Key Clinical Message
Treatment of patients with amelogenesis imperfecta extends over many years, from childhood to early adulthood. Their management at any age is complex and has to be adapted in relation to therapies validated in the general population.
Keywords: amelogenesis imperfecta, CAD‐CAM, dentistry, full‐mouth rehabilitation, prosthodontics, rare disease
1. INTRODUCTION
Amelogenesis imperfecta (AI) is a pathology caused by a heterogeneous group of genetic mutations affecting tooth enamel during odontogenesis and causing different forms of hypoplasia and hypomineralization. 1 It generally affects every tooth of the two dentitions with a great variability of phenotypic expression and can be isolated or syndromic. 2 It is also associated with impacted teeth and anomalies in tooth eruption, congenitally missing teeth, pulp calcification, root and coronal resorption, hypercementosis, root malformation, and taurodontism. 3 In some severe cases, permanent sensitivities and unusual aesthetic aspects have a major impact on the patient's daily life. 4
AI cases commonly prove to be complex and require interdisciplinary patient care to manage the different aspects of the disease. 5 , 6 , 7 , 8 , 9 , 10 In particular, conservative, prosthetic and orthodontic treatment should be conducted together. A decrease in the occlusal vertical dimension (VDO) is often observed due to quick wear and deterioration of the dental structure. 11
However, each case is unique. Only a few case reports of AI rehabilitation have been reported, and even fewer report significant follow‐up. 12 , 13 , 14 , 15 These cases mainly describe definitive rehabilitation when the patient is already an adult or young adult.
One of the main issues of rehabilitation is certainly the quality of adhesion obtained on altered enamel. The dental treatment of patients with AI can present challenges related to adhesive bonding due to qualitative enamel composition changes, resulting in weakened enamel‐resin bonds, depending on the AI phenotype. 16
The aim of this paper was to report the 12‐year management of an AI patient from a transitional phase in childhood to permanent dentition therapeutics in early adulthood. It describes interdisciplinary work, including pediatric dentistry, orthodontics, and prosthetic full‐mouth definitive rehabilitation, with an increased VDO. Most of this treatment was carried out chairside. We also included a deep reflection on the adhesive problem.
2. CASE HISTORY
2.1. Childhood
A 9‐year‐old female patient in good health was referred by her dentist to Bretonneau Hospital (AP‐HP, Paris) in 2011 for global dental care. The patient related that severe sensitivities were triggered by the slightest thermic stimuli, which complicated daily basic activities, such as drinking water or brushing teeth. She complained about the aesthetic aspect of her smile, which was very different from that of her fellow pupils and for which she get bullied.
The diagnosis of hypocalcified AI was made in 2011 by the pediatric dentistry team and was based on clinical factors without any molecular diagnosis. All the permanent teeth had erupted, except the second permanent molars, and presented both qualitative and quantitative impairments of enamel. This yellow‐brown enamel flaked and exposed the underlying dentin in many areas (Figure 1A). The gingival phenotype was thin, and the impossibility for the patient to effectively brush her teeth led to substantial plaque accumulation with gingivitis, and many early carious lesions. The DFMT index was 8‐0‐0 = 8.
FIGURE 1.

First consult, intraoral view of maximal intercuspal occlusion (A); composite bonding with strip crowns (B); orthopedic interception with tongue positioner (C), 2011.
A substantial anterior open bite was associated with a bilateral skeletal class II occlusion and tongue thrust.
The initial orthopantomogram showed that tooth 23 was impacted and that the enamel had the same radiopacity as the dentin (Figure 2).
FIGURE 2.

Orthopantomogram, first consult at the Hospital in 2011. Enamel is less opaque than the dentin. Tooth 23 is impacted. Several malpositions are observed.
The transitional therapy must ensure an adequate aesthetic and functional result until the end of skeletal growth when the definitive restorations can be placed.
Anterior teeth were restored with composite resin (A2 Ceram.X Mono, Dentsply‐Sirona, York, PA) and celluloid strip crowns were inserted (Figure 1B) using the classical adhesive protocol. Posterior teeth were restored with cemented stainless‐steel crowns (SSCs). The whole treatment lasted 8 months.
To start to manage the open bite and tongue thrust, an orthopedic interception device was introduced for 2 years from 2011 to 2013 (Figure 1C). The patient was seen every year for follow‐up, composite reparation or SSC placement on recently erupted teeth. From 2015 to 2018, the patient was lost to follow‐up.
2.2. Orthodontics
The patient returned to the hospital in 2018 for a check‐up, and orthodontic, treatment was initiated to meet the patient's aesthetic demands and objective functional considerations. The patient wore braces for 1 year (2018–2019) to correct the class II occlusion and open bite (Figure 3). However, the class II occlusion was only partially corrected. At the same time she underwent tongue rehabilitation with a physiotherapist to remove the tongue thrust. The space between teeth 22 and 24 was partially closed. Orthodontic traction accentuated a type I Cairo recession on tooth 41 (Figure 3B), and severe gingivitis remained. An orthodontic retainer was bonded from canine to canine to keep the teeth in place.
FIGURE 3.

Before orthodontic treatment. Intraoral view of maximal intercuspal occlusion, overbite and tongue thrust (A); after orthodontic treatment (B).
3. METHODS
In April 2020, the definitive restoration treatment was performed. The patient was 19 years old.
During the first consultation with the restorative dentistry team (Figures 3B and 4), we observed maladjusted restorations and porous apparent enamel at the neck of the teeth, which appeared secondarily after natural complete eruption of the teeth. Teeth 17 and 47 (Figure 5) 38 and 48 were impacted and extracted at an early stage of this treatment. Tooth 23 (Figure 5), on the other hand, was left impacted; at the time, the patient did not want to undergo such an invasive intervention. Choice was made to leave the maxillary third molar impacted as well, as it would be easier to extract them later when they would continue their eruption.
FIGURE 4.

First consult with the restorative dentistry team. Extraoral front view at rest (A); extraoral front view with natural smile (B); extraoral profile view with natural smile (C); intraoral mandibular view with SSCs on molars and composite resin on other teeth (D); maxillary view (E); intraoral left profile view, overjet increased by 4 mm, normal overbite and reduced crown height in the posterior region, Class II occlusion (F); intraoral right profile view, class II occlusion (G).
FIGURE 5.

Radiographic examination. Maladjusted crowns, impacted canine (23) and endodontic treatment on tooth 12 are observed.
Tooth 27 was completely erupted, whereas tooth 37, partially erupted, seemed to be pursuing its evolution on the successive orthopantomogram and was treated with fluoride regularly to prevent sensitivities (Fluor Protector, Ivoclar, Schaan, Liechtenstein) until an effective restoration could be placed.
We observed diastemas between teeth 13–14, 43–44 (Figure 4G) 34–35, and 22–24 (Figure 4F). The overall aspect of the smile was harmonious. The maxillary interincisal point deviated 2 mm to the left and was not perfectly vertical but rather slightly oblique to the right. The mandibular interincisal point deviated 1 mm to the right. We observed 70% exposure of the maxillary incisors and 100% exposure of the mandibular incisors (Figure 4B), narrow buccal corridors, an inverted incisal line, and an uneven maxillary cervical line, notably significantly lower in the posterior area. However, this maxillary cervical line remained covered during a natural smile and was not modified. We observed parallelism between the incisal edge of the maxillary incisors and the lower lip, and the depth of the smile stopped at the second premolar. The occluso‐functional analysis in static conditions revealed a bilateral class II occlusion with an overjet increase of 4 mm, normal overbite and reduced crown height in the posterior region, particularly in sector 2 (Figure 4F), leading to a reduced VDO.
The kinetic examination highlighted a straight mouth opening with repetitive occlusion on maximum intercuspation. The anterior guidance was primarily carried by the lateral incisors.
The patient was referred for genotyping. A heterozygous mutation of the FAM83H gene (NM_198488) was found.
After oral hygiene instructions were given to the patient and management of plaque control was performed, a thorough clinical and radiographic examination allowed us to determine a 3 mm increase in VDO. This measure corresponded to the minimum increase to provide adequate occlusal space for the restorative material and to improve anterior teeth aesthetics by reducing the overjet. A mandibular and maxillary wax‐up were performed by the dental technician, respecting the requirements of VDO increase and aesthetics considerations discussed with the patient. The wax‐up was milled out of PMMA (Multistratum Flexible, Zirkonzahn, Gais, Italy) and transferred into the mouth as mock‐ups during a trial appointment. The patient expressed satisfaction with the anterior mock‐up regarding the aesthetic of the teeth shape and integration in the face. The posterior mock‐up was validated for the VDO increase as well.
The patient's posterior quadrants were restored first at the chosen increased VDO. The sectors were restored two by two with chair‐side CAD/CAM milled temporary PMMA restorations, first on the left side (sectors 2 and 3) and then on the right side (sectors 1–4), with placement of bite ramps in between the appointments. Restorations were removed and carious tissues were excavated. We performed minimally invasive chamfer homothetic peripheral preparations with an overall thickness reduction of approximately 0.8 mm (Figure 6). Some teeth did not need any occlusal reduction as the prosthetic space was wide enough. After optical impressions (Cerec Primescan®, Dentsply Sirona, York, PA), temporary crowns were designed and milled out of PMMA (Telio CAD®, Ivoclar Vivadent, Schaan, Liechtenstein) with CEREC SW 5.1 software (Dentsply Sirona, York, PA) according to the initial wax‐up, and cemented with polycarboxylate temporary cement (Durelon, 3M ESPE, St. Paul, MN) (Figure 6C).
FIGURE 6.
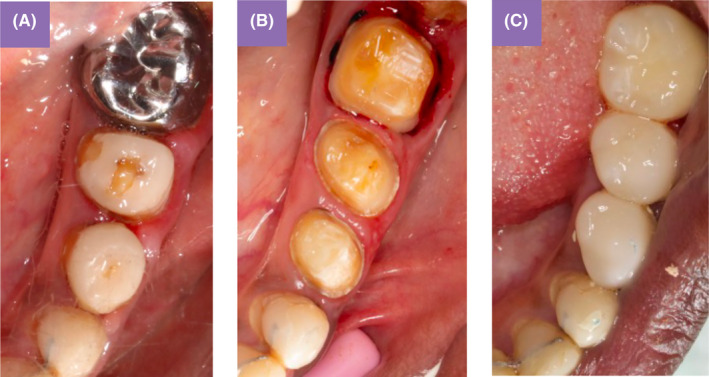
Teeth preparation and temporary crowns. Initial situation Sector 3 (A); minimally invasive chamfer homothetic peripheral preparations (B); temporary restorations in PMMA at increased VDO (C).
New optical impressions were taken of the preparations and the temporary restorations to use as a biocopy for the definitive restorations.
The definitive crowns were also designed on CEREC SW 5.1 software and milled out of 4Y‐TZP/5Y‐TZP zirconia (Emax ZirCAD MT, Ivoclar Vivadent, Schaan, Liechtenstein) (Figure 7) and cemented with glass ionomer cement (Fuji Plus®, GC Corporation, Tokyo, Japan) in two appointments with occlusal adjustments. Occlusion settings were performed in the maximal intercuspal position with intra‐ and inter‐arch stability.
FIGURE 7.

Definitive crowns milled out of zirconia (4Y‐TZP /5Y‐TZP, Emax ZirCAD MT, Ivoclar Vivadent), Sectors 2 and 3.
After achieving stable posterior occlusal support, the next phase involved restoring the anterior quadrants to address both aesthetic and functional requirements and establish proper anterior guidance. Subgingival preparations were performed for enhanced aesthetic integration and to prevent any dentinal exposure that could lead to sensitivities.
Subsequently, following a similar procedure to that of the posterior quadrants, we proceeded with a temporary stage using PMMA crowns, followed by an impression session. The aesthetic was checked and validated again with the patient at this stage. The maxillary incisors were modeled relatively longer to achieve better anterior guidance.
Two sessions were scheduled for cementing the six maxillary and six mandibular anterior crowns using resin‐modified glass‐ionomer luting cement (Fuji Plus, GC Corporation, Tokyo, Japan). Both static and dynamic occlusion adjustments were carefully performed to ensure optimal function.
4. CONCLUSION AND RESULTS
The patient was scheduled for a follow‐up appointment 1 month after the crowns were placed (Figure 8). The patient felt perfectly comfortable with the treatment. At this appointment, a 3D‐printed rigid bruxism splint (KeySplint Soft, Keystone Industries, Gibbstown, NJ, USA) for the mandible was provided to the patient after occlusal adjustments (Figure 9). The treatment lasted 6 months, from the first consultation to the 1‐month follow‐up appointment. Then, the patient was seen every 6 months for 2 years for regular scaling and check‐ups. At the 2‐year follow‐up (Figures 10, 11, 12, 13), no instances of sensitivity, restoration loss, marginal discoloration, or material staining were observed. Additionally, there were no occurrences of chipping, excessive wear, periodontal damage, or secondary caries. Tooth 37 (Figures 8B and 13B), after 2 years did not pursue its evolution and a decision was made to extract it as no sustainable restoration could be placed. This 12‐year dental care journey started when the patient was 9 years old, after many years of diagnosis wandering, and ended as she was attending university. AI presents challenges at all stages of life, especially in childhood and adolescence, due to the rapid and successive evolution in dentition. Each case is unique and should be managed with an interdisciplinary approach, evaluating the most adequate treatment at each stage and always being aware of evolving materials and technologies that could offer promising therapeutic options in the near future. Regular follow‐ups, approximately every 6 months, will be the key to the long‐term success of this treatment.
FIGURE 8.

One month follow‐up. Extraoral front zoom view with natural harmonious smile (A); intraoral view in maximal intercuspal position (B); maxillary occlusal view (C); mandibular occlusal view (D).
FIGURE 9.

3D‐printed rigid bruxism splint (KeySplint Soft®, Keystone industries) for the mandible, occlusal view with occlusal contacts marked.
FIGURE 10.

Two‐year follow‐up. Extraoral front view with natural smile (A) extra‐oral front view with forced smile still not exposing the anterior cervical line (B).
FIGURE 11.

Two‐year follow‐up. Extraoral front zoom view with natural harmonious smile.
FIGURE 12.

Two‐year follow‐up. Intraoral right profile view (A); intraoral view in maximal intercuspal position (B); intraoral left profile view (C).
FIGURE 13.

2‐year follow‐up. Maxillary occlusal view (A); mandibular occlusal view (B).
5. DISCUSSION
Early management of AI is crucial to improve the long‐term oral health and quality of life of affected individuals. The patient was diagnosed at 9 years old, which is quite young; some patients experience a very late diagnosis due to difficulties detecting the presence of AI and determining its clinical subtype. 17
In AI cases, it is appropriate to suggest that parents seek a genetic consultation for their child and consider including them in research programs. 18 However, the patient's parents did not express the desire to include their daughter in these programs at the early stage of treatment, and the genetic diagnosis occurred only at the end of the definitive rehabilitation as the patient reached the age of majority. An heterozygous mutation was found in the FAM83H gene, which is reported to be involved in autosomal dominant hypocalcified amelogenesis imperfecta (ADHCAI), 19 , 20 as the dominant allele. This gene shows incomplete penetrance. 21
5.1. Transitional stage
Regarding the use of strip crowns in the early transitional stage, tooth reconstruction using this technique is undoubtedly a quick, cost‐effective, and efficient solution. 22 , 23 Parents are usually satisfied with aesthetics 24 and it allows maximal tissue preservation. However, it inevitably presents long‐term challenges. Polymerization of the composite cannot be performed under an operative field 25 and the composite is polymerized as a single mass, resulting in significant polymerization shrinkage stress with a high C‐factor. 26 The approximate cervical adaptation and frequent subgingival composite overhangs sustain inflammation and make oral hygiene maintenance difficult.
All these factors ultimately lead to a higher susceptibility to bacterial reinfiltration, thus increasing the risk of dental tissue loss, which is already reduced in patients with this condition.
Therefore, with the recent advancements in materials and protocols, new alternatives should be considered. The current possibility of performing indirect restorations in a single appointment through digital technology eliminates the need for provisional restorations and makes appointment durations compatible with the limited patience of children. 27 Another promising option is injectable composite therapeutics, which allow full mouth rehabilitation to be performed in a single appointment, producing instant results. 28
5.2. Adhesive problems and treatment choices
As the quality of a durable bonding greatly depends on the bonded substrate, one of the main challenges of AI full‐mouth rehabilitation lies in adhesion to the altered enamel. Yaman et al. found that the microtensile bond strength of etch‐and‐rinse and self‐etch adhesives to hypoplastic AI enamel was nearly 40% lower than that of sound enamel. 29 Similarly, for enamel affected by hypocalcified Faria‐e‐Silva et al. observed a comparable decrease in the microshear bond strength of an etch‐and‐rinse adhesive. 30
Some enamel treatments have been developed to address this issue, such as applying 5% sodium hypochlorite after etching and before adhesive application 31 , 32 , 33 or using self‐etch adhesives only to limit enamel aggression. 34 However, current studies on these two protocols show that they do not yield significant improvements.
Given this assessment, any bonded restoration in patients with this condition presents issues for long‐term success. In this case, the tissue loss after caries excavation necessarily guided us toward crown peripheral preparations, mostly juxta‐ or subgingival. Despite good plaque control, residual gingival inflammation was still observed. Gingival inflammation is more frequently observed among patients with AI and, more specifically, among individuals with the hypocalcified subtype. 6 The rubber dam placement was compromised, and consequently, moisture was hard to control. Considering all these factors, we opted for cementation. Additionally, cementation would facilitate future reinterventions due to the simplicity of removing the previous restorations.
Regarding the choice of material for the definitive restoration, ceramic 35 was immediately selected, and zirconia appeared to be the most adequate solution. By the end of the treatment, the patient would be 20, and her periodontium would be mature. The goal was to preserve dental structures to the maximum extent possible so that each reintervention is minimally invasive and allows the teeth to remain on the arch for as long as possible. Zirconia allows for conservative preparations (Figure 6B). 36 The preparations of approximately 0.8 mm were compatible with cementation, whereas thinner zirconias should have been bonded, to maximize fracture toughness. 37
With a focus on tissue preservation enabled by high mechanical values and driven by the goal of achieving satisfactory aesthetics at the end of treatment, we opted for 4Y‐TZP/5Y‐TZP zirconia (Emax ZirCAD MT®, Ivoclar Vivadent, Schaan, Liechtenstein).
It would have been advisable to treat the RT1‐type recession (Cairo) on tooth 41 with a graft before preparing for the crown. There is indeed a risk that the recession may worsen in the coming years if oral hygiene deteriorates. However, this additional procedure was declined by the patient. Nevertheless, to ensure better biocompatibility of the anterior restoration, the cervical area of the zirconia was mechanically polished, providing better biocompatibility than a glazed preparation. 38
5.3. Chair‐Side CAD/CAM interest
The noteworthy aspect of this case is that it was mostly completed using chairside CAD/CAM technology. This approach has been proven to be highly beneficial for patients, who had experienced repeated dental treatments since childhood. 39 The use of chairside CAD/CAM enabled us to achieve a rapid and effective final outcome in relatively few sessions. 40 In general, this technology leads to reduced production costs 41 and improvements in time efficiency 42 and satisfies the patient's perception of a modern treatment concept. 43 Collaborating closely with the patient during the planning and design stages of the restorations, which is challenging when relying on a dental technician, was pivotal. The patient's active involvement played a crucial role in the seamless integration of the new restorations and contributed to the psychological success of the treatment. Although the sessions were lengthy, the immediate improvement in function and aesthetics seemed to outweigh any discomfort experienced by the patient.
5.4. Patient satisfaction and treatment prognosis
Our intervention appeared to have exerted a considerable psychosocial influence, as the patient reported a swift enhancement in her quality of life and overall social and mental well‐being. Consequently, effective management of the dental repercussions of AI appears to have a positive impact on all aspects of patients' health. 44 , 45 The long‐term success of the treatment will depend on the regularity of patient follow‐up and maintenance. The main short‐ to medium‐term failure risks include secondary caries recurrence under restorations and the risk of ceramic fractures. The carious risk will be clinically and radiographically monitored at each follow‐up appointment every 6 months for 2 years and then annually. To lower the risk of ceramic fractures, an occlusal splint is worn every night by the patient. 46
AUTHOR CONTRIBUTIONS
Élisa Caussin: Conceptualization; data curation; investigation; writing – original draft. Frédéric Courson: Data curation; investigation; writing – review and editing. Elisabeth Dursun: Conceptualization; methodology; supervision; validation; writing – review and editing. Yohann Brukarz: Investigation. Daniel Dot: Writing – review and editing. Catherine Chaussain: Investigation; writing – review and editing. Jean‐Pierre Attal: Supervision; writing – review and editing. Philippe François: Conceptualization; data curation; formal analysis; investigation; methodology; supervision; validation; writing – review and editing.
FUNDING INFORMATION
This research received no external funding.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
PATIENT CONSENT STATEMENT
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient, adult at the end of treatment, to publish this paper.
CLINICAL TRIAL REGISTRATION
This article describes the treatment of a young patient based on a therapeutic approach widely described in the literature. It is by no means an experimental clinical trial, but a case report.
Caussin É, Courson F, Dursun E, et al. Interdisciplinary full mouth rehabilitation of a patient with amelogenesis imperfecta from childhood to young adult‐hood: A 12‐year case report. Clin Case Rep. 2024;12:e8704. doi: 10.1002/ccr3.8704
DATA AVAILABILITY STATEMENT
The data that support the clinical case are available on request from the corresponding author. The data are not publicly available due to privacy or ethical re‐strictions.
REFERENCES
- 1. Crawford PJM, Aldred M, Bloch‐Zupan A. Amelogenesis imperfecta. Orphanet J Rare Dis. 2007;2(1):17. doi: 10.1186/1750-1172-2-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Witkop CJ. Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification. J Oral Pathol Med. 1988;17(9–10):547‐553. doi: 10.1111/j.1600-0714.1988.tb01332.x [DOI] [PubMed] [Google Scholar]
- 3. Ortiz L, Pereira AM, Jahangiri L, Choi M. Management of amelogenesis imperfecta in adolescent patients: clinical report. J Prosthodont. 2019;28(6):607‐612. doi: 10.1111/jopr.13069 [DOI] [PubMed] [Google Scholar]
- 4. Coffield KD, Phillips C, Brady M, et al. The psychosocial impact of developmental dental defects in people with hereditary amelogenesis imperfecta. J Am Dent Assoc. 2005;136(5):620‐630. doi: 10.14219/jada.archive.2005.0233 [DOI] [PubMed] [Google Scholar]
- 5. Millet C, Duprez JP, Khoury C, Morgon L, Richard B. Interdisciplinary care for a patient with amelogenesis imperfecta: a clinical report. J Prosthodont. 2014;24(5):424‐431. doi: 10.1111/jopr.12242 [DOI] [PubMed] [Google Scholar]
- 6. Quandalle C, Boillot A, Fournier B, et al. Gingival inflammation, enamel defects, and tooth sensitivity in children with amelogenesis imperfecta: a case‐control study. J Appl Oral Sci. 2020;28:e20200170. doi: 10.1590/1678-7757-2020-0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suchancova B, Holly D, Janska M, et al. Amelogenesis imperfecta and the treatment plan–interdisciplinary team approach. Bratisl Med J. 2014;115(1):44‐48. doi: 10.4149/bll_2014_010 [DOI] [PubMed] [Google Scholar]
- 8. Ramos AL, Pascotto RC, Filho LI, Hayacibara RM, Boselli G. Interdisciplinary treatment for a patient with open‐bite malocclusion and amelogenesis imperfecta. Am J Orthod Dentofacial Orthop. 2011;139(4):S145‐S153. doi: 10.1016/j.ajodo.2009.05.031 [DOI] [PubMed] [Google Scholar]
- 9. Patel M, McDonnell ST, Iram S, Chan MFWY. Amelogenesis imperfecta–lifelong management. Restorative management of the adult patient. Br Dent J. 2013;215(9):449‐457. doi: 10.1038/sj.bdj.2013.1045 [DOI] [PubMed] [Google Scholar]
- 10. Mathews DP, Kokich VG. Interdisciplinary management of a complex esthetic dilemma. Compend Contin Educ Dent. 2021;42(1):34‐37. [PubMed] [Google Scholar]
- 11. Möhn M, Bulski JC, Krämer N, Rahman A, Schulz‐Weidner N. Management of amelogenesis imperfecta in childhood: two case reports. Int J Environ Res Public Health. 2021;18(13):7204. doi: 10.3390/ijerph18137204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathews DP, Knight DJ, O'Connor RV, Kokich VG. Interdisciplinary treatment of a patient with amelogenesis imperfecta: case report with a 35‐year follow‐up. J Esthet Restor Dent. 2021;33(7):968‐975. doi: 10.1111/jerd.12804 [DOI] [PubMed] [Google Scholar]
- 13. Sreedevi S, Sanjeev R, Ephraim R, Joseph M. Interdisciplinary full mouth rehabilitation of a patient with amelogenesis imperfecta: a case report with 8 years follow‐up. J Int Oral Health. 2014;6(6):90‐93. [PMC free article] [PubMed] [Google Scholar]
- 14. Sabandal MMI, Dammaschke T, Schäfer E. Restorative treatment in a case of amelogenesis imperfecta and 9‐year follow‐up: a case report. Head Face Med. 2020;16(1):28. doi: 10.1186/s13005-020-00243-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trentesaux T, Rousset MM, Dehaynin E, Laumaillé M, Delfosse C. 15‐year follow‐up of a case of amelogenesis imperfecta: importance of psychological aspect and impact on quality of life. Eur Arch Paediatr Dent. 2013;14(1):47‐51. doi: 10.1007/s40368-012-0008-1 [DOI] [PubMed] [Google Scholar]
- 16. Strauch S, Hahnel S. Restorative treatment in patients with amelogenesis imperfecta: a review. J Prosthodont. 2018;27(7):618‐623. doi: 10.1111/jopr.12736 [DOI] [PubMed] [Google Scholar]
- 17. Adorno‐Farias D, Ortega‐Pinto A, Gajardo P, et al. Diversity of clinical, radiographic and genealogical findings in 41 families with amelogenesis imperfecta. J Appl Oral Sci. 2019;27:e20180359. doi: 10.1590/1678-7757-2018-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aldred MJ, Crawford PJM, Savarirayan R, Savulescu J. It's only teeth–are there limits to genetic testing? Clin Genet. 2003;63(5):333‐339. doi: 10.1034/j.1399-0004.2003.00045.x [DOI] [PubMed] [Google Scholar]
- 19. Sabandal MMI, Schäfer E. Amelogenesis imperfecta: review of diagnostic findings and treatment concepts. Odontology. 2016;104(3):245‐256. doi: 10.1007/s10266-016-0266-1 [DOI] [PubMed] [Google Scholar]
- 20. Zhang C, Song Y, Bian Z. Ultrastructural analysis of the teeth affected by amelogenesis imperfecta resulting from FAM83H mutations and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(2):e69‐e76. doi: 10.1016/j.oooo.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 21. Bai RQ, He WB, Peng Q, et al. A novel FAM83H variant causes familial amelogenesis imperfecta with incomplete penetrance. Mol Genet Genomic Med. 2022;10(4):e1902. doi: 10.1002/mgg3.1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dursun E, Savard E, Vargas C, et al. Management of amelogenesis imperfecta: a 15‐year case history of two siblings. Oper Dent. 2016;41(6):567‐577. doi: 10.2341/15-372-t [DOI] [PubMed] [Google Scholar]
- 23. Kupietzky A. Bonded resin composite strip crowns for primary incisors: clinical tips for a successful outcome. Pediatr Dent. 2002;24(2):145‐148. [PubMed] [Google Scholar]
- 24. Kupietzky A, Waggoner WF. Parental satisfaction with bonded resin composite strip crowns for primary incisors. Pediatr Dent. 2004;26(4):33740. [PubMed] [Google Scholar]
- 25. Gill A, Garcia M, An SW, Scott J, Seminario AL. Clinical comparison of three esthetic full‐coverage restorations in primary maxillary incisors at 12 months. Pediatr Dent. 2020;42(5):367‐372. [PubMed] [Google Scholar]
- 26. Somani R, Som NK, Jaidka S, Hussain S. Comparative evaluation of microleakage in various placement techniques of composite restoration: an in vitro study. Int J Clin Pediatr Dent. 2020;13(3):264‐268. doi: 10.5005/jp-journals-10005-1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davidovich E, Dagon S, Tamari I, Etinger M, Mijiritsky E. An innovative treatment approach using digital workflow and CAD‐CAM part 2: the restoration of molar incisor hypomineralization in children. Int J Environ Res Public Health. 2020;17(5):1499. doi: 10.3390/ijerph17051499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hulac S, Kois JC. Managing the transition to a complex full mouth rehabilitation utilizing injectable composite. J Esthet Restor Dent. 2023;35(5):796‐802. doi: 10.1111/jerd.13065 [DOI] [PubMed] [Google Scholar]
- 29. Yaman BC, Ozer F, Cabukusta CS, Eren MM, Koray F, Blatz MB. Microtensile bond strength to enamel affected by hypoplastic amelogenesis imperfecta. J Adhes Dent. 2014;16(1):7‐14. doi: 10.3290/j.jad.a30554 [DOI] [PubMed] [Google Scholar]
- 30. Faria‐e‐Silva AL, De Moraes RR, Menezes MDS, et al. Hardness and microshear bond strength to enamel and dentin of permanent teeth with hypocalcified amelogenesis imperfecta. Int J Paediatr Dent. 2011;21(4):314‐320. doi: 10.1111/j.1365-263x.2011.01129.x [DOI] [PubMed] [Google Scholar]
- 31. Venezie RD, Vadiakas G, Christensen JR, Wright JT. Enamel pretreatment with sodium hypochlorite to enhance bonding in hypocalcified amelogenesis imperfecta : case report and SEM analysis. Pediat Dent. 1994;16(6):433‐436. [PubMed] [Google Scholar]
- 32. Bayrak S, Tuloglu N, Tunc ES. Effects of deproteinization on bond strength of composite to primary teeth affected by amelogenesis. Pediatr Dent. 2019;41(4):304‐308. [PubMed] [Google Scholar]
- 33. Sönmez IS, Aras S, Tunç ES, Küçükeşmen C. Clinical success of deproteinization in hypocalcified amelogenesis imperfecta. Quintessence Int. 1985;40(2):11318. [PubMed] [Google Scholar]
- 34. Sapir S, Shapira J. Clinical solutions for developmental defects of enamel and dentin in children. Pediatr Dent. 2007;29(4):330‐336. [PubMed] [Google Scholar]
- 35. Ohrvik HG, Hjortsjö C. Retrospective study of patients with amelogenesis imperfecta treated with different bonded restoration techniques. Clin Exp Dent Res. 2019;6(1):16‐23. doi: 10.1002/cre2.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zarone F, Di Mauro MI, Ausiello P, Ruggiero G, Sorrentino R. Current status on lithium disilicate and zirconia: a narrative review. BMC Oral Health. 2019;19(1):134. doi: 10.1186/s12903-019-0838-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lawson NC, Jurado CA, Huang CT, et al. Effect of surface treatment and cement on fracture load of traditional zirconia (3Y), translucent zirconia (5Y), and lithium disilicate crowns. J Prosthodont. 2019;28(6):659‐665. doi: 10.1111/jopr.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brunot‐Gohin C, Duval JL, Verbeke S, et al. Biocompatibility study of lithium disilicate and zirconium oxide ceramics for esthetic dental abutments. J Periodontal Implant Sci. 2016;46(6):362‐371. doi: 10.5051/jpis.2016.46.6.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lundgren GP, Wickström A, Hasselblad T, Dahllöf G. Amelogenesis imperfecta and early restorative crown therapy: an interview study with adolescents and young adults on their experiences. PLoS ONE. 2016;11(6):e0156879. doi: 10.1371/journal.pone.0156879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Joda T, Zarone F, Ferrari M. The complete digital workflow in fixed prosthodontics: a systematic review. BMC Oral Health. 2017;17(1):124. doi: 10.1186/s12903-017-0415-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joda T, Brägger U. Digital vs. conventional implant prosthetic workflows: a cost/time analysis. Clin Oral Implants Res. 2014;26(12):1430‐1435. doi: 10.1111/clr.12476 [DOI] [PubMed] [Google Scholar]
- 42. Joda T, Brägger U. Time‐efficiency analysis comparing digital and conventional workflows for implant crowns: a prospective clinical crossover trial. Int J Oral Maxillofac Implants. 2015;30(5):1047‐1053. doi: 10.11607/jomi.3963 [DOI] [PubMed] [Google Scholar]
- 43. Joda T, Brägger U. Patient‐centered outcomes comparing digital and conventional implant impression procedures: a randomized crossover trial. Clin Oral Implants Res. 2015;27(12):e185‐e189. doi: 10.1111/clr.12600 [DOI] [PubMed] [Google Scholar]
- 44. Koruyucu M, Bayram M, Tuna EB, Gencay K, Seymen F. Clinical findings and long‐term managements of patients with amelogenesis imperfecta. Eur J Dent. 2014;8(4):546‐552. doi: 10.4103/1305-7456.143640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lundgren GP, Karsten A, Dahllöf G. Oral health‐related quality of life before and after crown therapy in young patients with amelogenesis imperfecta. Health Qual Life Outcomes. 2015;13(1):197. doi: 10.1186/s12955-015-0393-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Faus‐Matoses V, Ruiz‐Bell E, Faus‐Matoses I, Özcan M, Salvatore S, Faus‐Llácer VJ. An 8‐year prospective clinical investigation on the survival rate of feldspathic veneers: influence of occlusal splint in patients with bruxism. J Dent. 2020;99:103352. doi: 10.1016/j.jdent.2020.103352 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the clinical case are available on request from the corresponding author. The data are not publicly available due to privacy or ethical re‐strictions.


