Abstract
Background:
To investigate the effect of concurrent strength combined with endurance training on the lipid and glucose profile of type 2 diabetes mellitus (T2DM) using Meta-analysis.
Methods:
The literature was searched from PubMed, Web of Science, EBSCO, and China National Knowledge Infrastructure(CNKI) databases for relevant randomized controlled trials with dates from the date of establishment to June 2023, and the included studies were individually assessed according to the Cochrane Risk of Bias tool in the Cochrane Systematic Assessor’s Handbook, and the data were analyzed using RevMan 5.4 analysis software to analyze and process the data.
Results:
A total of 9 articles were included, including 589 subjects, including 308 in the experimental group and 281 in the control group. The results of Meta analysis showed that concurrent strength combined with endurance training improved TC (SMD = −1.12, 95% CI = [−1.81, −0.44], P < 0.01), TG (SMD = −0.46, 95% CI = [−0.85, −0.07], P < 0.05), LDL-C (SMD = −1.3, 95% CI = [−2.09, −0.50], P < 0.01), HDL-C (SMD = 0.61, 95% CI = [0.05, 1.17], P < 0.05), FBG (SMD = −0.65, 95% CI = [−1.27, −0.04], P < 0.05), HOMA-IR (SMD = −1.23, 95% CI = [−2.40, −0.06], P < 0.05).
Conclusion:
Concurrent strength combined with endurance training has a positive effect on the improvement of lipid and glucose profile in patients with type 2 diabetes.
Keywords: concurrent strength and endurance training, lipid and glucose profile, meta-analysis, T2DM
1. Introduction
With the improvement of people living standards, the prevalence of metabolic diseases such as diabetes and obesity is increasing. It not only affects people health, but also makes medical expenses increase greatly. According to the International Diabetes Federation, by 2045, about 700 million people will have diabetes, with an estimated annual investment of $850 billion.[1] Studies have shown that disorders of lipid and glucose metabolism are important risk factors and major causes of diabetes and obesity.[2] Among them, T2DM is a common chronic metabolic disorder characterized by insulin resistance and impaired glucose metabolism. Patients with T2DM are often associated with dyslipidemia, which further increases their risk of cardiovascular disease. Physical activity is widely recognized as a crucial component in the management of T2DM, and numerous studies have shown that exercise can effectively regulate glucose and lipid metabolism disorders in T2DM patients, with aerobic endurance training often recommended for T2DM patients to improve insulin sensitivity and reduce cardiovascular risk factors. Meanwhile, aerobic training tends to be more common than resistance training in T2DM management in clinical practice.[3–6] Concurrent aerobic combined with resistance training, which combines resistance exercise with aerobic training, may provide more benefits than aerobic training alone. In addition, concurrent strength combined with endurance training has a significant effect on improving cardiorespiratory endurance and enhancing muscle strength,[7] which may have a beneficial effect on lipid and glucose profile in patients with T2DM.
Although several studies have investigated the effects of concurrent strength combined with endurance training on lipid and glucose profile in patients with T2DM, inconsistent results exist. Zaki et al study indicates that concurrent aerobic and resistance training can improve metabolic markers, body composition, lipid profile, inflammation, and cardiorespiratory fitness in patients with T2DM, suggesting its potential inclusion in the management of T2DM.[8] Additionally, Zhao et al’s research demonstrates that combined exercise has a significant impact on improving blood glucose control, influencing weight loss, and enhancing insulin sensitivity in T2D patients with overweight/obesity.[9] Despite the well-described benefits of combined trainin, Bassi et al’s study suggests that simultaneous aerobic and resistance training for 3 months may not significantly improve HOMA-IR and cholesterol indicators in type 2 diabetes patients.[10] Therefore, a comprehensive meta-analysis is necessary to synthesize the available evidence and provide a clearer understanding of the overall effects of this combined training modality. The aim of this meta-analysis was to assess the effects of concurrent strength combined with endurance training on lipid and glucose profile in patients with T2DM. By pooling and analyzing the available data, we aimed to determine the magnitude and significance of these effects and to identify potential modifiers that may affect the results.
2. Materials and methods
This study aimed to conduct a systematic review and meta-analysis to investigate the effects of concurrent strength combined with endurance training on lipid and glucose profile in individuals with T2DM. The study followed the PRISMA 2020 statement for conducting systematic reviews and utilized the PICOS framework for formulating the research question and study design.[11] The PICOS framework included population (Type 2 Diabetes), Intervention (concurrent strength combined with endurance training), Control (healthy subjects without training), Outcomes (TC,TG,LDL-C,HDL-C,FBG, HOMA-IR and HbA1c), and study design (published randomized controlled trials).
2.1. Search strategy
This study was conducted in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The literature search databases included PubMed, Web of Science, EBSCO, and CNKI. When searching, keywords such as “concurrent training,” “concurrent strength combined with endurance training,” “concurrent resistance combined with endurance training,” “type 2 diabetes mellitus,” “type 2 diabetes,” and “type II diabetes,” the relevant literature was reviewed by combining the search strategy of subject terms and free words. The search date was from the database creation to June 2023. All English and Chinese literature on the effects of contemporaneous strength combined with endurance training on lipid and glucose profile in a type 2 diabetic population was collected.
2.2. Selection and exclusion criteria of literature
Inclusion criteria: The subjects were the literature of T2DM. Literature in which the intervention was a combination of concurrent strength and endurance training. The type of study is (randomized controlled trials, RCT). The outcome indicators were those that included at least 2 or more of (fasting blood glucose, FBG), (glycosylated hemoglobin, HbA1c), (homeostatic model assessment for insulin resistance, HOMA-IR),(triglyceride, TG),(total cholesterol, TC), (low-density lipoprotein cholesterol, LDL-C) and (high-density lipoprotein cholesterol, HDL-C).
Exclusion criteria: Full text is not available and data is incomplete. Conference papers, dissertations, reviews. Study subjects were unable to exercise normally due to various diseases. Non-English and Chinese literature. Basic animal research.
2.3. Studies selection and data extraction
Relevant literature retrieved from each database was imported into EndnoteX9 literature management software for de-duplication. Duplicates were first removed, and then the titles and abstracts of the literature were initially screened according to the inclusion and exclusion criteria, respectively, to exclude literature that did not meet the requirements, and further determined for inclusion in the study after reading the full text. This process was performed independently by 2 researchers, and for literature that could not be determined for inclusion or exclusion, the 2 evaluators were required to negotiate a solution, and when disagreement occurred, the articles were reevaluated. If disagreement persisted, a third for investigator ruling was passed.
The extracts mainly included the first author and year of publication of the literature; sample size, number of men and women, and age; form of exercise training, intervention modality (intervention content, intervention cycle, weekly intervention frequency, and duration of each intervention); outcome evaluation index, etc. The literature was not included if the full text was not available and the data were incomplete.
2.4. Quality assessment
The risk of bias in the included study literature was evaluated separately according to the Cochrane Systematic Assessor Handbook 5.1.0 Risk of Bias Assessment Tool, which included 7 entries: Random sequence generation (selection bias), Allocation concealment (selection bias), Blinding of participants and personnel (performance bias), Blinding of outcome assessment (detection bias), Incomplete outcome data (attrition bias), Selective reporting (reporting bias) and Other bias. The investigators classified the articles as low risk bias, high risk bias, and uncertain bias according to the systematic evaluation manual. Risk bias assessment was done independently by 2 reviewers, and conflicts were resolved by a third reviewer.
2.5. Statistical analysis
The data were analyzed using RevMan 5.4 with weighted mean difference (WMD) and 95% confidence interval (Cl) as effect sizes. Heterogeneity was analyzed using the consistency coefficient I2. If I2 > 50%, P < .10, it indicates a large heterogeneity between the 2, and a random-effects model was used for analysis; on the contrary, a fixed-effects model was used. When statistical heterogeneity existed but there was no clinical heterogeneity between study groups, a random-effects model was used for analysis. If the heterogeneity was too pronounced to identify its source, only descriptive analyses were used.
3. Results
3.1. General results of the selected research literature
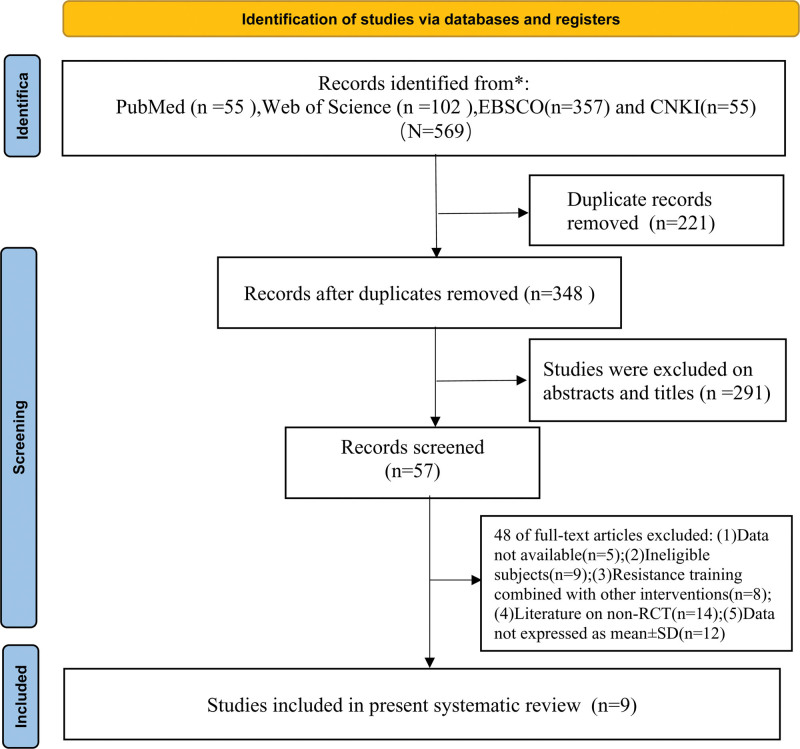
A total of 569 papers were retrieved through the database, and of the 569 papers retrieved, 221 duplicates were excluded after importing into EndnoteX9 software, and 291 papers were excluded after reading the titles and abstracts. The remaining 57 papers were thoroughly reviewed according to the inclusion and exclusion criteria. Nine studies were eventually screened for inclusion in the final meta-analysis, containing 8 in English and 1 in Chinese, and the specific literature screening process is shown in Figure 1.
Figure 1.
Flow chart of literature screening.
3.2. General characteristics of the selected research literature
A total of 9 studies were included in this study, all of which were RCT trials, and the sample sizes of the experimental and control groups were reported in the included literature. 589 subjects were included in the 9 studies, 308 in the experimental group and 281 in the control group. The basic characteristics of the included literature are shown in Table 1.
Table 1.
General characteristics of selected research literature.
| First author | Year | Sample size (EG/CG) | Gender M/F |
Age (year) | intervention modality | Duration (Week) |
Frequency | Outcome |
|---|---|---|---|---|---|---|---|---|
| Annibalini[12] | 2017 | 8/8 | All males | EG:57 ± 9.1 CG:60 ± 6.8 |
Aerobic training consists of running on a treadmill continuously for 30-60 minutes at an intensity of 40-65% of HRR. Strength training involves exercises such as squats, lat pulldowns, and bench presses, performed for 12-20 repetitions per set at an intensity of 40-60% of 1RM. Each exercise is performed for 2-4 sets, and there is no intervention in the control group. | 16 | 3/W | ①②③④⑤ |
| Balducci[13] | 2010 | 22/20 | EG:14/8 CG:11/9 |
EG:60.6 ± 9.3 CG:61.1 ± 7.1 |
Aerobic exercise consists of running for 40 minutes at an intensity of 70-80% of VO2max. Strength training involves exercises such as bench press and squats, performed for 20 minutes at an intensity of 80% of 1RM. The control group does not engage in physical activity, and there is no intervention in the control group. | 48 | 2/W | ②③④⑤⑥⑦ |
| Balducci[14] | 2012 | 161/142 | EG:94/67CG85/57 | EG:59.5 ± 8.3 CG:58.4 ± 8.9 |
Aerobic exercise is performed at 70% of VO2max, while strength training is conducted at 60% of 1RM. Control group performed normal daily activities. | 48 | 2/W | ①②③④⑤⑦ |
| Bassi[10] | 2015 | 21/20 | / | 51 ± 7 | The intervention includes 30 minutes of aerobic exercise and 30 minutes of resistance training. The aerobic exercise is performed at an intensity corresponding to 60-70% of peak VO2, based on heart rate. Each training session lasts for 30 minutes. The resistance training is conducted using weights corresponding to 60-80% of predicted 1RM, with three sets of 10-12 repetitions. | 12 | 3/W | ②⑤⑥ |
| Jorge[15] | 2011 | 12/12 | EG:4/8 CG:4/8 |
EG:57.9 ± 9.82 CG:53.42 ± 9.82 |
The intervention consists of 30 minutes of aerobic training and 30 minutes of strength training targeting major muscle groups. The control group does not receive any intervention. | 12 | 3/W | ①②③④⑤⑥⑦ |
| Saeidi[16] | 2021 | 11/11 | All males | 40-60 | Weeks 1-2: 10 minutes of aerobic exercise, running at 60% of VO2peak, and strength training at 60-65% of 1RM for 12-15 repetitions per set, with 3 sets. Weeks 3-4: 15 minutes of aerobic exercise, running at 60% of VO2peak, and strength training at 60-65% of 1RM for 12-15 repetitions per set, with 3 sets. Weeks 5-6: 20 minutes of aerobic exercise, running at 70% of VO2peak, and strength training at 70% of 1RM for 8-10 repetitions per set, with 3 sets. Weeks 7-8: 25 minutes of aerobic exercise, running at 70% of VO2peak, and strength training at 70% of 1RM for 8-10 repetitions per set, with 3 sets. Weeks 8-12: 30 minutes of aerobic exercise, running at 70% of VO2peak, and strength training at 70% of 1RM for 8-10 repetitions per set, with 3 sets. The control group does not receive any intervention. | 12 | 3/W | ①③④⑤⑦ |
| Tan[17] | 2012 | 15/10 | EG:8/7 CG:5/5 |
EG:65.9 ± 4.2 CG:64.8 ± 7.3 |
The intervention consists of a 10-minute warm-up, followed by 30 minutes of aerobic training at 55-70% of HRmax. This is followed by 10 minutes of leg strength training at 50-70% of 1RM, with 10-12 repetitions per set and 2 sets per exercise. The session concludes with a 10-minute stretching and relaxation routine. The control group does not receive any intervention. | 24 | 3/W | ①②③④⑤⑦ |
| Wang[18] | 2015 | 46/46 | EG:24/22CG:27/19 | EG:47.35 ± 12.79 CG:51.40 ± 13.55 |
The intervention includes aerobic exercise for 30-35 minutes at an intensity of 40%-60% of VO2max. Resistance training involves exercises such as bench press, shoulder press, and lat pulldowns, performed at an intensity of 50%-60% of 1RM, with 3 sets of 8-12 repetitions per set. The control group does not receive any intervention. | 12 | 3/W | ①②③④⑤⑥⑦ |
| Zarei[19] | 2021 | 12/12 | All males | EG:48.7 ± 10.1 CG:49.8 ± 8.2 |
The intervention begins with a 10-minute warm-up, followed by 20-30 minutes of aerobic exercise at 55%-70% of HRmax. This is followed by 40-45 minutes of strength training at 55%-70% of 1RM, with 3 sets per exercise such as squats, bench press, etc., and 10-12 repetitions per set. The session concludes with a 5-minute cool-down and relaxation period. The control group does not engage in any exercise intervention. | 12 | 3/W | ①②③④⑤⑥⑦ |
Note: e.g. = experimental group; CG = control group; M:F(male):(female); W = week(s); ①FBG = fasting blood glucose; ②HbA1c = glycosylated hemoglobin; ③HDL-C = high-density lipoprotein cholesterol; ④LDL-C = low-density lipoprotein cholesterol; ⑤TC = total cholesterol; ⑥HOMA-IR = homeostatic model assessment for insulin resistance; ⑦TG = triglyceride.
3.3. Quality evaluation of the selected literature
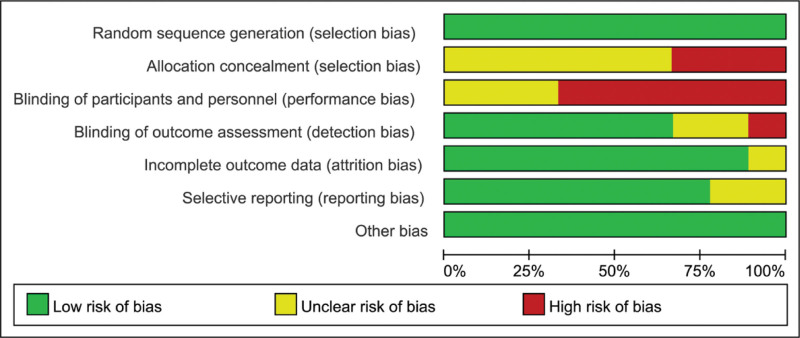
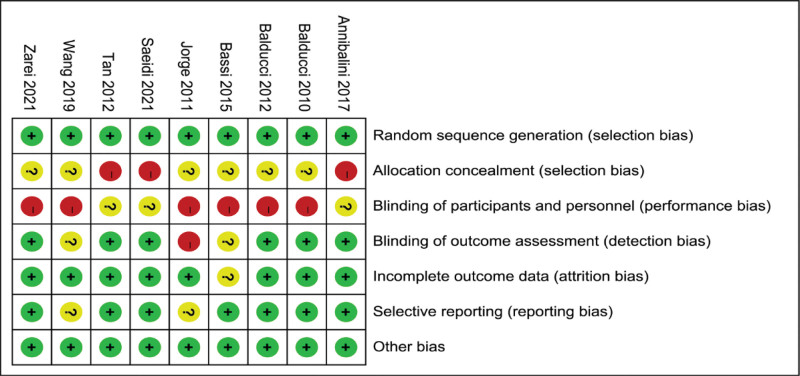
The risk of bias in the included study literature was individually assessed according to the Cochrane Systematic Assessor Handbook 5.1.0 risk of bias assessment tool. The results of the assessment are shown in Figures 2 and 3. The evaluation included 7 entries: Random sequence generation (selection bias), Allocation concealment (selection bias), Blinding of participants and personnel (performance bias), Blinding of outcome assessment (detection bias), Incomplete outcome data (attrition bias), Selective reporting (reporting bias) and Other bias. Since the interventions in this study were all exercise interventions, a complete double-blind design could not be achieved during the experiment, and 9 papers were finally included, all of which were low risk among random sequence generation; 3 were high risk and 6 were uncertain among Allocation concealment; 6 of the 9 papers were high risk and 3 were uncertain Blinding of participants and personnel; 1 was high risk and 2 were uncertain among Blinding of outcome assessment, the rest were low risk; 1 of the incomplete outcome data were uncertain and the rest were low risk; 2 of the selective reporting were uncertain and the rest were low risk; all other biases were low risk.
Figure 2.
Risk of bias graph.
Figure 3.
Risk of bias summary.
3.4. Meta-analysis of the effect of concurrent strength combined with endurance training on TC level in the T2DM
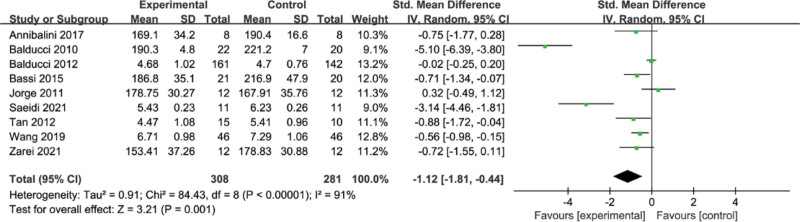
A total of 9 papers reported the effect of concurrent training on TC, and the results of the meta-analysis are shown in Figure 4, with heterogeneity among the 9 studies (I2 = 91%, P < .00001) and a random-effects model with a combined effect size of standardized mean difference (SMD) = −1.12, 95% CI = [−1.81, −0.44] (P = .001), indicating that concurrent training on T2DM patients with significant improvement in TC.
Figure 4.
Meta-analysis of the effect of concurrent strength combined with endurance training on TC level in the T2DM. T2DM = type 2 diabetes mellitus, TC = total cholesterol.
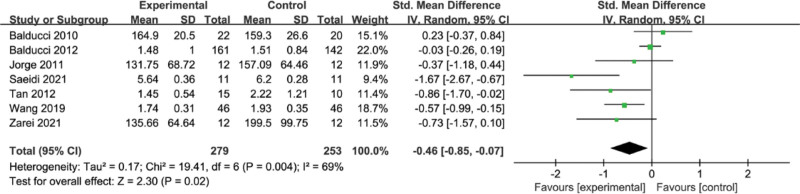
3.5. Meta-analysis of the effect of concurrent strength combined with endurance training on TG level in the T2DM
The results of the meta-analysis are shown in Figure 5. There was heterogeneity among the 7 studies (I2 = 69%, P = .004), and the combined effect size was SMD = −0.46, 95% CI = [−0.85, −0.07] (P = .02) using a random-effects model, indicating a significant improvement in TG with concurrent training.
Figure 5.
Meta-analysis of the effect of concurrent strength combined with endurance training on TG level in the T2DM. T2DM = type 2 diabetes mellitus, TG = triglycerides.
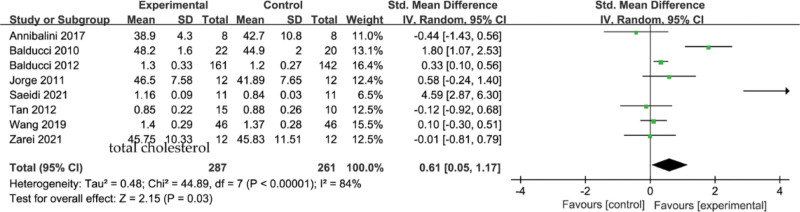
3.6. Meta-analysis of the effect of concurrent strength combined with endurance training on HDL-C level in the T2DM
A total of 8 studies were included in the analysis, and the results of the meta-analysis are shown in Figure 6, with a high heterogeneity among the 8 studies (I2 = 84%, P < .00001) and a statistically significant combined effect size of SMD = 0.61, 95% CI [0.05,1.17], Z = 2.15, P = .03, using a random-effects model, suggesting that the effect of concurrent training on HDL-C effect reached significance level.
Figure 6.
Meta-analysis of the effect of concurrent strength combined with endurance training on HDL-C level in the T2DM. HDL-C = high-density lipoprotein cholesterol, T2DM = type 2 diabetes mellitus.
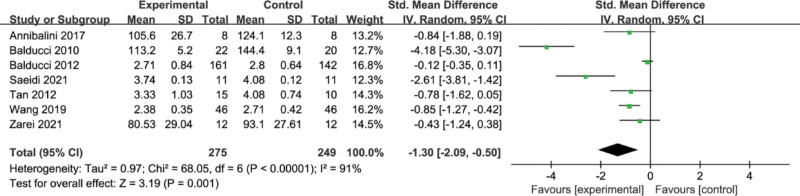
3.7. Meta-analysis of the effect of concurrent strength combined with endurance training on LDL-C level in the T2DM
A total of 7 papers reported the effect of concurrent training on LDL-C. The results of the meta-analysis are shown in Figure 7, with high heterogeneity among the 7 studies (I2 = 91%, P < .00001) and a random-effects model with a combined effect size of SMD = −1.30, 95% CI = [−2.09, −0.50] (P = .001), indicating that concurrent training had a significant effect on significant improvement in LDL-C.
Figure 7.
Meta-analysis of the effect of concurrent strength combined with endurance training on LDL-C level in the T2DM. LDL-C = low-density lipoprotein cholesterol, T2DM = type 2 diabetes mellitus.
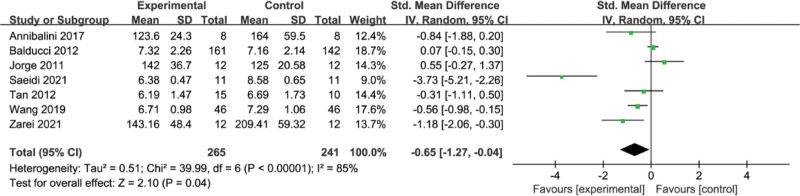
3.8. Meta-analysis of the effect of concurrent strength combined with endurance training on FBG level in the T2DM
A total of 7 studies were included in the analysis, and the results of the meta-analysis are shown in Figure 8, with a high heterogeneity among the 7 studies (I2 = 85%, P < .00001) and a statistically significant combined effect size of SMD = −0.65, 95% CI = [−1.27,−0.04] using a random-effects model, Z = 2.10, (P = .04), suggesting that concurrent training had a significant level of effect on FBG.
Figure 8.
Meta-analysis of the effect of concurrent strength combined with endurance training on FBG level in the T2DM. FBG = fasting blood glucose, T2DM = type 2 diabetes mellitus.
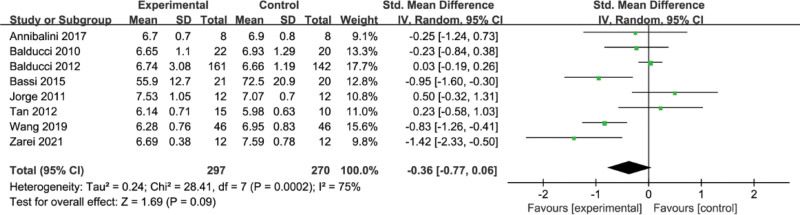
3.9. Meta-analysis of the effect of concurrent strength combined with endurance training on HbA1c level in the T2DM
A total of 7 papers reported the effect of concurrent training on HbA1c, and the results of the meta-analysis are shown in Figure 9, with high heterogeneity between the 7 studies, (I2 = 75%, P = .0002), using a random-effects model with a combined effect size of SMD = −0.36, 95% CI [−0.77, 0.06], (P = .09) not statistically significant, indicating that there was no significant difference in the effect of concurrent training on HbA1c.
Figure 9.
Meta-analysis of the effect of concurrent strength combined with endurance training on HbA1c level in the T2DM. HbA1c = glycosylated hemoglobin, T2DM = type 2 diabetes mellitus.
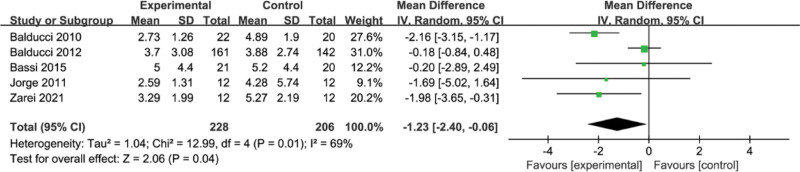
3.10. Meta-analysis of the effect of concurrent strength combined with endurance training on HOMA-IR level in the T2DM
A total of 5 studies were included in this study for analysis, and meta-analysis results showed a high level of heterogeneity between studies (I2 = 69%, P = .01) as shown in Figure 10, using a random-effects model with a combined effect size of SMD = −1.23, 95% CI = [−2.40,−0.06], which was statistically significant, Z = 2.06, P = .04, suggesting that concurrent training on HOMA-IR effect reached significance level.
Figure 10.
Meta-analysis of the effect of concurrent strength combined with endurance training on HOMA-IR level in the T2DM. HOMA-IR = homeostatic model assessment for insulin resistance, T2DM = type 2 diabetes mellitus.
4. Discussion
4.1. Effect of concurrent training on lipid profile
Diabetic patients are often accompanied by abnormalities in lipid metabolism, usually manifested by abnormal elevations in TC, TG with LDL-C and abnormal decreases in HDL-C.[20,21] This study showed that concurrent strength combined with endurance training had a significant effect on improving HDL-C, lowering LDL-C, TG, and TC. HDL-C has a certain protective effect on cardiovascular, and excess lipids in the blood are metabolized by HDL-C.[22] In the present study, concurrent strength combined with endurance training had a significant effect on improving HDL-C. Tiainen[23] found that HDL-C increased by 3.5% and TG decreased by 6% after 6 months of adherence to exercise. Meanwhile, Theodorou found a significant improvement in HDL-C by 8.6% after an exercise intervention in an elderly population.[24] Their findings were similar to the present study.
Lowering LDL-C helps prevent the occurrence of cardiovascular disease risk,[25] and this study similarly confirmed the positive effect of concurrent strength combined with endurance training on LDL-C. In Yang et al studied[26] the effect of concurrent strength combined with endurance training on serum cholesterol in middle-aged obese women. Subjects performed 45 minutes of aerobic exercise and 20 minutes of resistance training and showed significant improvements in both TG and LDL-C after 3 months. Meanwhile, recent studies have found that high TG levels are closely associated with the development of atherosclerotic cardiovascular disease.[27] The present study can show that concurrent strength combined with endurance training has a significant effect on improving TG. Regarding the role of concurrent strength combined with endurance training in improving lipids, some studies have shown that endurance training in simultaneous training achieves lipid improvement by regulating the levels and activities of related enzymes in the process of lipid metabolism.[28] However, plasma lipoprotein lipase promotes the hydrolysis of TG so that it is broken down to produce glycerol and fatty acids, and prolonged exercise accelerates the mobilization of fat in muscle so that fat is oxidized and used as an energy substance and TG is consumed.[29] Strength training during the same period of training also promotes lipolysis and increases blood circulation, while enhancing lipocalin activity to regulate lipid metabolism.[30,31]
4.2. Effect of concurrent training on glucose profile
In the meta-analysis conducted, concurrent strength combined with endurance training showed some improvement in FBG. One study showed[32] that both aerobic and resistance training improved glycemic control in type 2 diabetes, but aerobic combined with resistance training showed the greatest improvement. HbA1c is an important indicator of recent glycemic control, and good or bad glycemic control directly affects the appearance of some complications. Kelly[33] also found through their study that exercise has a significant effect on reducing HbA1c%. Concurrent strength combined with endurance training has been shown to improve muscle strength, maximal oxygen uptake, insulin sensitivity, and HbA1c.[34] Other studies have indicated that the improvement in HbA1c% after exercise did not significantly decrease. This may be influenced by the duration of intervention and the type of exercise modality. Furthermore, individuals with higher baseline HbA1c% values were found to have greater improvements compared to those with lower baseline values.[35,36] In this study, concurrent strength combined with endurance training did not show statistically significant improvement in HbA1c. This may be influenced by the duration of intervention and the type of exercise modality. HOMA-IR is not a disease but rather a state or condition in which the body tissue response to insulin is reduced. HOMA-IR serves as a basis for certain diseases, and type 2 diabetes is characterized by β-cell failure and a decrease in β-cell mass, which also contribute to HOMA-IR and insulin deficiency.[37–39] In this study, concurrent training had a significant effect on improving HOMA-IR. It has been shown that a single exercise session played a role in improving insulin resistance.[40] Improvement in HOMA-IR also occurred with a long period of exercise intervention. Researchers have found that insulin in skeletal muscle can regulate increased concentrations of glucose transporter protein[41] and increased glycogen synthase[42] in humans during prolonged regular exercise. Also, long-term aerobic exercise increases capillary density,[43] which can also improve the efficiency of glucose delivery to muscle, thus changing HOMA-IR.[44,45]
5. Conclusions
Concurrent strength combined with endurance training has a positive effect on the improvement of lipid and glucose profile in patients with type 2 diabetes.
6. Limitations of the study
There are limitations in this study: The included literature had variations in the exercise intervention protocols, with intervention durations ranging from 12 to 48 weeks and frequencies of 2 to 3 times per week, which may increase the heterogeneity of the study; The 9 studies included in this research had small sample sizes, which may introduce certain biases in the effects of the indicators and affect the intervention results. In future studies, it is recommended to select high-quality literature with rigorous experimental design, large sample sizes, and methodological standards, and perform meta-analysis based on addressing specific research questions.
Acknowledgments
The first author (Y.S.) extends heartfelt gratitude to all participants in this study, enabling the successful completion of this research. Additionally, the first author expresses sincere appreciation to parents, wife, and children for their consistent support, love, and encouragement. Furthermore, the author would like to thank Professor Hezhang Yun from Zhejiang Guangsha Vocational and Technical University of Construction for providing valuable feedback and meticulously reviewing this manuscript.
Author contributions
Conceptualization: Hezhang Yun, Yaowei Sun, Bin Lu, Wenbo Su.
Data curation: Yaowei Sun, Bin Lu, Jing Zheng.
Software: Xu Song, Xueyan Shang, Jing Wang.
Visualization: Xu Song, Jing Zheng, Jing Wang.
Writing – original draft: Yaowei Sun, Bin Lu, Wenbo Su, Hezhang Yun.
Writing – review & editing: Hezhang Yun.
Abbreviations:
- CI
- confidence intervals
- FBG
- fasting blood glucose
- HbA1c
- glycosylated hemoglobin
- HDL-C
- high-density lipoprotein cholesterol
- HOMA-IR
- homeostatic model assessment for insulin resistance
- LDL-C
- low-density lipoprotein cholesterol
- SMD
- standardized mean difference
- T2DM
- type 2 diabetes mellitus
- TC
- total cholesterol
- TG
- triglycerides
YS, BL, & WS contributed equally to this work.
Ethics Approval/Institutional Review Board: It not necessary, because no animal and human experiments have been done.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Sun Y, Lu B, Su W, Song X, Shang X, Zheng J, Wang J, Yun H. Comprehensive assessment of the effects of concurrent strength and endurance training on lipid profile, glycemic control, and insulin resistance in type 2 diabetes: A meta-analysis. Medicine 2024;103:12(e37494).
Contributor Information
Yaowei Sun, Email: sunyaow123@outlook.com.
Bin Lu, Email: 2021t1467@pwu.edu.ph.
Wenbo Su, Email: suwb@lzu.edu.cn.
Xu Song, Email: songxu0411@163.com.
Xueyan Shang, Email: 15212840001@163.com.
Jing Zheng, Email: WANGJING@zjnu.education.cn.
Jing Wang, Email: WANGJING@zjnu.education.cn.
References
- [1].Barella LF, Jain S, Kimura T, et al. Metabolic roles of G protein-coupled receptor signaling in obesity and type 2 diabetes. FEBS J. 2021;288:2622–44. [DOI] [PubMed] [Google Scholar]
- [2].Cai Z, Deng X, Zhao L, et al. The relationship between Schistosoma and glycolipid metabolism. Microb Pathog. 2021;159:105120. [DOI] [PubMed] [Google Scholar]
- [3].Thent ZC, Das S, Henry LJ. Role of exercise in the management of diabetes mellitus: the global scenario. PLoS One. 2013;8:e80436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Haus JM, Solomon TP, Marchetti CM, et al. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab. 2010;95:323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Solomon TP, Sistrun SN, Krishnan RK, et al. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol (Bethesda, Md. : 1985) 2008;104:1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–7. [DOI] [PubMed] [Google Scholar]
- [7].Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. [DOI] [PubMed] [Google Scholar]
- [8].Zaki S, Sharma S, Vats H. Effectiveness of concurrent exercise training in people with type 2 diabetes: a systematic review and meta-analysis. Physiother Theory Pract. 2023:1–22. [DOI] [PubMed] [Google Scholar]
- [9].Zhao X, He Q, Zeng Y, et al. Effectiveness of combined exercise in people with type 2 diabetes and concurrent overweight/obesity: a systematic review and meta-analysis. BMJ Open. 2021;11:e046252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bassi D, Mendes RG, Arakelian VM, et al. Potential effects on cardiorespiratory and metabolic status after a concurrent strength and endurance training program in diabetes patients - a randomized controlled trial. Sports Med Open. 2015;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Systematic Rev. 2021;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Annibalini G, Lucertini F, Agostini D, et al. Concurrent aerobic and resistance training has anti-inflammatory effects and increases both plasma and leukocyte levels of IGF-1 in late middle-aged type 2 diabetic patients. Oxid Med Cell Longev. 2017;2017:3937842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Balducci S, Zanuso S, Nicolucci A, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20:608–17. [DOI] [PubMed] [Google Scholar]
- [14].Balducci S, Zanuso S, Cardelli P, et al. Italian Diabetes Exercise Study (IDES) Investigators. Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the Italian Diabetes and Exercise Study (IDES). PLoS One. 2012;7:e49297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jorge ML, de Oliveira VN, Resende NM, et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60:1244–52. [DOI] [PubMed] [Google Scholar]
- [16].Saeidi A, Soltani M, Daraei A, et al. The effects of aerobic-resistance training and broccoli supplementation on plasma dectin-1 and insulin resistance in males with type 2 diabetes. Nutrients 2021;13:3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tan S, Li W, Wang J. Effects of six months of combined aerobic and resistance training for elderly patients with a long history of type 2 diabetes. J Sports Sci Med. 2012;11:495–501. [PMC free article] [PubMed] [Google Scholar]
- [18].Wang GF, Huang YZ, He MW, et al. Effects of resistance training combined with aerobic exercise and diet adjustment on complications and quality of life in patients with type 2 diabetes mellitus. J Pathol Clin Res. 2019;39:1465–70. [Google Scholar]
- [19].Zarei M, Khodakheyr JN, Rashidlamir A, et al. The effect of combined resistance aerobic exercise training on concentrations of asprosin and complement C1q tumor necrosis factor-related protein-1 in men with type 2 diabetes. Sport Sci Health. 2021;17:863–71. [Google Scholar]
- [20].Gong J, Fang K, Dong H, et al. Effect of fenugreek on hyperglycaemia and hyperlipidemia in diabetes and prediabetes: a meta-analysis. J Ethnopharmacol. 2016;194:260–8. [DOI] [PubMed] [Google Scholar]
- [21].Yun H, Su W, Zhao H, et al. Effects of different exercise modalities on lipid profile in the elderly population: a meta-analysis. Medicine (Baltim). 2023;102:e33854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scalsky RJ, Chen YJ, Desai K, et al. Baseline cardiometabolic profiles and SARS-CoV-2 infection in the UK Biobank. PLoS One. 2021;16:e0248602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tiainen S, Luoto R, Ahotupa M, et al. 6-mo aerobic exercise intervention enhances the lipid peroxide transport function of HDL. Free Radic Res. 2016;50:1279–85. [DOI] [PubMed] [Google Scholar]
- [24].Theodorou AA, Panayiotou G, Volaklis KA, et al. Aerobic, resistance and combined training and detraining on body composition, muscle strength, lipid profile and inflammation in coronary artery disease patients. Res Sports Med. 2016;24:171–84. [DOI] [PubMed] [Google Scholar]
- [25].Nurmohamed NS, Navar AM, Kastelein JJP. New and emerging therapies for reduction of LDL-Cholesterol and Apolipoprotein B: JACC Focus Seminar 1/4. J Am Coll Cardiol. 2021;77:1564–75. [DOI] [PubMed] [Google Scholar]
- [26].Yang SJ, Hong HC, Choi HY, et al. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clin Endocrinol (Oxf). 2011;75:464–9. [DOI] [PubMed] [Google Scholar]
- [27].Laufs U, Parhofer KG, Ginsberg HN, et al. Clinical review on triglycerides. Eur Heart J. 2020;41:99–109c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Egli L, Lecoultre V, Theytaz F, et al. Exercise prevents fructose-induced hypertriglyceridemia in healthy young subjects. Diabetes. 2013;62:2259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Earnest CP, Artero EG, Sui X, et al. Maximal estimated cardiorespiratory fitness, cardiometabolic risk factors, and metabolic syndrome in the aerobics center longitudinal study. Mayo Clin Proc. 2013;88:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Augusto Libardi C, Bonganha V, Soares Conceição M, et al. The periodized resistance training promotes similar changes in lipid profile in middle-aged men and women. J Sports Med Phys Fitness. 2012;52:286–92. [PubMed] [Google Scholar]
- [31].Varady KA, Jones PJ. Combination diet and exercise interventions for the treatment of dyslipidemia: an effective preliminary strategy to lower cholesterol levels? J Nutr. 2005;135:1829–35. [DOI] [PubMed] [Google Scholar]
- [32].Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–69. [DOI] [PubMed] [Google Scholar]
- [33].Kelley GA, Kelley KS. Effects of aerobic exercise on lipids and lipoproteins in adults with type 2 diabetes: a meta-analysis of randomized-controlled trials. Public Health. 2007;121:643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cuff DJ, Meneilly GS, Martin A, et al. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–82. [DOI] [PubMed] [Google Scholar]
- [35].Arora E, Shenoy S, Sandhu JS. Effects of resistance training on metabolic profile of adults with type 2 diabetes. Indian J Med Res. 2009;129:515–9. [PubMed] [Google Scholar]
- [36].Sigal RJ, Kenny GP. Combined aerobic and resistance exercise for patients with type 2 diabetes. JAMA. 2010;304:2298–9. [DOI] [PubMed] [Google Scholar]
- [37].Ferrannini E. The stunned beta cell: a brief history. Cell Metab. 2010;11:349–52. [DOI] [PubMed] [Google Scholar]
- [38].Kasuga M. Insulin resistance and pancreatic beta cell failure. J Clin Invest. 2006;116:1756–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. [DOI] [PubMed] [Google Scholar]
- [40].Mikines KJ, Sonne B, Farrell PA, et al. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988;254(3 Pt 1):E248–59. [DOI] [PubMed] [Google Scholar]
- [41].Zisman A, Peroni OD, Abel ED, et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med. 2000;6:924–8. [DOI] [PubMed] [Google Scholar]
- [42].Dela F, Larsen JJ, Mikines KJ, et al. Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training. Diabetes. 1995;44:1010–20. [DOI] [PubMed] [Google Scholar]
- [43].Harris BA. The influence of endurance and resistance exercise on muscle capillarization in the elderly: a review. Acta Physiol Scand. 2005;185:89–97. [DOI] [PubMed] [Google Scholar]
- [44].Coggins M, Lindner J, Rattigan S, et al. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes. 2001;50:2682–90. [DOI] [PubMed] [Google Scholar]
- [45].Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–23. [DOI] [PubMed] [Google Scholar]