Abstract
Purpose –
Growth hormone receptor knockout (GHR-KO) pigs have recently been developed, which serve as a large animal model of Laron syndrome (LS). GHR-KO pigs, like individuals with LS, are obese but lack some comorbidities of obesity. The purpose of this study was to examine the histological and transcriptomic phenotype of adipose tissue (AT) in GHR-KO pigs and humans with LS.
Methods –
Intraabdominal (IA) and subcutaneous (SubQ) AT was collected from GHR-KO pigs and examined histologically for adipocyte size and collagen content. RNA was isolated and cDNA sequenced, and the results were analyzed to determine differentially expressed genes that were used for enrichment and pathway analysis in pig samples. For comparison, we also performed limited analyses on human AT collected from a single individual with and without LS.
Results –
GHR-KO pigs have increased adipocyte size, while the LS AT had a trend towards an increase. Transcriptome analysis revealed 55 differentially expressed genes present in both depots of pig GHR-KO AT. Many significant terms in the enrichment analysis of the SubQ depot were associated with metabolism, while in the IA depot, IGF and longevity pathways were negatively enriched. In pathway analysis, multiple expected and novel pathways were significantly affected by genotype, i.e. KO vs controls. When GH related gene expression was analyzed, SOCS3 and CISH showed species-specific changes.
Conclusion –
AT of GHR-KO pigs has several similarities to that of humans with LS in terms of adipocyte size and gene expression profile that help describe the depot-specific adipose phenotype of both groups.
Keywords: Growth Hormone, Transcriptome, Laron Syndrome, Pig, Adipose
Introduction
Growth hormone (GH) insensitivity, also referred to as Laron syndrome (LS) in humans, is a condition characterized by decreased sensitivity to GH, leading to decreased circulating insulin-like growth factor 1 (IGF1) levels resulting in increased GH levels [1]. While a mouse model of LS (Ghr−/−) was developed over 20 years ago and is extremely well characterized [2, 3], models in higher mammals have been lacking until the GHR-deficient (GHR-KO) pig was recently developed [4].
GH is notable for its metabolic effects that contribute to its ability to promote growth. A major site of GH’s metabolic effects is adipose tissue (AT), where GH promotes lipolysis and inhibits lipogenesis. Accordingly, in LS and Ghr−/− mice, obesity is a prominent phenotype [5-8], although the metabolic dysfunction that typically accompanies an obese state is not present. That is, individuals with LS are resistant to developing cancer [9-12]. Further, Ghr−/− mice have increased insulin sensitivity, reduced tumor incidence [13] and dramatically increased lifespan [14]. Humans with LS and Ghr−/− mice also have increased circulating adiponectin, a beneficial adipokine [15, 16]. Depot differences have been reported, with increased AT mass and adipocyte cell size specifically in the inguinal subcutaneous (SubQ) depot in mice [17] and with greater regional fat deposition in the arms of patients with LS [7]. Ghr−/− mouse AT also has decreased markers of senescence [18], does not develop inflammation [19], and improves glucose metabolism when transplanted into normal mice [20]. Thus, the metabolic dysfunction that often accompanies increased AT mass is not found in either LS or Ghr−/− mice, making the study of this tissue of high interest.
GHR-KO pigs have some phenotypic similarities to Ghr−/− mice, with decreased body length and weight and increased body fat, but, as a recently developed model, they have not been extensively characterized. For example, to date, the AT phenotype of GHR-KO pigs has not been investigated beyond mass measurement. Therefore, in the current study, we measured the cell size and examined the transcriptome in the SubQ and intraabdominal (IA) depots of GHR-KO pig AT. In addition, we performed limited analysis on fat biopsies from an individual with LS and a matched control to determine if GH resistance/insensitivity alters AT similarly in both species.
Materials and methods
Human AT biopsy
SubQ and visceral AT were collected from a female patient with LS undergoing sleeve bariatric surgery. Another female patient, undergoing the same procedure and with tissues collected from the same surgeon, was used as a control. Patients had similar glucose and insulin levels and BMI (Table 1). Samples of SubQ AT were taken from the lower abdominal wall, and visceral AT was extracted from the greater omentum during the surgery. For histological analysis, samples were fixed in 4% neutral buffered formalin for 24 hours followed by paraffin embedding. For RNA sequencing, samples were flash frozen and stored at −80°C until further processing. All human experiments were approved by the IRBs of the Schneider Children’s and Rabin Medical Centers and Ohio University.
Table 1.
Patient characteristics
| Age | Height cm |
Weight kg |
BMI | Glucose mg/dl |
Insulin μU/ml |
|
|---|---|---|---|---|---|---|
| LS Pt | 29 | 138 | 82.7 | 43.3 | 104 | 10 |
| Ctr | 46 | 164 | 104.4 | 39.5 | 107 | 15 |
Pig Necropsy
GHR-KO pigs were generated as described previously [4]. Briefly, CRISPR/Cas technology was used to introduce a frameshift mutation in exon 3 of the GHR gene, generating pigs lacking functional GHR. GHR-KO pigs were euthanized at 7-8 months of age under anesthesia by IV injection of T61. SubQ AT was sampled from the back and IA adipose was sampled from the perirenal depot, as previously described [21]. For histological analysis, samples from females were fixed in 4% neutral buffered formalin for 24 hours followed by paraffin embedding. For RNA sequencing, samples from males and females were flash frozen and stored at −80°C.
Histology and cell sizing, and Picrosirius Red Staining
Paraffin-embedded adipose samples (females only, Control vs. GHR-KO pigs n=4 each) were sliced into 5-μm sections and stained with H&E (hematoxylin and eosin). Slides were observed at 200x magnification on a Nikon Eclipse E600 microscope and images were captured using a Nikon DS-Fi1 camera. Ten non-overlapping fields were used to determine mean adipocyte size for each sample, as described previously [22, 23]. For each group, an average was calculated using the mean adipocyte size, and a two-way ANOVA was used to determine significance. For fibrosis measurement, 5-μm sections were stained with picrosirius red (PSR) and imaged as above. Images were blinded and cropped to remove non-tissue areas before analysis using FIJI 2.2.0 with the Quantified Stained Liver Tissue macro [24].
RNA isolation and sequencing
RNA was isolated from frozen AT (n=3 per genotype per sex) using a QIAGEN RNeasy lipid tissue mini kit according to manufacturer’s instructions. Library prep was carried out using a Takara-Clontech Smarter kit, and samples were sequenced on an Illumina NovaSeq S4 (2x150) with a target of 30 million reads per library. Basecalls and demultiplexing were performed with Illumina’s bcl2fastq software and a custom python demultiplexing program with a maximum of one mismatch in the indexing read. RNA-seq reads were then aligned to the Ensembl release 100 primary assembly with STAR version 2.7.5a [25]. Gene counts were derived from the number of uniquely aligned unambiguous reads by Subread:featureCount version 2.2.6 [26]. Sequencing performance was assessed for the total number of aligned reads, total number of uniquely aligned reads, and features detected. The ribosomal fraction, known junction saturation, and read distribution over known gene models were quantified with RSeQC version 3.0.1 [27].
All gene counts were then imported into the R/Bioconductor package edgeR [28], and TMM normalization size factors were calculated to adjust for samples for differences in library size. Ribosomal genes and genes that were not expressed greater than one count-per-million in at least two samples were excluded from further analysis. The TMM size factors and the matrix of counts were then imported into the R/Bioconductor package Limma [29]. Weighted likelihoods based on the observed mean-variance relationship of every gene and sample were then calculated for all samples with the voomWithQualityWeights [29]. The performance of all genes was assessed with plots of the residual standard deviation of every gene to their average log-count with a robustly fitted trend line of the residuals. Differential expression analysis was then performed to assess differences between conditions, and the results were filtered for only those genes with Benjamini-Hochberg false-discovery rate adjusted p-values less than or equal to 0.05.
Because the human samples had n=1, no differential expression analysis was possible. The results from the human sample RNA sequencing are thus reported as cpm (counts per million) values.
Downstream analyses
To examine global gene expression between samples, PCA plots were generated using edgeR and limma. To filter out genes with that were universally not expressed, genes with cpm values >0.5 in fewer than 4 samples were excluded. Plots were graphed using ggplot2 [30]. Venn diagrams and heatmaps of differentially expressed genes were generated using VennDiagram [31] and ComplexHeatmap [32], respectively.
Enrichment analysis was performed with ClusterProfiler [33] using the GSEA (gene set enrichment analysis) method on the GO (gene ontology) and KEGG pathway databases. Pathway analysis was performed using Ingenuity Pathway Analysis on the differentially expressed genes (FDR<0.05, log2 fold change >1.5).
Results
Cell Size and Tissue Fibrosis
The mean cell area of GHR-KO female pig SubQ and IA AT was compared to WT female controls (n=4 each). GHR-KO females had increased mean cell area (Figure 2A, 2-way ANOVA genotype p=0.013), while there was no significant effect of depot (p=0.76) or interaction (p=0.93). Although conducted with a single replicate, the human fat tissue (Figure 2B) showed a similar trend, with a higher mean cell area in both depots compared to controls. A histogram of the frequency of cell sizes in each tissue also showed a similar trend between the pig (Figure 2D) and human (Figure 2E) samples, with the GHR-KO/LS samples having a wider distribution of cell sizes compared to their respective controls. Due to the low sample number of human samples (n=1), the following analyses were performed on pig samples only, unless stated otherwise. Fibrosis in the pig tissue was analyzed using PSR staining. There was no significant effect of genotype on the amount of fibrosis in the GHR-KO AT, but the SubQ had significantly more fibrosis than the IA, regardless of genotype (Figure 2C).
Figure 2.
Histology results. A. Average adipocyte area of pig samples, n=2-4. B. Average adipocyte cell area of human samples, n=1. C. Picrosirius red staining quantification of adipose sample from pigs, n=3-4. D. Frequency Distribution (histogram) of adipocyte cell area showing how the cell area data from Fig 2A is distributed. E. Frequency Distribution (histogram) of adipocyte cell area showing how the cell area data from Fig 2B is distributed. 2 way ANOVA p values are shown on Fig 2A and Fig 2C.
RNA Sequencing
Genotype comparison (within depot)
Genotype comparisons (pig GHR-KO vs WT) were made within each depot (n=3 per sex per genotype), generating pairwise comparisons of GHR-KO SubQ vs. WT SubQ and GHR-KO IA vs. WT IA. A principal component analysis of global gene expression in each depot (Figure 3A-B) did not show a separation between the genotypes. In terms of the differentially expressed genes (DEGs), within the SubQ depot there were 266 DEGs (log2 fold change >1.5 and adjusted p value <0.05; Top 20 most significant listed in Table 2A-B) while in the IA there were 52 DEGs (Figure 3C; Top 20 most significant listed in Table 3A-B.). There were 55 genes differentially expressed in both depots, and none of them had opposing changes, meaning that the genes were either upregulated in both depots or downregulated in both depots (Figure 3D). Due to the nature of the genetic manipulation, GHR gene expression was not significantly decreased in the GHR-KO pigs, but the frameshift mutation in these animals was detectable in the RNAseq data.
Figure 3.
Pig Genotype Comparison. A. Principal components analysis of the pig SubQ, showing the global gene expression. B. Principal components analysis of the pig IA, showing the global gene expression. C. Differential gene expression results of the genotype comparison, showing the number of significant DEGs (FDR<0.05, absolute log2 fold change >1.5) in each depot alone and present in both depots. D. Heatmap of the 55 DEGs present in both depots, showing the log2 fold change of each gene in Ghr−/− pigs compared to controls. n= 3 per group.
Table 2A.
Top 20 most significant upregulated genes in SubQ
| Ensembl ID | Gene Symbol | Biotype | Description | logFC | adj.P.Val |
|---|---|---|---|---|---|
| ENSSSCG00000008097 | TTL | protein coding | tubulin tyrosine ligase | 1.528246 | 6.12E-04 |
| ENSSSCG00000036201 | NPR3 | protein coding | natriuretic peptide receptor 3 | 1.944644 | 9.10E-04 |
| ENSSSCG00000051234 | lncRNA | 2.489461 | 1.09E-03 | ||
| ENSSSCG00000022258 | LOC100512873 | protein coding | antileukoproteinase | 3.021375 | 1.15E-03 |
| ENSSSCG00000043622 | protein coding | novel gene | 1.788859 | 4.29E-03 | |
| ENSSSCG00000036724 | CRYAB | protein coding | crystallin alpha B | 2.086655 | 4.29E-03 |
| ENSSSCG00000038429 | lncRNA | 2.155715 | 4.29E-03 | ||
| ENSSSCG00000002297 | RDH12 | protein coding | retinol dehydrogenase 12 | 2.534244 | 4.29E-03 |
| ENSSSCG00000003854 | ECHDC2 | protein coding | enoyl-CoA hydratase domain containing 2 | 1.287019 | 5.10E-03 |
| ENSSSCG00000022945 | UCHL1 | protein coding | ubiquitin C-terminal hydrolase L1 | 3.913681 | 6.50E-03 |
| ENSSSCG00000016877 | NNT | protein coding | nicotinamide nucleotide transhydrogenase | 1.095242 | 6.90E-03 |
| ENSSSCG00000008617 | FAM49A | protein coding | family with sequence similarity 49 member A | 1.161917 | 7.88E-03 |
| ENSSSCG00000007748 | PSPH | protein coding | phosphoserine phosphatase | 1.399001 | 7.88E-03 |
| ENSSSCG00000010471 | KIF11 | protein coding | kinesin family member 11 | 1.714148 | 7.88E-03 |
| ENSSSCG00000010814 | ESRRG | protein coding | estrogen related receptor gamma | 1.777834 | 7.88E-03 |
| ENSSSCG00000015023 | ALG9 | protein coding | ALG9 alpha-1,2-mannosyltransferase | 1.826432 | 7.88E-03 |
| ENSSSCG00000012693 | CT55 | protein coding | cancer/testis antigen 55 | 2.385056 | 8.14E-03 |
| ENSSSCG00000003402 | PGD | protein coding | phosphogluconate dehydrogenase | 1.705047 | 8.85E-03 |
| ENSSSCG00000008284 | LOC100513982 | protein coding | retinol dehydrogenase 12-like | 2.621829 | 8.85E-03 |
| ENSSSCG00000043880 | protein coding | novel gene | 1.350474 | 8.90E-03 |
Table 2B.
Top 20 most significant downregulated genes in SubQ
| Ensembl ID | Gene Symbol | Biotype | Description | logFC | adj.P.Val |
|---|---|---|---|---|---|
| ENSSSCG00000003788 | PTGER3 | protein coding | prostaglandin E receptor 3 | −2.75184 | 1.28E-05 |
| ENSSSCG00000040581 | CISH | protein coding | cytokine inducible SH2 containing protein | −3.96202 | 1.29E-04 |
| ENSSSCG00000028304 | ZFP36L1 | protein coding | ZFP36 ring finger protein like 1 | −1.32246 | 1.33E-04 |
| ENSSSCG00000003839 | PLPP3 | protein coding | phospholipid phosphatase 3 | −1.37672 | 8.71E-04 |
| ENSSSCG00000035057 | RUNDC1 | protein coding | RUN domain containing 1 | −1.54587 | 1.15E-03 |
| ENSSSCG00000038598 | ADRB2 | protein coding | adrenoceptor beta 2 | −1.49218 | 1.25E-03 |
| ENSSSCG00000025924 | IGFBP5 | protein coding | insulin-like growth factor-binding protein 5 | −2.33435 | 4.29E-03 |
| ENSSSCG00000016032 | TFPI | protein coding | tissue factor pathway inhibitor | −2.15092 | 4.29E-03 |
| ENSSSCG00000034167 | SLC5A3 | protein coding | solute carrier family 5 member 3 | −1.12151 | 4.29E-03 |
| ENSSSCG00000012572 | COL4A5 | protein coding | collagen type IV alpha 5 chain | −1.7042 | 7.88E-03 |
| ENSSSCG00000015413 | FGL2 | protein coding | fibrinogen like 2 | −1.531 | 7.88E-03 |
| ENSSSCG00000010440 | PTEN | protein coding | phosphatase and tensin homolog | −1.12742 | 7.88E-03 |
| ENSSSCG00000004039 | SLC22A3 | protein coding | solute carrier family 22 member 3 | −1.57169 | 8.01E-03 |
| ENSSSCG00000008624 | LPIN1 | protein coding | lipin 1 | −1.19195 | 8.52E-03 |
| ENSSSCG00000046255 | lncRNA | −1.78363 | 1.13E-02 | ||
| ENSSSCG00000026981 | MRPS6 | protein coding | mitochondrial ribosomal protein S6 | −1.30598 | 1.13E-02 |
| ENSSSCG00000000456 | SLC16A7 | protein coding | solute carrier family 16 member 7 | −1.14069 | 1.17E-02 |
| ENSSSCG00000002257 | MCTP2 | protein coding | multiple C2 and transmembrane domain containing 2 | −2.34592 | 1.36E-02 |
| ENSSSCG00000000857 | IGF1 | protein coding | insulin like growth factor 1 | −1.43342 | 1.44E-02 |
| ENSSSCG00000015735 | PTPN18 | protein coding | protein tyrosine phosphatase non-receptor type 18 | −1.20938 | 1.52E-02 |
Table 3A.
Top 20 most significant upregulated genes in IA
| ENSEMBL ID | Gene Symbol | Biotype | Description | logFC | adj.P.Val |
|---|---|---|---|---|---|
| ENSSSCG00000035218 | ADA2 | protein coding | adenosine deaminase 2 | 1.926652 | 8.78E-04 |
| ENSSSCG00000036201 | NPR3 | protein coding | natriuretic peptide receptor 3 | 1.695686 | 8.78E-04 |
| ENSSSCG00000032153 | C19orf12 | protein coding | chromosome 6 C19orf12 homolog | 1.287153 | 4.07E-03 |
| ENSSSCG00000043700 | lncRNA | 2.169118 | 5.98E-03 | ||
| ENSSSCG00000036724 | CRYAB | protein coding | crystallin alpha B | 2.191103 | 7.64E-03 |
| ENSSSCG00000005267 | ANXA1 | protein coding | annexin A1 | 1.197932 | 1.09E-02 |
| ENSSSCG00000016691 | JAZF1 | protein coding | JAZF zinc finger 1 | 1.032916 | 1.10E-02 |
| ENSSSCG00000021933 | CLEC5A | protein coding | C-type lectin domain containing 5A | 2.716904 | 1.24E-02 |
| ENSSSCG00000022945 | UCHL1 | protein coding | ubiquitin C-terminal hydrolase L1 | 2.656012 | 1.24E-02 |
| ENSSSCG00000006398 | SLAMF8 | protein coding | SLAM family member 8 | 2.769771 | 1.27E-02 |
| ENSSSCG00000008796 | RBM47 | protein coding | RNA binding motif protein 47 | 1.372184 | 1.44E-02 |
| ENSSSCG00000025652 | CDH1 | protein coding | cadherin 1 | 3.07402 | 1.45E-02 |
| ENSSSCG00000039985 | CES1 | protein coding | liver carboxylesterase-like | 2.424625 | 1.45E-02 |
| ENSSSCG00000038429 | lncRNA | 2.150642 | 1.45E-02 | ||
| ENSSSCG00000045478 | lncRNA | 1.707369 | 1.45E-02 | ||
| ENSSSCG00000043622 | protein coding | novel gene | 1.686322 | 1.45E-02 | |
| ENSSSCG00000022258 | LOC100512873 | protein coding | antileukoproteinase | 2.345292 | 1.46E-02 |
| ENSSSCG00000010253 | HK1 | protein coding | hexokinase 1 | 1.10378 | 1.46E-02 |
| ENSSSCG00000032857 | S100A12 | protein coding | S100 calcium binding protein A12 | 1.810676 | 1.60E-02 |
| ENSSSCG00000046684 | lncRNA | 1.172827 | 1.82E-02 | ||
| ENSSSCG00000015770 | VEGFC | protein coding | vascular endothelial growth factor C | 1.091051 | 1.90E-02 |
| ENSSSCG00000013839 | RASAL3 | protein coding | RAS protein activator like 3 | 1.951044 | 1.93E-02 |
Table 3B.
Top 20 most significant downregulated genes in IA
| Ensembl ID | Gene Symbol | Biotype | Description | logFC | adj.P.Val |
|---|---|---|---|---|---|
| ENSSSCG00000040581 | CISH | protein coding | cytokine inducible SH2 containing protein | −4.18029 | 1.24E-07 |
| ENSSSCG00000003788 | PTGER3 | protein coding | prostaglandin E receptor 3 | −2.50455 | 2.58E-05 |
| ENSSSCG00000028304 | ZFP36L1 | protein coding | ZFP36 ring finger protein like 1 | −1.11422 | 8.78E-04 |
| ENSSSCG00000035057 | RUNDC1 | protein coding | RUN domain containing 1 | −1.73967 | 8.78E-04 |
| ENSSSCG00000044825 | lncRNA | −1.74563 | 1.47E-03 | ||
| ENSSSCG00000022301 | EIF4EBP1 | protein coding | eukaryotic translation initiation factor 4E binding protein 1 | −2.58523 | 1.47E-03 |
| ENSSSCG00000010440 | PTEN | protein coding | phosphatase and tensin homolog | −1.35233 | 2.09E-03 |
| ENSSSCG00000008624 | LPIN1 | protein coding | lipin 1 | −1.42616 | 2.09E-03 |
| ENSSSCG00000015355 | DGKB | protein coding | diacylglycerol kinase beta | −2.20784 | 2.09E-03 |
| ENSSSCG00000012018 | CHODL | protein coding | chondrolectin | −1.64619 | 3.33E-03 |
| ENSSSCG00000038598 | ADRB2 | protein coding | adrenoceptor beta 2 | −1.595 | 4.07E-03 |
| ENSSSCG00000032374 | SULT1B1 | protein coding | sulfotransferase family cytosolic 1B member 1 | −1.9875 | 4.45E-03 |
| ENSSSCG00000007000 | FAT1 | pseudogene | FAT atypical cadherin 1 | −1.41143 | 4.59E-03 |
| ENSSSCG00000015873 | ACVR1C | protein coding | activin A receptor type 1C | −1.71365 | 4.59E-03 |
| ENSSSCG00000002257 | MCTP2 | protein coding | multiple C2 and transmembrane domain containing 2 | −2.61248 | 4.59E-03 |
| ENSSSCG00000009361 | POSTN | protein coding | periostin | −3.72303 | 4.59E-03 |
| ENSSSCG00000025206 | RNF19B | protein coding | ring finger protein 19B | −1.19718 | 5.98E-03 |
| ENSSSCG00000009789 | HCAR1 | protein coding | hydroxycarboxylic acid receptor 1 | −2.11288 | 5.98E-03 |
| ENSSSCG00000038516 | lncRNA | −3.56733 | 5.98E-03 | ||
| ENSSSCG00000041790 | pseudogene | −1.64504 | 6.04E-03 |
Enrichment and pathway analysis
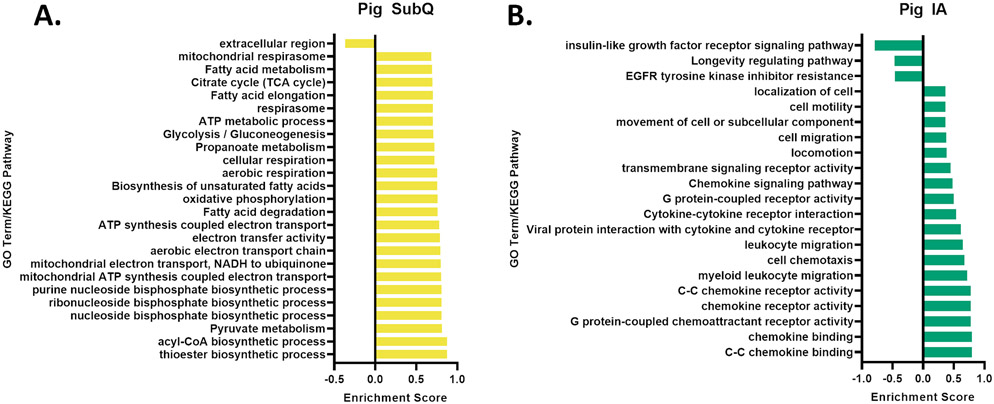
Gene set enrichment analysis (GSEA) was used to describe the gene alterations in this experiment through Gene Ontology (GO) terms or KEGG pathways as a whole without limiting the analysis to DEGs. The only enrichment term with a negative enrichment in the GHR-KO SubQ is the “extracellular region” GO term (Figure 4A). Among the terms with the highest positive enrichment scores in the GHR-KO SubQ, many were associated with cellular respiration, fatty acid metabolism, and glucose metabolism (Figure 4A; top 25 largest enrichment score shown, all significant results shown in Supplemental Table 1). In contrast, GHR-KO IA tissue had three terms with negative enrichment scores, “Insulin-like growth factor receptor signaling pathway”, “Longevity regulating pathway”, and “EGFR tyrosine kinase inhibitor resistance.” The terms with significant positive enrichment scores were associated with cell movement and general cytokine and chemokine signaling (Figure 4B). No significant terms were shared between SubQ and IA.
Figure 4.
Enrichment Results. A. GSEA results, showing the only significant negatively enriched GO term or KEGG pathway and the 24 most significant positively enriched terms or pathways in pig SubQ. B. GSEA results showing all of the significant terms in pig IA.
Ingenuity Pathway Analysis (IPA) was also performed to make use of the large Ingenuity Pathway Knowledge Base. Genes were filtered to >1.5 log2 fold change and adjusted p value <0.05 for significance. Fifty pathways were significantly altered in both depots (Figure 5), with 15 pathways having positive Z scores in both depots, 16 having negative Z scores in both, and 19 pathways having divergent Z scores. Of these divergent pathways, 18 were decreased in SubQ and increased in IA, while only one (triacylglycerol biosynthesis) was increased in SubQ and decreased in IA. Notable pathways decreased in both depots were AMPK signaling, mTOR signaling, and white AT browning. Increased pathways included insulin secretion signaling, senescence pathway, and PTEN signaling. Notable divergent pathways include MAPK signaling (p38 and LPS-stimulated), mitochondrial dysfunction, and renin-angiotensin signaling, each of which was decreased in SubQ and increased in IA AT of GHR-KO pigs compared to controls.
Figure 5.
Pathway analysis using Ingenuity Pathway analysis. Z Scores of all pathways that were significantly altered in both depots are shown.
Depot comparison (within genotype)
Depot comparisons (SubQ vs. IA) were made within each genotype, generating pairwise comparisons of GHR-KO SubQ vs. GHR-KO IA and WT SubQ vs. WT IA. Principal component analysis on global gene expression showed a better separation of depots in the GHR-KO samples than the WT samples (Figure 6A-B), which was borne out by the DEG results. In the GHR-KO AT, there were 32 differentially expressed genes (DEGs; log2 fold change >1 and adjusted p value <0.05) while in the WT there were no DEGs (Figure 6C). Of the DEGs in GHR-KO AT, 29 were upregulated and 3 were downregulated in the SubQ compared to IA (Figure 6D). Many of the DEGs are not fully annotated, but most of the unannotated genes are predicted to encode long noncoding RNAs.
Figure 6.
Pig Depot Comparison. A. Principal components analysis of the pig Ghr−/− samples, showing the global gene expression. B. Principal components analysis of the pig WT samples, showing the global gene expression. C. Differential gene expression results of the depot comparison showing the number of significant DEGs (FDR<0.05, absolute log2 fold change >1.5) in each genotype alone and present in both genotypes. D. Heatmap of the 32 DEGs present in Ghr−/−, showing the log2 fold change of each gene in Ghr−/− SubQ compared to Ghr−/− IA. n=3 per group.
Comparison to Human Results
Due to the low number (n=1) of human samples, no DEGs could be determined. Thus, to compare the human gene expression results to the pig results, the raw CPM number was used. The cpm of each Laron sample was reported, while in the pigs the mean cpm of each group was used in a similar manner. Due to the limitations in these data, this analysis focused on prominent genes in the GH/IGF-1 pathway (Figure 7A). Notably, gene expression of IGFBP3 and IGFALS was not detected in the pig samples. No genotype changes were consistent across depots and species (Figure 7B).
Figure 7.
Human and pig comparison. A. Diagram of key genes of the GH signaling pathway. B. Heatmap showing the expression of key GH genes across depot, genotype, and species expressed as log2 CPM (counts per million). Genes shown in gray were not detected in the pig transcriptome analysis.
Discussion
GHR-KO pigs have recently been developed [4] and serve as a large animal model of LS. In this study, we examined the AT phenotype of these pigs and compared the results to human samples from an individual with and without LS. GHR-KO pigs had increased adipocyte size in both depots examined, which matched the trend from the human samples. Similar to that of Ghr−/− mice [34, 35], adipocytes in the SubQ depot in the pigs were increased in size to a greater extent than adipocytes in the visceral depot suggesting that SubQ is more sensitive to the effects (or lack thereof) of GH action. Known GH target genes were downregulated in the GHR-KO pigs, consistent with the absence of GHR due to the frameshift mutation in the GHR gene. Interestingly, long noncoding RNAs (lncRNAs) were among the most significantly altered genes, including some that were changed in both depots. Enrichment and pathway analysis also showed expected changes to growth and metabolism related genes, but these changes were not consistent across depots.
The mean adipocyte size was increased in GHR-KO pigs in both the SubQ and IA depots. In Ghr−/− mice, either globally [15, 17] or specific to AT [36] or adipocytes [22], mean adipocyte size is also increased, in agreement with increased adipose mass. In the human samples, the mean adipocyte size also had a trend towards an increase in both depots, suggesting that this effect is consistent across species, and GH deficient humans also have increased adipocyte size, indicating that the effect is not limited to GH insensitivity [37]. This is likely due to the loss of GH’s lipolytic and antilipogenic effects on the tissue. As AT expands, it can do so through hypertrophy (increased cell size) or hyperplasia (increased cell number). AT dysfunction occurs when the adipocytes cannot expand as quickly as necessary [38], which may occur due to excess extracellular matrix deposition or fibrosis [39]. However, in this experiment, fibrosis of the AT was unchanged between genotypes, indicating that fibrosis does not play a major role in the adipose cell size in this model, despite the downregulation of the extracellular matrix term in the RNA sequencing enrichment analysis for the SubQ depot. However, the pig AT samples were from relatively young animals in which levels of fibrosis would be expected to be minimal.
The SubQ depot in pigs had a larger number of significantly altered genes than the IA depot in response to GHR disruption. This is consistent with previous studies examining the effects of GH alterations on AT [34, 35]. GH alterations in mice have a stronger effect in SubQ compared to IA depots in relation to depot mass [34], adipocyte size [17], fibrosis [35], and many other characteristics. This could be associated with the SubQ-specific obesity profile of Ghr−/− mice and may partially explain the improved insulin sensitivity and increased lifespan of Ghr−/− mice compared to controls. GHR-KO pigs also have an increase in SubQ fat [4] and normal glucose tolerance despite their obesity [40], indicating possible shared mechanisms between the species.
Known GH target genes were many of the most significantly altered genes in GHR-KO pig AT. These include IGF1, IGFBP5, CISH, and AKT1, all of which were significantly downregulated in both depots. CISH was the first and second most significantly downregulated gene in IA and SubQ, respectively. This is a somewhat expected result, as CISH is a member of the suppressors of cytokine signaling (SOCS) family, many of which are negative regulators of GH signaling. It is somewhat surprising, however, that SOCS3 is not the most changed SOCS gene, as Socs3 knockout mice have the strongest growth phenotype [41], and the human data in this experiment show a stronger effect on SOCS3 than CISH. Obesity related genes were also altered, including a strong downregulation of PTGER3 in both depots. Ptger3 disruption has previously been shown to increase fat mass on a high fat diet in mice [42].
Previous studies have examined the transcriptome of AT in mice in response to GH alterations, including Ghr−/− (multiple depots) [43], bGH (SubQ and Epidydimal) [44] and GH-injected GH knockout (SubQ only) [45]. The Ghr−/− and bGH experiments did not share any DEGs with this study with the exception of the Igf1 gene, albeit in opposite directions. The SubQ depot from GH-injected GHKO shares 7 DEGs with this study: Igf1, Cryab, Npr3, Lpin1, Pik3r1, Ptger3, and Cish. Because GH-injected GHKO mice have increased GH action, it would be expected that direction of change would be opposite of the GHR-KO pigs. Five of the genes follow this pattern, but LPIN1 and PTGER3 are decreased in both groups, meaning their changes don’t consistently correlate with GH action.
Many of the most significantly altered genes encode lncRNAs. Research is sparse about these genes in pigs, but some have been reported to be altered in other experiments. For example, ENSSSCG00000051234 increases with obesity [46] in minipigs, matching the increase seen the SubQ of GHR-KO pigs in this study. The lncRNA ENSSSCG00000038429 was increased in both depots of GHR-KO pigs and has previously been shown to be positively correlated with the ratios of linoleic to α-linolenic acid and omega-6 to omega-3 fatty acids in the muscle of pigs [47]. Although extensive metabolomic data are reported for these GHR-KO pigs [48], no measurements for these fatty acids are reported. As our data indicates that there may be some changes in gene expression levels resulting in different fatty acid ratios in the GHR-KO pigs, this analysis may be worth exploring in further experiments.
Gene set enrichment analysis was used to determine which GO terms or KEGG pathways were significantly enriched. The enrichment results in the SubQ showed a decreased enrichment of genes involved in the extracellular region, despite the unchanged fibrosis observed in the tissue. This may reflect changes to non-collagen genes in the ECM or perhaps the younger age of the animals. There were many significantly enriched terms, including terms associated with metabolism of both fats and carbohydrates as well as aerobic respiration. Interestingly, all of these terms are increased despite the increased fat mass, which may suggest that the AT in GHR-KO pigs is metabolically healthy despite obesity and supports the hypothesis that SubQ AT is metabolically healthier than IA. The IA depot enrichment analysis had decreased enrichment of the IGF receptor signaling pathway and the longevity regulating pathway, both of which are expected results in a GH-altered animal. The majority of the positively enriched terms are associated with leukocyte migration, an unexpected result indicating that the IA tissue of GHR-KO pigs has altered immune function. GHR-KO pigs have no striking differences in serum lymphocytes [49], and in a previous study examining immune cells in the AT of Ghr−/− mice [19], no change in immune cells was observed in the epidydimal depot at either age examined. This indicates that there may be a difference between the adipose phenotype of GHR-KO pigs and Ghr−/− mice, or it may be a reflection that the depots used between studies were not directly comparable.
When examining the pathway analysis results of this experiment, there were 50 pathways significantly altered in both tissues. Notable pathways that decreased in both depots were the mTOR signaling pathway, autophagy, and white AT browning pathway. Pathways increased in both depots include the insulin secretion signaling pathway and the senescence pathway. Previous data show decreased senescence with decreased GH action [18, 50], so this is an unexpected result, while the decreased WAT browning with decreased GH action agrees with previous reports [51]. Further examination into these results is warranted especially due to the lack of longevity data available for the GHR-KO pigs. Nineteen pathways were oppositely changed between the two depots, with triacylglycerol biosynthesis increased in SubQ and decreased in IA, and all of the other pathways decreased in SubQ and increased in IA. This is notable because one would expect both depots to have increased triacylglycerol synthesis because the GHR-KO pigs have increased fat mass and adipocyte size in both depots. Examination of the individual genes in the pathway shows that LPIN1 and PLPP3 are downregulated in GHR-KO in both depots, while the IA lacks the increases in AGPAT1, ELOVL1, ELOVL6, GPAM, GPAT4, and SIRT2 transcripts that are present in the SubQ. Another notable result is mitochondrial dysfunction, which is increased in IA but decreased in SubQ, as Ghr−/− mice have been shown to have increased mitochondrial biogenesis in diverse tissues [52] and GH releasing hormone (Ghrh)-KO mice have changes to mitochondrial function in BAT [53].
A notable and unexpected result came from the depot comparison. Although the SubQ and IA depots had differing numbers of DEGs, a direct comparison of depot differences within each genotype (e.g. GHR-KO SubQ vs. GHR-KO IA) showed very few DEGs, with 32 in the GHR-KO pigs and 0 DEGs in the WT pigs. These results indicate that GHR disruption enhances the differences between depots in pigs. However, in the pig the two depots examined were geographically close together, with SubQ sample being taken from just outside and the IA sample taken just inside the peritoneum. There were no significant results in the enrichment or pathway analysis of the 32 DEGs (data not shown). Further investigation is necessary to clarify whether GHR disruption drives differences between depots in pigs.
In the comparison of counts per million (cpm) values between the pig and human samples, no clear pattern emerged. GH1 expression was extremely low, as expected, in all samples except the pig WT IA samples. GHR mRNA expression was not altered in the human LS patient, similar to the GHR-KO pig samples. This is likely due to the nature of LS, as it usually arises from a gene mutation, not a deletion. Because of this, GHR mRNAs can still be detected although they do not produce a functional GHR protein. Another notable result is that SOCS3 has stronger expression in the human samples (along with a corresponding trend towards decreased expression in the LS samples) while CISH is more highly expressed in pigs, with a strong decrease in the GHR-KO pig samples. This may reflect a species difference in the downstream regulation of GHR signaling, but it may also reflect a breed specific effect, as CISH expression has previously been shown to differ between pig breeds [54].
In summary, the AT of GHR-KO pigs has increased cell size, with no change in fibrosis. Despite the limited annotation of the pig genome compared to humans and the small sample size of the human data (n=1), transcriptome analysis produced a number of interesting results, as this is the first study to examine the AT transcriptome in an individual with LS. GHR-KO pigs had a higher number of DEGs in SubQ compared to IA WAT and the DEGs shared between depots were changed in the same direction. Enrichment and pathway analyses showed expected as well as novel pathway alterations, while reinforcing the different responses of each depot to GHR knockout. Depot comparisons (within genotype) showed that only Ghr−/− mice had DEGs between the two depots. Transcriptome alterations were not consistent between pig and human data, which may reflect species differences in GH response, but may also reflect the limitations of the study. In addition to their value as a large animal model of LS, GHR-KO pigs are already being used for xenotransplantation of organs to humans [55, 56], thus, understanding the differences between GHR-KO pig and analogous human tissue is necessary.
Figure 1.
Graphical summary of the methods. Pig (n=4 per group) and human (n=1 per group) AT samples were used for histological analysis and RNA Sequencing.
Acknowledgements
We wish to acknowledge the help of Dr Andrei Keidar of the Surgical Department B, at the Beilinson Medical Center for providing human adipose tissue.
Funding:
This work was supported in part by NIH grant AG059779, Ohio University Heritage College of Osteopathic Medicine, The Diabetes Institute at Ohio University, Deutsche Forschungsgemeinschaft (DFG), Grant/Award Numbers: HI 2206/2-1, TRR 127, TRR 205; Deutsches Zentrum für Diabetesforschung (DZD); and the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll.
Footnotes
Conflicts of interest: The authors report no conflict of interest.
Ethics approval: All animal procedures were approved by the responsible animal welfare authority (Regierung von Oberbayern; permission 55.2-1-54-2532-70-12) and performed according to the German Animal Welfare Act and Directive 2010/63/EU on the protection of animals used for scientific purposes.
The studies in humans were approved by the ethics committee of the Schneider Children’s and Rabin Medical Centers
Availability of data and material: Upon request
Code availability: Upon request
References
- 1.Laron Z and Werner H, Laron syndrome - A historical perspective. Rev Endocr Metab Disord, 2021. 22(1): p. 31–41. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y., et al. , A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci U S A, 1997. 94(24): p. 13215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young J., et al. , Mouse models of growth hormone insensitivity. Rev Endocr Metab Disord, 2021. 22(1): p. 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinrichs A., et al. , Growth hormone receptor-deficient pigs resemble the pathophysiology of human Laron syndrome and reveal altered activation of signaling cascades in the liver. Mol Metab, 2018. 11: p. 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laron Z., et al. , Effects of insulin-like growth factor on linear growth, head circumference, and body fat in patients with Laron-type dwarfism. Lancet, 1992. 339(8804): p. 1258–61. [DOI] [PubMed] [Google Scholar]
- 6.Laron Z and Klinger B, Body fat in Laron syndrome patients: effect of insulin-like growth factor I treatment. Horm Res, 1993. 40(1-3): p. 16–22. [DOI] [PubMed] [Google Scholar]
- 7.Laron Z., et al. , Body composition in untreated adult patients with Laron syndrome (primary GH insensitivity). Clin Endocrinol (Oxf), 2006. 65(1): p. 114–7. [DOI] [PubMed] [Google Scholar]
- 8.Laron Z and Kopchick JJ, Laron syndrome - from man to mouse : lessons from clinical and experimental experience. 2011, Berlin ; London: Springer. xiv, 531 p. [Google Scholar]
- 9.Shevah O and Laron Z, Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res, 2007. 17(1): p. 54–7. [DOI] [PubMed] [Google Scholar]
- 10.Steuerman R, Shevah O, and Laron Z, Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol, 2011. 164(4): p. 485–9. [DOI] [PubMed] [Google Scholar]
- 11.Guevara-Aguirre J., et al. , Insights from the clinical phenotype of subjects with Laron syndrome in Ecuador. Rev Endocr Metab Disord, 2021. 22(1): p. 59–70. [DOI] [PubMed] [Google Scholar]
- 12.Guevara-Aguirre J., et al. , Cancer in growth hormone excess and growth hormone deficit. Endocr Relat Cancer, 2023. 30(10). [DOI] [PubMed] [Google Scholar]
- 13.Ikeno Y., et al. , Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci, 2009. 64(5): p. 522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coschigano KT, et al. , Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology, 2000. 141(7): p. 2608–13. [DOI] [PubMed] [Google Scholar]
- 15.Berryman DE, et al. , Growth hormone and adipose tissue: beyond the adipocyte. Growth Horm IGF Res, 2011. 21(3): p. 113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanety H., et al. , Total and high molecular weight adiponectin are elevated in patients with Laron syndrome despite marked obesity. Eur J Endocrinol, 2009. 161(6): p. 837–44. [DOI] [PubMed] [Google Scholar]
- 17.Berryman DE and List EO, Growth Hormone's Effect on Adipose Tissue: Quality versus Quantity. Int J Mol Sci, 2017. 18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stout MB, et al. , Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY), 2014. 6(7): p. 575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young JA, et al. , GHR(−/−) Mice are protected from obesity-related white adipose tissue inflammation. J Neuroendocrinol, 2020. 32(11): p. e12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennis MT, et al. , The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience, 2017. 39(1): p. 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albl B., et al. , Tissue Sampling Guides for Porcine Biomedical Models. Toxicol Pathol, 2016. 44(3): p. 414–20. [DOI] [PubMed] [Google Scholar]
- 22.List EO, et al. , Adipocyte-Specific GH Receptor-Null (AdGHRKO) Mice Have Enhanced Insulin Sensitivity With Reduced Liver Triglycerides. Endocrinology, 2019. 160(1): p. 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchoukalova YD, et al. , Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring), 2010. 18(10): p. 1875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasband WS Quantifying Stained Liver Tissue. 1997-2018. [cited 2023; Available from: https://imagej.nih.gov/ij/docs/examples/stained-sections/index.html. [Google Scholar]
- 25.Dobin A., et al. , STAR: ultrafast universal RNA-seq aligner. Bioinformatics, 2013. 29(1): p. 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao Y, Smyth GK, and Shi W, featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics, 2014. 30(7): p. 923–30. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Wang S, and Li W, RSeQC: quality control of RNA-seq experiments. Bioinformatics, 2012. 28(16): p. 2184–5. [DOI] [PubMed] [Google Scholar]
- 28.Robinson MD, McCarthy DJ, and Smyth GK, edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics, 2010. 26(1): p. 139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie ME, et al. , limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res, 2015. 43(7): p. e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wickham H., ggplot2 : Elegant Graphics for Data Analysis, in Use R!,. 2016, Springer International Publishing : Imprint: Springer,: Cham. p. 1 online resource (XVI, 260 pages 232 illustrations, 140 illustrations in color. [Google Scholar]
- 31.Chen H and Boutros PC, VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics, 2011. 12: p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Z, Eils R, and Schlesner M, Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics, 2016. 32(18): p. 2847–9. [DOI] [PubMed] [Google Scholar]
- 33.Wu T., et al. , clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb), 2021. 2(3): p. 100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berryman DE, et al. , Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res, 2004. 14(4): p. 309–18. [DOI] [PubMed] [Google Scholar]
- 35.Householder LA, et al. , Increased fibrosis: A novel means by which GH influences white adipose tissue function. Growth Horm IGF Res, 2018. 39: p. 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.List EO, et al. , The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol, 2013. 27(3): p. 524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaves VE, Junior FM, and Bertolini GL, The metabolic effects of growth hormone in adipose tissue. Endocrine, 2013. 44(2): p. 293–302. [DOI] [PubMed] [Google Scholar]
- 38.Sun K., et al. , Fibrosis and adipose tissue dysfunction. Cell Metab, 2013. 18(4): p. 470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troike KM, et al. , Impact of Growth Hormone on Regulation of Adipose Tissue. Compr Physiol, 2017. 7(3): p. 819–840. [DOI] [PubMed] [Google Scholar]
- 40.Hinrichs A., et al. , MECHANISMS IN ENDOCRINOLOGY: Transient juvenile hypoglycemia in growth hormone receptor deficiency - mechanistic insights from Laron syndrome and tailored animal models. Eur J Endocrinol, 2021. 185(2): p. R35–R47. [DOI] [PubMed] [Google Scholar]
- 41.Qian Y., et al. , Mice with gene alterations in the GH and IGF family. Pituitary, 2022. 25(1): p. 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ceddia RP, et al. , The PGE2 EP3 Receptor Regulates Diet-Induced Adiposity in Male Mice. Endocrinology, 2016. 157(1): p. 220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stout MB, et al. , Transcriptome profiling reveals divergent expression shifts in brown and white adipose tissue from long-lived GHRKO mice. Oncotarget, 2015. 6(29): p. 26702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duran-Ortiz S., et al. , Differential gene signature in adipose tissue depots of growth hormone transgenic mice. J Neuroendocrinol, 2020. 32(11): p. e12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young JA, et al. , Transcriptome profiling of insulin sensitive tissues from GH deficient mice following GH treatment. Pituitary, 2021. 24(3): p. 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marmol-Sanchez E., et al. , Modeling microRNA-driven post-transcriptional regulation using exon-intron split analysis in pigs. Anim Genet, 2022. 53(5): p. 613–626. [DOI] [PubMed] [Google Scholar]
- 47.Valdes-Hernandez J., et al. , Global analysis of the association between pig muscle fatty acid composition and gene expression using RNA-Seq. Sci Rep, 2023. 13(1): p. 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riedel EO, et al. , Functional changes of the liver in the absence of growth hormone (GH) action - Proteomic and metabolomic insights from a GH receptor deficient pig model. Mol Metab, 2020. 36: p. 100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schilloks MC, et al. , Effects of GHR Deficiency and Juvenile Hypoglycemia on Immune Cells of a Porcine Model for Laron Syndrome. Biomolecules, 2023. 13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aguiar-Oliveira MH and Bartke A, Growth Hormone Deficiency: Health and Longevity. Endocr Rev, 2019. 40(2): p. 575–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson CN, et al. , Growth hormone activated STAT5 is required for induction of beige fat in vivo. Growth Horm IGF Res, 2018. 42-43: p. 40–51. [DOI] [PubMed] [Google Scholar]
- 52.Gesing A., et al. , Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Gerontol A Biol Sci Med Sci, 2011. 66(10): p. 1062–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman JM, et al. , Transcriptomic and metabolomic profiling of long-lived growth hormone releasing hormone knock-out mice: evidence for altered mitochondrial function and amino acid metabolism. Aging (Albany NY), 2020. 12(4): p. 3473–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ropka-Molik K., et al. , Comprehensive analysis of the whole transcriptomes from two different pig breeds using RNA-Seq method. Anim Genet, 2014. 45(5): p. 674–84. [DOI] [PubMed] [Google Scholar]
- 55.Hinrichs A., et al. , Growth hormone receptor knockout to reduce the size of donor pigs for preclinical xenotransplantation studies. Xenotransplantation, 2021. 28(2): p. e12664. [DOI] [PubMed] [Google Scholar]
- 56.Griffith BP, et al. , Genetically Modified Porcine-to-Human Cardiac Xenotransplantation. N Engl J Med, 2022. 387(1): p. 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]