Abstract
Background
Stage III non-small cell lung cancer is a heterogeneous disease. Several international guidelines recommend neoadjuvant treatment before surgery; however, upfront surgery is the preferred approach for technically resectable non-small cell lung cancer in East Asia. The aim of this retrospective study was to evaluate the long-term outcomes of curative-intent upfront surgery in stage IIIA/B non-small cell lung cancer.
Methods
Patients who underwent curative-intent upfront surgery with stage cIIIA/B non-small cell lung cancer were identified. The clinical and pathological variables and survival outcomes were evaluated.
Results
Overall, 664 patients were identified, of whom 320 (48.8%) had N2 disease, 66.7% were males, 49.4% had a smoking history, and 61.2% had lung adenocarcinoma. Lobectomy was the most performed surgical procedure (84.9%). A total of 40 patients (6.02%) had positive margins (R1/R2). The grade III adverse event rate was 2.0% (13 of 664). The median follow-up was 30.6 (range 1.9–97.7) months. At follow-up, the mortality rate was 13.3% (88 of 664) and 37.2% of patients (247 of 664) had recurrence. Lung (101 of 247 (40.9%)) and brain (53 of 247 (21.5%)) were the most common sites of recurrence. The median overall survival was 60.0 (95% c.i. 51.5 to 67.6) months, with overall survival probability at 1, 2, 3, and 5 years being 89.6%, 77.8%, 67.2%, and 49.0% respectively. The R0 cohort showed an improved median overall survival compared with the R1/R2 cohort (67.4 versus 26.5 months respectively; P = greater than 0.001). The multivariable analysis revealed that age greater than or equal to 65 years (HR 1.51, 95% c.i. 1.08 to 2.12; reference = age less than 65 years), tumour size (greater than or equal to 5 cm (HR 2.13, 95% c.i. 1.41 to 3.21) and greater than or equal to 3 cm but less than 5 cm (HR 1.15, 95% c.i. 0.78 to 1.71); reference = less than 3 cm), and adjuvant treatment (chemotherapy (HR 0.69, 95% c.i. 0.49 to 0.96) and targeted therapy (HR 0.30, 95% c.i. 0.12 to 0.76); reference = none) significantly predicted overall survival.
Conclusion
Upfront surgery is an option for the management of stage IIIA/B non-small cell lung cancer.
This study provides important real-world data, with a sufficient sample size, that upfront surgery for stage IIIA/B non-small cell lung cancer could have an acceptable perioperative morbidity rate and favourable long-term survival, particularly when combined with adjuvant therapies. This study identifies specific patient characteristics that may make them more suitable candidates for upfront surgery, such as younger age, smaller tumour size, limited nodal involvement, and no distant metastases. A comprehensive literature review on the survival rates of upfront surgery in non-small cell lung cancer facilitates easy comparison with relevant research findings in this field.
Introduction
Stage III non-small cell lung cancer (NSCLC) is a heterogeneous disease1 and accounts for 22% of all cases of NSCLC2,3. Patients can present with disease that is potentially surgically resectable or with metastatic involvement4,5. Currently, most guidelines, including the National Comprehensive Cancer Network (NCCN)6, European Society for Medical Oncology (ESMO)7, American Society for Clinical Oncology (ASCO)8, and American College of Chest Physicians (ACCP)9 guidelines, recommend neoadjuvant therapy for stage IIIA-N2 NSCLC. ‘Upfront surgery’ refers to surgical resection as the initial treatment without prior radiation therapy or chemotherapy. This approach can provide radical cure and has been widely accepted as an optional first-line treatment for technically resectable stage III NSCLC in East Asia10–12, but not in all parts of the world.
There are no phase III RCTs directly comparing outcomes of upfront surgery followed by adjuvant chemotherapy versus induction therapy followed by surgery in stage III NSCLC, and the selection of the neoadjuvant therapy is based on extrapolation of phase I/II RCT data and expert consensus. The NATCH trial13 demonstrated identical disease survival rates for neoadjuvant versus adjuvant chemotherapy; however, it was conducted for stage IA (greater than 2 cm) to II NSCLC. Several studies14–18 failed to demonstrate the survival benefit of induction treatment when compared with upfront surgery. A study17 analysed the data of 1356 patients from the National Cancer Database (NCDB), demonstrating that postoperative chemotherapy showed comparable survival outcomes to preoperative chemotherapy in stage III/N2 NSCLC (HR 1.05; P = 0.438). Bertolaccini et al.16 concluded that upfront surgery, as the initial treatment for stage III/N2 NSCLC, yielded favourable recurrence-free survival (RFS), similar to induction chemotherapy followed by surgery (P = 0.93). Moreno et al.18 compared the impact of different treatment modalities in T3 (greater than 7 cm) N1 NSCLC and found that the 5-year overall survival (OS) rates were similar across neoadjuvant chemoradiation with surgery, neoadjuvant chemotherapy with surgery, surgery with adjuvant chemoradiation, and surgery with adjuvant chemotherapy modalities (40%, 44%, 40%, and 38% respectively). A meta-analysis by Lim et al.15 indicated that for stage IIIA-N2 NSCLC, the 5-year OS rate was 40.1% for the surgery-first approach followed by adjuvant chemotherapy and 39.3% for the chemotherapy-first approach followed by surgery. Surgical interventions after chemotherapy can be technically more challenging19 and neoadjuvant therapy might delay surgery and risk disease progression11.
Upfront surgery still remains a less commonly utilized approach for stage IIIA/B NSCLC, with definitive chemoradiotherapy (CRT) or CRT with durvalumab (PACIFIC pattern20) considered as the current standard of care. There is a lack of consensus regarding optimal candidate selection for upfront surgery and adjuvant treatment strategies for patients with stage IIIA-IIIB NSCLC, as well as insufficient research to reveal the long-term survival outcomes of upfront surgery in this patient cohort. The aim of this study was to assess the long-term outcomes of upfront surgery for patients with stage IIIA/B NSCLC in real-world practice, to identify prognostic factors that influence postoperative survival, and to discuss the selection criteria and definition of resectability for identifying optimal candidates who could benefit from upfront surgery.
Methods
Study design and patient inclusion/exclusion
This was a retrospective study of patients with stage cIIIA/B NSCLC (AJCC seventh or AJCC eighth) treated with upfront surgery between 1 January 2012 and 31 December 2019, from the structured electronic medical record (LinkDoc database) at The First Affiliated Hospital of Guangzhou Medical University. The inclusion criteria were as follows: patients who did not receive neoadjuvant therapy; patients greater than 18 years old; and patients who underwent surgical resection (lobectomy, bronchial sleeve resection, wedge resection, segmental resection, extended lobe resection, bi-lobectomy, or pneumonectomy). The exclusion criteria were as follows: patients enrolled in other clinical trials; patients with other malignancies at the time of diagnosis; and patients with missing data for treatment regimens.
Informed consent was waived due to the retrospective nature of the study. This study was approved by the ethics committee of The First Affiliated Hospital of Guangzhou Medical University (No. 2020-122).
Upfront surgery strategy
All patients underwent a standard preoperative staging workup that included pretreatment tumour biopsy (through endobronchial ultrasound (EBUS) or percutaneous biopsy), chest CT, bronchoscopy, enhanced brain MRI or CT, bone scintigraphy, abdominal ultrasonography or CT, and various cardiopulmonary examinations. Additionally, PET/CT was selectively used for patients presenting with bulky mediastinal masses or with discrete lymph nodes that could not be distinguished or measured.
A multidisciplinary team consisting of a medical oncologist, a thoracic surgeon, and a radiologist was responsible for assessing resectability for patients with stage IIIA/B NSCLC. If there were no indications of extra-nodal tumour invasion, there was no evidence of direct tumour invasion into the great vessels, diaphragm, heart, trachea, and carina, and the involved lymph node was clearly distinguishable from surrounding tissues by CT/PET-CT, the tumour was considered resectable. Those patients with a tumour deemed resectable underwent lobectomy with systematic mediastinal lymph node dissection (greater than or equal to 6 lymph nodes and greater than or equal to 3 nodal stations21), with extension to anatomical pulmonary resection (such as bi-lobectomy or pneumonectomy) performed as needed. For patients with poor lung function, limited resection, such as wedge resection or segmentectomy with systemic lymphadenectomy, was deemed acceptable. According to prior research22–24 and following the GOLD report25, poor lung function was defined as post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) less than 70%. Initial resection was conducted using video-assisted thoracoscopic surgery (VATS), with conversion to hybrid VATS or open surgery undertaken if necessary. All patients’ pathological stages were restaged according to the seventh edition26 of the Lung Cancer Stage Classification27. In our institution, surgery is rarely offered for patients diagnosed with N3 disease. Those scheduled for adjuvant therapy, without supraclavicular disease, with good cardiopulmonary performance, and with non-bulky nodal disease are candidates for surgery.
After upfront surgery, patients were assigned to one of several adjuvant treatment regimens based on driver mutation evaluation of postoperative pathological specimens, including chemotherapy, targeted therapy, CRT, anti-angiogenic therapy in combination with chemotherapy, or combined chemotherapy and targeted therapy. The administration of adjuvant therapy was not obligatory for patients who demonstrated intolerance to chemotherapy or other adjuvant treatments.
Data collection and statistical analysis
Information regarding demographics, diagnosis, treatment, and surgical and oncological outcomes was extracted from the structured electronic medical record (LinkDoc database). The extracted data included: age, sex, smoking history, year of initial NSCLC diagnosis, histological subtype, pathological N status, clinical stage, year and type of resection, surgical margin, adjuvant therapy, date of recurrence, type of recurrence after resection, and date of death. OS was defined as the interval from the date of diagnosis to the date of death. RFS was taken as the time from the date of surgical resection until the first recurrence (locoregional or distant failure). Grade 3 or higher postoperative adverse effects were defined based on the Common Terminology Criteria for Adverse Events (CTCAE) version 3.028.
The study estimated OS and RFS using the Kaplan–Meier method and stratified by resection margin (also proportionality assessed). For quantitative data, mean(s.d.) is used for normally distributed quantitative data and median (interquartile range (i.q.r.)) is used for skewed or non-normally distributed quantitative data. Cox proportional hazard models were used to identify potential prognostic factors for OS. A log rank test was used to compare survival between groups. Logistic regression was used to analyse relevant factors influencing complete resection. P < 0.050 was considered statistically significant.
Results
Patient characteristics
A total of 664 patients with stage cIIIA/B NSCLC met the eligibility criteria. The demographic and clinical characteristics of eligible patients are listed in Table 1. The majority of patients were male (65.7%), were younger than 65 years (70.5%), had lung adenocarcinoma (61.2%), and were cN2 (46.8%). Of these patients, 225 (33.9%) had a tumour size of less than 3 cm, 250 (37.7%) had a tumour size of greater than or equal to 3 cm but less than 5 cm, and 189 (28.4%) had a tumour size greater than or equal to 5 cm. A total of 118 patients (17.8%) underwent PET/CT for preoperative staging.
Table 1.
Baseline characteristics and surgical/pathological outcomes of included stage III patients (n = 664)
| Variable | Value |
|---|---|
| Sex | |
| Male | 410 (65.71) |
| Female | 214 (34.29) |
| Age (years), mean(s.d.) | 58.8(9.94) |
| <65 | 440 (70.51) |
| ≥65 | 184 (29.49) |
| BMI (kg/m 2 ), mean(s.d.) | 23.12(3.061) |
| Race, Chinese, % | 100.0 |
| Smoking history* | |
| Current/past | 160 (29.04)/112 (20.33) |
| Never | 279 (50.64) |
| Histological subtype | |
| LUAD | 382 (61.22) |
| LUSQ | 128 (20.51) |
| Other | 114 (18.27) |
| cT status | |
| T1 | 225 (33.89) |
| T2 | 250 (37.65) |
| T3–T4 | 189 (28.46) |
| Maximum tumour diameter (cm) | |
| Mean(s.d.) | 4.03(2.20) |
| Median (i.q.r.) | 3.50 (2.50–5.00) |
| cN status (based on preoperative staging) | |
| N0 | 62 (9.34) |
| N1 | 269 (30.51) |
| N2 | 320 (48.19) |
| N3 | 13 (1.96) |
| EGFR status† | |
| Positive | 110 (16.57) |
| Negative | 158 (23.80) |
| PD-L1 expression‡ | |
| <1% | 91 (64.08) |
| 1% to <25% | 29 (20.42) |
| 25% to <50% | 7 (4.93) |
| ≥50% | 15 (10.56) |
Values are n (%) unless otherwise indicated. *Smoking history data were unavailable for 48 patients. †EGFR status examination was not performed for 396 patients. ‡PD-L1 expression examination was not performed for 522 patients. LUAD, lung adenocarcinoma; LUSQ, lung squamous cell carcinoma; EGFR, epidermal growth factor receptor; PD-L1, programmed death ligand 1.
Perioperative outcomes
See Table 2. A total of 624 patients (94.0%) had an R0 resection and positive margins were identified in 40 cases (6.0%), including 10 (1.5%) with microscopically positive (R1) margins and 30 (4.5%) with macroscopically positive (R2) margins. Lobectomy was performed in 84.9% of cases, sleeve lobectomy in 7.2% of cases, pneumonectomy in 4.5% of cases, wedge resection in 2.6% of cases, and segmentectomy in 0.8% of cases. The 30-day perioperative mortality rate was 1.36%, with a median duration of hospital stay of 17.0 (i.q.r. 14.0–22.0) days. Within the patient cohort, 13.10% experienced adverse events, with nausea (2.3%), anaemia (1.8%), and leucopenia (1.4%) being the most common. Other adverse events included hyperglycaemia (1.2%), polypnoea (0.9%), chest pain (0.9%), myelosuppression (0.6%), vomiting (0.6%), coughing (2.1%), and other events (1.4%). Grade III adverse events were reported in 2.0% of patients.
Table 2.
Perioperative outcomes of all included patients (n = 664)
| Variable | Value |
|---|---|
| Surgical procedure | |
| Pneumonectomy | 30 (4.5) |
| Lobectomy | 564 (84.9) |
| Sleeve lobectomy | 48 (7.2) |
| Segmentectomy | 5 (0.8) |
| Wedge resection | 17 (2.6) |
| Surgical margin | |
| R0 | 624 (94.0) |
| R1 | 10 (1.5) |
| R2 | 30 (4.5) |
| pN status (based on surgical specimens) | |
| N0 | 51 (7.68) |
| N1 | 69 (10.39) |
| N2 | 521 (78.46) |
| Single-station metastasis | 278 (53.35) |
| Multi-station metastasis | 243 (46.64) |
| N3 | 23 (3.46) |
| pTNM stage | |
| IIIA | 569 (91.19) |
| IIIB | 55 (8.81) |
| IIIC | 0 |
| Duration of perioperative hospital stay (days), median (i.q.r.) | 17.0 (14.0–22.0) |
| Major postoperative complications | |
| Intraoperative blood loss ≥500 ml | 7 (1.1) |
| Prolonged air leak* | 71 (10.7) |
| Postoperative pneumonia | 46 (6.9) |
| Cardiac events | |
| Cardiac arrhythmia | 9 (1.4) |
| Postoperative atrial fibrillation | 2 (0.3) |
| Myocardial infarction | 2 (0.3) |
| Wound infection | 3 (0.5) |
| Acute respiratory failure | 2 (0.3) |
| Pulmonary thromboembolism | 3 (0.5) |
| Chylothorax | 3 (0.5) |
| Bronchopleural fistula | 1 (0.2) |
| Broncho-oesophageal fistula | 1 (0.2) |
| Vocal cord palsy/recurrent laryngeal nerve paralysis | 3 (0.5) |
| Grade III adverse event | 13 (2.0) |
Values are n (%) unless otherwise indicated. *A prolonged air leak was defined as a leak lasting more than 5 days. R0, R0 resection with negative margin; R1, R1 resection with microscopically positive margins; R2, R2 resection with macroscopically positive margins.
Predictors for incomplete resection
Logistic regression was conducted to investigate the determinants of resection margins, comparing patients who underwent complete resection and incomplete resection. A total of four patients who underwent wedge resections due to palliative surgery were excluded from the analysis. When considering T and N stages separately, surgical procedure, T stage, N stage, and histological pathology were found to be associated with R1/R2 resection. Specifically, the OR for T4 compared with T1 was 21.46 (95% c.i. 1.74 to 265; P = 0.017), for N3 compared with N0 was 36.55 (95% c.i. 1.52 to 876; P = 0.026), for N2 compared with N0 was 5.06 (95% c.i. 0.72 to 35.7; P = 0.104), and for adenocarcinoma compared with squamous cell carcinoma was 0.21 (95% c.i. 0.055 to 0.829; P = 0.026). Sleeve resection, compared with lobectomy, had an OR of 10.53 (95% c.i. 2.49 to 44.49; P = 0.001) (Table S1). When considering TNM staging, stage IIIB, compared with stage IIIA, had an OR of 4.79 (95% c.i. 1.45 to 15.76; P = 0.010), indicating a stronger association with R1/R2 resection. Similarly, surgical procedure and histological type yielded consistent results, with an OR of 0.22 (95% c.i. 0.059 to 0.790; P = 0.020) for adenocarcinoma compared with squamous cell carcinoma and an OR of 6.05 (95% c.i. 1.60 to 22.85; P = 0.008) for wedge resection compared with lobectomy. However, BMI was found to be unrelated to incomplete resection (Table S2).
Survival outcomes and recurrence pattern
Postoperative outcomes are shown in Table 3. After surgery, the following adjuvant therapies were received: 330 patients (49.70%) received chemotherapy; 65 patients (9.79%) received targeted therapy; 24 patients (3.61%) received chemotherapy combined with anti-angiogenic therapy; 23 patients (3.46%) received chemotherapy combined with radiotherapy; 8 patients (1.20%) received chemotherapy combined with targeted therapy; and 23 patients (3.46%) received other therapy.
Table 3.
Postoperative outcomes of all included patients (n = 664)
| Variable | Value |
|---|---|
| Adjuvant therapy | 473 (71.23) |
| Chemotherapy | 330 (49.70) |
| Targeted therapy | 65 (9.79) |
| Chemotherapy + anti-angiogenic therapy | 24 (3.61) |
| Chemotherapy + radiotherapy | 23 (3.46) |
| Chemotherapy + targeted therapy | 8 (1.20) |
| Other | 23 (3.46) |
| Recurrence rate at the last follow-up time | 247 (37.20) |
| Recurrence site (for all patients with recurrence) | |
| Lung | 111 (50.45) |
| Brain | 55 (25.00) |
| Skeleton | 43 (19.54) |
| Liver | 19 (8.64) |
| Lymph node | 15 (6.82) |
| Other | 41 (18.64) |
| Recurrence-free survival probability, % | |
| 1.0 year | 74.1 |
| 2.0 years | 57.4 |
| 3.0 years | 43.5 |
| 5.0 years | 26.4 |
| Overall survival (months), median (95% c.i.) | 60.0 (51.5,67.6) |
| Overall survival probability, % | |
| 1.0 year | 89.6 |
| 2.0 years | 77.8 |
| 3.0 years | 67.2 |
| 5.0 years | 49.0 |
| Survival status | |
| Death | 202 (30.42) |
| Live | 372 (56.02) |
| Missing | 90 (13.55) |
Values are n (%) unless otherwise indicated.
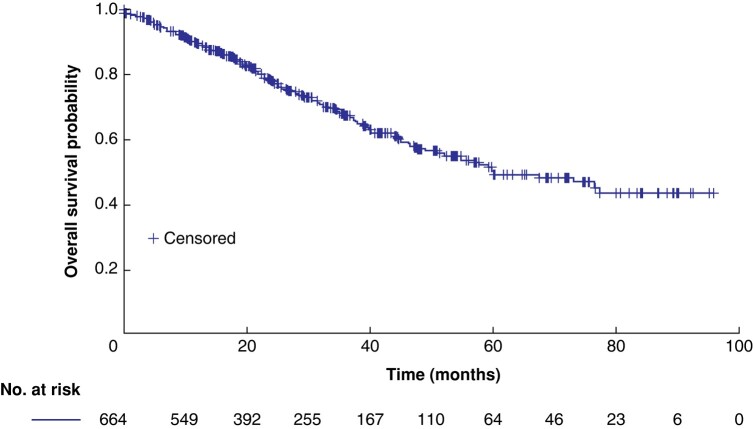
The median follow-up was 30.6 (range 1.9–97.7) months. At follow-up, the mortality rate was 13.3% (88 of 664) and 37.2% of patients (247 of 664) had evidence of disease recurrence. Recurrence was observed in 247 patients (37.20%); 101 of 247 patients (40.9%), 53 of 247 patients (21.5%), 45 of 247 patients (18.2%), and 19 of 247 patients (7.7%) developed lung, brain, skeleton, and liver metastasis respectively. The RFS probability at 1, 2, 3, and 5 years was 74.1%, 57.4%, 43.5%, and 26.4% respectively. Additionally, the median OS was 60.0 (95% c.i. 51.5 to 67.6) months (Fig. 1), with OS probability at 1, 2, 3, and 5 years being 89.6%, 77.8%, 67.2%, and 49.0% respectively. Survival status data revealed that 30.42% of patients had died at the time of analysis and that 56.02% of patients were alive at the time of analysis; data were missing for 13.55% of patients. R0 resection patients exhibited a median OS of 67.4 (95% c.i. 55.5 to 75.2) months, with 1-, 2-, 3-, and 5-year OS rates of 90.5%, 79.0%, 68.3%, and 50.2% respectively. In contrast, non-R0 resection patients had a median OS of 26.5 (95% c.i. 14.8 to 37.8) months, with 1-, 2-, and 3-year OS rates of 75.5%, 57.8%, and 48.9% respectively. Log rank test results indicated a significant disparity in OS between the two groups (P = greater than 0.001). Among R0 resection patients, the median disease-free survival was 30.8 (95% c.i. 25.7 to 34.7) months, with 1-, 2-, 3-, and 5-year disease-free survival rates of 74.7%, 58.2%, 44.4%, and 28.0% respectively. Meanwhile, non-R0 resection patients exhibited a median progression-free survival of 21.4 (95% c.i. 11.2 to 28.8) months, with 1-, 2-, and 3-year progression-free survival rates of 65.1%, 45.1%, and 30.1% respectively. However, the log rank test revealed no statistically significant difference in disease-free survival/progression-free survival between the two groups (P = 0.08).
Fig. 1.
Kaplan–Meier curve of overall survival for the included patients
Independent prognostic factors after upfront surgery
Multivariable regression analysis was used to explore the factors affecting RFS and OS for patients who received upfront surgery, adjusted for age, sex, smoking status, histology, tumour size, surgical factors, pN/pT status, and adjuvant treatment (Table 4). The multivariable analysis revealed that age greater than or equal to 65 years (HR 1.51, 95% c.i. 1.08 to 2.12; reference = age less than 65 years), tumour size (greater than or equal to 5 cm (HR 2.13, 95% c.i. 1.41 to 3.21) and greater than or equal to 3 cm but less than 5 cm (HR 1.15, 95% c.i. 0.78 to 1.71); reference = less than 3 cm) and adjuvant treatment (chemotherapy (HR 0.69, 95% c.i. 0.49 to 0.96) and targeted therapy (HR 0.30, 95% c.i. 0.12 to 0.76); reference = none) significantly predicted OS.
Table 4.
Multivariable Cox analyses for recurrence-free survival and overall survival of included patients
| Recurrence-free survival | Overall survival | |||
|---|---|---|---|---|
| HR (95% c.i.) | P | HR (95% c.i.) | P | |
| Age (reference = <65 years) | 0.1308 | 0.0174 | ||
| ≥65 years | 1.230 (0.940,1.608) | 1.508 (1.075,2.115) | ||
| Sex (reference = female) | 0.8596 | 0.6176 | ||
| Male | 0.970 (0.695,1.354) | 1.120 (0.718,1.748) | ||
| Smoking status (reference = never a smoker) | 0.6545 | 0.9865 | ||
| Past smoker | 1.039 (0.717,1.505) | 0.987 (0.621,1.569) | ||
| Current smoker | 1.156 (0.830,1.611) | 0.966 (0.630,1.482) | ||
| Histology (reference = LUAD) | 0.0577 | 0.1625 | ||
| LUSQ | 0.736 (0.533,1.017) | 1.156 (0.789,1.692) | ||
| LASC | 1.109 (0.525,2.345) | 1.446 (0.600,3.486) | ||
| Other | 0.641 (0.434,0.948) | 0.594 (0.337,1.046) | ||
| Tumour size (reference = <3 cm) | 0.0016 | 0.0003 | ||
| ≥3 cm but <5 cm | 1.136 (0.855,1.510) | 1.153 (0.778,1.707) | ||
| ≥5 cm | 1.723 (1.258,2.358) | 2.130 (1.413,3.209) | ||
| Surgical procedure (reference = pneumonectomy) | 0.4444 | – | 0.5678 | |
| Segmentectomy | 0.274 (0.036,2.117) | |||
| Sleeve lobectomy | 0.738 (0.356,1.534) | 1.108 (0.438,2.804) | ||
| Wedge resection | 1.186 (0.522,2.694) | 2.247 (0.779,6.484) | ||
| Lobectomy | 0.950 (0.526,1.718) | 1.277 (0.574,2.840) | ||
| Surgical margin (reference = R1/R2 resection) | 0.4907 | 0.2163 | ||
| R0 resection | 0.834 (0.499,1.396) | 0.684 (0.375,1.249) | ||
| pN status (reference = pN0) | 0.2175 | 0.5473 | ||
| pN1 | 0.854 (0.484,1.506) | 1.203 (0.590,2.454) | ||
| pN2 | 1.121 (0.706,1.777) | 1.450 (0.778,2.706) | ||
| pN3 | 1.820 (0.874,3.787) | 1.832 (0.721,4.659) | ||
| Adjuvant treatment (reference = none) | 0.0835 | 0.0163 | ||
| Chemotherapy | 0.797 (0.612,1.040) | 0.686 (0.492,0.959) | ||
| Targeted therapy | 0.512 (0.296,0.887) | 0.297 (0.117,0.756) | ||
| Other | 0.854 (0.576,1.265) | 0.599 (0.356,1.009) | ||
LUAD, lung adenocarcinoma; LUSQ, lung squamous cell carcinoma; LASC, lung adenosquamous carcinoma; R0, R0 resection with negative margin; R1, R1 resection with microscopically positive margins; R2, R2 resection with macroscopically positive margins.
Discussion
This observational, retrospective study reports real-world data for long-term survival outcomes and identifies potential prognostic factors that influence the postoperative survival of stage IIIA/B NSCLC patients, treated with upfront curative-intent surgery at a single thoracic oncology centre. In the present study, with a median follow-up of 30.6 months, the median OS was(95% c.i. 51.5 to 67.6) months, with OS probability at 1.0, 2.0, 3.0, and 5.0 years being 89.6%, 77.8%, 67.2%, and 49.0% respectively. These results exceed the median OS of 52.5 (95% c.i. 43.1 to 61.9) months reported by Jung et al.12 based on a Korean cohort of stage III surgically resected NSCLC and are comparable to the median OS of 63 months observed in a German cohort of patients with radically resected NSCLC, including those with stage IIIB disease29. The present study’s 5-year OS rate is consistent with those reported for large real-world cohorts of stage cN2/pN2 NSCLC patients who underwent upfront surgery during a similar interval, with 5-year OS rates ranging from 43%10,30 to 44.7%11,31. The improved survival may be attributed to the higher number of patients aged younger than 65 years and a greater number of patients who received adjuvant treatments, such as targeted therapy or chemotherapy. The literature comparing the outcomes of upfront surgery for stage III NSCLC is summarized in Table 5, highlighting that studies (with similar research intervals) with high adjuvant treatment rates have favourable 5-year OS rates (43–71%10,11,30,31,40,42), 5-year RFS rates (21–50.6%10,11,39,42), and median OS (44.5–57.8 months10,11,42,44).
Table 5.
World literature comparing the outcomes of upfront surgery for stage III NSCLC
| Reference (publication year) | Adjuvant treatment rate, % | Tumour stage | Number of patients | Outcome | Months (median) | Five-year survival rate, % |
|---|---|---|---|---|---|---|
| Roth et al.32 (1998) | 0 | cIIIA | 21 | OS | 7.0 | 0 |
| Rosell et al.33 (1999) | S + R 100 | cIIIA | 30 | OS | 10.0 | 0 |
| Andre et al.34 (2000) | S + R 65 | cN2/pN2 | 562 | OS | pN2L2 16; cN2 14 | mN2L1 34; mN2L2 11; cN2L1 8; cN2L2 3* |
| Ichinose et al.30 (2001) | S + C 41; S + R 15 | pIIIA-N2 | 402 | OS | – | Total 31; pN2L1 43; pN2L2 17 |
| Nagai et al.35 (2003) | 0 | cIIIA | 31 | OS | Total 16.0 | Total 22 |
| Casali et al.36 (2005) | S + C 100 | pN2 | 183 | OS | Total 24.0; pN2L1 25.2; pN2L2 16.0 |
Total 20; pN2L1 23.8; pN2L2 14.7 |
| Ratto et al.37 (2009) | S + C 5.4; S + R 39.4; S + CR 25.6 | cN2 | 192 | OS | 18.7 | – |
| Hishida et al.38 (2014) | S + C 6.67 | pIIIA-N2 | 45 | RFS | – | 22.3 |
| OS | – | 23.6 | ||||
| Hancock et al.39 (2015) | S + C 22.4; S + R 12.5; S + CR 35.1 | pIII NSCLC with R1 resection | 177 | OS | – | No adjuvant 12; S + C 18; S + R 9; S + CR 30 |
| Maniwa et al.40 (2016) | 45.8 | cN2 | 29 | OS | – | 71.1 |
| DFS | – | 50.6 | ||||
| Zheng et al.10 (2016) | S + C 84.6; S + R 23.5 | pIIIA | 668 | PFS | 17.1 | 21 |
| OS | 44.5 | 43 | ||||
| Maniwa et al.41 (2018) | S + C 47.8; S + T 21.3 | cN2 | 94 | OS | Total 58.0; cN2pN2 48.0 | Total 47.9; cN2pN0/1 74.9; cN2pN2 41.2 |
| Yuan et al.42 (2019) | S + C 95.9; S + CR 33.3 | pIIIA-N2 | 363 | RFS | – | S + C 23.1; S + CR 15.5 |
| OS | S + C 50.0; S + CR 50.0 | S + C 47.0; S + CR 44.5 | ||||
| Yun et al.11 (2019) | Total 82.6; S + C 23.9; S + R 17.4; S + CR 35.1 | pN2 | 706 | RFS | 23 | 33.8 |
| OS | 52 | 44.7 | ||||
| Taber and Pfannschmidt43 (2020) | Total 29.3; S + C 17.9; S + R 2.9; S + CR 8.5 | cIIIA | 250 | DFS | – | 24.9 |
| OS | – | 30.0 | ||||
| cIIIB | 94 | DFS | – | 8.3 | ||
| OS | – | 8.3 | ||||
| Jazieh et al.44 (2021) | 100 | IIIA | 114 | PFS | 18.8 | – |
| OS | 57.8 | – | ||||
| IIIB | 13 | PFS | 13.8 | – | ||
| OS | 34.7 | – | ||||
| Bertolaccini et al.16 (2022) | S ± C NR | pN2 | 126 | OS | R0 66.0 | R0 66.3 |
| RFS | – | R0 22 | ||||
| Bitenc et al.31 (2022) | S ± C/R NR | cIIIA/pIIIA | 85 | OS | cIIIA not reached; pIIIA 51.6 | cIIIA 55.3; pIIIA 49.9 |
| Hayakawa et al.45 (2023) | S ± C NR | cN2pN2 | 43 | RFS | – | No adjuvant 28.0; S+ C 56.5 |
| OS | – | No adjuvant 41.9; S + C 82.2 | ||||
| Fu et al.46 (2023) | S + C 85.7; S + R 23.4 | pIIIA-N2 | 475 | OS | R0 48.1; R1/2 28.0; pN2L1 61.0; pN2L2 35.0 | 42.2 |
| RFS | R0 18.0; R1/2 10.6; pN2L1 21.7; pN2L2 12.0 | 21.3 | ||||
| Campisi et al.47 (2023) | S + C 71.2; S + CR 21.2 | cN2pN2 | 52 | OS | 37.00 | 30.8 |
| RFS | 27.96 | – | ||||
| The present study (2024) | Total 71.23; S + C 49.70; S + T 9.79 | cIIIA/IIIB | 664 | RFS | R0 30.8; R1/2 21.4 | R0 28.0; R1/2 0 |
| OS | Total 60.0; R0 67.4; R1/2 26.5 | Total 49.0; R0 50.2; R1/2 0 |
*Patients with minimal N2 (mN2) are those with no preoperative detection of N2 disease, and patients with cN2 are those in whom N2 disease is detected before surgery by CT and confirmed by mediastinoscopy. Multiple lymph node levels involved is defined as L2, and a single lymph node level involved is defined as L1. OS, overall survival; S, surgery; R, adjuvant radiotherapy; C, adjuvant chemotherapy; CR, complete response; RFS, recurrence-free survival; NSCLC, non-small cell lung cancer; R1, R1 resection with microscopically positive margins; DFS, disease-free survival; PFS, progression-free survival; T, adjuvant targeted therapy; NR, not reported; R0, R0 resection with negative margin; R2, R2 resection with macroscopically positive margins.
The main challenge regarding upfront surgery for stage III NSCLC is selecting suitable patients and optimizing the adjuvant strategy. The involvement of lymph nodes, as determined by imaging or intraoperative examination48, can impact the extent and feasibility of surgical intervention and may require modification of the planned surgical procedure. Whereas some centres consider ipsilateral non-bulky single nodal station involvement or discrete non-diffuse mediastinal nodes as a surgical indication49, others advocate a more aggressive surgical intervention for NSCLC with mediastinal nodes over 10 mm50. According to Bertolaccini et al.16, upfront surgery may be appropriate for individuals with single-station mediastinal nodal involvement and no evidence of extra-nodal tumour invasion. Maniwa et al.40 specified four criteria for performing upfront surgery for patients with cN2 NSCLC (single-station N2 disease, non-bulky N2 disease, N2 disease with regional mode of spread, and N2 disease without N1 disease) and these patients had a 5-year OS rate of 71.1%. Hishida et al.38 also stated that true single-station pathological N2 and negative subcarinal node status were independent prognostic factors that favoured survival after initial resection for clinical single-station N2/pathological N2 patients, with an HR of 0.35 (P = 0.008) and 0.34 (P = 0.022) respectively. Bulky multi-station N2 disease is frequently not amenable to surgery. In the present study, approximately 51.71% of patients who underwent upfront surgery had multi-station N2 metastasis; this multi-station rate is consistent with previous literature30,42 and indicates that surgery is still feasible in these patients.
Although it is technically feasible to remove large and locally invasive tumours, there is limited evidence supporting extended resections for stage N3 NSCLC51. In the present study, 13 patients (1.96%) with N3 disease received upfront surgery. The best approach for stage N3 NSCLC is still uncertain, especially for patients who are fit for surgery. It is plausible that these patients could be treated similarly to those with operable stage IIIA (N2) NSCLC and achieve satisfactory outcomes. A previous study52 has demonstrated that, for patients with cN3 or pN3 stage NSCLC, surgery was associated with improved long-term survival compared with chemoradiation (HR 0.76, 95% c.i. 0.58 to 0.99). Similarly, an analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated that patients with N3 stage NSCLC who received surgery had significantly better survival (HR 0.71, 95% c.i. 0.64 to 0.79; P < 0.001) compared with those who did not53. These studies suggest that, in carefully selected patients with limited N3 disease, surgery may offer superior survival and can be considered as part of a multimodal therapeutic approach.
Although surgical resection is the mainstay treatment for NSCLC, positive margins occur in between 10%54 and 12.6%55 and are associated with worse long-term outcomes, essentially decreasing the 5-year OS by half39,56–58. Riquet et al.58 reported a median OS of 17 months for R1 resection and 51 months for R0 resection. R0 resection is crucial for upfront surgery. In the present study, the incidence of R1/R2 resection was 6.02%, which is consistent with previous literature39,58, and was significantly associated with worse survival compared with complete resection (median OS of 26.5 versus 67.4 months; P < 0.001). In addition, stage IIIB (particularly involving stage T4 and N3), lung squamous cell carcinoma, and sleeve resection all exhibited a heightened incidence of positive margins, aligning with findings from prior studies58,59. The identification of these factors that associated with incomplete resection may help guide clinical decision-making for upfront surgery and improve surgical outcomes.
While upfront surgery alone has demonstrated unfavourable 5-year OS rates, typically 14–30%50,60–62 for stage III NSCLC (Table 5), the addition of adjuvant treatment has been shown to lead to further improvement in survival. Examples include studies reporting enhanced median OS and 5-year rates (around 43.0%10 to 44.7%11) in stage cN2 and pIIIA NSCLC patients who received adjuvant treatments. Trials such as EVIDENCE63, ADAURA64, and IMpower01065 explored the effectiveness of adjuvant icotinib, osimertinib, and atezolizumab respectively, showing significant improvements in disease-free survival compared with chemotherapy. The encouraging results from trials involving adjuvant immunotherapy65,66 and targeted therapy63,64, surpassing adjuvant chemotherapy, suggest a potential shift toward a more efficacious treatment strategy involving upfront surgery followed by adjuvant therapy in the future.
Currently, most guidelines6–8 recommend induction therapy for stage IIIA-N2 NSCLC. For example, the ASCO guidelines8 recommend that patients with stage III N2 NSCLC scheduled to undergo surgical resection should receive neoadjuvant chemotherapy or neoadjuvant concurrent chemoradiation. The authors’ team67 has also found that neoadjuvant chemotherapy followed by surgery and adjuvant radiotherapy outperformed surgery alone and surgery followed by adjuvant radiotherapy through a network meta-analysis. However, not all patients can benefit from neoadjuvant treatment, with the pCR rate ranging from 0%68 to 53%69, depending on the specific regimen (chemotherapy/CRT70, targeted therapy68, or immunotherapy/immunochemotherapy71,72), and the persistent N273 rate ranging from approximately 39.4%74 to 53%73,75. This suggests that nearly half of patients do not respond positively or, at the very least, do not experience a complete response to neoadjuvant treatment. The benefits observed in this subset of patients may amplify the overall benefits for those who undergo neoadjuvant chemotherapy, as substantiated by the provided references. Given that persistent N2 implies minimal benefit from induction therapy76 (in stage cN2 NSCLC patients, those who exhibit persistent ypN2 after neoadjuvant therapy have comparable postoperative survival to those who undergo upfront surgery34), it is logical to consider the possibility of shifting surgery before chemotherapy for these patients. To select suitable candidates for upfront surgery, in addition to meeting the general surgical indications8 (such as achieving complete resection (R0) of both the primary tumour and the involved lymph nodes, N3 lymph nodes not involved, and an expected low perioperative mortality), the utilization of predictive efficacy biomarkers can also be instrumental in determining the need for neoadjuvant therapy77.
This supports the principles of personalized medicine, aiming to identify individuals within the stage III NSCLC patient population who may not directly benefit from neoadjuvant treatment. Moving surgery upfront under such circumstances could be justifiable to avoid surgery delays that could result in disease progression78 or lead to the experience of side effects79 that might preclude surgery (Fig. S1). Similarly, the international guidelines for the management of malignant pleural mesothelioma (MPM)80 state that chemotherapy has limited efficacy and surgery may be appropriate for carefully and highly selected patients. Although these guidelines predominantly address MPM, the perception of the surgical role remains consistent, highlighting surgery as a viable option when deemed suitable, particularly in situations where systemic therapy may offer limited efficacy.
Overall, the present study indicates that, for patients with stage IIIA/IIIB NSCLC, upfront surgery can serve as an alternative therapeutic approach, provided that a comprehensive evaluation of technical resectability is performed and that adjuvant therapy is administered. Several limitations should be acknowledged. First, selection bias is inherent in a retrospective study from a single institution. Secondly, the heterogeneity of the surgical procedure might bias the results. Thirdly, clinical staging of the mediastinum is challenging and can be inaccurate.
Supplementary Material
Acknowledgements
The authors thank LinkDoc Technology Co. Ltd, Beijing, China for patient screening and executing data extraction from the structured automatic electronic medical record (LinkDoc database) in this study. H. Deng, J. Liu, X. Cai, and S. Jiang contributed equally to the present study.
Contributor Information
Hongsheng Deng, Department of Thoracic Surgery and Oncology, The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, Guangzhou, China.
Jun Liu, Department of Thoracic Surgery and Oncology, The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, Guangzhou, China.
Xiuyu Cai, Department of General Internal Medicine, Sun Yat-sen University Cancer Centre, State Key Laboratory of Oncology in South China, Collaborative Innovation Centre for Cancer Medicine, Guangzhou, China.
Shunjun Jiang, Department of Thoracic Surgery and Oncology, The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, Guangzhou, China; Department of Pharmacy, The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, Guangzhou, China.
Weixiang Lu, Department of Thoracic Surgery and Oncology, The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, Guangzhou, China.
Qing Ai, Department of Thoracic Surgery and Oncology, The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, Guangzhou, China.
Jianfu Li, Department of Thoracic Surgery and Oncology, The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, Guangzhou, China.
Shan Xiong, Department of Thoracic Surgery and Oncology, The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, Guangzhou, China.
Xiangyun Qin, LinkDoc Technology Co. Ltd, Beijing, China.
Wenhua Liang, Department of Thoracic Surgery and Oncology, The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, Guangzhou, China.
Jianxing He, Department of Thoracic Surgery and Oncology, The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, Guangzhou, China.
Funding
The authors have no funding to declare.
Disclosure
X. Qin is currently employed by LinkDoc Technology Co. Ltd, Beijing, China. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
Data are available upon reasonable request.
References
- 1. Huber RM, De Ruysscher D, Hoffmann H, Reu S, Tufman A. Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur Respir Rev 2019;28:190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Cancer Institute . Surveillance, Epidemiology, and End Results Program (SEER) Cancer Statistics Review 1975–2015. 2019
- 3. Liu J, O'Donnell JS, Yan J, Madore J, Allen S, Smyth MJ et al. Timing of neoadjuvant immunotherapy in relation to surgery is crucial for outcome. Oncoimmunology 2019;8:e1581530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evison M. The current treatment landscape in the UK for stage III NSCLC. Br J Cancer 2020;123:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Schil PE, Berzenji L, Yogeswaran SK, Hendriks JM, Lauwers P. Surgical management of stage IIIA non-small cell lung cancer. Front Oncol 2017;7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2022;20:497–530 [DOI] [PubMed] [Google Scholar]
- 7. Remon J, Soria JC, Peters S. Early and locally advanced non-small-cell lung cancer: an update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol 2021;32:1637–1642 [DOI] [PubMed] [Google Scholar]
- 8. Daly ME, Singh N, Ismaila N, Antonoff MB, Arenberg DA, Bradley J et al. Management of stage III non-small-cell lung cancer: ASCO guideline. J Clin Oncol 2022;40:1356–1384 [DOI] [PubMed] [Google Scholar]
- 9. Kozower BD, Larner JM, Detterbeck FC, Jones DR. Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S–e399S [DOI] [PubMed] [Google Scholar]
- 10. Zheng D, Ye T, Hu H, Zhang Y, Sun Y, Xiang J et al. Upfront surgery as first-line therapy in selected patients with stage IIIA non-small cell lung cancer. J Thorac Cardiovasc Surg 2018;155:1814–1822.e4 [DOI] [PubMed] [Google Scholar]
- 11. Yun JK, Bok JS, Lee GD, Kim HR, Kim Y-H, Kim DK et al. Long-term outcomes of upfront surgery in patients with resectable pathological N2 non-small-cell lung cancer. Eur J Cardiothorac Surg 2020;58:59–69 [DOI] [PubMed] [Google Scholar]
- 12. Jung HA, Sun JM, Lee SH, Ahn JS, Ahn MJ, Park K. Ten-year patient journey of stage III non-small cell lung cancer patients: a single-center, observational, retrospective study in Korea (realtime autOmatically updated data warehOuse in healTh care; UNIVERSE-ROOT study). Lung Cancer 2020;146:112–119 [DOI] [PubMed] [Google Scholar]
- 13. Felip E, Rosell R, Maestre JA, Rodríguez-Paniagua JM, Morán T, Astudillo J et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138–3145 [DOI] [PubMed] [Google Scholar]
- 14. Savic M, Kontic M, Ercegovac M, Stojsic J, Bascarevic S, Moskovljevic D et al. Comparison of mediastinal lymph node status and relapse pattern in clinical stage IIIA non-small cell lung cancer patients treated with neoadjuvant chemotherapy versus upfront surgery: a single center experience. Thorac Cancer 2017;8:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lim E, Harris G, Patel A, Adachi I, Edmonds L, Song F. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380–1388 [DOI] [PubMed] [Google Scholar]
- 16. Bertolaccini L, Prisciandaro E, Guarize J, Girelli L, Sedda G, Filippi N et al. Long-term clinical outcomes and prognostic factors of upfront surgery as a first-line therapy in biopsy-proven clinical N2 non-small cell lung cancer. Front Oncol 2022;12:933278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boffa DJ, Hancock JG, Yao X, Goldberg S, Rosen JE, Kim AW et al. Now or later: evaluating the importance of chemotherapy timing in resectable stage III (N2) lung cancer in the National Cancer Database. Ann Thorac Surg 2015;99:200–208 [DOI] [PubMed] [Google Scholar]
- 18. Moreno AC, Morgensztern D, Boffa DJ, Decker RH, Yu JB, Detterbeck FC et al. Treating locally advanced disease: an analysis of very large, hilar lymph node positive non-small cell lung cancer using the National Cancer Data Base. Ann Thorac Surg 2014;97:1149–1155 [DOI] [PubMed] [Google Scholar]
- 19. Sonobe M, Yutaka Y, Nakajima D, Hamaji M, Menju T, Ohsumi A et al. Salvage surgery after chemotherapy or chemoradiotherapy for initially unresectable lung carcinoma. Ann Thorac Surg 2019;108:1664–1670 [DOI] [PubMed] [Google Scholar]
- 20. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–1929 [DOI] [PubMed] [Google Scholar]
- 21. Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25–33 [DOI] [PubMed] [Google Scholar]
- 22. Huang W, Deng H, Lan Y, Wang R, Ge F, Huo Z et al. Spontaneous ventilation video-assisted thoracic surgery for mediastinal tumor resection in patients with pulmonary function deficiency. Ann Transl Med 2020;8:1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang R, Wang Q, Jiang S, Chen C, Zheng J, Liu H et al. Spontaneous ventilation video-assisted thoracoscopic surgery for non-small-cell lung cancer patients with poor lung function: short- and long-term outcomes. Front Surg 2022;9:800082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer 2002;37:95–101 [DOI] [PubMed] [Google Scholar]
- 25. Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Respirology 2023;28:316–338 [DOI] [PubMed] [Google Scholar]
- 26. Lababede O, Meziane M, Rice T. Seventh edition of the cancer staging manual and stage grouping of lung cancer. Chest 2011;139:183–189. 10.1378/chest.10-10991 [DOI] [PubMed] [Google Scholar]
- 27. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest 2017;151:193–203 [DOI] [PubMed] [Google Scholar]
- 28. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–181 [DOI] [PubMed] [Google Scholar]
- 29. Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787–792 [DOI] [PubMed] [Google Scholar]
- 30. Ichinose Y, Kato H, Koike T, Tsuchiya R, Fujisawa T, Shimizu N et al. Completely resected stage IIIA non-small cell lung cancer: the significance of primary tumor location and N2 station. J Thorac Cardiovasc Surg 2001;122:803–808 [DOI] [PubMed] [Google Scholar]
- 31. Bitenc M, Cufer T, Kern I, Miklavcic M, Petrovic S, Groznik V et al. Real-life long-term outcomes of upfront surgery in patients with resectable stage I-IIIA non-small cell lung cancer. Radiol Oncol 2022;56:346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roth JA, Atkinson EN, Fossella F, Komaki R, Bernadette Ryan M, Putnam JB Jr et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer 1998;21:1–6 [DOI] [PubMed] [Google Scholar]
- 33. Rosell R, Gómez-Codina J, Camps C, Javier Sánchez J, Maestre J, Padilla J et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer 1999;26:7–14 [DOI] [PubMed] [Google Scholar]
- 34. Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol 2000;18:2981–2989 [DOI] [PubMed] [Google Scholar]
- 35. Nagai K, Tsuchiya R, Mori T, Tada H, Ichinose Y, Koike T et al. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg 2003;125:254–260 [DOI] [PubMed] [Google Scholar]
- 36. Casali C, Stefani A, Natali P, Rossi G, Morandi U. Prognostic factors in surgically resected N2 non-small cell lung cancer: the importance of patterns of mediastinal lymph nodes metastases. Eur J Cardiothorac Surg 2005;28:33–38 [DOI] [PubMed] [Google Scholar]
- 37. Ratto GB, Costa R, Maineri P, Alloisio A, Bruzzi P, Dozin B. Is there a subset of patients with preoperatively diagnosed N2 non-small cell lung cancer who might benefit from surgical resection? J Thorac Cardiovasc Surg 2009;138:849–858 [DOI] [PubMed] [Google Scholar]
- 38. Hishida T, Yoshida J, Ohe Y, Aokage K, Ishii G, Nagai K. Surgical outcomes after initial surgery for clinical single-station N2 non-small-cell lung cancer. Jpn J Clin Oncol 2014;44:85–92 [DOI] [PubMed] [Google Scholar]
- 39. Hancock JG, Rosen JE, Antonicelli A, Moreno A, Kim AW, Detterbeck FC et al. Impact of adjuvant treatment for microscopic residual disease after non-small cell lung cancer surgery. Ann Thorac Surg 2015;99:406–413 [DOI] [PubMed] [Google Scholar]
- 40. Maniwa T, Takahashi S, Isaka M, Endo M, Ohde Y. Outcomes of initial surgery in patients with clinical N2 non-small cell lung cancer who met 4 specific criteria. Surg Today 2016;46:699–704 [DOI] [PubMed] [Google Scholar]
- 41. Maniwa T, Shintani Y, Okami J, Kadota Y, Takeuchi Y, Takami K et al. Upfront surgery in patients with clinical skip N2 lung cancer based on results of modern radiological examinations. J Thorac Dis 2018;10:6828–6837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan C, Tao X, Zheng D, Pan Y, Ye T, Hu H et al. The lymph node status and histologic subtypes influenced the effect of postoperative radiotherapy on patients with N2 positive IIIA non-small cell lung cancer. J Surg Oncol 2019;119:379–387 [DOI] [PubMed] [Google Scholar]
- 43. Taber S, Pfannschmidt J. Validation of the 8th lung cancer TNM classification and clinical staging system in a German cohort of surgically resected patients. Innov Surg Sci 2020;5:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jazieh AR, Onal HC, Tan DSW, Soo RA, Prabhash K, Kumar A et al. Real-world treatment patterns and clinical outcomes in patients with stage III NSCLC: results of KINDLE, a multicountry observational study. J Thorac Oncol 2021;16:1733–1744 [DOI] [PubMed] [Google Scholar]
- 45. Hayakawa T, Isaka M, Konno H, Mizuno T, Kawata T, Kenmotsu H et al. Survival outcome of upfront surgery for clinical single-station N2 non-small cell lung cancer. Jpn J Clin Oncol 2023;53:429–435 [DOI] [PubMed] [Google Scholar]
- 46. Fu F, Sun W, Bai J, Deng C, Zheng D, Li Y et al. Long-term outcomes of selected patients with IIIA–N2 non-small cell lung cancer receiving upfront surgical resection. Ann Surg Oncol 2023;30:8261–8270 [DOI] [PubMed] [Google Scholar]
- 47. Campisi A, Catelli C, Gabryel P, Giovannetti R, Dell’Amore A, Kasprzyk M et al. Upfront surgery for N2 NSCLC: a large retrospective multicenter cohort study. Gen Thorac Cardiovasc Surg 2023;71:715–722 [DOI] [PubMed] [Google Scholar]
- 48. Zou JY, Zhao WH, Chen JL, Du XK, Hu XW, Ye ZY. [The role of EBUS-TBNA in the systematic evaluation of lymph node staging and resectability analysis in non-small cell lung cancer]. Zhonghua Zhong Liu Za Zhi 2019;41:792–795 [DOI] [PubMed] [Google Scholar]
- 49. Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA et al. Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S–e340S [DOI] [PubMed] [Google Scholar]
- 50. Watanabe Y, Shimizu J, Oda M, Hayashi Y, Watanabe S, Tatsuzawa Y et al. Aggressive surgical intervention in N2 non-small cell cancer of the lung. Ann Thorac Surg 1991;51:253–261 [DOI] [PubMed] [Google Scholar]
- 51. Bryan DS, Donington JS. The role of surgery in management of locally advanced non-small cell lung cancer. Curr Treat Options Oncol 2019;20:27. [DOI] [PubMed] [Google Scholar]
- 52. Raman V, Jawitz OK, Yang CJ, Voigt SL, Wang H, D’Amico TAet al. Outcomes of surgery versus chemoradiotherapy in patients with clinical or pathologic stage N3 non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1680–1692.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Han C, Wu Y, Sun X, Chong Y, Kang K, Liu Z et al. Outcome of non-small cell lung cancer patients with N3 stage: survival analysis of propensity score matching with a validated predictive nomogram. Front Surg 2021;8:666332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gagliasso M, Migliaretti G, Ardissone F. Assessing the prognostic impact of the International Association for the Study of Lung Cancer proposed definitions of complete, uncertain, and incomplete resection in non-small cell lung cancer surgery. Lung Cancer 2017;111:124–130 [DOI] [PubMed] [Google Scholar]
- 55. Osarogiagbon RU, Lin CC, Smeltzer MP, Jemal A. Prevalence, prognostic implications, and survival modulators of incompletely resected non-small cell lung cancer in the U.S. National Cancer Data Base. J Thorac Oncol 2016;11:e5–e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Berghmans T, Pasleau F, Paesmans M, Bonduelle Y, Cadranel J, Cs Toth I et al. Surrogate markers predicting overall survival for lung cancer: ELCWP recommendations. Eur Respir J 2012;39:9–28 [DOI] [PubMed] [Google Scholar]
- 57. Rasing MJA, Peters M, Moreno AC, Hofman EFN, Herder GJM, Welvaart PWN et al. Predicting incomplete resection in non-small cell lung cancer preoperatively: a validated nomogram. Ann Thorac Surg 2021;111:1052–1058 [DOI] [PubMed] [Google Scholar]
- 58. Riquet M, Achour K, Foucault C, Le Pimpec Barthes F, Dujon A, Cazes A. Microscopic residual disease after resection for lung cancer: a multifaceted but poor factor of prognosis. Ann Thorac Surg 2010;89:870–875 [DOI] [PubMed] [Google Scholar]
- 59. Hofmann HS, Taege C, Lautenschläger C, Neef H, Silber RE. Microscopic (R1) and macroscopic (R2) residual disease in patients with resected non-small cell lung cancer. Eur J Cardiothorac Surg 2002;21:606–610 [DOI] [PubMed] [Google Scholar]
- 60. Brandt WS, Yan W, Leeman JE, Tan KS, Park BJ, Adusumilli PS et al. Postoperative radiotherapy for surgically resected ypN2 non-small cell lung cancer. Ann Thorac Surg 2018;106:848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mountain CF. Expanded possibilities for surgical treatment of lung cancer. Survival in stage IIIa disease. Chest 1990;97:1045–1051 [DOI] [PubMed] [Google Scholar]
- 62. Naruke T, Goya T, Tsuchiya R, Suemasu K. The importance of surgery to non-small cell carcinoma of lung with mediastinal lymph node metastasis. Ann Thorac Surg 1988;46:603–610 [DOI] [PubMed] [Google Scholar]
- 63. He J, Su C, Liang W, Xu S, Wu L, Fu X et al. Icotinib versus chemotherapy as adjuvant treatment for stage II-IIIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): a randomised, open-label, phase 3 trial. Lancet Respir Med 2021;9:1021–1029 [DOI] [PubMed] [Google Scholar]
- 64. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 2020;383:1711–1723 [DOI] [PubMed] [Google Scholar]
- 65. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344–1357 [DOI] [PubMed] [Google Scholar]
- 66. Paz-Ares L, O’Brien MER, Mauer M, Dafni U, Oselin K, Havel L et al. VP3-2022: Pembrolizumab (pembro) versus placebo for early-stage non-small cell lung cancer (NSCLC) following complete resection and adjuvant chemotherapy (chemo) when indicated: randomized, triple-blind, phase III EORTC-1416-LCG/ETOP 8-15 — PEARLS/KEYNOTE-091 study. Ann Oncol 2022;33:451–453 [Google Scholar]
- 67. Zhao Y, Wang W, Liang H, Yang CJ, D’Amico T, Ng CSH et al. The optimal treatment for stage IIIA-N2 non-small cell lung cancer: a network meta-analysis. Ann Thorac Surg 2019;107:1866–1875 [DOI] [PubMed] [Google Scholar]
- 68. Sun L, Guo YJ, Song J, Wang YR, Zhang SL, Huang LT et al. Neoadjuvant EGFR-TKI therapy for EGFR-mutant NSCLC: a systematic review and pooled analysis of five prospective clinical trials. Front Oncol 2020;10:586596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhai H, Li W, Jiang K, Zhi Y, Yang Z. Neoadjuvant nivolumab and chemotherapy in patients with locally advanced non-small cell lung cancer: a retrospective study. Cancer Manag Res 2022;14:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosner S, Liu C, Forde PM, Hu C. Association of pathologic complete response and long-term survival outcomes among patients treated with neoadjuvant chemotherapy or chemoradiotherapy for NSCLC: a meta-analysis. JTO Clin Res Rep 2022;3:100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu Y, Verma V, Gay CM, Chen Y, Liang F, Lin Q et al. Neoadjuvant immunotherapy for advanced, resectable non-small cell lung cancer: a systematic review and meta-analysis. Cancer 2023;129:1969–1985 [DOI] [PubMed] [Google Scholar]
- 72. Rothschild SI, Zippelius A, Eboulet EI, Savic Prince S, Betticher D, Bettini A et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer—a multicenter single-arm phase II trial. J Clin Oncol 2021;39:2872–2880 [DOI] [PubMed] [Google Scholar]
- 73. Kamel MK, Rahouma M, Ghaly G, Nasar A, Port JL, Stiles BM et al. Clinical predictors of persistent mediastinal nodal disease after induction therapy for stage IIIA N2 non-small cell lung cancer. Ann Thorac Surg 2017;103:281–286 [DOI] [PubMed] [Google Scholar]
- 74. Hu XF, Duan L, Jiang GN, Chen C, Fei KE. Surgery following neoadjuvant chemotherapy for non-small-cell lung cancer patients with unexpected persistent pathological N2 disease. Mol Clin Oncol 2016;4:261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim HK, Cho JH, Choi YS, Zo JI, Shim YM, Park K et al. Outcomes of neoadjuvant concurrent chemoradiotherapy followed by surgery for non-small-cell lung cancer with N2 disease. Lung Cancer 2016;96:56–62 [DOI] [PubMed] [Google Scholar]
- 76. Betticher DC, Hsu Schmitz SF, Tötsch M, Hansen E, Joss C, von Briel C et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752–1759 [DOI] [PubMed] [Google Scholar]
- 77. Deng H, Zhao Y, Cai X, Chen H, Cheng B, Zhong R et al. PD-L1 expression and tumor mutation burden as pathological response biomarkers of neoadjuvant immunotherapy for early-stage non-small cell lung cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2022;170:103582. [DOI] [PubMed] [Google Scholar]
- 78. Gard G, Voskoboynik M. Setting the stage: delay to surgery may upstage patients with non-small cell lung cancer. Chest 2019;156:633–634 [DOI] [PubMed] [Google Scholar]
- 79. Jia XH, Xu H, Geng LY, Jiao M, Wang WJ, Jiang LL et al. Efficacy and safety of neoadjuvant immunotherapy in resectable nonsmall cell lung cancer: a meta-analysis. Lung Cancer 2020;147:143–153 [DOI] [PubMed] [Google Scholar]
- 80. Scherpereel A, Opitz I, Berghmans T, Psallidas I, Glatzer M, Rigau D et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J 2020;55:1900953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.
References
- 1. Huber RM, De Ruysscher D, Hoffmann H, Reu S, Tufman A. Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur Respir Rev 2019;28:190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Cancer Institute . Surveillance, Epidemiology, and End Results Program (SEER) Cancer Statistics Review 1975–2015. 2019
- 3. Liu J, O'Donnell JS, Yan J, Madore J, Allen S, Smyth MJ et al. Timing of neoadjuvant immunotherapy in relation to surgery is crucial for outcome. Oncoimmunology 2019;8:e1581530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evison M. The current treatment landscape in the UK for stage III NSCLC. Br J Cancer 2020;123:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Schil PE, Berzenji L, Yogeswaran SK, Hendriks JM, Lauwers P. Surgical management of stage IIIA non-small cell lung cancer. Front Oncol 2017;7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2022;20:497–530 [DOI] [PubMed] [Google Scholar]
- 7. Remon J, Soria JC, Peters S. Early and locally advanced non-small-cell lung cancer: an update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol 2021;32:1637–1642 [DOI] [PubMed] [Google Scholar]
- 8. Daly ME, Singh N, Ismaila N, Antonoff MB, Arenberg DA, Bradley J et al. Management of stage III non-small-cell lung cancer: ASCO guideline. J Clin Oncol 2022;40:1356–1384 [DOI] [PubMed] [Google Scholar]
- 9. Kozower BD, Larner JM, Detterbeck FC, Jones DR. Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S–e399S [DOI] [PubMed] [Google Scholar]
- 10. Zheng D, Ye T, Hu H, Zhang Y, Sun Y, Xiang J et al. Upfront surgery as first-line therapy in selected patients with stage IIIA non-small cell lung cancer. J Thorac Cardiovasc Surg 2018;155:1814–1822.e4 [DOI] [PubMed] [Google Scholar]
- 11. Yun JK, Bok JS, Lee GD, Kim HR, Kim Y-H, Kim DK et al. Long-term outcomes of upfront surgery in patients with resectable pathological N2 non-small-cell lung cancer. Eur J Cardiothorac Surg 2020;58:59–69 [DOI] [PubMed] [Google Scholar]
- 12. Jung HA, Sun JM, Lee SH, Ahn JS, Ahn MJ, Park K. Ten-year patient journey of stage III non-small cell lung cancer patients: a single-center, observational, retrospective study in Korea (realtime autOmatically updated data warehOuse in healTh care; UNIVERSE-ROOT study). Lung Cancer 2020;146:112–119 [DOI] [PubMed] [Google Scholar]
- 13. Felip E, Rosell R, Maestre JA, Rodríguez-Paniagua JM, Morán T, Astudillo J et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138–3145 [DOI] [PubMed] [Google Scholar]
- 14. Savic M, Kontic M, Ercegovac M, Stojsic J, Bascarevic S, Moskovljevic D et al. Comparison of mediastinal lymph node status and relapse pattern in clinical stage IIIA non-small cell lung cancer patients treated with neoadjuvant chemotherapy versus upfront surgery: a single center experience. Thorac Cancer 2017;8:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lim E, Harris G, Patel A, Adachi I, Edmonds L, Song F. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380–1388 [DOI] [PubMed] [Google Scholar]
- 16. Bertolaccini L, Prisciandaro E, Guarize J, Girelli L, Sedda G, Filippi N et al. Long-term clinical outcomes and prognostic factors of upfront surgery as a first-line therapy in biopsy-proven clinical N2 non-small cell lung cancer. Front Oncol 2022;12:933278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boffa DJ, Hancock JG, Yao X, Goldberg S, Rosen JE, Kim AW et al. Now or later: evaluating the importance of chemotherapy timing in resectable stage III (N2) lung cancer in the National Cancer Database. Ann Thorac Surg 2015;99:200–208 [DOI] [PubMed] [Google Scholar]
- 18. Moreno AC, Morgensztern D, Boffa DJ, Decker RH, Yu JB, Detterbeck FC et al. Treating locally advanced disease: an analysis of very large, hilar lymph node positive non-small cell lung cancer using the National Cancer Data Base. Ann Thorac Surg 2014;97:1149–1155 [DOI] [PubMed] [Google Scholar]
- 19. Sonobe M, Yutaka Y, Nakajima D, Hamaji M, Menju T, Ohsumi A et al. Salvage surgery after chemotherapy or chemoradiotherapy for initially unresectable lung carcinoma. Ann Thorac Surg 2019;108:1664–1670 [DOI] [PubMed] [Google Scholar]
- 20. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–1929 [DOI] [PubMed] [Google Scholar]
- 21. Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25–33 [DOI] [PubMed] [Google Scholar]
- 22. Huang W, Deng H, Lan Y, Wang R, Ge F, Huo Z et al. Spontaneous ventilation video-assisted thoracic surgery for mediastinal tumor resection in patients with pulmonary function deficiency. Ann Transl Med 2020;8:1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang R, Wang Q, Jiang S, Chen C, Zheng J, Liu H et al. Spontaneous ventilation video-assisted thoracoscopic surgery for non-small-cell lung cancer patients with poor lung function: short- and long-term outcomes. Front Surg 2022;9:800082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer 2002;37:95–101 [DOI] [PubMed] [Google Scholar]
- 25. Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Respirology 2023;28:316–338 [DOI] [PubMed] [Google Scholar]
- 26. Lababede O, Meziane M, Rice T. Seventh edition of the cancer staging manual and stage grouping of lung cancer. Chest 2011;139:183–189. 10.1378/chest.10-10991 [DOI] [PubMed] [Google Scholar]
- 27. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest 2017;151:193–203 [DOI] [PubMed] [Google Scholar]
- 28. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–181 [DOI] [PubMed] [Google Scholar]
- 29. Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787–792 [DOI] [PubMed] [Google Scholar]
- 30. Ichinose Y, Kato H, Koike T, Tsuchiya R, Fujisawa T, Shimizu N et al. Completely resected stage IIIA non-small cell lung cancer: the significance of primary tumor location and N2 station. J Thorac Cardiovasc Surg 2001;122:803–808 [DOI] [PubMed] [Google Scholar]
- 31. Bitenc M, Cufer T, Kern I, Miklavcic M, Petrovic S, Groznik V et al. Real-life long-term outcomes of upfront surgery in patients with resectable stage I-IIIA non-small cell lung cancer. Radiol Oncol 2022;56:346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roth JA, Atkinson EN, Fossella F, Komaki R, Bernadette Ryan M, Putnam JB Jr et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer 1998;21:1–6 [DOI] [PubMed] [Google Scholar]
- 33. Rosell R, Gómez-Codina J, Camps C, Javier Sánchez J, Maestre J, Padilla J et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer 1999;26:7–14 [DOI] [PubMed] [Google Scholar]
- 34. Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol 2000;18:2981–2989 [DOI] [PubMed] [Google Scholar]
- 35. Nagai K, Tsuchiya R, Mori T, Tada H, Ichinose Y, Koike T et al. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg 2003;125:254–260 [DOI] [PubMed] [Google Scholar]
- 36. Casali C, Stefani A, Natali P, Rossi G, Morandi U. Prognostic factors in surgically resected N2 non-small cell lung cancer: the importance of patterns of mediastinal lymph nodes metastases. Eur J Cardiothorac Surg 2005;28:33–38 [DOI] [PubMed] [Google Scholar]
- 37. Ratto GB, Costa R, Maineri P, Alloisio A, Bruzzi P, Dozin B. Is there a subset of patients with preoperatively diagnosed N2 non-small cell lung cancer who might benefit from surgical resection? J Thorac Cardiovasc Surg 2009;138:849–858 [DOI] [PubMed] [Google Scholar]
- 38. Hishida T, Yoshida J, Ohe Y, Aokage K, Ishii G, Nagai K. Surgical outcomes after initial surgery for clinical single-station N2 non-small-cell lung cancer. Jpn J Clin Oncol 2014;44:85–92 [DOI] [PubMed] [Google Scholar]
- 39. Hancock JG, Rosen JE, Antonicelli A, Moreno A, Kim AW, Detterbeck FC et al. Impact of adjuvant treatment for microscopic residual disease after non-small cell lung cancer surgery. Ann Thorac Surg 2015;99:406–413 [DOI] [PubMed] [Google Scholar]
- 40. Maniwa T, Takahashi S, Isaka M, Endo M, Ohde Y. Outcomes of initial surgery in patients with clinical N2 non-small cell lung cancer who met 4 specific criteria. Surg Today 2016;46:699–704 [DOI] [PubMed] [Google Scholar]
- 41. Maniwa T, Shintani Y, Okami J, Kadota Y, Takeuchi Y, Takami K et al. Upfront surgery in patients with clinical skip N2 lung cancer based on results of modern radiological examinations. J Thorac Dis 2018;10:6828–6837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan C, Tao X, Zheng D, Pan Y, Ye T, Hu H et al. The lymph node status and histologic subtypes influenced the effect of postoperative radiotherapy on patients with N2 positive IIIA non-small cell lung cancer. J Surg Oncol 2019;119:379–387 [DOI] [PubMed] [Google Scholar]
- 43. Taber S, Pfannschmidt J. Validation of the 8th lung cancer TNM classification and clinical staging system in a German cohort of surgically resected patients. Innov Surg Sci 2020;5:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jazieh AR, Onal HC, Tan DSW, Soo RA, Prabhash K, Kumar A et al. Real-world treatment patterns and clinical outcomes in patients with stage III NSCLC: results of KINDLE, a multicountry observational study. J Thorac Oncol 2021;16:1733–1744 [DOI] [PubMed] [Google Scholar]
- 45. Hayakawa T, Isaka M, Konno H, Mizuno T, Kawata T, Kenmotsu H et al. Survival outcome of upfront surgery for clinical single-station N2 non-small cell lung cancer. Jpn J Clin Oncol 2023;53:429–435 [DOI] [PubMed] [Google Scholar]
- 46. Fu F, Sun W, Bai J, Deng C, Zheng D, Li Y et al. Long-term outcomes of selected patients with IIIA–N2 non-small cell lung cancer receiving upfront surgical resection. Ann Surg Oncol 2023;30:8261–8270 [DOI] [PubMed] [Google Scholar]
- 47. Campisi A, Catelli C, Gabryel P, Giovannetti R, Dell’Amore A, Kasprzyk M et al. Upfront surgery for N2 NSCLC: a large retrospective multicenter cohort study. Gen Thorac Cardiovasc Surg 2023;71:715–722 [DOI] [PubMed] [Google Scholar]
- 48. Zou JY, Zhao WH, Chen JL, Du XK, Hu XW, Ye ZY. [The role of EBUS-TBNA in the systematic evaluation of lymph node staging and resectability analysis in non-small cell lung cancer]. Zhonghua Zhong Liu Za Zhi 2019;41:792–795 [DOI] [PubMed] [Google Scholar]
- 49. Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA et al. Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S–e340S [DOI] [PubMed] [Google Scholar]
- 50. Watanabe Y, Shimizu J, Oda M, Hayashi Y, Watanabe S, Tatsuzawa Y et al. Aggressive surgical intervention in N2 non-small cell cancer of the lung. Ann Thorac Surg 1991;51:253–261 [DOI] [PubMed] [Google Scholar]
- 51. Bryan DS, Donington JS. The role of surgery in management of locally advanced non-small cell lung cancer. Curr Treat Options Oncol 2019;20:27. [DOI] [PubMed] [Google Scholar]
- 52. Raman V, Jawitz OK, Yang CJ, Voigt SL, Wang H, D’Amico TAet al. Outcomes of surgery versus chemoradiotherapy in patients with clinical or pathologic stage N3 non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1680–1692.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Han C, Wu Y, Sun X, Chong Y, Kang K, Liu Z et al. Outcome of non-small cell lung cancer patients with N3 stage: survival analysis of propensity score matching with a validated predictive nomogram. Front Surg 2021;8:666332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gagliasso M, Migliaretti G, Ardissone F. Assessing the prognostic impact of the International Association for the Study of Lung Cancer proposed definitions of complete, uncertain, and incomplete resection in non-small cell lung cancer surgery. Lung Cancer 2017;111:124–130 [DOI] [PubMed] [Google Scholar]
- 55. Osarogiagbon RU, Lin CC, Smeltzer MP, Jemal A. Prevalence, prognostic implications, and survival modulators of incompletely resected non-small cell lung cancer in the U.S. National Cancer Data Base. J Thorac Oncol 2016;11:e5–e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Berghmans T, Pasleau F, Paesmans M, Bonduelle Y, Cadranel J, Cs Toth I et al. Surrogate markers predicting overall survival for lung cancer: ELCWP recommendations. Eur Respir J 2012;39:9–28 [DOI] [PubMed] [Google Scholar]
- 57. Rasing MJA, Peters M, Moreno AC, Hofman EFN, Herder GJM, Welvaart PWN et al. Predicting incomplete resection in non-small cell lung cancer preoperatively: a validated nomogram. Ann Thorac Surg 2021;111:1052–1058 [DOI] [PubMed] [Google Scholar]
- 58. Riquet M, Achour K, Foucault C, Le Pimpec Barthes F, Dujon A, Cazes A. Microscopic residual disease after resection for lung cancer: a multifaceted but poor factor of prognosis. Ann Thorac Surg 2010;89:870–875 [DOI] [PubMed] [Google Scholar]
- 59. Hofmann HS, Taege C, Lautenschläger C, Neef H, Silber RE. Microscopic (R1) and macroscopic (R2) residual disease in patients with resected non-small cell lung cancer. Eur J Cardiothorac Surg 2002;21:606–610 [DOI] [PubMed] [Google Scholar]
- 60. Brandt WS, Yan W, Leeman JE, Tan KS, Park BJ, Adusumilli PS et al. Postoperative radiotherapy for surgically resected ypN2 non-small cell lung cancer. Ann Thorac Surg 2018;106:848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mountain CF. Expanded possibilities for surgical treatment of lung cancer. Survival in stage IIIa disease. Chest 1990;97:1045–1051 [DOI] [PubMed] [Google Scholar]
- 62. Naruke T, Goya T, Tsuchiya R, Suemasu K. The importance of surgery to non-small cell carcinoma of lung with mediastinal lymph node metastasis. Ann Thorac Surg 1988;46:603–610 [DOI] [PubMed] [Google Scholar]
- 63. He J, Su C, Liang W, Xu S, Wu L, Fu X et al. Icotinib versus chemotherapy as adjuvant treatment for stage II-IIIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): a randomised, open-label, phase 3 trial. Lancet Respir Med 2021;9:1021–1029 [DOI] [PubMed] [Google Scholar]
- 64. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 2020;383:1711–1723 [DOI] [PubMed] [Google Scholar]
- 65. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344–1357 [DOI] [PubMed] [Google Scholar]
- 66. Paz-Ares L, O’Brien MER, Mauer M, Dafni U, Oselin K, Havel L et al. VP3-2022: Pembrolizumab (pembro) versus placebo for early-stage non-small cell lung cancer (NSCLC) following complete resection and adjuvant chemotherapy (chemo) when indicated: randomized, triple-blind, phase III EORTC-1416-LCG/ETOP 8-15 — PEARLS/KEYNOTE-091 study. Ann Oncol 2022;33:451–453 [Google Scholar]
- 67. Zhao Y, Wang W, Liang H, Yang CJ, D’Amico T, Ng CSH et al. The optimal treatment for stage IIIA-N2 non-small cell lung cancer: a network meta-analysis. Ann Thorac Surg 2019;107:1866–1875 [DOI] [PubMed] [Google Scholar]
- 68. Sun L, Guo YJ, Song J, Wang YR, Zhang SL, Huang LT et al. Neoadjuvant EGFR-TKI therapy for EGFR-mutant NSCLC: a systematic review and pooled analysis of five prospective clinical trials. Front Oncol 2020;10:586596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhai H, Li W, Jiang K, Zhi Y, Yang Z. Neoadjuvant nivolumab and chemotherapy in patients with locally advanced non-small cell lung cancer: a retrospective study. Cancer Manag Res 2022;14:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosner S, Liu C, Forde PM, Hu C. Association of pathologic complete response and long-term survival outcomes among patients treated with neoadjuvant chemotherapy or chemoradiotherapy for NSCLC: a meta-analysis. JTO Clin Res Rep 2022;3:100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu Y, Verma V, Gay CM, Chen Y, Liang F, Lin Q et al. Neoadjuvant immunotherapy for advanced, resectable non-small cell lung cancer: a systematic review and meta-analysis. Cancer 2023;129:1969–1985 [DOI] [PubMed] [Google Scholar]
- 72. Rothschild SI, Zippelius A, Eboulet EI, Savic Prince S, Betticher D, Bettini A et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer—a multicenter single-arm phase II trial. J Clin Oncol 2021;39:2872–2880 [DOI] [PubMed] [Google Scholar]
- 73. Kamel MK, Rahouma M, Ghaly G, Nasar A, Port JL, Stiles BM et al. Clinical predictors of persistent mediastinal nodal disease after induction therapy for stage IIIA N2 non-small cell lung cancer. Ann Thorac Surg 2017;103:281–286 [DOI] [PubMed] [Google Scholar]
- 74. Hu XF, Duan L, Jiang GN, Chen C, Fei KE. Surgery following neoadjuvant chemotherapy for non-small-cell lung cancer patients with unexpected persistent pathological N2 disease. Mol Clin Oncol 2016;4:261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim HK, Cho JH, Choi YS, Zo JI, Shim YM, Park K et al. Outcomes of neoadjuvant concurrent chemoradiotherapy followed by surgery for non-small-cell lung cancer with N2 disease. Lung Cancer 2016;96:56–62 [DOI] [PubMed] [Google Scholar]
- 76. Betticher DC, Hsu Schmitz SF, Tötsch M, Hansen E, Joss C, von Briel C et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752–1759 [DOI] [PubMed] [Google Scholar]
- 77. Deng H, Zhao Y, Cai X, Chen H, Cheng B, Zhong R et al. PD-L1 expression and tumor mutation burden as pathological response biomarkers of neoadjuvant immunotherapy for early-stage non-small cell lung cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2022;170:103582. [DOI] [PubMed] [Google Scholar]
- 78. Gard G, Voskoboynik M. Setting the stage: delay to surgery may upstage patients with non-small cell lung cancer. Chest 2019;156:633–634 [DOI] [PubMed] [Google Scholar]
- 79. Jia XH, Xu H, Geng LY, Jiao M, Wang WJ, Jiang LL et al. Efficacy and safety of neoadjuvant immunotherapy in resectable nonsmall cell lung cancer: a meta-analysis. Lung Cancer 2020;147:143–153 [DOI] [PubMed] [Google Scholar]
- 80. Scherpereel A, Opitz I, Berghmans T, Psallidas I, Glatzer M, Rigau D et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J 2020;55:1900953. [DOI] [PubMed] [Google Scholar]