Abstract
Background
Few studies have examined prostate cancer incidence and aggressiveness in urban-rural Appalachian populations. We examined these rates in urban-rural Appalachia and non-Appalachia Pennsylvania (PA), and the association between these areas and more aggressive prostate cancer at diagnosis.
Methods
Men, aged ≥ 40 years, with a primary prostate cancer diagnosis were identified from the 2004–2014 Pennsylvania Cancer Registry. Age-adjusted incidence rates for prostate cancer and more aggressive prostate cancer at diagnosis were calculated by urban-rural Appalachia status. Multivariable Poisson regressions were conducted. Multiple logistic regressions were used to examine the association between the geographical areas and more aggressive prostate cancer, after adjusting for confounders.
Results
There were 94,274 cases, aged 40–105 years, included. Urban non-Appalachia had the highest 2004–2014 age-adjusted incidence rates of prostate cancer and more aggressive prostate cancer (293.56 and 96.39 per 100,000 men, respectively) and rural Appalachia had the lowest rates (256.48 and 80.18 per 100,000 men, respectively). Among the cases, urban Appalachia were more likely (OR=1.12; 95% CI=1.08–1.17) and rural Appalachia were less likely (OR= 0.92; 95% CI=0.87–0.97) to have more aggressive prostate cancer at diagnosis compared to urban non-Appalachia.
Conclusions
Lower incidence rates and the proportion of aggressive disease in rural Appalachia may be due to lower prostate cancer screening rates. More aggressive prostate cancer at diagnosis among the cases in urban Appalachia may be due to exposures that are prevalent in the region.
Impact
Identifying geographical prostate cancer disparities will provide information to design programs aimed at reducing risk and closing the disparity gap.
Introduction
Prostate cancer is the most common malignancy and the second leading cause of cancer-related deaths among U.S. men(1). Even though prostate cancer incidence (166.31 to 108.40 per 100,000 men) and mortality (26.19 to 19.39 per 100,000 men) rates have decreased in the U.S. from 2004 to 2016(2), some men remain at higher risk for aggressive prostate cancer that can lead to poor outcomes such as metastasis and prostate cancer-related death. Black men are known to have higher prostate cancer incidence rates and more aggressive disease compared to other racial groups(1,3,4), demonstrating a racial disparity for this disease. As for geographical disparities, areas that have lower socioeconomic status and poor access to medical care have been found to have higher prostate cancer incidence rates for both rural and urban populations compared to areas that have a higher socioeconomic status and where medical care is more accessible(5). However, few studies have examined geographical disparities for prostate cancer in the Appalachian region, a geopolitical designation defined by the Appalachia Regional Commission, that roughly follows the spine of the Appalachian mountains(6). The Appalachian region is 42% rural and is characterized by lower income, less accessibility to medical care, and lower high school graduation rates compared to the rest of the U.S(6,7).
Studies have found that Appalachian populations had higher overall cancer risk compared to other parts of the U.S.(5,8,9). For prostate cancer, two studies examined age-adjusted prostate cancer incidence rates and found lower rates in Appalachia compared to non-Appalachia U.S. populations. When examined by different geographical regions in Appalachia, one study reported Northern Appalachia having slightly higher prostate cancer incidence rates compared non-Appalachia U.S. incidence rates in 2001–2003(8); another study reported Southern Appalachia having slightly higher prostate cancer incidence rates compared non-Appalachia U.S. incidence rates in 2004–2011(9). According to the Appalachian Community Cancer Network, Appalachia Pennsylvania (PA), which is part of Northern Appalachia, had a lower age-adjusted prostate cancer incidence rate (incidence rates: 148.5 in Appalachia PA versus 164.3 in non-Appalachia PA per 100,000 men) but a higher proportion of late stage of disease at diagnosis (distant stage) compared to non-Appalachia PA in 2002–2006(7). However, these rates have not been examined by urban-rural regions in PA; therefore, possible geographical disparities in Appalachia versus non-Appalachia PA by urban-rural regions remain not known.
To investigate possible geographical prostate cancer disparities in PA, we examined whether the incidence rates of prostate cancer and more aggressive prostate cancer and the proportion of more aggressive prostate cancer at diagnosis among cases differed between Appalachia and non-Appalachia by urban-rural regions. We also examined whether having more aggressive prostate cancer at diagnosis was associated with residing in rural or urban Appalachia PA compared to urban non-Appalachia PA.
Materials and Methods
Study population
We conducted a cross-sectional study among men, aged ≥ 40 years, identified from the Pennsylvania Cancer Registry (PCR) who had a primary, clinical diagnosis of prostate cancer between 2004 and 2014. Men were included if they had a Gleason score (GS) ≥ 6. Men who had an unknown GS but a known tumor stage ≥ T3 were also included. Demographic (i.e., age, race, ethnicity, and insurance) and clinical/pathologic information (i.e., serum prostate-specific antigen [PSA], GS, tumor stage, lymph node positive status, and definitive treatment) were extracted from the PCR. Definitive treatment was defined as primary site surgery and/or radiation in order to capture the most common prostate cancer treatments with curative intent.
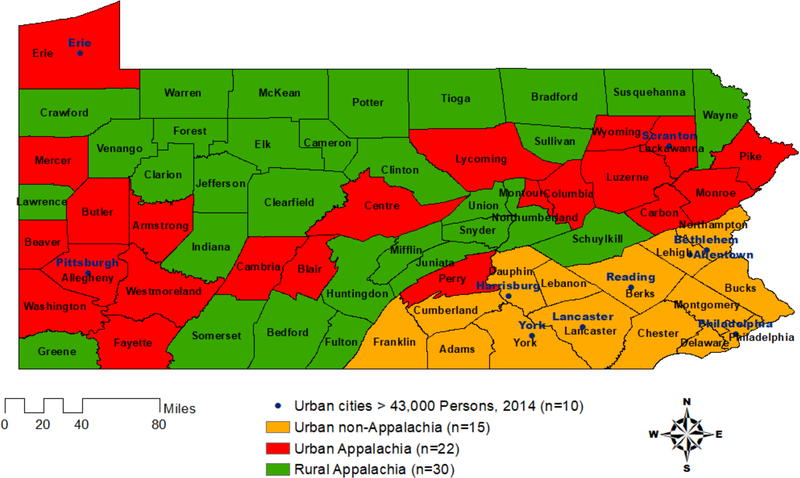
Appalachian counties by urban-rural regions were classified into three geographical strata using the Appalachian Regional Commission definition(6) and the U.S. Department of Agriculture’s Rural-Urban Continuum Code (RUCC)(10), where counties coded with a RUCC < 4 represent metro (urban) areas(11): rural Appalachia, urban Appalachia, and urban non-Appalachia (Figure 1). PA does not have any rural non-Appalachian counties in the state; as a result, all rural regions are located within Appalachia. Prostate cancer aggressiveness was classified into two disease risk groups defined by pathologic GS and tumor stage or GS at biopsy and clinical tumor stage if pathologic results were missing: 1) less aggressive prostate cancer (GS 6 or GS 7 [3 + 4], tumor stage T1-T2, and no distant metastasis) and 2) more aggressive prostate cancer (GS ≥ 7 [4+3] or tumor stage ≥ T3 or distant metastasis).
Figure 1. Appalachia and Non-Appalachia Counties in Pennsylvania by Urban-Rural Regions.
The Appalachian Regional Commission definition and the U.S. Department of Agriculture’s Rural-Urban Continuum Code (RUCC) were used to define Appalachia areas by rurality; and, n equals the number of urban cities > 43,000 persons in 2014 and the number of counties that are classified as urban non-Appalachia, urban Appalachia, and rural Appalachia PA.
The reporting of all cancer cases to the PCR is mandated by the state; therefore, informed consent of prostate cancer cases was waived. This study was conducted in accordance with recognized ethical guidelines and approved by the Pennsylvania Department of Health and the Institutional Review Board of the Pennsylvania State University College of Medicine.
Data Analysis
Summary statistics for demographic and clinical characteristics were calculated and compared among the three geographical areas (rural Appalachia, urban Appalachia, and urban non-Appalachia) using Chi-square test for categorical variables and one-way ANOVA for continuous variables. Age-adjusted incidence rates for prostate cancer and more aggressive prostate cancer at diagnosis were calculated(12–16). The 2000 U.S. Standard Population was adjusted for the application of corresponding distribution weights to an age-specific subgroup of the population (age groups: 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, and 85+ years)(17). For these rate calculations, only the male population was used for prostate cancer, and either the ‘white alone male population’ or the ‘black or African American alone male population’ census estimates were used accordingly for incidence rates stratified by race (Poisson regression below); otherwise, the ‘total male population’ was used. Age-adjusted incidence rates of more aggressive prostate cancer were calculated similarly, having the numerator considered only prostate cancer cases defined as aggressive. Age-adjusted incidence rates of distant metastasis at diagnosis (a subset of more aggressive prostate cancer) were calculated using the same methodology. The proportion of more aggressive prostate cancer at diagnosis was calculated as the number of more aggressive prostate cancer cases divided by the total number of prostate cancer cases at diagnosis, overall and stratified by age group and geographical area. Incidence rate ratios (RRs) and their 95% confidence interval (CI) were calculated(18,19).
Multivariable Poisson regressions were conducted to examine the association between geographical areas and the incidence rates of prostate cancer and more aggressive prostate cancer, after adjusting for age group (40–49, 50–59, 60–69, and 70+ years), race, and year of diagnosis using the 2004–2014 U.S. Census data(12,13) in which the incidence RRs and their 95% CI were calculated. Multiple logistic regressions were used to examine the association between geographical areas and more aggressive prostate cancer at the time of diagnosis among the cases, after adjusting for age, race, ethnicity, insurance status, serum PSA, positive lymph node status, and year of prostate cancer diagnosis, in which the odds ratios (ORs) and their 95% CI were calculated, overall and stratified by race (black and white cases).
All data analyses were performed in R version 3.6.1(20) using a two-sided significance level of 0.05.
Results
Of the 102,194 prostate cancer cases reported with a primary diagnosis in the 2004–2014 PCR, there were 94,274 eligible men, aged 40 to 105 years, identified. The majority of the cases were of white race (83.9%). There were 56,121 and 30,931 cases classified as having less and more aggressive prostate cancer, respectively (Table 1). Cases from rural Appalachia were older in age, less likely to have more aggressive prostate cancer at diagnosis, and less likely to receive definitive prostate cancer treatment compared to urban Appalachia and urban non-Appalachia. Cases from urban Appalachia were more likely to have more aggressive prostate cancer at diagnosis compared to rural Appalachia and urban non-Appalachia. Cases from urban non-Appalachia had a lower serum PSA level and were more likely to receive definitive prostate cancer treatment compared to rural and urban Appalachia.
Table 1.
Study Characteristics of 2004–2014 Prostate Cancer Cases in Pennsylvania, According to Geographical Area of Residence at the Time of Diagnosis
| Overall (N=94,274) | Rural Appalachia (N=11,941) | Urban Appalachia (N=31,886) | Urban Non-Appalachia (N=50,447) | P-Value | |
|---|---|---|---|---|---|
|
| |||||
| Age at Diagnosis in Years | <0.001 | ||||
| Mean (SD, Range) | 66.38 (9.38, 40–105) | 67.22 (9.29, 40–105) | 66.77 (9.46, 40–105) | 65.94 (9.32, 40–99) | |
| Median (25th–75th Percentile) | 66 (60–73) | 67 (61–74) | 66 (60–73) | 66 (59–73) | |
|
| |||||
| Race N (%) | <0.001 | ||||
| White | 79066 (83.87) | 11544 (96.68) | 29010 (90.98) | 38512 (76.34) | |
| Black | 10067 (10.68) | 163 (1.37) | 1923 (6.03) | 7981 (15.82) | |
| Other | 1022 (1.08) | 46 (0.39) | 189 (0.59) | 787 (1.56) | |
| Unknown | 4119 (4.37) | 188 (1.57) | 764 (2.40) | 3167 (6.28) | |
|
| |||||
| Ethnicity N (%) | <0.001 | ||||
| Hispanic | 1309 (1.39) | 53 (0.44) | 178 (0.56) | 1078 (2.14) | |
| Non-Hispanic | 80271 (85.15) | 10444 (87.46) | 26380 (82.73) | 43447 (86.12) | |
| Unknown | 12694 (13.47) | 1444 (12.09) | 5328 (16.71) | 5922 (11.74) | |
|
| |||||
| Insurance N (%) | 0.53 | ||||
| Insured | 76737 (81.40) | 9898 (82.89) | 26615 (83.47) | 40224 (79.74) | |
| Uninsured | 443 (0.47) | 62 (0.52) | 143 (0.45) | 238 (0.47) | |
| Unknown | 17094 (18.13) | 1981 (16.59) | 5128 (16.08) | 9985 (19.79) | |
|
| |||||
| Serum PSA (ng/mL) | <0.001 | ||||
| Mean (SD, Range) | 12.21 (19.03, 0.1–98.0) | 12.55 (19.00, 0.1–98.0) | 12.54 (19.33, 0.1–98.0) | 11.93 (18.84, 0.1–98.0) | |
| Median (25th–75th Percentile) | 6.00 (4.40, 9.80) | 6.10 (4.50, 10.20) | 6.10 (4.50, 10.00) | 5.80 (4.30, 9.50) | |
| Unknown N | 11963 | 1250 | 4226 | 6487 | |
|
| |||||
| Gleason Score N (%) | <0.001 | ||||
| 6 | 40762 (43.24) | 5464 (45.76) | 13301 (41.71) | 21997 (43.60) | |
| 7 | 37531 (39.81) | 4467 (37.41) | 12672 (39.74) | 20392 (40.42) | |
| 8–10 | 15525 (16.47) | 1944 (16.28) | 5773 (18.11) | 7808 (15.48) | |
| Unknown | 456 (0.48) | 66 (0.55) | 140 (0.44) | 250 (0.50) | |
|
| |||||
| Tumor Stage N (%) | <0.001 | ||||
| T1 | 37957 (40.26) | 5138 (43.03) | 13437 (42.14) | 19382 (38.42) | |
| T2 | 41341 (43.85) | 5117 (42.85) | 13544 (42.48) | 22680 (44.96) | |
| T3 | 8539 (9.06) | 983 (8.23) | 2912 (9.13) | 4644 (9.21) | |
| T4 | 1338 (1.42) | 186 (1.56) | 481 (1.51) | 671 (1.33) | |
| Unknown | 5099 (5.41) | 517 (4.33) | 1512 (4.74) | 3070 (6.09) | |
|
| |||||
| Distant Metastasis N (%) | <0.001 | ||||
| Yes | 3580 (3.80) | 482 (4.04) | 1384 (4.34) | 1714 (3.40) | |
| No | 82355 (87.36) | 10272 (86.02) | 27765 (87.08) | 44318 (87.85) | |
| Unknown | 8339 (8.85) | 1187 (9.94) | 2737 (8.58) | 4415 (8.75) | |
|
| |||||
| Stage of Aggressiveness N (%) | <0.001 | ||||
| Less Aggressive | 56121 (59.53) | 7226 (60.51) | 18536 (58.13) | 30359 (60.18) | |
| More Aggressive | 30931 (32.81) | 3670 (30.73) | 11039 (34.62) | 16222 (32.16) | |
| Unknown | 7222 (7.66) | 1045 (8.75) | 2311 (7.25) | 3866 (7.66) | |
|
| |||||
| Lymph Node Positive N (%) | 0.11 | ||||
| No | 81875 (86.85) | 10236 (85.72) | 27499 (86.24) | 44140 (87.50) | |
| Yes | 2327 (2.47) | 291 (2.44) | 828 (2.60) | 1208 (2.39) | |
| Unknown | 10072 (10.68) | 1414 (11.84) | 3559 (11.16) | 5099 (10.11) | |
|
| |||||
| Definitive Treatment Received N (%) | <0.001 | ||||
| Yes | 69334 (73.55) | 8102 (67.85) | 22610 (70.91) | 38622 (76.56) | |
| No | 22707 (24.09) | 3658 (30.63) | 8641 (27.10) | 10408 (20.63) | |
| Unknown | 2233 (2.37) | 181 (1.52) | 635 (1.99) | 1417 (2.81) | |
|
| |||||
| Definitive Treatment Regimen N (%) | <0.001 | ||||
| Radiation Only | 35373 (37.52) | 4244 (35.54) | 11440 (35.88) | 19689 (39.03) | |
| Primary Site Surgery Only | 31985 (33.93) | 3628 (30.38) | 10606 (33.26) | 17751 (35.19) | |
| Both | 1976 (2.10) | 230 (1.93) | 564 (1.77) | 1182 (2.34) | |
| Neither | 22707 (24.09) | 3658 (30.63) | 8641 (27.10) | 10408 (20.63) | |
| Unknown | 2233 (2.37) | 181 (1.52) | 635 (1.99) | 1417 (2.81) | |
Note: SD = Standard Deviation; PSA = prostate-specific antigen; N = number of cases; Rural refers to Non-Metro Rural-Urban Continuum Code (RUCC); Urban refers to Metro RUCC; Other races include Asian, American Indian, and Pacific Islander; Tumor stage was based on TNM 7; Primary site surgery refers only to total organ resection (radical prostatectomy NOS, total prostatectomy NOS, prostatectomy with resection in continuity with other organs, prostatectomy NOS); Definitive treatment received refers to any treatment regimen involving radiation or primary site surgery; and, “Unknown” refers to variable documented as unknown or missing data.
The 2004–2014 age-adjusted incidence rates of prostate cancer and more aggressive prostate cancer in PA were 276.68 per 100,000 men and 92.43 per 100,000 men (adjusted to 2000 U.S. standard population), respectively. Urban non-Appalachia had the highest 2004–2014 age-adjusted incidence rates of prostate cancer and more aggressive prostate cancer (293.56 and 96.39 per 100,000 men, respectively) followed by urban Appalachia (260.46 and 91.53 per 100,000 men, respectively) and rural Appalachia (256.48 and 80.18 per 100,000 men, respectively). As for race, black men had higher 2004–2014 overall age-adjusted incidence rates of prostate cancer and more aggressive prostate cancer (400.63 and 153.11 per 100,000 men, respectively) compared to white men (256.34 and 84.94 per 100,000 men, respectively) regardless of geographical area, with urban non-Appalachia having the highest rates among black men (black men: 412.49 and 155.48 per 100,000 men, respectively; white men: 262.08 and 84.52 per 100,000 men, respectively) followed by urban Appalachia (black men: 364.00 and 147.22 per 100,000 men, respectively; white men: 250.65 and 87.85 per 100,000 men, respectively) and rural Appalachia (black men: 362.65 and 130.25 per 100,000 men, respectively; white men: 252.12 and 78.96 per 100,000 men, respectively). Urban Appalachia had the highest 2004–2014 age-adjusted incidence rate of distant metastasis at diagnosis (11.86 per 100,000 men) followed by rural Appalachia (10.95 per 100,000 men) and urban non-Appalachia (10.48 per 100,000 men).
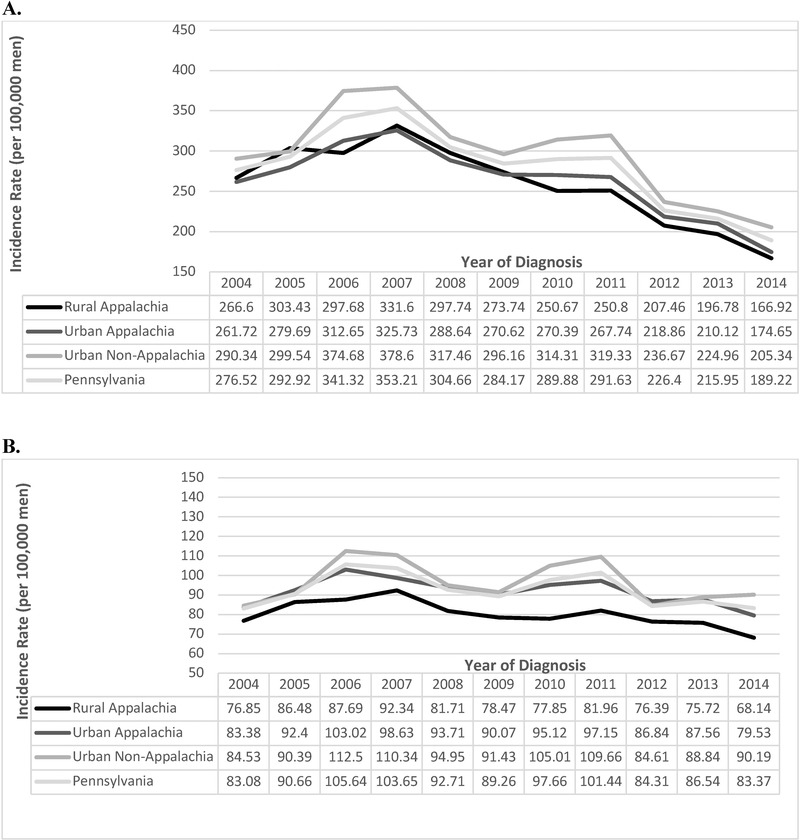
Age-adjusted incidence rates of prostate cancer decreased from 2004 to 2014 for each geographical area (p-values for trend < 0.01), with a 37%, 33%, and 29% decrease for rural Appalachia, urban Appalachia, and urban non-Appalachia, respectively (Figure 2). Age-adjusted incidence rates for more aggressive prostate cancer in 2014 compared to 2004 were lower for rural and urban Appalachia (RR= 0.89; 95% CI= 0.73–1.05 and RR = 0.95; 95% CI= 0.86–1.05, respectively) and higher for urban non-Appalachia (RR = 1.07; 95% CI = 0.99–1.14) (Figure 2); however, these comparisons were not statistically significant.
Figure 2. Age-Adjusted Incidence Rates of Prostate Cancer and More Aggressive Prostate Cancer by Year of Diagnosis in Appalachia and Non-Appalachia Pennsylvania by Urban-Rural Regions, 2004–2014.
A. Age-Adjusted Incidence Rates of Prostate Cancer. B. Age-Adjusted Incidence Rates of More Aggressive Prostate Cancer.
The associations between geographical areas and the 2004–2014 incidence rates of prostate cancer and aggressive prostate cancer were examined, after adjusting for age group, race, and year of diagnosis (Supplementary Table 1). The 2004–2014 incidence rate of prostate cancer was statistically significantly lower in urban Appalachia compared to urban non-Appalachia (RR = 0.95; 95% CI = 0.92–0.98). However, the 2004–2014 incidence rate of aggressive prostate cancer was higher in urban Appalachia compared to urban non-Appalachia (RR = 1.02; 95% CI = 0.97–1.08); this association was not statistically significant. The 2004–2014 incidence rates of prostate cancer and aggressive prostate cancer were lower in rural Appalachia compared to urban non-Appalachia (RR = 0.96; 95% CI= 0.92–1.00 and RR = 0.93; 95% CI= 0.86–1.01, respectively); these associations were not statistically significant.
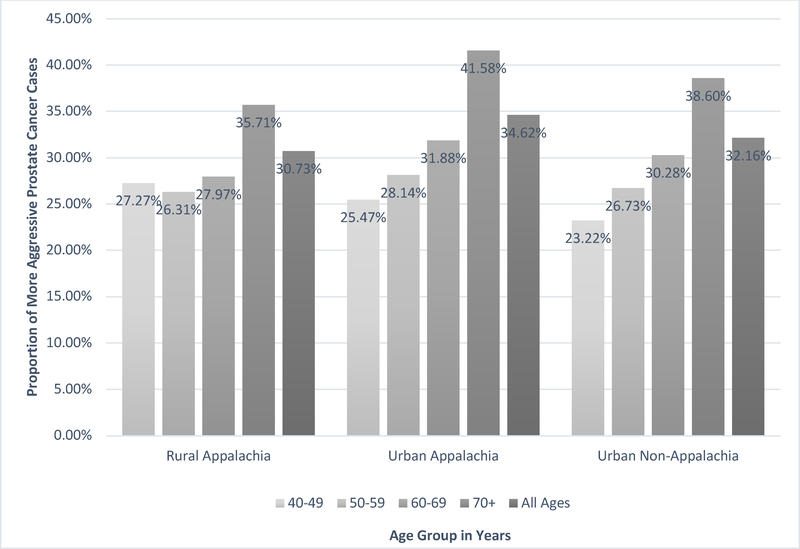
Urban Appalachia had a statistically significantly higher proportion of more aggressive prostate cancer at diagnosis (34.62%) compared to urban non-Appalachia (32.16%) and rural Appalachia (30.73%) in 2004–2014 (p-values < 0.001) (Figure 3). The proportion of more aggressive prostate cancer increased with older age groups in urban Appalachia and urban non-Appalachia. For rural Appalachia, men ages 40–49 years had a slightly higher proportion of aggressive prostate cancer (27.27%) compared to men ages 50–59 years (26.31%); however, the comparison between these two age groups was not statistically significant (p-value=0.74). Men ages ≥ 70 years had a significantly higher proportion of more aggressive prostate cancer compared to younger men (40–49, 50–59, and 60–69 years) for each geographical area (p-values < 0.01).
Figure 3.
The Proportion of More Aggressive Prostate Cancer at Diagnosis, According to Age and Geographical Area in Pennsylvania, 2004–2014.
Among the prostate cancer cases after adjusting for potential confounders, urban Appalachia was more likely to have aggressive prostate cancer at diagnosis compared to urban non-Appalachia overall (OR= 1.12; 95% CI= 1.08–1.17) and stratified by race (ORBlack= 1.18; 95% CI= 1.03–1.35; ORWhite= 1.12; 95% CI= 1.07–1.17) (Table 2). Rural Appalachia was less likely to have more aggressive prostate cancer at diagnosis compared to urban non-Appalachia overall (OR= 0.92; 95% CI= 0.87–0.97), among white men (OR= 0.92; 95% CI= 0.86–0.97), and among black men (OR= 0.86; 95% CI= 0.54–1.33); however, the association was not statistically significant among black men.
Table 2.
Multiple Logistic Regression Analysis of More Aggressive compared to Less Aggressive Prostate Cancer Cases at Diagnosis
| All Cases (N=57,885: 20,481 More Aggressive; 37,404 Less Aggressive) | White Cases (N=50,640: 17,676 More Aggressive; 32,964 Less Aggressive) | Black Cases (N=6,568: 2,547 More Aggressive; 4,021 Less Aggressive) | |
|---|---|---|---|
| Variables | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Age at Diagnosis in Years A | 1.03 (1.03, 1.03) | 1.03 (1.03, 1.04) | 1.02 (1.01, 1.02) |
| Serum PSAA (ng/mL) | 1.03 (1.03, 1.03) | 1.03 (1.03, 1.03) | 1.03 (1.03, 1.03) |
| Race | |||
| White | Reference | ------------------- | ------------------- |
| Black | 1.14 (1.07, 1.21) | ------------------- | ------------------- |
| Other | 1.13 (0.96, 1.34) | ------------------- | ------------------- |
| Ethnicity | |||
| Non-Hispanic | Reference | Reference | Reference |
| Hispanic | 1.01 (0.87, 1.17) | 1.07 (0.91, 1.26) | 0.60 (0.30, 1.15) |
| Insurance | |||
| Insured | Reference | Reference | Reference |
| Uninsured | 1.65 (1.29, 2.10) | 1.65 (1.26, 2.16) | 1.69 (0.93, 3.08) |
| Lymph Node Positive | |||
| No | Reference | Reference | Reference |
| Yes | 22.69 (18.45, 28.25) | 23.76 (18.97, 30.19) | 16.54 (9.84, 30.15) |
| Geographical Region | |||
| Urban Non-Appalachia | Reference | Reference | Reference |
| Rural Appalachia | 0.92 (0.87, 0.97) | 0.92 (0.86, 0.97) | 0.86 (0.54, 1.33) |
| Urban Appalachia | 1.12 (1.08, 1.17) | 1.12 (1.07, 1.17) | 1.18 (1.03, 1.35) |
| Year at Diagnosis | |||
| 2004 | Reference | Reference | Reference |
| 2005 | 0.77 (0.63, 0.94) | 0.79 (0.64, 0.99) | 0.62 (0.36, 1.07) |
| 2006 | 0.76 (0.63, 0.92) | 0.79 (0.64, 0.97) | 0.60 (0.36, 1.01) |
| 2007 | 0.73 (0.61, 0.89) | 0.76 (0.61, 0.93) | 0.60 (0.36, 1.01) |
| 2008 | 0.75 (0.62, 0.90) | 0.75 (0.61, 0.93) | 0.71 (0.43, 1.19) |
| 2009 | 0.82 (0.68, 0.99) | 0.82 (0.67, 1.02) | 0.76 (0.45, 1.27) |
| 2010 | 0.87 (0.72, 1.05) | 0.90 (0.73, 1.11) | 0.68 (0.41, 1.15) |
| 2011 | 0.93 (0.77, 1.13) | 0.93 (0.76, 1.15) | 0.88 (0.53, 1.48) |
| 2012 | 0.97 (0.80, 1.18) | 1.01 (0.82, 1.25) | 0.77 (0.47, 1.30) |
| 2013 | 1.10 (0.91, 1.34) | 1.14 (0.93, 1.41) | 0.85 (0.51, 1.43) |
| 2014 | 1.27 (1.05, 1.54) | 1.32 (1.07, 1.63) | 0.98 (0.59, 1.63) |
Note: OR = odds ratio; CI = confidence interval; PSA = prostate-specific antigen
= one unit increase; “All Cases” include other races and therefore the total number of all cases is greater than the sum of black and white cases; and, Unknown/missing values for the study variables were removed from the analysis.
Discussion
In the present study, urban non-Appalachia PA had the highest incidence rates of prostate cancer and aggressive prostate cancer compared to rural and urban Appalachia PA between 2004 and 2014. During this time period, the incidence rates of prostate cancer decreased for the three geographical areas which are consistent with a decrease in the overall U.S incidence rates of prostate cancer during this same time period(2). As for more aggressive prostate cancer, the age-adjusted incidence rates in 2014 compared to 2004 were lower for rural and urban Appalachia but higher for urban non-Appalachia; however, these rates were not statistically significant. Regardless of geographical area, black men had higher 2004–2014 age-adjusted incidence rates of prostate cancer and aggressive prostate cancer in comparison to white men. Among the prostate cancer cases, urban Appalachia were more likely and rural Appalachia were less likely to have more aggressive prostate cancer at diagnosis compared to urban non-Appalachia. The differences in the incidence rates and proportions of prostate cancer among these three geographical areas may be attributed to a combination of factors such as socioeconomics, access to screening services, and/or exposures that are prevalent in the area.
Studies have shown that rural Appalachia carries a higher burden of cancer compared to urban non-Appalachia(5,9). For prostate cancer, few studies have examined their burden in Appalachia, in particular, by urban-rural regions. In the present study, rural Appalachia PA had the lowest incidence rates of prostate cancer and more aggressive prostate cancer compared to both urban Appalachia and non-Appalachia PA; and, they were less likely to have more aggressive prostate cancer at diagnosis compared to urban non-Appalachia among the cases, after adjusting for confounders. Even among black men, rural Appalachia PA had lower incidence rates of prostate cancer and aggressive prostate cancer at diagnosis compared to urban Appalachia and non-Appalachia PA. One possible reason for the lower incidence rates could be that fewer men are being screened for prostate cancer in rural Appalachia compared to the urban areas. Appalachia has been reported to have lower breast and cervical cancer screening rates (2.5% lower) compared to the rest of the U.S.(21); and, lower colorectal cancer screening rates have been reported in rural Appalachia Kentucky (44%)(22). As for prostate cancer, screening rates for all Appalachia compared to the rest of the U.S. have not been reported. However, in PA, the Appalachian Community Cancer Network found that men, aged ≥ 50 years, in Appalachia PA were less likely to have had a serum PSA test or digital rectal examination (1–2% difference) compared to men of similar age from non-Appalachia PA(7). This finding suggests that there may be missed prostate cancer cases in Appalachia PA due to the lack of screening. It is not known whether the lower incidence rates of prostate cancer and aggressive prostate cancer in rural Appalachia are due to the cost of getting screened or the distance to travel to a medical facility that offers screening. A study found that both rural and urban prostate cancer patients who lived farther away from a treatment facility were less likely to receive radiation compared to surgery, especially among rural patients who lived > 75 miles away compared to ≤ 25 miles(23). As for costs, a study found that individuals with no insurance were less likely to have cancer screening(24). In the present study, there was no statistically significant association between insurance status and geographical area, as almost all of the study population was insured. However, other economic variables such as household income or being medically underinsured were not collected by the PCR and therefore these variables were not examined. As a result, economic inferences as a probable cause for a lower screening rate in rural Appalachia were not explored in this study, nor was the association between the distance to a medical facility and the incidence of prostate cancer.
The incidence rates of prostate cancer decreased from 2004 to 2014 for every geographical area, with rural Appalachia PA showing a greater decrease (37.4%) compared to urban Appalachian (33.3%) and urban non-Appalachia (29.3%) PA. This decrease supports a previous study that showed a decline in overall age-adjusted cancer incidence rates in urban Appalachia and urban non-Appalachia U.S. populations, 6% and 9% decrease, respectively, between 2000 and 2011(5). However, in this previous study(5), an increase of 6% in overall cancer incidence rate was observed for rural Appalachia which differed from the decreased prostate cancer incidence rate observed in the present study. The greater percent decrease of prostate cancer incidence rates in rural Appalachia PA could be due to lower rates of prostate cancer and/or lower prostate cancer screening rates in this geographical area.
As for aggressive prostate cancer, rural and urban Appalachia PA had a lower age-adjusted incidence rate in 2014 compared to 2004; however, urban non-Appalachia had a higher rate in 2014 compared to 2004, with an increase in rates from 84.61 per 100,000 men in 2012 to 90.19 per 100,000 in 2014. In 2008 and 2012, the U.S. Preventative Service Task Force’s (USPSTF) recommended against using serum PSA for prostate cancer screening for ages ≥ 75 years(25) and all ages(26), respectively which may have influenced screening practices. After the 2012 USPSTF recommendation, several studies reported a decrease in prostate cancer screening either by digital rectal examination or PSA, resulting in a decrease in the number of prostate biopsies performed(27). In addition, studies have reported an increase in the incidence rates of late stage prostate cancer and metastatic prostate cancer in the U.S. after the 2008 USPSTF’s recommendation against prostate cancer screening(28,29); however, these rates were not reported by urban/rural or Appalachia status. The decrease in the incidence rates of more aggressive prostate cancer in rural Appalachia may reflect the lower screening rates. The increase in incidence rates of more aggressive prostate cancer in urban non-Appalachia, in particular, after 2012, may be due to an increase in late stage disease at diagnosis.
Urban Appalachia had a very slightly higher 2004–2014 age-adjusted incidence rate of distant metastasis at diagnosis, followed closely by rural Appalachia compared to urban non-Appalachia. Conservatively speaking, Appalachia PA presented with slightly more advanced stages of disease at diagnosis based on distant metastasis, which is generally consistent with previous study findings that found more advanced stages of prostate cancer in Appalachia populations compared to non-Appalachia populations (7,30). The higher incidence rate of distant of metastasis at diagnosis in Appalachia PA in the present study may reflect the lack of prostate cancer screening at earlier stages of disease in this population.
Among the prostate cancer cases, urban Appalachia PA were more likely to have aggressive prostate cancer at diagnosis compared to urban non-Appalachia PA, after adjusting for age, race, ethnicity, insurance status, serum PSA, positive lymph node status, and year of diagnosis. Black race, smoking, and obesity have been suggested as possible risk factors for aggressive prostate cancer (3,31,32). Smoking and obesity, which are documented as being higher in Appalachia compared to non-Appalachia populations(7) are not collected in the PCR; therefore, these variables could not be considered. Increasing age is also associated with more aggressive disease(33) which is consistent with the present study’s findings of more aggressive disease among men ≥ 70 years of age. Even after for controlling for age and race, urban Appalachia (i.e., Alleghany County - Pittsburgh, PA; Erie County – Erie, PA; and, Lackawanna County – Scranton, PA) were more likely to have more aggressive prostate cancer at diagnosis among the cases (Figure 1). A possible reason for more aggressive disease in urban Appalachia could be due to exposures that are or have been prevalent to those areas. Appalachia areas, both rural and urban, are known to be rich in fossil fuels which include coal, oil, and gas(34). As a result, their economy has been supported by occupational industries that use these resources(6,7). Few studies have examined the relationship between these fossil fuels and various cancers, including prostate cancer. The limited number of studies that investigated associations between occupational and residential exposures of fossil fuels and prostate cancer risk and prostate cancer mortality have reported conflicting findings(35–38). In the present study, a higher proportion of more aggressive disease was observed among younger aged men at diagnosis (40–49 years) in rural Appalachia compared to men of similar age from urban Appalachia and non-Appalachia. This higher proportion of aggressive disease at diagnosis among younger aged men suggests that other factors may play a role in prostate cancer risk. Persistent exposures to environmental factors that are prevalent in Appalachia, such as fossil fuels, and their relationship to prostate cancer risk and progression warrant further investigation.
To our knowledge, this is the first study to report the incidence rates of prostate cancer and more aggressive prostate cancer as well as the proportion of aggressive prostate cancer at diagnosis in Appalachia compared to non-Appalachia by urban-rural regions in PA. However, there were some limitations associated with utilizing PCR data. The PCR does not collect comorbidity or behavioral variables such as smoking or obesity or other aggressive prostate cancer risk factors. Therefore, these potential confounding factors could not be included in the analyses. Missing data for some study variables resulted in a loss of prostate cancer cases that could be used for selected analyses. For a few cases, GS patterns 1 and 2 from the PCR did not always added up to the total GS; and, treatment and their corresponding date were not always consistent with other treatment status indicators, which may have led to misclassification.
In conclusion, a possible higher availability of prostate cancer screening in the urban compared to rural areas resulting in a greater prostate cancer detection rate may explain the lower incidence rates of prostate cancer and more aggressive prostate cancer in rural Appalachia PA. Exposures that are common in Appalachia or unknown factors may explain more aggressive prostate cancer in urban Appalachia PA. Determining whether prostate cancer disparities exist in rural Appalachia populations due to the lack of screening services as well as investigating whether exposures that are common in Appalachia compared non-Appalachia areas may contribute to prostate cancer risk and aggressiveness warrant further investigation in order to address geographical disparities for prostate cancer.
Supplementary Material
Acknowledgements
This work was supported by the Eberly Medical Research Endowment Innovation Fund at the Pennsylvania State University College of Medicine (ACM and MW); the National Center for Advancing Translational Sciences (UL1 TR002014 - MW); and, the Highmark Incorporation Grant at Penn State Cancer Institute (RJH, NRG, EW, and MW). A special thanks to James Rubertone for data extraction and other inquiries related to the Pennsylvania Cancer Registry.
Abbreviation list
- CI
confidence interval
- GS
Gleason score
- OR
odds ratio
- PA
Pennsylvania
- PCR
Pennsylvania Cancer Registry
- RR
rate ratio
- RUCC
Rural-Urban Continuum Code
- PSA
serum prostate-specific antigen
- USPSTF
U.S. Preventative Service Task Force
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2019. Atlanta: American Cancer Society 2019. [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2016. Bethesda, MD: National Cancer Institute; 2019. [Google Scholar]
- 3.Wood HM, Reuther AM, Gilligan TD, Kupelian PA, Modlin CS Jr., Klein EA. Rates of biochemical remission remain higher in black men compared to white men after radical prostatectomy despite similar trends in prostate specific antigen induced stage migration. Journal of Urology 2007;178(4 Pt 1):1271–6. [DOI] [PubMed] [Google Scholar]
- 4.Godley PA, Schenck AP, Amamoo MA, Schoenbach VJ, Peacock S, Manning M, et al. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. Journal of the National Cancer Institute 2003;95(22):1702–10. [DOI] [PubMed] [Google Scholar]
- 5.Yao N, Alcala HE, Anderson R, Balkrishnan R. Cancer Disparities in Rural Appalachia: Incidence, Early Detection, and Survivorship. The Journal of rural health : official journal of the American Rural Health Association and the National Rural Health Care Association 2017;33(4):375–81 doi 10.1111/jrh.12213. [DOI] [PubMed] [Google Scholar]
- 6.Appalachia Regional Commission. The Appalachian Region. Appalachia Regional Commission; <https://www.arc.gov/appalachian_region/TheAppalachianRegion.asp>. Accessed 2019. [Google Scholar]
- 7.Appalachia Community Cancer Network. The Cancer Burden in Appalachia. 2009.
- 8.Wingo PA, Tucker TC, Jamison PM, Martin H, McLaughlin C, Bayakly R, et al. Cancer in Appalachia, 2001–2003. Cancer 2008;112(1):181–92 doi 10.1002/cncr.23132. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RJ, Ryerson AB, Singh SD, King JB. Cancer Incidence in Appalachia, 2004–2011. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2016;25(2):250–8 doi 10.1158/1055-9965.epi-15-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Department of Agriculture. 2013. Rural-Urban Continuum Codes. United States Department of Agriculture; <https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/>. Accessed 2019. [Google Scholar]
- 11.Rural Institute. Defining Rural. In RTC:Rural. Research and Training Center on Disability in Rural Communities; <http://rtc.ruralinstitute.umt.edu/resources/defining-rural/>. Accessed 2019. [Google Scholar]
- 12.U.S. Census Bureau. Intercensal Estimates of the Resident Population by Age, Sex, Race, and Hispanic Origin for Counties in Pennsylvania: April 1, 2000 to July 1, 2010. In: U.S. Census Bureau PD, editor2012. [Google Scholar]
- 13.U.S. Census Bureau. Annual County Resident Population Estimates by Age, Sex, Race, and Hispanic Origin: April 1, 2010 to July 1, 2017. In: U.S. Census Bureau PD, editor2018. [Google Scholar]
- 14.Washington State Department of Health. Guidelines for Using and Developing Rates for Public Health Assessment. Washington State Department of Health; 2012. [Google Scholar]
- 15.Klein R, Schoenborn C. Age adjustment using the 2000 projected U.S. population. Hyattsville, Maryland: National Center for Health Statistics; 2001. [PubMed] [Google Scholar]
- 16.New Mexico Departmemt of Health. 2018. Age-adjusted Rates. New Mexico Department of Health; <https://ibis.health.state.nm.us/resource/AARate.html>. Accessed 2019. [Google Scholar]
- 17.National Cancer Institute. 2012 Standard Populations - 19 Age Groups. National Cancer Institute Surveillance, Epidemiology, and End Results Program; <https://seer.cancer.gov/stdpopulations/stdpop.19ages.html>. Accessed 2019. [Google Scholar]
- 18.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Statistics in medicine 1997;16(7):791–801 doi . [DOI] [PubMed] [Google Scholar]
- 19.Chiang C Standard error of the age-adjusted death rate. Vital Statistics Special Reports Volume 47: US Department of Health, Education and Welfare; 1961. p 271–85. [Google Scholar]
- 20.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 21.Hall HI, Uhler RJ, Coughlin SS, Miller DS. Breast and Cervical Cancer Screening among Appalachian Women. Cancer Epidemiology Biomarkers & Prevention 2002;11(1):137–42. [PubMed] [Google Scholar]
- 22.Dignan M, Shelton B, Slone SA, Tolle C, Mohammad S, Schoenberg N, et al. Effectiveness of a primary care practice intervention for increasing colorectal cancer screening in Appalachian Kentucky. Preventive medicine 2014;58:70–4 doi 10.1016/j.ypmed.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muralidhar V, Rose BS, Chen YW, Nezolosky MD, Nguyen PL. Association Between Travel Distance and Choice of Treatment for Prostate Cancer: Does Geography Reduce Patient Choice? International journal of radiation oncology, biology, physics 2016;96(2):313–7 doi 10.1016/j.ijrobp.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Royse D, Dignan M. Appalachian knowledge of cancer and screening intentions. Journal of cancer education : the official journal of the American Association for Cancer Education 2009;24(4):357–62 doi 10.1080/08858190902876577. [DOI] [PubMed] [Google Scholar]
- 25.Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149(3):185–91. [DOI] [PubMed] [Google Scholar]
- 26.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157(2):120–34 doi 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 27.Gejerman G, Ciccone P, Goldstein M, Lanteri V, Schlecker B, Sanzone J, et al. US Preventive Services Task Force prostate-specific antigen screening guidelines result in higher Gleason score diagnoses. Investigative and clinical urology 2017;58(6):423–8 doi 10.4111/icu.2017.58.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly SP, Anderson WF, Rosenberg PS, Cook MB. Past, Current, and Future Incidence Rates and Burden of Metastatic Prostate Cancer in the United States. European urology focus 2018;4(1):121–7 doi 10.1016/j.euf.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004–2014. Annals of epidemiology 2018;28(5):328–30 doi 10.1016/j.annepidem.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myint ZW, O’Neal R, Chen Q, Huang B, Vanderpool R, Wang P. Disparities in prostate cancer survival in Appalachian Kentucky: a population-based study. Rural and remote health 2019;19(2):4989 doi 10.22605/rrh4989. [DOI] [PubMed] [Google Scholar]
- 31.Zapata DF, Howard LE, Aronson WJ, Kane CJ, Terris MK, Amling CL, et al. Smoking is a predictor of adverse pathological features at radical prostatectomy: Results from the Shared Equal Access Regional Cancer Hospital database. Int J Urol 2015;22(7):658–62 doi 10.1111/iju.12773. [DOI] [PubMed] [Google Scholar]
- 32.Vidal AC, Freedland SJ. Obesity and Prostate Cancer: A Focused Update on Active Surveillance, Race, and Molecular Subtyping. Eur Urol 2016. doi 10.1016/j.eururo.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cary C, Odisho AY, Cooperberg MR. Variation in prostate cancer treatment associated with population density of the county of residence. Prostate cancer and prostatic diseases 2016;19:174 doi 10.1038/pcan.2015.65 https://www.nature.com/articles/pcan201565#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 34.Trippi M, Kinney S, Gunther G, Ryder R, Ruppert L. Digital Data in Support of Studies and Assessments of Coal and Petroleum Resources in the Appalachian Basin. U.S. Geological Survey; 2014. [Google Scholar]
- 35.Wong O, Raabe GK. A critical review of cancer epidemiology in the petroleum industry, with a meta-analysis of a combined database of more than 350,000 workers. Regulatory toxicology and pharmacology : RTP 2000;32(1):78–98 doi 10.1006/rtph.2000.1410. [DOI] [PubMed] [Google Scholar]
- 36.Mueller GS, Clayton AL, Zahnd WE, Hollenbeck KM, Barrow ME, Jenkins WD, et al. Manuscript title: Geospatial analysis of Cancer risk and residential proximity to coal mines in Illinois. Ecotoxicology and environmental safety 2015;120:155–62 doi 10.1016/j.ecoenv.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 37.Huvinen M, Pukkala E. Cancer incidence among Finnish ferrochromium and stainless steel production workers in 1967–2011: a cohort study. BMJ open 2013;3(11):e003819 doi 10.1136/bmjopen-2013-003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gun RT, Pratt N, Ryan P, Roder D. Update of mortality and cancer incidence in the Australian petroleum industry cohort. Occupational and environmental medicine 2006;63(7):476–81 doi 10.1136/oem.2005.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.