Abstract
Objective
To assess expression pattern and subcellular compartmentalization of 5-lipoxygenase in cutaneous, UV radiation–induced, and oral squamous cell carcinomas (SCCs) in cats and determine the effects of cyclooxygenase or 5-lipoxygenase inhibition on proliferation or apoptosis in a feline oral squamous cell carcinoma (SCCF1) cell line.
Sample
60 archived paraffin-embedded samples of SCCs from 60 cats and SCCF1 cells.
Procedures
Retrospective immunohistochemical analysis of the archived samples of SCCs (20 cutaneous, 20 UV radiation–induced, and 20 oral tumors) was performed. Cell culture proliferation assays involving SCCF1 cells were performed, and tepoxalin-induced apoptosis and signaling were examined via western blotting and annexin V staining.
Results
Immunohistochemically, staining for 5-lipoxygenase was most frequently of greatest intensity in oral SCCs, whereas staining of cutaneous and UV radiation–induced lesions had less consistent 5-lipoxygenase expression. Exposure of SCCF1 cells to the 5-lipoxygenase inhibitor tepoxalin resulted in apoptosis; the effect appeared to be mediated via alteration of cell signaling rather than via suppression of lipid mediators that are typically produced as a result of 5-lipoxygenase activity.
Conclusions and Clinical Relevance
In cats, expression of 5-lipoxygenase in SCCs appeared to differ depending on tumor location. The influence of tepoxalin-induced 5-lipoxygenase inhibition on a 5-lipoxygenase–expressing cell line coupled with the notable expression of 5-lipoxygenase in oral SCCs suggested that 5-lipoxygenase inhibition may have therapeutic benefits in affected cats. Although the safety of tepoxalin in cats has yet to be investigated, 5-lipoxygenase inhibitors should be evaluated for use as a potential treatment for SCCs in that species.
Squamous cell carcinoma is the most common epithelial neoplasm of the skin and oral cavity of cats. Treatment depends on the anatomic location and the inciting cause. Squamous cell carcinomas in cats can be categorized into 3 forms: oral, UV radiation–induced, and spontaneous cutaneous neoplasms, although in some cases, cutaneous neoplasm can be virally induced. Of the 3 forms, oral SCC has the worst prognosis for multiple reasons, including inadequate margins available for surgical resection in many patients as well as a potentially higher incidence of regional metastasis and extensive local invasion.1,2 Cutaneous tumors are typically thought to be locally invasive but late to metastasize.1 Squamous cell carcinoma in the digits may be more aggressive than those in other dermal locations. Of the SCCs in dermal locations, UV radiation–induced SCC is the most common, observed primarily in nonpigmented and sparsely haired pinnae, eyelid margins, and nasal plana of cats and which often responds well to surgical intervention.1 Little research has been performed to evaluate outcome and metastatic rates of these various neoplasms on the basis of anatomic location in cats, to our knowledge; however, in human medicine, anatomic location of oral SCC is an indicator for specific treatment protocols and prognosis.3
Several retrospective studies4-8 have been conducted to assess COX-2 expression in oral SCCs in humans, dogs, and cats. Results of a study4 in humans suggest that lipoxygenase expression (20/20 individuals) and often COX-2 (35/45 individuals) expression are up-regulated in oral SCCs. Immunohistochemical analysis of oral SCCs in cats has revealed immunoreactivity to COX-2 in approximately 28 of 34 (82%) oral tumors7; in another study,8 37 of 55 (67%) oral tumors reacted positively for COX-2. These findings in cats correlate well with results of human and dog studies.4-6 However, immunohistochemical staining for COX-2 may not be a reflection of enzymatic activity, and functional assays are required to determine its potential use as a pharmacological target.9
During chemically induced carcinogenesis, there is consistent upregulation of COX-2 and 5-lipoxygenase in preneoplastic lesions in the hamster pouch model of human oral SCC.10-12 In those experiments, PGE2 and LTB4 increased at the level of the epithelium and appeared to be part of the induction of uncontrolled proliferation.10,12 The use of NSAIDs that inhibit COX and lipoxygenase in animals with experimentally induced preneoplastic lesions can reduce tumor size, reduce or eliminate carcinoma formation, and reduce multiplicity11-14
Studies15,16 examining the use of COX-2–selective inhibitors in humans with oral SCCs or tobacco smoke–induced tissue injury have revealed that, in saliva and urine, PGE2 concentrations decrease and LTB4 concentrations increase with treatment, suggesting a shunting of arachidonic acid to 5-lipoxygenase upon COX inhibition. Hence, dual COX and 5-lipoxygenase inhibition in tumors may be more efficacious in slowing neoplastic growth than COX inhibition alone17 by decreasing the 5-lipoxygenase lipid mediators such as LTB4 and 5-oxo-ETE. These eicosanoids are promitogenic and are also potential therapeutic targets.18-20
Unfortunately, NSAIDs are generally not tolerated well by cats. Certain COX inhibitors, primarily piroxicam, have been used in cats and appear to be tolerated, but other selective COX-2 inhibitors have low margins of safety.21 5-Lipoxygenase expression in oral SCCs in cats has not been studied. Therefore, the purpose of the study reported here was to assess expression pattern and subcellular compartmentalization of 5-lipoxygenase in cutaneous, UV radiation–induced, and oral SCCs in cats and determine the effects of 5-lipoxygenase or COX inhibition on proliferation or apoptosis in the SCCF1 cell line. Additionally, the mechanism of cell death induced by the 5-lipoxygenase inhibitor tepoxalin was compared with the mechanisms of action of other 5-lipoxygenase inhibitors.
Materials and Methods
Histologic examinations
Sixty cases of SCC in cats were identified from the Cornell University College of Veterinary Medicine anatomic pathology archives for the years 1998 through 2009. Each tumor was derived from a different cat. Twenty tumors of each category of SCC (cutaneous, UV radiation–induced, and oral) were chosen for sectioning. Oral SCCs were defined as any tumor originating from the gingival, tongue, or oral mucosa. Cutaneous tumors were defined as those that originated from any area on the haired portion of the cat, except for the ear margins, and for which any evidence of virally induced squamous cell disease was excluded. The UV radiation–induced tumors were defined as tumors along the ear margins or nasal planum of light colored or white cats. For each case, tumor type and anatomic location were confirmed by a veterinary pathologist (JP-K) after review of the patient’s clinical history, gross description of the tumor, and histologic examination of H&E-stained tissue sections. Ultraviolet radiation–induced SCCs were from pinnae, eyelids, or nasal plana of white or lightly pigmented cats. All specimens were fixed in neutral-buffered 10% formalin and embedded in paraffin blocks. Paraffin-embedded tissues were sectioned at 4 μm and mounted onto slides for immunohistochemical analysis.
Immunohistochemical analysis
A standard immunohistochemical analysis protocol based on a previous study22 performed at our laboratory was used. Briefly, tissue sections were deparaffinized through a graded alcohol series. Endogenous peroxidases were quenched in 1% hydrogen peroxide in methanol for 10 minutes. Sections were rinsed in PBS solution and incubated for 30 minutes in goat serum (1:10 dilution) for 30 minutes. Primary anti–5-lipoxygenase rabbit polyclonal rabbit antibodya was used at a 1:100 dilution. Control slides were treated in parallel with an equal concentration of control rabbit antibody IgGb and treated identically to the immunohistochemical slides assessed for 5-lipoxygenase. Experimental and control slides were incubated for 3 hours at room temperature (approx 25°C) in a humid chamber. Sections were rinsed with PBS–0.1% detergent solution 3 times, incubated with a biotinylated goat anti-rabbit antibodyb at room temperature for 30 minutes, and then rinsed 3 times in PBS-detergent solution. Further incubation was performed in prediluted avidin horseradish peroxidase–labeled streptavidin conjugate solutionc for 30 minutes followed by 3 rinses in PBS–0.1% detergent solution. Each slide was exposed to diaminobenzidine tetrahydrochloride for approximately 60 to 90 seconds, washed in distilled water, counterstained with Gill’s hematoxylin stain, and covered with a coverslip. A veterinary pathologist (JP-K) then examined each experimental slide and the corresponding control slide and subjectively rated the staining intensity of each slide by use of a score. The staining intensity score was rated as follows: 1 = weak staining intensity, 2 = moderate staining intensity, and 3 = marked staining intensity. Any subcellular compartmental staining was categorized as cytoplasmic, perinuclear, or nuclear. Cytoplasmic, perinuclear, or nuclear staining of the sections were each further categorized as focal if < 10% of neoplastic cells were stained, patchy if 11% to 50% of cells were stained, and diffuse if > 50% of cells were stained. This system was adapted from previously published criteria established by Hayes et al.7
To examine whether staining intensity was associated with cell proliferation, the number of active mitoses were counted in 5 random hpfs (400X) for each tumor section; the mean of those 5 values was calculated and used as a mitotic index for each tumor.
Cell culture
Dulbecco modified Eagle mediumc with 10% heat-inactivated FBSc with 1% antimicrobial and antimycotic solutiond was used for cell proliferation assays. Growth curve assays involving SCCF1 cellse (originating from a feline oral SCC) were performed in 2% FBS supplemented with Dulbecco modified Eagle medium.23 Nonsteroidal anti-inflammatory drugs used were global COX inhibitors piroxicamd and RWJ 20142f (ie, TAM), the 5-lipoxygenase inhibitor tepoxalin,f and the dual COX and 5-lipoxygenase inhibitor licofelone.a All NSAIDs were reconstituted in DMSO to make 20mM stock solutions that were stored at −80°C until use in vitro.
MTT proliferation assays and cell counts in the presence of NSAIDs
Squamous cell carcinoma feline 1 cells were plated at a density of 5 X 103 cells/well in 96-well plates and incubated overnight (approx 18 hours). The following day the cells were treated in quadruplicate with each NSAID (piroxicam, TAM, tepoxalin, licofelone, and TAM-tepoxalin in combination) or vehicle control (DMSO) in a dilution series (0.4 to 50μM) for 48 hours. To assess cell viability, MTTd proliferation assays were performed spectophotometrically at a wavelength of 540 nm as previously described.24 In addition, to confirm MTT proliferation assay results, cells were plated in 24-well plates at a concentration of 160,000 cells/well and cell counts were assessed after similar treatments for 48 hours by use of manual hemocytometry after trypan blue staining. Furthermore, as a comparison, non–5-lipoxygenase–expressing A-72 canine thymic fibroblastsg were examined via MTT proliferation assay by use of the same treatment protocol and medium as used for the SCCF1 cells. Each experiment with each NSAID was performed in triplicate, and the mean optical density was calculated.
MTT proliferation assays and cell counts in the presence of tepoxalin
Squamous cell carcinoma feline 1 cells were plated at a density of 1 X 103 cells/well in 96-well tissue culture–treated plates in Dulbecco modified Eagle medium with 2% FBS and 1% antimicrobial and antimycotic solution and incubated overnight. Then, various concentrations of tepoxalin from 0.31 to 50μM were used to treat wells in quadruplicate (day 0). 4,5-Dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide assays were performed every 2 days for 6 days with medium and drug changes performed every other day until completion of the experiment. Each experiment was performed in triplicate, and the mean optical density was calculated at each time point.
LTB4 ELISA and LTB4 and 5-oxo-ETE MTT assays
Squamous cell carcinoma feline 1 cells were plated at a density of 5 X 103 cells/well in 96-well plates and allowed to incubate in 2% FBS-supplemented medium overnight. The following day, cells were treated in quadruplicate with tepoxalin or vehicle control (DMSO) in a dilution series (0.4 to 50μM) for 48 hours. Immediately after that addition, tepoxalin, LTB4, 5-oxo ETE,a or vehicle control (ethanol) was added to wells to a final concentration of 500 or 100nM. The following day, medium was removed and the treatment was repeated (treatment duration, 48 hours). 4,5-Dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide assays were performed as described, and each experiment was performed in triplicate, and the mean optical density was calculated. Additionally, samples of conditioned medium in which SCCF1 cells were grown for 24 and 48 hours with and without 5μM tepoxalin treatment were assayed for LTB4 concentrations by use of a commercial LTB4 ELISAa according to the manufacturer’s instructions.
Cell lysis and western blotting
Squamous cell carcinoma feline 1 cells were grown and treated for variable amounts of time with vehicle DMSO or 10μM tepoxalin. Samples of cells were lysed and assessed before treatment (0 hours) and at 12, 24, 36, and 48 hours. Cells were lysed according to a protocol described by Antonyak et al.25 Cell lysates were collected, and protein determination by use of the Bradford technique was performed on each sample. Lysates were equilibrated to a common volume (in μg/μL) in lysis buffer and loading buffer. Western blot analysis was performed by use of 8%, 10%, or 15% SDS-PAGE and loaded with 30 μg of protein/well, followed by transfer to a polyvinylidene difluoride membrane and then immunoblotting. Lysates were immunoblotted with anti-5-lipoxygenase antibodies,a anti-caspase-3 rabbit polyclonal antibodies,h anti-Bcl-2–associated X protein (ie, Bax) rabbit polyclonal antibodies,h anti-ser473pAkt rabbit polyclonal antibodies,h anti-Akt rabbit polyclonal antibodies,h and anti-β-actin mouse monoclonal antibody.d After membrane blocking with 10% dried nonfat milk, cells were incubated with a primary antibody overnight at 4°C. Membranes were washed twice with Tris-buffered saline solution and 0.1% Tween 20, then incubated at room temperature for 1 hour with a 1:5,000 dilution of sheep anti-rabbit horseradish peroxidase–linked and donkey anti-mouse horseradish peroxidase–labeled antibodies.i Blots were again washed 3 times with Tris-buffered saline solution and examined by use of an imaging station.j At the onset of this experiment, an initial western blot was also performed to examine 5-lipoxygenase expression, which was assessed in comparison with a commercially available human his-tagged 5-lipoxygenase.a
Annexin V apoptosis
Squamous cell carcinoma feline 1 cells were plated on glass cell culture 2-well chamber slidesk to achieve approximately 25% to 30% confluence and incubated and supplemented with 2% FBS. Cells were incubated overnight, then treated with tepoxalin (10μM) or DMSO as a vehicle control. Before (0 hours) and after a 24-, 36-, or 48-hour period of treatment, cells were washed once with ice-cold PBS solution, which was then replaced with 0.5 mL of annexin binding buffer (10mM HEPES, 140mM NaCl, and 2.5mM CaCl2; pH, 7.4) in each well. Twenty microliters of annexin V Alexa 488l was instilled in each well and incubated for 15 minutes at 37°C. Two microliters of 7-AADc was instilled in each well and incubated an additional 5 minutes on ice. Wells were washed once with ice-cold annexin binding buffer, then the well apparatus was removed. Each slide was air-dried and immediately covered with a coverslip with fluorescent mounting medium.m Coverslips were affixed with clear nail polish. Cells were imaged with a fluorescent microscopen and software.o Images were merged and optimized with management software.p
Statistical analysis
To assess 5-lipoxygenase–positive versus 5-lipoxygenase–negative tumors, a χ2 analysis of staining proportions was performed to determine significant differences in staining across the 3 categories of SCC. Staining intensity was also assessed by use of an ANOVA with a Tukey post hoc analysis to determine differences between tumor categories. Spearman nonparametric correlation was performed to examine the relationship between staining intensity and mitotic index. Cell cytotoxicosis was evaluated by use of an ANOVA with a Tukey post hoc comparison for each treatment, compared with vehicle control–treated cells. Day 6 growth curve data were assessed by use of an ANOVA with a Tukey post hoc comparison to evaluate differences between treatment and vehicle control groups for all concentrations of tepoxalin. For all lipid mediator plus tepoxalin inhibition dual treatments, mean optical density for each lipid mediator treatment concentration was compared with vehicle control (ethanol) data at each concentration by use of an ANOVA with a Tukey post hoc comparison. All annexin apoptosis data were evaluated by use of a 2-way ANOVA and Bonferroni post test to compare the relative percentage of cells undergoing apoptosis in DMSO control and tepoxalin-treatment conditions across time points. All statistical testing and graphing were performed by use of a curve-fitting and data analysis graphing toolq and a spreadsheet programr or 2-D graphing and statistics software.s For all comparisons, a value of P < 0.05 was considered significant.
Results
Immunohistochemical analysis
Of the cutaneous, UV radiation–induced, and oral SCCs analyzed, some or all tumors of each form were positive for 5-lipoxygenase immunohistochemically. All 20 oral SCCs, 17 of 20 UV radiation–induced SCCs, and 12 of 20 cutaneous SCCs were positive for 5-lipoxygenase. Many tumors had 5-lipoxygenase–specific staining within the cytoplasm with less perinuclear or nuclear staining in general (Figure 1). Examination of sections at high magnification revealed compartmental staining in the nucleus and perinuclear areas with homogeneous cytoplasmic staining. Treatment with the rabbit control antibody resulted in no staining in any tumor section. χ2 Analysis of the numbers of 5-lipoxygenase–positive and 5-lipoxygenase–negative tumors within classification groups revealed a significant (P < 0.05) difference in the proportion of immunoreactive oral SCCs and cutaneous SCCs only.
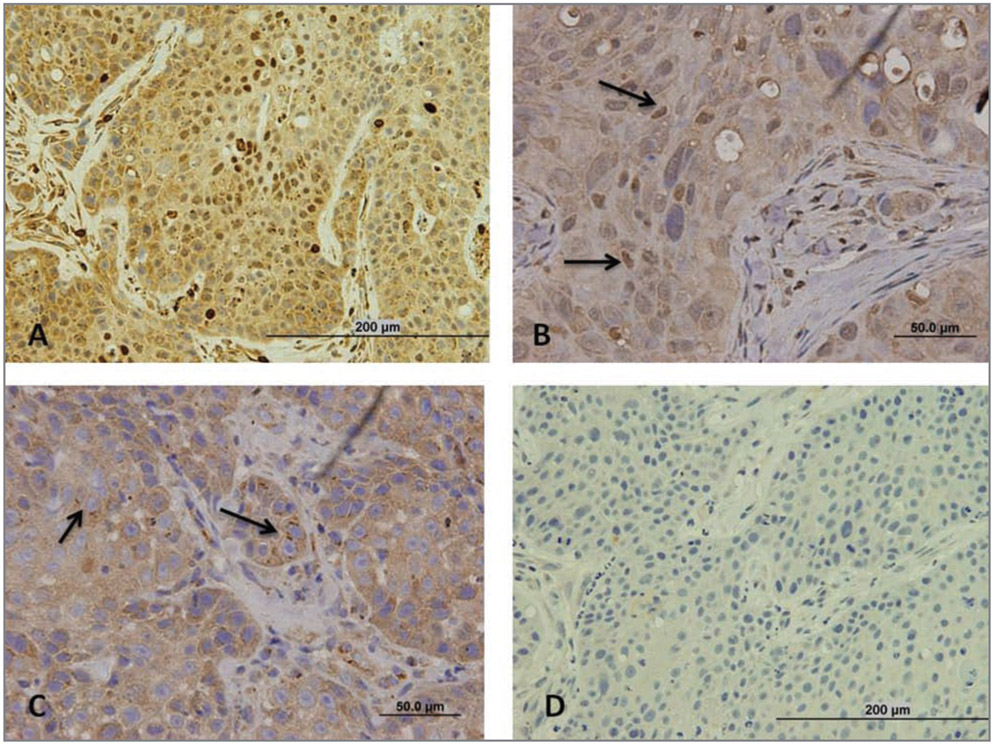
Figure 1—
Representative photomicrographs of sections of oral or UV radiation–induced SCCs in 3 cats to illustrate results of immunohistochemical staining for 5-lipoxygenase. Sections from each were processed with anti–5-lipoxygenase rabbit polyclonal rabbit antibody or a negative control rabbit antibody, and intensity and subcellular compartmentalization of 5-lipoxygenase–specific staining were assessed. Staining intensity was subjectively scored as follows: 1 = weak staining intensity, 2 = moderate staining intensity, and 3 = marked staining intensity. Subcellular compartmental staining was categorized as cytoplasmic, perinuclear, or nuclear, which were each further categorized as focal if < 10% of neoplastic cells were stained, patchy if 11% to 50% of cells were stained, and diffuse if > 50% of cells were stained. A—Section of an oral SCC that underwent immunohistochemical staining with anti–5-lipoxygenase antibody.The staining intensity score for this tumor is 3, and there is uniform cytoplasmic staining and nuclear staining. Bar = 200 μm. B—Section of another oral SCC that underwent immunohistochemical staining with anti–5-lipoxygenase antibody. Notice the intense cytoplasmic staining and patchy nuclear staining of variable intensity (arrows). Bar = 50 μm. C—Section of a UV radiation–induced SCC that underwent immunohistochemical staining with anti–5-lipoxygenase antibody. Notice the intense cytoplasmic 5-lipoxygenase–specific staining and patchy punctuate perinuclear staining (arrows). Bar = 50 μm. D—Section of the same oral SCC in panel A that underwent immunohistochemical staining with control rabbit antibody. Bar = 200 μm.
Most (18/20) of the oral SCCs had moderate to marked immunoreactivity, and most (9/12) of the 5-lipoxygenase–positive cutaneous SCCs had weak or equivocal immunoreactivity. None of the cutaneous SCCs had marked staining intensity. Of the 5-lipoxygenase–positive UV radiation-induced SCCs, most (12/17) had moderate or marked staining intensity. Based on staining intensity scores assigned by use of the 3-point scale, there was a significant difference (P < 0.05) in staining intensity among all 3 forms of SCC (Table 1). However, when mitotic index was quantified and correlated to intensity of staining in each classification group, no association could be made.
Table 1—
Mean ± SD mitotic index and intensity of 5-lipoxygenase–specific staining in sections of oral, UV radiation–induced, and cutaneous SCCs in cats (20 tumors of each neoplastic form from 60 cats [1 tumor/cat]).
| Variable | Oral | UV radiation–induced |
Cutaneous | ANOVA P value |
|---|---|---|---|---|
| Mitotic index | 1.2 ± 1.1 | 1.1 ± 1.0 | 1.9 ± 2.1 | 0.639 |
| Stain intensity | 2.3 ± 0.6 | 1.6 ± 0.9 | 0.8 ± 0.7 | < 0.001 |
| Correlation P value | 0.201 | 0.914 | 0.143 | — |
One section from each cat was evaluated and scored. Staining intensity was subjectively scored as follows: 1 = weak staining intensity, 2 = moderate staining intensity, and 3 = marked staining intensity. A value of P < 0.05 was considered significant.
— = Not applicable.
The subcellular compartmentalization of 5-lipoxygenase–specific staining in each of the 3 forms of SCC was evaluated and categorized as nuclear, perinuclear, or cytoplasmic. For each 5-lipoxygenase–positive tumor, staining was present in 1 or more of these compartments. Of the 12 5-lipoxygenase–positive cutaneous SCCs, 5 had nuclear, 1 had perinuclear, and 12 had cytoplasmic staining. Of the seventeen 5-lipoxygenase–positive UV radiation–induced SCCs, 6 had nuclear, 5 had perinuclear, and 16 had cytoplasmic staining. Of the oral SCCs, 6 had nuclear, 12 had perinuclear, and 19 had cytoplasmic staining. The distribution of nuclear, perinuclear, or cytoplasmic staining in sections was each examined and categorized on the basis of the percentage of cells affected (ie, focal, < 10% of cells; patchy, 11% to 50% of cells; and diffuse, > 50% of cells). With regard to cytoplasmic staining, all tumors that were 5-lipoxygenase positive had > 50% of cells affected. There was extreme variability in nuclear and perinuclear staining intensities within each tumor regardless of classification, and each was categorized as patchy (11% to 50% of cells affected) in all 5-lipoxygenase–positive tumors. Therefore, nuclear and perinuclear staining in tumor sections were each categorized as either present or absent and the tumors as 5-lipoxygenase positive or negative only.
NSAID MTT proliferation assays and western blot analysis for 5-lipoxygenase
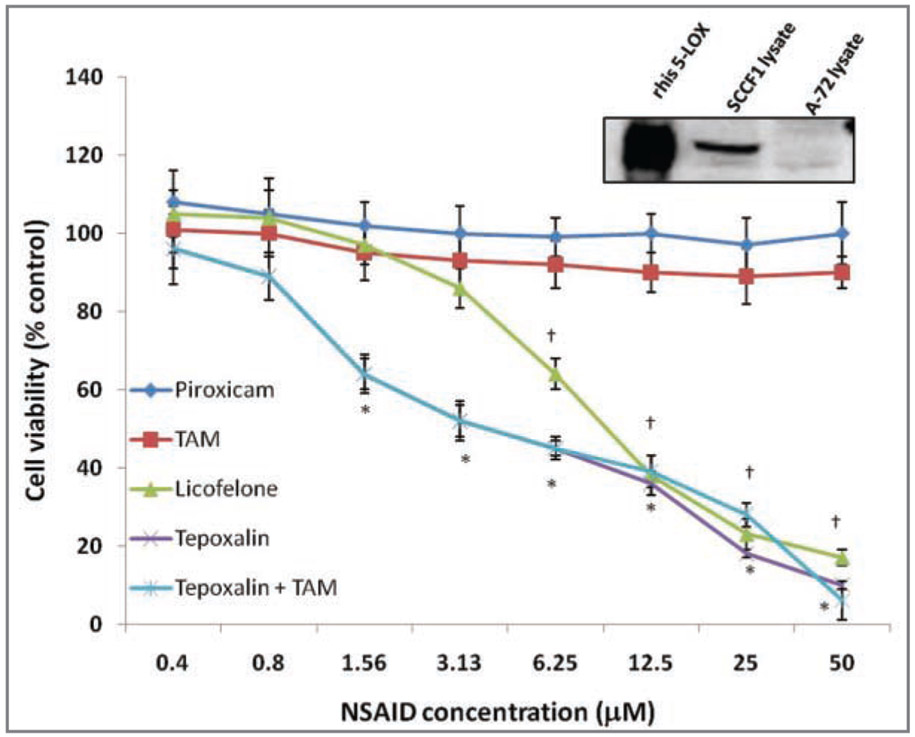
Squamous cell carcinoma feline 1 cell viability did not significantly decrease following treatment with piroxicam or TAM at any concentration from 0.39 to 50μM, compared with viability of vehicle control (DMSO)–treated cells (Figure 2). Treatment with 5-lipoxygenase inhibitors (tepoxalin and licofelone) significantly (P < 0.05) decreased cell viability; the lowest concentration at which this effect was apparent was 1.56μM for tepoxalin and 6.25μM for licofelone. Addition of tepoxalin at equal concentrations with TAM did not enhance the effects of tepoxalin alone. Basal expression of 5-lipoxygenase in the SCCF1 cells was evaluated in comparison with human 5-lipoxygenase control protein and expression of 5-lipoxygenase in A-72 thymic fibroblasts; a lack of 5-lipoxygenase expression in A-72 cells was confirmed. A-72 thymic fibroblasts were also examined in 48-hour MTT proliferation assays similar to the assays performed with SCCF1 cells. There was no significant decrease in A-72 cell proliferation following treatment with any of the NSAIDs except tepoxalin at a concentration of 50μM; with this treatment, percentage cell viability was 63.4 ± 10.8% (data not shown). Cell counts for the SCCF1 with serial dilutions of tepoxalin treatment revealed a decrease in cell viability and overall cell loss consistent with cell death, with a nearly identical pattern of change as that indicated by the MTT data (data not shown).
Figure 2—
Representative results of western blot analysis for 5-lipoxygenase in SCCF1 and A-72 thymic fibroblast lysates, compared with 2 μg of recombinant his-5-lipoxygenase (rhis–5-LOX) and mean ± SD viability of SCCF1 cells treated for 48 hours with various concentrations of piroxicam, TAM, licofelone, tepoxalin, and tepoxalin with TAM, or a vehicle control (DMSO). For the western blot analysis, 30 μg of each lysate was loaded into each lane. Cell viability was determined by use of MTT assays, and results are expressed as a percentage of vehicle control–treated cell viability. *At this concentration, percentage viability of tepoxalin-treated and tepoxalin-TAM–treated cells differed significantly (P < 0.05) from the findings for the vehicle control–treated cells. †At this concentration, percentage viability of licofelone-treated cells differed significantly (P < 0.05) from the findings for the vehicle control–treated cells.
Tepoxalin MTT growth curves
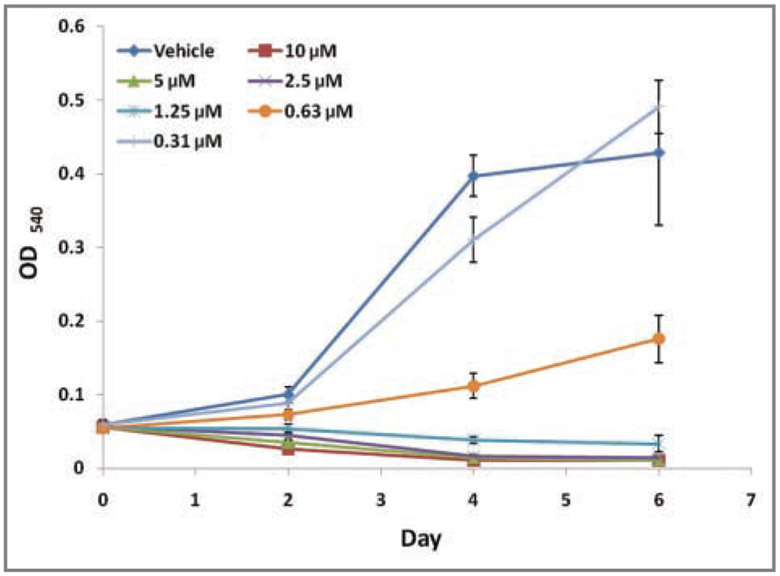
Growth curve data were generated on days 2, 4, and 6 with analysis of percentage inhibition in SCCF1 cell viability on day 6. There was marked growth retardation with various concentrations of tepoxalin and significant reductions in cell growth by day 6 at a concentration of 630nM and above (Figure 3). Actual reductions in cell viability, compared with baseline MTT readings on day 0 (P < 0.05), were detected at concentrations > 1.25μM, suggesting an overall loss of cell mass with treatment.
Figure 3—
Growth curves for SCCF1 cells in the presence of various concentrations of tepoxalin or a vehicle control (DMSO) by use of MTT assays. Optical density readings at a wavelength of 540 nm were obtained via spectophotometry at day 0 (prior to exposure to tepoxalin) and every other day thereafter during serial dilution treatment with tepoxalin or vehicle control. At day 6, all concentrations of tepoxalin (0.625 to 10μM) resulted in significant (P < 0.05) decreases in cell proliferation, compared with findings for vehicle control–treated cells.
LTB4 ELISA and LTB4 and 5-oxo-ETE MTT assays
Squamous cell carcinoma feline 1 cell viability was assessed after exposure to concentrations of tepoxalin that cause a decrease in cell viability (≥ 0.156μM) and followed by the addition of common promitogenic 5-lipoxygenase–dependent lipid mediators into the culture medium to determine whether the proliferative capacity could be recovered after tepoxalin-induced inhibition. Addition of 100 and 500nM of LTB4 did not cause a significant increase in cell growth, regardless of the concentration of tepoxalin used. 5-Oxo-eicosatetranone additions (100 and 500nM) also resulted in no recovery of SCCF1 cell growth under the influence of tepoxalin. Further analysis of LTB4 in samples of culture medium by use of the LTB4 ELISA kit revealed that LTB4 concentrations were less than the lower limit of detection (< 8 pg/mL), suggesting that SCFF1 cells do not secrete notable amounts of LTB4.
SCCF1 cell apoptosis, Akt western blot analysis, and annexin staining
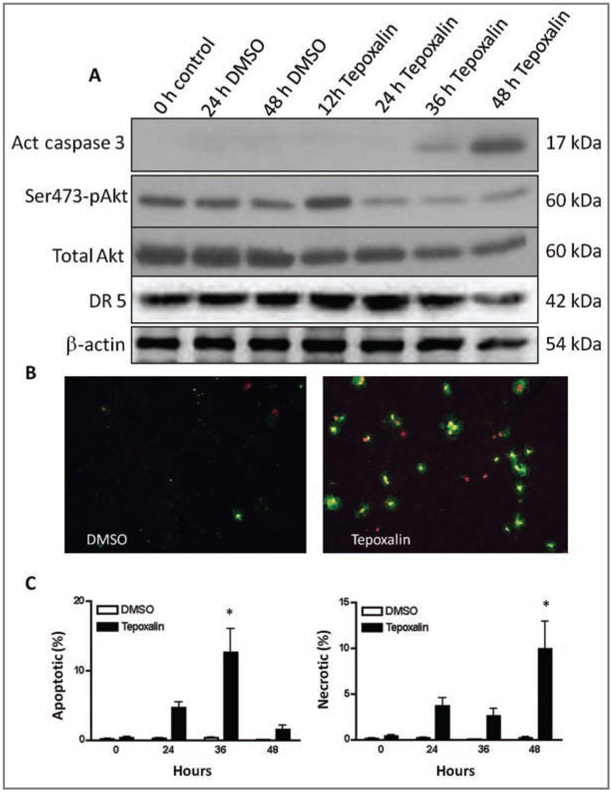
Results of western blot analysis for markers of apoptosis indicated that caspase-3, the terminal caspase, was activated after 48 hours of treatment of SCCF1 cells with 10μM tepoxalin in low serum conditions (Figure 4), suggesting either intrinsic or extrinsic activation of apoptosis. Via fluorescence microscopy, the presence or absence of annexin V and 7-AAD staining confirmed that there was a mild increase in apoptosis after a 24-hour period of treatment with tepoxalin, compared with DMSO-treated cells at the same time point, and that there was a prominent increase (P < 0.05) in annexin staining after 36 hours coinciding with the timing for caspase-3 activation. Although the percentage of apoptotic cells decreased by 48 hours, the percentage of necrotic (ie, annexin V– positive and 7-AAD–positive) cells was increased (P < 0.05), suggesting that postapoptotic nuclear staining caused by permeability changes secondary to apoptosis had occurred. Western blot analysis of Akt phosphorylation at serine 473, a marker for promitogenic activity and cell survival, revealed a modest decrease in phosphorylation after 24 hours of tepoxalin treatment, suggesting a potential role for lipoxygenase inhibition in Akt phosphorylation. However, there was a modest decrease in total Akt protein expression over time as well. For death receptor 5 protein, a signal for tumor necrosis factor-α death receptor activation, there was no prominent increase, suggesting no evidence for the extrinsic cascade during induction of apoptosis. Mitochondrial Bax protein expression or cleavage products did not change (data not shown).
Figure 4—
Evidence supporting the apoptotic effects of tepoxalin on SCCF1 cells in culture. A—Results of western blot analysis for markers of apoptosis in SCCF1 cells that were treated with tepoxalin (10μM) or vehicle control (DMSO) for 48 hours, with β-actin as a marker of equal loading. Samples of cells were lysed and were assessed before treatment (0 hours) and at 12, 24, 36, and 48 hours, and representative blots are illustrated. There is late activation of caspase-3 (Act caspase 3) and diminished Akt serine 473 phosphorylation (Ser473-pAkt) prior to caspase activation. As well as less phosphorylation of Akt, the amount of total Akt decreases with time. The amount of death receptor 5 (DR 5) is stable until apoptosis occurs at 36 to 48 hours. B—Photomicrographs (obtained by use of a fluorescence microscope) of preparations of SCCF1 cells that were treated with tepoxalin (10μM; right panel) or vehicle control (DMSO; left panel) for 48 hours and stained with annexin V Alexa 488 and 7-AAD. Annexin V (positive green) and 7-AAD (positive red) staining reveals apoptotic (annexin V–negative and 7-AAD–negative) and subsequent necrotic (annexin V–positive and 7-AAD–positive) cells treated with tepoxalin (right panel) or vehicle control (left panel). C—Graphs depicting mean ± SD percentages of apoptotic (left panel) or necrotic (right panel) SCCF1 cells after 24, 36, and 48 hours of exposure to tepoxalin or the vehicle control (DMSO). *At this time point, the value for tepoxalin-treated cells is significantly (P < 0.01) different from the value for vehicle control–treated cells.
Discussion
To our knowledge, few studies26,27 have been performed to investigate the role of 5-lipoxygenase in neoplastic disease because of extensive focus on COX-mediated PGE2 expression, given that its inhibition can affect neoplastic growth. However, recent evidence suggests that products of 5-lipoxygenase eicosanoid metabolism can promote tumor cell proliferation in pancreatic, lung, and prostate neoplasms has sparked interest in 5-lipoxygenase inhibition.18-20 Preliminary data from clinical trials involving an LTB4 receptor antagonist for treatment of pancreatic neoplasia have indicated potential efficacy.28 In an oral carcinogenesis study,10 5-lipoxygenase was identified as an important enzyme in tumor induction and proliferation. Therefore, it is presumed that 5-lipoxygenase has fundamental activities during hyperplasia or uncontrolled cell proliferation.
In our study, data obtained for oral SCCs in cats confirmed findings in oral SCCs in humans.10 All 20 oral SCCs from cats examined in the present study appeared to have some degree of immunoreactivity and > 50% of tumor cells were 5-lipoxygenase positive. In contrast, 17 of 20 UV radiation–induced SCCs and 12 of 20 cutaneous SCCs had detectable immunoreactivity against 5-lipoxygenase. Additionally, most of the 5-lipoxygenase–positive cutaneous SCCs (9/12) had weak staining intensity, whereas most of the 5-lipoxygenase–positive oral SCCs (18/20) and UV radiation–induced SCCs (12/17) had moderate to marked staining intensity. Hence, expression and extent of upregulation of 5-lipoxygenase in SCCs may be related to their anatomic location, and there may be a potentially greater role for 5-lipoxygenase inhibition in oral versus cutaneous SCC lesions. This is encouraging, but no apparent correlation between staining intensity mitotic index could be made, regardless of anatomic location. However, investigations with more sensitive biomarkers of proliferative index, such as Ki67 staining, are warranted, and results may prove more fruitful in this regard.
The subcellular compartmentalization of 5-lipoxygenase in the 3 forms of SCC were markedly variable. Regardless of classification group, most SCCs had cytoplasmic staining in the cytoplasm; however, perinuclear and nuclear staining predominated in oral SCCs. 5-Lipoxygenase may have different roles depending on cellular localization. Nuclear localization of 5-lipoxygenase is required for eicosanoid synthesis.29 Alternatively, cytoplasmic expression may function to alter cellular proteins and induce oxidative reactions at cysteine residues in proteins like Src and the phosphatase and tensin homologue.29-31 In the present study, investigation of 5-lipoxygenase inhibition by use of the 5-lipoxygenase inhibitor tepoxalin in the SCCF1 cells proved interesting in many respects. Tepoxalin is a unique veterinary-approved NSAID that is classified as a dual COX and 5-lipoxygenase inhibitor. Although safety and efficacy data for tepoxalin in cats have not been published, to our knowledge, there is sufficient evidence in dogs and other species to support that tepoxalin is a potent long-duration COX inhibitor and a short-duration lipoxygenase inhibitor.32-34 Initially after ingestion, tepoxalin acts as a 5-lipoxygenase inhibitor with a serum half-life of 2 to 4 hours; the drug rapidly undergoes hepatic conversion to an active COX inhibitor with a 12-hour serum half-life.33 Tepoxalin in dogs has a wide margin of safety, and typical doses of approximately 5 to 10 mg/kg of body weight are administered. Long-term administration of doses of 50 and 100 mg of tepoxalin/kg in dogs was associated with only mild gastrointestinal adverse effects.34 In cats, there has been no peer-reviewed examination of the anti-inflammatory properties of tepoxalin other than pharmaceutical company data,t making oral administration of tepoxalin in cats a potential area of research.
In the cell culture system used in the present study, it was evident that when the effects of TAM were compared with piroxicam at doses of up to 50μM, piroxicam or TAM (both COX inhibitors) did not diminish cell viability However, tepoxalin in the low micromolar range inhibited cell viability. This is interesting because piroxicam has been recommended as an adjuvant treatment of oral SCC in cats.8 Cyclooxygenase inhibitors, although not traditionally thought to diminish cell proliferation in therapeutic doses, may have a role in decreasing tumor angiogenesis and metastasis.17 This suggests that tepoxalin has the potential to have not only the antiangiogenic effects of COX inhibition, but also an ability to retard tumor cell proliferation. Additionally, the known COX and 5-lipoxygenase inhibitor licofelone35 was effective in the present study, yet tepoxalin was slightly superior at decreasing tumor cell viability. Growth curve kinetics for SCCF1 cells treated with tepoxalin were encouraging; cell viability was diminished by tepoxalin exposure in the high nanomolar concentration range, which is therapeutically achievable assuming similar pharmacokinetics in cats.
5-Lipoxygenase is traditionally a modifier of arachidonic acid as an initiator for leukotriene synthesis, the progenitor of which is 5 hydroxyeicosatetranoic acid. Depending on cell type, 5 hydroxyeicosatetranoic acid is modified into leukotrienes or to 5-oxo-ETE.36 In oral carcinomas in humans and experimentally induced carcinomas in hamsters, LTB4 and 5 hydroxyeicosatetranoic acid are increased, suggesting a role in cancer cell survival or proliferation for these eicosanoids; however, the elevations in PGE2 are far greater, unless COX inhibition drives arachidonic acid toward 5-lipoxygenase metabolism.12,14,15 If eicosanoid autocrine stimulation were a proliferative mechanism in SCCF1 cells, addition of LTB4 or 5-oxo-ETE into the cell culture system under conditions of modest tepoxalin-mediated inhibition would recover proliferative capacity. Yet, with various concentrations of tepoxalin, there was no obvious recovery of cell viability in our SCCF1 cell lines, as detected in other LTB4- and 5-oxo-ETE–dependent human cell lines.37 Furthermore, when testing conditioned medium in which SCCF1 cells were growing, LTB4 could not be detected.
This lack of LTB4 may be related to the lack of nuclear localization of 5-lipoxygenase in SCCs, most of which had predominantly cytoplasmic 5-lipoxygenase–specific staining. Findings of recent studies30,31 suggest that 5-lipoxygenase may also have functions in the cytoplasm via modification of proteins through alteration of cysteine residues. One possible mechanism that has been identified is the alteration of the tumor suppressor phosphatase and tensin homologue. The action of 5-lipoxygenase on phosphatase and tensin homologue suppresses its ability to dephosphorylate Akt, thereby allowing hyperphosphorylation of Akt and promoting cell survival and proliferation.30 Therefore, inhibition of 5-lipoxygenase may alter cell signaling dynamics in addition to decreasing eicosanoid production. 5-Lipoxygenase inhibitors such as MK-886 and AA861 alter signaling pathways to induce apoptosis. MK-886 induces the intrinsic apoptotic cascade through alteration of mitochondrial permeability by altering Bcl-2 (antiapototic) and Bax (proapoptotic) at the mitochondrial membrane, causing caspase-mediated apoptosis.38,39 AA861 induces death receptor 5 protein, which augments the extrinsic pathway of apoptosis.40
It is evident that 10μM tepoxalin in low serum conditions will induce apoptosis of SCCF1 cells within 36 to 48 hours through caspase-3 activation and phosphatidylserine exposure of the cell surface. However, immunoblotting for Bax revealed no alterations in Bax expression or cleavage, and SCCF1 cells expressed abundant death receptor 5 protein; thus, death receptor 5 protein upregulation was an unlikely mechanism for induction of apoptosis. There was a modest decrease in Akt phosphorylation of serine 473 early in the course of tepoxalin treatment, suggesting that decreased Akt phosphorylation may cause a shift toward an apoptotic response. This decrease in phosphorylation was in accordance with a decrease in the total amount of Akt protein. These results are provocative, but further studies need to be performed to fully understand the mechanisms behind retardation of cell growth and induction of apoptosis by tepoxalin. Unfortunately, because of the lack of a known 5-lipoxygenase sequence in cats, small interfering RNA experiments performed in our laboratory were unsuccessful in determining the extent of 5-lipoxygenase in SCCF1 cell survival and sensitivity to tepoxalin.
Overall, it is evident that SCCs in cats, regardless of anatomic location, have enhanced 5-lipoxygenase expression, compared with normal (nonneoplastic) squamous epithelium. The intensity of 5-lipoxygenase–specific staining was significantly greater in oral lesions than it was in cutaneous or UV radiation–induced lesions. Of the 3 forms of SCC, a greater proportion of oral tumors had perinuclear 5-lipoxygenase–specific staining. Compared with previous reports7-8 regarding COX–specific staining in SCCs, it is evident that 5-lipoxygenase is more prevalent, suggesting a role for dual inhibitors of COX and 5-lipoxygenase in SCCs, particularly oral SCCs. Initial evaluation of the effects of tepoxalin, a dual COX and 5-lipoxygenase inhibitor, in SCCF1 cells line revealed growth retardation at therapeutic concentrations and a proapoptotic response at slightly higher concentrations. Use of tepoxalin as an antiproliferative drug may be related not to eicosanoid metabolism but potentially to other 5-lipoxygenase functions in cell signaling or other undefined mechanisms. This surprising cell culture response to tepoxalin warrants further investigation of its mechanism of action, toxic effects, and pharmacokinetics in cats with a view to its potential use as a treatment option in cats with oral SCC.
Acknowledgments
Supported by the Winn Feline Foundation.
Abbreviations
- 7-AAD
7-Aminoactinomycin D
- COX
Cyclooxygenase
- DMSO
Dimethyl sulfoxide
- ETE
Eicosatetranone
- FBS
Fetal bovine serum
- LTB
Leukotriene B4
- MTT
4,5-Dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide
- PGE2
Prostaglandin E2
- SCC
Squamous cell carcinoma
- SCCF1
Squamous cell carcinoma feline 1
- TAM
Tepoxalin active metabolite
Footnotes
Cayman Chemical, Ann Arbor, Mich.
Vector Labs, Burlingame, Calif.
Invitrogen Inc, Carlsbad, Calif.
Sigma, St Louis, Mo.
Provided by Dr. Tom Rosol, The Ohio State University, Columbus, Ohio.
Provided by Dr. Alan Weingarten, Schering Plough Pharmaceuticals, Kenilworth, NJ.
Provided by Cornell Comparative Oncology Program, Ithaca, NY.
Cell Signaling Technology Inc, Danvers, Mass.
Amersham Inc, Piscataway, NJ.
Versadoc with Quant One Software, Bio-Rad Laboratories Inc, Hercules, Calif.
Nunc Dual Chamber slides, Thermo-Fisher Scientific Inc, Rochester, NY.
Alexa Fluor 488, Invitrogen Inc, Carlsbad, Calif.
Kirkegarrd and Perry Labs, Gaithersburg, Md.
Olympus Life Sciences, Center Valley, Pa.
DP Controller, version 2.1.1, Olympus Life Sciences, Center Valley, Pa.
DP Manager, version 2.1.1, Olympus Life Sciences, Center Valley, Pa.
KaleidaGraph, version 4.0, Synergy Software, Northampton, Mass.
Excel 2007, Microsoft Corp, Redmond, Wash.
GraphPad Prism, version 5.0, GraphPad Sofware Inc, La Jolla, Calif.
Weingarten A, Schering Plough Pharmaceuticals, Kenilworth, NJ: Personal communication, 2007.
References
- 1.Vail DM, Withrow SJ. Tumors of the skin and subcutaneous tissues. In: Withrow SJ, Vail DM, eds. Small animal clinical oncology. 4th ed. St Louis: Saunders, 2007;375–401. [Google Scholar]
- 2.Head KW, Else RW, Dubielzieg RR. Tumors of the alimentary tract. In: Meuten DJ, ed. Tumors in domestic animals. 4th ed. Ames, Iowa: Blackwell Publishing, 2002;401–482. [Google Scholar]
- 3.Gil Z, Fiss DM. Contemporary management of head and neck tumors. Isr Med Assoc J 2009;11:296–300. [PubMed] [Google Scholar]
- 4.Pannone G, Bufo P, Grothey A, et al. Cyclooxygenase-2 expression in oral squamous cell carcinoma. Int J Immunopath Pharmacol 2004;17:273–282. [DOI] [PubMed] [Google Scholar]
- 5.Pestili de Almeida EM, Piche C, Sirios J, et al. Expression of cyclo-oxygenase 2 in naturally occurring squamous cell carcinomas in dogs. J Histochem Cytochem 2001;49:867–875. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed SI, Khan KN, Sellers RS, et al. Expression of cyclooxygenase-1 and 2 in naturally-occurring canine cancer. Prostaglandins Leukot Essent Fatty Acids 2004;70:479–483. [DOI] [PubMed] [Google Scholar]
- 7.Hayes A, Scase J, Miller S, et al. COX-1 and COX-2 expression in feline oral squamous cell carcinoma. J Comp Pathol 2006;135:93–99. [DOI] [PubMed] [Google Scholar]
- 8.DiBernardi L, Dore M, Davis JA, et al. Study of feline oral squamous cell carcinoma: potential target for cyclooxygenase inhibitor treatment. Prostaglandins Leukot Essent Fatty Acids 2007;76:245–250. [DOI] [PubMed] [Google Scholar]
- 9.Heller DA, Fan TM, deLorimier LP, et al. In vitro cyclooxygense-2 protein expression and enzymatic activity in neoplastic cells. J Vet Intern Med 2007;21:1048–1055. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Sood S, Wang S, et al. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventative effects of Zileuton and Celecoxib. Clin Cancer Res 2005;11:2089–2096. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z, Sood S, Li N, et al. Involvement of the 5-lipoxygenase/leukotriene A4 hydrolase pathway in 7,12–DMBA-induced oral carcinogenesis in hamster cheek pouch and inhibition of carcinogenesis by its inhibitors. Carcinogenesis 2006;27:1902–1908. [DOI] [PubMed] [Google Scholar]
- 12.Yang P, Sun Z, Chan D, et al. Zyflamend reduces LTB4 formation and prevents oral carcinogenesis in a DMBA-induced hamster cheek pouch model. Carcinogenesis 2008;29:2182–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeder CP, Yang P, Newman RA, et al. Eicosanoid metabolism in squamous cell carcinoma cell lines derived from primary and metastatic head and neck cancer and its modulation by Celecoxib. Curr Biol Ther 2004;9:847–852. [DOI] [PubMed] [Google Scholar]
- 14.Porteder H, Matejka M, Ulrish W, et al. The cyclo-oxygenase and lipoxygenase pathways in human oral cancer tissue. J Maxillofac Surg 1984;12:145–147. [DOI] [PubMed] [Google Scholar]
- 15.Metzger K, Angres G, Maier H, et al. Lipoxygenase products in human saliva: patients with oral cancer compared to controls. Free Radic Biol Med 1995;18:185–192. [DOI] [PubMed] [Google Scholar]
- 16.Duffield Lillico AJ, Boyle JO, Zhou XK, et al. Levels of prostaglandin E metabolite and leukotriene E4 are increased in the urine of smokers: evidence that celecoxib shunts arachidonic acid into the 5-lipoxygenase pathway. Cancer Prev Res 2009;2:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furstenberger G, Krieg P, Muller-Decker K, et al. What are cyclooxygenases and lipoxygenases doing in the driver’s seat of carcinogenesis. Int J Cancer 2006;119:2247–2254. [DOI] [PubMed] [Google Scholar]
- 18.O’Flaherty JT, Rogers LC, Pauma CM, et al. 5-oxo-ETE analogs and the proliferation of cancer cells. Biochim Biophys Acta 2005;1736:228–236. [DOI] [PubMed] [Google Scholar]
- 19.Grant GE, Rokach J, Powell WS. 5-oxo-ETE and the OXE receptor. Prostaglandins Other Lipid Med 2009;89:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rovati GE, Capra V. Cysteinyl-leukotriene receptors and cellular signals. Sci World J 2007;7:1375–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lascelles BDX, Court MH, Hardle EM, et al. Nonsteroidal anti-inflammatory drugs in cats: a review. Vet Anesth Analg 2007;34:228–250. [DOI] [PubMed] [Google Scholar]
- 22.Wakshlag JJ, McNeill CJ, Antonyak MA, et al. Expression and activity of transglutaminase II in spontaneous tumors of dogs and cats. J Comp Pathol 2006;134:202–210. [DOI] [PubMed] [Google Scholar]
- 23.Tannehill-Gregg S, Kergosein E, Rosol TJ. Feline head and neck squamous cell carcinoma cell line: characterization, production of parathyroid hormone-related protein and regulation by transforming growth factor-β3. In Vitro Cell Dev Biol 2001;37:676–683. [DOI] [PubMed] [Google Scholar]
- 24.Wakshlag JJ, Antonyak MA, Boehm JE, et al. Effects of tissue transglutaminase on beta amyloid 1-42 induced apoptosis. Protein J 2006;25:83–94. [DOI] [PubMed] [Google Scholar]
- 25.Antonyak MA, Miller AM, Jansen JM, et al. Augmentation of tissue transglutaminase expression and activation by epidermal growth factor inhibit doxorubicin-induced apoptosis in human breast cancer cells. J Biol Chem 2004;279:41461–41467. [DOI] [PubMed] [Google Scholar]
- 26.Lin DT, Subbaramaiah K, Shah JP, et al. Cyclooxygenase-2: a novel molecular target for the prevention and treatment of head and neck cancer. Head Neck 2002;24:792–799. [DOI] [PubMed] [Google Scholar]
- 27.Fecker LF, Stockfleth E, Nidi I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol 2007;156:25–33. [DOI] [PubMed] [Google Scholar]
- 28.Ding XZ, Talamonti MS, Bell RH, et al. A novel anti-pancreatic cancer agent, LY293111. Anticancer Drugs 2005;16:467–473. [DOI] [PubMed] [Google Scholar]
- 29.Radmark O, Werz O, Steinhiler D, et al. 5-lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci 2007;32:332–341. [DOI] [PubMed] [Google Scholar]
- 30.Covey TM, Edes K, Fitzpatrick FA. Akt activation by arachidonic acid metabolism occurs via oxidation and inactivation of PTEN tumor suppressor. Oncogene 2007;26:5784–5792. [DOI] [PubMed] [Google Scholar]
- 31.Giannoni E, Fiaschi T, Ramponi G, et al. Redox regulation of anoikis resistance of metastatic prostate cancer cells: key role for Src and EGFR-mediated pro-survival signals. Oncogene 2009;28:2074–2086. [DOI] [PubMed] [Google Scholar]
- 32.DeBoever S, Neirinkx E, Baert K, et al. Pharmacokinetics of tepoxalin and its active metabolite in broiler chickens. J Vet Pharmacol Therap 2008;12:97–100. [DOI] [PubMed] [Google Scholar]
- 33.Argentieri DC, Ritchie DM, Ferro MP, et al. Tepoxalin: a dual cyclooxygenase/5-lipoxygenase inhibitor of arachidonic acid metabolism and potent anti-inflammatory activity and a favorable gastrointestinal profile. J Pharmacol Exp Ther 1994;271:1399–1408. [PubMed] [Google Scholar]
- 34.Knight EV, Keenan CM, Smith IL, et al. Preclinical toxicity evaluation of tepoxalin, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, in Sprague-Dawley rats and Beagle dogs. Fundam Appl Toxicol 1996;33:38–48. [DOI] [PubMed] [Google Scholar]
- 35.Cicero AFG, Laghi L. Activity and potential role of licofelone in the management of osteoarthritis. Clin Interv Aging 2007;2:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundaram S, Ghosh J. Expression of 5-oxo-ETE receptor in prostate cancer cells: critical role in survival. Biochem Biophys Res Comm 2006;339:93–98. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi T, Nishiyama K, Shirahama T. Inhibition of 5-lipoxygenase pathway suppresses the growth of bladder cancer cells. Int J Urol 2006;13:1086–1091. [DOI] [PubMed] [Google Scholar]
- 38.Cianshi F; Cortesini C, Magnelli L, et al. Inhibition of 5-lipoxygenase by MK-886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol Cancer Ther 2006;5:2716–2726. [DOI] [PubMed] [Google Scholar]
- 39.Fan XM, Tu SP, Lam SK, et al. Five-lipoxygenase activating protein inhibitor MK-886 induces apoptosis in gastric cancer through upregulation of p27kip1 and bax. J Gastroenterol Hepatol 2004;19:31–37. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida T, Shiraishi T, Horinaka M, et al. Lipoxygenase inhibitors induce death receptor5/Trail-R2 expression and sensitize malignant tumor cells to TRAIL-induced apoptosis. Cancer Sci 2006;98:1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]