Abstract
Diabetic foot ulcer is a debilitating complication of long‐standing diabetes mellitus. Patients lose their earning potential, face repeated hospitalizations, and are forced to bear heavy treatment costs. This places an enormous financial burden on the patients and their families. This study seeks to ascertain the out‐of‐pocket expenditure among these patients and correlate it with their risk factor profile. In this hospital‐based cross‐sectional study, a total of 154 patients with diabetic foot ulcers or amputations have been studied with an elaborate patient questionnaire and relevant clinical examinations. The costs incurred and the risk factors of the patients were analyzed for statistical association. The median total annual out‐of‐pocket expenditure for the management of diabetic foot ulcers among the study participants was found to be ₹29 775 (₹9650–₹81 120) ($378.14 [$122.56–$1030.22]). Out of the total expenditure, 58.49% went towards direct medical costs, 5.64% towards direct non‐medical costs, and 35.88% for indirect costs. Medications, ulcer dressing and periodic debridement have accounted for 79.26% of direct medical costs. Transportation (61.37%) and patient's loss of income (89.45%) account for the major costs under the direct non‐medical and indirect cost categories, respectively. A high ulcer grade and area, long ulcer duration, and past history of ulcers have higher expenditure. Patients seeking treatment from private establishments and those engaged in professional/skilled occupations have higher expenses. Adequate dressing of foot ulcers and proper footwear are associated with lower treatment expenditure. 68.8% of the participants have faced catastrophic expenditure due to treatment costs of diabetic foot ulcers. Adequate glycaemic control and proper foot care are necessary. Patients must seek medical care at the earliest in case of foot ulceration. Clinicians must provide proper wound care, institute effective antibiotics, and manage the complications. Government and insurance schemes are required to alleviate the patients' financial burden.
Keywords: diabetes mellitus, diabetic foot ulcer, financial burden, out of pocket expenditure, tertiary care hospital
1. INTRODUCTION
Diabetes mellitus is one of the leading causes of global health concern in the 21st century. According to the International Diabetes Federation (IDF), 537 million adults live with diabetes worldwide (as of 2021). This figure is projected to rise to 643 million by 2030 and 783 million by 2045. 1 According to the World Health Organization (WHO), diabetes is the ninth leading cause of death worldwide, which resulted in 1.6 million deaths in 2019. 2 In India, diabetes has attained epidemic proportions, justifying its position as the ‘Diabetes Capital of the World’ alongside China. 3 Amidst the vast diabetic population in India, previous studies have reported alarming proportions of diabetic complications such as retinopathy (32.5%), nephropathy (30.2%), peripheral neuropathy (26.8%), coronary heart disease (25.8%), and peripheral vascular disease (28%). 4
Diabetic foot ulcer (DFU) is one such yet serious complication of long‐standing diabetes. They are found in 4.54% of patients newly diagnosed with type 2 diabetes mellitus in India. 5 The annual incidence and prevalence of DFU in population‐based studies is 1%–4.1%, and 4.5%–10% respectively, with an overall lifetime incidence of up to 25%. 6 Of the people with diabetes in India, 25% develop DFUs, of which 50% become infected, requiring hospitalization, while 20% need amputation. 7 DFU is a leading cause of non‐traumatic amputation with rates soaring 20–30 times more than non‐diabetic counterparts. 8 Foot ulcers significantly reduce the quality of life and make the individual susceptible to infections and repeated hospitalizations. 9 Despite amputations, the patients experience recurrences and increased chances of mortality.
DFUs cause enormous financial burden to the patient and their family. Studies by Kumpatla et al. 10 and Shobhana et al. 11 have indicated that patients with DFUs spent four times more than those without DFUs during the course of their treatment. According to Angadi and Tejaswi, 12 the mean 3‐months direct and indirect expenditures were ₹431.40 and ₹611.98, respectively. Medications (mean ₹1165.80) and investigations (mean ₹113.16) were the major direct expenditures, while the indirect expenditure was largely due to loss of income for patients and caregivers, partly due to the rendered disability and prolonged healing times of DFUs.
India is the most expensive country for DFU care, as 5.7 years (68.8 months) of an average patient's income is required to pay for complete DFU therapy. The treatment cost for neuropathic ulcers, infected neuropathic foot, diabetic foot salvage, limb amputation, salvage followed by amputation and neuro‐ischemic foot were $56, $165, $1080, $960, $2650, respectively. 7 In India, DFUs are a significant financial burden owing to the recurring healthcare interventions required to manage them. The dearth of affordable insurance schemes and policies further escalates the colossal economic burden. 13
Multiple studies have highlighted prominent risk factors that predispose to DFUs and aid their progression and recurrence. These include male gender, duration of diabetes (more than 10 years), peripheral neuropathy, diabetic retinopathy and nephropathy, peripheral vascular disease, foot deformity, smoking, history of prior ulcers or amputations, poor glycaemic control, obesity, genetic and nutritional factors. 14 , 15 A host of socioeconomic and environmental factors like improper footwear and foot care practices, poor hygiene, delay in seeking medical attention, resorting to indigenous remedies, lack of health education and healthcare services also govern the prevalence and progression of DFUs. 6 Healthcare professionals must thus adopt a holistic approach to identify and manage patients with DFUs at an early stage.
The first step towards this is understanding the natural history of progression of the condition and its associated financial burden. However, there exists a lacuna in the literature with regards to the current trends of expenditure among DFU patients, especially among the lower‐ and middle‐class population of the nation. These sections tend to have a long treatment history alternating between Government and private healthcare establishments which have different expenditure patterns. The lack of evidence in the medical literature pertaining to this natural course of treatment hinders our complete understanding of the situation.
This study thus seeks to ascertain the financial burden along the course of treatment on DFU patients in and before arriving at our tertiary care facility and correlate it with their risk factor profile, thus allowing medical practitioners and policy‐makers to identify and prioritize patients and ameliorate their financial burden.
1.1. Objectives
1.1.1. Primary objective
To estimate the out‐of‐pocket expenditure among patients with DFUs in a tertiary care hospital and correlate it with their risk factor profile.
1.1.2. Secondary objective
To determine the proportion of DFU patients with financial burden exceeding the critical point of catastrophic expenditure.
2. METHODOLOGY
2.1. Study design and setting
This study is a Facility‐based Cross‐sectional study conducted over 2 months (between July 2022 and September 2022). It was conducted among the patients in the in‐patient department (IPD) and out‐patient department (OPD) of the Departments of Diabetology and General Surgery at Rajiv Gandhi Government General Hospital, Chennai, Tamil Nadu, India .
2.2. Operational definitions
Diabetic foot ulcer (DFU) refers to destruction, infection and loss of tissues of the foot in individuals with long‐standing diabetes mellitus, associated with neuropathy and/or peripheral artery disease in the lower extremity. 16
Out‐of‐pocket expenditure refers to the expenses the patients and their families had borne from their financial resources (apart from the insurance coverage) in order to fulfil the expenses required to manage the patient's diabetic foot ulcer.
Catastrophic out‐of‐pocket expenditure refers to an annual financial burden of more than or equal to 10% of the family's annual income. This definition has been assumed in contrast to the WHO definition of 40% or more of the ‘capacity to pay’ as the latter parameter cannot be calculated among the study population.
Lower limb/extremity amputation refers to complete surgical removal of any part or whole of the lower extremity, irrespective of the cause. 17
2.3. Sample size
The sample size was calculated based on the study by Muhammad et al. 13 where the prevalence of out‐of‐pocket expenditure was 90% (p).
The formula used is .
Keeping the precision (d) at 5%, non‐response rate of 10%, Z = 1.96 and α = 95%, the calculated sample size is 154 patients.
2.4. Sampling procedure
Consecutive sampling method will be employed among the patients at the study setting. Data will be collected from the questionnaires (in both English and local language), case records and laboratory investigations of the patients.
2.5. Eligibility criteria
2.5.1. Inclusion criteria
The participants included in the study are those patients with DFU receiving treatment for a minimum of 3 months and/or have undergone amputation(s) for the same in the past year.
2.5.2. Exclusion criteria
The patients previously diagnosed with other peripheral vascular diseases, patients with diabetes suffering from non‐ulcerative foot complications like cellulitis, and patients who have not consented to participate were excluded from the study.
2.6. Study procedure
2.6.1. Ethics statement
The study was conducted after obtaining approval from the Institutional Ethics Committee (IEC) of Rajiv Gandhi Government General Hospital, Chennai, Tamil Nadu, India.
2.6.2. Selection of study participants
In‐patients and out‐patients in the Departments of Diabetology and General Surgery were scrutinized regularly in a systematic manner during the study period. Patients with DFUs satisfying the inclusion and exclusion criteria were identified and approached.
2.6.3. Informed consent
Patients were explained about the nature, purpose, and procedure of the study in the local language. Written informed consent was then obtained after placing due emphasis on the confidentiality of their details and their freedom to refuse.
2.6.4. Data collection
A brief history regarding the chief complaints and the site, size and duration of the foot ulcer was elicited from all study subjects. Clinical examination of the foot ulcer was done and graded based on the Wagner Classification System. 18
The presence of posterior tibial artery or dorsalis pedis pulse, Doppler study or relevant clinical features was used to assess the absence of peripheral vascular diseases. Anthropometric measurements (height and weight) were obtained from the patients to calculate the body mass index (BMI).
Study questionnaire
All participants were administered a questionnaire in the language of their choice (English or local language). The questionnaire was developed based on the references from the previous literature. The study was pre‐tested among 15 patients with DFUs. The modified and finalized questionnaire was translated into the local language (Tamil) and was back‐translated to English afterwards. Cronbach alpha was calculated to be 0.8, indicating a good reliability and internal consistency of the assessment instrument. Sections of questionnaire includes demographic, socio‐economic, expenditure, risk factor details and quality of life assessment (by Barthel Index questionnaire). 19
Other investigations
Glycaemic levels (random blood glucose or fasting/postprandial blood glucose levels), haemoglobin, and anaemia status were obtained to evaluate diabetes mellitus. Peripheral neuropathy was assessed by 128 Hz Tuning Fork Test, pin prick sensation test, ankle reflex test, monofilament sensation test, or relevant clinical features. Swabs were collected from ulcers of willing patients with ulcer grade ≥2 (Wagner Classification) for evaluating the microbiological profile and antimicrobial sensitivity.
2.7. Analysis plan
Data entry was done in the Microsoft Excel 2007 software, and analysis was done on JAMOVI version 2.2.2. Each patient's out‐of‐pocket expenditure was calculated and correlated. The critical point of catastrophic expenditure was identified based on the cumulative results of the study. Based on the normality of data distribution, continuous variables were expressed as either mean ± SD or median ± interquartile range. Categorical variables were expressed in percentages with 95% confidence interval. Appropriate tests of significance were done. A p‐value <0.05 was considered statistically significant. Costs expressed in US Dollars are based on the average exchange rate (2022) of ₹1 = $0.0127.
2.8. Protection of human participants
The confidentiality of participants' details was strictly maintained in this study. No names would be mentioned during publication. The participants were allotted a unique study reference number during data collection, and only this reference number was used during analysis.
3. RESULTS
3.1. Demographic profile of the participants
The total number of participants in the study is 154. Out of this, 107 (69.5%) were male and 47 (30.5%) were female. The mean age for the study population is 54.06 years with a standard deviation of 10.43 years (males: 54.73 ± 9.46 years, females: 52.55 ± 12.33 years). The minimum and maximum age of participants are 25 and 78 years, respectively. 65.6% of study participants received school education of different levels, while 24.7% were illiterate. 44.2% of the patients were involved in unskilled labour, while 27.9% were unemployed. Most of the study participants belonged to the upper lower class (67.5%), according to the Modified Kuppuswamy Scale (2022).
Among the study population, 46 patients (29.9%) approached only Government set‐ups for treatment. Thirty‐five patients (22.7%) approached both Government and Private clinics, but continued treatment in a Government hospital. Thirty‐one patients (20.1%) used both facilities equally, while 42 patients (27.3%) used both but relied mainly on Private establishments for treatment. One hundred and three patients (66.9%) were admitted in the hospital as in‐patients, while 51 patients (33.1%) visited the departments as out‐patients. One hundred and four (67.5%) had enrolled in an insurance program, majority (66.23%) of which was the state government insurance programme.
3.2. Out‐of‐pocket expenditure for diabetic foot ulcers
3.2.1. Estimation of annual out‐of‐pocket expenditure
The median annual out‐of‐pocket expenditure was estimated to be ₹29 775 (₹9650–₹81 120) ($378.14 [$122.56–$1030.22]). The minimum and maximum expenditure values stand at ₹600.00 ($7.62) and ₹1 183 250 ($15 027.26), respectively (Figure 1).
FIGURE 1.
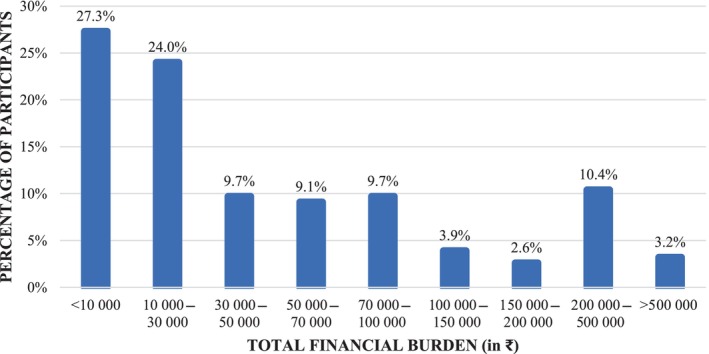
Annual out‐of‐pocket expenditures among study participants (n = 154).
Out of the total expenditure, 58.49% went towards direct medical costs, 5.64% towards Direct non‐medical costs and 35.88% for Indirect costs. From the Table 1, it can be inferred that Medications, ulcer dressing and periodic debridement accounts for a major portion (79.26%) of Direct medical costs. Transportation (61.37%) and Patient's loss of income (89.45%) account for the major cost under the Direct non‐medical and Indirect categories, respectively.
TABLE 1.
Detailed split‐up of out‐of‐pocket expenditure under each category in rupees (₹) (n = 154).
| Expenditure categories | Mean | Median | IQR |
|---|---|---|---|
| Direct medical costs | 53 168 | 10 275 | 2100–50 750 |
| Consultation | 151 | 0 | 0 |
| Investigations | 220 | 0 | 0 |
| Medications, dressings and debridement | 42 142 | 8350 | 1000–37 500 |
| Admission | 6050 | 0 | 0 |
| Surgery | 4606 | 0 | 0 |
| Orthopaedic equipment (if any) | 0 | 0 | 0 |
| Direct non‐medical costs | 5125 | 2050 | 900–5313 |
| Transportation | 3145 | 775 | 313–2000 |
| Food | 1413 | 600 | 0–1475 |
| Hospital rent | 0 | 0 | 0 |
| Miscellaneous costs | 567 | 0 | 0–100 |
| Indirect costs | 32 615 | 29 775 | 9650–81 120 |
| Patient's loss of income | 29 174 | 0 | 0–10 000 |
| Attender's loss of income | 3441 | 0 | 0–2188 |
Abbreviation: IQR, interquartile range.
3.2.2. Proportion of family income spent on DFU management
The median total annual family income of the study participants is ₹120 000 (90 000–180 000) ($1524 [1143–2286]). The average proportion of annual family income spent for management of DFU and its complications among patients is found to be 52.55%. It is alarming to note that 106 patients (68.8%) of patients were bound to spend more than 10% of their annual income for treating DFU, thus accounting for catastrophic expenditure.
3.2.3. Evaluation of quality of life in patients with DFUs
Despite being a non‐monetary measure, quality of life reflects on the ability of the patient to generate income in future (earning member) and lead a normal life without the need of significant medical interventions in future (dependent members). The mean Barthel Index score for the participants was 68.61 ± 28.45 (out of 100). The mean score is 56 ± 28 (partially dependent, Sinoff interpretation 20 ) among patients who have undergone amputation, as compared to a score of 75 ± 27 (minimally dependent) among non‐amputated counterparts.
3.3. Correlation between risk factors and financial burden
3.3.1. Socio‐demographic factors
A higher out‐of‐pocket expenditure (OOPE) has been observed among patients in professional/semi‐professional occupations, and those seeking treatment from private establishments. No significant correlation exists between OOPE and the age, gender, educational status, socio‐economic status, admission status, and the health insurance enrolment of the patients (Table 2).
TABLE 2.
Relationship between socio‐demographic factors and out‐of‐pocket expenditure (n = 154).
| Socio‐demographic factors | Percentage of patients | Total financial burden (₹), median (IQR) | p‐Value | |
|---|---|---|---|---|
| Age groups (in years) | <50 | 31.2 | 33 950 (11 900–79.980) | 0.409 a |
| ≥50 | 68.8 | 28 250 (7000–83 800) | ||
| Gender | Male | 69.5 | 30 000 (9800–98 705) | 0.2 a |
| Female | 30.5 | 24 400 (4400–72 725) | ||
| Educational status | Illiterate | 24.7 | 32 200 (5800–91 250) | 0.358 b |
| School education | 65.6 | 31 800 (10 700–79 600) | ||
| Higher education | 9.7 | 17 600 (5775–32 925) | ||
| Occupational status | Unemployed | 27.9 | 24 400 (7000–64 125) | 0.041 b ( ε 2 = 0.0539) |
| Unskilled | 44.2 | 22 700 (8975–78 400) | ||
| Skilled and semi‐skilled | 20.1 | 31 800 (9800–104 675) | ||
| Professional and semi‐professional | 7.8 | 264 500 (50 525–587 050) | ||
| Socio‐economic status | Lower | 9.1 | 10 275 (2450–28 688) | 0.138 b |
| Upper lower | 67.5 | 32 750 (10 700–79 980) | ||
| Lower middle | 17.5 | 23 000 (7250–48 880) | ||
| Upper middle | 5.8 | 99 500 (12 100–434 000) | ||
| Upper | 0.0 | ‐ | ||
| Location of treatment | Government only | 29.9 | 11 900 (6200–41 230) | 0.001 b ( ε 2 = 0.102) |
| Both, but mainly government | 22.7 | 22 700 (9775–79 600) | ||
| Equally both government and private | 20.1 | 32 200 (14 250–125 875) | ||
| Mainly private but both | 27.3 | 58 350 (30 263–163 100) | ||
| Admission/out‐patient | Admission | 66.9 | 29 800 (9800–76 030) | 0.685 b |
| Out‐patient | 33.1 | 28 550 (9325–84 675) | ||
| Health insurance scheme | Yes | 67.5 | 30 175 (8650–81 790) | 0.704 b |
| No | 32.5 | 29 775 (9963–68 645) | ||
Note: Statistically significant associations (p‐value 〈 0.05) have been given in bold for ease of the readers.
Abbreviation: IQR, interquartile range.
Mann–Whitney U‐test.
Kruskal–Wallis test.
3.3.2. Foot ulcer characteristics
A higher financial burden is significantly correlated with a greater ulcer area (with a large effect size), higher ulcer grade and a longer duration of ulceration (both with moderate effect sizes) (Table 3).
TABLE 3.
Relationship between foot ulcer characteristics and out‐of‐pocket expenditure (n = 154).
| Characteristics of foot ulcer | Percentage of patients | Total financial burden (₹), median (IQR) | p‐Value | |
|---|---|---|---|---|
| Trigger factor | Spontaneous | 58.4 | 19 800 (7225–79 200) | 0.390 a |
| Trauma | 37.7 | 36 150 (13 900–85 550) | ||
| Puncture | 1.3 | 51 795 (28 738–74 853) | ||
| Bite | 2.6 | 46 500 (32 200–60 800) | ||
| Ulcer area (in cm2) | 0–25 | 66.2 | 22 425 (7413–51 480) | <0.001 a (ε 2 = 0.142) |
| 25–50 | 11.0 | 107 350 (29 750–200 900) | ||
| 50–75 | 10.4 | 57 375 (4125–228 425) | ||
| 75–100 | 1.9 | 238 000 (168 750–239 000) | ||
| >100 | 10.4 | 78 000 (32 200–192 763) | ||
| Ulcer grade (according to Wagner's Classification) | 1 | 9.1 | 6525 (1550–25 250) | 0.002 a ( ε 2 = 0.114) |
| 2 | 13.6 | 64 500 (20 200–78 000) | ||
| 3 | 40.9 | 29 750 (10 250–83 800) | ||
| 4 | 32.5 | 28 550 (6400–101 375) | ||
| 5 | 3.9 | 284 875 (48 350–569 635) | ||
| Duration since onset (in months) | 0–2 | 51.9 | 19 725 (6100–51 710) | <0.001 a ( ε 2 = 0.113) |
| 2–4 | 25.9 | 29 775 (13 450–108 250) | ||
| 4–6 | 6.5 | 46 050 (28 550–63 540) | ||
| >6 | 15.6 | 83 335 (67 525–248 900) | ||
| Amputation | Not amputated | 65.6 | 24 400 (9650–83 800) | 0.548 b |
| Amputated | 34.4 | 32 200 (11 650–79 600) | ||
Abbreviation: IQR, interquartile range.
Kruskal–Wallis test.
Mann–Whitney U‐test.
3.3.3. Diabetes‐related risk factors
In our study, the history of diabetes mellitus, random blood glucose levels (with cut‐off of 200 mg/dL), compliance to diabetic medications, and BMI levels have proved to be of lesser significance in assessing the financial burden due to DFUs/amputation (Table 4).
TABLE 4.
Relationship between diabetes‐related risk factors and out‐of‐pocket expenditure on diabetic foot ulcers (n = 154).
| Risk factors | Number of participants (N = 154) | Total financial burden (₹), median (IQR) | p‐Value | |
|---|---|---|---|---|
| History of diabetes mellitus (in years) | <15 | 136 (88.3%) | 28 250 (9000–67 450) | 0.06 a |
| ≥15 | 18 (11.7%) | 47 000 (14 550–123 300) | ||
| Random blood glucose (in mg/dL) | <200 | 26 (16.9%) | 48 715 (26 125–57 815) | 0.672 a |
| ≥200 | 128 (83.1%) | 28 400 (9650–83 130) | ||
| Diabetic medication compliance | Regular | 24 (15.6%) | 17 600 (4410–48 455) | 0.06 a |
| Irregular | 130 (84.4%) | 30 900 (10 025–85 550) | ||
| BMI | Underweight | 22 (14.3%) | 41 025 (12 088–80 340) | 0.334 b |
| Normal | 91 (59.1%) | 31 800 (13 900–90 855) | ||
| Overweight | 26 (16.9%) | 16 700 (6200–64 500) | ||
| Obese | 15 (9.7%) | 11 900 (7000–67 450) | ||
Note: Statistically significant associations (p‐value 〈 0.05) have been given in bold for ease of the readers.
Abbreviations: BMI, body mass index; IQR, interquartile range.
Mann–Whitney U‐test.
Kruskal–Wallis test.
3.3.4. Risk factors related to other disease complications
A significant correlation appears to exist between OOPE and history of past foot ulcers with a small effect size. Less statistical correlation is observed between OOPE and co‐existent comorbidities, peripheral neuropathy, retinopathy, nephropathy, peripheral vascular disease, lower limb amputations, anatomical foot deformities, and anaemia (Table 5).
TABLE 5.
Relationship between disease‐related risk factors and out‐of‐pocket expenditure on diabetic foot ulcers (n = 154).
| Risk factors | Present | Absent | p‐Value | ||
|---|---|---|---|---|---|
| Number of participants (N = 154) | Total financial burden (₹), median (IQR) | Number of participants (N = 154) | Total financial burden (₹), median (IQR) | ||
| Comorbidities | 57 (37%) | 31 800 (10 900–78 000) | 97 (63%) | 28 250 (9650–83 800) | 0.899 a |
| Past foot ulcers | 92 (59.7%) | 33 125 (13 450–1,02638) | 62 (40.3%) | 17 600 (7000–74 750) | 0.029 a ( ε 2 = 0.208) |
| Past lower limb amputations | 34 (22.1%) | 46 000 (20 650–92 750) | 120 (77.9%) | 23 000 (7250–81 120) | 0.090 a |
| Anatomical foot deformities | 6 (3.9%) | 7250 (6463–62 653) | 148 (96.1%) | 29 900 (9800–83 800) | 0.307 a |
| Peripheral Vascular Disease | 38 (24.7%) | 31 375 (17 488–91 250) | 116 (75.3%) | 28 550 (8975–79 600) | 0.402 a |
| Peripheral neuropathy | 107 (69.5%) | 32 750 (11 750–83 800) | 47 (30.5%) | 22 700 (7050–71 500) | 0.055 a |
| Nephropathy | 28 (18.2%) | 33 125 (20 000–67 450) | 126 (81.8%) | 26 325 (7413–83 130) | 0.226 a |
| Retinopathy | 10 (6.5%) | 41 100 (18 438–1,46 438) | 144 (93.5%) | 29 150 (8975–79 980) | 0.209 a |
| Anaemia | 147 (95.5%) | 28 550 (9650–80 360) | 7 (4.5%) | 64 500 (34 095–3,17 975) | 0.206 a |
Note: Statistically significant associations (p‐value 〈 0.05) have been given in bold for ease of the readers.
Abbreviation: IQR, interquartile range.
Mann–Whitney U‐test.
3.3.5. Patient‐related risk factors
In our study, a higher OOPE is significantly associated with a history of long‐term alcoholism, irregular changing of ulcer dressing, and wearing improper footwear. It is also inferred that smoking and proper foot care practices have low statistical correlation with OOPE towards diabetic foot ulcers among the study participants (Table 6).
TABLE 6.
Relationship between patient‐related risk factors and out‐of‐pocket expenditure on diabetic foot ulcers (n = 154).
| Risk factors | Number of participants (N = 154) | Total financial burden (₹), median (IQR) | p‐Value | |
|---|---|---|---|---|
| Alcoholism | Yes | 81 (52.6%) | 36 100 (11 900–102 000) | 0.016 a ( ε 2 = 0.225) |
| No | 73 (47.4%) | 22 150 (6200–67 450) | ||
| Smoking | Yes | 37 (24%) | 32 200 (9800–64 500) | 0.866 a |
| No | 117 (76%) | 28 550 (9650–83 800) | ||
| Foot ulcer dressing | Yes | 102 (66.2%) | 32 750 (11 950–94 820) | 0.015 a ( ε 2 = 0.241) |
| No | 52 (33.8%) | 14 550 (6888–54 500) | ||
| Frequency of dressing | Twice a day | 3 (1.9%) | 22 700 (20 075–22 700) | 0.002 b ( ε 2 = 0.149) |
| Daily | 52 (33.8%) | 29 150 (6800–60 800) | ||
| Alternative days | 34 (22.1%) | 76 500 (24 313–1,1 675) | ||
| Once in 2 days | 4 (2.6%) | 93 475 (79 600–120 613) | ||
| Once in 3 days | 4 (2.6%) | 45 385 (9650–81 120) | ||
| Occasionally | 1 (0.6%) | 517 350 | ||
| Only a few times till now | 5 (3.2%) | 17 600 (6200–17 600) | ||
| N/A | 51 (33.1%) | 14 550 (6525–58 000) | ||
| Type of footwear | MCR chappals | 87 (56.5%) | 17 850 (6200–51 700) | 0.001 b ( ε 2 = 0.156) |
| Foam chappals | 13 (8.4%) | 83 800 (64 500–311 500) | ||
| Shoes | 7 (4.5%) | 29 750 (22 700–174 650) | ||
| Sandals with straps | 28 (18.2%) | 31 800 (11 900–119 350) | ||
| Specialized diabetic footwear | 10 (6.5%) | 106 400 (31 375–194 113) | ||
| Worn out footwear of any kind | 1 (0.6%) | 1100 | ||
| Others | 2 (1.3%) | 10 700 | ||
| None | 6 (3.9%) | 46 050 (45 975–72 353) | ||
| Proper foot care practices | Yes | 88 (57.1%) | 32 200 (9600–98 308) | 0.390 a |
| No | 66 (42.9%) | 25 625 (9650–61 850) | ||
Note: Statistically significant associations (p‐value 〈 0.05) have been given in bold for ease of the readers.
Abbreviations: IQR, interquartile range; MCR, micro cellular rubber.
Mann–Whitney U‐test.
Kruskal–Wallis test.
3.3.6. Microbiological risk factors
Among the study participants, the microbiology report was available only from 37 patients (24%). A significant associated was noted between the nature of microbe in the ulcer swab and the OOPE among the study subjects. Among bacteria, Gram negative bacteria have a higher OOPE as compared to the Gram positive microbes (Table 7).
TABLE 7.
Relationship between microbiological profile and out‐of‐pocket expenditure on diabetic foot ulcers.
| Organism in swab culture | Number of participants | Total financial burden (₹), median (IQR) |
|---|---|---|
| Gram positive bacteria | 3 (1.9%) | 1190 (1190–6395) |
| Gram negative bacteria | 20 (13%) | 41 550 (9800–83 800) |
| Virulent poly‐microbial culture | 3 (1.9%) | 249 800 (186 550–249 800) |
| Fungi | 2 (1.3%) | 587 050 |
| No growth on culture | 9 (5.8%) | 20 200 (19 400–24 400) |
| N/A | 117 (76%) | 29 800 (8900–79 600) |
Note: p‐Value = 0.006 (Kruskal–Wallis test).
Abbreviation: IQR, interquartile range.
Antimicrobial susceptibility profile
The antimicrobial susceptibility profile was collected from 28 patients (22.58%) after excluding the cases where no growth was observed on culture. A significant statistical association is visible between the OOPE and susceptibilities to Cephalosporins, Piperacillin‐Tazobactam and Meropenam with small effect sizes, and antifungals (Amphotericin/caspofungin/micafungin) with a large effect size. The other antimicrobial agents tested revealed lesser statistical significance in deciding OOPE for DFUs (Table 8).
TABLE 8.
Relationship between antimicrobial susceptibility profile and out‐of‐pocket expenditure on diabetic foot ulcers.
| Antimicrobial drug | Susceptible | Resistant | p‐Value a | ||
|---|---|---|---|---|---|
| Number of participants | Total financial burden (₹), median (IQR) | Number of participants | Total financial burden (₹), median (IQR) | ||
| Tetracycline | 25 (89.29%) | 51 000 (9800–249 800) | 3 (10.71%) | 36 100 (23 850–79 700) | 0.549 |
| Amikacin | 19 (67.86%) | 14 550 (6200 – 103 550) | 9 (32.14%) | 65 000 (47 000 – 249 800) | 0.073 |
| Ceftazidime/cefoxitin/cefepime/cefotaxim | 13 (46.43%) | 9800 (6200–83 800) | 15 (53.57%) | 78 000 (41 550–249 800) | 0.025 ( ε 2 = 0.0484) |
| Ciprofloxacin | 14 (50%) | 64 500 (6388–343 075) | 14 (50%) | 41 550 (10 375–161 138) | 0.539 |
| Erythromycin/gentamicin | 23 (82.14%) | 51 000 (10 950–249 800) | 5 (17.86%) | 11 600 (1190–123 300) | 0.254 |
| Cotrimoxazole | 22 (78.57%) | 49 000 (7100–249 800) | 6 (21.43%) | 41 225 (13 438–74 750) | 0.542 |
| Oxacillin/penicillin | 24 (85.71%) | 58 000 (11 525–249 800) | 4 (14.29%) | 6395 (1190 – 39 525) | 0.085 |
| Piperacillin–Tazobactam | 21 (75%) | 17 450 (6200 – 83 800) | 7 (25%) | 173 750 (94 150 – 249 800) | 0.030 ( ε 2 = 0.0457) |
| Meropenam | 21 (75%) | 17 450 (6200 – 83 800) | 7 (25%) | 173 750 (94 150 – 249 800) | 0.030 ( ε 2 = 0.0457) |
| Colistin | 26 (92.86%) | 49 000 (9800 – 230 788) | 2 (7.14%) | 38 550 (25 325 – 51 775) | 0.532 |
| Amphotericin/caspofungin/micafungin | 0 (0%) | ‐ | 2 (7.14%) | 587 050 | 0.019 b ( ε 2 = 0.974) |
Abbreviation: IQR, interquartile range.
Kruskal–Wallis test.
Mann–Whitney U‐test.
4. DISCUSSION
DFU is one of the most dreaded complications of long‐standing diabetes mellitus with a devastating financial burden. The study seeks to assess the out‐of‐pocket expenditure incurred by patients with DFUs in a Government tertiary care setup, while identifying critical point of catastrophic expenditure within the patient population. The findings presented in this study shed light on the profound impact of DFUs on the economic situation of patients and their families, highlighting the urgent need for timely management and financial support for these patients.
The demographic characteristics of the study population reveal a population largely affected by the long‐term implications of diabetes mellitus (40–60 years' age group; 7:3 male preponderance), concurrent with the study by Hopkins et al. 21 The prevalence of DFUs was particularly high among individuals in the lower socio‐economic strata (Socio‐economic classes of patients: upper‐lower = 67.5%, lower‐middle = 17.5%, lower = 9.1%), reinforcing the association between lower economic status and increased vulnerability to this complication. This aligns with previous research by Bal et al., 15 confirming the socio‐economic divide in diabetes‐related complications.
The natural history of DFUs and patients' response to them has demonstrated a characteristic pattern. Most of these foot ulcers originated as a spontaneous blister (58.4%) or following trauma (37.7%) with an onset of less than 6 months (84.41% of cases). In most cases, patients have been complacent about seeking medical attention early in the clinical course. Non‐healing or progression of DFUs has forced them to seek medical care, and the first point‐of‐treatment for 70.1% of the patients was a local private clinic. Patients approached a Government hospital only after a long delay or after incurring a substantial financial burden from their initial treatment at private establishments. By the time patients approached a tertiary care set‐up, the foot ulcers were of large‐size (mean foot ulcer area = 37.45 ± 49.51 cm2) and advanced grade (Wagner's grade ≥ 3 in 77.3% cases), owing to the delay in adequate treatment.
Insights into the cumulative financial burden among these patients reveals an alarming picture. Despite being a tertiary care set‐up where a majority of medical costs are absorbed by the Government, the median total annual out‐of‐pocket expenditure for the management of diabetic foot ulcers among the study participants was found to be ₹29 775 (₹9650–₹81 120) ($378.14 [$122.56–$1030.22]). Out of the total out‐of‐pocket expenditure, 58.49% went towards direct medical costs, 5.64% towards direct non‐medical costs and 35.88% for indirect costs. Lower grade ulcers were managed with medications, frequent ulcer dressings, and periodic debridement (which accounted for 79.26% of direct medical costs), while higher grade ulcers, especially those with gangrenous and infective changes were considered for amputations. Transportation (61.37%) accounted for the majority of direct non‐medical costs. The indirect costs were mainly formed by the patients' loss of income (89.45%). This pattern of expenditure is in synchrony with the study by Angadi and Tejaswi, 12 thus reinforcing the common financial trend displayed among the patient population.
Expenditure of this magnitude is detrimental to the financial stability of the patients' families. 68.8% of participants have faced catastrophic expenditure (>10% of annual income) due to management of DFUs. This is a heavy burden that collectively compromises the quality of life for the patient's family as a whole. Even after treatment, patients have experienced difficulty and dependence (mean Barthel Index score = 68.61 ± 28.45), thus hampering their earning potential in future. This is partly due to the rendered disability and prolonged healing times of DFUs.
Though present among a considerable portion of families, health insurance schemes did not comprehensively cover the expenses associated with DFU management. 67.5% of the families had enrolled for a health insurance scheme, most of whom were under the umbrella of the state insurance scheme, which only covers expenses due to amputations. However, only 34.4% of the participants had undergone lower limb amputation and thus in a position to avail the insurance benefits. This observation concurs with the study by Muhammad et al., 13 where out‐of‐pocket payment accounted for 90% of the payment, despite health insurance coverage. This study highlights the deficiency in the current insurance schemes and underscores the need for schemes that adequately address the financial aspects of DFU management holistically.
The relationship between diabetic and comorbidity history with the financial burden aligns with previous research. 14 , 15 Longer duration of diabetes and poorly‐controlled glycaemic levels point towards a higher expenditure. Participants with additional comorbid conditions spent 1.48 times more on foot ulcer management than their non‐comorbid counterparts. These findings emphasize the multifaceted nature of DFU management and the need for tailored interventions based on patients' medical history and comorbidities.
The effectiveness of DFU treatment rests on proper wound care and adequate infection control. Frequent ulcer dressing and proper footwear were associated with a lower out‐of‐pocket expenditure. The study highlights the importance of proper foot care practices in preventing further deterioration of foot ulcers and reducing financial burden. The choice of the correct antimicrobial agent is of paramount importance. Diligent administration of broad‐spectrum antibiotics early in the clinical course drastically brings down the expenditure.
Interestingly, the organisms identified in the ulcer swab also appear to be associated with the ulcer severity, 22 and thus the ‘financial prognosis’ of the patient. The ratio of mean annual financial burden seen among ulcers with Gram positive, Gram negative and virulent poly‐microbial cultures has been found to be 1: 22.3: 44.6.
While the study provides valuable insights, it is not without limitations. The recall bias among patients in recalling expenditures over a year could have influenced the accuracy of the expenditure data. Additionally, the study's location within a Government setup might have led to underestimating the financial burden, considering the higher costs associated with private facilities. Further studies are required to comprehensively address these limitations and broaden the insights provided in this study.
5. CONCLUSION
In the light of the findings from this study, it is apparent that DFUs exert a substantial out‐of‐pocket financial burden on patients. Factors such as prolonged diabetes duration, comorbidities, alcoholism, and inadequate foot care practices have been identified as significant contributors to this financial strain. Educating diabetic patients about glycaemic control and proper foot care is a crucial preventive strategy, particularly for those at heightened risk. Seeking medical attention early in the clinical course of DFUs must be advised to all diabetic patients. Encouraging patients, especially those from lower socio‐economic backgrounds, to seek care at Government hospitals holds promise in alleviating their overall financial burden. Establishing specialized wards dedicated to the comprehensive management of diabetic foot ulcers within healthcare facilities is recommended. Clinicians should advocate for meticulous ulcer care practices, including regular dressing changes and the appropriate use of broad‐spectrum antibiotics. Revamping Government and Private insurance schemes to encompass a broader scope of direct and indirect expenses is essential. This study vividly highlights the considerable financial hardship borne by individuals grappling with diabetic foot ulcers, particularly in Government tertiary care. The implications of these findings underscore the imperative for holistic healthcare policies that embrace both medical and financial dimensions of DFU management. Future research endeavours should concentrate on devising interventions that mitigate the financial burden, enhance healthcare accessibility, and cultivate preventative measures to curb diabetic foot ulcers.
FUNDING INFORMATION
This study has received a grant of Rs. 50 000 ($600) from the Indian Council of Medical Research (ICMR) under the Short‐Term Studentship (STS) 2022 programme (reference ID: 2022‐05696).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
INFORMED CONSENT
Written consent was obtained from all participants whose data were used in this study.
ACKNOWLEDGMENTS
We would like to thank Dr. Costantia Dovita S. MBBS., MD, and Dr. Sankarmani Ramasamy Mathivanan MBBS, MD, from the Institute of Community Medicine, Madras Medical College, for guiding us in the preparation of the manuscript and the statistical analysis.
Seshadri H, Karthikeyan V, Rudrakumar M, et al. Out‐of‐pocket expenditure among patients with diabetic foot ulcer in a tertiary care hospital of south India: A cross‐sectional study. Int Wound J. 2024;21(4):e14552. doi: 10.1111/iwj.14552
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 10th ed.; 2021. Accessed August 14 2023. International Diabetes Federatio. https://www.diabetesatlas.org [Google Scholar]
- 2. World Health Organization . The top 10 causes of death. Accessed August 14, 2023. http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 3. Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69(11):2932‐2938. doi: 10.4103/ijo.IJO_1627_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agrawal RP, Ola V, Bishnoi P, Gothwal S, Sirohi P, Agrawal R. Prevalence of micro and macrovascular complications and their risk factors in type‐2 diabetes mellitus. J Assoc Physicians India. 2014;62(6):504‐508. [PubMed] [Google Scholar]
- 5. Das A, Pendsey S, Abhyankar M, Malabade R. Management of diabetic foot in an Indian clinical setup: an opinion survey. Cureus. 2020;12(6):e8636. doi: 10.7759/cureus.8636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rastogi A, Bhansali A. Diabetic foot infection: an Indian scenario. J Foot Ankle Surg. 2016;3(2):71‐79. doi: 10.5005/jp-journals-10040-1052 [DOI] [Google Scholar]
- 7. Ghosh P, Valia R. Burden of diabetic foot ulcers in India: evidence landscape from published literature. Value Health. 2017;20(9):A485. [Google Scholar]
- 8. World Health Organization . Global report on diabetes. 2016. Accessed August 14, 2023. https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1&isAllowed=y
- 9. Raghav A, Khan ZA, Labala RK, Ahmad J, Noor S, Mishra BK. Financial burden of diabetic foot ulcers to world: a progressive topic to discuss always. Ther Adv Endocrinol Metab. 2018;9(1):29‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumpatla S, Kothandan H, Tharkar S, Viswanathan V. The costs of treating long‐term diabetic complications in a developing country: a study from India. J Assoc Physicians India. 2013;61(2):102‐109. [PubMed] [Google Scholar]
- 11. Shobhana R, Rao PR, Lavanya A, Vijay V, Ramachandran A. Foot care economics–cost burden to diabetic patients with foot complications: a study from southern India. J Assoc Physicians India. 2001;49:530‐533. [PubMed] [Google Scholar]
- 12. Angadi N, Tejaswi Y. Assessment of economic burden for the management of diabetic foot ulcer in patients attending tertiary care teaching hospital, Davangere. Int J Med Sci Public Health. 2019;8(8):677‐681. [Google Scholar]
- 13. Muhammad F, Pedro L, Suleiman H, et al. Cost of illness of diabetic foot ulcer in a resource limited setting: a study from northwestern Nigeria. J Diabetes Metab Disord. 2018;17(2):93‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shahbazian H, Yazdanpanah L, Latifi SM. Risk assessment of patients with diabetes for foot ulcers according to risk classification consensus of International Working Group on Diabetic Foot (IWGDF). Pak J Med Sci. 2013;29(3):730‐734. doi: 10.12669/pjms.293.3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bal B, Khanna G, Bhardwaj A, Singh K. Evaluation of risk factors for lower extremity amputation in diabetic foot ulcer: a hospital based observational study in Northern India. Int J Res Med Sci. 2019;7:1190. doi: 10.18203/2320-6012.ijrms20191323 [DOI] [Google Scholar]
- 16. van Netten JJ. Definitions and criteria for diabetic foot disease – IWGDF guidelines. Accessed August 14, 2023. https://iwgdfguidelines.org/wp-content/uploads/2019/05/definitions-and-criteria-final.pdf
- 17. Unwin N. Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia. Br J Surg. 2000;87(3):328‐337. [DOI] [PubMed] [Google Scholar]
- 18. Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2(2):64‐122. doi: 10.1177/107110078100200202 [DOI] [PubMed] [Google Scholar]
- 19. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61‐65. [PubMed] [Google Scholar]
- 20. Sinoff G, Ore L. The Barthel activities of daily living index: self‐reporting versus actual performance in the old‐old (> or = 75 years). J Am Geriatr Soc. 1997;45(7):832‐836. doi: 10.1111/j.1532-5415.1997.tb01510.x [DOI] [PubMed] [Google Scholar]
- 21. Hopkins RB, Burke N, Harlock J, Jegathisawaran J, Goeree R. Economic burden of illness associated with diabetic foot ulcers in Canada. BMC Health Serv Res. 2015;22(15):13. doi: 10.1186/s12913-015-0687-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pal B, Gupta S. A study on the relation of the severity of diabetic foot ulcers with the type of bacterial flora isolated from the wounds. Int Surg J. 2016;3(1):189‐194. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


