Abstract
Introduction:
Carotid endarterectomy (CEA) for asymptomatic carotid artery disease is advised for patients with low perioperative stroke risk and life expectancy of three to five years. We sought to explore the role of risk stratification and postoperative medical management in identifying appropriate asymptomatic candidates for CEA in the end-stage kidney disease (ESKD) population.
Methods:
We identified ESKD patients on dialysis from the United States Renal Data System (USRDS) that underwent CEA (2008 – 2014) for asymptomatic carotid artery disease. We used the Liu comorbidity index as well as a novel risk prediction model based on Cox-proportional hazards model to stratify patients. The primary outcome evaluated was three-year survival, and Kaplan-Meier methods were used to generate survival estimates.
We further conducted a sub-analysis of patients with Medicare part D data to determine postoperative usage of the following medications: statins, antiplatelets, and antihypertensives. We evaluated the association of medication utilization and three-year survival using Kaplan-Meier methods and Cox proportional hazards modelling.
Results:
We analyzed 1,813 patients meeting inclusion criteria. The population was predominantly older (mean age 70.2±9.1), White (84.8%) and had a high prevalence of cardiovascular comorbidities, such as hypertension (90.7%), diabetes (62.5%) and CHF (35.4%). Among the entire cohort, 23.0% had a Liu comorbidity index ≤ 8, 35.0% had index 9 to 12, and 42.0% had index >12. Increasing Liu comorbidity index was associated with worse survival (p<0.01), however even the group with Liu index ≤ 8 had poor three-year survival of 58.8 (53.9 – 63.4) %.
The Cox proportional hazards model identified variables for inclusion in the risk model such as age>80 (aHR=2.49, 95% CI [1.87-3.33], p<0.001), congestive heart failure (aHR=1.31, 95% CI [1.14-1.51], p<0.001) and Liu comorbidity index > 12 (aHR=1.89, 95% C.I. [1.56 – 2.28], p<0.001). The risk score generated ranged from 0 to 6.5, and patients were divided into three groups: score ≤2 (43.4%), 2 to 4 (41.2%), and >4 (15.4%). Increasing risk score was associated with worse survival (p<0.01) but even the “low-risk” group had three-year survival of 58.5 (54.9 – 61.9)%.
Sub-analysis of the 1,249 (68.8% of total) patients with part D data found that statins and calcium channel blocker use was associated with improved survival, although observed rates for patients on drug were still low.
Conclusion:
The overall long-term survival of ESKD patients undergoing CEA for asymptomatic carotid artery disease is low. Risk stratification and analysis of postoperative medical management did not identify a subgroup of patients with adequate three-year survival. Hence, the preventive benefits of CEA are not realized in these patients.
Keywords: dialysis, database, carotid endarterectomy, outcomes, medical management
1. INTRODUCTION
Multiple trials have demonstrated the utility of carotid revascularization to reduce strokes related to carotid artery disease in appropriately selected patients.1-3 However, most of these large randomized studies exclude patients with limited life expectancy, including end-stage kidney disease (ESKD) patients. The European Society for Vascular Surgery and Society for Vascular Surgery guidelines recommend that patients with asymptomatic carotid disease selected for carotid endarterectomy (CEA) must have a low perioperative stroke risk (<3%) and a three- to five-year life expectancy.4,5 Earlier analyses from the United States Renal Data System (USRDS) suggest that the poor survival of ESKD patients precludes them from the benefits of a CEA.6,7 Some authors suggest a non-operative approach is appropriate for this patient subpopulation.7,8 Nonetheless, enthusiasm to perform CEAs persists, and many patients being revascularized are asymptomatic at baseline.8 Risk stratification is advised to select good-risk patients who will benefit from operative intervention, and multiple publications describe risk factors for mortality.7,9
Beyond the operating theatre, medical management of ESKD patients receiving a CEA requires careful thought. Medical management in this population is complicated by pharmacodynamics, fluid shifts during dialysis sessions, and polypharmacy to treat comorbid conditions.10 Current guidelines recommend statins, antiplatelets, and control of hypertension and diabetes to slow the progression of atherosclerosis and limit stroke-related morbidity.4,5 Current practices in ESKD patients are extrapolated from data for patients with normal renal function, as there is no specific prospective data.11 Optimal medical therapy may be one potential avenue to ensure a sustained benefit of this operation for ESKD patients.
Given the limitations of the existing published literature, we designed a retrospective review of ESKD patients undergoing CEA for asymptomatic carotid artery disease in the USRDS, an extensive administrative database with robust follow-up. The aim of our study was two-fold a) to use preoperative patient factors to identify a “low-risk” patient sub-group with good survival & b) to explore the role of postoperative medical management in improving survival of these patients. In both cases, the goal was to potentially identify a group of ESKD patients where the benefits of CEA may be realized.
2. METHODS
2.1. Study Cohort
The University of Pittsburgh Institutional Review Board approved the study. The database used for this analysis was the USRDS. Since the data in USRDS are deidentified, no individual patient consent is required. The data reported here have been supplied by USRDS.12 The interpretation and reporting of these data are the authors' responsibility and in no way should be seen as an official policy or interpretation of the U.S. government.
The study population consisted of patients receiving CEA between 2008 and 2014 after dialysis initiation for asymptomatic carotid artery disease. Patients were identified using claims for Current Procedural Terminology ® (CPT) codes (35390 or 35301) in the Physician Supplier files. Inclusion criteria for our analysis included valid CMS-2728 forms, Medicare coverage at the time of operation, no history of recorded pre-dialysis CEA, and at least six months of lead time before the operation. We identified asymptomatic patients as those with no neurological symptoms in the six months before operation. Neurological symptoms were defined using claims for International Classification of Diseases, 9th Revision, Clinical Modification, and 10th Revision (ICD-9; ICD-10CM) codes summarized in Supplementary Table 1.
2.2. Outcomes of interest
The primary outcome evaluated was survival at three years. Death in the USRDS is linked to the Social Security Death Index. If a patient did not experience the event of interest, they were censored at the last available claim.
2.3. Covariates
Relevant covariates were extracted from the CMS-2728 forms, using the oldest entry if multiple forms were present. Demographic information such as age at procedure, race, ethnicity, and sex were recorded. Similarly, comorbid conditions such as hypertension, diabetes mellitus, cerebrovascular disease, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD) and peripheral vascular disease (PVD) were noted. Ambulatory status and residential location were also extracted. For calculating the Liu comorbidity index, preoperative claims were checked for validated and published ICD codes for the relevant comorbid conditions (Supplementary Table 2).13
2.4. Statistical Analysis
Primary Analysis: Risk Factor Identification and Association with Survival
Normally distributed continuous variables were summarized as means and standard deviations. Categorical variables were summarized using frequencies and percentages. Kaplan Meier methods (with 95% confidence intervals [CI]) with logrank testing were used to visualize survival after CEA. Patients were stratified by Liu comorbidity index, an existing and validated score, to evaluate survival differences among groups of patients based on their Liu index.
Cox proportional hazards models were used to identify factors associated with survival. Variables with a p-value of less than 0.20 were entered into a stepwise multivariate model to identify factors independently associated with survival. The effect size generated was an adjusted hazards ratio (aHR) with 95% CI. Variables from the Cox-proportional hazards model were used to construct a logistic regression model with three-year mortality as the outcome. Area under the reviewer-operating curve was used to assess the predictive ability of this model.
Using the factors independently associated with survival in the Cox model, we constructed a risk score to predict three-year survival. The risk scoring system was created by dividing the effect sizes of each variable by the smallest effect size in the model. The presence of a risk factor would contribute 1, 1.5 or 2 points as per the model created. We divided patients into risk groups based on their risk score. Kaplan-Meier estimates for postoperative survival were generated to evaluate survival differences (with 95% CI) among patients grouped by their risk score.
Sub-Analysis: Postoperative Medication Use and Survival
We performed a sub-analysis of patients with available part D claims before the operation was selected to ensure consistent access to medications. Patient characteristics were summarized as previously described and compared between the primary cohort and subgroup with part D data. Continuous variables were compared using t-tests, and categorical variables were compared using Chi-squared tests. The exposure of interest, medication usage, was assessed with Medicare part D data. We queried the database for medications commonly prescribed by vascular surgeons, including antiplatelets, beta-blockers, statins, diuretics, calcium channel blockers (CCBs), and ACE inhibitors/ angiotensin receptor blockers (ACEi-ARBs). The drugs explored in each class are summarized in Supplementary Table 3. Patients were considered exposed to the medication if they had a medicine possession ratio (MPR) greater than 50%. The MPR has been previously used to indicate continued medication adherence to physician instruction in the analysis of claims data, and is calculated by dividing the number of drug days (days where part D claims for medication were available) by the total follow-up days.14 Kaplan Meier methods with logrank testing were used to establish differences in primary outcomes, stratified by prescription. Cox proportional hazards models were used to assess the role of each medication, after adjusting for variables selected a priori such as age at procedure, sex, race, and Liu comorbidity index.
A P-value less than 0.05 was considered statistically significant for comparison. All statistical analysis was conducted using Stata version 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.).
3. RESULTS
3.1. Patient Characteristics
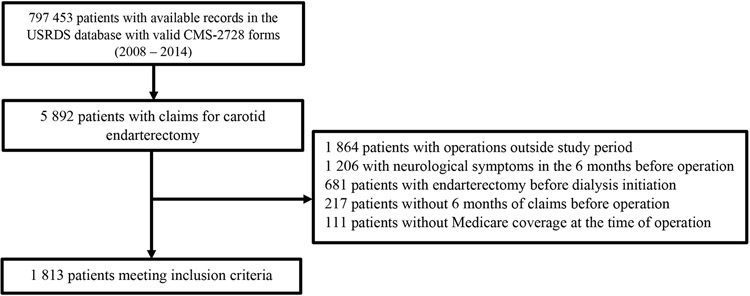
We identified 1,813 patients meeting inclusion criteria out of 797,453 patients initiating dialysis from 2008 to 2014 (Figure 1). Patient demographics and comorbidities are summarized in Table 1. The patient population was predominantly older (mean age 70.2±9.1), White (84.8%) and had a high prevalence of cardiovascular comorbidities, such as hypertension (90.7%), diabetes (62.5%) and CHF (35.4%).
Figure 1:
Patient selection for analysis
Table 1:
Patient Characteristics
| Variable | N=1,813 |
|---|---|
| Age at operation, mean (s.d) | 70.2 (9.1) |
| Less than 60, n (%) | 234 (12.9%) |
| 60 to 80, n (%) | 1,332 (73.5%) |
| More than 80, n (%) | 247 (13.6%) |
| Male, n (%) | 1,076 (59.3%) |
| Race, n (%) | |
| White | 1,537 (84.8%) |
| Black | 217 (12.0%) |
| Other | 59 (3.3%) |
| Hispanic ethnicity, n (%) | 193 (10.6%) |
| Body mass index, mean (s.d) | 29.2 (7.0) |
| Body mass index > 30, n (%) | 707 (39.4%) |
| Tobacco use, n (%) | 182 (10.1%) |
| Congestive heart failure, n (%) | 637 (35.4%) |
| Atherosclerotic heart disease, n (%) | 598 (33.2%) |
| Peripheral vascular disease, n (%) | 438 (24.3%) |
| Hypertension, n (%) | 1,631 (90.7%) |
| Diabetes, n (%) | 1,124 (62.5%) |
| Amputation, n (%) | 62 (3.4%) |
| COPD, n (%) | 243 (13.5%) |
| Functional dependence, n (%) | 158 (8.8%) |
| Institutional care, n (%) | 66 (3.7%) |
| Nephrology care before dialysis initiation, n (%) | 1,227 (75.0%) |
| AV Access used for dialysis at initiation, n (%) | 374 (22.5%) |
| Liu comorbidity score, mean (s.d) | 11.2 (3.8) |
| ≤ 8, n (%) | 417 (23.0%) |
| 9 to 12, n (%) | 635 (35.0%) |
| >12, n (%) | 761 (42.0%) |
Abbreviations: COPD – chronic obstructive pulmonary disease; s.d – standard deviation
3.2. Survival Analysis of the Entire Cohort and Estimates for Groups by Liu Comorbidity Index
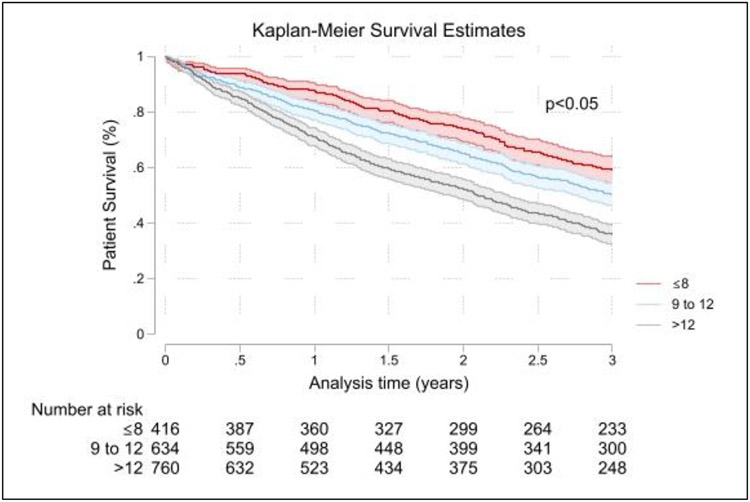
The mean and median follow-up of these patients was 33.8±24.0 months and 31.2 months (IQR 13.2–48.7 months), respectively. In the complete cohort, Kaplan-Meier survival estimates showed survival at one, two and three years of 78.1 (76.2 – 80.0)%, 61.7 (59.4 – 64.0) %, and 46.3 (43.9 – 48.6) %, respectively. Based on the Liu comorbidity index, patients with an index >12 and 9 to 12 had significantly worse survival at all time points than those with Liu comorbidity index≤8 (Table 2; Figure 2). However, even for the group with Liu comorbidity index≤8, three-year survival was poor at 58.8% (53.9 – 63.4%).
Table 2:
Survival estimate for groups on the basis of Liu score
| Liu Score Category |
Survival Estimates, (%) | ||
|---|---|---|---|
| One-Year | Two-Year | Three-Year | |
| ≤ 8 | 87.7 (84.1 – 90.5) | 73.7 (69.2 – 77.7) | 58.8 (53.9 – 63.4) |
| 9 to 12 | 80.3 (77.0 – 83.2)* | 65.1 (61.2 – 68.7)* | 50.4 (46.4 – 54.3)* |
| >12 | 71.1 (67.7 – 74.2)* | 52.2 (48.5 – 55.8)* | 35.7 (32.2 – 39.2)* |
p<0.05
Figure 2:
Kaplan-Meier survival curves for patients grouped by Liu comorbidity index
Cox-proportional hazards analysis generated a multivariate model, presented in Table 3, demonstrating factors independently associated with worse survival. Notably, age above 80 (aHR=2.49, 95% CI [1.87-3.33], p<0.001), CHF (aHR=1.31, 95% CI [1.14-1.51], p<0.001) and Liu comorbidity index > 12 (aHR=1.89, 95% C.I. [1.56 – 2.28], p<0.001) were associated with worse survival.
Table 3:
Cox-proportional hazards model for three-year survival after carotid endarterectomy
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR [95% C.I.] | P-value | aHR [95% C.I.] | P-value | |
| Age at operation | ||||
| Less than 60 | 1 | Ref | 1 | Ref |
| 60 to 80 | 1.58 [1.26 – 1.98] | <0.001* | 1.55 [1.21 – 1.99] | 0.001* |
| More than 80 | 2.66 [2.05 – 3.46] | <0.001* | 2.49 [1.87 – 3.33] | <0.001* |
| Male | ||||
| Race | 1.01 [0.89 – 1.15] | 0.882 | ||
| White | 1 | Ref | 1 | Ref |
| Black | 0.83 [0.68 – 1.02] | 0.078 | 0.86 [0.69 – 1.07] | 0.181 |
| Other | 1.10 [0.78 – 1.56] | 0.586 | ||
| Hispanic ethnicity | 0.82 [0.66 – 1.02] | 0.08 | ||
| Body mass index > 30 | 0.90 [0.79 – 1.03] | 0.12 | ||
| Tobacco use | 1.06 [0.86 – 1.13] | 0.574 | ||
| Congestive heart failure | 1.50 [1.32 – 1.71] | <0.001* | 1.31 [1.14 – 1.51] | <0.001* |
| Atherosclerotic heart disease | 1.26 [1.09 – 1.45] | 0.001* | ||
| Peripheral vascular disease | 1.26 [1.09 – 1.45] | 0.002* | ||
| Hypertension | 1.13 [0.90 – 1.41] | 0.301 | ||
| Diabetes | 0.99 [0.86 – 1.13] | 0.842 | ||
| Amputation | 1.40 [1.02 – 1.93] | 0.036* | ||
| COPD | 1.28 [1.08 – 1.53] | 0.006* | ||
| Functional dependence | 1.30 [1.05 – 1.62] | 0.015* | ||
| Institutional care | 1.69 [1.24 – 2.31] | 0.001* | 1.44 [1.03 – 2.03] | 0.033* |
| Nephrology care before dialysis initiation | 1.21 [1.03 – 1.43] | 0.02* | 1.21 [1.02 – 1.43] | 0.026* |
| AV Access used for dialysis at initiation | 0.97 [0.83 – 1.14] | 0.743 | ||
| Liu comorbidity score | ||||
| ≤ 8 | 1 | Ref | 1 | Ref |
| 9 to 12 | 1.32 [1.09 – 1.59] | 0.004* | 1.19 [0.97 – 1.46] | 0.092 |
| >12 | 1.99 [1.67 – 2.37] | <0.001* | 1.89 [1.56 – 2.28] | <0.001* |
Abbreviations: aHR – adjusted hazards ratio; CI – confidence interval; COPD – chronic obstructive pulmonary disease; HR – hazards ratio
3.3. Novel Risk Score Generation and Survival Estimates for Patients Stratified by Risk Score
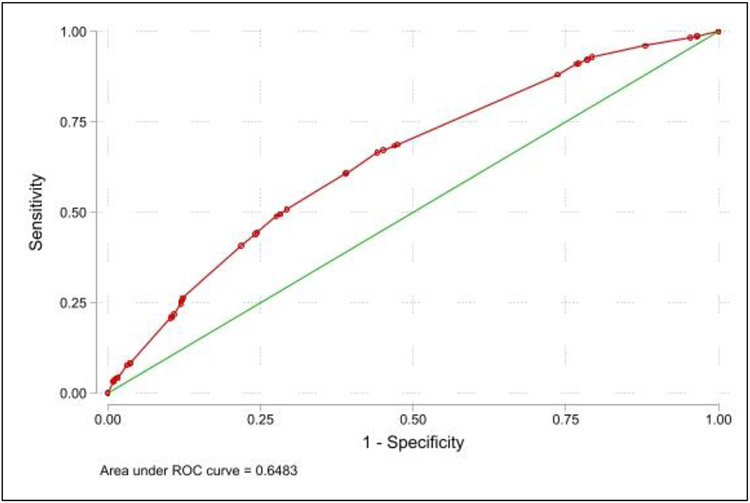
The logistic regression model for three-year survival is demonstrated in Table 4. The model had an area under the reviewer-operative curve of 0.648, demonstrating poor predictive ability (Figure 3). The hazards ratios from the Cox model were used to create a risk scoring system by dividing the effect sizes of each variable by the smallest effect size in the model i.e nephrology care before dialysis initiation: aHR=1.21, 95% C.I. [1.02 – 1.43]. The presence of a risk factor would contribute 1, 1.5 or 2 points as per the model created.
Table 4:
Risk model generated based on factors independently associated with survival, and associated logistic regression model
| Variable | Multivariate Cox: aHR [95% C.I.] |
Logistic Regression Model, aOR [95% C.I.] |
Points Assigned |
|---|---|---|---|
| Age at operation: 60 to 80 | 1.55 [1.21 – 1.99] | 1.82 [1.33 – 2.50] | +1 |
| Age at operation: More than 80 | 2.49 [1.87 – 3.33] | 3.22 [2.13 – 4.83] | +2 |
| Congestive heart failure | 1.31 [1.14 – 1.51] | 1.45 [1.17 – 1.80] | +1 |
| Institutional care | 1.44 [1.03 – 2.03] | 1.40 [0.78 – 2.53] | +1 |
| Nephrology care before dialysis initiation | 1.21 [1.02 – 1.43] | 1.48 [1.17 – 1.87] | +1 |
| Liu comorbidity score: >12 | 1.89 [1.56 – 2.28] | 1.97 [1.60 – 2.43] | +1.5 |
Abbreviations: aHR – adjusted hazards ratio; aOR – adjusted odds ratio; CI – confidence interval;
Figure 3:
Reviewer-operating curve for logistic regression model predicting three-year survival in the patient cohort
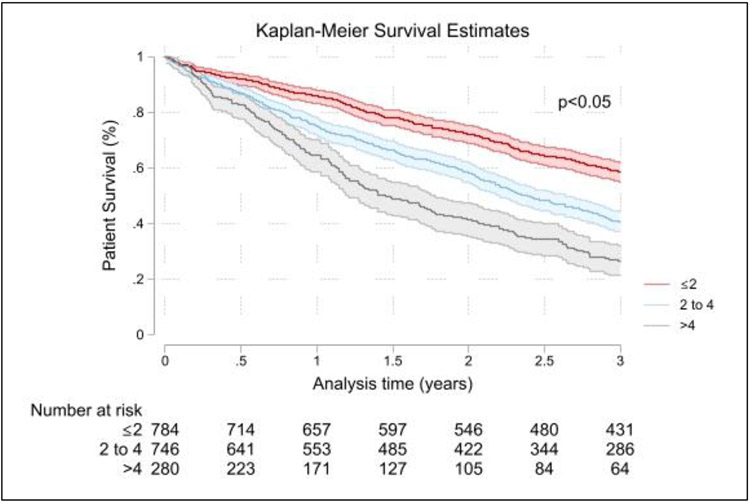
The risk score ranged from minimum 0 to a maximum of 6.5, and the mean and median risk score in the cohort was 2.70±1.23 and 2.5(2.0 – 3.5), respectively. Patients were grouped into groups based on their risk score i.e score ≤2 (43.4%), 2 to 4 (41.2%), and >4 (15.4%). Survival estimates at one, two and three years for these patients are summarized in Table 5 and Figure 4. Patients with a total risk score > 4 and between 2 to 4, had worse survival at all time points than patients with total score ≤2. Even in the “low-risk” group, overall three-year survival was 58.5 (54.9 – 61.9) %.
Table 5:
Survival estimate for groups on the basis of risk score categorization.
| Risk Score Category |
Survival Estimates, (%) | ||
|---|---|---|---|
| One-Year | Two-Year | Three-Year | |
| ≤ 2 | 85.7 (83.1 – 88.0) | 72.1 (68.8 – 75.1) | 58.5 (54.9 – 61.9) |
| 2 to 4 | 75.1 (71.8 – 78.1)* | 58.2 (54.6 – 61.7)* | 40.6 (37.0 – 44.1)* |
| >4 | 64.7 (58.7 – 70.1)* | 41.3 (35.3 – 47.2)* | 26.4 (21.2 – 31.9)* |
p<0.05
Figure 4:
Kaplan-Meier survival curves for patients stratified by novel risk score
3.4. Sub-Analysis for Patients with Available Medicare Part D Data
Of the entire cohort of 1,813 patients, 1,249 (68.8%) had available Medicare part D data with mean and median follow-up of 34.6±23.9 months and 32.7 months (IQR 14.0 – 50.4 months), respectively. Patient characteristics between the complete cohort and the cohort with part D data were compared in Supplementary Table 4. Patients with part D data were likely to be younger (68.9±9.1 years vs 70.2±90.1 years) at the time of CEA, less likely to be male (54.5% vs 59.3%, p=0.008) and more likely to be of Hispanic ethnicity (13.5% vs 10.6%, p=0.018). The distribution of comorbid cardiovascular conditions was similar between both groups, except obesity which was more prevalent among patients with part D data (58.2% vs 39.4%, p<0.001). Overall, the Liu comorbidity index was also higher in patients with part D data (11.8±3.6 vs 11.2±3.8, p<0.001).
Medication usage in the patient cohort was as follows: 29.1% ACEi - ARBs, 28.1% antiplatelet, 29.5% beta-blocker, 31.9% CCB, 17.0% diuretic and 56.0% statin. Survival analysis demonstrated improved survival at three years with CCBs (56.8% on CCB vs 44.9% not on CCB, p<0.001) and statins (52.3% on statins vs 43.4% not on statins, p<0.001) on unadjusted analysis. The protective effect remains significant on adjusted analysis (CCB: aHR=0.76, [0.64-0.91], p=0.003; statin: aHR=0.74, [0.64–0.88], p<0.001). Of note, antiplatelet use was not associated with improved survival (45.1% on drug vs 49.8% not on drug; aHR=1.09, [0.92 – 1.29], p=0.243). These data are presented in Table 6.
Table 6.
Survivor estimates, univariate, and multivariate time-to-event analyses for three-year survival
| Medication | Survivor estimates | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| On drug,% (95 C.I.) | Not on drug% (95 C.I.) | P-value | HR | 95% CI | P-value | aHR | 95% CI | P-value | |
| ACEi/ARB | 49.4 (44.1 - 54.5) | 48.0 (44.6 - 51.4) | 0.684 | 0.96 | (0.81 - 1.15) | 0.684 | 1.02 | (0.86 - 1.22) | 0.803 |
| Antiplatelet | 45.1 (39.8 - 50.3) | 49.8 (46.4 - 53.0) | 0.243 | 1.11 | (0.93 - 1.31) | 0.243 | 1.09 | (0.92 - 1.29) | 0.343 |
| Beta blocker | 48.3 (43.0 - 53.3) | 48.5 (45.1 - 51.8) | 0.704 | 0.97 | (0.81 - 1.15) | 0.704 | 0.94 | (0.79 - 1.12) | 0.508 |
| CCB | 56.8 (51.7 - 61.6) | 44.5 (41.1 - 47.9) | <0.001 | 0.72 | (0.61 - 0.86) | <0.001* | 0.76 | (0.64 - 0.91) | 0.003* |
| Diuretic | 49.9 (42.8 - 56.6) | 48.1 (45.0 - 51.2) | 0.692 | 0.96 | (0.77 - 1.18) | 0.692 | 0.96 | (0.77 - 1.19) | 0.728 |
| Statin | 52.3 (48.5 - 56.0) | 43.4 (39.1 - 47.6) | <0.001 | 0.77 | (0.66 - 0.91) | 0.001* | 0.75 | (0.64 - 0.88) | <0.001* |
Abbreviations: ACE – Angiotensin converting enzyme, ARB – Angiotensin receptor blocker, aHR – adjusted hazard ratio; CCB – calcium channel blocker; CI – confidence interval; HR – hazard ratio
p<0.05
4. DISCUSSION
Using the USRDS, we explored factors associated with survival in ESKD patients with asymptomatic carotid artery disease undergoing CEA. Using the established Liu comorbidity index and a novel risk score generated using our dataset, we demonstrated that even in patients with minimal risk factors, long-term survival is poor. Second, we analyzed postoperative medical management in a subset of this cohort and found a protective role of statins and CCBs against mortality. However even in patients on the aforementioned drugs, we observed low rates of three-year survival. Our findings demonstrate that a CEA may not benefit any subgroup of ESKD patients, despite risk stratification or postoperative management.
Current guidelines recommend CEA for asymptomatic carotid disease only for patients expected to live at least three to five years and have a low risk for perioperative strokes.4 As in the general surgical patient population, impaired renal function predicts lower survival in carotid revascularization patients.15 Hence, the intended benefits of a CEA, which aims to prevent strokes over the remaining life expectancy, may not be realized in ESKD patients.7 Nonetheless, national trends demonstrate that many patients with renal insufficiency receive carotid revascularization annually, a large proportion of whom are asymptomatic. Adil et al. report that only 6.9% of operations captured by the National Inpatient Sample were performed for symptomatic patients.8 Our findings and those of Yuo et al. suggest that nearly half of the operative candidates are symptomatic.7 Despite recommendations by multiple guidelines, published trends indicate that “high-risk” asymptomatic patients are frequently taken for CEA.4,5,16
Risk stratification is universally key to improving patient outcomes for moderate-risk and high-risk operations. Retrospective series provide valuable information regarding risk factors for mortality and adverse events that may be used to guide clinical decision making. Given that the Liu comorbidity index has been created specifically for patients on dialysis, we explored application of this score in our cohort.13 While it has previously been shown to be associated with survival, we were unable to identify a cohort with adequate survival for three years postoperatively.17 The factors that were incorporated into our risk model have previously been associated with poor survival as well, such as increased age and need for nursing home care.6,7 Congestive heart failure is similarly well-documented as a life-limiting condition, and has been noted to triple the risk of perioperative adverse events after CEA.9 A 2016 publication using the Medicare-linked Vascular Quality Initiative data stated that the large majority of patients receiving CEA for asymptomatic carotid artery stenosis was justified on the basis of adequate postoperative survival, and identified a small high-risk group. Notably, the majority of this group had impaired renal function (87.2% of 39 patients included) and showed high two-year mortality of 44% and significantly higher costs to the healthcare system.18 Our efforts to further stratify among dialysis patients and identify potential candidates for CEA demonstrate that the overall mortality in this patient population should be prohibitive to an operative intervention. Other approaches such as medical management or trans-carotid stent placement, may better serve these patients.19
Antiplatelet and statin therapy and control of hypertension and diabetes are recommended for all patients after CEA to ensure the benefits of the operation.4 Medical management of ESKD patients is complicated by altered pharmacodynamics, the impact of renal replacement sessions, polypharmacy to manage multiple co-existing comorbid conditions, and high potential for drug-drug interactions.10,20 Antiplatelet use must be considered cautiously as ESKD predisposes patients to thrombosis and bleeding. Moreover, earlier analysis of the dialysis population suggests that these medications are not associated with the survival benefit observed in the general population.21 Our findings also show that the benefits of antiplatelet therapy are limited after CEA in dialysis patients. Even the role of statin therapy is debatable. Statins are recommended for patients with vascular disease to slow the progression of cardiovascular disease, but requiring renal replacement indicates an irreversible disease severity. There are multiple retrospective analyses from large databases that explore their use. Sung et al. and Huang et al. in their study of patients with ESKD and advanced CKD, suggest that statins provide a survival benefit.22,23 However, Cheng et al. show that there is no advantage associated with this practice.24 Currently, statin use is prevalent as there is no high-quality evidence to modify the current recommendations for this patient population.11 A strength of our study is the ability to capture medication utilization, however, our methods may capture a patient population with better health-seeking behaviors. Additionally, our study demonstrates that medication compliance remains low in this patient population, however this may also be representative of provider variation in prescriptions.
Antihypertensives are underutilized in ESKD patients, with beta-blockers and CCBs being used commonly.25 The benefits of CCBs in the ESKD population have also been noted in earlier publications. Fuji et al. utilized prospective data from patients initiating dialysis and demonstrated that CCB use is protective against all-cause and cardiovascular-cause mortality, an effect compounded with simultaneous use of a renin-angiotensin system blocker.26 Tanaka et al. did not find any such difference in mortality between patients using CCBs and those not.27 The benefits of CCBs observed may be attributable to more consistent blood pressure control than ARBs.28 However, in our cohort, even with compliance, groups using calcium channel blockers or statins consistently had three-year survival estimates less than 60%. Hence, these results suggest that the survival in these patients remains poor, despite medical therapy, and as such the preventive benefits of CEA may not be realized.
Our study has limitations. The USRDS is an administrative database generated from claims and data entry at critical points such as the first dialysis event. Inconsistencies in data entry or inaccuracies in the entry of CPT and ICD codes may affect the validity of our findings. Anatomical information about carotid disease is unavailable in this dataset. Therefore, we cannot comment on essential aspects of the operation such as degree of stenosis, operation duration, and other nuances in operative technique. It is entirely possible that incorporation of these variables in our models may allow for better identification of good candidates for operation, however this would be a topic for future studies with more granular data. Thirdly, administrative data do not capture the reasons for the prescriptions being provided. Additionally, aspirin use is not captured using part D claims data since this medication is easily available over the counter and at a low cost, that it may not be represented in billing claims, hence antiplatelet use represents use of non-aspirin antiplatelets. Lastly our study only analyses mortality whereas quality of life may also play a role in the patient’s choice for operative intervention. A primary strength of our study is that a large number of operative patients on dialysis were analyzed and had robust follow-up, for both mortality and medication utilization.
5. CONCLUSIONS
The overall long-term survival of ESKD patients undergoing CEA for asymptomatic carotid artery disease is low. Risk stratification and analysis of postoperative medical management did not identify a subgroup of patients with adequate three-year survival. Hence, the preventive benefits of CEA are not realized in these patients, and they may be better served with non-operative management. Further studies are needed with granular data regarding anatomical and operative variables to potentially identify a subgroup where the benefits may be realized.
Supplementary Material
HIGHLIGHTS.
We explored the role of risk stratification and medical management of end-stage kidney disease patients undergoing carotid endarterectomy for asymptomatic carotid artery disease.
Using the validated Liu comorbidity index, we found even patients with Liu index<8 had poor three-year survival.
Using a novel risk model, patient survival was poor even in the low-risk group.
Postoperative statin and calcium channel blocker use was associated with improved survival.
We were unable to identify good-risk patients with adequate three-year survival, suggesting that endarterectomy for asymptomatic disease is not suitable in the ESKD population.
ACKNOWLEDGEMENTS:
We have no pertinent acknowledgments
Funding Acknowledgement:
This research was supported by a start-up grant from the University of Pittsburgh awarded to Dr Theodore Yuo MD MSc. The funding source did not play a role in the study design, data analysis, presentation of results, or manuscript preparation.
ABBREVIATIONS
- ACEi-ARBs
Angiotensin-converting enzyme inhibitors/ angiotensin receptor blockers
- aHR
adjusted hazards ratio
- CCB
calcium channel blocker
- CEA
carotid endarterectomy
- CHF
congestive heart failure
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CPT
Current Procedural Terminology ®
- ESKD
end-stage kidney disease
- ICD
International Classification of Diseases
- MPR
medicine possession ratio
- PVD
peripheral vascular disease
- USRDS
United States Renal Data System
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflicting Interests: The authors declare that there is no conflict of interest.
Presentation Information: This study was presented in the Poster Competition at 2022 Eastern Vascular Society in Philadelphia, PA (29th September to 1st October, 2022)
REFERENCES
- 1.Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, et al. The North American Symptomatic Carotid Endarterectomy Trial : surgical results in 1415 patients. Stroke. 1999. Sep;30(9):1751–8. [DOI] [PubMed] [Google Scholar]
- 2.Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus Endarterectomy for Treatment of Carotid-Artery Stenosis. New England Journal of Medicine. 2010. Jul 1;363(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995. May 10;273(18):1421–8. [PubMed] [Google Scholar]
- 4.Naylor AR, Rantner B, Ancetti S, de Borst GJ, De Carlo M, Halliday A, et al. European society for vascular surgery (ESVS) 2023 Clinical practice guidelines on the management of atherosclerotic carotid and vertebral artery disease. Eur J Vasc Endovasc Surg. 2022. May 19;S1078-5884(22)00237–4. [DOI] [PubMed] [Google Scholar]
- 5.AbuRahma AF, Avgerinos ED, Chang RW, Darling RC, Duncan AA, Forbes TL, et al. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J Vasc Surg. 2022. Jan;75(1S):4S–22S. [DOI] [PubMed] [Google Scholar]
- 6.Cooper M, Arhuidese IJ, Obeid T, Hicks CW, Canner J, Malas MB. Perioperative and Long-term Outcomes After Carotid Endarterectomy in Hemodialysis Patients. JAMA Surg. 2016. Oct 1;151(10):947–52. [DOI] [PubMed] [Google Scholar]
- 7.Yuo TH, Sidaoui J, Marone LK, Makaroun MS, Chaer RA. Revascularization of asymptomatic carotid stenosis is not appropriate in patients on dialysis. J Vasc Surg. 2015. Mar;61(3):670–4. [DOI] [PubMed] [Google Scholar]
- 8.Adil MM, Saeed F, Chaudhary SA, Malik A, Qureshi AI. Comparative Outcomes of Carotid Artery Stent Placement and Carotid Endarterectomy in Patients with Chronic Kidney Disease and End-Stage Renal Disease. J Stroke Cerebrovasc Dis. 2016. Jul;25(7):1721–7. [DOI] [PubMed] [Google Scholar]
- 9.Paraskevas KI, Gloviczki P. Prognostic factors of long-term survival to guide selection of asymptomatic patients for carotid endarterectomy. Int Angiol. 2020. Feb;39(1):29–36. [DOI] [PubMed] [Google Scholar]
- 10.Weir MR, Fink JC. Safety of medical therapy in patients with chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2014. May;23(3):306–13. [DOI] [PubMed] [Google Scholar]
- 11.Laufs U, Custodis F, Böhm M. Who does not need a statin: too late in end-stage renal disease or heart failure? Heart. 2008. Sep;94(9):1138–40. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Renal Data System. 2021 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2021.; [Google Scholar]
- 13.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010. Jan;77(2):141–51. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs K, Julyan M, Lubbe MS, Burger JR, Cockeran M. Medicine possession ratio as proxy for adherence to antiepileptic drugs: prevalence, associations, and cost implications. Patient Prefer Adherence. 2016;10:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AbuRahma AF, Srivastava M, Stone PA, Chong B, Jackson W, Dean LS, et al. The effect of chronic renal insufficiency by use of glomerular filtration rate versus serum creatinine level on late clinical outcome of carotid endarterectomy. J Vasc Surg. 2015. Mar;61(3):675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonati LH, Kakkos S, Berkefeld J, de Borst GJ, Bulbulia R, Halliday A, et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. European Stroke Journal. 2021. Jun 1;6(2):I–XLVII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuğcu M, Kasapoğlu U, Şahin G, Apaydın S. The Factors Affecting Survival in Geriatric Hemodialysis Patients. Int J Nephrol. 2018;2018:5769762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallaert JB, Newhall KA, Suckow BD, Brooke BS, Zhang M, Farber AE, et al. Relationships between 2-Year Survival, Costs, and Outcomes following Carotid Endarterectomy in Asymptomatic Patients in the Vascular Quality Initiative. Ann Vasc Surg. 2016. Aug;35:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsayed N, Vasudevan RS, Zarrintan S, Barleben A, Kashyap VS, Malas MB. TransCarotid Artery Revascularization Can Be Safely Performed in Patients Undergoing Dialysis. Ann Vasc Surg. 2023. May;92:57–64. [DOI] [PubMed] [Google Scholar]
- 20.Ponchia PI, Ahmed R, Farag M, Alkhalil M. Antiplatelet Therapy in End-stage Renal Disease Patients on Maintenance Dialysis: a State-of-the-art Review. Cardiovasc Drugs Ther. 2022. Jul 22; [DOI] [PubMed] [Google Scholar]
- 21.Trespalacios FC, Taylor AJ, Agodoa LY, Abbott KC. Incident acute coronary syndromes in chronic dialysis patients in the United States. Kidney Int. 2002. Nov;62(5):1799–805. [DOI] [PubMed] [Google Scholar]
- 22.Sung FC, Jong YC, Muo CH, Hsu CC, Tsai WC, Hsu YH. Statin Therapy for Hyperlipidemic Patients With Chronic Kidney Disease and End-Stage Renal Disease: A Retrospective Cohort Study Based on 925,418 Adults in Taiwan. Front Pharmacol. 2022;13:815882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang TM, Wu VC, Lin YF, Wang JJ, Shiao CC, Chen L, et al. Effects of Statin Use in Advanced Chronic Kidney Disease Patients. J Clin Med. 2018. Sep 17;7(9):E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng YL, Yang HY, Wu CY, Tsai CY, Chen CY, Hsiao CC, et al. Does Statin Therapy Reduce the Risks of Mortality and Major Adverse Cardiac and Cerebrovascular Events in Young Adults with End-Stage Renal Disease? Population-Based Cohort Study. J Clin Med. 2021. May 13;10(10):2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang TI, Zheng Y, Montez-Rath ME, Winkelmayer WC. Antihypertensive Medication Use in Older Patients Transitioning from Chronic Kidney Disease to End-Stage Renal Disease on Dialysis. Clin J Am Soc Nephrol. 2016. Aug 8;11(8):1401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujii M, Inaguma D, Koide S, Ito E, Takahashi K, Hayashi H, et al. Relationship Between Patterns in Antihypertensive Drugs Medication and Mortality in Incident Dialysis Patients: A Multicenter Prospective Cohort Study. Ther Apher Dial. 2019. Aug;23(4):353–61. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Yamashita T, Koyama M, Moniwa N, Ohno K, Mitsumata K, et al. Impact of use of angiotensin II receptor blocker on all-cause mortality in hemodialysis patients: prospective cohort study using a propensity-score analysis. Clin Exp Nephrol. 2016. Jun;20(3):469–78. [DOI] [PubMed] [Google Scholar]
- 28.Takenaka T, Sueyoshi K, Arai J, Watanabe Y, Takane H, Ohno Y, et al. Calcium channel blockers suppress daily variations of blood pressure in hypertensive patients with end-stage renal diseases. Clin Exp Hypertens. 2014;36(2):78–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.