Abstract
Introduction
It remains unclear whether enlarged perivascular spaces (EPVS) predict poor clinical outcomes in patients with acute ischaemic stroke (AIS) or transient ischaemic attack (TIA).
Method
Data were obtained from the Third China National Stroke Registry study. We estimated EPVS in basal ganglia (BG) and centrum semiovale (CSO) using a semiquantified scale (Grade from 0 to 4). Using Cox and logistic regression analyses, the associations of EPVS with 3-month and 1-year adverse outcomes (including recurrent stroke, ischaemic stroke, haemorrhagic stroke, combined vascular event, disability and mortality) were explored. Sensitivity analyses of any association of cerebral small vessel disease at baseline and development of a small arterial occlusion (SAO) were conducted.
Result
Among 12 603 patients with AIS/TIA, median age was 61.7±11.6 years, and 68.2% were men. After adjusting for all potential confounders, frequent-to-severe BG-EPVS was associated with a decreased risk of recurrent ischaemic stroke (HR 0.71, 95% CI 0.55 to 0.92, p=0.01) but an increased risk of haemorrhagic stroke (HR 1.99, 95% CI 1.11 to 3.58, p=0.02) at 1 year after AIS/TIA, compared with none-to-mild BG-EPVS. Patients with frequent-to-severe CSO-EPVS had a decreased risk of disability (OR 0.76, 95% CI 0.62 to 0.92, p=0.004) and all-cause death (HR 0.55, 95% CI 0.31 to 0.98, p=0.04) within 3-month but not 1-year follow-ups, compared with those with none-to-mild BG-EPVS. Sensitivity analyses showed that both BG-EPVS (HR 0.43, 95% CI 0.21 to 0.87, p=0.02) and CSO-EPVS (HR 0.58, 95% CI 0.35 to 0.95, p=0.03) were associated with a decreased risk of subsequent ischaemic stroke in patients with SAO during 1-year follow-up.
Conclusion
BG-EPVS increased the risk of haemorrhagic stroke in patients already with AIS/TIA within 1 year. Therefore, caution is recommended when selecting antithrombotic agents for secondary stroke prevention in patients with AIS/TIA and more severe BG-EPVS.
Keywords: Magnetic Resonance Imaging, Stroke, hemorrhagic stroke, enlarged eprivascular spaces, transient ischemic attack
WHAT IS ALREADY KNOWN ON THIS TOPIC
Enlarged perivascular spaces (EPVS) are prognostically significant in patients with cerebral small vessel disease, but it is unclear whether EPVS predict clinical outcomes in patients with acute ischaemic stroke (AIS) or transient ischaemic attack (TIA).
WHAT THIS STUDY ADDS
EPVS in basal ganglia (BG) was related to the increased risk of haemorrhagic stroke in patients already with AIS/TIA within 1 year.
However, this relationship did not observe in centrum semiovale.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The results could be useful in selecting antithrombotic agents for secondary stroke prevention in patients with AIS/TIA and more severe EPVS in BG.
Introduction
To avoid early and long-term complications in patients already with an acute ischaemic stroke (AIS) or transient ischaemic attack (TIA) is a challenge.1 About 5%–20% of these patients experienced recurrent stroke events2 and 60%–90% had poor functional outcomes.3 To reduce these complications, a more comprehensive evaluation of the risk factors and stratified risk management will be helpful.
Cerebral small vessel disease (CSVD), characterised by typical neuroimaging markers, including white matter hyperintensity (WMH), lacune, cerebral microbleeds (CMB) and enlarged perivascular spaces (EPVS), causes about a quarter of stroke.4 More severe burden of CSVD is related to higher risk of adverse clinical outcomes.5 Prevalent CSVD markers, such as WMH, lacune, and CMB, are also associated with poor clinical outcomes after AIS/TIA.6 Another marker, EPVS, especially located in basal ganglia (BG), is a traceable predictor of poststroke cognitive impairment.7 However, the associations between EPVS and stroke recurrence, disability and mortality have not been well explored in patients with AIS/TIA.
PVS are fluid cavities that surround the small blood vessels in the brain parenchyma and visualise as EPVS on Magnetic Resonance Imaging (MRI) when PVS dilate.8 EPVS are commonly located in the BG and centrum semiovale (CSO).8 Recent advance indicated that cerebrospinal fluid (CSF) surrounding the brain parenchyma entered into brain tissue along PVS within minutes of ischaemia and caused brain oedema.9 It highlighted the potential implication of EPVS during the ischaemic process. Previous studies focused on the role of EPVS on clinical outcomes were limited to specific populations (such as patients with haemorrhage stroke), incomplete outcome assessment, relatively small sample size and short follow-up period.5 10 11 Furthermore, the relationships of EPVS with post-AIS/TIA outcomes remain poorly defined in patients with baseline CSVD burden or small arterial occlusion (SAO).
In this study, using data from a prospective multicentre study enrolled patients with AIS/TIA in China, we aimed to determine the associations of EPVS in BG and CSO with clinical outcomes, including stroke recurrence, disability and morality, at 90-day and 1-year follow-ups. Moreover, we also intended to investigate the associations of CSVD at the baseline and development of an SAO.
Methods
Study population
We derived data from the Third China National Stroke Registry (CNSR-III) study. Details about the CNSR-III study have been published elsewhere.12 Briefly, CNSR-III cohort is a nationwide multicentre clinical registry study of AIS/TIA between August 2015 and March 2018 in China. A total of 15 166 patients were enrolled in the CNSR-III study following these criteria: (1) diagnosed with AIS or TIA, (2) hospitalised within 7 days from symptom onset and (3) >18 years old. In the present study, patients were excluded if they had: (1) unavailable MRI data (n=2154) and (2) unqualified MRI data for CSVD assessment (EPVS, n=191; lacune, n=199; WMH, n=16).
Standard protocol approvals and patient consent
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. All participants or their legal guardians signed a written informed consent prior to their enrolment.
Data collection
The baseline data were collected by well-trained research coordinators during the face-to-face baseline surveys. All variables were collected using a standardised protocol by well-trained investigators. The clinical information included demographic characteristics (age and sex), medical histories (diabetes mellitus, hypertension, hyperlipidaemia, stroke, TIA, coronary artery disease (CAD), heart failure and peripheral artery disease), medication (antiplatelet, anticoagulation, statins and hypoglycaemic treatments), current alcohol and tobacco intake statuses, index event, and physical examinations (body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), modified Rankin Scale (mRS) Score, National Institutes of Health Stroke Scale (NIHSS) Score) and Trial of Org 10 172 in Acute Stroke Treatment (TOAST) classification. The protocol of brain structural imaging, including T1 weighted (T1w), T2 weighted (T2w), fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC), T2*/susceptibility weighted imaging (SWI) or computerised tomography (if contraindicated to MRI), was completed for each participant. All imaging data were collected in Digital Imaging and Communications in Medicine format on disks in all participating sites and further analysed by professional neurologists at the neuroimaging research centre at Beijing Tiantan Hospital. Deep and periventricular WHM (D-WMH and PV-WMH) were assessed according to the Fazekas Scale.13 14 The presence of lacune and CMB were defined using the standards for reporting vascular changes on neuroimaging (STRIVE) criteria.14 The total burden of CSVD was estimated by a previously published scale (points from 0 to 4), which was considered WMH, EPVS, lacune and CMB.15 If the total burden was more than 0 points, CSVD was considered present on baseline MRI.
EPVS assessment
According to the STRIVE criteria,14 MRI-visible EPVS was defined as small, sharply delineated structures of CSF intensity on T1w, T2w, FLAIR images, which followed the orientation of the perforating vessels, and ran perpendicular to the brain surface. EPVS was always less than 3 mm wide and appeared round or ovoid in axial sections (in the BG) or linear in longitudinal sections (in the CSO). The isolated single giant Virchow-Robin space was not estimated in the current study. BG-EPVS and CSO-EPVS were separately assessed because previous evidence indicated that they represented distinct pathophysiologies. EPVS was counted with the previously validated scale applied to standard axial images: grade 0 (none)=0 EPVS, grade 1 (mild)=1–10 EPVS, grade 2 (moderate)=11–20 EPVS, grade 3 (frequent)=21–40 EPVS and grade 4 (severe)>40 EPVS.16 The number of EPVS was assessed on each side of the brain and counted in the slice with the highest number. When the scores were asymmetric between two cerebral hemispheres, EPVS was counted on the side with the higher number. The assessment of EPVS was performed by two experienced neurologists (YT and YY) who were blinded to patients’ clinical information. The inconsistent results were further assessed by another senior neurologist (JJ) who was blinded to initial results. The kappa coefficient of EPVS between raters was 0.89.
To generate groups of a similar size for meaningful subsequent statistical analyses, all patients were categorised into three groups according to the severity of EPVS: none to mild (equivalent to rating scale grade 0–1), moderate (rating scale grade 2) and frequent to severe (rating scale grade 3–4).17
Follow-up outcomes
Details about the follow-up procedure of the CNSR-III study have been described elsewhere.12 The primary outcome was recurrent stroke (including subsequent ischaemic stroke and new haemorrhagic stroke) during follow-up period. The secondary outcomes included subsequent ischaemic stroke, new haemorrhagic stroke (including intracerebral haemorrhage (ICH) and subarachnoid haemorrhage), recurrent combined vascular event (CVE, including stroke, myocardial infarction and cardiovascular death) and all-cause death. Additionally, disability, defined as mRS scores 3–5, was a functional outcome. The specific definitions of follow-up outcomes are presented in online supplemental appendix S1. The clinical outcomes mentioned above were observed at 3 months and 1 year after AIS/TIA onset by face-to-face or telephone interviews. Confirmation of an outcome event was sought from the treating hospital and was judged by an independent endpoint judgement committee.
svn-2022-002157supp001.pdf (102.2KB, pdf)
Statistical analysis
Categorical variables are presented as frequencies with percentages, and continuous variables are presented as the means with SDs or medians with IQRs. To investigate differences in baseline characteristics between groups defined by BG-EPVS and CSO-EPVS, we performed χ2 tests for categorical variables and one-way analyses of variance for continuous variables following a normal distribution (such as age and BMI) or Kruskal-Wallis tests for other continuous variables as appropriate, respectively.
Using Cox proportional hazards regression analyses, we investigate the associations between EPVS and recurrence and all-cause death in patients with AIS/TIA in 3-month and 1-year follow-ups, respectively. The HRs with 95% CIs were calculated. And logistic regression analyses were applied to explore the relationships of EPVS with disability in patients with AIS/TIA in 3-month and 1-year follow-ups, respectively. The ORs with 95% CIs were calculated. In model 1, we did not adjust for any variables; in model 2, we adjusted for age and sex; in model 3, we selected all potential confounding factors in univariable analyses, including age, sex, BMI, SBP, DBP, mRS, NIHSS, current alcohol and tobacco intakes, diabetes, hypertension, hyperlipidaemia, stroke, CAD, index event, TOAST, lacune, PV-WMH and D-WMH. Additionally, to eliminating the potential effects of collinearity between EPVS and other CSVD markers, we further removed PV-WMH, D-WMH and lacune in model 4. The Kaplan-Meier survival curve was further conducted to depict the cumulative risk of new stroke event and analysed with the use of the log-rank test.
To better examine the robustness of the role of EPVS, sensitivity analyses were conducted in different study sample. First, to minimise potential influence of different aetiologies of AIS/TIA, we selected patients who had a diagnosis of SAO according to the TOAST classification, and investigated the associations between EPVS and clinical outcomes in patients with SAO. Second, to examine whether the associations between EPVS and clinical outcomes differed in patients with CSVD, we selected patients who had present CSVD burden on baseline MRI and explored the associations between EPVS and clinical outcomes in patients with CSVD.
In our analyses, a two-sided p<0.05 was defined to indicate nominally statistical significance for a potential, but yet to be confirmed, association. An observed two-sided p<0.017 (Bonferroni-corrected significance threshold calculated as 0.05 divided by 3 (for 3 groups) was considered as statistically significant evidence for an association between EPVS and clinical outcome. All statistical analyses were conducted using SAS software (V.9.4, SAS Institute, Cary, North Carolina, USA).
Data availability statement
All data generated or analysed during this study are included in this published article and available on reasonable requests to the corresponding author.
Results
Baseline characteristics and clinical outcomes of patients with AIS/TIA
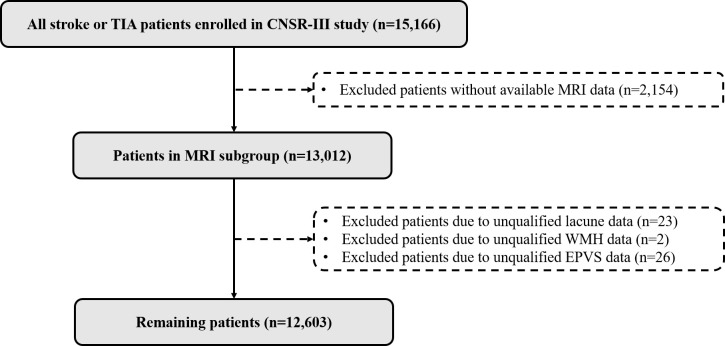
As shown in figure 1, a total of 12 603 participants with AIS/TIA who had baseline EPVS data were enrolled in the current study. Their average age was 61.7±11.6 years and 68.2% were men. The baseline characteristics between patients included and those excluded from the CNSR-III study are shown in online supplemental table S1.
Figure 1.
The flowchart of patients’ selection from the CNSR-III study. CNSR-III, the China National Stroke Registry III; EPVS, enlarged perivascular spaces; TIA, transient ischaemic attack; WMH, white matter hyperintensity; MRI, Magnetic Resonance Imaging.
svn-2022-002157supp002.pdf (568.9KB, pdf)
Patients with frequent-to-severe BG-EPVS were older, predominantly men, and had a lower BMI, higher SBP, lower DBP, more severe D-WMH and PV-WMH, a higher prevalence of hypertension, on antihypertension treatment, history of stroke, CAD, lacune, a lower prevalence of current alcohol and tobacco intakes, history of diabetes, hyperlipidaemia, on hypoglycaemic treatment, and a higher proportion of current AIS events (especially of SAO), compared with those with none-to-mild and moderate BG-EPVS (table 1). Patients’ baseline characteristics according to severity of CSO-EPVS are presented in online supplemental table S2.
Table 1.
Baseline characteristics of patients with AIS/TIA according to BG-EPVS
| Characteristics | None/mild (n=8634) | Moderate (n=3135) | Frequent/severe (n=834) | P value |
| Age, years, mean (SD) | 60.2±11.2 | 66.1±9.9 | 70.5±9.2 | <0.001 |
| BMI, kg/m2, mean (SD) | 24.8±3.3 | 24.7±3.4 | 24.3±3.4 | <0.001 |
| Sex, male (%) | 5808 (67.3) | 2218 (70.8) | 573 (68.7) | 0.002 |
| Alcohol intake, n (%) | 1244 (14.4) | 438 (14.0) | 93 (11.2) | 0.03 |
| Tobacco intake, n (%) | 2848 (33.0) | 890 (28.4) | 194 (23.3) | <0.001 |
| SBP, mm Hg, median (IQR) | 147.5 (134.0–163.0) | 150.0 (137.0–165.0) | 150.0 (137.5–164.0) | <0.001 |
| DBP, mm Hg, median (IQR) | 86.0 (79.0–95.0) | 87.0 (79.5–96.5) | 85.5 (79.0–94.0) | 0.03 |
| History of disease, n (%) | ||||
| Diabetes mellitus | 2030 (23.5) | 701 (22.4) | 135 (16.2) | <0.001 |
| Hypertension | 5097 (59.0) | 2225 (71.0) | 591 (70.9) | <0.001 |
| Hyperlipidaemia | 716 (8.3) | 243 (7.8) | 45 (5.4) | 0.01 |
| Stroke | 1635 (18.9) | 868 (27.7) | 261 (31.3) | <0.001 |
| TIA | 233 (2.7) | 88 (2.8) | 19 (2.3) | 0.70 |
| Coronary artery disease | 797 (9.2) | 385 (12.3) | 95 (11.4) | <0.001 |
| Heart failure | 43 (0.5) | 27 (0.9) | 5 (0.6) | 0.08 |
| Peripheral artery disease | 67 (0.8) | 21 (0.7) | 6 (0.7) | 0.84 |
| Medication use during hospitalisation or at discharge, n (%) | ||||
| Antiplatelet treatment | 8465 (98.0) | 3063 (97.7) | 819 (98.2) | 0.46 |
| Anticoagulation treatment | 860 (10.0) | 319 (10.2) | 97 (11.6) | 0.31 |
| Statins treatment | 8422 (97.5) | 3058 (97.5) | 803 (96.3) | 0.08 |
| Hypoglycaemic treatment | 2359 (27.3) | 811 (25.9) | 156 (18.7) | <0.001 |
| Antihypertension treatment | 4348 (50.4) | 1932 (61.6) | 501 (60.1) | <0.001 |
| NIHSS on admission, median (IQR) | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) | 3.0 (2.0–5.0) | 0.98 |
| mRS, median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | <0.001 |
| Index event, n (%) | <0.001 | |||
| Ischaemic stroke | 8014 (92.8) | 2973 (94.8) | 796 (95.4) | |
| TIA | 620 (7.2) | 162 (5.2) | 38 (4.6) | |
| TOAST, n (%) | <0.001 | |||
| Large artery atherosclerosis | 2295 (26.6) | 825 (26.3) | 210 (25.2) | |
| Cardioembolism | 466 (5.4) | 193 (6.2) | 70 (8.4) | |
| Small vascular occlusion | 1859 (21.5) | 783 (25.0) | 223 (26.7) | |
| Other determination | 141 (1.6) | 26 (0.8) | 4 (0.5) | |
| Undetermined | 3873 (44.9) | 1308 (41.7) | 327 (39.2) | |
| CSVD markers | ||||
| D-WMH, median (IQR) | 1.0 (1.0–2.0) | 2.0 (1.0–2.0) | 2.0 (1.0–3.0) | <0.001 |
| PV-WMH, median (IQR) | 1.0 (1.0–2.0) | 2.0 (1.0–2.0) | 2.0 (2.0–3.0) | <0.001 |
| Lacune, n (%) | 3521 (40.8) | 2068 (66.0) | 637 (76.4) | <0.001 |
AIS, acute ischaemic attack; BG-EPVS, EPVS in basal ganglia; BMI, body mass index; CSVD, cerebral small vessel disease; DBP, diastolic blood pressure; D-WMH, deep-WMH; EPVS, enlarged perivascular spaces; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale score; PV-WMH, periventricular-WMH; SBP, systolic blood pressure; TIA, transient ischaemic attack; TOAST, Trial of Org 10 172 in Acute Stroke Treatment; WMH, white matter hyperintensity.
Among 12 603 patients with AIS/TIA, 761 (6.0%) and 1189 (9.4%) had experienced recurrent stroke, 1515 (12.3%) and 1218 (10.2%) developed disabilities and 153 (1.2%) and 382 (3.0) died at 3-month and 1-year follow-ups, respectively.
The associations between BG-EPVS and clinical outcomes in patients with AIS/TIA
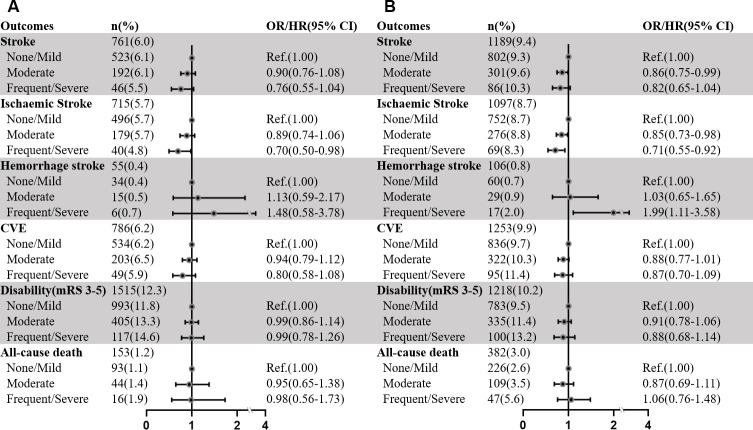
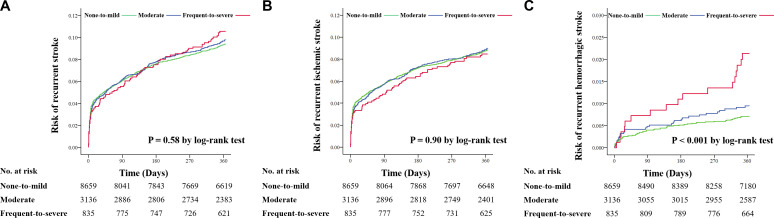
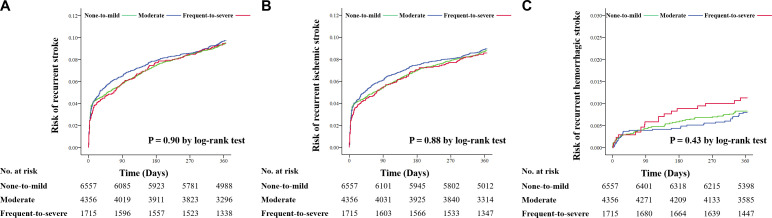
The associations between BG-EPVS and clinical outcomes in patients with AIS/TIA are shown in figure 2 and online supplemental table S3. After adjusting for all potential confounders in model 3, patients with frequent-to-severe BG-EPVS had a decreased risk of recurrent ischaemic stroke during 3-month (HR 0.70, 95% CI 0.50 to 0.98, p=0.04) and 1-year (HR 0.71, 95% CI 0.55 to 0.92, p=0.01) follow-ups. However, after removing PV-WMH, D-WMH and lacune as covariates in model 4, these relationships disappeared. Frequent-to-severe BG-EPVS was significantly associated with an increased risk of new haemorrhage stroke (model 3: HR 1.99, 95% CI 1.11 to 3.58, p=0.02; model 4: HR 3.06, 95% CI 1.73 to 5.42, p<0.001) within 1 year after adjusting for all potential covariates. The Kaplan-Meier curve estimated the cumulative risk of 1-year haemorrhagic stroke (p for log rank test<0.001; figure 3). No significant associations between BG-EPVS and recurrent stroke, CVE, disability or all-cause death were yielded during the 3-month and 1-year follow-ups after AIS/TIA.
Figure 2.
Multivariable analyses of the associations between BG-EPVS and clinical outcomes in patients with AIS/TIA during 3-month (A) and 1-year (B) follow-ups. Multivariable Cox or logistic regression model included age, sex, BMI, SBP, DBP, mRS, NIHSS, alcohol intake, tobacco intake, diabetes, hypertension, hyperlipidaemia, stroke, CAD, index event, TOAST, lacune, PV-WMH and D-WMH. AIS, acute ischaemic stroke; BMI, body mass index; BG-EPVS, EPVS in basal ganglia; CAD: coronary artery disease; CVE, combined vascular event; CSO-EPVS, EPVS in centrum semiovale; DBP, diastolic blood pressure; D-WMH, deep-WMH; EPVS, enlarged perivascular spaces; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale score; PV-WMH, periventricular-WMH; Ref, reference; SBP, systolic blood pressure; TIA, transient ischaemic attack; TOAST, Trial of Org 10 172 in Acute Stroke Treatment; WMH, white matter hyperintensity.
Figure 3.
Cumulative risk of new stroke event according to BG-EPVS using Kaplan-Meier survival graph at 1-year cumulative risks of new stroke (A), ischaemic stroke (B), haemorrhagic stroke (C) according to BG-EPVS during 1-year follow-up. BG-EPVS, enlarged perivascular spaces in basal ganglia.
The associations between CSO-EPVS and clinical outcomes in patients with AIS/TIA
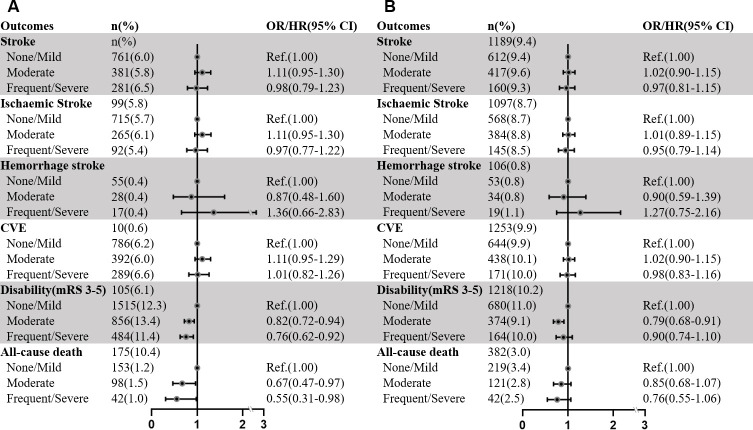
The associations between CSO-EPVS and clinical outcomes in patients with AIS/TIA are presented in figures 4 and 5 and online supplemental table S4. After adjustment for potential confounding factors, patients with frequent-to-severe CSO-EPVS had a decreased risk of disability (model 3: OR 0.76, 95% CI 0.62 to 0.92, p=0.004; model 4: OR 0.76, 95% CI 0.63 to 0.92, p=0.004) and all-cause death (model 3: HR 0.55, 95% CI 0.31 to 0.98, p=0.04) within 3 months. There was no association between CSO-EPVS and subsequent recurrent events during 3-month or 1-year follow-ups.
Figure 4.
Multivariable analyses of associations between CSO-EPVS and clinical outcomes in patients with AIS/TIA during 3-month (A) and 1-year (B) follow-ups. Multivariable Cox or logistic regression model included age, sex, BMI, SBP, DBP, mRS, NIHSS, alcohol intake, tobacco intake, diabetes, hypertension, hyperlipidaemia, stroke, CAD, index event, TOAST, lacune, PV-WMH and D-WMH. AIS, acute ischaemic stroke; BMI, body mass index; BG-EPVS, EPVS in basal ganglia; CAD: coronary artery disease; CVE, combined vascular event; CSO-EPVS, EPVS in centrum semiovale; DBP, diastolic blood pressure; D-WMH, deep-WMH; EPVS, enlarged perivascular spaces; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale score; PV-WMH, periventricular-WMH; Ref, reference; SBP, systolic blood pressure; TIA, transient ischaemic attack; TOAST, Trial of Org 10 172 in Acute Stroke Treatment; WMH, white matter hyperintensity.
Figure 5.
Cumulative risk of new stroke event according to CSO-EPVS using Kaplan-Meier survival graph at 1-year cumulative risks of new stroke (A), ischaemic stroke (B), haemorrhagic stroke (C) according to CSO-EPVS during 1-year follow-up. CSO-EPVS, enlarged perivascular spaces in centrum semiovale.
Sensitivity analyses
The associations between EPVS and clinical outcomes in 2839 patients with SAO are shown in online supplemental tables S5 and S6 after excluding patients with other stroke aetiologies (n=8944) according to the TOAST classification and TIA (n=820) (online supplemental figure S1). Both BG-EPVS (model 3: HR 0.43, 95% CI 0.21 to 0.87, p=0.02) and CSO-EPVS (model 3: HR 0.58, 95% CI 0.35 to 0.95, p=0.03) were nominally associated with decreased risks of new ischaemic stroke at 1 year after AIS/TIA after adjusting for potential confounders.
The associations between EPVS and clinical outcomes in 4785 patients with baseline CSVD burden are presented in online supplemental tables S7 and S8 after excluding patients with no CMB data (n=5454), absent CSVD burden (0 score; n=2069) and TIA (n=295) (online supplemental figure S2). There were no significant links between EPVS and clinical outcomes in patients with present CSVD after adjusting for possible confounders.
Discussion
In this study, we systematically investigated whether MRI-visible EPVS (both in BG and CSO) was associated with poor clinical outcomes in patients with AIS/TIA during the 3-month and 1-year follow-ups. Our results showed that frequent-to-severe BG-EPVS was independently associated with an increased risk of new haemorrhagic stroke in patients with AIS/TIA within 1 year as compared with none-to-mild BG-EPVS. Moreover, we found patients with frequent-to-severe BG-EPVS had a decreased risk of new ischaemic stroke after 1-year AIS/TIA. Furthermore, we discovered AIS/TIA patients with frequent-to-severe CSO-EPVS had decreased risks of disability and all-cause mortality within 3 months but not within 1 year.
Different CSVD markers (including EPVS, WMH, lacune and CMB) had distinct aspects, heterogeneous aetiologies and underlying pathogenetic mechanisms of CSVD.4 18 The coexistence of different CSVD characteristics may represent an overall status of cerebral microcirculation and total burden of cerebral small vessel injury.5 14 15 Total CSVD burden, commonly seen in patients who had a stroke and TIA (eg, 57.8% in the current study), was associated with poor stroke outcomes.5 Moreover, individual CSVD marker, such as WMH and lacune, also contributed to worse outcomes after AIS/TIA.5 However, to the best of our knowledge, there were limited evidence regarding the associations between EPVS and stroke recurrence, disability, and mortality, even though EPVS has emerged as a predictor of poststroke cognitive impairment and a potential MRI marker of the presence of the glymphatic system in the last few years.19
Our finding of BG-EPVS and increased risk of subsequent haemorrhagic stroke at 1 year after AIS/TIA, independent of major vascular risk factors and other CSVD markers, is clinically significant. At 3-month follow-up, patients with frequent-to-severe BG-EPVS exhibited a higher incidence of new haemorrhagic stroke than those with none-to-mild BG-EPVS, but did not reach statistically significance. One prospective research published by Lau et al indicated that AIS/TIA patients with frequent-to-severe BG-EPVS had a 2.6-fold increased risk of developing an ICH after AIS/TIA.5 Best et al found that moderate-to-severe BG-EPVS was related to the incidence of anticoagulant-related ICH in AIS/TIA patients with atrial fibrillation.10 However, Song et al founded moderate-to-severe BG-EPVS was not independently related to haemorrhagic transformation after AIS,20 which was contrary to the results of our present study and other studies. Unlike the above research concentrated mainly on haemorrhagic transformation following AIS (median time from stroke onset to haemorrhage 3.5 days, IQR, 2–6 days),20 we focused on subsequent haemorrhagic stroke after AIS/TIA within 3-month and 1-year follow-up periods, Lau et al focused on the development of ICH in patients with AIS/TIA after a mean follow-up of 42±23 months,5 and Best et al focused on anticoagulant-related ICH (median time from anticoagulation initiation to haemorrhage 272 days, IQR, 211–657 days).10 Additionally, variation in study population and sample size may be another possible explanation for the difference. Neither our study nor Lau et al’s research showed an association between CSO-EPVS and recurrent haemorrhagic stroke in patients with AIS/TIA.5 However, previous studies indicated that more severe CSO-EPVS was associated with a high risk of ICH in healthy population and in patients with cognitive dysfunction.21 22 Therefore, there was likely limited predictive ability of CSO-EPVS for new haemorrhagic stroke in patients with AIS/TIA. Previous cross-sectional evidence suggested that BG-EPVS was associated with deep ICH, while CSO-EPVS was associated with lobar ICH and cerebral amyloid angiopathy.23–25 Furthermore, in patients with cognitive impairment, BG-EPVS was related to deep CMBs, while CSO-EPVS was related to lobar CMBs and cortical superficial siderosis.17 22 26 Hence, EPVS in different anatomical locations may be involved in different pathophysiological processes evolving into future haemorrhagic stroke.
The above findings provided the evidence linking EPVS to haemorrhagic cerebrovascular disease, although the potential mechanisms were not entirely clear. PVS provide channels for flow of CSF and interstitial fluid. Dilated PVS may represent extravasation and accumulation of fluid across damaged blood vessel wall.27 Dilated PVS possibly recruited inflammatory cells and toxic substance,28 29 which may damage the integrity of blood–brain barrier (BBB) and impair the efficiency of glymphatic transport.30 31 Furthermore, another potential explanation was that EPVS and the incidence of ICH may be mediated by shared possible pathogenesis mechanisms. For example, arterial stiffness was proved to simultaneously relate to BG-EPVS and deep ICH.32–34 By decreasing attenuation of cardiac impulse and altering transmission of pulsatile force to cerebral small vessels, which was deemed as crucial drivers of glymphatic transport, arterial stiffness may increase the risk of ICH and promote enlargement of PVS.32–34 In clinical practice, the implication of EPVS on increased risk of haemorrhagic stroke implied that more caution should be exercised for individuals with more severe EPVS (especially in BG) when selecting antithrombotic drugs for secondary stroke prevention after AIS/TIA. For example, cilostazol, a phosphodiesterase 3 inhibitor, was recommended to suppress poststroke ICH by protecting the integrity of BBB.35 36
In this study, we further explored whether BG-EPVS was associated with recurrent ischaemic event in patients with AIS/TIA. No suggestive links were found in univariable regression analyses and log-rank tests. Since lower prevalence of diabetes and hyperlipidaemia in patients with a greater burden of BG-EPVS at baseline, which played vital roles in subsequent ischaemic events after AIS/TIA, multivariable regression models were conducted. Interestingly, we found the association between BG-EPVS and decreased risk of recurrent ischaemic stroke in patients with AIS/TIA after adjusting potential covariables. Since patients with frequent-to-severe BG-EPVS had a higher proportion of antihypertension intake and blood presume-lowering treatment was significant to prevent recurrent stroke in patients with a history of AIS/TIA, it may be a possible reason of the decreased risk of new ischaemic stroke after AIS/TIA. Moreover, we speculated the contradictory appeared related to differences between models. In univariable analyses and log-rank tests, the association between exposure and outcome incorporated effects and interactions of covariables, thus presenting a composite result.37 There was only an independent association of EPVS with outcome after eliminating effects of confounders in multivariable analyses.37 A previous study did not show any significant relationship between large PVS and incident stroke in a community-based population.38 In contrast, Lau et al found AIS/TIA patients with frequent-to-severe BG-EPVS presented at 1.8-fold increased risk of recurrent ischaemic stroke.5 Selvarajah et al suggested EPVS was significantly associated with estimated stroke risk.39 In our study, patients with moderate-to-severe BG-EPVS had a higher proportion of history of stroke. Furthermore, Doubal et al demonstrated that EPVS, especially BG-EPVS, was associated with more lacunar strokes than large vessel stroke.16 Consistent with our findings, patients with frequent-to-severe BG-EPVS had higher proportions of SAO (26.7% vs 21.5%) and lacune (76.4% vs 40.8%) than those with none-to-mild BG-EPVS. In clinical practice, acute or chronic medication management was essential to recurrent ischaemic events but not haemorrhage after AIS/TIA, although we did not observe any significant differences of antiplatelet, anticoagulation and statins treatments in patients with different severity of BG-EPVS. Given the potential influence of EPVS on stroke, further studies should be performed to determine the associations between EPVS, antithrombotic drugs and future stroke events in patients with AIS/TIA.
Our study has found that frequent-to-severe CSO-EPVS was independently associated with decreased risks of disability and mortality within 3 months but not within 1 year. Perhaps, CSO-EPVS somehow slowed down the deterioration or fluctuation of neurological deficits in the acute and subacute stages.40 Although CSVD (especially WMH and lacune) may portend unfavourable functional outcome and death,40 PVS may play a vital role in the development of cerebral oedema during the acute ischaemic phase.9
Our study has several limitations. First, a semiquantitative visual rating scale by observation and comparison was used rather than by an objective measurement to assess the severity of EPVS. Automated quantification with accurate segmentation of EPVS, such as by machine-learning technology, should be considered in future research. Second, since the first MRI examination after acute cerebrovascular events did not routinely include SWI or T2*-weighted gradient-echo sequences in emergency clinic, there were 5454 patients with no data on CMB. Hence, CMB did not appear as a covariate in the multivariable Cox and logistic regression models. Third, as second MRI or CT scan data in patients with clinical outcomes were not collected, we cannot identify the position of subsequent haemorrhagic stroke and dynamic changes of EPVS. Moreover, our present study does not include all residual confounding factors, but this unfortunately is a limitation of any observational study design. And we cannot completely eliminate the potential confounding effects of vascular risk factors and treatments, although limited evidence supporting a relationship of EPVS with vascular risk factors.41 Furthermore, Bonferroni’s correction is very strict, therefore more cautions need to be taken when interpreting our present findings, and the potential type I and II errors cannot be fully eliminated. Last, only Chinese population included in the CNSR-III study, the extrapolation of the current results in other populations needs further validation.
Conclusion
BG-EPVS was associated with the development of haemorrhagic stroke within 1 year after AIS/TIA. The robustness of this relationship and potential clinical implication in predicting and preventing recurrent haemorrhagic stroke in this patient population should be further explored in a larger cohort.
Acknowledgments
We thank all the participants in the study.
Footnotes
Twitter: @yilong
Contributors: Conceptualisation: YT and Yilong Wang. Methodology: MW and YP. Investigation: XM, XZ and LL. Writing—original draft: YT. Writing—reviewing & editing: Yilong Wang. Supervision: Yongjun Wang. Guarantor: Yilong Wang.
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81825007), Beijing Outstanding Young Scientist Program (No. BJJWZYJH01201910025030), National Key R&D Program of China (2016YFC0901002), Youth Beijing Scholar Program (No. 010), "National Ten-Thousand Talent Plan"—Leadership of Scientific and Technological Innovation, Capital's Funds for Health Improvement and Research (2022-2-2045), Beijing Talent Project - Class A: Innovation and Development (2018A12), National Key R&D Program of China (2017YFC1307900).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. The CNSR-III study was conducted according to the Declaration of Helsinki and was approved by the ethics committees of Beijing Tiantan Hospital (IRB number: KY2015-001-01) and all participating sites. Participants gave informed consent to participate in the study before taking part.
References
- 1. Wang Y-J, Li Z-X, Gu H-Q, et al. China stroke statistics 2019: a report from the National center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, National center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and Institute for global neuroscience and stroke collaborations. Stroke Vasc Neurol 2020;5:211–39. 10.1136/svn-2020-000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lioutas V-A, Ivan CS, Himali JJ, et al. Incidence of transient ischemic attack and association with long-term risk of stroke. JAMA 2021;325:373–81. 10.1001/jama.2020.25071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Y-J, Li Z-X, Gu H-Q, et al. China stroke statistics: an update on the 2019 report from the National center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, National center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and Institute for global neuroscience and stroke collaborations. Stroke Vasc Neurol 2022;7:415–50. 10.1136/svn-2021-001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi Y, Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol 2016;1:83–92. 10.1136/svn-2016-000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lau KK, Li L, Schulz U, et al. Total small vessel disease score and risk of recurrent stroke: validation in 2 large cohorts. Neurology 2017;88:2260–7. 10.1212/WNL.0000000000004042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Debette S, Schilling S, Duperron M-G, et al. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol 2019;76:81–94. 10.1001/jamaneurol.2018.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arba F, Quinn TJ, Hankey GJ, et al. Enlarged perivascular spaces and cognitive impairment after stroke and transient ischemic attack. Int J Stroke 2018;13:47–56. 10.1177/1747493016666091 [DOI] [PubMed] [Google Scholar]
- 8. Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020;16:137–53. 10.1038/s41582-020-0312-z [DOI] [PubMed] [Google Scholar]
- 9. Mestre H, Du T, Sweeney AM, et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 2020;367:eaax7171. 10.1126/science.aax7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Best JG, Barbato C, Ambler G, et al. Association of enlarged perivascular spaces and anticoagulant-related intracranial hemorrhage. Neurology 2020;95:e2192–9. 10.1212/WNL.0000000000010788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng L, Leng X, Nie X, et al. Small vessel disease burden may not portend unfavorable outcome after thrombectomy for acute large vessel occlusion. Eur Radiol 2022;32:7824–32. 10.1007/s00330-022-08795-3 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Jing J, Meng X, et al. The third China national stroke registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol 2019;4:158–64. 10.1136/svn-2019-000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fazekas F, Chawluk JB, Alavi A, et al. Mr signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 1987;149:351–6. 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 14. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Staals J, Makin SDJ, Doubal FN, et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014;83:1228–34. 10.1212/WNL.0000000000000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doubal FN, MacLullich AMJ, Ferguson KJ, et al. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010;41:450–4. 10.1161/STROKEAHA.109.564914 [DOI] [PubMed] [Google Scholar]
- 17. Banerjee G, Kim HJ, Fox Z, et al. MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden. Brain 2017;140:1107–16. 10.1093/brain/awx003 [DOI] [PubMed] [Google Scholar]
- 18. Shu M-J, Zhai F-F, Zhang D-D, et al. Metabolic syndrome, intracranial arterial stenosis and cerebral small vessel disease in community-dwelling populations. Stroke Vasc Neurol 2021;6:589–94. 10.1136/svn-2020-000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Georgakis MK, Fang R, Düring M, et al. Cerebral small vessel disease burden and cognitive and functional outcomes after stroke: a multicenter prospective cohort study. Alzheimers Dement 2023;19:1152–63. 10.1002/alz.12744 [DOI] [PubMed] [Google Scholar]
- 20. Song Q, Cheng Y, Wang Y, et al. Enlarged perivascular spaces and hemorrhagic transformation after acute ischemic stroke. Ann Transl Med 2021;9:1126. 10.21037/atm-21-1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yakushiji Y, Charidimou A, Hara M, et al. Topography and associations of perivascular spaces in healthy adults: the Kashima scan study. Neurology 2014;83:2116–23. 10.1212/WNL.0000000000001054 [DOI] [PubMed] [Google Scholar]
- 22. Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology 2013;80:1551–6. 10.1212/WNL.0b013e31828f1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charidimou A, Boulouis G, Pasi M, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017;88:1157–64. 10.1212/WNL.0000000000003746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koo HW, Jo KI, Yeon JY, et al. Clinical features of high-degree centrum semiovale-perivascular spaces in cerebral amyloid angiopathy. J Neurol Sci 2016;367:89–94. 10.1016/j.jns.2016.05.040 [DOI] [PubMed] [Google Scholar]
- 25. Charidimou A, Meegahage R, Fox Z, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. Journal of Neurology, Neurosurgery & Psychiatry 2013;84:624–9. 10.1136/jnnp-2012-304434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shams S, Martola J, Charidimou A, et al. Topography and determinants of magnetic resonance imaging (MRI) -visible perivascular spaces in a large memory clinic cohort. J Am Heart Assoc 2017;6:e006279. 10.1161/JAHA.117.006279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown R, Benveniste H, Black SE, et al. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 2018;114:1462–73. 10.1093/cvr/cvy113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bechmann I, Priller J, Kovac A, et al. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci 2001;14:1651–8. 10.1046/j.0953-816x.2001.01793.x [DOI] [PubMed] [Google Scholar]
- 29. Perosa V, Oltmer J, Munting LP, et al. Perivascular space dilation is associated with vascular amyloid-β accumulation in the overlying cortex. Acta Neuropathol 2022;143:331–48. 10.1007/s00401-021-02393-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wardlaw JM, Doubal F, Armitage P, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol 2009;65:194–202. 10.1002/ana.21549 [DOI] [PubMed] [Google Scholar]
- 31. Koundal S, Elkin R, Nadeem S, et al. Optimal mass transport with lagrangian workflow reveals advective and diffusion driven solute transport in the glymphatic system. Sci Rep 2020;10. 10.1038/s41598-020-59045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riba-Llena I, Jiménez-Balado J, Castañé X, et al. Arterial stiffness is associated with basal ganglia enlarged perivascular spaces and cerebral small vessel disease load. Stroke 2018;49:1279–81. 10.1161/STROKEAHA.118.020163 [DOI] [PubMed] [Google Scholar]
- 33. Acampa M, Guideri F, Di Donato I, et al. Arterial stiffness in patients with deep and lobar intracerebral hemorrhage. J Stroke 2014;16:184–8. 10.5853/jos.2014.16.3.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iliff JJ, Wang M, Zeppenfeld DM, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 2013;33:18190–9. 10.1523/JNEUROSCI.1592-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takagi T, Imai T, Mishiro K, et al. Cilostazol ameliorates collagenase-induced cerebral hemorrhage by protecting the blood-brain barrier. J Cereb Blood Flow Metab 2017;37:123–39. 10.1177/0271678X15621499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takagi T, Hara H. Protective effects of cilostazol against hemorrhagic stroke: current and future perspectives. J Pharmacol Sci 2016;131:155–61. 10.1016/j.jphs.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 37. Lo SK, Li IT, Tsou TS, et al. Non-significant in univariate but significant in multivariate analysis: a discussion with examples. Changgeng Yi Xue Za Zhi 1995;18:95–101. [PubMed] [Google Scholar]
- 38. Zhang J, Han F, Liang X, et al. Lacune and large perivascular space: two kinds of cavities are of different risk factors and stroke risk. Cerebrovasc Dis 2020;49:522–30. 10.1159/000508732 [DOI] [PubMed] [Google Scholar]
- 39. Selvarajah J, Scott M, Stivaros S, et al. Potential surrogate markers of cerebral microvascular angiopathy in asymptomatic subjects at risk of stroke. Eur Radiol 2009;19:1011–8. 10.1007/s00330-008-1202-8 [DOI] [PubMed] [Google Scholar]
- 40. Huo Y-C, Li Q, Zhang W-Y, et al. Total small vessel disease burden predicts functional outcome in patients with acute ischemic stroke. Front Neurol 2019;10:808. 10.3389/fneur.2019.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laveskog A, Wang R, Vetrano DL, et al. Associations of vascular risk factors and APOE genotype with perivascular spaces among community-dwelling older adults. J Am Heart Assoc 2020;9:e015229. 10.1161/JAHA.119.015229 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2022-002157supp001.pdf (102.2KB, pdf)
svn-2022-002157supp002.pdf (568.9KB, pdf)
Data Availability Statement
All data generated or analysed during this study are included in this published article and available on reasonable requests to the corresponding author.
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.