Obesity is a risk factor for hypertension and cardiovascular disease. In SURMOUNT-1, tirzepatide, the glucose-dependent insulinotropic polypeptide/GLP-1 (glucagon-like peptide-1) receptor agonist approved in the United States for treatment of type 2 diabetes and obesity, provided substantial weight loss and reduced office blood pressure (BP).1 This study assessed the effect of tirzepatide on 24-hour BP, measured by ambulatory BP monitoring (ABPM), in people with the disease of obesity but without type 2 diabetes.
SURMOUNT-1 (N=2539; NCT04184622: https://clinicaltrials.gov/study/NCT04184622) was a randomized, placebo-controlled trial investigating the effects of once-weekly tirzepatide (5, 10, and 15 mg) in adults with a body mass index (BMI) ≥27 kg/m2.1 A subset of participants underwent 24-hour ABPM at baseline and week 36 as part of a prospectively planned substudy. Key inclusion criteria for the substudy were BP <140/90 mm Hg and stable (≥3 months) antihypertensive therapy, if used. BP was measured every 30 minutes during daytime (07:00–22:00 hours) and every 60 minutes during nighttime (22:00–07:00 hours) over a 24- to 27-hour period using an Oscar 2 Monitor (SunTech Medical). Only participants with ≥70% valid readings on ABPM and a minimum of 20 daytime and 7 nighttime readings were included in the analyses.2
Mixed model repeated measures were used to compare changes in ABPM parameters from baseline to week 36 between tirzepatide and placebo with an unstructured covariance matrix.
The substudy enrolled 600 participants (155 placebo, 145 tirzepatide 5 mg, 152 tirzepatide 10 mg, and 148 tirzepatide 15 mg); 68.7% female, 66.8% White, and 25.0% Hispanic. Mean (SD) age was 45.5 (12.9) years, and BMI was 37.4 (6.8) kg/m2; 30.0% of participants reported hypertension at baseline, and 29.0% reported the use of ≥1 antihypertensive medication. Overall, 494 participants had valid ABPM data at baseline and week 36.
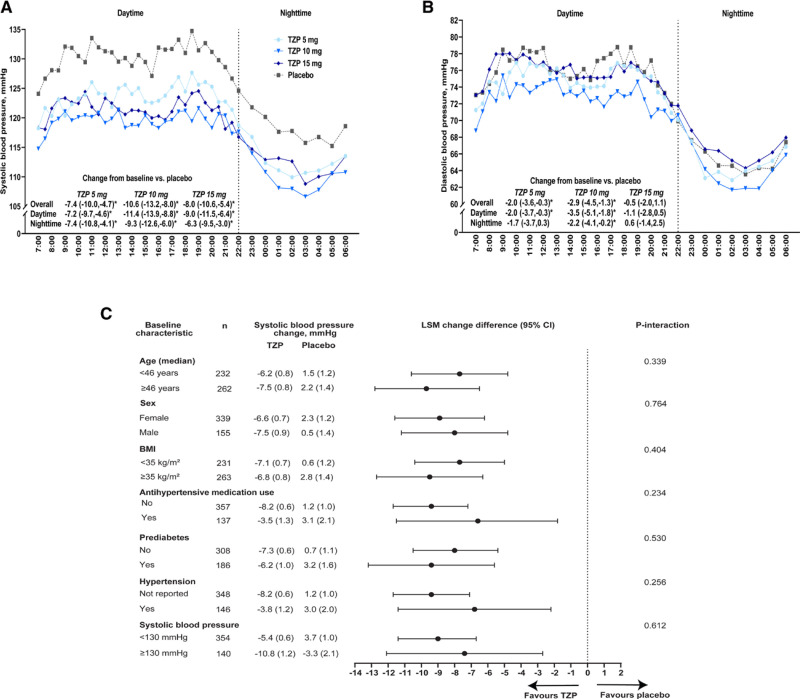
The baseline mean (SD) 24-hour systolic BP was 124.6 (10.4) mm Hg, with no significant between-group differences. Treatment with each tirzepatide dose reduced 24-hour systolic BP at 36 weeks compared with placebo. The placebo-adjusted systolic BP change from baseline was −7.4 (95% CI, −10.0 to −4.7) mm Hg for 5-mg tirzepatide, −10.6 (95% CI, −13.2 to −8.0) mm Hg for 10-mg tirzepatide, and −8.0 (95% CI, −10.6 to −5.4) mm Hg for 15-mg tirzepatide (Figure [A]). Results were consistent for both day and nighttime BP, with significant reductions versus placebo for each tirzepatide dose (Figure [A]). There were no significant interactions by baseline age, sex, BMI, systolic BP, the presence of hypertension, antihypertensive medication use, or glycemic status (prediabetes: yes/no) for the change in 24-hour mean systolic BP (Figure [C]). For pooled tirzepatide doses, change in 24-hour systolic BP correlated with change in body weight (r=0.31; P<0.0001). Mediation analyses indicated that changes in ambulatory systolic BP were partially mediated by weight changes (percentage mediated, 70.0% [95% CI, 47.0–102.6]).
Figure.
24-hour ambulatory systolic and diastolic blood pressure at week 36 among SURMOUNT-1 participants. Mean 24-hour ambulatory systolic (A) and diastolic (B) blood pressure with least squares mean (LSM; 95% CI) change difference from baseline vs placebo and (C) 24-hour ambulatory systolic blood pressure LSM (SE) and LSM difference (95% CI) between pooled tirzepatide (TZP) doses and placebo among subgroups at week 36. BMI indicates body mass index. n indicates the number of participants in the subgroup with baseline and week 36 value. *P<0.05 vs placebo.
At baseline, the mean (SD) overall 24-hour diastolic BP from ABPM was 72.1 (7.7) mm Hg, with no between-group differences. At week 36, 24-hour diastolic BP decreased from baseline versus placebo in participants who received tirzepatide 5 mg (−2.0 [95% CI, −3.6 to −0.3] mm Hg) and 10 mg (−2.9 [95% CI, −4.5 to −1.3] mm Hg) but not 15 mg (−0.5 [95% CI, −2.0 to 1.1] mm Hg; Figure [B]).
The mean 24-hour heart rate was 77.4 (8.7) beats per minute at baseline and did not differ between treatment groups. At week 36, heart rate increased with tirzepatide versus placebo by 2.1 (95% CI, 0.3–3.9), 2.3 (95% CI, 0.6–4.1), and 5.4 (95% CI, 3.6–7.1) beats per minute, respectively, with tirzepatide 5, 10, and 15 mg.
This study demonstrated the BP-lowering effects of tirzepatide in people with BMI ≥27 kg/m2, during both daytime and nighttime. Reductions in 24-hour systolic BP were consistent across subgroups of participants stratified by clinically relevant factors, including age, sex, BMI, and hypertension-related factors. This study demonstrates that tirzepatide improves 24-hour BP in obesity-related hypertension. While being consistent with in-office measurement,1 the current study uses a method that is superior to office BP alone for predicting cardiovascular risk.3 Furthermore, nighttime systolic BP, which is a stronger predictor for cardiovascular death and all-cause death than daytime and 24-hour systolic BP, was also significantly reduced by tirzepatide.3 Correlation and mediation analyses indicated that tirzepatide-induced body weight reduction effects were associated with BP reductions, but tirzepatide, as a GLP-1/glucose-dependent insulinotropic polypeptide receptor agonist, may also have effects on BP independent of weight loss.
An increase in the heart rate was observed, as expected from GLP-1 receptor agonist studies in people living with obesity.4,5 In SURMOUNT-1, after 72 weeks, changes in pulse from baseline were 0.6, 2.3, and 2.6 beats per minute in tirzepatide 5-, 10-, and 15-mg groups, respectively, compared with 0.1 beats per minute in the placebo group.1 This suggests that the heart rate increase may attenuate with continued treatment over a longer time frame than this 36-week data.
Strengths of this study include the use of 24-hour ABPM, providing a more comprehensive assessment of trends than in-office BP measurements. Limitations include that ABPM was only conducted in a subset of the SURMOUNT-1 population. Additionally, BP was only measured at baseline and 1 other time point, and measurements were only taken once per hour at night to minimize participant burden. Changes in food intake and 24-hour urine sodium excretion were not assessed. Thus, the contribution of dietary modifications, including salt intake, to BP changes cannot be estimated.
These data provide further evidence for the potential benefits of tirzepatide on cardiometabolic health and cardiovascular outcomes.
ARTICLE INFORMATION
Acknowledgments
Eli Lilly and Company and the authors would like to thank the clinical trial participants and their caregivers, without whom this work would not be possible.
Sources of Funding
The study and this work were supported by Eli Lilly and Company.
Disclosures
J.A. de Lemos reports participation on a data safety monitoring board or advisory board for Eli Lilly and Company, Novo Nordisk, AstraZeneca, and Amgen and travel support from Eli Lilly and Company. B. Linetzky, L. Fan, A. Hemmingway, N.N. Ahmad, M.C. Bunck, and A. Stefanski are employees and shareholders of Eli Lilly and Company. C.W. le Roux reports grants from the Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. C.W. le Roux serves on advisory boards and speakers panels of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly and Company, Johnson & Johnson, Glia, Irish Life Health, and Boehringer Ingelheim, Currax, Zealand Pharma, and Rhythm Pharma. C.W. le Roux is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. C.W. le Roux was the chief medical officer and the director of the Medical Device Division of Keyron in 2021; both of these are unremunerated positions. C.W. le Roux was a previous investor in Keyron that develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. No patients have been included in any of Keyron’s studies, and they are not listed on the stock market. C.W. le Roux was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September 2021. C.W. le Roux continues to provide scientific advice to Keyron for no remuneration. C.W. le Roux provides obesity clinical care in the Beyond BMI clinic and is a shareholder in the clinic. L.J. Laffin reports grants or contracts from AstraZeneca, Mineralys Therapeutics, and Arrowhead Pharmaceuticals; royalties or licenses from Elsevier and Belvoir Media Group; consulting fees from Medtronic and Eli Lilly and Company; payment or honoraria from the American Heart Association and Cardiometabolic Health Congress; participation on a data safety monitoring board or advisory board for CRISPR Therapeutics; and stock or stock options in Gordy Health and LucidAct Health. W. Vongpatanasin reports no conflict of interest.
Nonstandard abbreviations and acronyms
- ABPM
- ambulatory blood pressure monitoring
- BMI
- body mass index
- BP
- blood pressure
- GLP-1
- glucagon-like peptide-1
For Sources of Funding and Disclosures, see page e43.
REFERENCES
- 1.Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, et al. ; SURMOUNT-1 Investigators. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–216. doi: 10.1056/NEJMoa2206038 [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35–e66. doi: 10.1161/HYP.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staplin N, de la Sierra A, Ruilope LM, Emberson JR, Vinyoles E, Gorostidi M, Ruiz-Hurtado G, Segura J, Baigent C, Williams B. Relationship between clinic and ambulatory blood pressure and mortality: an observational cohort study in 59 124 patients. Lancet. 2023;401:2041–2050. doi: 10.1016/S0140-6736(23)00733-X [DOI] [PubMed] [Google Scholar]
- 4.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, et al. ; STEP 1 Study Group. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 5.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DCW, le Roux CW, Violante Ortiz R, Jensen CB, et al. ; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]