Abstract
Background
Constipation has been recently recognized as a complication associated with motor and autonomic dysfunction in patients with motor neuron disease (MND), typified by amyotrophic lateral sclerosis (ALS). However, the long-term characteristics of constipation remain unclear in patients with MND. We longitudinally investigated the prevalence and risk factors of constipation in a consecutive cohort of patients with MND.
Methods
Data from Japanese patients with MND enrolled in a single-center registry from June 2017 to December 2021 were retrospectively investigated. The diagnosis of ALS was based on the updated Awaji criteria, and other MND subtypes were also included. The presence or absence of constipation symptoms was determined by referring to the Rome III criteria. The clinical backgrounds and symptoms of patients with and without constipation were compared.
Results
Among 155 consecutive patients (female, 63; age, 66.5 ± 12.4 years), 30.3% had constipation at diagnosis and 52.9% after a median follow-up of 18 months. Univariate analysis showed that female sex, use of tracheostomy and invasive ventilation, and delivery of enteral nutrition were more frequent in the constipation group. The Revised Amyotrophic Lateral Sclerosis Functional Rating Scale score was significantly lower in the constipation group, especially for the sub-items related to physical motor function. Multivariate analysis showed that the use of enteral nutrition was an independent risk of constipation, with an odds ratio of 3.69 (95% CI, 1.49–9.17; p = 0.005).
Conclusion
Constipation had a high prevalence in patients with MND with impaired motor function. Controlling defecation is important in patients with MND, especially during enteral nutrition.
Keywords: Amyotrophic lateral sclerosis, Constipation, Enteral nutrition, Motor neuron disease
1. Introduction
Motor neuron disease (MND), mainly represented by amyotrophic lateral sclerosis (ALS), is a neurodegenerative disease that predominantly affects upper motor neurons, lower motor neurons, or both, usually resulting in generalized muscle weakness [1]. Survival is usually limited to 3–5 years mainly due to respiratory failure, while respiratory support with tracheostomy and invasive ventilation (TIV) leads to long-term survival [2]. Although traditionally under-recognized, non-motor symptoms have now been associated with ALS [3,4]. In ALS patients with TIV, non-motor manifestations occurred more frequently with longer periods of TIV or with more severe communication impairment [5]. Of the non-motor features, autonomic symptoms such as urinary urgency, frequent urination [6], and gastrointestinal disturbances are common. Bowel problems can cause tremendous anxiety and distress and reduce the quality of life in patients with neurodegenerative diseases [7]. The prevalence of constipation, the major gastrointestinal issue, ranges from 46.0 to 68.3% in patients with ALS [[8], [9], [10]]. Age, family history, total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) score, site of onset, presence of depression, and sleep disorders have been associated with constipation risk in patients with ALS [10]. However, previous reports have been limited by the relatively small number of cases and scarcity of information on longitudinal changes. Thus, we aimed to investigate the prevalence and risk factors of constipation in patients with MND with long-term survival via longitudinal analyses.
2. Methods
2.1. Study design and data collection
We retrospectively examined 155 consecutive patients diagnosed with ALS and other atypical variants of MND at Tokushima University Hospital from June 2017 to December 2021. We analyzed the medical information at the time of diagnosis. For cases for which follow-up was possible, we referred to the most recent medical information. ALS diagnoses were issued by board-certified neurologists based on the updated Awaji criteria [11], and patients with definite, probable, probable-laboratory supported, and possible ALS were included. Cases with progressive muscular atrophy, bulbar ALS, flail arm syndrome, or flail leg syndrome were also included. Disease severity in patients was evaluated via ALSFRS-R [12,13]. Patients who reported at least one of the following symptoms with reference to the Rome III criteria [14], that is, habitual use of constipation drugs, use of manual maneuvers, and <3 bowel movements/week, were judged to have constipation symptoms. Medical information on the types of administered constipation drugs was collected retrospectively. We compared the clinical characteristics, symptom onset of ALS, and ALSFRS-R contents between the constipation and non-constipation groups at the final follow-up. The onset types were defined by the sites that were dominantly impaired at the early stage of the disease and were divided into spinal versus bulbar types. Patients were also classified by clinical phenotypes (classic, bulbar, flail arm, flail leg, predominantly upper motor neuron [PUMN], respiratory failure, and dropped neck/trunk type) based on established ALS phenotypes [15].
2.2. Statistical analyses
The results are expressed as means ± standard deviations or median and inter-quartile range (IQR) for quantitative variables and as counts and percentages for categorical variables. Univariate analyses were performed with the Student's t-test and Mann–Whitney U test for continuous variables with normal or non-normal distribution, respectively, and the χ2 test for categorical variables. All statistical tests were 2-sided, and the significance (p) level was set at 0.05. We used a logistic regression model in multivariate analyses, and variables with p-values less than 0.05 from the univariate analysis were included. The results of the multivariate analysis were expressed as adjusted odds ratios and 95% confidence intervals (CI). All statistical analyses were performed with SPSS 26.0 (IBM, Armonk, New York).
3. Results
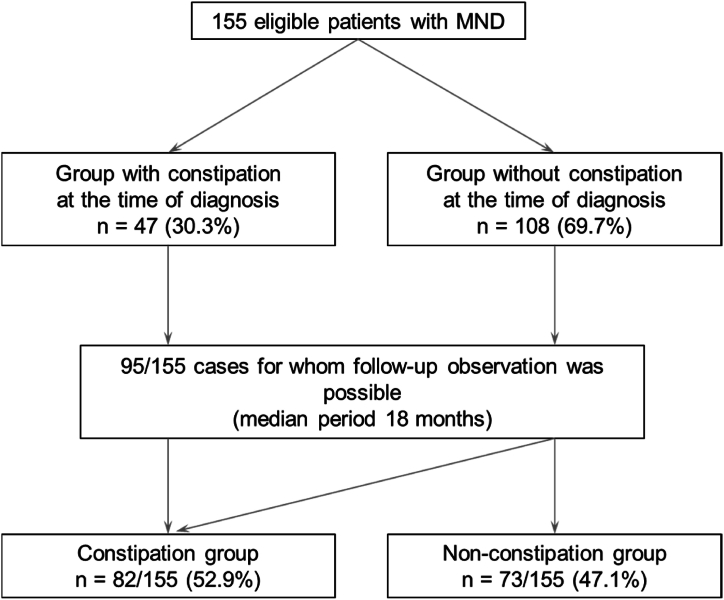
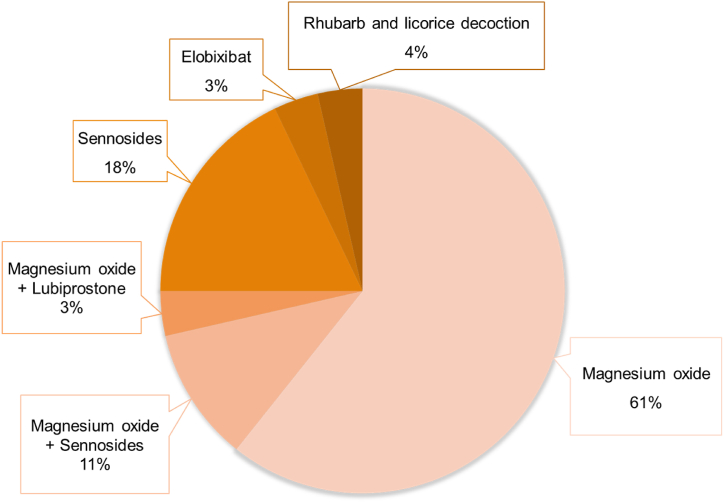
One hundred and fifty-five patients (female, 63; age at onset, 64.9 ± 12.8 years) were enrolled according to the inclusion criteria and their data were analyzed. Constipation symptoms were present in 30.3% (47/155) of the patients at the time of MND diagnosis. 95/155 patients were followed up for a median duration of 18 months, and 52.9% (82/155) eventually had constipation symptoms (defined as the “constipation group”, Fig. 1). The baseline characteristics of the patients are summarized in Table 1. Comparing the constipation and non-constipation groups, female sex (40.8% versus 31.5%; p = 0.029), use of tracheostomy and invasive ventilation (TIV) (17.1% versus 4.1%; p = 0.010), and enteral nutrition (40.2% versus 13.7%; p < 0.001) were more frequent in the constipation group. The most common sites of onset were upper limb (36.8%), others (27.7%), and the bulbar region (22.6%). There was no significant association between onset site and prevalence of constipation. Overall, the ALSFRS-R score was significantly lower in the constipation than in the non-constipation group (36.61 versus 39.88; p = 0.027). In the ALSFRS-R sub-items, the constipation group had lower scores for the following items related to physical motor function: cutting food and handling utensils, dressing and hygiene, turning in bed and adjusting bed clothes, walking, and climbing stairs. The multivariate analysis included variables with significant differences in the univariate analysis (Table 2). Only the use of enteral nutrition was significantly associated with constipation in patients with MND, with an odds ratio of 3.69 (95% CI, 1.49–9.17; p = 0.005). Twenty-eight patients with constipation were interviewed regarding their medication (Fig. 2). Magnesium oxide was prescribed to 75% of the patients, followed by sennosides to 29%.
Fig. 1.
Study flowchart Abbreviations: MND, motor neuron disease.
Table 1.
Demographic and clinical characteristics of patients with MND.
| Total |
Constipation group |
Non- constipation group |
p value | |
|---|---|---|---|---|
| n = 155 | n = 82 | n = 73 | ||
| Clinical characteristics | ||||
| Age at onset, years | 64.9 ± 12.8 | 65.2 ± 14.3 | 64.5 ± 11.1 | 0.728 |
| Age at diagnosis, years | 66.5 ± 12.4 | 67.1 ± 13.7 | 65.9 ± 11.0 | 0.545 |
| Sex, female | 63 (40.6%) | 40 (48.8%) | 23 (31.5%) | 0.029* |
| Follow-up period, months (IQR) | 18 (9–30.5) | 19 (10–32) | 17 (8.75–29) | 0.269 |
| Family history with MND | 4 (2.6%) | 2 (2.4%) | 2 (2.7%) | 1.000 |
| Smoking | 84 (54.2%) | 42 (51.2%) | 42 (57.5%) | 0.431 |
| Trauma history | 87 (56.1%) | 46 (56.1%) | 41 (56.2%) | 0.993 |
| Sports history | 115 (74.2%) | 55 (67.1%) | 59 (80.8%) | 0.053 |
| Surgical history | 100 (64.5%) | 55 (67.1%) | 45 (61.6%) | 0.481 |
| NPPV | 27 (17.4%) | 16 (19.5%) | 8 (11.0%) | 0.142 |
| TIV | 17 (11.0%) | 14 (17.1%) | 3 (4.1%) | 0.010* |
| Enteral nutrition | 43 (27.7%) | 33 (40.2%) | 10 (13.7%) | <0.001* |
| Death | 25 (16.1%) | 11 (13.4%) | 14 (19.2%) | 0.330 |
| Onset types | 0.850 | |||
| Spinal | 120 (77.4%) | 64 (78.0%) | 56 (76.7%) | |
| Bulbar | 35 (22.6%) | 18 (22.0%) | 17 (23.3%) | |
| Clinical phenotypes | 0.919 | |||
| Classic | 76 (49.0%) | 42 (51.2%) | 34 (46.6%) | |
| Bulbar | 35 (22.6%) | 18 (22.0%) | 17 (23.3%) | |
| Flail arm | 18 (11.6%) | 7 (8.5%) | 11 (15.1%) | |
| Flail leg | 10 (6.5%) | 6 (7.3%) | 4 (5.5%) | |
| PUMN | 4 (2.6%) | 2 (2.4%) | 2 (2.7%) | |
| Respiratory failure | 2 (1.3%) | 1 (1.2%) | 1 (1.4%) | |
| Dropped neck/trunk type | 10 (6.5%) | 6 (7.3%) | 4 (5.5%) | |
| ALSFRS-R at diagnosis | ||||
| Speech | 3.06 ± 1.10 | 3.26 ± 1.10 | 0.261 | |
| Salvation | 3.41 ± 1.01 | 3.45 ± 0.99 | 0.816 | |
| Swallowing | 3.16 ± 1.02 | 3.22 ± 1.06 | 0.718 | |
| Handwriting | 3.09 ± 1.08 | 3.30 ± 0.91 | 0.183 | |
| Cutting food and handling utensils | 2.50 ± 1.39 | 3.05 ± 1.24 | 0.009* | |
| Dressing and hygiene | 2.37 ± 1.21 | 2.74 ± 1.08 | 0.045* | |
| Turning in bed and adjusting bed clothes | 2.90 ± 1.21 | 3.33 ± 1.03 | 0.020* | |
| Walking | 2.87 ± 1.11 | 3.38 ± 0.89 | 0.002* | |
| Climbing stairs | 2.15 ± 1.57 | 2.89 ± 1.37 | 0.002* | |
| Dyspnea | 3.63 ± 1.06 | 3.66 ± 1.02 | 0.889 | |
| Orthopnea | 3.71 ± 0.94 | 3.79 ± 0.83 | 0.543 | |
| Respiratory insufficiency | 3.77 ± 0.87 | 3.79 ± 0.83 | 0.848 | |
| Total | 38.2 ± 9.2 | 36.6 ± 9.3 | 39.9 ± 8.8 | 0.027* |
Abbreviations: ALSFRS-R, the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; IQR: Interquartile range; MND, motor neuron disease; NPPV, non-invasive positive pressure ventilation; PUMN, predominantly upper motor neuron; TIV, tracheostomy and invasive ventilation.
Table 2.
Multivariate analysis of factors predicting constipation in patients with MND.
| Odds ratio | 95% CI | p value | |
|---|---|---|---|
| Sex, female | 1.86 | 0.88–3.91 | 0.105 |
| TIV | 1.48 | 0.33–6.56 | 0.610 |
| Enteral nutrition | 3.69 | 1.49–9.17 | 0.005* |
| Cutting food and handling utensils | 0.70 | 0.45–1.10 | 0.126 |
| Dressing and hygiene | 1.35 | 0.73–2.48 | 0.340 |
| Turning in bed and adjusting bed clothes | 0.93 | 0.55–1.56 | 0.780 |
| Walking | 0.79 | 0.36–1.73 | 0.561 |
| Climbing stairs | 0.87 | 0.55–1.39 | 0.566 |
Abbreviations: CI, confidence interval; MND, motor neuron disease; TIV, tracheostomy and invasive ventilation.
Fig. 2.
Drugs reported to be used to treat constipation in patients with MND (n = 28).
4. Discussion
In this partial longitudinal study of 155 Japanese patients with MND, constipation was present in 30.3% at diagnosis and in 52.9% after a median follow-up of 18 months. Univariate analysis showed a significantly higher percentage of women and more frequent use of TIV and delivery of enteral nutrition in the constipation group. A previous study based on the Rome III criteria reported a prevalence of constipation in the Japanese population of 28.0% [16], which is similar to the prevalence of constipation in patients with MND at the time of diagnosis in our study. Our longitudinal study showed that the final prevalence of constipation was 52.9%, which is higher than that observed at the time of diagnosis. Similarly, Samara et al. reported an increase in constipation from 33% before to 64.7% after the diagnosis of MND in a cohort of 66 patients [17]; these findings indicate that the course of MND influences the prevalence of constipation. The relationship between the onset type of MND and susceptibility to constipation has not yet been clarified. In the literature, limb onset is one of the factors associated with constipation compared to the bulbar onset [10]. Our study found no difference in the prevalence of constipation among onset sites. Conversely, the constipation group had lower ALSFRS-R scores in the items related to limb motor function. This suggests that reduced physical activity increases the risk of constipation, similar to the results of studies in the general population [18].
Constipation symptoms in MND have been thought to occur as a result of prolonged bed rest due to decreased motor function, the use of anticholinergic drugs, and delivery of enteral nutrition [9,10]. In our study, the multivariate analysis revealed that enteral nutrition was an independent risk factor for constipation, with a high odds ratio of 3.69. In a cohort of 30 enterally fed patients with ALS, the prevalence of constipation reached 76.9%, and other abdominal symptoms, such as abdominal pain and diarrhea, were readily observed [19]. Enteral nutrition is an effective way to provide nutrition to patients who have difficulty with oral intake. However, gastrointestinal complications, such as constipation, gastric residue, vomiting, and diarrhea, can occur. In patients with advanced-stage MND who require enteral nutrition, it is necessary to manage constipation, taking measures such as adjusting fluid intake, using fiber-enriched formulas, and administering appropriate pharmacotherapy [20]. There are considerable variations among countries in the use of TIV in patients with MND. The rate of TIV use is 29.3% in Japan [21], which is higher than that in Europe (5–10%) [22,23] and the United States (4–8%) [24,25]. Enteral nutrition is inevitable in patients with long-term MND using TIV and coping with constipation as a complication is important.
In recent years, there have been some reports on autonomic dysfunction in MND. Dubbioso R et al. demonstrated that autonomic symptoms were present in most ALS patients at the time of diagnosis, in a prospective study using autonomic assessment through questionnaires and other functional evaluation [26]. They also showed that the severity of autonomic symptoms progressed over time and was an independent predictor of disease progression, and that urinary complaints were associated with shorter survival. Although their study and ours used different methodologies, our study may support the relationship between gastrointestinal autonomic dysfunction, including constipation, and disease progression of MND. The association between enteral nutrition, which applies to clinical stage 4A of the modified King's classification [27], and constipation by multivariate analysis could be linked to the higher percentage of autonomic symptoms in advanced clinical stages. Further research in the MND population with larger numbers of patients are needed to clarify whether gastrointestinal autonomic dysfunction suggests multiple system neurodegeneration in MND and also influences disease progression.
Although based on information from a limited number of cases, our survey found that magnesium oxide was frequently used in patients with MND. Polyethylene glycol is frequently used for constipation management in Europe and the United States, whereas magnesium oxide is “strongly recommended” in the guidelines and is often used as a first-line drug in Japan [28]. Although magnesium oxide is relatively safe, it can induce hypermagnesemia in patients with renal insufficiency or in those undergoing long-term treatment [29]. Severe hypermagnesemia can lead to respiratory failure, complete cardiac obstruction, and cardiac arrest [30]. In patients with MND, serum creatinine concentration decreases due to skeletal muscle atrophy, making it difficult to measure renal function using creatinine, and other less convenient methods such as serum cystatin C are used. Therefore, patients with MND receiving magnesium oxide are at risk of progressive deterioration of renal function, resulting in hypermagnesemia. To avoid this risk, serum magnesium concentrations should be measured periodically during administration of magnesium oxide. New drugs such as a type-2 chloride channel activator, a guanylate cyclase 2C receptor agonist, and an inhibitor of the ileal bile acid transporter have recently been launched to treat constipation, and their use may be a good option.
This study has several limitations. First, it was a single-center observational study and no healthy control group was included. Second, not all patients in the cohort were observed longitudinally. Prospective studies in more homogeneous populations, excluding other autonomic disfunction such as diabetes mellitus, may help elucidate the association between gastrointestinal autonomic symptoms and disease progression in MND. Third, although the definition of constipation followed the Rome III criteria, it was based on retrospective excerpts from clinical information. Although not used in this study, questionnaires on autonomic symptoms of functional assessment of gastrointestinal disorders may provide a more multifaceted research perspective.
5. Conclusion
Among 155 Japanese patients with MND, approximately 30% had constipation at diagnosis and 53% after a median follow-up of 18 months. Multivariate analysis showed that administration of enteral nutrition was an independent risk factor of constipation, and attention should be paid to defecation control.
Statements
Funding Sources
This study was supported by Grants-in-Aid from the Research Committee of CNS Degenerative Diseases, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health, Labour and Welfare Sciences Research Grants, the Ministry of Health, Labor and Welfare, Japan.
Ethics approval and informed consent
This study complied with the declaration of Helsinki and has been approved by the ethics committee of Tokushima University Hospital (Ethics approval No: S3009-5). Consent for publication was obtained from all participants.
Data availability statement
The dataset supporting the findings of this study is available from the corresponding author upon reasonable and justified request.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Yuki Yamamoto: Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Koji Fujita: Writing – review & editing, Validation. Hiroki Yamazaki: Investigation, Data curation. Shotaro Haji: Methodology, Data curation. Yusuke Osaki: Methodology, Data curation. Yuishin Izumi: Supervision.
Declaration of Competing Interest
None
References
- 1.Brown R.H., Al-Chalabi A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2017;377(2):162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi N., Atsuta N., Yokoi D., Nakamura R., Nakatochi M., Katsuno M., Izumi Y., Kanai K., Hattori N., Taniguchi A., Morita M., Kano O., Shibuya K., Kuwabara S., Suzuki N., Aoki M., Aiba I., Mizoguchi K., Oda M., Kaji R., Sobue G. Prognosis of amyotrophic lateral sclerosis patients undergoing tracheostomy invasive ventilation therapy in Japan. Journal of neurology, neurosurgery, and psychiatry. 2020;91(3):285–290. doi: 10.1136/jnnp-2019-322213. [DOI] [PubMed] [Google Scholar]
- 3.Mahoney C.J., Ahmed R.M., Huynh W., Tu S., Rohrer J.D., Bedlack R.S., Hardiman O., Kiernan M.C. Pathophysiology and treatment of non-motor dysfunction in amyotrophic lateral sclerosis. CNS Drugs. 2021;35(5):483–505. doi: 10.1007/s40263-021-00820-1. [DOI] [PubMed] [Google Scholar]
- 4.Piccione E.A., Sletten D.M., Staff N.P., Low P.A. Autonomic system and amyotrophic lateral sclerosis. Muscle Nerve. 2015;51(5):676–679. doi: 10.1002/mus.24457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakayama Y., Shimizu T., Matsuda C., Haraguchi M., Hayashi K., Mochizuki Y., Nagao M., Kawata A., Isozaki E. Non-motor manifestations in ALS patients with tracheostomy and invasive ventilation. Muscle Nerve. 2018;57(5):735–741. doi: 10.1002/mus.26004. [DOI] [PubMed] [Google Scholar]
- 6.Arlandis S., Vázquez-Costa J.F., Martínez-Cuenca E., Sevilla T., Boronat F., Broseta E. Urodynamic findings in amyotrophic lateral sclerosis patients with lower urinary tract symptoms: results from a pilot study. Neurourol. Urodyn. 2017;36(3):626–631. doi: 10.1002/nau.22976. [DOI] [PubMed] [Google Scholar]
- 7.Coggrave M., Norton C., Cody J.D. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst. Rev. 2014;1 doi: 10.1002/14651858.CD002115.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toepfer M., Folwaczny C., Klauser A., Riepl R.L., Müller-Felber W., Pongratz D. Gastrointestinal dysfunction in amyotrophic lateral sclerosis, Amyotrophic lateral sclerosis and other motor neuron disorders : official publication of the World Federation of Neurology. Research Group on Motor Neuron Diseases. 1999;1(1):15–19. doi: 10.1080/146608299300079484. [DOI] [PubMed] [Google Scholar]
- 9.Nübling G.S., Mie E., Bauer R.M., Hensler M., Lorenzl S., Hapfelmeier A., Irwin D.E., Borasio G.D., Winkler A.S. Increased prevalence of bladder and intestinal dysfunction in amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2014;15(3–4):174–179. doi: 10.3109/21678421.2013.868001. [DOI] [PubMed] [Google Scholar]
- 10.Niu T., Zhou X., Li X., Liu T., Liu Q., Li R., Liu Y., Dong H. Development and validation of a dynamic risk prediction system for constipation in patients with amyotrophic lateral sclerosis. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.1060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geevasinga N., Loy C.T., Menon P., de Carvalho M., Swash M., Schrooten M., Van Damme P., Gawel M., Sonoo M., Higashihara M., Noto Y., Kuwabara S., Kiernan M.C., Macaskill P., Vucic S. Awaji criteria improves the diagnostic sensitivity in amyotrophic lateral sclerosis: a systematic review using individual patient data. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2016;127(7):2684–2691. doi: 10.1016/j.clinph.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Cedarbaum J.M., Stambler N., Malta E., Fuller C., Hilt D., Thurmond B., Nakanishi A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) Journal of the neurological sciences. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi Y., Tashiro K., Itoyama Y., Nakano I., Sobue G., Nakamura S., Sumino S., Yanagisawa N. [Study of functional rating scale for amyotrophic lateral sclerosis: revised ALSFRS(ALSFRS-R) Japanese version], No to shinkei. Brain and nerve. 2001;53(4):346–355. [PubMed] [Google Scholar]
- 14.Longstreth G.F., Thompson W.G., Chey W.D., Houghton L.A., Mearin F., Spiller R.C. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 15.Chiò A., Calvo A., Moglia C., Mazzini L., Mora G. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. Journal of neurology, neurosurgery, and psychiatry. 2011;82(7):740–746. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- 16.Tamura A., Tomita T., Oshima T., Toyoshima F., Yamasaki T., Okugawa T., Kondo T., Kono T., Tozawa K., Ikehara H., Ohda Y., Fukui H., Watari J., Miwa H. Prevalence and Self-recognition of Chronic constipation: results of an Internet survey. Journal of neurogastroenterology and motility. 2016;22(4):677–685. doi: 10.5056/jnm15187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samara V.C., Jerant P., Gibson S., Bromberg M. Bowel, bladder, and sudomotor symptoms in ALS patients. Journal of the neurological sciences. 2021;427 doi: 10.1016/j.jns.2021.117543. [DOI] [PubMed] [Google Scholar]
- 18.Yurtdaş G., Acar-Tek N., Akbulut G., Cemali Ö., Arslan N., Beyaz Coşkun A., Zengin F.H. Risk factors for constipation in adults: a Cross-Sectional study. J. Am. Coll. Nutr. 2020;39(8):713–719. doi: 10.1080/07315724.2020.1727380. [DOI] [PubMed] [Google Scholar]
- 19.Ramírez Puerta R., Yuste Ossorio E., Narbona Galdó S., Pérez Izquierdo N., Peñas Maldonado L. [Amyotrophyc lateral sclerosis; gastrointestinal complications in home enteral nutrition] Nutr. Hosp. 2013;28(6):2014–2020. [PubMed] [Google Scholar]
- 20.Muscaritoli M., Kushta I., Molfino A., Inghilleri M., Sabatelli M., Rossi Fanelli F. Nutritional and metabolic support in patients with amyotrophic lateral sclerosis. Nutrition. 2012;28(10):959–966. doi: 10.1016/j.nut.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Atsuta N., Watanabe H., Ito M., Tanaka F., Tamakoshi A., Nakano I., Aoki M., Tsuji S., Yuasa T., Takano H., Hayashi H., Kuzuhara S., Sobue G. Age at onset influences on wide-ranged clinical features of sporadic amyotrophic lateral sclerosis. Journal of the neurological sciences. 2009;276(1–2):163–169. doi: 10.1016/j.jns.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Tollefsen E., Midgren B., Bakke P., Fondenes O. Amyotrophic lateral sclerosis: gender differences in the use of mechanical ventilation. Eur. J. Neurol. 2010;17(11):1352–1357. doi: 10.1111/j.1468-1331.2010.03036.x. [DOI] [PubMed] [Google Scholar]
- 23.Chiò A., Calvo A., Ghiglione P., Mazzini L., Mutani R., Mora G. Tracheostomy in amyotrophic lateral sclerosis: a 10-year population-based study in Italy. Journal of neurology, neurosurgery, and psychiatry. 2010;81(10):1141–1143. doi: 10.1136/jnnp.2009.175984. [DOI] [PubMed] [Google Scholar]
- 24.Vender R.L., Mauger D., Walsh S., Alam S., Simmons Z. Respiratory systems abnormalities and clinical milestones for patients with amyotrophic lateral sclerosis with emphasis upon survival. Amyotroph Lateral Scler. : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2007;8(1):36–41. doi: 10.1080/17482960600863951. [DOI] [PubMed] [Google Scholar]
- 25.Qadri S., Langefeld C.D., Milligan C., Caress J.B., Cartwright M.S. Racial differences in intervention rates in individuals with ALS: a case-control study. Neurology. 2019;92(17):e1969–e1974. doi: 10.1212/WNL.0000000000007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubbioso R., Provitera V., Pacella D., Santoro L., Manganelli F., Nolano M. Autonomic dysfunction is associated with disease progression and survival in amyotrophic lateral sclerosis: a prospective longitudinal cohort study. Journal of neurology. 2023;270(10):4968–4977. doi: 10.1007/s00415-023-11832-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roche J.C., Rojas-Garcia R., Scott K.M., Scotton W., Ellis C.E., Burman R., Wijesekera L., Turner M.R., Leigh P.N., Shaw C.E., Al-Chalabi A. A proposed staging system for amyotrophic lateral sclerosis. Brain : a journal of neurology. 2012;135(Pt 3):847–852. doi: 10.1093/brain/awr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori H., Tack J., Suzuki H. Magnesium oxide in constipation. Nutrients. 2021;13(2) doi: 10.3390/nu13020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori H., Suzuki H., Hirai Y., Okuzawa A., Kayashima A., Kubosawa Y., Kinoshita S., Fujimoto A., Nakazato Y., Nishizawa T., Kikuchi M. Clinical features of hypermagnesemia in patients with functional constipation taking daily magnesium oxide. J. Clin. Biochem. Nutr. 2019;65(1):76–81. doi: 10.3164/jcbn.18-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khairi T., Amer S., Spitalewitz S., Alasadi L. Severe Symptomatic hypermagnesemia associated with over-the-Counter Laxatives in a patient with renal failure and Sigmoid Volvulus. Case reports in nephrology. 2014;2014 doi: 10.1155/2014/560746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the findings of this study is available from the corresponding author upon reasonable and justified request.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.