cells, the role of NO after viral infection remains to be unveiled. NO produced by human B cells seemed to inhibit Epstein-Barr virus reactivation (20). Moreover, NO donors can inhibit human immunodeficiency virus type 1 (HIV-1) replication in human peripheral blood mononuclear cells (5). Nevertheless, Koka et al. (11) suggest that some pathologic effects that appeared in the central nervous system after HIV-1 infection could be due to the toxic effect of NO. NO constitutively produced by activated human promonocytic U937 cells plays a role in resistance to H-1 autonomous parvovirus infection (17). Infection with HSV-1 of U937 cells differentiated with the phorbol ester 12-myristate 13-acetate induced the production of significant levels of NO; however, this NO production did not change viral production (16).
Despite the protective effect of NO against certain viral infections, a number of recent studies indicate a harmful role of NO in many systems. Thus, NO seems to play an important role in the development of pneumonia triggered by influenza virus in mice (2) and in pathogenesis in mice infected with the tick-borne encephalitis flavivirus (13). Furthermore, it has been reported that infection of mice with coxsackievirus B3 (CVB3) induced NO in the heart, aggravating the course of the viral myocarditis (21). These results are in conflict with those of Lowenstein et al. (19), who observed that NO ameliorated the effect of CVB3 infection in mice. In a recent work, Adler et al. (1) showed that HSV-1-induced pneumonia in mice could be suppressed by the inhibitor of inducible nitric oxide synthase (iNOS), Nω-monomethyl-l-arginine (l-NMMA). Considering all these controversial results, the question of whether NO acts as an inhibitor of viral replication or as a harmful agent remains unanswered.
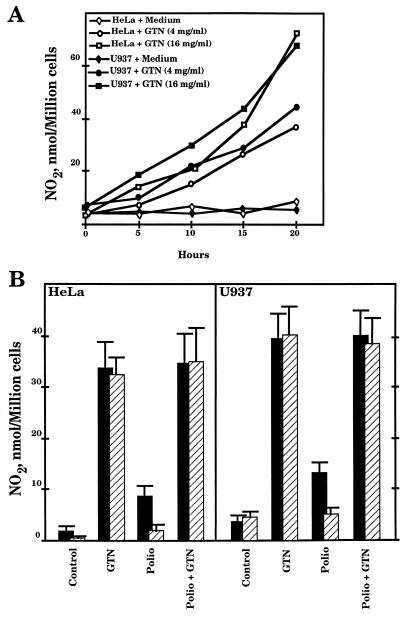
We have studied the effect of NO on poliovirus infection. To this end, human promonocytic U937 cells were cultured in RPMI 1640 (Life Technologies, Paisley, United Kingdom) and supplemented with 10% heat-inactivated fetal calf serum. HeLa cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% newborn calf serum. Poliovirus type 1 (Mahoney strain) was propagated in HeLa cells. Initially, the accumulation of NO in both human cell cultures after addition of the NO donor glycerin trinitrate (GTN) (Merck, Darmstadt, Germany) was studied. Fig. 1A shows dose-dependent levels of NO, which increased during the course of incubation, detected in both HeLa and U937 cells. For further assays, cells were preincubated with 4 mg of GTN/ml for 12 h, since higher concentrations produced cytotoxicity, as observed by trypan blue staining (data not shown). In order to study the poliovirus-induced NO, HeLa and U937 cells were infected at multiplicities of infection (MOI) of 0.5 and 5 PFU/cell, respectively. The formation of NO was measured as described by Green et al. (7). In each individual experiment, aliquots of U937 or HeLa supernatants (0.1 ml), uninfected or infected with poliovirus, were incubated, in triplicate, in flat-bottom 96-well culture plates and mixed with the same amount of Greiss reagent (0.1% naphthyl-ethylenediamine dihydrochloride [Sigma] in distilled water and 1% sulfanilamide [Sigma] in 5% phosphoric acid [vol/vol]). Subsequently, this mixture was incubated for 10 min at room temperature and the optical density at 550 nm was measured in an MR 5000 microplate reader (Dynatech, Billingshurst, West Sussex, United Kingdom). As illustrated in Fig. 1B, the infection induced a slight but significant production of NO. Incubation with 2 mM l-NMMA (Calbiochem-Novabiochem Corporation, San Diego, Calif.) decreased the production of NO induced by the viral infection (Fig. 1B). Addition of monomethyl-d-arginine (Calbiochem) as a control of specificity did not exert any effect on the accumulation of NO (data not shown). Furthermore, infection of GTN-pretreated cells did not modify the levels of NO produced with GTN alone, suggesting that maximal levels of NO had been reached or that exogenous NO addition could inhibit cellular iNOS.
FIG. 1.
Poliovirus induces NO production in human cells. (A) Treatment of HeLa and U937 cell cultures with GTN produces NO accumulation. Cells (105 per ml) were incubated at 37°C in the presence or absence of the NO donor. At the indicated times, NO production was assayed as detailed in the text. (B) HeLa or U937 cells (105) were infected with poliovirus at MOIs of 0.5 and 5 PFU/cell, respectively. Subsequently, cultures were incubated at 37°C in the presence or absence of 4 mg of GTN/ml. In parallel, cultures were preincubated for 4 h with 2 mM l-NMMA (striped bars). At 20 h p.i. the accumulation of NO was assayed. Values are means ± standard deviations of three experiments, performed in triplicate.
The implication of this endogenous NO production in HeLa and U937 cells after poliovirus infection is shown in Table 1. Treatment of the cultures with 2 mM l-NMMA altered neither the production of infectious poliovirus particles nor the cellular death observed by plaque assay and cell counting, respectively. On the other hand, and in agreement with previous findings (12), exogenous NO supplied by 12 h of pretreatment with 4 mg of GTN/ml produced an increase of cell viability and a 3.9- or 15-fold decrease in the PFU produced in HeLa and U937 cells, respectively, analyzed by means of a plaque assay performed on HeLa cell monolayers. This reduction of infectious particles was not due to a direct inhibitory effect of GTN on poliovirus input, since pretreatment of 5 × 106 poliovirus particles with 16 mg of GTN/ml for 5 h did not alter the subsequent infectivity of the virus (data not shown). Altogether, these results indicate that the addition of NO decreases poliovirus infection in both the HeLa and U937 human cell lines. However, the low level of endogenous NO production induced after the infection does not seem to be sufficient to alter the course of poliovirus infection. Morphological studies confirmed these results (data not shown).
TABLE 1.
Pretreatment of HeLa and U937 cells with GTN protects from poliovirus infectiona
| Cells | h p.i. | Virus productionb (PFU/cell) | Cell viabilityc (%) |
|---|---|---|---|
| HeLa | 24 | 63.6 ± 13.8 | <5 |
| HeLa + l-NMMA | 24 | 58.7 ± 8.4 | <5 |
| HeLa + GTN | 24 | 16.2 ± 2.3* | 28 |
| U937 | 54 | 24.0 ± 3.9 | 9 |
| U937 + l-NMMA | 54 | 28.4 ± 3.2 | 11 |
| U937 + GTN | 54 | 1.6 ± 0.3* | 46 |
Cells were left untreated or preincubated for 4 or 12 h with 2 mM l-NMMA or 4 mg of GTN/ml, respectively. Subsequently, all cells were infected at an MOI of 1 PFU/cell. After 1 h of viral adsorption, cells were washed to remove nonadsorbed viral particles (zero time) and incubated at 37°C for the indicated times.
The production of infectious virus was measured by plaque assay. *, significantly different from values for untreated cells (P < 0.001).
Survival of infected cells was determined by the trypan blue exclusion technique and is expressed relative to survival of mock-infected cultures. Values are means from three independent experiments.
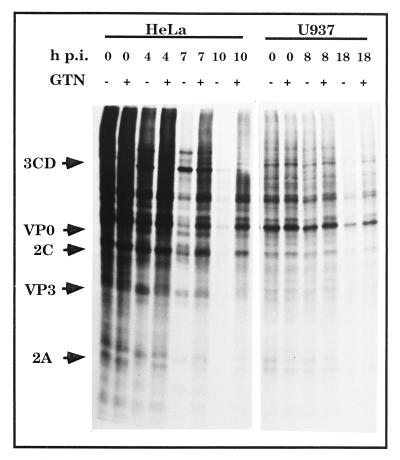
We further studied the effect exerted by NO on the course of protein synthesis during poliovirus infection by polyacrylamide gel electrophoretic assay. In vivo labeling of newly synthesized proteins was carried out by giving 1-h pulses with 20 μCi of l-(35S) Pro-mix (approximately 70% l-[35S]methionine [>1,000 Ci/mmol] and 30% l-[35S]cysteine; Amersham Life Science, Little Chalfont, Buckinghamshire, United Kingdom)/ml in methionine- and cysteine-free medium. At 4 h postinfection (p.i.), some viral proteins could be detected in infected HeLa cells (MOI, 5). The inhibition of the cellular protein was more evident at later times after infection. This shutoff was almost total at 10 h p.i. (Fig. 2). Preincubation with 4 mg of GTN/ml delayed the induction of this shutoff. Cellular protein synthesis was detected even at 10 h p.i. Cells incubated for longer times after infection underwent total cellular destruction in all cultures (data not shown). In the case of the U937 cell line, previous work from our laboratory demonstrated a weaker effect of poliovirus, and a longer time of infection was necessary to achieve cellular destruction (18). Moreover, this cellular death was not followed by detectable levels of viral protein synthesis. Figure 2 confirms this weaker induction of shutoff after infection for U937 cells. However, even under these conditions, preincubation with GTN protected the cells. Altogether, these results show the protection afforded by NO against poliovirus infection.
FIG. 2.
NO delays shutoff induction in poliovirus-infected HeLa and U937 cells. Cultures were preincubated in the presence (+) or absence (−) of 4 mg of GTN/ml for 12 h at 37°C. After this time, cells were infected with poliovirus at 5 PFU/cell. Then cells were incubated at 37°C. At the indicated time points, protein labeling was performed for 1 h. Proteins were resolved by polyacrylamide gel electrophoresis as described in reference 18. Arrows indicate the positions of some poliovirus proteins.
These results represent the first indication of poliovirus-mediated NO production. Nevertheless, the level of NO detected did not seem to be sufficient to ameliorate the cytopathic effect produced by the virus. Activation of iNOS after picornavirus infection has been described only for murine systems, and the role of this endogenously produced NO remains unclear (8, 19, 21, 23). Thus, murine L-929 cells produced NO after encephalomyocarditis virus infection without counteracting viral replication (8). Another picornavirus, the cardiovirus of Theiler’s murine encephalomyelitis, is an important model of virus-induced demyelinating disease. Although infection of the susceptible SJL strain of mice with Theiler’s murine encephalomyelitis virus increased expression of iNOS, NO did not play a direct role in the late phase of demyelination (23). Furthermore, two independent groups have shown iNOS induction in the hearts of mice infected with the enterovirus CVB3 (19, 21). However, Mikami et al. (21) could not determine whether NO plays a cytotoxic or a cytoprotective role in the pathogenic mechanisms of myocardial dysfunction.
The infection of U937 cells by poliovirus described herein produced detectable levels of NO without the need of previous cellular activation. This finding contrasts with a previous study in which commitment to a more mature state of U937 cells was needed for the production of NO after infection by HSV-1 (16). This might suggest different pathways of iNOS induction triggered by virus infection. The role of this NO produced in vitro remains unknown. Further investigation should tackle the questions of whether infection of primary human cultures leads to the activation of iNOS and what role, if any, NO plays in an in vivo context. Regarding this point, Tucker et al. (25) suggest that NO could protect some types of cells against viral infection just until the specific immune response controls the infection.
In conclusion, it is clear from the present study that NO can delay poliovirus infection and that this picornavirus induces detectable production of NO, although probably not in sufficient amounts for the establishment of an antiviral state, at least in these culture systems.
Acknowledgments
We are indebted to M. A. Alonso for critical reading of the manuscript.
Financial support was provided by the Plan Nacional (project no. BIO 94-0148) and by an Institutional Grant to the Centro de Biología Molecular by the Fundación Ramón Areces.
REFERENCES
- 1.Adler H, Beland J L, Del-Pan N C, Kobzik L, Brewer J P, Martin T R, Rimm I J. Suppression of herpes simplex virus type 1 (HSV-1)-induced pneumonia in mice by inhibition of inducible nitric oxide synthase (iNOS, NOS2) J Exp Med. 1997;185:1533–1540. doi: 10.1084/jem.185.9.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng Y M, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akarid K, Sinet M, Desforges B, Gougerot-Pocidalo M A. Inhibitory effect of nitric oxide on the replication of a murine retrovirus in vitro and in vivo. J Virol. 1995;69:7001–7005. doi: 10.1128/jvi.69.11.7001-7005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi Z, Reiss C S. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukrinsky M I, Nottet H S, Schmidtmayerova H, Dubrovsky L, Flanagan C R, Mullins M E, Lipton S A, Gendelman H E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croen K D. Evidence for an antiviral effect of nitric oxide: inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green L C, Wagner D A, Glogowsky J, Skipper P L, Wishnok J S, Tannenbaum J S. Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. Anal Biochem. 1982;126:131–136. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 8.Guillemard E, Geniteau-Legendre M, Kergot R, Lemaire G, Petit J F, Labarre C, Quero A M. Activity of nitric oxide-generating compounds against encephalomyocarditis virus. Antimicrob Agents Chemother. 1996;40:1057–1059. doi: 10.1128/aac.40.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris N, Buller R M L, Karupiah G. Gamma interferon-induced, nitric oxide-mediated inhibition of vaccinia virus replication. J Virol. 1995;69:910–915. doi: 10.1128/jvi.69.2.910-915.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karupiah G, Xie Q W, Buller R M L, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science (Washington, DC) 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 11.Koka P, He K, Zack J A, Kitchen S, Peacock W, Fried I, Tran T, Yashar S S, Merrill J E. Human immunodeficiency virus 1 envelope proteins induce interleukin 1, tumor necrosis factor α, and nitric oxide in glial cultures derived from fetal, neonatal, and adult human brain. J Exp Med. 1995;182:941–952. doi: 10.1084/jem.182.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komatsu T, Bi Z, Reiss C S. Interferon-γ-induced type I nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68:101–108. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 13.Kreil T R, Eibl M M. Nitric oxide and viral infection: NO antiviral activity against a flavivirus in vitro, and evidence for contribution to pathogenesis in experimental infection in vivo. Virology. 1996;219:304–306. doi: 10.1006/viro.1996.0252. [DOI] [PubMed] [Google Scholar]
- 14.Lane T E, Paoletti A D, Buchmeier M J. Disassociation between the in vitro and in vivo effects of nitric oxide on a neurotropic murine coronavirus. J Virol. 1997;71:2202–2210. doi: 10.1128/jvi.71.3.2202-2210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y-L, Huang Y-L, Ma S-H, Yeh C-T, Chiou S-Y, Chen L-K, Liao C-L. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Guerrero J A, Alonso M A. Nitric oxide production induced by herpes simplex virus type 1 does not alter the course of the infection in human monocytic cells. J Gen Virol. 1997;78:1977–1980. doi: 10.1099/0022-1317-78-8-1977. [DOI] [PubMed] [Google Scholar]
- 17.López-Guerrero J A, Rayet B, Tuynder M, Rommelaere J, Dinsart C. Constitutive activation of U937 promonocytic cell clones selected for their resistance to parvovirus H-1 infection. Blood. 1997;89:1642–1653. [PubMed] [Google Scholar]
- 18.López-Guerrero J A, Carrasco L, Martínez-Abarca F, Fresno M, Alonso M A. Restriction of poliovirus RNA translation in a human monocytic cell line. Eur J Biochem. 1989;186:577–582. doi: 10.1111/j.1432-1033.1989.tb15247.x. [DOI] [PubMed] [Google Scholar]
- 19.Lowenstein C J, Hill S L, Lafond-Walker A, Wu J, Allen G, Landavere M, Rose N R, Herskowitz A. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Invest. 1996;97:1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannick J B, Asano K, Izumi K, Kieff E, Stamler J S. Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein-Barr virus reactivation. Cell. 1994;79:1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 21.Mikami S, Kawashima S, Kanazawa K, Hirata K, Katayama Y, Hotta H, Hayashi Y, Ito H, Yokoyama M. Expression of nitric oxide synthase in a murine model of viral myocarditis induced by coxsackievirus B3. Biochem Biophys Res Commun. 1996;220:983–989. doi: 10.1006/bbrc.1996.0519. [DOI] [PubMed] [Google Scholar]
- 22.Nathan C, Xie Q W. Nitric oxide synthases: roles, tolls and control. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 23.Oleszak E L, Katsetos C D, Kuzmak J, Varadhachary A. Inducible nitric oxide synthase in Theiler’s murine encephalomyelitis virus infection. J Virol. 1997;71:3228–3235. doi: 10.1128/jvi.71.4.3228-3235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolph M S, Cowden W B, Medveczky C J, Ramshaw I A. A recombinant vaccinia virus encoding inducible nitric oxide synthase is attenuated in vivo. J Virol. 1996;70:7678–7685. doi: 10.1128/jvi.70.11.7678-7685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tucker P C, Griffin D E, Choi S, Bui N, Wesselingh S. Inhibition of nitric oxide synthesis increases mortality in Sindbis virus encephalitis. J Virol. 1996;70:3972–3977. doi: 10.1128/jvi.70.6.3972-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]