Abstract
Background:
We evaluated existing data on the prophylactic efficacy of atovaquone-proguanil (AP) in order to determine whether prophylaxis in travellers can be discontinued on the day of return from a malaria-endemic area instead of seven days after return as per currently recommended post-travel schedule.
Methods:
PubMed and Embase databases were searched to identify relevant studies. This PROSPERO-registered systematic review followed PRISMA guidelines. The search strategy included terms or synonyms relevant to AP combined with terms to identify articles relating to prophylactic use of AP and inhibitory and half-life properties of AP. Studies considered for inclusion were: randomized controlled trials, cohort studies, quasi-experimental studies, open-label trials, patient-control studies, cross-sectional studies; as well as case-series and non-clinical studies. Data on study design, characteristics of participants, interventions, and outcomes were extracted. Primary outcomes considered relevant were prophylactic efficacy and prolonged inhibitory activity and half-life properties of AP.
Results:
The initial search identified 1,482 publications, of which 40 were selected based on screening. Following full text review, 32 studies were included and categorized into two groups, namely studies in support of the current post-travel regimen (with a total of 2,866 subjects) and studies in support of an alternative regimen (with a total of 533 subjects).
Conclusion:
There is limited direct and indirect evidence to suggest that an abbreviated post-travel regimen for AP may be effective. Proguanil, however, has a short half-life and is essential for the synergistic effect of the combination. Stopping AP early may result in mono-prophylaxis with atovaquone and possibly select for atovaquone-resistant parasites. Furthermore, the quality of the studies in support of the current post-travel regimen outweighs the quality of the studies in support of an alternative short, post-travel regimen, and the total sample size of the studies to support stopping AP early comprises a small percentage of the total sample size of the studies performed to establish the efficacy of the current AP regimen. Additional research is required — especially from studies evaluating impact on malaria parasitaemia and clinical illness and conducted among travellers in high malaria risk settings — before an abbreviated regimen can be recommended in current practice.
PROSPERO registration number: CRD42017055244.
Keywords: Atovaquone-proguanil, chemoprophylaxis, abbreviated regime, systematic review
1. Introduction
Atovaquone-proguanil (AP; marketed as Malarone® or Malanil® or as generic brands such as Atovaquone Plus®) is a convenient choice for malaria drug prophylaxis in short-term travel [1,2]. ‘Short-term’ is considered to be a travel of three weeks or less [3]. The current approved regimen of AP for malaria chemoprophylaxis is daily administration of one tablet of 250 mg atovaquone/100 mg proguanil hydrochloride beginning one to two days before entry into a malaria-endemic area, continued during exposure, and discontinued seven days after leaving the endemic area [1]. This drug is highly effective in preventing clinical malaria episodes, but non-compliance and non-adherence, in a proportion of patients due to (mainly gastrointestinal) adverse events, are major contributors to a reduced effectiveness.
AP is approved for causal prophylaxis against P. falciparum and does not prevent the formation of dormant liver stages (hypnozoites) by P. vivax and P. ovale, as illustrated by several case-reports [4–7]. Pre-sumptive primaquine treatment may be required to eliminate the hypnozoites in order to prevent relapses due to these malaria species.
Atovaquone belongs to the hydroxynapthoquinone class of compounds and inhibits the parasite mitochondrial electron transport and ATP synthesis, whereas the active proguanil metabolite, cycloguanil, inhibits plasmodial dihydrofolate reductase. Proguanil works synergistically with atovaquone, as it lowers the effective concentration of atovaquone needed to collapse mitochondrial potential [8,9]. Both drugs are active against erythrocytic and pre-erythrocytic stages of Plasmodium species, and thus AP exhibits causal prophylactic activity against liver stages and activity against plasmodial blood stages [10,11]. Because of this causal prophylactic activity, AP can be discontinued seven days after return from a malaria-endemic area instead of one month in the case of antimalarials with only suppressive prophylaxis against blood stages of malaria.
The elimination half-life of proguanil is only 12–15 h in both adults and children, while the half-life of atovaquone is two to three days in adults and one to two days in children [8]. However, Edstein and colleagues determined the half-life of atovaquone to be 5.9 days in a study with three volunteers [12], thus giving rise to concerns of a drug partners mismatch time window, which has only very rarely been reported to impact the clinical course of patients [13].
Nixon et al. reviewed pharmacokinetic and –dynamic properties of this slow-acting drug (atovaquone) [14]. Molecular surveillance data from Gabon and Ethiopia [15] demonstrated that in the absence of drug pressure, the occurrence of potentially drug resistance-conveying polymorphisms remain an exception. Over 500 samples from treatment failures and other imported isolates to Europe were screened for single-point, potentially resistance-conferring polymorphisms in the cytochrome b gene. This showed that the prevalence of those mutations in the European gene pool is well below 1% [16].
AP is well tolerated by the majority of users; however, adverse reactions when used as prophylactic agent against malaria are nausea, vomiting, abdominal pain, headache, and diarrhea [8]. When compared to other antimalarials currently used for malaria prophylaxis, AP has been found to have fewer reported adverse events in randomized trials [17,18].
A recently performed study by Leshem and colleagues did not detect failures among 485 travellers who discontinued prophylaxis one day after return from a malaria-endemic area, mostly in Eastern Africa; however, several methodological shortcomings were acknowledged [19,20]. These included the choice of a region with limited risk of exposure to malaria, insufficient level of evidence that the drugs were taken appropriately, and possible recall bias. Apart from clinical studies, several pharmacological studies also support the proposal to shorten the AP regimen, citing the long half-life properties of atovaquone with schizonticidal effects [11,12]. However, the absence of comprehensive funding opportunities needed to conduct a study of considerable complexity and study subject numbers makes it challenging to provide a comprehensive, definitive recommendation. Very few clinical and pharmacological studies have been performed that have focused on providing evidence for an abridged AP malaria chemoprophylaxis regimen [19].
The objective of this systematic review is to determine the prophylactic efficacy when discontinuing AP in travellers one day after return from a malaria-endemic area instead of after seven days. In order to assess whether the currently available evidence supports shortening post-travel duration of AP, we reviewed and weighed current clinical and pharmacological data with regard to the prophylactic activity and prolonged inhibitory activity or half-life properties of AP. Finally, we suggest a methodologically feasible study approach in order to answer future questions with regard to malaria prophylaxis.
2. Methods
In this systematic review, we evaluate existing data with regard to the prophylactic efficacy of AP, in order to determine whether prophylaxis in travellers can be discontinued on the day of return from a malaria-endemic area instead of seven days later. However, because of the limited research performed on this topic, we also included studies with alternative regimens of AP chemoprophylaxis, whilst in an endemic area, in support of the prolonged antimalarial activity of AP.
2.1. Search strategy and study selection
The electronic PubMed and Embase databases were consulted to identify relevant studies. Because AP was registered in 1998, we included studies published between 1995 and the present. . Relevant studies identified by additional reading/citation were also considered for inclusion. The PROSPERO protocol was registered at http://www.crd.york.ac.uk (CRD42017055244). The PRISMA guidelines for systematic reviews were followed in most aspects [21]. The few deviations from PRISMA guidelines are discussed below.
The search strategy included terms or synonyms relevant to AP combined with terms to identify articles related to prophylactic use of AP, or pharmacokinetic properties of AP. The full search strategy is provided in Appendix 1 and Appendix 2. This search strategy was verified by a clinical librarian. Screening on title/abstract and full text was performed independently by two reviewers. Discrepancies were resolved by discussion. A recent update of the PubMed and Embase search was performed on the 6th of September 2017. No language restrictions were applied, though no studies meeting the inclusion criteria but not written in English were identified.
2.2. Eligibility: inclusion and exclusion criteria
The PICO format was used to determine the inclusion criteria: (P) Participants: travellers to malaria-endemic areas, in which travellers were defined as children and adults (both pregnant and non-pregnant); (I) Intervention: discontinuation of daily administered AP prophylaxis one day upon return from a malaria-endemic area; (C) Comparison: discontinuation of daily administered AP prophylaxis seven days after return from a malaria-endemic area; (O) Outcome: parasitaemia. Studies with focus on alternative regimens of AP, defined as discontinuation one to seven days after return from a malaria-endemic area, or an outcome other than parasitaemia such as adverse events, were also considered for inclusion. The outcomes considered for non-clinical (e.g. pharmacological or experimental) studies were the half-life properties of AP or an outcome related to elimination half-life (i.e. an outcome suggesting the prolonged inhibitory activity of AP).
Criteria for exclusion were: a focus on malaria treatment (except when there was an emphasis on the duration of the prolonged inhibitory activity or half-life properties of AP), a focus on adherence to prophylaxis, a focus on adverse effects, a focus on resistance (patterns), a focus on prescribing patterns, or when no abstract or PDF file was available.
The following study designs were considered for inclusion: randomized controlled trials, prospective cohort studies, retrospective cohort studies, quasi-experimental studies, open-label trials, patient-control studies, cross-sectional studies; case-series, and non-clinical studies. Pharmacological and experimental studies were considered as non-clinical, and only papers with a focus on the prolonged inhibitory activity or half-life properties of AP were considered and included as non-clinical studies.
2.3. Data extraction
The following data were extracted: first author, publication date, study design, total number of participants (together with the inclusion and exclusion criteria), characteristics of participants (age, sex, country), intervention and comparison, (primary) outcomes and results. Primary outcomes considered relevant were prophylactic efficacy (e.g. parasitaemia) or half-life properties in the case of non-clinical (e.g. pharmacological or experimental) studies. Data extraction was reviewed independently by a second reviewer, and any discrepancies were resolved by discussion.
2.4. Quality assessment
The methodological quality of the eligible randomized studies was rated by using the Jadad criteria [22]. The methodological quality of the eligible non-randomized studies was rated by using the Joanna Briggs Institute Critical Appraisal tools [23]. The Joanna Briggs Institute Critical Appraisal tools were chosen for their comprehensive scope, but were considered inappropriate for the critical appraisal of randomized studies. Studies without a matching critical appraisal checklist are discussed in the results and discussion sections.
2.5. Data analysis
The regular (i.e. current) regimen and alternative regimens are defined as discontinuation seven days and discontinuation between one to seven days after return from a malaria-endemic area, respectively. To avoid eliminating relevant studies, we used non-specific inclusion criteria and limited exclusion criteria. The available data were too heterogenous to support a meta-analysis.
2.6. Deviations from PRISMA guidelines
The systematic review deviates from the PRISMA guidelines at several aspects. See Appendix 3 for the PRISMA 2009 checklist. The deviations included the absence of a risk of bias assessment due to the heterogeneity in study selection, and therefore the impossibility of comparing the results.
3. Results
The initial search identified 1,482 studies of which 40 studies were included after thorough analysis based on title and abstract. The PRISMA flow diagram is shown in Fig. 1. The 40 studies were screened on full text, after which 32 studies were found eligible. We also identified three additional studies by additional reading/citation that had initially been excluded based on title and abstract. This resulted in a total number of 32 eligible studies. Few studies evaluated a parasitaemia outcome. The reasons for exclusion of the eight studies were the limited number of patients in four case series, focus on malaria cases alone in one of the case series, using data of already included studies in a comparative study, the absence of an outcome of interest due to the uncertainty of the number of malaria cases in AP subjects in one study, and a focus on evaluation of treatment in another study. The eligible studies for the regular and alternative regimen will be discussed separately.
Fig. 1.
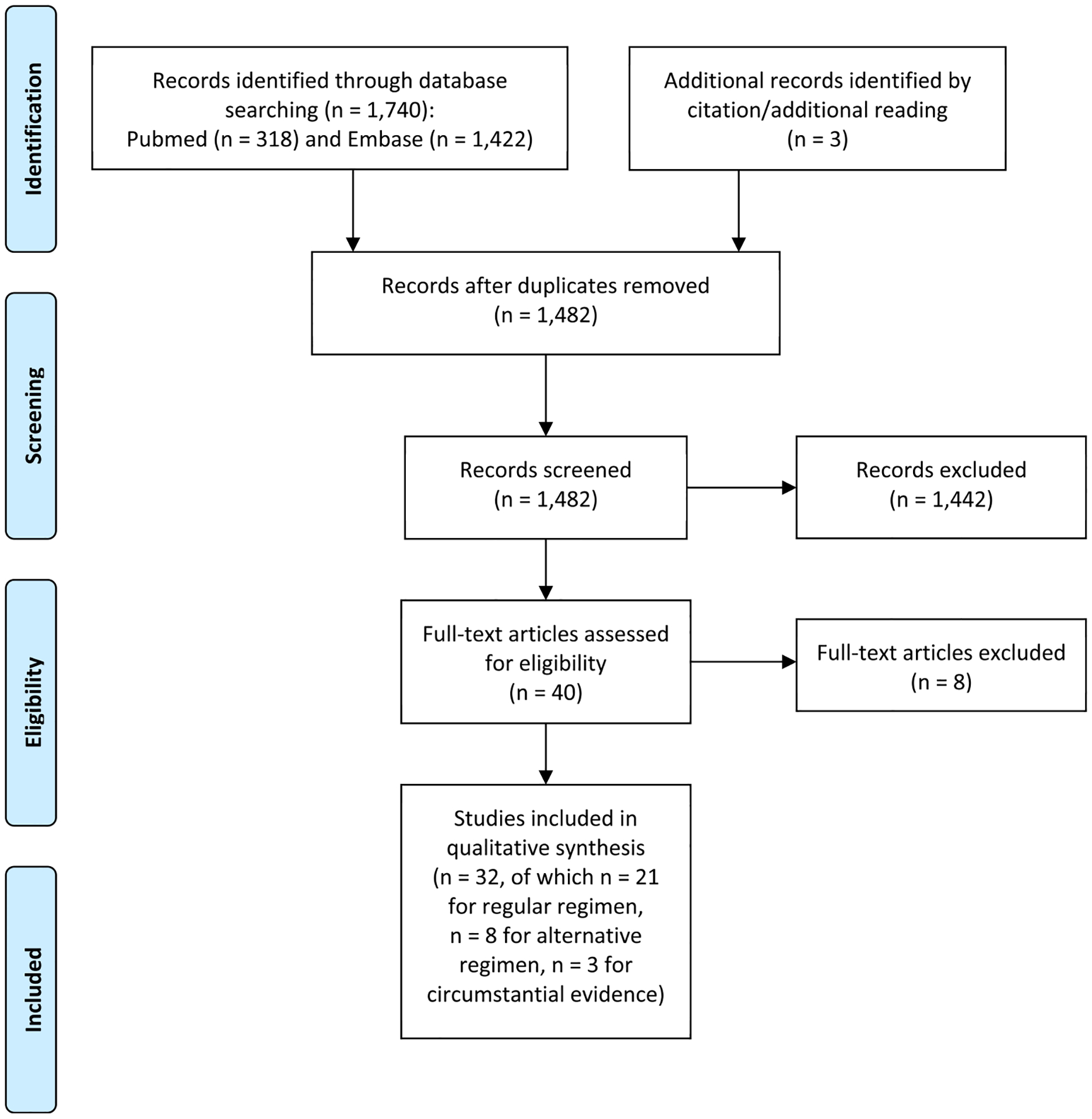
Study selection (PRISMA flow diagram).
3.1. Overview of the studies in support of the current post-travel regimen
3.1.1. Study designs
The total number of eligible studies that provide data about the effectiveness of the current regimen for AP prophylaxis is 21 (see Table 1 for an overview of the eligible studies). Of the 21 studies, there are 12 randomized studies and nine observational studies. Seven studies used a randomized, double-blind, placebo-controlled design [10,24–29]. Three studies used a randomized, double-blind design without a placebo arm [17,18,30]. One randomized study challenged volunteers with P. falciparum-infected mosquitoes [10]. Another randomized study was based on direct venous inoculation of P. falciparum sporozoites with an additional parallel open-label control cohort of AP [31]. One study was a randomized open-label study [32]. The observational study designs included three retrospective studies [2,33,34], two prospective observational studies [35,36], one eligible open-label trial [37], and one open case-control study [38]. Two studies were considered cross-sectional studies [39,40].
Table 1.
Characteristics of the studies providing evidence for the current post-travel regimen.
| Author, year of publication, country | Study design | Number of participants | Studied antimalarial agent | Measure of outcome and results | Critical appraisal score |
|---|---|---|---|---|---|
| Sulyok et al., 2017 [31] Institute of Tropical Medicine in Tubingen, Germany |
Single center, double-blind, randomized, placebo-controlled phase 1 clinical trial with a parallel open label cohort of AP recipients based on direct venous inoculation of P. falciparum- sporozoites on day 0. Follow-up of 60 days. | 6 healthy, malaria-naive adults with a median age of 25.5 (23.5–29.8), of which 4 men. | AP 250/100 mg delivered for 9 days on starting on 1 day before challenge | Blood smear and PCR performed daily from day 6 till day 28. No AP recipient acquired malaria. | 5/5 following Jadad checklist |
| Soto et al., 2006 [24] Colombia |
Phase IV, randomized, double-blind, placebo-controlled study. Drug intake through 10–16 weeks of residence and 7 days after leaving endemic area with 4 weeks follow-up. | 180 non-immune adults. All male, mean age of 19 years (range 17–27). ITT: 120 AP and 60 placebo. PP1: 110 AP and 57 placebo. PP2: 97 AP and 46 placebo. |
AP 250/100 mg vs. placebo | Blood smear and physical examination during weekly visits. Protective efficacy of AP in PP1 for all malaria and P. vivax 97% (LL 95% CI = 74%) and 97% (LL 95% CI = 69%), respectively. Protective efficacy in PP2 of AP for all malaria and P. vivax was 100% (LL 95% CI = 63) and 100% (LL 95% CI = 58), respectively. No cases of falciparum malaria reported. | 4/5 following Jadad checklist |
| Camus et al., 2004 [32] 13 travel clinics and infectious disease units in Canada, Denmark, France, Germany, The Netherlands and United Kingdom |
International randomized open label study. Follow-up at 7, 28 (clinic visit), and 60 days. | 221 non-immune paediatric travellers, of which 110 AP and 111 CP subjects completed the trial, aged 2–17 years with 43% female. | AP 250/100 mg or paediatric formulation of 62.5/25 mg vs. CP | Blood smear during visits. No subjects were diagnosed with malaria at any time during the study. | 4/5 following Jadad checklist |
| Schlagenhauf et al., 2003 [17] Travel clinics in Switzerland, Germany and Israel |
Randomized, double-blind, four-arm parallel study. Check-up 17 days before travel, 1–3 weeks during travel and 4 weeks of follow-up. | 623 non-immune (adult) travellers, both sexes, 18–70 years, travelling to sub-Saharan Africa. Analysis included 154 AP, 138 mefloquine, 135 CP, and 142 doxycycline subjects. | AP 250/100 mg vs. mefloquine vs. CP vs. doxycycline | Self-reported AEs assessed by an investigator during visits by using questionnaires for AEs, mood/feeling and QoL. No cases of malaria reported for any study arm. | 5/5 following Jadad checklist |
| Faucher et al., 2002 [26] Lambaréné, Gabon |
Randomized, double blind, placebo-controlled study. Study duration of 12 weeks with 4 weeks of follow-up. | 330 children (54% female), of which 165 received AP, aged between 4 and 16 years living in an endemic area. Stratified according to weight. 150 subjects were included in the AP efficacy analysis. | AP (according to weight) vs. placebo | Blood smear during weekly visits (only P. falciparum was assessed). Prophylactic efficacy of AP was 97% (95% CI = 79–100). | 4/5 following Jadad checklist |
| Ling et al., 2002 [25] Papua, Indonesia |
Randomized, placebo controlled, double-blind trial. Study duration of 20 weeks with 4 weeks of follow-up. | 299 non-immune (adults) subjects living in non-endemic areas, of which 150 AP subjects included in the efficacy analysis. Aged between 12 and 65 years with a mean age of 32 years, 35% female. | AP 250/100 mg vs. placebo | Blood smears during daily visits. Protective efficacy of AP was 84% (95% CI = 44–95) for vivax malaria, 96% (95% CI = 72–99) for falciparum malaria and 93% (95% CI = 77–98) for all malaria. | 5/5 following Jadad checklist |
| Berman et al., 2001 [10] Walter Reed Army Institute of Research United States |
Randomized, double-blind, placebo-controlled study based on a human challenge model by P. falciparum-infected mosquitoes on day 2. Follow-up of 8 weeks. | 12 AP subjects with a mean age of 38 years (SD 6.7), 10 were male. 4 placebo subjects with a mean age of 37 years (SD 4), 2 were male. | AP 250/100 mg or matching placebo delivered on day 1–8 | Blood smear daily from study day 8–21, and weekly from week 4–8. Combined with PCR. No AP recipient acquired malaria (P < .001). Protective efficacy of 100%. | 5/5 following Jadad checklist |
| Overbosch et al., 2001 [30] 15 travel clinics in The Netherlands, Germany, United Kingdom, Canada and South Africa |
Randomized double-blind study. Follow-up 7, 28 (active visit), and 60 days after travel. | 976 non-immune (adult) travellers to malaria endemic areas were included in the ITT analysis, of which 92% aged between 12 and 65 years old and 45% female. 486 subjects included in the AP efficacy analysis. | AP 250/100 or 62.5/25 mg vs. mefloquine | Self-reported AEs assessed by an investigator. Minimal and maximum efficacy was 100% (95% CI = 48–100) and 100% (95% CI = 99–100), respectively. No confirmed cases of malaria. | 4/5 following Jadad checklist |
| Hogh et al., 2000 [18] 21 travel clinics in Denmark, United Kingdom, France, Germany, The Netherlands, South Africa and Canada |
Randomized, double-blind study. Follow-up 7, 28 (active clinic visit), and 60 days after travel. | 1022 non-immune (adult) travellers to malaria endemic areas were included in the ITT analysis. Subjects were 13–74 years old, of which 48% female. 501 subjects were included in the AP efficacy analysis. | AP 250/100 mg vs. CP | Blood smear and ELISA assessed by an investigator during visits. Estimated minimum efficacy for prevention of falciparum malaria was 100% (95% CI = 59–100) in the AP group. | 5/5 following Jadad checklist |
| Sukwa et al., 1999 [27] Zambia |
Randomized, double-blind, placebo-controlled, two-arm parallel study. Study duration of 10 weeks with 4 weeks of follow-up. | 213 adults, aged 18–65 years, both sexes, were included in the efficacy analysis. 102/213 subjects received AP. | AP 250/100 mg vs. placebo | Blood smear during weekly visits. Success rate of 98% for P. falciparum (P = .001). Efficacy rate for P. falciparum was 95% (95% CI = 79–100). | 4/5 following Jadad checklist |
| Shanks et al., 1998 [28] Western Kenya |
Randomized, double-blind, placebo-controlled, three-armed, and parallel-grouped trial. Study duration of 10 weeks with 4 weeks of follow-up. | 162 semi-immune (adult) Kenyan subjects aged 18–65, both sexes. The efficacy analysis comprised 54 subjects in each group. | AP 500/200 mg vs. AP 250/100 mg vs. placebo | Blood smear and assessment by an investigator during weekly visits. Prophylactic efficacy and success rate in both the high and low dose treatment groups were 100% (95% CI = 77–100). Success rates between placebo and AP treatments were highly significant (P = .001). | ≤ 4/5 following Jadad checklist |
| Lell et al., 1998 [29] Lambaréné, Gabon |
Randomized, double-blind, placebo-controlled study. Study duration of 12 weeks with 4 weeks of follow-up. | 265 school children, of which 125 received AP, aged between 4 and 16 years, and living in a hyper-endemic area. Stratified according to weight: 11–20, 21–30, 31–40 and > 40 kg. 115 subjects were included in the AP efficacy analysis. | AP (according to their weight) vs. placebo | Blood smear at weekly visits. Prophylactic efficacy of AP was 100% (95% CI = 83–100). No cases of malaria in the AP group (P = .001). | 5/5 following Jadad checklist |
| Bloechliger et al., 2014 [2] United Kingdom |
Descriptive retrospective drug utilization study between 2001 and 2012 | Mostly prescribed to age group 18–65 years (77.5%) with a mean age of 38, median age of 36, range 108 (1–109). 47.2% male. 47.5% < 4 weeks on prophylaxis. | 108,344 AP prescriptions of which 99.9% prescribed as chemoprophylaxis | Data derived from the UK based CPRD. Estimated malaria incidence rate of 13 per 100,000 person-years. | 6/8 following checklist for cross-sectional studies |
| Gimnig et al., 2013 [35] Western Kenya |
Prospective cohort study of human landing catches four nights a week during a six week period | 152 collectors with a median age of 29 (18.6–51.5) were compared to 147 non-collectors with a median age of 28 (18.1–52.2) conducting human landing catches | Collectors were provided AP for seven weeks | Blood smear and questionnaire during follow-up visit every 2 weeks for 12 weeks. No case of malaria occurred in collectors during the human landing catches. Overall the survival of collectors was different to that of non-collectors (P < .0001). Prevalence of P. falciparum during follow-up was 5 in the collectors group and 32 in the matched non-collectors. Collectors were 96.6% less likely to become infected with malaria than non-collectors (P < .001). | 9/11 following checklist for cohort studies |
| Kato et al., 2013 [39] Travel clinic in Japan |
Questionnaire-based analysis between 2009 and 2011 | 278 travellers provided AP with a mean age of 39.2 (SD 12.7), median age of 36 (12–74), of which 174 men. The mean duration of administration was 20.0 ± 9.6 days in the AP group. | AP 250/100 mg vs. mefloquine | Questionnaires upon return. One reported case of malaria in the AP group | 4/8 following checklist for cross-sectional studies |
| Mavrogordato et al., 2012 [40] Scientific expedition in Ethiopia |
Observational study based on questionnaires following a two month scientific expedition | 31 subjects completed the survey with an age range of 22–74 years (mean 54), of whom 11 took AP. | AP | Blood smear when malaria was suspected and contacted upon return and two years after return. None of the subjects in the AP group developed malaria. | 5/7 applicable items, (5/8 of total score) following checklist for cross-sectional studies |
| Zuckerman et al., 2009 [33] United Kingdom |
Retrospective observational study between 2001 and 2007 | Returning UK travellers | 1.26 million prescriptions of AP by estimation | Surveillance data derived from the Health Protection Agency website and prescription data from the Cegedim Strategic Data UK. Total of 2.9 (1.3 by removing confounding factors) reported cases of P. falciparum malaria per 100,000 prescriptions. | 5/8 following checklist for cross-sectional studies |
| Andersson et al., 2008 [34] Swedish Armed Forces unit in Liberia |
Descriptive retrospective compilation study design with follow-up of 6 months after return | Only contingent 2, 4, and 5 were asked exactly the same questions and therefore only those answers are included in this report (n = 690) with an 88% response rate. Total: 609 (4.8% female), average age 28.2 (SD 7.1), AP 26.4% | AP or mefloquine | Questionnaires upon return. No reported cases of falciparum malaria in the AP group resulted in 100% effectiveness for long-term prophylaxis. | 6/8 following checklist for cross-sectional studies |
| van Genderen et al., 2007 [36] Travel clinic in The Netherlands | Prospective observational study with 57 person-years of follow-up from 1999 until 2004 | Non-immune adult travellers or expatriates to sub-Saharan Africa using AP > 28 days of travel. 169 evaluable subjects (41% female) used AP for 2974 weeks. | AP 250/100 mg | AEs were recorded on questionnaire at 3, 6, 9 and 12 months of use. Prophylactic efficacy of 97% against P. falciparum. | 5/8 following checklist for cross-sectional studies |
| Kofoed et al., 2003 [38] Gastrointestinal and Parasitic Infections clinic in Denmark |
Open case-control study with returning travellers from 1997 to 1999 | 320 cases of which 200 cases with P. falciparum were notified. 3 cases used AP as prophylaxis compared to 42 controls. Non-infected travellers were matched by age, sex, and destination. | AP | Questionnaires upon information on use of prophylaxis and length of stay. Falciparum malaria was described in 3 subjects using AP. Estimated efficacy of AP in fully compliant users was 1:1943 (falciparum malaria cases per prescription). | 7/10 following checklist for case-control studies |
| van der Berg et al., 1999 [37] 4 research sites in South Africa |
Open-label trial, 10 weeks duration | 175 healthy, non-immune subjects between 16 and 65 years. 149 men with a mean age of 29, 26 women with a mean age of 34. 113 included in efficacy analyses. | AP 250/100 mg | Blood smear at any time malaria was expected and at week 10. ICT was used on site for P. falciparum. Clinic visits at week 5 and 10. Prophylaxis success 97% (95% CI = 92–99) and none of the subjects in the efficacy analysis developed malaria. | 5/6 applicable items (5/9 of total score) following checklist for quasi-experimental studies |
AP, atovaquone-proguanil; CP, chloroquine-proguanil; UK, United Kingdom; CPRD, Clinical Practice Research Datalink; PCR, polymerase chain reaction; ELISA; enzyme-linked immunosorbent assay; ICT, immunochromatographic test; AE(s), adverse event(s); QoL, quality of life; ITT, intention to treat; PP1 is defined as being compliant and visiting for weekly blood smear; PP2 is defined as having adequate drug concentrations; CI, confidence interval; SD, standard deviation; LL, lower limit.
3.1.2. Participants′ characteristics
The demographic characteristics varied, but the studies mostly included adults. The demographic characteristics of the patients in the different treatment arms in each of the randomized studies were similar. Three studies were performed solely in paediatric participants between three and sixteen years of age [26,29,32]. Major similarities in exclusion criteria for the randomized studies were childbearing potential or pregnancy, concomitant use of drugs with antimalarial potential, previous malarial infection, recent travel to a malaria endemic area, severe adverse events (e.g. hypersensitivity), and co-morbidities such as HIV/AIDS, other immune-deficiencies or glucose-6-phosphate dehydrogenase (G6PD) deficiency. Clinical, physical, or laboratory abnormalities were also considered for exclusion. The exclusion criteria for the open label trial reported by van der Berg and colleagues were similar to those for the randomized studies described above [37]. Van Genderen and colleagues excluded participants aged less than 18 years old [36].
Four of the randomized studies were performed in individuals living in a malaria-endemic area [26–29]. In contrast, eight studies were performed in non-immune participants [10,17,18,24,25,30–32]. Six of the observational studies included travellers [2,33,36–39].
3.1.3. Types of interventions
The randomized studies compared AP to placebo or several antimalarial drugs. Seven of the randomized studies compared AP to placebo [10,24–29], two studies compared AP to chloroquine/proguanil [18,32], one study compared AP to mefloquine [30], and one four-arm parallel study compared AP, mefloquine, doxycycline and chloroquine/proguanil to each other [17]. Also, an AP group served as an additional open-label control group in one of the randomized studies [31].
Of the observational studies three studied AP alone [35–37], one study compared AP to mefloquine and chloroquine/proguanil [38], two studies compared AP to mefloquine [34,39], and one study compared AP to multiple antimalarial regimens [40]. One study compared the prescribing patterns for several antimalarial drugs [2], while another study evaluated the effectiveness of different antimalarial regimens based on prescribing and surveillance data [33]. Daily dosing of 250/100 mg AP was assumed when no information about dosage was stated.
3.1.4. Types of outcome measurement
Parasitaemia was the primary outcome in eight of the randomized studies [10,24–29,31], whereas four of the randomized studies used adverse events as primary outcome [17,18,30,32]. In the latter group, efficacy of malaria chemoprophylaxis was a secondary outcome in two studies [18,30]. Three of the randomized studies were not powered to determine the efficacy for malaria prevention or to compare the difference in efficacy rates between the treatment groups [17,18,32].
Parasitaemia alone [35,40] and parasitaemia and safety [37] were the primary outcomes in two and one study each, respectively. Questionnaires were used in four of the observational studies, in which presence of malaria infection [38], adverse events [36,39], and effectiveness and adverse events were the primary outcomes [34]. One of the retrospective studies extracted data from the UK based Clinical Practice Research Datalink (CPRD) [2] while another used surveillance data from a Health Protection Agency website and prescribing data obtained from the Cegedim Strategic data UK [33], both providing effectiveness estimates of antimalarial regimens.
3.1.5. Methodological quality
Ratings of the methodological quality of the studies are shown in Table 1. The randomized studies were of excellent methodological quality. Randomization was described by all studies, as were blinding methods. Ten studies employed a double-blind design and compared AP to placebo using identical capsules containing either AP or placebo [10,17,18,24–30]. A group taking AP served as an additional open-label cohort in one of the randomized studies [31]. During completion of critical appraisal checklists, two additional points were provided for the non-applicable items during the critical appraisal of the randomized open-label study of Camus and colleagues. Confounding factors were avoided by excluding participants with a history of malaria, living in a malaria-endemic area, and concurrent use of drugs with antiplasmodial activity. Five of the randomized studies above used a curative treatment phase before randomization to eliminate pre-existing malarial parasites [25–29]. Five studies analysed the results according to a per-protocol analysis (PP); no crossing-over was described between the treatment arms [17,26–29]. Five studies analysed the results according to an intention-to-treat analysis (ITT) [10,18,25,30,32]. Two studies provided both a PP and ITT analysis [24,31]. The use of a PP analysis leads to a possible overestimation of the efficacy [41].
Six observational studies were rated by using the checklist for cross-sectional studies due to the use of questionnaires or use of data on prescribing patterns from a clinical database [2,33,34,36,39,40]. The cohort study was rated according to the cohort study checklist [35]. The case-control study [38] and open-label trial [37] were rated by using the checklist for case-control studies and quasi-experimental studies, respectively. All the rated observational studies showed medium-to-high quality. Most studies did not identify or deal with confounding factors, with the exception of one of the retrospective studies, which identified and corrected for confounding factors in the analysis [33]. The resulting effectiveness might still have been an overestimation since only returning travellers were considered for analysis. Most studies rated by means of the cross-sectional checklist did not measure the exposure in a reliable and valid way; because there was no direct observation to ensure the drugs were taken appropriately [33,34,36,39]. For example, the study reported by van Genderen and colleagues lacked supervision of drug intake and lacked confirmation of the self-reported malaria cases, and therefore induced potential recall bias and possible underestimation of the efficacy [36]. The case-control study also lacked observation of drug intake [38]. In the study of Kato and colleagues there was an absence of confirmation of the self-reported malaria cases [39]. The open-label trial lacked a control group and a treatment phase to eliminate any pre-existing parasites [37]. The prospective cohort study cleared subjects from any pre-existing parasites using a treatment phase before administration of AP [35].
3.1.6. Results presented
Nine randomized studies provided results by calculating the efficacy of AP [10,18,24–30]. The remaining two randomized studies were not powered to determine the efficacy of the antimalarial prophylaxis, but no cases of malaria were identified [17,32]. The randomized study with the open-label control cohort of AP measured the level of parasitaemia in subjects receiving AP [31].
The study reported by van der Berg and colleagues provided success rates [37]. The case-control study calculated the efficacy by determining the number of malaria cases per prescription [38]. Reported cases of malaria were used in order to estimate an overall protective efficacy in the prospective observational study [36]. Four studies presented the number of malaria cases [34,35,39,40]. One of the retrospective studies presented the estimated number of malaria cases per 100,000 prescriptions [33]. One of the descriptive retrospective studies presented the results by determining the incident rate of malaria per person-years [2]. The descriptive drug utilization study done by Bloechliger and colleagues estimated the incident rate of malaria in an exploratory analysis. However, methodological shortcomings (e.g. inadequate reporting on malaria cases) and lack of information about exposure were acknowledged and rendered an interpretation of the results impossible.
3.2. Overview of the studies in support of a short post-travel regimen
3.2.1. Study designs
The total number of eligible studies in support of the alternative regimen for AP prophylaxis was 11, including two randomized studies, three observational studies, and six pharmacological studies (see Tables 2 and 3 for an overview of the eligible studies). The non-clinical experimental studies were considered as circumstantial evidence (Table 3). Two studies were randomized, placebo-controlled, double-blind trials [11,42]. The eligible observational study designs were one quasi-experimental study [43], one retrospective cohort study [20], and one observational open-label study [44]. Three short reports were included [12,45,46]. Finally, three non-clinical experimental studies (i.e. serological studies) were included [47–49].
Table 2.
Characteristics of the studies in support of an abridged post-travel regimen.
| Author, year of publication, country | Study design | Number of participants | Studied antimalarial agent | Measure of outcome and results | Critical appraisal score |
|---|---|---|---|---|---|
| Deye et al., 2012 [42] United States, Walter Reed Army Institute of Research |
Randomized, placebo-controlled, double trial including challenge by P. falciparum-infected mosquitoes on day 0. The control cohort enrolled as an open-label study. Follow-up duration of 90 days. | 36 participants, of which 35 included in the ITT analysis: 23 men and 12 non-pregnant/non-lactating women, with a mean age 31.2 years (SD 8.6, range 20–50). 33 subjects were included in the ATP analysis. AP 250/100 mg d-1 group: 5 men, 1 woman, mean age of 35.7 years (SD 9.4, range 22–48) AP 250/100 mg d + 4 group: 1 men, 4 women, mean age of 31.2 years (SD 8.2, range 24–44) |
30 subjects randomized: 6 subjects received AP 250/100 mg on d-1, 6 received AP 250/100 mg on d+4, 6 received AP 250/100 mg on d-7, 6 received AP 500/200 mg on d-7, and 6 received AP 1000/400 mg on d-7. The control group comprised of 6 infectivity controls. | Blood smear on days 6–20 and day 23 or when malaria infection was expected. Close observation from day 9–20. ATP analysis: 6/6 subjects receiving AP 250/100 mg on d-1 and 4/4 receiving AP 250/100 mg on d + 4 were 100% protected. Single-dose AP proves to be 100% effective prophylaxis against P. falciparum at weekly dosing or when used as post-exposure prophylaxis 4 days after challenge. | < 5/5 following Jadad checklist |
| Shapiro et al., 1999 [11] National Institutes of Health (Bethesda, MD) - sponsored outpatient General Clinical Research Center of the Johns Hopkins Hospital, United States |
Randomized, double-blind, placebo-controlled study including challenge by P. falciparum-infected mosquitoes. Follow-up of 12 weeks after challenge. Day of challenge is designated study day 0. | 16 subjects (6 high dose, 6 low dose, and 4 placebo) with an average age of 30.1 years (range 22–44), of which 1 woman. | 750 mg atovaquone vs. 250 mg atovaquone plus placebo tablets vs. placebo. All dosed prior to sporozoite challenge. | Blood smear and assessment visits daily (days 5–21), every other day (days 22–35), and then weekly until 12 weeks after challenge. PCR and culture were performed to confirm parasitaemia. 250 mg of atovaquone protected 6/6 subjects, and either of the atovaquone regimens provides effective (100%) prophylaxis compared to placebo (P = .005, 95% CI of protection = 61–100%). | 4/5 following Jadad checklist |
| Lachish et al., 2016 [43] Infectious diseases clinic Israel, long-term expatriates in Angola and Equatorial Guinea |
Observational surveillance study using a quasi-experimental set-up with 1368 person-months of follow-up | 122 long-term expatriates travelling to work in West Africa, of which 33 subjects took AP twice weekly. 14 expatriates (all male, median age of 24 years) switched from no-prophylaxis to AP in the Angola jungles for 10 months. 108 subjects (m:f = 1:1; age range 1.5–71; 28 of 107 were ≤12 old) lived for different periods (median stay = 19.45 months) in Equatorial Guinea. | AP twice weekly vs. mefloquine vs. group refusing to take prophylaxis. | Adherence was observed by paramedics and self-reporting. Malaria incidence was lower in AP group (0/391 person-months, 95% CI = 1.4–∞, P = .01) than in no-prophylaxis group (11.7/1000 person-months). After adjustment, treatment was associated with ~ 20 times decreased odds for malaria compared with no prophylaxis: OR = 0.05 (95% CI = 0.006–0.42; P = .006). |
5/9 following checklist for quasi-experimental studies |
| Leshem et al., 2014 [20] Active surveillance in travel clinic Israel |
Retrospective cohort study between 2010 and 2011 | 485 travellers to sub-Saharan Africa of which 421 subjects used a short-course of AP prophylaxis. 219 (52%) male, mean age of 38.1 ± 20.3 years (median age 43 years, range 4–76), and mean travel duration of 10.1 ± 7.8 days (median 10 days, range 2–77). | 421/485 (87%) discontinued AP 1 day after leaving endemic area (cumulative exposure of 4337 days) compared to 9 (2%) travellers (cumulative period of 95 travel days) continued taking AP for 2–7 days after leaving endemic area; and 55 (11%) travellers (cumulative period of 547 travel days) did not take AP prophylaxis at all or discontinued AP prior to leaving the endemic country. | Active surveillance during 2010–2011 by retrospective telephone survey 1–6 months after travellers’ return. None of the 485 travellers reported malaria infection. | 2/7 applicable items, (2/11 of total score) following checklist for cohort studies |
| Petersen et al., 2003 [44] Gastrointestinal and Parasitic Infections clinic |
Observational open-label study with no possibility of follow-up due to the anonymous questionnaires. | 184 (61%) non-immune soldiers filled in the questionnaires. 176 (96%) were male with a mean age of 30 years. | AP prophylaxis without control group. Analysis therefore compared self-reported symptoms in soldiers who were compliant (i.e. taking at least 3 of every 4 tablets during the 6-month period) with soldiers who reported taking fewer. | Post-travel questionnaires. 44 subjects were total compliant, 37 took 3/4 pills, 29 took 2/4 pills, and 63 took 1/4 pills or fewer. 11 took none at all. No cases of falciparum malaria were recorded. | 5/8 following checklist for cross-sectional studies |
| Edstein et al., 2005 [12] Data extrapolated from a study described elsewhere |
Short report extrapolated from a study described elsewhere [53] | 3 Caucasian volunteers | AP treatment with 1000/400 mg daily for 3 days | Mean plasma atovaquone concentrations-versus-time curves measured by HPLC and bioassay measurement on blood samples 6, 20, and 35 days after onset of treatment. Average elimination half-life was 5.9 days by HPLC and 4.9 days by bioassay. | Not applicable |
| Polhemus et al., 2008 [45] United states, Walter Reed Army Institute of Research | Short report extrapolated from a study described elsewhere [54] | 80 malaria immune (adult) subjects (69 male, 11 female) in areas of Kenya | AP treatment with 1000/400 mg daily for 3 days | Passive detection of infection began immediately. Active detection of infection by scheduled weekly malaria blood smear began at week 2 post-completion of AP. Time to first falciparum parasitaemia was 32 days (95% CI = 45 days). | Not applicable |
| Shanks et al., 1999 [46] Western Kenya | Short report extrapolated from a study elsewhere [28] | 65 adult volunteers in western Kenya | AP treatment with 1000/400 mg daily for 3 days | Time to first falciparum parasitaemia was 32 days | Not applicable |
AP, atovaquone-proguanil; PCR, polymerase chain reaction; HPLC, high-pressure liquid chromatography; ITT, intention to treat; ATP, according to protocol; OR, odds ratio; SD, standard deviation; CI, confidence interval.
Table 3.
Characteristics of the studies with circumstantial pharmacological evidence in support of a short post-travel regimen.
| Author, year of publication, country | Study design | Number of participants | Studied antimalarial agent | Measure of outcome and results |
|---|---|---|---|---|
| Butcher et al., 2003 [47] Department of Biologic Sciences, Imperial College of Science Technology and Medicine, London |
Non-clinical experimental (i.e. serological) study with P. falciparum transmission and asexual experiments by feeding mosquitoes AP treated sera | 3 AP treated subjects were bled for sera on day 0, 7, 14, 21, 28, 42, and 56. 2 atovaquone only treated subjects were bled for sera on day 0, 7, 14, 21, 28 or 35, and 42 or 49. |
AP treatment with 1000/400 mg daily for 3 days on day 0. Secondly, 2 volunteers were treated with atovaquone only at a dosage equivalent to that of AP taken on day 0. | Transmission inhibition: sera 4–28 days after AP treatment totally blocked infection of the mosquitoes, and significant inhibition of oocyst development (P = .05) was still present at day 42 (mean: 12.5% SEM ± 1.94, of day 0 value). Asexual stage inhibition: sera inhibitory up to day 28: mean 11.4% (SEM ± 0.3) of growth compared to day 0 sera (P = .05). On day 42, the mean parasitaemia still was only 43.9% (SEM ± 17.7) of the day 0 value. |
| Butcher et al., 2000 [49] Department of Biologic Sciences, Imperial College of Science Technology and Medicine, London |
Non-clinical experimental (i.e. serological) study with P. berghei transmission and asexual experiments by feeding mosquitoes AP treated sera | 4 AP treated subjects were bled for sera on day 0 and subsequently for up to 10 weeks. 3 atovaquone or proguanil only subjects were bled for sera accordingly. |
AP treatment with 1000/400 mg daily for 3 days on day 0. Secondly, 3 volunteers were treated with atovaquone or proguanil only at a dosage equivalent to that of AP taken on day 0. |
Gametocyte-oocyst development tested at a dilution of 1:100 was totally inhibitory up to day 14 but varied from 0 to 100% [with a mean (S.E.) percentage of 43 (29.7)] on day 21. At day 56 there was no significant difference compared to the day-0 level. Gametocyte-ookinete development on days 3, 7, 14 and > 14 and tested at a dilution of 1:100 was 50%, 68%, 84% and 100% of the day-0 levels, respectively. Ookinete-oocyst development on days 3, 7, 14, 21, and 56 tested at a dilution of 1:100 was 4%, 8%, 33%, 25% and 100% of the day-0 levels, respectively. Asexual stage: on day 7 there was a 26-fold decrease in schizonts. Further data was not available. |
| Enosse et al., 2000 [48] United Kingdom and Matola, Mozambique |
Non-clinical, randomized, experimental (i.e. serological) study with P. falciparum and P. berghei transmission and asexual experiments by feeding mosquitoes AP treated sera | Field studies on P. falciparum infectivity: 33 AP treated subjects in Matola were bled for sera on day 0, 4, 7, 14, and 21. P. berghei infectivity: 4 AP treated British subjects were bled for sera on day 0 and subsequently for up to 12 weeks. |
AP treatment with 1000/400 mg daily for 3 days on day 0 vs. chloroquine. | Field studies on P. falciparum infectivity: asexual stages declined to 0 on day 4 in 14/15 subjects, 0 on day 14 for 26 subjects, and became positive on day 21 for 2/16 subjects. Gametocyte numbers in AP treated subjects declined on day 4, 7, 14, and 21–68%, 31%, 4%, and 0%, respectively. The oocysts declined to 30% on day 4, 0% on day 7, and rose to 30% on day 21. The mean prevalence of infected mosquitos was 20%, 1%, 0%, 2%, and 3% on day 0, 4, 7, 14, and 21, respectively. P. berghei infectivity: infectivity in AP treated subjects, tested at a 1:100 dilution, was completely inhibited up to day 28 with significant inhibition still present at day 42 and 56 (P = .03), namely 19% and 10%, respectively. |
AP, atovaquone-proguanil; SEM, mean expressed as a percentage of mean pretreatment day 0 sera; S.E., standard error.
3.2.2. Participants’ characteristics
Both children and adults were represented. The demographic characteristics of the included participants were similar. The two randomized studies with sporozoite challenge excluded participants who concomitantly used drugs with antiplasmodial activity or when the participants had any history of malaria, travelled to a malaria-endemic region in the past year or lived in a malaria-endemic area [11,42]. The randomized studies also excluded participants with clinical, physical or laboratory abnormalities, those with an underlying blood disorder, or those with childbearing potential or pregnancy. The retrospective cohort study by Leshem and colleagues included travellers but excluded persons visiting friends and relatives (VFR) [20].
The participants in the randomized studies and observational open-label study were non-immune to malaria [11,42,44]. Lachish and colleagues included long-term expatriates defined as travelling to work for more than six months in West Africa [43]. The subjects in the short reports were semi-immune to malaria [12,45,46]. Two serological studies used P. berghei in the transmission model whilst the other serological study used P. falciparum [47–49]. No previous malaria infection has been described in the serological studies of Butcher and colleagues [47,49]. The subjects of Enosse and colleagues were from a malaria-endemic area [48].
3.2.3. Types of interventions
The two randomized studies with sporozoite challenge studied different dosages of AP. Both studies were placebo controlled [11,42]. The observational study with quasi-experimental set-up compared twice-weekly dosing of AP to mefloquine once weekly, and a group refusing to take any chemoprophylaxis at all [43]. The retrospective cohort study studied the discontinuation of AP prophylaxis one day after return from a malaria-endemic area [20]. The open-label study studied AP alone, comparing those who complied with those who did not [44]. Two short reports provided information about the time until first parasitaemia after treatment with AP [45,46]. The short report of Edstein and colleagues studied the half-life of atovaquone after treatment with AP for three days [12]. Three experimental, non-clinical (serological) studies studied the inhibition of malarial transmission after treatment with AP [47–49]. Dosing of 250/100 mg AP was assumed when no information about dosage was stated.
3.2.4. Types of outcome measurement
Microscopic parasitaemia was the primary outcome for four studies [11,42,45,46], of which two were randomized studies, and two short reports. Polymerase chain reaction (PCR) was also performed in the randomized studies with sporozoite challenge, but not in real time. Lachish and colleagues used incidence rates as outcome, but the method of outcome measurement was not clearly stated apart from observation of adherence by paramedics or self-reporting [43]. Leshem and colleagues used active surveillance by retrospective telephone survey one to six months after travellers’ return [20]. Petersen and colleagues determined the long-term safety and compliance as primary outcome [44]. Edstein and colleagues measured the mean plasma concentrations of atovaquone by using high-pressure liquid chromatography (HLPC) to determine the half-life [12]. The experimental non-clinical (i.e. serological) studies determined the parasite count after dissection of the mosquitoes that fed on the participants [47–49].
3.2.5. Methodological quality
Ratings of the methodological quality of the studies are shown in Table 2. The randomized studies showed excellent methodological quality. Randomization was described by both studies, as well as blinding methods. Both studies were conducted using a double-blind design, with identical capsules containing either AP or placebo. Confounding factors were avoided by excluding participants with a history of malaria, those living in a malaria-endemic area, and concurrent use of drugs with antiplasmodial activity. The study of Deye and colleagues analysed the results according to protocol, but no crossing-over was described between the treatment arms [42]. The study of Shapiro and colleagues hinged on an intention-to-treat analysis [11].
The retrospective cohort study of Leshem and colleagues had several methodological shortcomings: inadequate power, possible recall bias, travel to a region with limited risk of exposure, no evidence of malaria exposure and insufficient data ensuring that the drugs were taken appropriately [20]. Outcomes were not measured in a valid way due to the absence of using a validated survey tool. No confounding factors were stated, but excluding VFRs can be seen as an attempt to eliminate confounding by semi-immunity. No information was provided on whether the participants were malaria parasite-free at study start.
The quasi-experimental study of Lachish and colleagues was of intermediate quality [43] as the major target travel region posed a limited risk of exposure. No clear comparison of the demographic characteristics of the three different study groups was possible. However, the authors did adjust for sex and location to compare the treatment groups, which is an indication of similarity between the participants. Secondly, the living conditions were similar. Unfortunately, no curative treatment was initiated to eliminate patent parasitaemia. No clear information was provided about outcome measurement. Again, this study was not powered to provide the efficacy of an alternative regimen of AP prophylaxis.
The observational open-label study of Petersen and colleagues was of intermediate quality, but lacked appropriate observation of drug intake [44]. No confounding factors were identified or dealt with; however, it needs to be noted that the study’s primary focus was adverse events rather than efficacy of the AP regimen.
The short reports and the three experimental non-clinical studies were not rated by means of a checklist [12,45–49].
3.2.6. Results
The randomized studies provided data on the effectiveness of the prophylactic regimen [11,42]. The quasi-experimental study of Lachish and colleagues determined the incidence of malaria infection in cases of malaria per person-months [43]. Both the retrospective cohort and the observational open-label studies yielded no cases of malaria [20,44]. Edstein and colleagues determined the half-life of atovaquone, whereas the other two short reports determined the time until first parasitaemia after AP treatment [12,45,46]. The three experimental (i.e. serological) non-clinical studies focused on the prolonged inhibition of transmission and asexual parasite development [47–49].
4. Discussion
This is the first systematic review to provide a comprehensive overview of evidence on the efficacy of both the recommended and alternative regimens of AP prophylaxis. The literature search yielded some limited clinical and non-clinical evidence suggesting that a short post-travel regimen of AP is potentially effective, but requires further investigation. The total sample size and quality of those studies comprise a relatively small percentage of the total sample size and quality of the studies with evidence in support of the current post-travel regimen; information which must be taken into account when weighing the evidence for a curtailed regimen.
4.1. Studies in support of the current post-travel regimen
The randomized studies performed with a seven day post-travel regimen show high efficacy as determined by the systematic review of Nakato and colleagues [50]. This previously conducted systematic review performed a meta-analysis of six of the twelve randomized studies included in our systematic review. The meta-analysis of the six studies found an efficacy of 95.8% (95% CI = 91.5–97.9) [24–29]. Five of the other randomized studies included in this systematic review, but not part of the meta-analysis of Nakato and colleagues, described no cases of falciparum malaria in AP recipients [10,17,30–32]. The last randomized study provided an estimated efficacy for prevention of P. falciparum of 100% (95% CI = 59–100) [18]. The single study of Berman and colleagues led to the clinical studies, as described above, and ultimately resulted in the implementation of the currently recommended post-travel regimen of seven days [10].
An interesting finding is that three of the randomized studies described that a percentage of the participants took less than 80% of the recommended doses in the post-travel period, but none of those participants developed malaria (3%, 12%, 7%, respectively) [18,30,32]. No information was provided about the total number of missed pills, or days on which pills were not taken. However, it raises the question whether it is necessary to fully adhere to the current post-travel regimen.
The descriptive retrospective drug utilization study was selected to demonstrate the effectiveness of AP when prescribed as prophylactic agent, namely 13 cases per 100,000 person years [2]. Another retrospective study determined the number of malaria cases to be 1.3 per 100,000 prescriptions of AP [33]. Both are proof that AP is a highly effective agent for the prevention of clinical malaria episodes, as is the study in which no cases of malaria were described in the collectors who were performing human landing catches while receiving AP [35]. The latter study highlights the efficacy of AP even in a high-risk setting. The open case-control study, in which three travellers used AP, estimated the number of malaria cases per prescription in fully compliant users to be 1 per 1943 [38]. Finally, the observational study with 57 person-years of follow-up [36] and the open-label trial with a ten-week duration [37] determined the efficacy and prophylactic success of AP prophylaxis against falciparum malaria both to be 97%; no cases of malaria were described in the latter study. The success rate consisted of people who did not develop parasitaemia or who withdrew due to a treatment-related adverse event. The prophylactic efficacy estimated in the study by van Genderen and colleagues may even be an underestimation since they were not able to verify the diagnosis of the self-reported malaria cases [36].
4.2. Studies in support of a short post-travel regimen
The randomized controlled clinical trials of Deye and colleagues with sporozoite challenge with very few subject numbers supports the hypothesis of weekly dosing of AP and stated that once weekly and post-exposure prophylaxis four days after challenge was 100% effective [42]. None of the participants developed malaria. The post-exposure dose four days after challenge is in line with the observational study of Lachish and colleagues with a twice-weekly dosing schedule in which no cases of malaria were recorded [43]. It needs to be stressed that the methodology for these studies was weak and exposure varied. The results are in line with the observational open-label study of Petersen and colleagues where no cases of falciparum malaria were recorded in participants who took one out of four pills (i.e. consistent with twice-weekly prophylaxis) [44].
In line with the information provided by Deye and colleagues, the randomized sporozoite challenge study of Shapiro and colleagues, again with very limited subject numbers, described no malaria cases when dosed one day before malaria challenge with a broad confidence interval (95% CI = 61–100%) [11]. This raises the hypothesis of an abbreviated post-travel course. The observational study of Leshem and colleagues found that none of participants who discontinued AP one day after return from a malaria-endemic area developed malaria [20].
Finally, several studies related to pharmacological aspects of AP have been included. The short report of Edstein and colleagues determined the half-life of atovaquone to be 5.9 days by HLPC in contrast to the currently accepted half-life of one to three days in adults [12]. The other two short reports both determined the time until first parasitaemia after malaria treatment with AP to be 32 days [45,46], which cannot be explained by the currently accepted elimination half-life. These results support the data on the half-life properties of atovaquone provided by Edstein and colleagues, and suggest that a regimen of AP taken less frequently than daily may be effective. This prolonged inhibitory activity was further illustrated by the complete inhibition of schizont formation until day 35 post-treatment [12]. However, a point of critique on the justification of a short course of AP based on this half-life is the questionable efficacy when subjects are in the end exposed to atovaquone alone due to the short half-life of proguanil. Additionally, regimens spreading out the AP doses could leave the travellers with primarily atovaquone and thus also potentially inducing atovaquone resistance. Taking these points into consideration, atovaquone-only exposure due to an abbreviated course of AP might ultimately result in AP resistance.
Non-clinical experimental (i.e. serological) studies were considered as circumstantial evidence to support the theory of a half-life of 5.9 days, because the inhibition of asexual blood stages (responsible for clinical malaria episodes) was less pronounced than the inhibition of sexual blood stages (responsible for transmission), suggesting a difference in sensitivity to AP. The results on inhibition on the different stages of the asexual and sexual blood stage development shown in Table 3 were also extracted to demonstrate the differences in inhibitory potential depending on the various stages.
The differences in the inhibition of sexual stage development of malaria are beyond the scope of this systematic review. However the prolonged inhibitory potential may suggest that concentrations of AP have inhibitory potential, which cannot be explained by our current understanding of the half-life properties.
The plasma levels after treatment with AP completely inhibited transmission until day 28 in P. falciparum and in the P. berghei model, respectively [47,48]. Again, the long inhibitory activity of AP cannot be explained by our current understanding of the half-life properties. The remaining P. berghei study showed an inhibitory potential of transmission until day 14, in contrast to the inhibitory potential of 28 days (when considering the gametocyte-oocyst stadium as transmission potential) [49]. However, the sensitivity differs between the different stages of the malaria cycle, and therefore this evidence should be considered only as circumstantial. One of the studies of Butcher and colleagues showed that atovaquone-only serum totally inhibited transmission up to, and including, day 28; suggesting that it is the persistence of atovaquone that is responsible for the prolonged schizonticidal effect [47], whilst the other study of Butcher and colleagues totally inhibited oocyst formation between days 3 and 21 [49]. The circumstantial results illustrate that AP is schizonticidal, but not gametocytocidal, because gametocytes quickly declined only to rise again after days to weeks.
4.3. Key findings and failure rates
The failure rates in the studies with a focus on the seven-day post travel regimen are higher in comparison to the studies in support of a short post-travel regimen (see Tables 4 and 5 for key findings and failure rates). However, the total number of subjects in studies in support of a short post-travel regimen is considerable smaller than the respective number in support of the current post-travel regimen. Secondly, the efficacy of the full course of AP is 95.8% [50] and so we should expect a few failures in the abbreviated regimen in case we expect a prolonged inhibitory potential of AP. Also, the observational studies with no reported malaria cases might not reflect the true efficacy of a short post-travel regimen due to flaws in their study methodology [20,43]. This should be taken into account when comparing and interpreting the regimens based on our findings. Finally, it should be stressed that the two randomized studies in support of a short post-travel regimen were performed under ideal conditions, that is, under the supervised administration of the drug together with a (fatty) meal, and, secondly, that the daily habits of the subjects were not disrupted in a way one could expect in travellers at the end or after a prolonged travel from endemic regions [11,42]. In the case of travellers, disrupted daily routines with irregular meals may result in the ingestion of AP while fasting, resulting in a decreased maximum concentration and therefore the possibility of prophylactic failure (see 4.3).
Table 4.
Failure rates of the studies in support of the current post-travel regimen.
| Author | Study size | Efficacy | Failures |
|---|---|---|---|
| Sulyok et al., 2017 [31] | 6 AP subjects | All subjects were protected | 0 |
| Soto et al., 2006 [24] | 120 AP subjects in ITT analysis 110 AP subjects in PP1 analysis 97 AP subjects in PP2 analysis 8/9 AP failures included in ITT analysis withdrew consent and were not administered any drug |
Protective efficacy of AP in PP1 for all malaria and P. vivax 97% (LL 95% CI = 74%) and 97% (LL 95% CI = 69%), respectively. Protective efficacy in PP2 of AP for all malaria and P. vivax was 100% (LL 95% CI = 63) and 100% (LL 95% CI = 58), respectively. No cases of falciparum malaria reported. |
0 falciparum malaria and 1 vivax malaria case in PP1 0 falciparum malaria and 0 vivax malaria in PP2 |
| Camus et al., 2004 [32] | 110 AP subjects | No subjects were diagnosed with malaria at any time during the study. | 0 |
| Schlagenhauf et al., 2003 [17] | 154 AP subjects | No cases of malaria reported for any study arm. | 0 |
| Faucher et al., 2002 [26] | 150 AP subjects included in efficacy analysis | Prophylactic efficacy of AP was 97% (95% CI = 79–100). | 1 case of falciparum malaria |
| Ling et al., 2002 [25] | 150 AP subjects included in efficacy analysis | Protective efficacy of AP was 84% (95% CI = 44–95) for vivax malaria, 96% (95% CI = 72–99) for falciparum malaria and 93% (95% CI = 77–98) for all malaria. | 1 case of falciparum malaria (co-infecton with a vivax malaria case) and 2 vivax malaria cases |
| Berman et al., 2001 [10] | 12 AP subjects | No AP recipient acquired malaria (P < .00l). Protective efficacy of 100%. | 0 |
| Overbosch et al., 2001 [30] | 486 AP subjects included in efficacy analysis | Minimal and maximum efficacy was 100% (95% CI = 48–100) and 100% (95% CI = 99–100), respectively. No confirmed cases of malaria. | 0 |
| Hogh et al., 2000 [18] | 501 AP subjects included in efficacy analysis | Estimated minimum efficacy for prevention of falciparum malaria was 100% (95% CI = 59–100) in the AP group. | 0 |
| Sukwa et al., 1999 [27] | 102 AP subjects included in efficacy analysis | Success rate of 98% for P. falciparum (P = .001). Efficacy rate for P. falciparum was 95% (95% CI = 79–100). | 2 cases of falciparum malaria |
| Shanks et al., 1998 [28] | 54 low dose AP subjects included in efficacy analysis | Prophylactic efficacy and success rate in both the high and low dose treatment groups were 100% (95% CI = 77–100). | 0 |
| Lell et al., 1998 [29] | 115 AP subjects included in efficacy analysis | Prophylactic efficacy of AP was 100% (95% CI = 83–100). No cases of malaria in the AP group (P = .001). | 0 |
| Bloechliger et al., 2014 [2] | 108,344 AP prescriptions of which 99.9% prescribed as chemoprophylaxis | Estimated malaria incidence rate of 13 per 100,000 person-years. | – |
| Gimnig et al., 2013 [35] | 152 AP subjects | All collectors provided with AP were protected during HLC | 0 |
| Kato et al., 2013 [39] | 278 AP subjects | 1 subject diagnosed with malaria | 1 |
| Mavrogordato et al., 2012 [40] | 11 AP subjects | None diagnosed with malaria | 0 |
| Zuckerman et al., 2009 [33] | 1.26 million prescriptions of AP by estimation | 2.9 (1.3 by removing confounding factors) cases of falciparum malaria per 100,000 prescriptions | – |
| Andersson et al., 2008 [34] | 161 AP subjects | No reported cases of falciparum malaria resulted in 100% effectiveness for long-term prophylaxis. | 0 |
| van Genderen et al., 2007 [36] | 169 AP subjects | Prophylactic efficacy of 97% against P. falciparum. | 5 |
| Kofoed et al., 2003 [38] | 45 AP subjects | Estimated efficacy of AP in fully compliant users was 1:1943 (falciparum malaria cases per prescription). | 3 cases of falciparum malaria |
| van der Berg et al., 1999 [37] | 113 AP subjects included in efficacy analysis | Prophylaxis success 97% (95% CI = 92–99). | 0 cases of malaria as a results of withdrawal of 3 subjects due to AEs |
| Total subjects: 2866 (including the PP2 cohort and excluding the studies with data on AP prescriptions) | Total failures: 13 cases excluding vivax malaria cases |
AP, atovaquone-proguanil; AE(s), adverse event(s); HLC, human landing catches; ITT, intention to treat; PP1 is defined as being compliant and visiting for weekly blood smear; PP2 is defined as having adequate drug concentrations; CI, confidence interval; LL, lower limit.
Table 5.
Failure rates of the studies in support of a short post-travel regimen.
| Author | Study size | Efficacy | Failures |
|---|---|---|---|
| Deye et al., 2012 [42] | 6 subjects received AP 250/100 mg on d-1 4 subjects received AP 250/100 mg on d + 4 |
Subjects receiving AP 250/100 mg on d-1 and AP 250/100 mg on d+4 were 100% protected. | 0 |
| Shapiro et al., 1999 [11] | 6 subjects received 250 mg atovaquone | 250 mg of atovaquone protected all subjects | 0 |
| Lachish et al., 2016 [43] | 33 subjects received AP twice weekly | Malaria incidence in AP group was 0/391 person-months | 0 |
| Leshem et al., 2014 [20] | 421 subjects used a short-course of AP prophylaxis | None of the subjects reported malaria infection. | 0 |
| Petersen et al., 2003 [44] | 184 subjects filled in the questionnaires, of which only the 63 subjects of the 1/4 group are included in the total amount of subjects. | 44 subjects were total compliant, 37 took 3/4 pills, 29 took 2/4 pills, and 63 took 1/4 pills or fewer. 11 took none at all. No cases of falciparum malaria were recorded. | 0 falciparum malaria cases during 6 months, but 1 subject developed P. ovale malaria 4 months after return |
| Edstein et al., 2005 [12] | – | – | – |
| Polhemus et al., 2008 [45] | – | – | – |
| Shanks et al., 1999 [46] | – | – | – |
| Butcher et al., 2003 [47] | – | – | – |
| Butcher et al., 2000 [49] | – | – | – |
| Enosse et al., 2000 [48] | – | – | – |
| Total subjects: 533 | Total failures: 0 cases excluding ovale malaria cases |
AP, atovaquone-proguanil.
4.4. Additional data on alternative prophylactic regimens
Caution is warranted when considering alternative prophylactic regimens, as illustrated by the following data on use of AP for prophylaxis among those with malaria from 2006 to 2014. Malaria is a mandatorily reportable disease in the U.S. The National Malaria Surveillance System (NMSS) collects information on malaria cases, including type of prophylaxis taken and adherence. From 2006 to 2014, there were 354 malaria cases that reported taking AP for malaria prophylaxis. Of these, 176 had acute malaria, defined as onset < 45 days after arrival, and took AP exclusively for prophylaxis. Information on adherence was available for 153 out of 176. While 53 out of 153 (35%) took AP with good adherence, most (100 out of 153, 65%) missed doses. Of these, 90 patients had additional data on missed doses. Eighteen of the 90 patients (20%) reported stopping AP prematurely after returning home. All of these patients travelled to Africa (West Africa-10, East Africa-4, Central Africa-3, unspecified sub-Saharan African country-1) with a median trip duration of 21 days (range 9–300 days). Two of these patients had severe malaria. This strong evidence against a shortened regimen highlights the need for additional research before short post-travel AP regimens can be recommended for use in routine practice.
Caution against changing the current regimen is further supported by the key issue in AP absorption, namely the need for AP intake with a fatty meal, as not doing so may result in sub-therapeutic drug concentrations and ultimately a fatal outcome due to prophylactic failure. Atovaquone has a very low aqueous solubility and to ascertain absorption, it needs to be taken with fatty food, as the ingestion with food leads to a 5-fold increase in maximum plasma concentration, compared to ingestion with water alone [8]. Illustrated by the following case report [51], we aim to demonstrate that even full adherence to AP might result into prophylactic failure when one does not co-administer AP with a fatty meal. The patient took AP on an empty stomach, which resulted in sub-therapeutic concentrations of atovaquone and proguanil by 1000-fold and 100-fold, respectively. The problem described above might have contributed to some of the failures in the included studies. The reason we present this key issue is that in the case of travellers, altered activity pattern with irregular meals may result in the ingestion of AP while fasting, resulting in a decreased maximum concentration, and therefore the possibility of prophylactic failure.
Another reason for presenting this data is that the two randomized studies that observed AP being effective after a single dose might have been an over-estimation, because the studies were performed in controlled settings with observed intake of the pills during or following a meal [11,42]. In the absence of this meal, one might question whether the same results with respect to effectiveness would have been obtained, as the maximum concentrations might have been decreased, the latter being probably more frequently the case in travellers.
4.5. Rationale for the current antimalarial regimens
The different regimens for prophylactic agents depend on the stages of the malaria parasite being targeted by the active drug compounds, and so the current prophylactic regimens are based upon the pharmacodynamic properties of the antimalarial agents. Because AP exerts causal prophylactic activity, it can be discontinued seven days after return from a malaria-endemic area instead of one month in the case of antimalarials with only suppressive effects against blood stages of malaria, such as chloroquine, mefloquine, and doxycycline [3]. This is to assure the eradication of any parasites released from the liver in the following month due to the fact that merozoites are released from the liver after approximately 7–23 days [52].
4.6. Strengths and limitations
The methodological strengths of this systematic review include the all-encompassing search strategy and the non-specific inclusion criteria. The search strategy included additional terms related to pharmacokinetic properties of AP in order to identify articles that might have been missed when only focusing on the terms related to prophylaxis and half-life properties of AP. The non-specific inclusion criteria provided the possibility to identify relevant articles without overlooking studies that would not have met more specific criteria. Studies considered for inclusion either provided evidence in support of the current regimen or the alternative regimen, which our hypothesis is based on. This gave us the possibility to compare the evidence.
Several limitations have to be acknowledged. Regarding internal validity; first of all, no meta-analysis was possible in our systematic review due to the enormous heterogeneity in eligible study designs, outcome measurements and presentation of results. This heterogeneity in eligible studies also led to the omission of performing a risk-of-bias assessment, because the results could not be compared. Secondly, our search only focused on the combination of both atovaquone and proguanil, and only one article with focus on solely atovaquone has been included by citation, but when bearing in mind that it is only atovaquone that is responsible for the prolonged inhibitory effect of AP, an additional search with a specific focus on atovaquone should have been part of the search strategy.
Regarding the external validity, a limitation in determining the efficacy is the fact that almost all controlled studies were of small sample size, and were therefore not powered to evaluate the prophylactic efficacy. In addition, exposure regions varied in the included studies. Because the exposure was not uniform, one might question whether the efficacy of the included studies can be compared at all. Further limitations include the fact that the observational studies of Lachish and Leshem were performed in a region with limited risk of exposure and both studies lacked a control group, as did the study of Petersen and colleagues [20,43,44]. The lack of a control group is a major limitation in observational studies. The disadvantage of the randomized challenge studies is the limited intensity of exposure compared to the randomized studies performed in highly endemic regions [11,42]. The effects of the short reports reflect both drug effect and immunity, and might therefore lead to over-estimate the results [12,45,46]. Taking the collective limitations related to external validity into account, the results in support of an alternative post-travel regimen may have been an overestimation.
4.7. Methodological approaches in determining alternative regimens for malaria prophylaxis
In of reviewing studies regarding malaria prophylaxis in general, and the use of AP prophylaxis in particular, we acknowledge the limitations when putting alternative regimens of malaria prophylaxis to the test. The methodological approaches of Leshem and Lachish are bold efforts to evaluate alternative, shorter prophylactic regimens; however, the limited risk of exposure (not a methodological error due to poor choices but dependent on the travel destinations chosen by the study cohort; with few subjects destined for highly endemic malaria areas such as West and Central Africa) and the lack of a control group made the results inferior to those of randomized controlled studies in support of the current post-travel regimen of AP. The pharmaceutical industry may have limited interest in pursuing randomized controlled trials to determine the efficacy of alternative prophylactic regimens. Therefore, the randomized controlled studies with P. falciparum challenge as performed by Deye and Shapiro could be seen as a solution in dealing with those limitations. Both the limited risk of exposure and lack of a control group would be resolved. In our opinion, this less complex methodological study design could replace the current gold standard of randomized controlled studies within the travel medicine community when considering putting alternative prophylactic approaches to the test. This being said, observational studies with a solid methodological approach might also provide relevant data on alternative AP regimens when the study methodology is solid with numerous subject numbers. A suggestion when studying alternative regimens of AP would therefore be to pay additional attention to several shortcomings that potentially lead to biased results or results distorted by confounding factors.
5. Conclusion
The efficacy of a post-travel AP chemoprophylaxis regimen of seven days in fully compliant volunteers has undoubtedly been established by high quality studies. Stopping AP chemoprophylaxis on return from travel is an attractive proposition but data demonstrating continued protection are scarce and of limited quality.
We conclude that there is some limited direct and indirect evidence to support the possibility of an alternative post-travel regimen for AP. However, the total sample size of the studies to support this possibility of which studies with focus on discontinuation one day after return were part, comprises a small percentage of the total sample size of the studies performed to establish the efficacy of the current AP regimen. On top of that, the methodological quality of the studies performed with a seven day post-travel regimen outweighs the quality of the studies with evidence for alternative regimens. Abbreviated AP regimens require a closer look and additional research with numerous subject numbers is required to fully support the hypothesis of a short post-travel regimen of AP before a short post-travel regimen can be implemented in current practice.
Acknowledgements
We thank Dr. Benjamin Jelle Visser (AMC Tropencentrum) for his valuable input during the screening process and helping hand in performing the initial literature screening, and Drs. S. Patrick Kachur, Malaria Branch, and Barbara J. Marsten, Division of Parasitic Diseases and Malaria, CDC, Atlanta, USA, for their highly valuable comments on the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendix 1. Pubmed search strategy
| Number | Searches |
|---|---|
| #1 | Atovaquone[mesh] OR atovaquone[tiab] |
| #2 | Proguanil[mesh] OR proguanil[tiab] |
| #3 | Atovaquone, proguanil drug combination[Supplementary Concept] OR malarone[tiab] OR hydroxynaphthoquinone[tiab] OR Mepron [tiab] OR Wellvone[tiab] OR atovaquone/proguanil[tiab] OR Atovaquone and Proguanil[tiab] OR Atovaquone + proguanil[tiab] |
| #4 | Chemoprevention[Mesh:NoExp] OR prevention and control[Subheading] OR chemoprophylaxis[tiab] OR prophylaxis[tiab] OR chemoprevention[tiab] OR pharmacokinetics[Mesh] OR pharmacokinetics[Subheading] OR pharmacokinetics[tiab] OR Half-Life [Mesh] OR half-life*[tiab] OR halflife*[tiab] OR duration[tiab] OR area under curve[mesh] OR absorption[mesh] OR PK[tiab] OR PPK [tiab] OR tmax[tiab] OR cmax[tiab] OR AUC[tiab] OR “area under the curve”[tiab] OR clearance[tiab] OR elimination[tiab] OR “volume of distribution”[tiab] OR “drug level”[tiab] OR absorption[tiab] OR serum concentration[tiab] OR plasma concentration[tiab] |
| #5 | #1 AND #2 |
| #6 | #3 OR #5 |
| #7 | #4 AND #6 |
Appendix 2. Embase search strategy
| Number | Searches |
|---|---|
| #1 | exp atovaquone/ |
| #2 | atovaquone.ti,ab,kw,hw,tn. |
| #3 | 1 or 2 |
| #4 | exp proguanil/ |
| #5 | proguanil.ti,ab,kw,hw,tn. |
| #6 | 4 or 5 |
| #7 | 3 and 6 |
| #8 | exp atovaquone plus proguanil/ |
| #9 | (malarone or hydroxynaphthoquinone or Mepron or Wellvone or “atovaquone/proguanil” or (Atovaquone adj Proguanil) or “Atovaquone+proguanil”).ti,ab,kw. |
| #10 | 8 or 9 |
| #11 | 7 or 10 |
| #12 | exp chemoprophylaxis/or exp “prevention and control”/or exp pharmacokinetics/or exp half life time/or exp area under the curve/or exp absorption/or prevention.fs. or (pharmacokinetics or half-life* or halflife* or duration or PK or PPK or tmax or cmax or AUC or “area under the curve” or clearance or elimination or “volume of distribution” or “drug level” or absorption or ((serum or plasma) adj concentration)).ti,ab,kw. |
| #13 | 11 and 12 |
Appendix 3. PRISMA 2009 checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3–4 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 4 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 5 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 5–6 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 5 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 5, 42–43 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 5–6 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 6 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 6 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | NA |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | NA |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | NA |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | NA |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | NA |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 8, 41 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 27–38 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | NA |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | NA |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | NA |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | NA |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | NA |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 15–18, 39–41 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 19–20 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 20–22 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 22 |
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of interest
None of the authors has any conflict of interest to declare.
References
- [1].Freedman DO, Chen LH, Kozarsky PE. Medical considerations before international travel. N Engl J Med 2016;375:247–60 10.1056/NEJMra1508815. [DOI] [PubMed] [Google Scholar]
- [2].Bloechliger M, Schlagenhauf P, Toovey S, Schnetzler G, Tatt I, Tomianovic D, et al. Malaria chemoprophylaxis regimens: a descriptive drug utilization study. Trav Med Infect Dis 2014;12:718–25 10.1016/j.tmaid.2014.05.006. [DOI] [PubMed] [Google Scholar]
- [3].Kurt TL. Malaria prevention in short-term travelers. N Engl J Med 2008;359:2293. author reply −4 10.1056/NEJMc081858. [DOI] [PubMed] [Google Scholar]
- [4].Morgan GS, Chiodini P, Evans M. Relapsing malaria: two cases of malaria presenting 8 months after return from Africa despite adherence to antimalarial chemoprophylaxis. Br J Gen Pract 2012;62:555–6 10.3399/bjgp12X657017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gallien S, Taieb F, Schlemmer F, Lagrange-Xelot M, Atlan A, Sarfati C, et al. Failure of atovaquone/proguanil to prevent Plasmodium ovale malaria in traveler returning from Cameroon. Trav Med Infect Dis 2008;6:128–9 10.1016/j.tmaid.2008.01.011. [DOI] [PubMed] [Google Scholar]
- [6].Maguire JD, Llewellyn DM. Relapsing vivax malaria after 6 months of daily atovaquone/proguanil in Afghanistan: the case for expanded use of primaquine as a causal prophylactic. J Trav Med 2007;14:411–4 10.1111/j.1708-8305.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- [7].Povinelli L, Monson TA, Fox BC, Parise ME, Morrisey JM, Vaidya AB. Plasmodium vivax malaria in spite of atovaquone/proguanil (malarone) prophylaxis. J Trav Med 2003;10:353–5 10.2310/7060.2003.9318. [DOI] [PubMed] [Google Scholar]
- [8].Boggild AK, Parise ME, Lewis LS, Kain KC. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). Am J Trop Med Hyg 2007;76:208–23 10.4269/ajtmh.2007.76.208. [DOI] [PubMed] [Google Scholar]
- [9].Thapar MM, Gupta S, Spindler C, Wernsdorfer WH, Bjorkman A. Pharmacodynamic interactions among atovaquone, proguanil and cycloguanil against Plasmodium falciparum in vitro. Trans Roy Soc Trop Med Hyg 2003;97:331–7 10.1016/S0035-9203(03)90162-3. [DOI] [PubMed] [Google Scholar]
- [10].Berman JD, Nielsen R, Chulay JD, Dowler M, Kain KC, Kester KE, et al. Causal prophylactic efficacy of atovaquone-proguanil (Malarone) in a human challenge model. Trans Roy Soc Trop Med Hyg 2001;95:429–32 10.1016/S0035-9203(01)90206-8. [DOI] [PubMed] [Google Scholar]
- [11].Shapiro TA, Ranasinha CD, Kumar N, Barditch-Crovo P. Prophylactic activity of atovaquone against Plasmodium falciparum in humans. Am J Trop Med Hyg 1999;60:831–6 10.4269/ajtmh.1999.60.831. [DOI] [PubMed] [Google Scholar]
- [12].Edstein MD, Kotecka BM, Anderson KL, Pombo DJ, Kyle DE, Rieckmann KH, et al. Lengthy antimalarial activity of atovaquone in human plasma following atovaquone-proguanil administration. Antimicrob Agents Chemother 2005;49:4421–2 10.1128/AAC.49.10.4421-4422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Plucinski MM, Huber CS, Akinyi S, Dalton W, Eschete M, Grady K, et al. Novel mutation in cytochrome B of plasmodium falciparum in one of two atovaquone-proguanil treatment failures in travelers returning from same site in Nigeria. Open Forum Infect Dis 2014;1:ofu059. 10.1093/ofid/ofu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nixon GL, Moss DM, Shone AE, Lalloo DG, Fisher N, O’Neill PM, et al. Antimalarial pharmacology and therapeutics of atovaquone. J Antimicrob Chemother 2013;68:977–85 10.1093/jac/dks504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gebru T, Hailu A, Kremsner PG, Kun JF, Grobusch MP. Molecular surveillance of mutations in the cytochrome b gene of Plasmodium falciparum in Gabon and Ethiopia. Malar J 2006;5:112. 10.1186/1475-2875-5-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wichmann O, Muehlberger N, Jelinek T, Alifrangis M, Peyerl-Hoffmann G, Muhlen M, et al. Screening for mutations related to atovaquone/proguanil resistance in treatment failures and other imported isolates of Plasmodium falciparum in Europe. J Infect Dis 2004;190:1541–6 10.1086/424469. [DOI] [PubMed] [Google Scholar]
- [17].Schlagenhauf P, Tschopp A, Johnson R, Nothdurft HD, Beck B, Schwartz E, et al. Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. Br Med J 2003;327:1078. 10.1136/bmj.327.7423.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hogh B, Clarke PD, Camus D, Nothdurft HD, Overbosch D, Gunther M, et al. Atovaquone-proguanil versus chloroquine-proguanil for malaria prophylaxis in non-immune travellers: a randomised, double-blind study. Malarone International Study Team. Lancet 2000;356:1888–94 10.1016/S0140-6736(00)03260-8. [DOI] [PubMed] [Google Scholar]
- [19].Grobusch MP. Malaria chemoprophylaxis with atovaquone-proguanil: is a shorter regimen fully protective? J Trav Med 2014;21:79–81 10.1111/jtm.12100. [DOI] [PubMed] [Google Scholar]
- [20].Leshem E, Meltzer E, Stienlauf S, Kopel E, Schwartz E. Effectiveness of short prophylactic course of atovaquone-proguanil in travelers to sub-saharan Africa. J Trav Med 2014;21:82–5 10.1111/jtm.12088. [DOI] [PubMed] [Google Scholar]
- [21].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J 2009;339:b2535. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Contr Clin Trials 1996;17:1–12 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- [23].Joanna Briggs Institute reviewers’ manual. 2016 edition Australia: The Joanna Briggs Institute; 2016. [Google Scholar]
- [24].Soto J, Toledo J, Luzz M, Gutierrez P, Berman J, Duparc S. Randomized, double-blind, placebo-controlled study of Malarone for malaria prophylaxis in non-immune Colombian soldiers. Am J Trop Med Hyg 2006;75:430–3 10.4269/ajtmh.2006.75.430. [DOI] [PubMed] [Google Scholar]
- [25].Ling J, Baird JK, Fryauff DJ, Sismadi P, Bangs MJ, Lacy M, et al. Randomized, placebo-controlled trial of atovaquone/proguanil for the prevention of Plasmodium falciparum or Plasmodium vivax malaria among migrants to Papua, Indonesia. Clin Infect Dis 2002;35:825–33 10.1086/342578. [DOI] [PubMed] [Google Scholar]
- [26].Faucher JF, Binder R, Missinou MA, Matsiegui PB, Gruss H, Neubauer R, et al. Efficacy of atovaquone/proguanil for malaria prophylaxis in children and its effect on the immunogenicity of live oral typhoid and cholera vaccines. Clin Infect Dis 2002;35:1147–54 10.1086/342908. [DOI] [PubMed] [Google Scholar]
- [27].Sukwa TY, Mulenga M, Chisdaka N, Roskell NS, Scott TR. A randomized, double-blind, placebo-controlled field trial to determine the efficacy and safety of Malarone (atovaquone/proguanil) for the prophylaxis of malaria in Zambia. Am J Trop Med Hyg 1999;60:521–5 10.4269/ajtmh.1999.60.521. [DOI] [PubMed] [Google Scholar]
- [28].Shanks GD, Gordon DM, Klotz FW, Aleman GM, Oloo AJ, Sadie D, et al. Efficacy and safety of atovaquone/proguanil as suppressive prophylaxis for Plasmodium falciparum malaria. Clin Infect Dis 1998;27:494–9 10.1086/514710. [DOI] [PubMed] [Google Scholar]
- [29].Lell B, Luckner D, Ndjave M, Scott T, Kremsner PG. Randomised placebo-controlled study of atovaquone plus proguanil for malaria prophylaxis in children. Lancet 1998;351:709–13 10.1016/s0140-6736(97)09222-2. [DOI] [PubMed] [Google Scholar]
- [30].Overbosch D, Schilthuis H, Bienzle U, Behrens RH, Kain KC, Clarke PD, et al. Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clin Infect Dis 2001;33:1015–21 10.1086/322694. [DOI] [PubMed] [Google Scholar]
- [31].Sulyok M, Ruckle T, Roth A, Murbeth RE, Chalon S, Kerr N, et al. DSM265 for Plasmodium falciparum chemoprophylaxis: a randomised, double blinded, phase 1 trial with controlled human malaria infection. Lancet Infect Dis 2017;17:636–44 10.1016/s1473-3099(17)30139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Camus D, Djossou F, Schilthuis HJ, Hogh B, Dutoit E, Malvy D, et al. Atovaquone-proguanil versus chloroquine-proguanil for malaria prophylaxis in nonimmune pediatric travelers: results of an international, randomized, open-label study. Clin Infect Dis 2004;38:1716–23 10.1086/421086. [DOI] [PubMed] [Google Scholar]
- [33].Zuckerman JN, Batty AJ, Jones ME. Effectiveness of malaria chemoprophylaxis against Plasmodium falciparum infection in UK travellers: retrospective observational data. Trav Med Infect Dis 2009;7:329–36 10.1016/j.tmaid.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [34].Andersson H, Askling HH, Falck B, Rombo L. Well-tolerated chemoprophylaxis uniformly prevented Swedish soldiers from Plasmodium falciparum malaria in Liberia, 2004–2006. Mil Med 2008;173:1194–8 10.7205/MILMED.173.12.1194. [DOI] [PubMed] [Google Scholar]
- [35].Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Ombok M, et al. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am J Trop Med Hyg 2013;88:301–8 10.4269/ajtmh.2012.12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van Genderen PJ, Koene HR, Spong K, Overbosch D. The safety and tolerance of atovaquone/proguanil for the long-term prophylaxis of plasmodium falciparum malaria in non-immune travelers and expatriates [corrected]. J Trav Med 2007;14:92–5 10.1111/j.1708-8305.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- [37].van der Berg JD, Duvenage CS, Roskell NS, Scott TR. Safety and efficacy of atovaquone and proguanil hydrochloride for the prophylaxis of Plasmodium falciparum malaria in South Africa. Clin Ther 1999;21:741–9 10.1016/S0149-2918(00)88325-3. [DOI] [PubMed] [Google Scholar]
- [38].Kofoed K, Petersen E. The efficacy of chemoprophylaxis against malaria with chloroquine plus proguanil, mefloquine, and atovaquone plus proguanil in travelers from Denmark. J Trav Med 2003;10:150–4 10.2310/7060.2003.35746. [DOI] [PubMed] [Google Scholar]
- [39].Kato T, Okuda J, Ide D, Amano K, Takei Y, Yamaguchi Y. Questionnaire-based analysis of atovaquone-proguanil compared with mefloquine in the chemoprophylaxis of malaria in non-immune Japanese travelers. J Infect Chemother 2013;19:20–3 10.1007/s10156-012-0446-z. [DOI] [PubMed] [Google Scholar]
- [40].Mavrogordato A, Lever AM. A cluster of Plasmodium vivax malaria in an expedition group to Ethiopia: prophylactic efficacy of atovaquone/proguanil on liver stages of P. vivax. J Infect 2012;65:269–74 10.1016/j.jinf.2012.04.015. [DOI] [PubMed] [Google Scholar]
- [41].Abraha I, Cherubini A, Cozzolino F, De Florio R, Luchetta ML, Rimland JM, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study. BMJ 2015;350 10.1136/bmj.h2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Deye GA, Miller RS, Miller L, Salas CJ, Tosh D, Macareo L, et al. Prolonged protection provided by a single dose of atovaquone-proguanil for the chemoprophylaxis of Plasmodium falciparum malaria in a human challenge model. Clin Infect Dis 2012;54:232–9 10.1093/cid/cir770. [DOI] [PubMed] [Google Scholar]
- [43].Lachish T, Bar-Meir M, Eisenberg N, Schwartz E. Effectiveness of twice a week prophylaxis with atovaquone-proguanil (Malarone(R)) in long-term travellers to West Africa. J Trav Med 2016;23 10.1093/jtm/taw064. [DOI] [PubMed] [Google Scholar]
- [44].Petersen E The safety of atovaquone/proguanil in long-term malaria prophylaxis of nonimmune adults. J Trav Med 2003;10(Suppl 1):S13–5. discussion S21 10.2310/7060.2003.35050. [DOI] [PubMed] [Google Scholar]
- [45].Polhemus ME, Remich S, Ogutu B, Waitumbi J, Lievens M, Ballou WR, et al. Malaria treatment with atovaquone-proguanil in malaria-immune adults: implications for malaria intervention trials and for pre-exposure prophylaxis of malaria. Antimicrob Agents Chemother 2008;52:1493–5 10.1128/AAC.01367-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shanks GD, Ragama BO, Oloo AJ. Time to reappearance of malaria parasites following various drug treatment regimens in a holoendemic area of western Kenya. Trans Roy Soc Trop Med Hyg 1999;93:304–5 10.1016/S0035-9203(99)90031-7. [DOI] [PubMed] [Google Scholar]
- [47].Butcher GA, Sinden RE. Persistence of atovaquone in human sera following treatment: inhibition of Plasmodium falciparum development in vivo and in vitro. Am J Trop Med Hyg 2003;68:111–4 10.4269/ajtmh.2003.68.111. [DOI] [PubMed] [Google Scholar]
- [48].Enosse S, Butcher GA, Margos G, Mendoza J, Sinden RE, Hogh B. The mosquito transmission of malaria: the effects of atovaquone-proguanil (Malarone) and chloroquine. Trans Roy Soc Trop Med Hyg 2000;94:77–82 10.1016/S0035-9203(00)90447-4. [DOI] [PubMed] [Google Scholar]
- [49].Butcher GA, Mendoza J, Sinden RE. Inhibition of the mosquito transmission of Plasmodium berghei by Malarone (atovaquone-proguanil). Ann Trop Med Parasitol 2000;94:429–36 10.1080/00034983.2000.11813561. [DOI] [PubMed] [Google Scholar]
- [50].Nakato H, Vivancos R, Hunter PR. A systematic review and meta-analysis of the effectiveness and safety of atovaquone proguanil (Malarone) for chemoprophylaxis against malaria. J Antimicrob Chemother 2007;60:929–36 10.1093/jac/dkm337. [DOI] [PubMed] [Google Scholar]
- [51].Boggild AK, Lau R, Reynaud D, Kain KC, Gerson M. Failure of atovaquone-proguanil malaria chemoprophylaxis in a traveler to Ghana. Trav Med Infect Dis 2015;13:89–93 10.1016/j.tmaid.2014.12.010. [DOI] [PubMed] [Google Scholar]
- [52].DiTusa C, Kozar MP, Pybus B, Sousa J, Berman J, Gettayacamin M, et al. Causal prophylactic efficacy of primaquine, tafenoquine, and atovaquone-proguanil against Plasmodium cynomolgi in a rhesus monkey model. J Parasitol 2014;100:671–3 10.1645/13-480.1. [DOI] [PubMed] [Google Scholar]
- [53].Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 2002;360:610–7 10.1016/s0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- [54].Polhemus ME, Remich SA, Ogutu BR, Waitumbi JN, Otieno L, Apollo S, et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLos One 2009;4:e6465. 10.1371/journal.pone.0006465. [DOI] [PMC free article] [PubMed] [Google Scholar]


