Abstract
Objective:
Fenestrated endovascular aneurysm repair (FEVAR) is a well-established treatment approach for juxtarenal and short neck infrarenal aortic aneurysms. Recommendations and clinical outcomes are lacking for offering FEVAR in patients with chronic kidney disease (CKD). We aimed to compare short and long-term outcomes for patients with none-to-mild versus moderate-to-severe CKD undergoing FEVAR.
Methods:
We retrospectively reviewed consecutive patients undergoing standard FEVAR with Cook devices at a single institution. The cohort was stratified by preoperative CKD stage none-to-mild or moderate-to-severe (CKD 1–2 and CKD 3–5, respectively). The primary outcome was postoperative acute kidney injury. Secondary outcomes included 30-day perioperative complications, 1- and 5-year rates of overall survival, dialysis, renal target artery patency, endoleak, and reintervention assessed by Kaplan-Meier method. Aneurysm sac regression, number of surveillance computed tomography scans, and CKD stage progression were assessed at latest follow up. Multivariate Cox proportional hazards modeling was used to evaluate the association of CKD stage 3 and stage 4–5 with all-cause mortality, controlling for differences in baseline characteristics.
Results:
From 2012–2022, 184 patients (of which 82% were male) underwent FEVAR with the Cook ZFEN device (mean follow-up 34.3 months). Group CKD 3–5 comprised 77 patients (42%), was older (75.2 vs 73.0 years, P=.04), had increased preoperative creatinine (1.6 vs 0.9 mg/dL, P<.01) and demonstrated increased renal artery ostial calcification (37.7% vs 21.5%, P=.02) compared with Group CKD 1–2. Perioperatively, CKD 3–5 sustained higher estimated blood loss (342 vs 228 ml, P=.01), longer operative times (186 vs 162 min, P=.04), and longer length of stay (3 vs 2 days, P<.01). Kaplan-Meier 1- and 5-year survival estimates were lower for CKD 3–5 (82.3% vs 95.1%, P<.01 and 55.4% vs 70.8%, P=.02). Fewer CKD 3–5 patients remained free from chronic dialysis at 1 year (94.4% vs 100%, P=.015) and 5 years (84.7% vs 100%, P<.01). There were no significant differences in postoperative AKI rate (CKD 1–2 6.5% vs CKD 3–5 14.3%, P=.13), long-term renal artery patency, reinterventions, type I or III endoleak, mean sac regression, or total follow-up CT scans between groups. CKD stage progression occurred in 47 patients (31%) at latest follow-up but did not differ between stratified groups (P=.17). On multivariable modeling, age (HR 1.05, 95%CI 1.01–1.09, P=.02) and CKD stage 4–5 (HR 6.39, 95%CI 2.26–18.05, P<.01) were independently associated with mortality.
Conclusions:
Preoperative CKD status did not negatively impact the durability nor technical success related to aneurysm outcomes after FEVAR. Worsening CKD stage was associated with lower 1- and 5-year overall survival and freedom from dialysis after FEVAR with no statistically significant differences in 30-day or long-term technical aneurysm outcomes.
1. Introduction
Fenestrated endovascular aneurysm repair (FEVAR), first described in 1999,1 has revolutionized the treatment of complex aortic aneurysms using minimally invasive, custom-made devices specific to patient anatomy.2–6 The Cook Zenith fenestrated endovascular graft (ZFEN; Cook Medical, Inc, Bloomington, IN) received FDA approval in 2012 and is the only commercially-available device to treat juxtarenal and short-neck infrarenal aneurysms.7,8 While endovascular repair is increasingly used to treat complex aneurysms in patients unfit to undergo open repair often with multiple medical comorbidities,9 guidelines are lacking for treating patients with chronic kidney disease (CKD), who often present with these complex aneurysms amenable to endovascular repair and are poor candidates for open repair.
CKD affects more than 15% of US population10 and is an established risk factor for poor outcomes after both open and endovascular abdominal aortic aneurysm repair.11 However, no specific Society for Vascular Surgery (SVS) guidelines exist for treating patients with moderate to severe CKD.11 Because FEVAR includes renal artery revascularization, improved understanding of renal-stratified outcomes is important for patient selection for this strategy. Therefore, this study aimed to compare short and long-term outcomes for patients with none-to-mild versus moderate-to-severe CKD and to determine the independent effect of moderate-to-severe CKD on long-term overall survival in patients undergoing FEVAR.
2. Methods
We performed a single-institution, retrospective review of a prospectively maintained database of all consecutive FEVAR performed from September 2012 to January 2022. Patients treated with an FDA-approved Cook ZFEN or clinical-trial Cook Zenith p-Branch device, which is a fenestrated endograft configured with three fenestrations and one scallop (Cook Medical, Inc) were included. Those with any adjunctive modifications (e.g., chimney, snorkel, periscope, or laser fenestration) were excluded from the analysis. This study was approved by the Stanford University Institutional Review Board for retrospective review of deidentified patient data.
The cohort was stratified by severity of CKD stage as none-to-mild CKD (defined as no CKD or stage 1–2) and moderate-to-severe CKD (defined as stage 3–5). CKD stage was determined using preoperative serum creatinine (sCr) and estimated glomerular filtration race (eGFR) calculated using the 2021 CKD-EPI-Creatinine Equation recommended by the National Kidney Foundation.12,13 CKD stages were defined based on eGFR as shown below:
Stage 1: Normal or increased (>90 mL/min/1.73m2)
Stage 2: Mild (60–89 mL/min/1.73m2)
Stage 3a: Moderate (45–59 mL/min/1.73m2)
Stage 3b: Moderate (30–44 mL/min/1.73m2)
Stage 4: Severe (15–29 mL/min/1.73m2)
Stage 5: Kidney failure (<15 mL/min/1.73m2 or dialysis)
In this study, CKD staging was determined based on preoperative eGFR alone and did not include preoperative urinalysis, which could suggest evidence of proteinuria and distinguish patients with normal eGFR but mild CKD. For this study, patients without CKD and CKD stage 1 and 2 were analyzed together as a single none-to-mild CKD group.
Baseline demographics, anatomic and device features, operative characteristics, 30-day postoperative events, and 1-year technical outcomes and survival, and long-term outcomes, including rates of dialysis, endoleak, sac regression, reintervention, and overall survival at latest follow-up, were obtained from the database. Comorbidities were assessed using the previously validated Society for Vascular Surgery (SVS) Comorbidity Severity Score.14 Operative technical success was defined as all target vessels patent without proximal endoleak on completion angiogram.
The primary outcome was rate of postoperative acute kidney injury (AKI), defined by RIFLE criteria as 1.5x increase in sCr from baseline to hospital discharge.15 Secondary outcomes included 30-day perioperative complications (myocardial infarction, stroke, bleeding, access complications, bowel ischemia, limb ischemia, new-onset dialysis, reintervention, and mortality) and 1-and 5-year overall survival and rates of dialysis, renal target artery patency, type I or III endoleak, and reintervention. These five-year outcomes were considered “long-term” outcomes in this cohort and consistent with the existing literature on FEVAR.16 Additional secondary endpoints assessed at latest follow-up included aneurysm sac regression, total number of surveillance computed tomography (CT) scans, and CKD stage progression. Progression of CKD stage was defined as worsening in CKD stage from baseline to latest follow-up by ≥1 stage (i.e., CKD stage 2 at baseline to stage 3 at latest follow-up). CKD stage at latest follow-up was determined using last available serum creatinine, from which eGFR was then calculated using age at latest follow up, as described above.
2.1. Statistical analysis
Analyses were stratified by severity of CKD: none-to-mild (CKD 1–2) or moderate-to-severe (CKD 3–5) groups. Univariate analysis (CKD 1–2 vs CKD 3–5) was performed with t-tests for normally distributed continuous variables, Wilcoxon rank sum test for non-normally distributed continuous variables, and chi-squared or Fisher’s exact test for categorical values. All 30-day outcomes were calculated as standard frequencies and proportions. One and 5-year outcomes, including overall survival, were estimated using the Kaplan-Meier time-to-event method with cumulative incidence reported and differences compared using the log-rank test. Multivariate Cox proportional hazards modeling was used to assess for independent effect of CKD stage ≥3 on all-cause mortality at latest follow up, controlling for a priori clinically relevant preoperative baseline characteristics and those associated with an increased risk of CKD and mortality on univariate testing (P< .1). Different from our univariate analyses, in the multivariate model as part of our secondary analysis, CKD stage 3 and CKD stages 4–5 were analyzed separately to determine the independent effect of each stage on mortality. A two-tailed p-value threshold of 0.05 was used to determine statistical significance. Analyses were performed using SAS Studio, version 3.81 (SAS Institute Inc., Cary, NC) and Stata SE, version 14.1 (StataCorp LLC, College Station, TX).
3. Results
3.1. Baseline characteristics
Of 244 consecutive FEVAR performed from September 2012 to January 2022, 184 were treated with FDA-approved Cook ZFEN or clinical-trial Cook Zenith p-Branch fenestrated devices without adjunctive modification and included in our analysis. Of these, 107 patients (58%) had none-to-mild CKD (CKD 1–2) at baseline, while 77 patients (42%) had moderate-to-severe CKD (CKD 3–5). Overall, the mean age was 73.9 ± 7.6 years and 82% of patients were male (Table I). Patients with CKD 3–5 were significantly older (75.2 ± 7.1 vs 73.0 ± 7.9 years, P=.04), with higher preoperative serum creatinine (1.6 ± 0.5 vs 0.9 ± 0.2 mg/dL, P<.01) and lower eGFR (44.0 ± 10.9 vs 81.0 ± 12.8 mL/min/1.73m2, P<.01) as expected, and with higher SVS Comorbidity Score (10.5 ± 4.6 vs 7.7 ± 4.5, P<.01). There were no statistically significant differences in any comorbidities other than CKD between groups. Preoperative medication use was similar, except for a higher rate of calcium channel blocker and diuretic prescriptions in the CKD3–5 group (40.3% vs 26.2%, P=.04 and 42.9% vs 21.5%, P<.01, respectively). There were no significant aneurysm or anatomic differences between groups except for a higher proportion of patients with renal artery ostial calcification on preoperative CT angiography in the CKD3–5 group (37.7% vs 21.5%, P=.02).
Table I.
Baseline demographics, comorbidities, medications, and anatomic characteristics stratified by none-to-mild vs moderate-to-severe CKD.
| Total (N=184) | CKD 1–2 (N=107) | CKD 3–5 (N=77) | P value | |
|---|---|---|---|---|
| Age, years | 73.9 ± 7.6 | 73.0 ± 7.9 | 75.2 ± 7.1 | .04 |
| Male sex | 150 (81.5) | 91 (85.1) | 59 (76.6) | .15 |
| BMI, kg/m2 | 27.6 ± 5.1 | 27.3 ± 5.0 | 28.1 ± 5.3 | .30 |
| Preoperative serum creatinine, mg/dL | 1.2 ± 0.5 | 0.9 ± 0.2 | 1.6 ± 0.5 | <.01 |
| Preoperative eGFR, mL/min/1.73m2 | 65.5 ± 21.9 | 81.0 ± 12.8 | 44.0 ± 10.9 | <.01 |
| Preoperative CKD stage ≥3 | 77 (41.8) | 0 (0) | 77 (100) | N/A |
| Stage 3a | 41 (53.2) | |||
| Stage 3b | 28 (36.4) | |||
| Stage 4 | 7 (9.1) | |||
| Stage 5 | 1 (1.3) | |||
| SVS Comorbidity Severity Score, Total | 8.9 ± 4.7 | 7.7 ± 4.5 | 10.5 ± 4.6 | <.01 |
| Cardiac score | 3.1 ± 3.2 | 3.1 ± 3.4 | 3.1 ± 3.0 | .99 |
| Pulmonary score | 1.0 ± 1.8 | 0.9 ± 1.8 | 1.2 ± 1.9 | .33 |
| Renal score | 1.0 ± 1.2 | 0.2 ± 0.6 | 2.2 ± 0.8 | <.01 |
| Hypertension score | 1.7 ± 1.1 | 1.6 ± 1.1 | 1.9 ± 1.0 | .07 |
| Age score | 2.0 ± 0.8 | 1.9 ± 0.8 | 2.2 ± 0.8 | .02 |
| Comorbidities | ||||
| Coronary artery disease | 102 (55.4) | 57 (53.3) | 45 (58.4) | .49 |
| Atrial Fibrillation | 28 (15.2) | 17 (15.9) | 11 (14.3) | .77 |
| Prior MI | 50 (27.2) | 30 (28.0) | 20 (26.0) | .76 |
| Congestive heart failure | 37 (20.1) | 22 (20.6) | 15 (19.5) | .86 |
| Hypertension | 166 (90.2) | 95 (88.8) | 71 (92.2) | .62 |
| Hyperlipidemia | 148 (80.4) | 84 (78.5) | 64 (83.1) | .44 |
| Current or prior smoking | 130 (70.7) | 77 (72.0) | 53 (68.8) | .65 |
| COPD | 53 (28.8) | 29 (27.1) | 24 (31.2) | .55 |
| Diabetes mellitus | 30 (16.3) | 14 (13.1) | 16 (20.8) | .16 |
| Prior stroke | 25 (13.6) | 14 (13.1) | 11 (14.3) | .81 |
| Peripheral arterial disease | 46 (25.0) | 25 (23.4) | 21 (27.3) | .55 |
| Medications | ||||
| Aspirin | 135 (73.4) | 80 (74.8) | 55 (71.4) | .61 |
| Clopidogrel | 32 (17.4) | 17 (15.9) | 15 (19.5) | .53 |
| Anticoagulation | 15 (8.2) | 10 (9.4) | 5 (6.5) | .59 |
| Statin | 141 (76.6) | 83 (77.6) | 58 (75.3) | .72 |
| Nitrates | 16 (8.7) | 8 (7.5) | 8 (10.4) | .60 |
| Calcium channel blocker | 59 (32.1) | 28 (26.2) | 31 (40.3) | .04 |
| ACE inhibitor/ARB | 95 (51.6) | 61 (57.0) | 34 (44.2) | .09 |
| Beta-blockers | 106 (57.6) | 59 (55.1) | 47 (61.0) | .42 |
| Diuretics | 56 (30.4) | 23 (21.5) | 33 (42.9) | .002 |
| Insulin | 2 (4.2) | 1 (3.3) | 1 (5.6) | 1.0 |
| Prior AAA repair | 25 (13.6) | 14 (13.1) | 11 (14.3) | .81 |
| Aneurysm size max diameter, mm | 61.5 ± 12.2 | 61.4 ± 12.2 | 61.7 ± 12.2 | .84 |
| Aneurysm extent | .87 | |||
| Infrarenal | 9 (4.9) | 6 (5.6) | 3 (3.9) | |
| Juxtarenal | 149 (81.0) | 88 (82.2) | 61 (79.2) | |
| Pararenal | 13 (7.1) | 6 (5.6) | 7 (9.1) | |
| Paravisceral | 2 (1.1) | 1 (0.9) | 1 (1.3) | |
| Thoracoabdominal | 10 (5.4) | 5 (4.7) | 5 (6.5) | |
| Pseudoaneurysm | 1 (0.5) | 1 (0.9) | 0 (0) | |
| Renal artery ostial calcification | 52 (28.3) | 23 (21.5) | 29 (37.7) | .02 |
| Native RRA diameter, mm | 5.9 ± 0.8 | 6.0 ± 0.6 | 5.8 ± 1.0 | .22 |
| Native LRA diameter, mm | 6.0 ± 0.8 | 6.0 ± 0.6 | 5.9 ± 1.0 | .12 |
| Infrarenal neck diameter, mm | 25.9 ± 6.0 | 26.1 ± 6.1 | 25.8 ± 6.0 | .85 |
| Infrarenal neck length, mm | 4.5 ± 3.8 | 4.9 ± 3.9 | 4.0 ± 3.5 | .13 |
| Infrarenal neck angle, degrees | 26.7 ± 17.1 | 25.7 ± 16.5 | 27.9 ± 17.8 | .39 |
| Suprarenal neck angle, degrees | 19.7 ± 16.5 | 18.9 ± 15.8 | 20.8 ± 17.4 | .43 |
| Proximal seal zone diameter,a mm | 25.4 ± 3.0 | 25.3 ± 2.7 | 25.5 ± 3.2 | .71 |
| Proximal seal zone length, mm | 33.2 ± 7.8 | 33.2 ± 6.8 | 33.2 ± 9.0 | .97 |
Values reported as number (%) or mean ± standard deviation.
BMI, body mass index; eGFR, estimated glomerular function; CKD, chronic kidney disease; SVS, Society for Vascular Surgery; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; AAA, abdominal aortic aneurysm; RRA, right renal artery; LRA, left renal artery.
Proximal seal zone diameter is defined as the native aortic diameter at the level of the superior mesenteric artery.
3.2. Operative characteristics
Operative details are presented in Table II. Patients with CKD 3–5 had significantly fewer target arteries incorporated into their repair (2.8 ± 0.5 vs 2.9 ± 0.5, P=.03), smaller renal artery stent diameters (right 6.1 ± 0.6 vs 6.3 ± 0.5, P=.03; left 6.2 ± 0.6 vs 6.4 ± 0.6, P=.03), higher blood loss (342 ± 330 mL vs 228 ± 239 mL, P=.01), longer operating room time (186 ± 83 min vs 162 ± 71 min, P=.04), and longer median [IQR] hospital length of stay (3 [2] days vs 2 [2] days, P<.01). Overall technical success was 97.3% and did not differ significantly between groups (CKD 1–2 99.1% vs CKD 3–5 94.8%, P=.16). There were no significant differences in device size, percutaneous or upper extremity access, fluoroscopy time, radiation dose, or contrast volume between CKD 1–2 and CKD 3–5 groups.
Table II.
Operative details and device characteristics
| Total (N=184) | CKD 1–2 (N=107) | CKD 3–5 (N=77) | P value | |
|---|---|---|---|---|
| Number of target vessels | 2.8 ± 0.5 | 2.9 ± 0.5 | 2.8 ± 0.5 | .03 |
| Device type | .04 | |||
| Cook ZFEN | 177 (96.2) | 100 (93.5) | 77 (100) | |
| Cook P-Branch | 7 (3.8) | 7 (6.54) | 0 (0) | |
| Device diameter, mm | 30.4 ± 3.4 | 30.2 ± 3.4 | 30.6 ± 3.4 | .44 |
| Percutaneous access only | 86 (46.7) | 56 (52.3) | 30 (39.0) | .07 |
| Brachial or axillary access | 8 (4.4) | 7 (6.5) | 1 (1.3) | .14 |
| Right renal artery stent diameter, mm | 6.3 ± 0.5 | 6.3 ± 0.5 | 6.1 ± 0.6 | .03 |
| Left renal artery stent diameter, mm | 6.4 ± 0.6 | 6.4 ± 0.6 | 6.2 ± 0.6 | .03 |
| Estimated blood loss, mL | 275 ± 285 | 228 ± 239 | 342 ± 330 | .01 |
| Median fluoroscopy time (IQR), min | 52.1 (34.0) | 50.9 (33.1) | 56.7 (32.9) | .19 |
| Median radiation dose (IQR), mGy | 3558 (3396) | 3312 (3318) | 3742 (3616) | .17 |
| Contrast volume, mL | 94 ± 40 | 95 ± 42 | 93 ± 38 | .64 |
| Operating room time, min | 172 ± 77 | 162 ± 71 | 186 ± 83 | .04 |
| Technical successa | 179 (97.3) | 106 (99.1) | 73 (94.8) | .16 |
| Median length of stay (IQR), days | 2 (2) | 2 (2) | 3 (2) | <.01 |
Values reported as number (%) or mean ± standard deviation, unless otherwise indicated.
CKD, chronic kidney disease; IQR, interquartile range.
Technical success defined as all target vessels patent without proximal endoleak on completion angiogram.
3.3. Primary outcome
The rate of postoperative AKI did not significantly differ between CKD 1–2 and CKD 3–5 groups (6.5% vs 14.3%, P=.13), though AKI occurred at more than double the rate in CKD 3–5 patients (Table III).
Table III.
Early perioperative outcomes (in hospital or <30 days)
| Total (N=184) | CKD 1–2 (N=107) | CKD 3–5 (N=77) | P value | |
|---|---|---|---|---|
| Acute kidney injury | 18 (9.8) | 7 (6.5) | 11 (14.3) | .13 |
| New dialysis | 2 (1.1) | 0 (0) | 2 (2.6) | .17 |
| Any major adverse event | 11 (6.0) | 6 (5.6) | 5 (6.5) | 1.0 |
| Myocardial infarction | 4 (2.2) | 3 (2.8) | 1 (1.3) | |
| Stroke | 0 (0) | |||
| Bleeding | 1 (0.5) | 1 (0.9) | 0 (0) | |
| Access complications | 1 (0.5) | 0 (0) | 1 (1.3) | |
| Paralysis | 0 (0) | |||
| Bowel ischemia | 3 (1.6) | 0 (0) | 3 (3.9) | |
| Limb ischemia | 2 (1.1) | 2 (1.9) | 0 (0) | |
| Reintervention | 11 (6.0) | 7 (6.5) | 4 (5.1) | .76 |
| Death | 4 (2.2) | 1 (0.9) | 3 (3.9) | .31 |
Values reported as number (%).
CKD, chronic kidney disease.
3.4. Secondary outcomes
Additional 30-day perioperative events were similar between groups (Table III). No significant differences were observed in rates of new onset dialysis, any major adverse event, reintervention, or mortality at 30 days. The overall 30-day mortality rate was 2.2% with 4 deaths occurring in 1 patient with baseline CKD stage 2, 2 patients with CKD stage 3, and 1 patient with CKD stage 4. On Kaplan-Meier analysis, overall survival at 1 and 5 years was significantly lower for the CKD 3–5 group compared to CKD 1–2 (82.3% vs 95.1%, P<.01; Fig 1A and 55.4% vs 70.8%, P=.02; Fig 1B). Freedom from new-onset chronic dialysis was also significantly lower for CKD 3–5 at 1 year (94.4% vs 100%, P=.02; Fig 2A) and 5 years (84.7% vs 100%, P<.01; Fig 2B). There were no significant differences in long-term technical outcomes, including renal artery patency, reinterventions, or type I or III endoleak at 1 and 5 years (Fig 3A–C).
Fig 1.
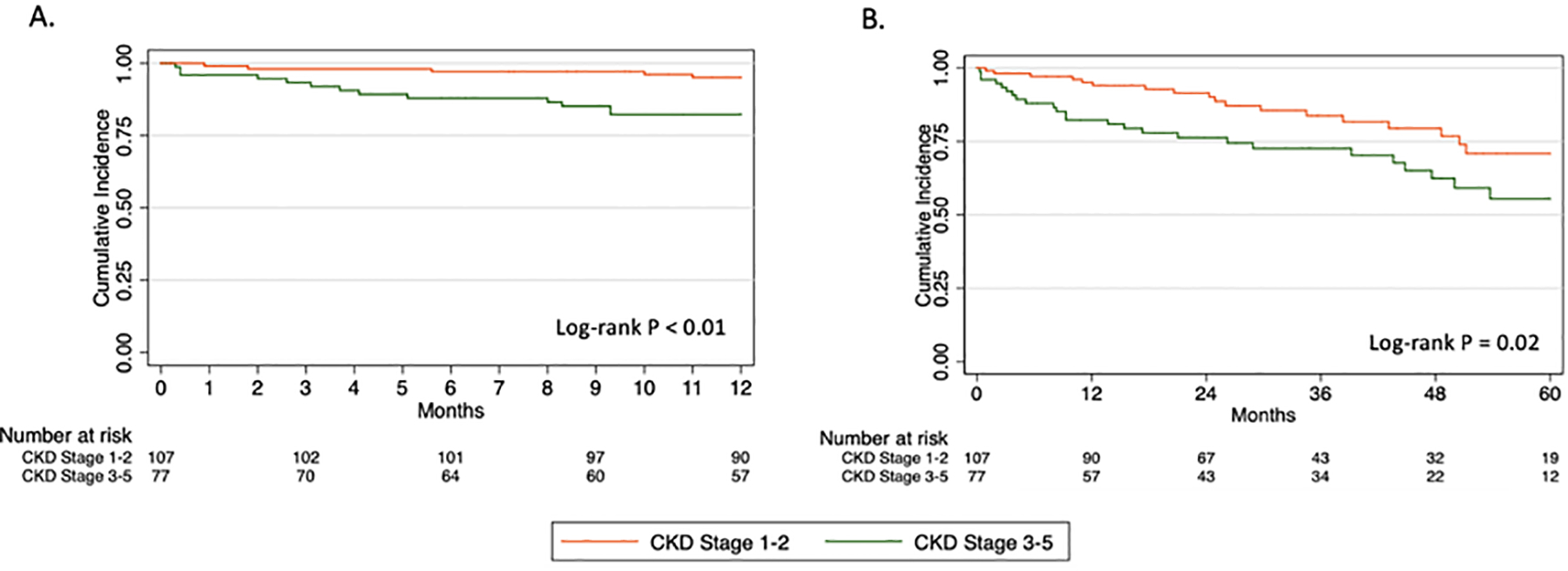
Kaplan-Meier estimates of overall survival at (A) 1 year and (B) 5 years.
Fig 2.
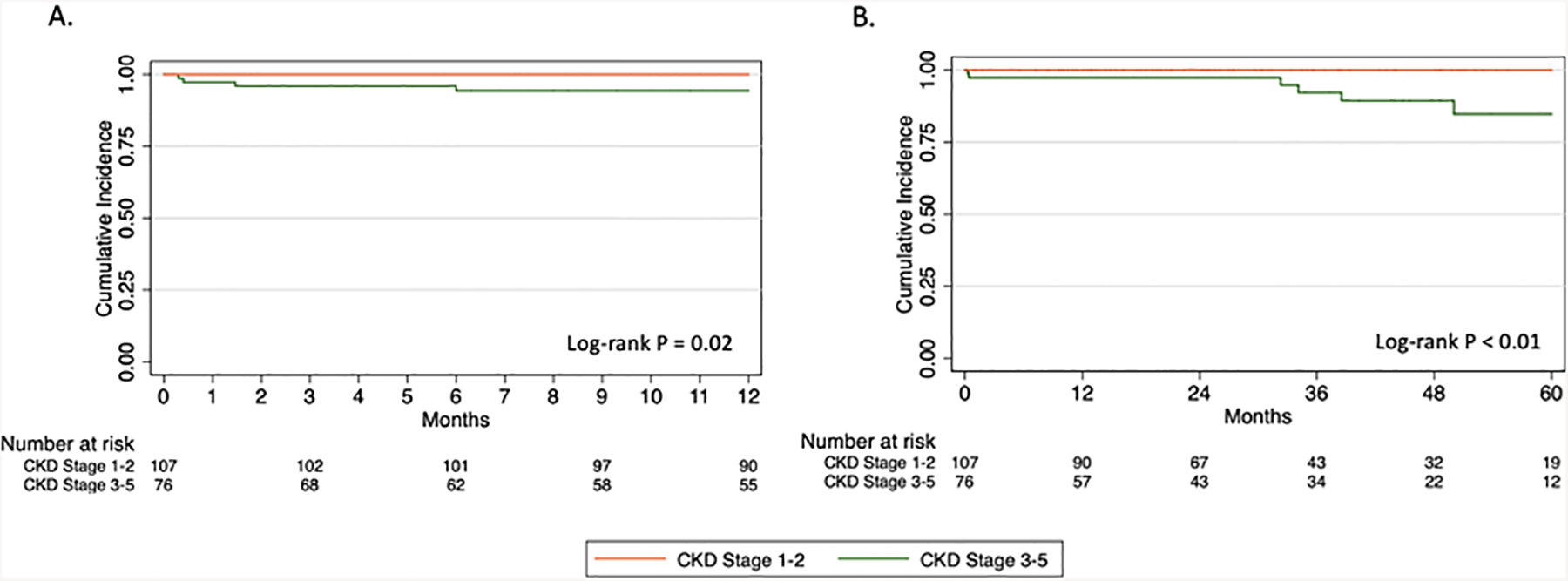
Kaplan-Meier estimates of freedom-from new-onset chronic dialysis at (A) 1 year and (B) 5 years.
Fig 3.
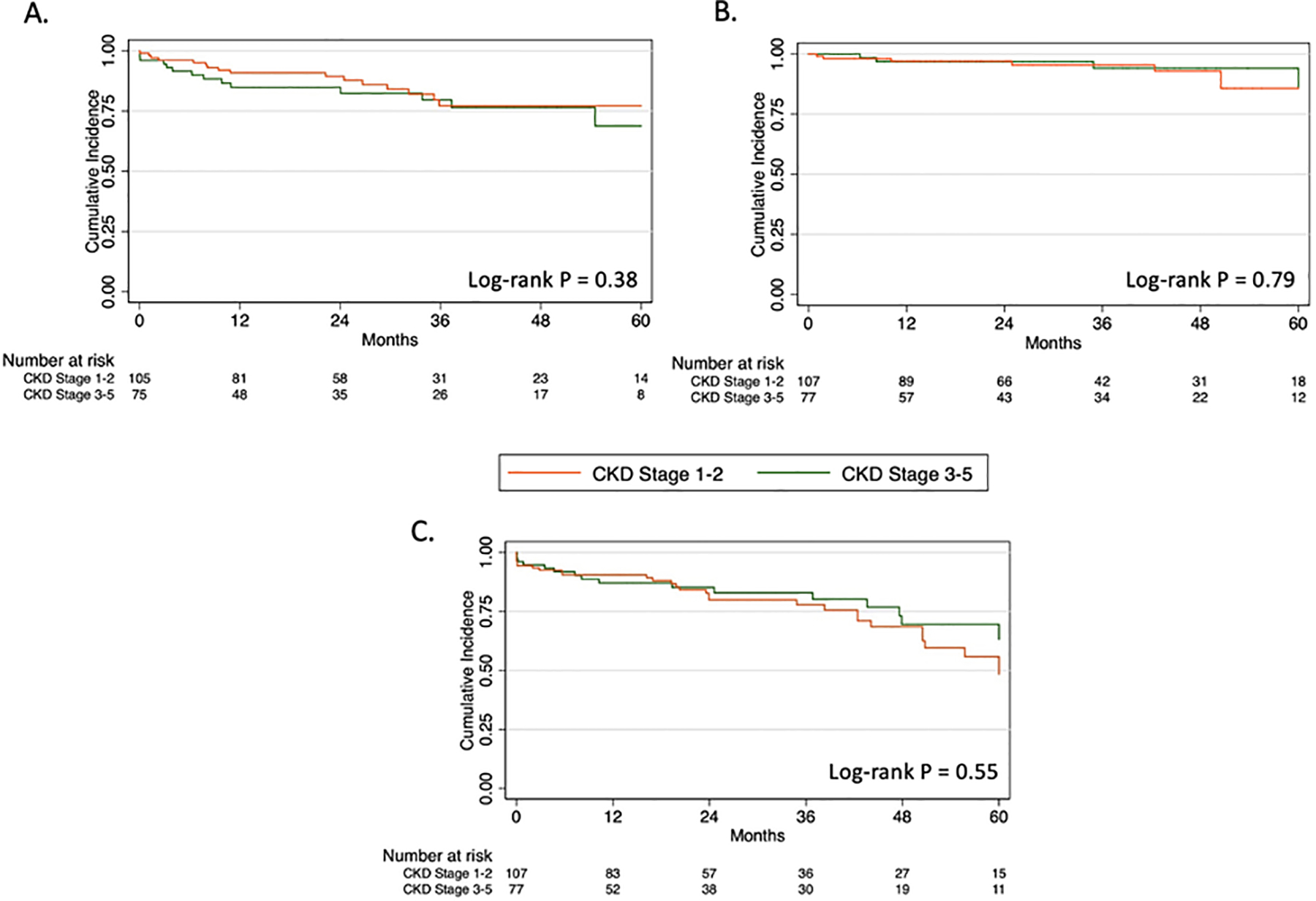
Kaplan-Meier estimates of (A) renal target artery patency, (B) freedom from type I or III endoleak, and (C) freedom from reintervention at 5 years.
For patients with available postoperative surveillance imaging (N=167), mean aneurysm sac regression at latest follow-up was 10.8 ± 10.3 mm for the overall cohort and did not significantly differ between groups (CKD 1–2 10.5 ± 10.9 mm vs CKD 3–5 11.3 ± 9.2 mm, P=.58). The mean number of CT scans during follow-up was not significantly different between CKD 1–2 vs CKD 3–5 groups (4 ± 2 scans vs 3 ± 2 scans, P=.11). For patients with at least one serum creatinine value available at ≥30 days (N=153), CKD stage progression occurred in 47 patients (30.7%) at latest follow-up but did not differ between stratified groups (CKD 1–2 36.0% vs CKD 3–5 23.4%; P=.17; Table IV). Of note, 24 patients (15.7%) were observed to have improved their CKD stage by ≥1 stage at latest follow-up. The mean follow-up time for the overall cohort was 34.3 ± 24.5 months (range 0 to 101 months or 8.4 years).
Table IV.
Change in CKD stage from baseline to latest follow up after FEVAR
| Total (N=153) | CKD 1–2 (N=89) | CKD 3–5 (N=64) | P value | |
|---|---|---|---|---|
| .17 | ||||
| No change | 82 (53.6) | 46 (51.7) | 36 (56.3) | |
| Worsened stage | 47 (30.7) | 32 (36.0) | 15 (23.4) | |
| Improved stage | 24 (15.7) | 11 (12.4) | 13 (20.3) |
Values reported as number (%).
CKD, chronic kidney disease.
3.5. Multivariate analysis of mortality
As part of our secondary outcome analysis, multivariable Cox proportional hazards modeling was used to determine the independent effect of CKD stage 3, CKD stage 4–5, and other baseline preoperative characteristics on all-cause mortality. Increased age (HR 1.05, 95% CI 1.01–1.09, P=.02) and CKD stage 4–5 (HR 6.39, 95% CI 2.26–18.05, P<.01) were independently associated with mortality (Table V). Of note, CKD stage 3, female sex, and renal artery ostial calcification on preoperative imaging did not appear significantly predictive of mortality.
Table V.
Multivariate Cox proportion hazards model to determine independent effect of CKD stage 3–5 and other baseline preoperative characteristics on all-cause mortality
| Variable | Hazard Ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Age, per year | 1.05 | 1.01–1.09 | .02 |
| Female sex | 0.99 | 0.46–2.10 | .97 |
| Renal artery ostial calcification on CTA | 1.42 | 0.73–2.76 | .31 |
| CKD stage 3 | 1.46 | 0.79–2.68 | .23 |
| CKD stage 4–5 | 6.39 | 2.26–18.05 | <.01 |
CTA, computed tomography angiography; CKD, chronic kidney disease.
3.6. Causes of mortality
There were 49 deaths during the follow-up period (26.6% of the total cohort) with 4 of these deaths occurring within 30 days of the index FEVAR procedure (2.2% of total cohort). Of the 49 total deaths, 6 were aortic aneurysm related. These included aortic graft infection (n=1), bowel ischemia (n=2), hemorrhagic shock (n=1), cardiogenic shock after reintervention for endoleak (n=1), and renal stent occlusion with attempted embolectomy at outside hospital complicated by perforation leading to hemorrhagic shock and multisystem organ failure (n=1).
The other 43 causes of death included unknown (n=23), cancer (n=4), MI or heart failure (n=8), proximal acute aortic dissection (n=1), endocarditis with embolic stroke (n=1), stroke (n=1), pulmonary embolism (n=1), pneumonia (n=1), sepsis (n=1), acute on chronic mesenteric ischemia (n=1), and motor vehicle accident (n=1). While there were no deaths specifically attributed to chronic kidney disease or dialysis needs in this cohort, we can only postulate that chronic renal dysfunction may have contributed to some of the disease states and causes of mortality as listed above.
4. Discussion
FEVAR has emerged as a first-line treatment strategy for complex aortic aneurysm pathology across many centers in the United States and even more so in Europe with high technical success, low perioperative mortality, and promising mid and long-term technical outcomes and survival with ongoing surveillance.4,17,18 However, rates of AKI after FEVAR vary from 5% to 30% based on the extent of repair.19 Identifying rates of postoperative AKI stratified by preoperative CKD stage may be useful for risk-stratification of patients prior to undergoing FEVAR.
In our series of 184 consecutive patients undergoing commercially available FEVAR, the rate of postoperative AKI was 10% and did not differ significantly between none-to-mild and moderate-to-severe CKD groups (6.5% vs 14.3%). Additional 30-day secondary outcomes and long-term technical outcomes were similar between CKD stage groups. However, as hypothesized, baseline CKD stage 3–5 was associated with lower overall survival and freedom-from dialysis across all time points out to 5 years. Moreover, on multivariate analysis, CKD stage 4–5 increased the hazard of death over six-fold, while CKD stage 3 was not independently associated with mortality after FEVAR.
The overall AKI rate of 10% after FEVAR in the present study is consistent with, if not slightly lower than, prior reports.20–24 Our group previously reported an AKI rate of 23% in 110 patients treated with FEVAR for juxtarenal or paravisceral aneurysms from 2012–2015 with mean follow up 11 months, in collaboration with a second institution.21 While rates of AKI were not reported based on baseline CKD stage, moderate CKD defined as stage ≥3 was present in 51% of patients preoperatively with mean eGFR 60.0 mL/min/1.73 m2. It is interesting to put these earlier findings in the context of this present study for a few reasons. First, this seems to indicate that there may be a learning curve effect with respect to the rate of postoperative AKI as patients treated by us from 2012–2015 at the start of our experience performing FEVAR had two-times as a high a rate of developing AKI as compared to all patients treated from 2012–2022 (23% vs 10%, respectively). However, in Tran et al.’s earlier study over 50% of patients had baseline CKD stage ≥3, while in our current study this rate was lower at 42% (77/184 patients) and our preoperative eGFR was correspondingly slightly higher at 66 mL/min/1.73 m2. As the present study has a lower overall rate of patients with moderate-to-severe CKD, together, this suggests a potential learning curve effect with respect to patient selection for FEVAR as well. While a specific learning curve analysis is out of the scope of this present study, this would be an interesting area of future work.
The association of baseline CKD stage with outcomes after FEVAR has been explored by only one prior European study to our knowledge, however with notable differences. D’Oria et al. reported their 8-year experience of fenestrated-branched endovascular aortic repair (F-BEVAR) for pararenal and thoraco-abdominal aortic aneurysms, including 202 consecutive elective and non-elective patients.25 They found an overall postoperative AKI rate of 18%, which differed for moderate-to-severe CKD patients as compared to those without preoperative CKD (37% vs. 12%, P<.01). Importantly, while their rate of AKI was higher than that of our study (18% vs 10%), D’Oria et al. included more complex aneurysms (pararenal 37% and thoracoabdominal 63%) and included urgent/emergent cases, both of which may be associated with worse renal outcomes. In our current study, most aneurysms were juxtarenal (81%) with only 7% pararenal and 5% thoracoabdominal. We further included only elective repairs, and thus our lower rate of AKI may be explained by the potentially lower-risk cohort at baseline. The authors utilized similar definitions for AKI (i.e., RIFLE) and CKD staging as our present study, however, with a slightly larger patient cohort, they found a significantly higher rate of postoperative AKI for patients with CKD stage ≥3 (19/51 patients, 37%) as compared to those without baseline CKD (18/151 patients, 12%) with over three-fold difference. We similarly found a two-fold higher rate of AKI in the CKD 3–5 group as comparted to CKD 1–2 group (11/77, 14% vs 7/107, 6.5%). However, this finding did not reach statistical significance, and thus we may have been underpowered to detect a significant difference between groups.
D’Oria et al. also reported a lower overall 3-year survival for patients with moderate-to-serve CKD as compared to those without baseline CKD (57% vs. 82%, P= .010), which is comparable to our findings of significantly lower 1- and 5-year survival for CKD 3–5 patients (82% vs 95% and 55% vs 71%, respectively). On multivariate analysis, CKD stage ≥3 was independently associated with all-cause mortality (HR 3.2, 95% CI 1.2–8.6, P=.020), however their model did not distinguish CKD stage 3 from CKD stage 4–5 as we do in the present study. The findings of our secondary analysis demonstrate that CKD stage 4–5 was independently associated with over 6-fold higher hazard of all-cause mortality, and CKD stage 3 alone did not appear to be an independent predictor of mortality.
An unexpected finding of our study relates to CKD stage progression following FEVAR. We found that 31% of patients experienced CKD stage progression, by one or more stages, at latest follow-up (mean follow up 34 months), but the rate of progression was similar between none-to-mild and moderate-to-severe CKD stage groups (36% vs 23% respectively, P =.17). Of note, 16% of the cohort had an improvement in their CKD stage from baseline to latest follow-up after FEVAR, and 54% had no change in CKD stage. Improvement in renal function may be secondary to the effects of the index revascularization and/or postoperative surveillance practices that can identify new lesions for early treatment. Tran et al. similarly found that during mean follow up of 11 months, 24% of patients had CKD stage progression by one stage, and 74% of patients had no change or improved renal function by CKD stage.21 D’Oria et al. did not report on rate of CKD stage progression specifically, however their study demonstrated that 3-year freedom from renal function decline, defined as eGFR decline >20% or progression in CKD stage, was significantly lower in patients with moderate-to-severe CKD as compared to no baseline CKD (43% vs 80%, P=.020).25 While this study’s endpoint is different from ours, and a direct comparison cannot be made, understanding the rate of long-term renal function decline in our cohort is an ongoing area of future work.
Our study has important implications for the ongoing study of FEVAR for the treatment of complex aortic aneurysms. Preoperative risk assessment practices based in shared surgeon-patient decision making are critical but currently lacking for fenestrated and branched endovascular repair.26 Prior studies have demonstrated that baseline chronic renal insufficiency (defined as eGFR <60 or CKD stage ≥ 3) significantly predicted late all-cause mortality among octogenarians undergoing complex EVAR.27 This study further establishes that preoperative CKD stage 4 or 5, specifically, is an independent predictor of long-term mortality. Future work is needed to differentiate outcomes between CKD stage 3A and 3B patients and those with and without baseline proteinuria, as well as to better characterize predictors of patients with worsening vs improving CKD stage following FEVAR. These findings are relevant to health care policy given the high cost of complex endovascular repair28 and the need for further secondary interventions following the index procedure in approximately 25% of patients.29,30 Patients with CKD have an increased risk of cardiovascular and all-cause mortality in addition to progression to end-stage renal disease as compared to the general population.31 The significantly worse outcomes in patients with CKD stage 4 and 5 should caution against elective FEVAR, similar to SVS guidelines that advise against carotid revascularization for asymptomatic patients with less than three years life expectancy.32
4.1. Study limitations
This was a single-center, prospective dataset but some data had to be collected retrospectively, which may have led to an unequal comparison in some instances. Second, the CKD 1–2 group was determined by eGFR only and included patients with both no CKD and mild CKD at baseline. These patients were not separated out further as this would require inclusion of preoperative urinalysis for proteinuria, which was not available in this current dataset. The CKD 1–2 group is a heterogeneous group of patients that cannot be further accurately separated into true no CKD and true mild CKD. Third, our definition of AKI used the maximum serum creatinine from the index hospitalization and did not include AKI occurring after discharge but within 30 days. This allowed us to capture postoperative creatinine for the entire cohort, however, our finding may thus underestimate the rate of AKI within 30 days, which has been used by some prior studies.19 Finally, the low event rate for our primary outcome of AKI is clinically encouraging but may represent a potential type II error. We found no statistically significant difference in rate of AKI between groups, though the CKD 3–5 group had more than twice the rate of AKI as compared to the CKD 1–2 group.
5. Conclusion
Over 40% of patients undergoing FEVAR had baseline CKD stage ≥3 with associated worse 1 and 5-year survival but acceptable 30-day and long-term technical outcomes—likely reflecting the underlying disease course of CKD. While no significant difference was observed in the primary outcome of postoperative AKI, patients with baseline CKD stage 3–5 had more than twice the rate of AKI. Nevertheless, careful patient selection remains critical, and for patients with CKD stage 4–5 (i.e., eGFR <30 mL/min/1.73m2), a higher threshold for elective FEVAR should be considered. Future studies aimed at validating these findings in larger cohorts will be essential to create clinical practice guidelines for aneurysm repair in patients with moderate-to-severe CKD.
Highlights:
Postoperative acute kidney injury (AKI) occurred in 10% of patients undergoing fenestrated endovascular aneurysm repair (FEVAR) and did not differ significantly for those with moderate-to-severe stage chronic kidney disease (CKD 3–5) compared to those with none-to-mild stage CKD at baseline (CKD 1–2).
CKD 3–5 patients had significantly lower 1 and 5-year survival but acceptable 30-day and long-term technical outcomes—likely reflecting the underlying disease course of CKD.
CKD stage 4–5 was independently associated with an over six-fold increased hazard of all-cause mortality; a higher threshold for elective FEVAR should be considered in these patients.
Acknowledgements:
S.S.D. is partially funded by the Enright Family Postdoctoral Research Scholar Initiative in 2023. This project was further partially supported by the Helen and Paul Baszucki Vascular Surgery Research Fund provided to Stanford Vascular Surgery in 2022–2023.
Funding:
S.S.D. is supported by the Stanford Health Services Research Training Program, grant number T32HS026128 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (HHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ or HHS.
Footnotes
Presented at the Vascular & Endovascular Surgery Society (VESS) 47th Annual Winter Meeting, Whistler, British Columbia, Canada, Feb 23 – 26, 2023.
References
- 1.Browne T, Hartley D, Purchas S, Rosenberg M, Van Schie G, Lawrence-Brown M. A Fenestrated Covered Suprarenal Aortic Stent. Eur J Vasc Endovasc Surg. 1999;18(5):445–449. doi: 10.1053/ejvs.1999.0924 [DOI] [PubMed] [Google Scholar]
- 2.Amiot S, Haulon S, Becquemin JP, et al. Fenestrated Endovascular Grafting: The French Multicentre Experience. Eur J Vasc Endovasc Surg. 2010;39(5):537–544. doi: 10.1016/j.ejvs.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 3.Verhoeven ELG, Vourliotakis G, Bos WTGJ, et al. Fenestrated Stent Grafting for Short-necked and Juxtarenal Abdominal Aortic Aneurysm: An 8-Year Single-centre Experience. Eur J Vasc Endovasc Surg. 2010;39(5):529–536. doi: 10.1016/j.ejvs.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Eagleton MJ, Follansbee M, Wolski K, Mastracci T, Kuramochi Y. Fenestrated and branched endovascular aneurysm repair outcomes for type II and III thoracoabdominal aortic aneurysms. J Vasc Surg. 2016;63(4):930–942. doi: 10.1016/j.jvs.2015.10.095 [DOI] [PubMed] [Google Scholar]
- 5.Schanzer A, Simons JP, Flahive J, et al. Outcomes of fenestrated and branched endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg. 2017;66(3):687–694. doi: 10.1016/j.jvs.2016.12.111 [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell TFX, Boitano LT, Deery SE, et al. Open Versus Fenestrated Endovascular Repair of Complex Abdominal Aortic Aneurysms. Ann Surg. 2020;271(5):969–977. doi: 10.1097/SLA.0000000000003094 [DOI] [PubMed] [Google Scholar]
- 7.Oderich GS, Correa MP, Mendes BC. Technical aspects of repair of juxtarenal abdominal aortic aneurysms using the Zenith fenestrated endovascular stent graft. J Vasc Surg. 2014;59(5):1456–1461. doi: 10.1016/j.jvs.2013.10.060 [DOI] [PubMed] [Google Scholar]
- 8.Simons JP, Shue B, Flahive JM, et al. Trends in use of the only Food and Drug Administration-approved commercially available fenestrated endovascular aneurysm repair device in the United States. J Vasc Surg. 2017;65(5):1260–1269. doi: 10.1016/j.jvs.2016.10.101 [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell TFX, Patel VI, Deery SE, et al. The state of complex endovascular abdominal aortic aneurysm repairs in the Vascular Quality Initiative. J Vasc Surg. 2019;70(2):369–380. doi: 10.1016/j.jvs.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2021. Published 2021. Accessed February 25, 2023. https://www.cdc.gov/kidneydisease/publications-resources/CKD-national-facts.html
- 11.Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2–77.e2. doi: 10.1016/j.jvs.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 12.Inker LA, Eneanya ND, Coresh J, et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CKD-EPI Creatinine Equation (2021). National Kidney Foundation. Published October 26, 2015. Accessed June 4, 2023. https://www.kidney.org/content/ckd-epi-creatinine-equation-2021
- 14.Chaikof EL, Fillinger MF, Matsumura JS, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35(5):1061–1066. doi: 10.1067/mva.2002.123991 [DOI] [PubMed] [Google Scholar]
- 15.Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007;33(3):409–413. doi: 10.1007/s00134-006-0478-x [DOI] [PubMed] [Google Scholar]
- 16.Sveinsson M, Sonesson B, Kristmundsson T, Dias N, Resch T. Long-term outcomes after fenestrated endovascular aortic repair for juxtarenal aortic aneurysms. J Vasc Surg. 2022;75(4):1164–1170. doi: 10.1016/j.jvs.2021.11.050 [DOI] [PubMed] [Google Scholar]
- 17.Verhoeven ELG, Katsargyris A, Oikonomou K, Kouvelos G, Renner H, Ritter W. Fenestrated Endovascular Aortic Aneurysm Repair as a First Line Treatment Option to Treat Short Necked, Juxtarenal, and Suprarenal Aneurysms. Eur J Vasc Endovasc Surg. 2016;51(6):775–781. doi: 10.1016/j.ejvs.2015.12.014 [DOI] [PubMed] [Google Scholar]
- 18.Greenberg RK, Sternbergh WC, Makaroun M, et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2009;50(4):730–737.e1. doi: 10.1016/j.jvs.2009.05.051 [DOI] [PubMed] [Google Scholar]
- 19.Dossabhoy SS, Simons JP, Crawford AS, et al. Impact of acute kidney injury on long-term outcomes after fenestrated and branched endovascular aortic aneurysm repair. J Vasc Surg. 2020;72(1):55–65.e1. doi: 10.1016/j.jvs.2019.09.034 [DOI] [PubMed] [Google Scholar]
- 20.Martin-Gonzalez T, Pinçon C, Maurel B, et al. Renal Outcomes Following Fenestrated and Branched Endografting. Eur J Vasc Endovasc Surg. 2015;50(4):420–430. doi: 10.1016/j.ejvs.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 21.Tran K, Fajardo A, Ullery BW, Goltz C, Lee JT. Renal function changes after fenestrated endovascular aneurysm repair. J Vasc Surg. 2016;64(2):273–280. doi: 10.1016/j.jvs.2016.01.041 [DOI] [PubMed] [Google Scholar]
- 22.Castagno C, Varetto G, Quaglino S, et al. Acute kidney injury after open and endovascular elective repair for infrarenal abdominal aortic aneurysms. J Vasc Surg. 2016;64(4):928–933.e1. doi: 10.1016/j.jvs.2016.02.048 [DOI] [PubMed] [Google Scholar]
- 23.de Souza LR, Oderich GS, Farber MA, et al. Editor’s Choice – Comparison of Renal Outcomes in Patients Treated by Zenith® Fenestrated and Zenith® Abdominal Aortic Aneurysm Stent grafts in US Prospective Pivotal Trials. Eur J Vasc Endovasc Surg. 2017;53(5):648–655. doi: 10.1016/j.ejvs.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 24.Saratzis A, Joshi S, Benson RA, et al. Editor’s Choice - Acute Kidney Injury (AKI) in Aortic Intervention: Findings From the Midlands Aortic Renal Injury (MARI) Cohort Study. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2020;59(6):899–909. doi: 10.1016/j.ejvs.2019.09.508 [DOI] [PubMed] [Google Scholar]
- 25.D’Oria M, Wanhainen A, Lindström D, Tegler G, Mani K. Editor’s Choice – Pre-Operative Moderate to Severe Chronic Kidney Disease is Associated with Worse Short-Term and Mid-Term Outcomes in Patients Undergoing Fenestrated-Branched Endovascular Aortic Repair. Eur J Vasc Endovasc Surg. 2021;62(6):859–868. doi: 10.1016/j.ejvs.2021.08.033 [DOI] [PubMed] [Google Scholar]
- 26.Georgiadis GS, Verhoeven E. Baseline Chronic Kidney Disease Exacerbated after Primary F-BEVAR. An Observed Association or Proven Causation Leading to Further Renal Function Decline and Increased Midterm Mortality? Eur J Vasc Endovasc Surg. 2022;63(2):357–359. doi: 10.1016/j.ejvs.2021.08.004 [DOI] [PubMed] [Google Scholar]
- 27.Tran K, Lee AM, McFarland GE, Sgroi MD, Lee JT. Complex endovascular aneurysm repair is associated with higher perioperative mortality but not late mortality compared with infrarenal endovascular aneurysm repair among octogenarians. J Vasc Surg. 2019;69(2):327–333. doi: 10.1016/j.jvs.2018.04.064 [DOI] [PubMed] [Google Scholar]
- 28.D’Oria M, Wanhainen A, DeMartino RR, Oderich GS, Lepidi S, Mani K. A scoping review of the rationale and evidence for cost-effectiveness analysis of fenestrated-branched endovascular repair for intact complex aortic aneurysms. J Vasc Surg. 2020;72(5):1772–1782. doi: 10.1016/j.jvs.2020.05.037 [DOI] [PubMed] [Google Scholar]
- 29.Dossabhoy SS, Sorondo SM, Tran K, Stern JR, Dalman RL, Lee JT. Reintervention does not affect long-term survival after fenestrated endovascular aneurysm repair. J Vasc Surg. 2022;76(5):1180–1188.e8. doi: 10.1016/j.jvs.2022.04.050 [DOI] [PubMed] [Google Scholar]
- 30.Zettervall SL, Tenorio ER, Schanzer A, et al. Secondary interventions after fenestrated/branched aneurysm repairs are common and nondetrimental to long-term survival. J Vasc Surg. 2022;75(5):1530–1538.e4. doi: 10.1016/j.jvs.2021.11.074 [DOI] [PubMed] [Google Scholar]
- 31.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol JASN. 2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085 [DOI] [PubMed] [Google Scholar]
- 32.Ricotta JJ, AbuRahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54(3):e1–e31. doi: 10.1016/j.jvs.2011.07.031 [DOI] [PubMed] [Google Scholar]


