Abstract
Background:
In patients with PD, stimulation above the STN may engage the pallidofugal fibers and directly suppress dyskinesia.
Objectives:
To evaluate the effect of interleaving stimulation through a dorsal DBS contact above the STN in a cohort of PD patients, and to define the volume of tissue activated with anti-dyskinesia effects.
Methods:
We analyzed the CAPSIT dyskinesia scale, UPDRS parts III and IV, and other endpoints in 20 patients with interleaving stimulation for management of dyskinesia. Individual models of volume of tissue activated and heat maps were used to identify stimulation sites with anti-dyskinesia effects.
Results:
The CAPSIT dyskinesia score improved 70.9±20.6% from baseline with non-interleaved settings (p<0.003). With interleaved settings, dyskinesia improved 82.0±27.3% from baseline (p<0.001) and 61.6±39.3% from the non-interleaved phase (p=0.006). The heat map showed a concentration of volume of tissue activated dorsally to the STN during the interleaved setting with anti-dyskinesia effect.
Conclusion:
Interleaved DBS using the dorsal contacts can directly suppress dyskinesia, probably due to involvement of the pallidofugal tract, allowing more conservative medication reduction.
Keywords: Parkinson’s Disease, Deep Brain Stimulation, Dyskinesia, Interleaving, Volume of Tissue activated
Introduction
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) can aggravate or induce dyskinesia1,2. In patients with Parkinson’s disease (PD), dyskinesia improvements are mostly due to reduction in the dopaminergic therapy3. Stimulation superior to the STN, a region enriched with pallidofugal fibers, can directly suppress dyskinesias4,5.
Interleaving stimulation (ILS) is a programming technique in which two independent settings are programmed on the same electrode, using different contacts, amplitudes, and pulse widths. These independent settings are delivered in an interleaved pattern, typically at 125 Hz6. ILS allows activation of multiple areas after conventional stimulation (non-interleaved) fails to achieve desired results6–9.
The aim of this study was to assess the effect of alternating stimulation from dorsal lead contacts above the STN with presumed engagement of pallidofugal fibers for specific suppression of dyskinesia, with a conventional STN contact to address the cardinal symptoms of PD.
Methods
Patient selection
Patients were selected during DBS programming visits and by review of health records. We included patients with STN DBS (uni- or bilateral) programmed with ILS for treatment of dyskinesia. We excluded patients with other neurosurgical interventions for PD and patients without available brain images.
DBS Programming
Initial programming was based on monopolar review to address PD symptoms with minimal side-effects10. On follow-up visits, increments in voltage were done in parallel with medication reduction11. In patients with bothersome dyskinesia (residual or stimulation-induced) we used ILS in monopolar configuration of the more dorsal contact contralateral to the dyskinesia or bilaterally in case of axial dyskinesia. We started with pulse width of 60 μs and low voltages (~1.0 V according to the thresholds). Stimulation amplitude was increased until the dyskinesia was visibly suppressed or side-effects occurred. In cases limited by side-effects, the contact immediately below (second more dorsal) was activated, or bipolar configuration was tried.
Data collection
The main clinical endpoints were the CAPSIT dyskinesia scale (ranging from 0 to 28)12, the Unified Parkinson’s Disease rating scale (UPDRS) part III (motor symptoms), and the therapy complications measured by UPDRS part IV, divided into subitems for dyskinesia and motor fluctuations.
Additional clinical endpoints were the tremor score, axial scores, levodopa equivalent daily dose (LEDD)13, total electrical energy delivered (TEED)14, and internal pulse generator (IPG) longevity.
Computational Modeling
Computational volumes of tissue activated during conventional and ILS settings were generated as previously described15. Briefly, all DBS leads were mapped to the left side to allow for direct comparison. The activated volumes were brought into a common space by registering each patient’s T1 MRI to the PD-25 template16, and applying the resulting non-linear transform to the volume of tissue activated. Stimulation location maps were generated by discretizing each transformed activation volume into a binary volume, and summing each voxel in our grid across all activation volumes. Three maps were generated: 1. volume of tissue activated with conventional settings, 2. volume of tissue activated with ILS, and 3. volume of tissue activated with ILS subtracting the volume of tissue activated with conventional stimulation for each patient.
Statistical analysis
T-tests were used to for parametric variables and the Wilcoxon test for non-parametric variables. We used one-way ANOVA for multiple comparisons, and Chi-square test for analysis of frequencies. A two-sided p-value <0.05 was adopted for statistical significance.
Results
Twenty patients with STN DBS were programmed using ILS settings for dyskinesia management. The demographic features are presented in the supplemental table 1, and the baseline characteristics in supplemental table 2.
Using conventional stimulation, there was a 56.0±22.0% improvement in UPDRS-III scores in the on-stimulation/off-medication condition relative to the off-medication baseline (p<0.001), and 62.5±19.2% improvement in on-stimulation/on-medication relative to off-medication baseline (p<0.001; Figure 1.A). The CAPSIT dyskinesia score improved 70.9±20.6% relative to baseline (p<0.003; Figure 1.B), and the patients reported improvement in complications of therapy (UPDRS-IV) relative to levodopa-induced dyskinesia (p=0.04), motor fluctuations (p=0.06), and total score (p=0.02; Figure 1.C). The LEDD after STN DBS with conventional settings was 979±472 mg/day, which represents a reduction of 346.9±391.3 mg/day (27±30%; p=0.002).
Figure 1. Left-Conventional settings:
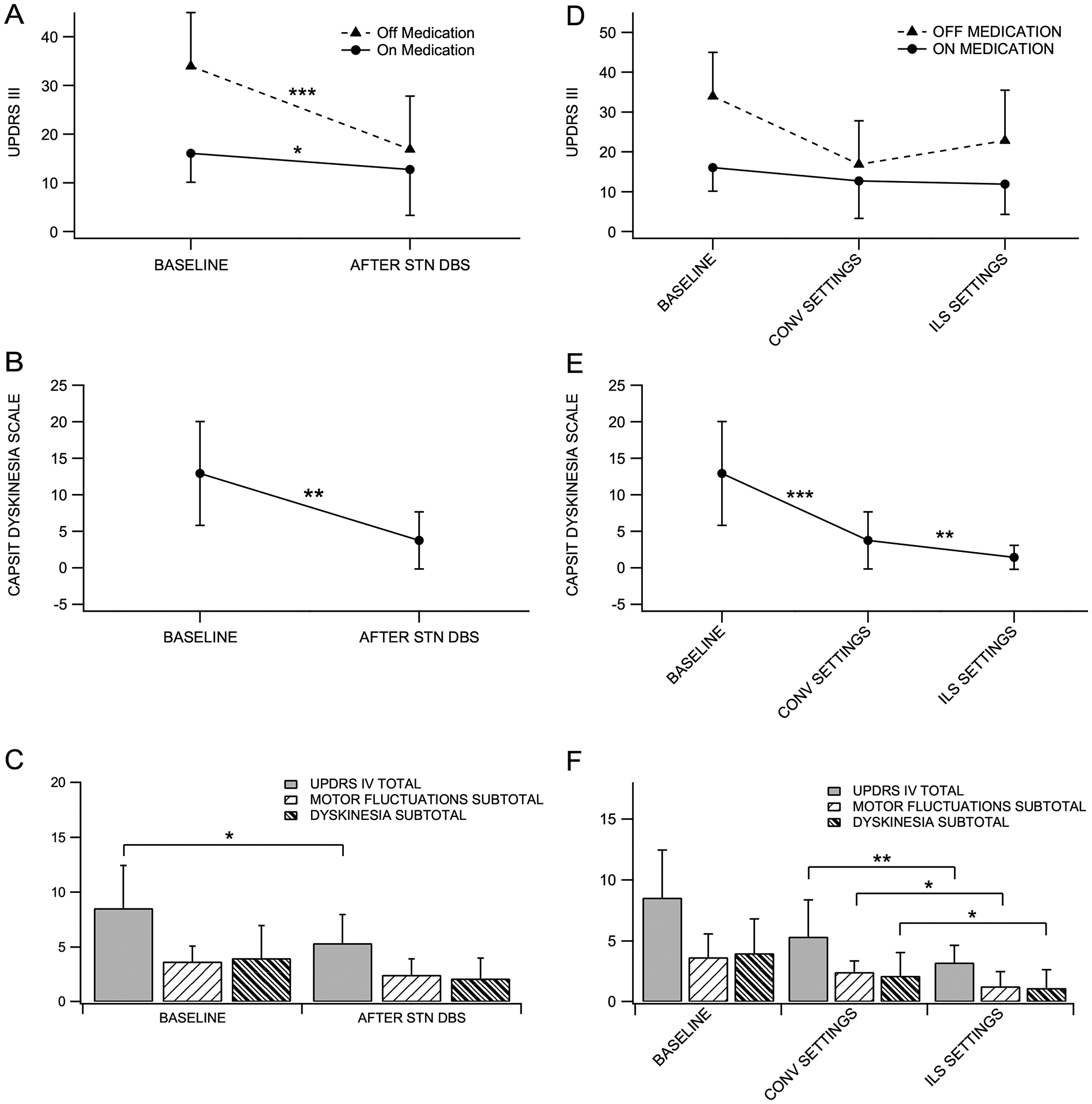
(A) UPDRS-III: Baseline off-medication 33.9 ± 11.0, on-medication 16.0 ± 5.9; Conventional DBS off-medication/on-stimulation 16.8 ± 10.9, on-medication/on-stimulation 12.7 ± 9.4. (B) CAPSIT Dyskinesia: Baseline on-medication 12.9 ± 7.1; conventional DBS on-medication/on-stimulation 3.7 ± 3.9. (C) UPDRS-IV: Baseline total score 8.5 ± 3.8, Dyskinesia score 4.0 ± 2.9; motor fluctuation score 3.6 ± 1.4. Conventional DBS Total score 5.3 ± 2.5, Dyskinesia score 2.1 ± 1.8, and motor fluctuation score 2.4 ± 1.4. Right-ILS: (D) UPDRS-III: Off-medication/on-stimulation 22.8 ± 12.6, on-medication/on-stimulation 11.8 ± 7.6. (E) CAPSIT Dyskinesia: On-medication/On-stimulation 1.4 ± 1.6. (F) UPDRS-IV: Total 3.2 ± 1.4; Dyskinesia 1.1 ± 1.5, Motor fluctuations 1.3 ± 1.2. Significance markers *p < 0.05; **p < 0.01; ***p < 0.001. Values are (mean ± standard deviation).
Conventional programming settings are presented in supplemental table 3. During conventional treatment, 15 (75%) patients reported bothersome dyskinesia. In this group, the CAPSIT dyskinesia score was 5.3±3.6, a reduction of 58.4±16.3% from baseline (p=0.003). Additionally, five (25%) patients developed stimulation-induced dyskinesia in the off-medication state during programming visits. To improve dyskinesia in these patients, an additional dorsal contact of the DBS electrode was activated using ILS as detailed in the supplemental material. Final ILS settings are presented in supplemental table 4.
Using ILS with activation of a more dorsal contact, the CAPSIT dyskinesia score improved 61.6±39.3% (2.3±3.7 points) relative to the conventional settings (p=0.006) and 82.0±27.3% relative to baseline (p<0.001; Figure 1.E). Patients also reported improvement in the UPDRS-IV in both domains, dyskinesia (p=0.03) and motor fluctuation (p=0.04), relative to conventional settings (Figure 1.F). There was no significant change in the UPDRS-III on-medication/on-stimulation (p=0.89), however, there was a mild worsening in the off-medication/on-stimulation scores (p=0.16; Figure 1.D). The average LEDD with ILS was 993±346 mg/day, which was not significantly changed from the conventional phase (p=0.75).
To clarify the source of motor worsening, we analyzed the changes in tremor and axial scores (Supplemental figure 1 and 2). There was no significant change in the tremor score in the off-medication/on-stimulation between the conventional and ILS condition (p=0.77). There was a trend for deterioration of 1.35±3.2 points in the axial score during the off-medication/on-stimulation condition with ILS (p=0.08).
Volume of Tissue Activated
The patient-specific volume activated with conventional stimulation (STN1) were situated within or bordering the STN. The volume activated with ILS used for dyskinesia suppression (STN2) were situated above and lateral to the STN (supplemental figures 3 represents the volume of tissue activated models of two selected patients)
The heat maps revealed a higher concentration of volume activated within or in the dorsal border of the STN during the conventional settings (STN1) (Figure 2. Conventional). The volume of tissue activated with ILS settings, originated by the overlap of STN1 + STN2 were spread around the STN with no single “hot spot” (Figure 2. ILS). The volume of tissue activated only during ILS were highly concentrated dorsally to the STN, suggesting that these areas were selectively stimulated by STN2 (Figure 2. ILS−Conventional).
Figure 2.
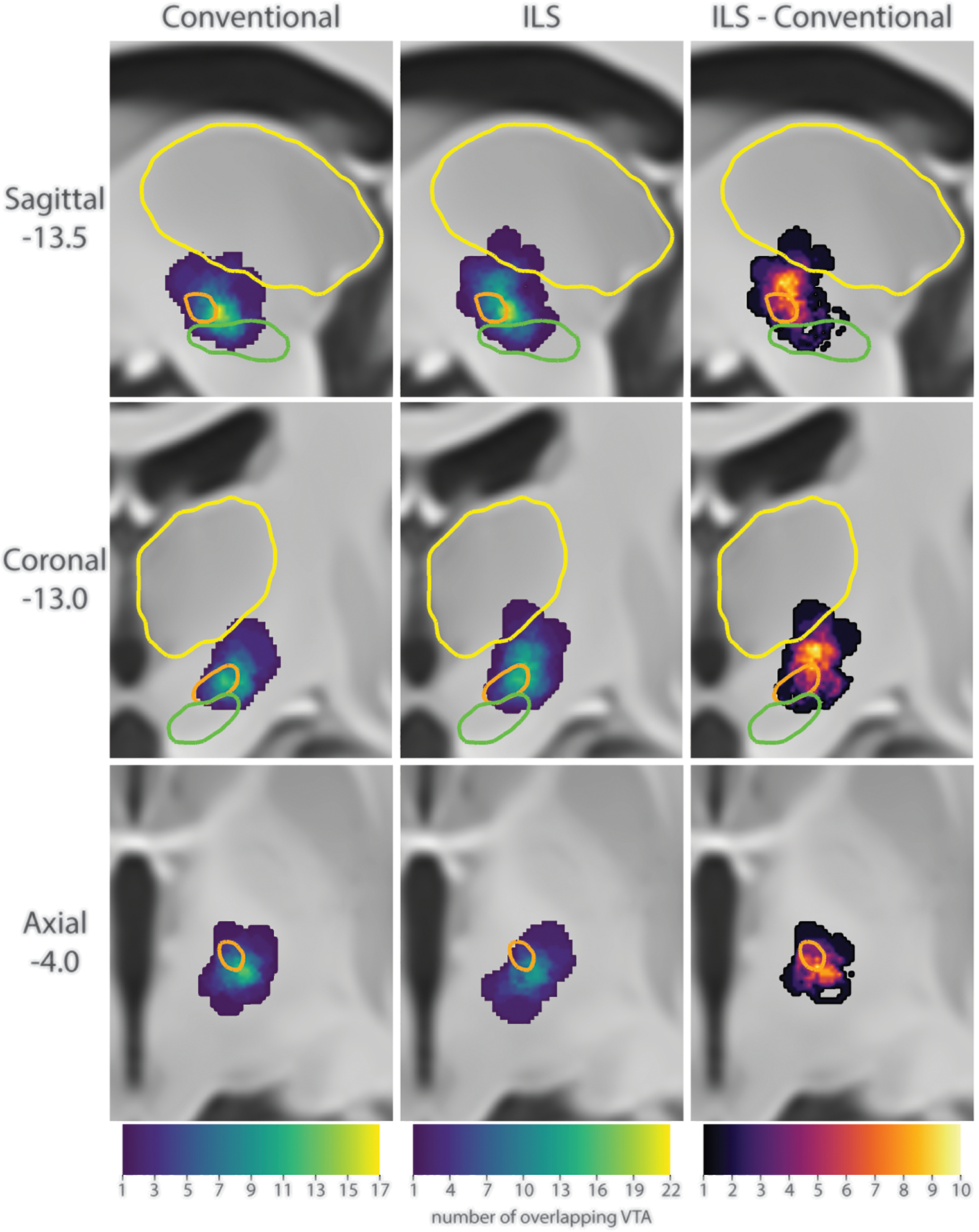
Comparison of the location of activation between conventional and ILS stimulation. Left: Sum of the VTA from the initial conventional programming setting. Center: Sum of the VTA from the ILS programming setting. Right: Sum of the areas of areas that were activated during ILS stimulation, but not during conventional stimulation. Colors: orange = STN, green = substantia nigra, yellow = thalamus.
Additional endpoints
The TEED during the conventional settings was 33.5±29.1 μJ. During the ILS the TEED delivered by STN1 was 42.0±39.9 μJ and by STN2 was 27.2±29.2 μJ. The average battery longevity was 4.0±0.9 years.
Adverse events
Six patients (30%) had gait deterioration after STN DBS. In one of these patients, the occurrence of falls was reduced by transitioning from ILS setting to low frequency stimulation. Three patients (15%) developed dysarthrophonia. Repeated reprogramming visits were not successful in relieving the speech problems.
Discussion
In our study, we found that engaging the pallidofugal fibers through a dorsal DBS contact using the ILS paradigm resulted in more robust dyskinesia suppression (~65%) in comparison to conventional settings. This effect was independent of medication reduction. We chose to transition to ILS instead of reducing dopaminergic therapy due to individual characteristics that raised concerns regarding mood17, apathy18,19, and axial symptoms20. To our knowledge, this is the first study focused on the potential of ILS as a dyskinesia-specific therapy in PD patients with STN DBS6–9.
Different mechanisms could potentially explain the anti-dyskinesia effect of the pallidofugal tract stimulation: the orthodromic activation of pallidothalamic fibers, which would inhibit the thalamus, and the antidromic stimulation of the GPi21,22. Studies in nonhuman primates23 documented the abolishment of hyperkinesia by lesions in the pallidum or pallidal outflow tract24. Subsequent lesioning studies in humans of the GPi, pallidal outflow tract, and pallidal receiving area of the thalamus resulted in similar suppression of dyskinesia25–27. The Zi also resides in this region, and has been suggested as a potential anti-dyskinesia target27,28. Further studies with tractography are essential to clarify these mechanisms.
The use of the volume of tissue activated models in our study allowed an “in vivo” evaluation of the area with anti-dyskinesia effect. The volume of tissue activated unique to STN2, responsible for dyskinesia suppression, was above and toward the lateral aspect of the STN. In this complex area between the dorsal STN border and the ventral thalamus lie several interconnected tracts, comprised in part by pallidofugal fibers en route to the thalamus (i.e., the pallidothalamic tract29), as well as the Zi30. The pallidothalamic tract is formed by the lenticular fasciculus (or Field H2 of Forel) and the ansa lenticularis of Von Monakow, both of which merge to form the thalamic fasciculus (Field H1 of Forel)29. The thalamic fasciculus enters the ventral thalamus, carrying the bundle of fibers originated both from parts of the GPi, the ventral and the doral29,30.
The advantage of ILS over other configurations4,5,31 is the use of independent parameters for each contact, allowing a more tailored stimulation field9,32. This is particularly important in the compact region above the STN, which is bordered laterally by the internal capsule. Problematic side effects, particularly with gait33 and speech34, have been reported with stimulation in this area. We also observed mild deterioration in axial scores, but cannot exclude the potential effect of disease progression, as there was a mean interval of 23 months between surgery and ILS in our sample35,36. Although a higher stimulation frequency (e.g., 180 Hz)37 may be required to control the parkinsonian tremor, the tremor score was unchanged with ILS in our sample; probably because we would immediately discontinue ILS in case of tremor recurrence during the programming visit. A frequency-mediated effect on dyskinesia, previously reported with stimulation reduction from 130 to 80Hz is unlikely to explain our results, as most patients were switched from 140 to 125 Hz38.
We acknowledge the limitations of this small retrospective cohort, including the high risk of performance and selection bias. Also, variability in patient-specific anatomy may not be adequately accounted for using atlas-based imaging analysis. Despite these issues, we demonstrated that, at least in selected individuals, the combined stimulation of the STN and subthalamic area through ILS is feasible and effective for the treatment of residual or stimulation induced dyskinesia.
Our results encourage the use of ILS settings not as a last resort, but as a way of refining stimulation to optimize benefit without additional side effects. As we enter a time of rapid technological advance, with the availability of directional current39 and multiple independent current control, our ability to selectively stimulate specific fiber tracts40,41 will further refine symptom-specific approaches.
Supplementary Material
Acknowledgements:
The authors acknowledge the essential contribution of Desiree Davis - RN, Meghan Zorn - PA, Perla Thulin - MD, and Paolo Moretti, MD for providing excellent care for patients with PD at the University of Utah. We also acknowledge the work of James Ballard - PT, DPT, and Kevin Duff - PhD for conducting motor evaluation and neuropsychological assessments of all DBS candidates at the University of Utah. We thank Holly Cushing - PA for contribution with data collection, and our patients for their willingness to collaborate in research.
Funding sources for study:
Nothing to declare.
Conflict of interest disclosure:
Camila C. Aquino:
| Employment | Department of Neurology, University of Utah |
| Grants | Scholarship from CAPES Foundation, Ministry of Education, Brazil. Brasilia, DF, 070040–020. |
David Hedges:
| Affiliation | Scientific Computing & Imaging (SCI) Institute, University of Utah, Salt Lake City, Utah, USA |
| Grants | National Institute of Nursing Research (NINR) R01 [NR014852]. |
Gordon Duffley:
| Affiliation | Scientific Computing & Imaging (SCI) Institute, University of Utah, Salt Lake City, Utah, USA |
| Grants | National Institute of Nursing Research (NINR) R01 [NR014852]. |
Johannes Vorwerk:
| Employment | Scientific Computing & Imaging (SCI) Institute, University of Utah, Salt Lake City, Utah, USA Institute of Electrical and Biomedical Engineering, UMIT - University for Health Sciences, Medical Informatics and Technology, Hall in Tirol, Austria |
| Grants | National Science Foundation (NSF): US IGNITE – 10037840 Austrian Wissenschaftsfonds (FWF): Project I 3790-B27 |
Paul A House:
| Nothing to disclose |
Henrique B. Ferraz:
| Employment | Universidade Federal de São Paulo |
John D. Rolston:
| Stock Ownership in medically-related fields | Axion Biosystems |
| Consultancies | Consultant for Medtronic and NeuroPace |
| Employment | Department of Neurosurgery, University of Utah |
| Grants | Supported by a career development award from NIH NCATS KL2TR002539 and funding from the Utah Science Technology and Research Initiative (USTAR) |
Christopher R. Butson:
| Intellectual Property Rights | Holds intellectual property related to DBS |
| Consultancies | Consultant for NeuroPace, Advanced Bionics, Boston Scientific, IntelectMedical, St. Jude Medical, Functional Neuromodulation |
| Employment | Scientific Computing & Imaging (SCI) Institute, University of Utah, Salt Lake City, Utah, USA |
Lauren E. Schrock:
| Employment | University of Minnesota |
Footnotes
Financial Disclosure/Conflict of Interest concerning the manuscript: Nothing to declare
Contributor Information
Camila C Aquino, Sleep and Movement Disorder Division, University of Utah.
Gordon Duffley, Scientific Computing and Imaging Institute, University of Utah.
David M. Hedges, Scientific Computing and Imaging Institute, University of Utah.
Johannes Vorwerk, Scientific Computing and Imaging Institute, University of Utah.
Paul A. House, Neurosurgical Associates, LLC, Murray, Utah.
Henrique B. Ferraz, Department of Neurology and Neurosurgery, Universidade Federal de Sao Paulo.
John D. Rolston, Dept. of Neurosurgery, Dept. of Biomedical Engineering, University of Utah.
Christopher R. Butson, Department of Biomedical Engineering, Scientific Computing and Imaging (SCI) Institute, Departments of Neurology, Neurosurgery & Psychiatry, University of Utah.
Lauren E. Schrock, Department of Neurology, University of Minnesota.
References
- 1.Limousin P, Pollak P, Hoffmann D, et al. Abnormal involuntary movements induced by subthalamic nucleus stimulation in parkinsonian patients. Mov. Disord 1996;11:231–235. [DOI] [PubMed] [Google Scholar]
- 2.Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 1998;339:1105–1111. [DOI] [PubMed] [Google Scholar]
- 3.Benabid AL, Benazzouz A, Limousin P, et al. Dyskinesias and the subthalamic nucleus. Ann Neurol. 2000;47:S189–92. [PubMed] [Google Scholar]
- 4.Herzog J, Pinsker M, Wasner M, et al. Stimulation of subthalamic fibre tracts reduces dyskinesias in STN-DBS. Mov. Disord 2007;22:679–684. [DOI] [PubMed] [Google Scholar]
- 5.Katayama Y, Oshima H, Kano T, et al. Direct effect of subthalamic nucleus stimulation on levodopa-induced peak-dose dyskinesia in patients with Parkinson’s disease. Stereotact Funct Neurosurg 2006;84:176–179. [DOI] [PubMed] [Google Scholar]
- 6.Miocinovic S, Khemani P, Whiddon R, et al. Outcomes, management, and potential mechanisms of interleaving deep brain stimulation settings. Parkinsonism and Related Disorders 2014;20:1434–1437. [DOI] [PubMed] [Google Scholar]
- 7.Kern DS, Picillo M, Thompson JA, et al. Interleaving Stimulation in Parkinson’s Disease, Tremor, and Dystonia. Stereotact Funct Neurosurg 2018;96:379–391. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez-Zamora A, Kahn M, Campbell J, et al. Interleaved programming of subthalamic deep brain stimulation to avoid adverse effects and preserve motor benefit in Parkinson’s disease. J Neurol 2015;262:578–584. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Zhou P, Jiang S, et al. Interleaving subthalamic nucleus deep brain stimulation to avoid side effects while achieving satisfactory motor benefits in Parkinson disease: A report of 12 cases. Medicine (Baltimore) 2016;95:e5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson’s disease. Mov. Disord 2006;21:S284–S289. [DOI] [PubMed] [Google Scholar]
- 11.Picillo M, Lozano AM, Kou N, et al. Programming Deep Brain Stimulation for Parkinson’s Disease: The Toronto Western Hospital Algorithms. Brain Stimulation 2016;9:425–437. [DOI] [PubMed] [Google Scholar]
- 12.Defer GL, Widner H, Marié RM, et al. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord. 1999;14:572–584. [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 14.Koss AM, Alterman RL, Tagliati M, Shils JL. Calculating total electrical energy delivered by deep brain stimulation systems. Ann Neurol. 2005;58:168–9. [DOI] [PubMed] [Google Scholar]
- 15.Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. NeuroImage 2007;34:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao Y, Fonov V, Beriault S, et al. Multi-contrast unbiased MRI atlas of a Parkinson’s disease population. Int J CARS 2014;10:329–341. [DOI] [PubMed] [Google Scholar]
- 17.Castrioto A, Lhommée E, Moro E, Krack P. Mood and behavioural effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol 2014;13:287–305. [DOI] [PubMed] [Google Scholar]
- 18.Thobois S, Ardouin C, Lhommée E, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain 2010;133:1111–1127. [DOI] [PubMed] [Google Scholar]
- 19.Thobois S, Lhommée E, Klinger H, et al. Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain 2013;136:1568–1577. [DOI] [PubMed] [Google Scholar]
- 20.Fasano A, Aquino CC, Krauss JK, et al. Axial disability and deep brain stimulation in patients with Parkinson disease. Nature Rev Neurol 2015;11:98–110. [DOI] [PubMed] [Google Scholar]
- 21.Johnson MD, Miocinovic S, McIntyre CC, Vitek JL. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics 2008;5:294–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopell BH, Rezai AR, Chang JW, Vitek JL. Anatomy and physiology of the basal ganglia: Implications for deep brain stimulation for Parkinson’s disease. Mov. Disord 2006;21:S238–S246. [DOI] [PubMed] [Google Scholar]
- 23.Whittier JR, Mettler FA. Studies on the subthalamus of the rhesus monkey. II. Hyperkinesia and other physiologic effects of subthalamic lesions, with special reference to the subthalamic nucleus of Luys. J. Comp. Neurol 1949;90:319–372. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter MB, Whittier JR, Mettler FA. Analysis of choreoid hyperkinesia in the rhesus monkey. Surgical and pharmacological analysis of hyperkinesia resulting from lesions in the subthalamic nucleus ol luys. J. Comp. Neurol 1950;92:293–331. [DOI] [PubMed] [Google Scholar]
- 25.HASSLER R, Mundinger F, RIECHERT T. Stereotaxis in Parkinson syndrome. With an atlas of the basal ganglio in parkinsonism. Berlin: Springler, 1979. [Google Scholar]
- 26.Guiot G, BRION S. Treatment of abnormal movement by pallidal coagulation. Rev. Neurol. (Paris) 1953;89:578–580. [PubMed] [Google Scholar]
- 27.Cooper IS. The vital probe: my life as a brain surgeon. Norton; New York. 1981 [Google Scholar]
- 28.Forel A Untersuchungen über die Haubenregion und ihre oberen Verknüpfungen im Gehirne des Menschen und einiger Säugethiere, mit Beiträgen zu den Methoden der Gehirnuntersuchung. Eur Arch Psychiatry Clin Neurosci 1877;7:393–495. [Google Scholar]
- 29.Gallay MN, Jeanmonod D, Liu J, Morel A. Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct 2008;212:443–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamani C, Saint-Cyr JA, Fraser J, et al. The subthalamic nucleus in the context of movement disorders. Brain 2004;127:4–20. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Chang WS, Jung HH, Chang JW. Effect of Subthalamic Deep Brain Stimulation on Levodopa-Induced Dyskinesia in Parkinson’s Disease. Yonsei Med J 2015;56:1316–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wojtecki L, Vesper J, Schnitzler A. Interleaving programming of subthalamic deep brain stimulation to reduce side effects with good motor outcome in a patient with Parkinson’s disease. Parkinsonism Relat Disord 2011;17:293–294. [DOI] [PubMed] [Google Scholar]
- 33.Fleury V, Pollak P, Gere J, et al. Subthalamic stimulation may inhibit the beneficial effects of levodopa on akinesia and gait. Mov. Disord 2016;31:1389–1397. [DOI] [PubMed] [Google Scholar]
- 34.Eklund E, Qvist J, Sandström L, et al. Perceived articulatory precision in patients with Parkinson’s disease after deep brain stimulation of subthalamic nucleus and caudal zona incerta. Clin Linguist Phon 2015;29:150–166. [DOI] [PubMed] [Google Scholar]
- 35.Merola A, Zibetti M, Angrisano S, et al. Parkinson’s disease progression at 30 years: a study of subthalamic deep brain-stimulated patients. Brain 2011;134:2074–2084. [DOI] [PubMed] [Google Scholar]
- 36.Fasano A, Romito LM, Daniele A, et al. Motor and cognitive outcome in patients with Parkinson’s disease 8 years after subthalamic implants. Brain 2010;133:2664–2676. [DOI] [PubMed] [Google Scholar]
- 37.Su D, Chen H, Hu W, et al. Frequency-dependent effects of subthalamic deep brain stimulation on motor symptoms in Parkinson’s disease: a meta-analysis of controlled trials. Scientific Reports 2018;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merola A, Zibetti M, Artusi CA, et al. 80 Hz versus 130 Hz subthalamic nucleus deep brain stimulation: effects on involuntary movements. Parkinsonism Relat Disord 2013;19:453–456. [DOI] [PubMed] [Google Scholar]
- 39.Pollo C, Kaelin-Lang A, Oertel MF, et al. Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain 2014;137:2015–2026. [DOI] [PubMed] [Google Scholar]
- 40.Slopsema JP, Peña E, Patriat R, et al. Clinical deep brain stimulation strategies for orientation-selective pathway activation. J. Neural Eng 2018;15:056029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunalan K, Chaturvedi A, Howell B, et al. Creating and parameterizing patient-specific deep brain stimulation pathway-activation models using the hyperdirect pathway as an example. PLoS ONE 2017;12:e0176132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


