ABSTRACT
The causative agent of human tuberculosis, Mycobacterium tuberculosis, has a complex lipid-rich diderm envelope, which acts as a major barrier protecting the bacterium against the hostile environment inside the host cells. For the transfer of diverse molecules across this complex cell envelope, M. tuberculosis has a series of general and specialized protein secretion systems, characterized by the SecA general secretion pathway, the twin-arginine translocation pathway, and five specific ESX type VII secretion systems. In this review, we focus on the latter systems, known as ESX-1 to ESX-5, which were first discovered almost 20 years ago during the in silico analysis of the genome sequence of M. tuberculosis H37Rv. Since then, these systems have been the subject of highly dynamic research due to their involvement in several key biological processes and host-pathogen interactions of the tubercle bacilli.
INTRODUCTION
The different bacterial species within the tree of life (1) possess a range of secretion systems, which play important roles in the transport of proteins across the various types of bacterial cell envelopes. Classically, Gram staining was used for differentiating Gram-positive and Gram-negative bacteria, but classifications on cell envelope architecture might come closer to the biological reality, and thus, bacteria may also be differentiated according to their cell envelopes into diderm-lipopolysaccharide (archetypal Gram-negative), monoderm (archetypal Gram-positive), and diderm-mycolate (archetypal acid-fast) bacteria (2). For Gram-negative bacteria a range of at least eight different secretion systems has been described (types I to VI, VIII, and IX) (3–5). While in monoderm bacteria secretion and export are synonymous, in diderm bacteria the secretion is completed only upon translocation of the substrates across the outer membrane (2). The here-reviewed mycobacterial ESAT-6 secretion (ESX) systems (6, 7), which were also named type VII secretion (T7S) systems (8), represent a particular class of protein export and/or secretion systems, for which at present only the inner-membrane translocation machinery has been explored in more detail (9, 10), whereas it remains unknown how ESX/T7S-exported proteins get transported through the mycobacterial outer membrane into the extracellular environment (11). Indeed, one of the remarkable characteristics of mycobacteria is their complex cell envelope, which is shared to some extent with other members of the Corynebacterineae, a suborder of the phylum Actinobacteria (1, 12, 13). Mycobacteria are surrounded by a diderm cell envelope, consisting of an inner membrane, a peptidoglycan layer, an arabinogalactan layer, an outer membrane, named mycomembrane, which is composed of covalently linked mycolic acids and extractable lipids, and a capsule (14, 15). This unusual cell envelope requires complex secretion systems for the export/secretion of proteins, such as those of the SecA and twin-arginine translocation pathways, as well as the specialized ESX/T7S systems (7, 8, 16), which were first discovered almost 20 years ago during in silico analyses of the genome sequence and the proteome of Mycobacterium tuberculosis H37Rv (17, 18). Moreover, T7S-like systems that share some core components of mycobacterial ESX/T7S systems exist in various genera of the phylum Firmicutes, representing many classical Gram-positive bacterial species (19), which, however, are not the subject of the current review.
M. tuberculosis possesses five ESX/T7S systems (ESX-1 to ESX-5) (7, 8, 11, 16). All five ESX systems share several common features: the presence of small secreted proteins (of about 100 amino acids) with a conserved Trp-X-Gly (WXG) motif (20), an FtsK-SpoIIIE ATPase, several transmembrane proteins, and a subtilisin-like mycosin (MycP) (11, 16) (Fig. 1). These systems, encoded in different sections of the mycobacterial chromosome, seem to have evolved by gene duplication and diversification from simpler systems that were shuffled around in different actinobacterial and mycobacterial species, often mediated by plasmids encoding ESX/T7S elements as well as elements of type IV secretion systems (21–23). ESX/T7S systems play an important role in the biology of M. tuberculosis, as well as in the interactions M. tuberculosis has with its host. Indeed, a number of secreted effectors, including EsxA (ESAT-6), EsxB (CFP-10), and ESX-1 secretion-associated proteins (Esp), such as EspA or EspC, as well as proteins that carry the characteristic N-terminal motifs Pro-Glu (PE) and Pro-Pro-Glu (PPE), have been suggested to intervene in host cellular and immune signaling pathways (11, 24, 25).
FIGURE 1.
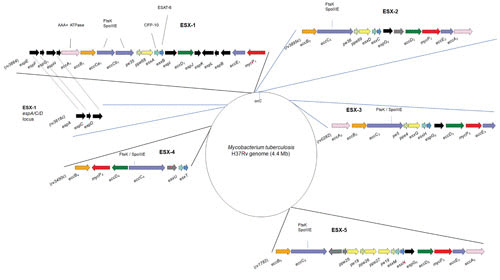
Genetic organization of the ESX loci. Shown is a schematic representation of the approximative genomic sites of the ESX-1 to ESX-5 clusters in the M. tuberculosis H37Rv genome. Gene nomenclature and gene color scheme were adapted from reference 16.
Here we focus on recent updates on the ESX/T7S systems of mycobacteria and summarize new findings on their structure, function, and role in host-pathogen interactions and briefly touch on their significance in translational research.
RECENT INSIGHTS INTO THE STRUCTURAL AND FUNCTIONAL CHARACTERISTICS OF ESX/T7S NANOMACHINES
Five ESX systems are encoded in the genome of M. tuberculosis (16, 18), and this number is the highest found in mycobacteria so far; other mycobacterial species show fewer systems (e.g., Mycobacterium marinum shows four systems and Mycobacterium abscessus shows three systems) (21, 22). While ESX-4, ESX-3, and ESX-1 are present in most fast-growing and slow-growing mycobacteria, ESX-2 and ESX-5 systems are found only in selected slow-growing mycobacteria and thus represent the most recently evolved systems (21, 22). For ESX-2, currently not much is known on its putative function. In contrast, for ESX-1, ESX-3, and ESX-5, recent research has determined that they all contribute to virulence of M. tuberculosis, although the insights into the exact molecular functions often remain vague; also, because many studies have been undertaken with different mycobacterial species (M. tuberculosis, M. marinum, M. abscessus, and/or Mycobacterium smegmatis), which may show some species-specific differences (reviewed in references 11 and 26 to 28). ESX-3 is important for metal homeostasis, pathogenicity, and immunogenicity (29–31). ESX-5 was suggested to be crucial for nutrient uptake and for the export of members of the PE and PPE protein families (9, 32, 33). These two large protein families have expanded during mycobacterial evolution (34, 35), and they include representative proteins that are associated with ESX/T7S systems and others with highly repetitive sequence motifs that are exported by ESX-5 and impact virulence and immunogenicity (32, 36–39). The ESX-5 nanomachine, which is integrated in the inner mycobacterial membrane, is composed of four proteins, namely, EccB5, EccC5, EccD5, and EccE5 (9, 10), that are organized in a hexameric complex, as recently determined by cryo-electron microscopy and single-particle analysis (10). This organization differs substantially from those of secretion systems of Gram-negative bacteria (10) (Fig. 2). Among the Ecc proteins (ESX conserved components), the structural and functional roles of EccC (FtsK/SpoIIIE ATPase) have been studied in more detail with the thermophilic actinobacterium Thermomonospora curvata (40). A certain flexibility of the cytosolic domains of EccC in interaction with effectors was suggested (10, 40), which is different from the cognate ATPase in type IV secretion systems (10).
FIGURE 2.
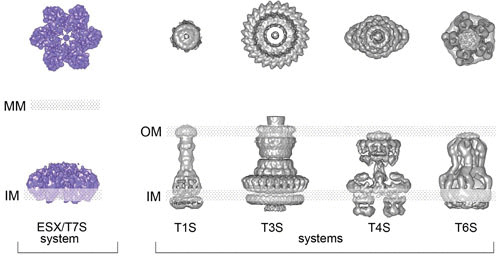
Representation of top and side views of the ESX/T7S system based on recent structural data generated by cryo-electron microscopy and single-particle analysis on an ESX-5 system from Mycobacterium xenopi, in comparison to selected examples of secretion systems from Gram-negative bacteria. The positions of the inner membrane (IM), outer membrane (OM), and mycomembrane (MM) are indicated. Adapted from reference 10, with permission.
Another conserved ESX component in mycobacterial ESX systems is the serine protease MycP, although this protein is not directly integrated in the EccBCDE complex (10, 41–43). Moreover, different proteins, such as EspA, EspC, EspD, and EccA1, may be essential to contributing to secretion and stabilizing the core ESX/T7S complex in the case of ESX-1 (44–47). How these related effectors are explicitly recognized and targeted towards the specific system in a single mycobacterial species with different ESX/T7S systems can be a matter of debate. Recently, it has become clear that some of the conserved ESX components could potentially exhibit chaperone-like activity (e.g., EspG or EccA) (48). It was suggested that besides their chaperone activity, these proteins are also involved in determining the secretion system specificity. Indeed, by substituting the binding domain of EspG, the ESX-1-dependent substrate can be rerouted to the ESX-5 system (49). In addition, it was suggested that EspL can have a role as a chaperone and is essential for ESX-1-dependent virulence (50). Therefore, scrutinizing the role of chaperones will certainly help to provide a better understanding of the ESX/T7S functions and mechanisms.
It is also intriguing that certain ESX/T7S systems may have a dual function. For example, the ESX-1 system, which is present in fast- and slow-growing mycobacterial species, is required for distributive conjugal transfer (DCT) of chromosomal DNA from donor into recipient strains of M. smegmatis (51, 52). This procedure apparently also involves the ESX-4 system of M. smegmatis (53). Moreover, it was shown that SigM, an extracytoplasmic function σ factor, is an activator of ESX-4 expression and necessary for DCT in the recipient strain of M. smegmatis (54). Intriguingly, experimental strain-to-strain transfer of chromosomal DNA was also observed in selected Mycobacterium canettii strains (55), representing a group of rare tubercle bacilli that are thought to resemble the ancestor of M. tuberculosis and have been isolated mainly from tuberculosis patients in the region of the Horn of Africa (East Africa) (56, 57). In contrast to M. canettii strains, DNA transfer between M. tuberculosis strains was not observed despite numerous trials (55), emphasizing the clonal population structure of M. tuberculosis strains (58, 59). While it is predicted that the interstrain DNA transfer between M. canettii strains might also involve an ESX-1 system in the recipient strain, in analogy to the situation in M. smegmatis, experimental confirmation for this hypothesis has not yet been reported.
ESX SYSTEMS IN HOST-PATHOGEN INTERACTIONS
The potential dual function of the ESX-1 system is best visible by the fact that in slow-growing mycobacteria, in contrast to fast-growing mycobacteria, the ESX-1 system is also involved in the pathogenic potential of the strains. It has been speculated that this phenotype might be associated with horizontal gene transfer of a putative genomic island harboring the ESX-1-associated espACD locus (60). M. tuberculosis and M. marinum mutants with deletion of ESX-1 are attenuated in their respective hosts (61–64), which is in line with the attenuation of “natural” ESX-1 deletion mutants, such as the Mycobacterium bovis BCG (bacillus Calmette-Guérin) vaccine, which has lost ESX-1 functions due to the deletion of the region of difference RD1 (65). The ESX-1 system was shown to be involved in bacterial phagosome-to-cytosol transition of M. tuberculosis and host cell death (66–68), an important cell biological process that has numerous consequences for the host cell, such as induction of the cGAS/STING/TBK1/IRF-3/type I interferon signalling axis and NLRP3 inflammasome activation (69–75). However, ESX-1 is not the only factor involved in the process; it has been shown that besides ESX-1, the mycobacterial virulence lipids phthiocerol dimycocerosates also contribute to phagosomal rupture (76–78). Moreover, recent studies have also demonstrated that the endosomal sorting complex required for the transport III (ESCRT-III) system promotes the repair of small perforations in the endolysosomal membrane (79). Intriguingly, certain ESX-3-secreted effectors can block ESCRT-dependent receptor trafficking to the lysosome (80). It was shown that effectors of ESX systems differentially respond to the ESCRT endomembrane damage response. In an ESX-1-dependent manner, the ESCRT machinery is recruited to phagosomes, while ESX-3 effectors (EsxG-EsxH) antagonize the host damage response by blocking the recruitment of HRS, ESCRT-III, and GAL3 (81) (Fig. 3).
FIGURE 3.
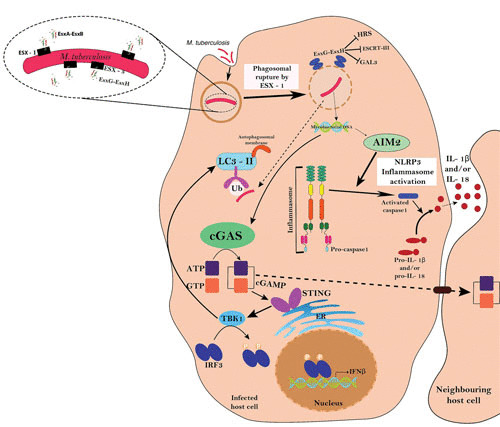
Interplay of ESX-1 and ESX-3 in host-pathogen interactions. ESX-1 is essential for the bacterial phagosome-to-cytosol transition by involving a cGAS/STING/TBK1/IRF-3/type I interferon signalling axis and AIM2 and NLRP3 inflammasome activities. In an ESX-1-dependent manner, the ESCRT machinery is recruited to phagosomes, while ESX-3 effectors (EsxG-EsxH) antagonize the host damage response by blocking the recruitment of HRS, ESCRT-III, and GAL3. The scheme is adapted from reference 11, with some additions from reference 81, with permission.
ESX-4 is one of the least well-characterized ESX systems, although it is considered the most ancestral esx locus in mycobacteria (21, 82). The ESX-4 loci usually lack pe/ppe and espG genes, which may be involved in host-pathogen interactions (34), as well as the eccE gene. However, the ESX-4 locus of M. abscessus is different from that of other species, as it does contain EccE4 (21). In a recent study, by using an M. abscessus genome-scale Himar mariner transposon library, it was shown that an intact ESX-4 system is needed for full virulence in this fast-growing mycobacterium and emerging human pathogen, whereby the ESX-4 function in infection was associated with phagosomal rupture and transition of bacteria to the cytosol of amoebae and human macrophages (83). As M. abscessus does not possess an ESX-1 system, in this particular case, ESX-4 might be considered a surrogate of ESX-1.
Because of extensive sequence similarities and immune cross-reactions among Esx and PE/PPE proteins secreted by the ESX/T7S systems, investigation of the secretion and regulation of these effectors is challenging. Recently, a technology termed multiplexed analysis of substrate secretion by transduced T cell hybridomas (MASSTT) was developed to explore the intra-host cell secretion profiles of various mycobacterial strains via fluorescence-mediated detection of specific M. tuberculosis major histocompatibility complex class II (MHC-II) epitopes by highly discriminative T cell hybridomas (84). This method thus allows investigators to follow the intracellular secretion profiles of selected mycobacterial ESX proteins, such as EsxA or EspC, as well as other secreted proteins, such as the members of the Ag85 complex. The secretion of the latter proteins (e.g., Ag85B) is regulated by the PhoP/PhoR two-component regulatory system and the small RNA Mcr7 (85). Interestingly, strains of different phylogenetic lineages showed distinct secretion levels of Ag85B proteins in a preliminary set of M. tuberculosis strains by the MASSTT assay (84), information that needs to be confirmed with a larger strain collection.
It is clear from the few examples mentioned here that ESX systems have a strong impact on mycobacterial host-pathogen interaction, although more work is needed to elucidate the various molecular mechanisms by which the effects are generated. New insights into these phenomena are also of interest for translational implications, as is shown by the example of attenuated whole-cell vaccines against tuberculosis. Loss of ESX-1 is one of the main reasons for the attenuation of the BCG vaccine (65), while ESX-1 effectors are important antigens in immune responses (6). Due to the absence of ESX-1, BCG does not gain access to the host cell cytosol and thus lacks the induction of certain immune signaling pathways (74, 86). Several recombinant BCG vaccine candidates have been constructed to overcome these limitations of BCG (75, 87–90). Alternatively, rationally attenuated M. tuberculosis vaccine candidates may also secrete particular ESX antigens that are absent from BCG (36, 91–93) and thereby may induce improved protection.
CONCLUDING COMMENTS AND PERSPECTIVES
In summary, we have presented here a few examples showing that mycobacterial ESX/T7S systems represent dynamic molecular machines which play important roles in various aspects of the biology of mycobacteria and the interaction with their hosts. Advances in structural biology together with the use of new approaches (e.g., MASSTT) will be very helpful for better understanding the functional details that are linked to their biological activities and for exploiting this knowledge for improved intervention strategies against tuberculosis.
ACKNOWLEDGMENTS
We are grateful to our many colleagues who were involved in the original work of the subjects reviewed in this article.
Research in the authors’ laboratories is in part supported by the European Union’s Horizon 2020 Research and Innovation Program (grant 643381 TBVAC2020), the Agence National de Recherche (ANR-10-LABX-62-IBEID, ANR-16-CE15-0003, and ANR-16-CE35-0009), the Fondation pour la Recherche Médicale (DEQ20130326471), and the Institut Pasteur. The research internship of F.V. at the Institut Pasteur in Paris was enabled and supported by the Pasteur International Network Programme and Campus France (grant 936638E).
Contributor Information
Farzam Vaziri, Institut Pasteur, Unit for Integrated Mycobacterial Pathogenomics, UMR3525 CNRS, 75015 Paris, France; Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, 13164 Tehran, Iran; Microbiology Research Center, Pasteur Institute of Iran, 13164 Tehran, Iran.
Roland Brosch, Institut Pasteur, Unit for Integrated Mycobacterial Pathogenomics, UMR3525 CNRS, 75015 Paris, France.
Maria Sandkvist, Department of Microbiology and Immunology, University of Michigan, Ann Arbor, Michigan.
Eric Cascales, CNRS Aix-Marseille Université, Mediterranean Institute of Microbiology, Marseille, France.
Peter J. Christie, Department of Microbiology and Molecular Genetics, McGovern Medical School, Houston, Texas
REFERENCES
- 1.Hug LA, Baker BJ, Anantharaman K, Brown CT, Probst AJ, Castelle CJ, Butterfield CN, Hernsdorf AW, Amano Y, Ise K, Suzuki Y, Dudek N, Relman DA, Finstad KM, Amundson R, Thomas BC, Banfield JF. 2016. A new view of the tree of life. Nat Microbiol 1:16048. 10.1038/nmicrobiol.2016.48. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Chagnot C, Zorgani MA, Astruc T, Desvaux M. 2013. Proteinaceous determinants of surface colonization in bacteria: bacterial adhesion and biofilm formation from a protein secretion perspective. Front Microbiol 4:303. 10.3389/fmicb.2013.00303. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerlach RG, Hensel M. 2007. Protein secretion systems and adhesins: the molecular armory of Gram-negative pathogens. Int J Med Microbiol 297:401–415. 10.1016/j.ijmm.2007.03.017. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Green ER, Mecsas J. 2016. Bacterial secretion systems: an overview. Microbiol Spectr 4:VMBF-0012-2015. 10.1128/microbiolspec.VMBF-0012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veith PD, Glew MD, Gorasia DG, Reynolds EC. 2017. Type IX secretion: the generation of bacterial cell surface coatings involved in virulence, gliding motility and the degradation of complex biopolymers. Mol Microbiol 106:35–53. 10.1111/mmi.13752. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Sørensen AL, Nagai S, Houen G, Andersen P, Andersen AB. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun 63:1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R. 2004. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol 12:500–508. 10.1016/j.tim.2004.09.007. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. 2007. Type VII secretion—mycobacteria show the way. Nat Rev Microbiol 5:883–891. 10.1038/nrmicro1773. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Houben EN, Bestebroer J, Ummels R, Wilson L, Piersma SR, Jiménez CR, Ottenhoff TH, Luirink J, Bitter W. 2012. Composition of the type VII secretion system membrane complex. Mol Microbiol 86:472–484. 10.1111/j.1365-2958.2012.08206.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Beckham KS, Ciccarelli L, Bunduc CM, Mertens HD, Ummels R, Lugmayr W, Mayr J, Rettel M, Savitski MM, Svergun DI, Bitter W, Wilmanns M, Marlovits TC, Parret AH, Houben EN. 2017. Structure of the mycobacterial ESX-5 type VII secretion system membrane complex by single-particle analysis. Nat Microbiol 2:17047. 10.1038/nmicrobiol.2017.47. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Gröschel MI, Sayes F, Simeone R, Majlessi L, Brosch R. 2016. ESX secretion systems: mycobacterial evolution to counter host immunity. Nat Rev Microbiol 14:677–691. 10.1038/nrmicro.2016.131. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Zuber B, Chami M, Houssin C, Dubochet J, Griffiths G, Daffé M. 2008. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J Bacteriol 190:5672–5680. 10.1128/JB.01919-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur D, Guerin ME, Skovierová H, Brennan PJ, Jackson M. 2009. Chapter 2: biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Adv Appl Microbiol 69:23–78. 10.1016/S0065-2164(09)69002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daffé M. 2015. The cell envelope of tubercle bacilli. Tuberculosis (Edinb) 95(Suppl 1):S155–S158. 10.1016/j.tube.2015.02.024. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Touchette MH, Seeliger JC. 2017. Transport of outer membrane lipids in mycobacteria. Biochim Biophys Acta Mol Cell Biol Lipids 1862:1340–1354. 10.1016/j.bbalip.2017.01.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Bitter W, Houben EN, Bottai D, Brodin P, Brown EJ, Cox JS, Derbyshire K, Fortune SM, Gao LY, Liu J, Gey van Pittius NC, Pym AS, Rubin EJ, Sherman DR, Cole ST, Brosch R. 2009. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog 5:e1000507. 10.1371/journal.ppat.1000507. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. 10.1038/31159. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Tekaia F, Gordon SV, Garnier T, Brosch R, Barrell BG, Cole ST. 1999. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber Lung Dis 79:329–342. 10.1054/tuld.1999.0220. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Unnikrishnan M, Constantinidou C, Palmer T, Pallen MJ. 2017. The enigmatic Esx proteins: looking beyond mycobacteria. Trends Microbiol 25:192–204. 10.1016/j.tim.2016.11.004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Pallen MJ. 2002. The ESAT-6/WXG100 superfamily—and a new Gram-positive secretion system? Trends Microbiol 10:209–212. 10.1016/S0966-842X(02)02345-4. [DOI] [PubMed] [Google Scholar]
- 21.Dumas E, Christina Boritsch E, Vandenbogaert M, Rodríguez de la Vega RC, Thiberge JM, Caro V, Gaillard JL, Heym B, Girard-Misguich F, Brosch R, Sapriel G. 2016. Mycobacterial pan-genome analysis suggests important role of plasmids in the radiation of type VII secretion systems. Genome Biol Evol 8:387–402. 10.1093/gbe/evw001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton-Foot M, Warren RM, Sampson SL, van Helden PD, Gey van Pittius NC. 2016. The plasmid-mediated evolution of the mycobacterial ESX (type VII) secretion systems. BMC Evol Biol 16:62. 10.1186/s12862-016-0631-2. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ummels R, Abdallah AM, Kuiper V, Aâjoud A, Sparrius M, Naeem R, Spaink HP, van Soolingen D, Pain A, Bitter W. 2014. Identification of a novel conjugative plasmid in mycobacteria that requires both type IV and type VII secretion. mBio 5:e01744-14. 10.1128/mBio.01744-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoop EJ, Bitter W, van der Sar AM. 2012. Tubercle bacilli rely on a type VII army for pathogenicity. Trends Microbiol 20:477–484. 10.1016/j.tim.2012.07.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.Queval CJ, Brosch R, Simeone R. 2017. The macrophage: a disputed fortress in the battle against Mycobacterium tuberculosis. Front Microbiol 8:2284. 10.3389/fmicb.2017.02284. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majlessi L, Prados-Rosales R, Casadevall A, Brosch R. 2015. Release of mycobacterial antigens. Immunol Rev 264:25–45. 10.1111/imr.12251. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Ates LS, Houben EN, Bitter W. 2016. Type VII secretion: a highly versatile secretion system. Microbiol Spectr 4:VMBF-0011-2015. 10.1128/microbiolspec.VMBF-0011-2015. [DOI] [PubMed] [Google Scholar]
- 28.Madacki J, Mas Fiol G, Brosch R. 2019. Update on the virulence factors of the obligate pathogen Mycobacterium tuberculosis and related tuberculosis-causing mycobacteria. Infect Genet Evol 72:67–77. 10.1016/j.meegid.2018.12.013. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Serafini A, Boldrin F, Palù G, Manganelli R. 2009. Characterization of a Mycobacterium tuberculosis ESX-3 conditional mutant: essentiality and rescue by iron and zinc. J Bacteriol 191:6340–6344. 10.1128/JB.00756-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegrist MS, Steigedal M, Ahmad R, Mehra A, Dragset MS, Schuster BM, Philips JA, Carr SA, Rubin EJ. 2014. Mycobacterial Esx-3 requires multiple components for iron acquisition. mBio 5:e01073-14. 10.1128/mBio.01073-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tufariello JM, Chapman JR, Kerantzas CA, Wong KW, Vilchèze C, Jones CM, Cole LE, Tinaztepe E, Thompson V, Fenyö D, Niederweis M, Ueberheide B, Philips JA, Jacobs WR, Jr. 2016. Separable roles for Mycobacterium tuberculosis ESX-3 effectors in iron acquisition and virulence. Proc Natl Acad Sci U S A 113:E348–E357. 10.1073/pnas.1523321113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bottai D, Di Luca M, Majlessi L, Frigui W, Simeone R, Sayes F, Bitter W, Brennan MJ, Leclerc C, Batoni G, Campa M, Brosch R, Esin S. 2012. Disruption of the ESX-5 system of Mycobacterium tuberculosis causes loss of PPE protein secretion, reduction of cell wall integrity and strong attenuation. Mol Microbiol 83:1195–1209. 10.1111/j.1365-2958.2012.08001.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 33.Ates LS, Ummels R, Commandeur S, van de Weerd R, Sparrius M, Weerdenburg E, Alber M, Kalscheuer R, Piersma SR, Abdallah AM, Abd El Ghany M, Abdel-Haleem AM, Pain A, Jiménez CR, Bitter W, Houben EN. 2015. Essential role of the ESX-5 secretion system in outer membrane permeability of pathogenic mycobacteria. PLoS Genet 11:e1005190. 10.1371/journal.pgen.1005190. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gey van Pittius NC, Sampson SL, Lee H, Kim Y, van Helden PD, Warren RM. 2006. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol Biol 6:95. 10.1186/1471-2148-6-95. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bottai D, Brosch R. 2009. Mycobacterial PE, PPE and ESX clusters: novel insights into the secretion of these most unusual protein families. Mol Microbiol 73:325–328. 10.1111/j.1365-2958.2009.06784.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Sayes F, Sun L, Di Luca M, Simeone R, Degaiffier N, Fiette L, Esin S, Brosch R, Bottai D, Leclerc C, Majlessi L. 2012. Strong immunogenicity and cross-reactivity of Mycobacterium tuberculosis ESX-5 type VII secretion: encoded PE-PPE proteins predicts vaccine potential. Cell Host Microbe 11:352–363. 10.1016/j.chom.2012.03.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 37.Fishbein S, van Wyk N, Warren RM, Sampson SL. 2015. Phylogeny to function: PE/PPE protein evolution and impact on Mycobacterium tuberculosis pathogenicity. Mol Microbiol 96:901–916. 10.1111/mmi.12981. [PubMed] [DOI] [PubMed] [Google Scholar]
- 38.Ates LS, Dippenaar A, Ummels R, Piersma SR, van der Woude AD, van der Kuij K, Le Chevalier F, Mata-Espinosa D, Barrios-Payán J, Marquina-Castillo B, Guapillo C, Jiménez CR, Pain A, Houben ENG, Warren RM, Brosch R, Hernández-Pando R, Bitter W. 2018. Mutations in ppe38 block PE_PGRS secretion and increase virulence of Mycobacterium tuberculosis. Nat Microbiol 3:181–188. 10.1038/s41564-017-0090-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- 39.Ates LS, Dippenaar A, Sayes F, Pawlik A, Bouchier C, Ma L, Warren RM, Sougakoff W, Majlessi L, van Heijst JWJ, Brossier F, Brosch R. 2018. Unexpected genomic and phenotypic diversity of Mycobacterium africanum lineage 5 affects drug resistance, protein secretion, and immunogenicity. Genome Biol Evol 10:1858–1874. 10.1093/gbe/evy145. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg OS, Dovala D, Li X, Connolly L, Bendebury A, Finer-Moore J, Holton J, Cheng Y, Stroud RM, Cox JS. 2015. Substrates control multimerization and activation of the multi-domain ATPase motor of type VII secretion. Cell 161:501–512. 10.1016/j.cell.2015.03.040. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Winden VJC, Damen MPM, Ummels R, Bitter W, Houben ENG. 2019. Protease domain and transmembrane domain of the type VII secretion mycosin protease determine system-specific functioning in mycobacteria. J Biol Chem 294:4806–4814. 10.1074/jbc.RA118.007090. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosserman RE, Champion PA. 2017. Esx systems and the mycobacterial cell envelope: what’s the connection? J Bacteriol 199:e00131-17. 10.1128/JB.00131-17. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Winden VJ, Ummels R, Piersma SR, Jiménez CR, Korotkov KV, Bitter W, Houben EN. 2016. Mycosins are required for the stabilization of the ESX-1 and ESX-5 type VII secretion membrane complexes. mBio 7:01471-16. 10.1128/mBio.01471-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fortune SM, Jaeger A, Sarracino DA, Chase MR, Sassetti CM, Sherman DR, Bloom BR, Rubin EJ. 2005. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci U S A 102:10676–10681. 10.1073/pnas.0504922102. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacGurn JA, Raghavan S, Stanley SA, Cox JS. 2005. A non-RD1 gene cluster is required for Snm secretion in Mycobacterium tuberculosis. Mol Microbiol 57:1653–1663. 10.1111/j.1365-2958.2005.04800.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 46.Chen JM. 2016. Mycosins of the mycobacterial type VII ESX secretion system: the glue that holds the party together. mBio 7:02062-16. 10.1128/mBio.02062-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lou Y, Rybniker J, Sala C, Cole ST. 2017. EspC forms a filamentous structure in the cell envelope of Mycobacterium tuberculosis and impacts ESX-1 secretion. Mol Microbiol 103:26–38. 10.1111/mmi.13575. [PubMed] [DOI] [PubMed] [Google Scholar]
- 48.Phan TH, Houben ENG. 2018. Bacterial secretion chaperones: the mycobacterial type VII case. FEMS Microbiol Lett 365:fny197. 10.1093/femsle/fny197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phan TH, Ummels R, Bitter W, Houben EN. 2017. Identification of a substrate domain that determines system specificity in mycobacterial type VII secretion systems. Sci Rep 7:42704. 10.1038/srep42704. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sala C, Odermatt NT, Soler-Arnedo P, Gülen MF, von Schultz S, Benjak A, Cole ST. 2018. EspL is essential for virulence and stabilizes EspE, EspF and EspH levels in Mycobacterium tuberculosis. PLoS Pathog 14:e1007491. 10.1371/journal.ppat.1007491. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coros A, Callahan B, Battaglioli E, Derbyshire KM. 2008. The specialized secretory apparatus ESX-1 is essential for DNA transfer in Mycobacterium smegmatis. Mol Microbiol 69:794–808. 10.1111/j.1365-2958.2008.06299.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Derbyshire KM, Gray TA. 2014. Distributive conjugal transfer: new insights into horizontal gene transfer and genetic exchange in mycobacteria. Microbiol Spectr 2:MGM2-0022-2013. 10.1128/microbiolspec.MGM2-0022-2013. [DOI] [PubMed] [Google Scholar]
- 53.Gray TA, Clark RR, Boucher N, Lapierre P, Smith C, Derbyshire KM. 2016. Intercellular communication and conjugation are mediated by ESX secretion systems in mycobacteria. Science 354:347–350. 10.1126/science.aag0828. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark RR, Judd J, Lasek-Nesselquist E, Montgomery SA, Hoffmann JG, Derbyshire KM, Gray TA. 2018. Direct cell-cell contact activates SigM to express the ESX-4 secretion system in Mycobacterium smegmatis. Proc Natl Acad Sci U S A 115:E6595–E6603. 10.1073/pnas.1804227115. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boritsch EC, Khanna V, Pawlik A, Honoré N, Navas VH, Ma L, Bouchier C, Seemann T, Supply P, Stinear TP, Brosch R. 2016. Key experimental evidence of chromosomal DNA transfer among selected tuberculosis-causing mycobacteria. Proc Natl Acad Sci U S A 113:9876–9881. 10.1073/pnas.1604921113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Supply P, Marceau M, Mangenot S, Roche D, Rouanet C, Khanna V, Majlessi L, Criscuolo A, Tap J, Pawlik A, Fiette L, Orgeur M, Fabre M, Parmentier C, Frigui W, Simeone R, Boritsch EC, Debrie AS, Willery E, Walker D, Quail MA, Ma L, Bouchier C, Salvignol G, Sayes F, Cascioferro A, Seemann T, Barbe V, Locht C, Gutierrez MC, Leclerc C, Bentley SD, Stinear TP, Brisse S, Médigue C, Parkhill J, Cruveiller S, Brosch R. 2013. Genomic analysis of smooth tubercle bacilli provides insights into ancestry and pathoadaptation of Mycobacterium tuberculosis. Nat Genet 45:172–179. 10.1038/ng.2517. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boritsch EC, Frigui W, Cascioferro A, Malaga W, Etienne G, Laval F, Pawlik A, Le Chevalier F, Orgeur M, Ma L, Bouchier C, Stinear TP, Supply P, Majlessi L, Daffé M, Guilhot C, Brosch R. 2016. pks5-recombination-mediated surface remodelling in Mycobacterium tuberculosis emergence. Nat Microbiol 1:15019. 10.1038/nmicrobiol.2015.19. [PubMed] [DOI] [PubMed] [Google Scholar]
- 58.Godfroid M, Dagan T, Kupczok A. 2018. Recombination signal in Mycobacterium tuberculosis stems from reference-guided assemblies and alignment artefacts. Genome Biol Evol 10:1920–1926. 10.1093/gbe/evy143. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orgeur M, Brosch R. 2018. Evolution of virulence in the Mycobacterium tuberculosis complex. Curr Opin Microbiol 41:68–75. 10.1016/j.mib.2017.11.021. [PubMed] [DOI] [PubMed] [Google Scholar]
- 60.Ates LS, Brosch R. 2017. Discovery of the type VII ESX-1 secretion needle? Mol Microbiol 103:7–12. 10.1111/mmi.13579. [PubMed] [DOI] [PubMed] [Google Scholar]
- 61.Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. 2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J Infect Dis 187:117–123. 10.1086/345862. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR, Jr. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A 100:12420–12425. 10.1073/pnas.1635213100. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stanley SA, Raghavan S, Hwang WW, Cox JS. 2003. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci U S A 100:13001–13006. 10.1073/pnas.2235593100. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao LY, Guo S, McLaughlin B, Morisaki H, Engel JN, Brown EJ. 2004. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol 53:1677–1693. 10.1111/j.1365-2958.2004.04261.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 65.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 46:709–717. 10.1046/j.1365-2958.2002.03237.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 66.van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. 2007. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129:1287–1298. 10.1016/j.cell.2007.05.059. [PubMed] [DOI] [PubMed] [Google Scholar]
- 67.Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, Brosch R, Enninga J. 2012. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog 8:e1002507. 10.1371/journal.ppat.1002507. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aguilo JI, Alonso H, Uranga S, Marinova D, Arbués A, de Martino A, Anel A, Monzon M, Badiola J, Pardo J, Brosch R, Martin C. 2013. ESX-1-induced apoptosis is involved in cell-to-cell spread of Mycobacterium tuberculosis. Cell Microbiol 15:1994–2005. 10.1111/cmi.12169. [PubMed] [DOI] [PubMed] [Google Scholar]
- 69.Wong KW, Jacobs WR, Jr. 2011. Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell Microbiol 13:1371–1384. 10.1111/j.1462-5822.2011.01625.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wassermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, Schmid-Burgk JL, Schmidt T, Hornung V, Cole ST, Ablasser A. 2015. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe 17:799–810. 10.1016/j.chom.2015.05.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS. 2015. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 17:811–819. 10.1016/j.chom.2015.05.004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collins AC, Cai H, Li T, Franco LH, Li XD, Nair VR, Scharn CR, Stamm CE, Levine B, Chen ZJ, Shiloh MU. 2015. Cyclic GMP-AMP synthase is an innate immune DNA sensor for Mycobacterium tuberculosis. Cell Host Microbe 17:820–828. 10.1016/j.chom.2015.05.005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Majlessi L, Brosch R. 2015. Mycobacterium tuberculosis meets the cytosol: the role of cGAS in anti-mycobacterial immunity. Cell Host Microbe 17:733–735. 10.1016/j.chom.2015.05.017. [PubMed] [DOI] [PubMed] [Google Scholar]
- 74.Kupz A, Zedler U, Stäber M, Perdomo C, Dorhoi A, Brosch R, Kaufmann SH. 2016. ESAT-6-dependent cytosolic pattern recognition drives noncognate tuberculosis control in vivo. J Clin Invest 126:2109–2122. 10.1172/JCI84978. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gröschel MI, Sayes F, Shin SJ, Frigui W, Pawlik A, Orgeur M, Canetti R, Honoré N, Simeone R, van der Werf TS, Bitter W, Cho SN, Majlessi L, Brosch R. 2017. Recombinant BCG expressing ESX-1 of Mycobacterium marinum combines low virulence with cytosolic immune signaling and improved TB protection. Cell Rep 18:2752–2765. 10.1016/j.celrep.2017.02.057. [PubMed] [DOI] [PubMed] [Google Scholar]
- 76.Augenstreich J, Arbues A, Simeone R, Haanappel E, Wegener A, Sayes F, Le Chevalier F, Chalut C, Malaga W, Guilhot C, Brosch R, Astarie-Dequeker C. 2017. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol 19:e12726. 10.1111/cmi.12726. [PubMed] [DOI] [PubMed] [Google Scholar]
- 77.Quigley J, Hughitt VK, Velikovsky CA, Mariuzza RA, El-Sayed NM, Briken V. 2017. The cell wall lipid PDIM contributes to phagosomal escape and host cell exit of Mycobacterium tuberculosis. mBio 8:e00148-17. 10.1128/mBio.00148-17. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barczak AK, Avraham R, Singh S, Luo SS, Zhang WR, Bray MA, Hinman AE, Thompson M, Nietupski RM, Golas A, Montgomery P, Fitzgerald M, Smith RS, White DW, Tischler AD, Carpenter AE, Hung DT. 2017. Systematic, multiparametric analysis of Mycobacterium tuberculosis intracellular infection offers insight into coordinated virulence. PLoS Pathog 13:e1006363. 10.1371/journal.ppat.1006363. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skowyra ML, Schlesinger PH, Naismith TV, Hanson PI. 2018. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 360:eaar5-78. 10.1126/science.aar5078. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehra A, Zahra A, Thompson V, Sirisaengtaksin N, Wells A, Porto M, Köster S, Penberthy K, Kubota Y, Dricot A, Rogan D, Vidal M, Hill DE, Bean AJ, Philips JA. 2013. Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog 9:e1003734. 10.1371/journal.ppat.1003734. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mittal E, Skowyra ML, Uwase G, Tinaztepe E, Mehra A, Köster S, Hanson PI, Philips JA. 2018. Mycobacterium tuberculosis type VII secretion system effectors differentially impact the ESCRT endomembrane damage response. mBio 9:01765-18. 10.1128/mBio.01765-18. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Houben EN, Korotkov KV, Bitter W. 2014. Take five—type VII secretion systems of mycobacteria. Biochim Biophys Acta 1843:1707–1716. 10.1016/j.bbamcr.2013.11.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 83.Laencina L, Dubois V, Le Moigne V, Viljoen A, Majlessi L, Pritchard J, Bernut A, Piel L, Roux AL, Gaillard JL, Lombard B, Loew D, Rubin EJ, Brosch R, Kremer L, Herrmann JL, Girard-Misguich F. 2018. Identification of genes required for Mycobacterium abscessus growth in vivo with a prominent role of the ESX-4 locus. Proc Natl Acad Sci U S A 115:E1002–E1011. 10.1073/pnas.1713195115. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sayes F, Blanc C, Ates LS, Deboosere N, Orgeur M, Le Chevalier F, Gröschel MI, Frigui W, Song OR, Lo-Man R, Brossier F, Sougakoff W, Bottai D, Brodin P, Charneau P, Brosch R, Majlessi L. 2018. Multiplexed quantitation of intraphagocyte Mycobacterium tuberculosis secreted protein effectors. Cell Rep 23:1072–1084. 10.1016/j.celrep.2018.03.125. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Solans L, Gonzalo-Asensio J, Sala C, Benjak A, Uplekar S, Rougemont J, Guilhot C, Malaga W, Martín C, Cole ST. 2014. The PhoP-dependent ncRNA Mcr7 modulates the TAT secretion system in Mycobacterium tuberculosis. PLoS Pathog 10:e1004183. 10.1371/journal.ppat.1004183. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simeone R, Sayes F, Song O, Gröschel MI, Brodin P, Brosch R, Majlessi L. 2015. Cytosolic access of Mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog 11:e1004650. 10.1371/journal.ppat.1004650. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 9:533–539. 10.1038/nm859. [PubMed] [DOI] [PubMed] [Google Scholar]
- 88.Bottai D, Frigui W, Clark S, Rayner E, Zelmer A, Andreu N, de Jonge MI, Bancroft GJ, Williams A, Brodin P, Brosch R. 2015. Increased protective efficacy of recombinant BCG strains expressing virulence-neutral proteins of the ESX-1 secretion system. Vaccine 33:2710–2718. 10.1016/j.vaccine.2015.03.083. [PubMed] [DOI] [PubMed] [Google Scholar]
- 89.Gengenbacher M, Nieuwenhuizen N, Vogelzang A, Liu H, Kaiser P, Schuerer S, Lazar D, Wagner I, Mollenkopf HJ, Kaufmann SH. 2016. Deletion of nuoG from the vaccine candidate Mycobacterium bovis BCG ΔureC::hly improves protection against tuberculosis. mBio 7:e00679-16. 10.1128/mBio.00679-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ates LS, Sayes F, Frigui W, Ummels R, Damen MPM, Bottai D, Behr MA, van Heijst JWJ, Bitter W, Majlessi L, Brosch R. 2018. RD5-mediated lack of PE_PGRS and PPE-MPTR export in BCG vaccine strains results in strong reduction of antigenic repertoire but little impact on protection. PLoS Pathog 14:e1007139. 10.1371/journal.ppat.1007139. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aguilo N, Gonzalo-Asensio J, Alvarez-Arguedas S, Marinova D, Gomez AB, Uranga S, Spallek R, Singh M, Audran R, Spertini F, Martin C. 2017. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nat Commun 8:16085. 10.1038/ncomms16085. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sayes F, Pawlik A, Frigui W, Gröschel MI, Crommelynck S, Fayolle C, Cia F, Bancroft GJ, Bottai D, Leclerc C, Brosch R, Majlessi L. 2016. CD4+ T cells recognizing PE/PPE antigens directly or via cross reactivity are protective against pulmonary Mycobacterium tuberculosis infection. PLoS Pathog 12:e1005770. 10.1371/journal.ppat.1005770. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marinova D, Gonzalo-Asensio J, Aguilo N, Martin C. 2017. MTBVAC from discovery to clinical trials in tuberculosis-endemic countries. Expert Rev Vaccines 16:565–576. 10.1080/14760584.2017.1324303. [PubMed] [DOI] [PubMed] [Google Scholar]


