Abstract
Type I interferons (IFN-I) play pivotal roles in tumor therapy for three decades, underscoring the critical importance of maintaining the integrity of the IFN-1 signaling pathway in radiotherapy, chemotherapy, targeted therapy, and immunotherapy. However, the specific mechanism by which IFN-I contributes to these therapies, particularly in terms of activating dendritic cells (DCs), remains unclear. Based on recent studies, aberrant DNA in the cytoplasm activates the cyclic GMP-AMP synthase (cGAS)- stimulator of interferon genes (STING) signaling pathway, which in turn produces IFN-I, which is essential for antiviral and anticancer immunity. Notably, STING can also enhance anticancer immunity by promoting autophagy, inflammation, and glycolysis in an IFN-I-independent manner. These research advancements contribute to our comprehension of the distinctions between IFN-I drugs and STING agonists in the context of oncology therapy and shed light on the challenges involved in developing STING agonist drugs. Thus, we aimed to summarize the novel mechanisms underlying cGAS-STING-IFN-I signal activation in DC-mediated antigen presentation and its role in the cancer immune cycle in this review.
Keywords: cGAS-STING pathway, Type I interferon, DC, Tumor immune cycle
Introduction
Immune therapy harnesses the body's innate immune system to combat cancer, particularly through immune checkpoint inhibitors (ICIs) such as CTLA-4, PD-1/PD-L1, or LAG-3 [1, 2]. Immunotherapy prognosis is favorably connected with T-cell infiltration into the tumor, and the efficacy of ICIs depends on pre-existing anticancer T-cell responses [1, 3, 4]. To activate T-cell immune responses, dendritic cells (DCs) must first internalize and process tumor antigens. Subsequently, these captured antigens must be presented to T cells in the tumor-draining lymph nodes, contingent upon the major histocompatibility complexes (MHC-I and MHC-II). This initiation leads to the activation of effector T cells, which then recognize, bind to, and eliminate target cancer cells. This sequence of events is referred to as the tumor immune cycle [5]. The complete tumor immune cycle is crucial for initiating antitumor immunity, with the breakdown of tumor cells and antigen presentation marking the first steps in this cycle.
Tumor cells exhibit diverse forms of cell death, including apoptosis, necrosis, autophagy, and ferroptosis, elicited by physical, chemical, or biological interventions. In recent years, immunogenic cell death (ICD) has emerged as a novel mechanism in the tumor immune cycle. Subsequent to ICD, deceased tumor cells release damage-associated molecular pattern molecules (DAMPs) such as calreticulin (CRT), heat-shock proteins, high-mobility group box 1, and adenosine triphosphate. This process recruits and activates various immune cells, thereby augmenting the processing of tumor-associated antigens (TAAs) by DCs. In addition to the release of DAMPs, recent studies have revealed the pivotal role of type I interferon (IFN-I) release in promoting tumor ICD, with a deficiency in the IFN-I pathway impeding DC activation [6].
IFN-I is a class of cytokines that can be triggered by receptors such as retinoic acid-inducible gene I (RIG-I), toll-like receptors (TLRs), and cGAS. They play a vital role in responding to bacteria, viruses, tumors, and cellular stress. Once activated, IFNs regulate the expression of numerous IFN-stimulated genes (ISGs) via the janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway, thereby promoting antitumor immune responses. Numerous studies have shown that IFNs are a successful treatment for a variety of malignant cancers. Their primary effectiveness is in effectively stimulating DCs, which in turn promotes antigen cross-presentation and maturation. IFNs also exert an influence on other immune cells, including T cells, macrophages, NK cells, and regulatory T cells (Tregs), thereby regulating tumor immunity (Fig. 1). Clinical data indicate a positive correlation between elevated intratumor expression of IFNs or ISGs and improved prognosis [7]. As a result, using or activating IFNs has become a very promising approach in tumor immunotherapy.
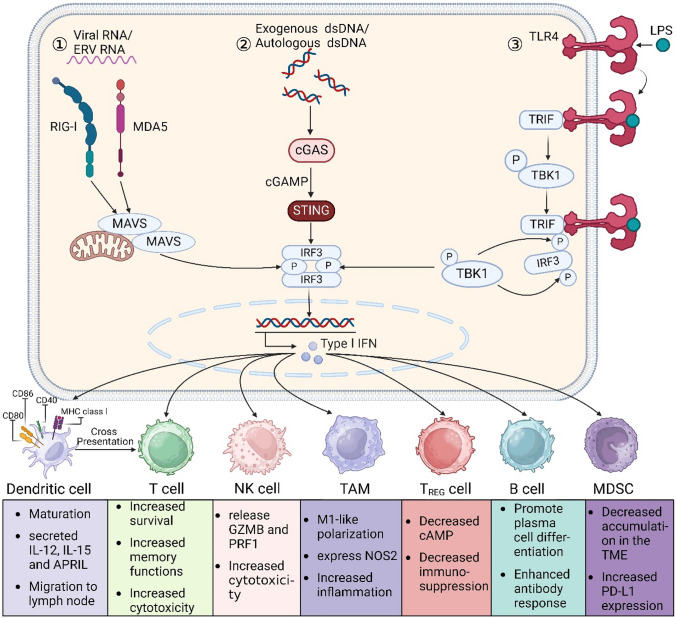
Fig. 1.
Immunomodulatory effects of IFN-I signaling. ①: RIG-I and MDA5, members of the PRR, are major cytoplasmic RNA sensors that, upon ligand binding, sequentially activate downstream axes to produce phosphorylated IRF3; ②: STING is phosphorylated by TBK1 in response to DNA stimulation. DNA in the cytoplasm activates cGAS to produce the second messenger cGAMP, which then binds to STING and activates STING, and active STING then directly recruits and activates TBK1, resulting in phosphorylated IRF3; ③: LPS stimulation activates TLR4, which in turn activates the adaptor protein MyD88 (not shown) and TRIF. TRIF activates TBK1, which in turn phosphorylates TRIF at consensus motifs. phosphorylated TRIF then recruits IRF3, which promotes phosphorylation of IRF3 by TBK1. Phosphorylated IRF3 dimerizes through the same positively charged surface. The IRF3 dimer then enters the nucleus and, together with NF-κB, initiates the production of type I interferons. After secretion, IFN-I interferons exert effects on various cells through different mechanisms. APRIL (official name TNFSF13), TNF superfamily member 13; cAMP cyclic AMP, GZMB granzyme B, EMT epithelial-to-mesenchymal transition, MDSC myeloid-derived suppressor cell, NK natural killer, NOS2 nitric oxide synthase 2, PRF1 perforin 1
The RIG-I/MDA5-MAVS and TLR-MyD88 pathways are activated by external viruses and bacteria, whereas the cGAS-STING pathway detects DNA from both external microorganisms and endogenous sources, such as nuclear and mitochondrial DNA [8]. When cGAS detects DNA, it generates cGAMP, which then activates STING and starts a cascade that results in the creation of IFN-I [9–11]. The cGAS-STING pathway enhances both innate and adaptive immunity by activating DCs, T cells, NK cells, and macrophages, with STING playing a crucial role as a transducer. Positive correlations between STING expression in tumors and immune cell infiltration have been observed in the cancer genome atlas program (TCGA) database [12]. STING can function in both IFN-dependent and -independent manners [13]. While research suggests that both cGAS-STING and IFN-I promote DC antigen cross-presentation, the specific mechanisms underlying DC activation remain unclear. Developing anticancer medications that target the cGAS-STING-IFN-I signaling axis requires an understanding of how cGAS-STING and IFN-I activate DCs.
In this review, we primarily address the methods by which the IFN-I-cGAS-STING signaling axis activates DCs in immunogenic tumors, especially considering the parallels and divergences between IFN and cGAS-STING activation processes. This evaluation is of paramount importance to gauge the profound impact of this pathway on innovative approaches to cancer treatment.
DCs
As a specialized class of antigen-presenting cells (APCs), DCs can be classified into three primary subtypes: conventional DCs (cDCs), plasmacytoid DCs (pDCs), and inflammatory DCs [14]. They play a pivotal role in engulfing tumor-specific antigens or tumor-associated antigens (TAAs) responsible for T cell-mediated tumor-specific cytotoxicity, thereby initiating the tumor immune cycle [15, 16]. Different DC subtypes possess unique mechanisms for antigen capturing and innate immune sensing, leading to variations in their abilities to acquire and process internalized antigens, produce cytokines, and activate T cells (Fig. 2) [15]. The composition of DCs within solid tumors varies depending on the tumor type, which in turn influences the antitumor T cell response [17]. Tumors with higher levels of DC infiltration often exhibit better responses to immunotherapies. Tumor-infiltrating DCs can exist in different functional states, which has significant implications for antitumor immune responses [18, 19]. In fact, numerous factors, including antigens, innate immune signals, adaptive immune cells, and environmental factors, can influence the state of DCs [20]. DCs recognize various pathogen-associated molecular patterns (PAMPs) and respond to the autocrine or paracrine release of cytokines independently of PAMPs for activation. Although interactions with CD4+ and CD8+ T cells can also induce DC activation [21–24], the main topics of this review are cDCs and pDCs, their functions in tumor immunity, and the ways in which they activate the innate immune system.
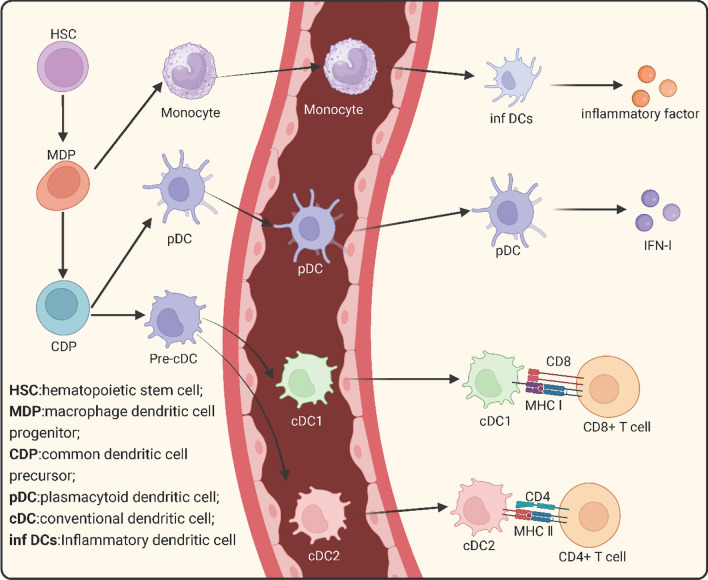
Fig. 2.
Classification and functional diagram of DC. In the bone marrow, hematopoietic stem cells (HSCs) give rise to macrophage-dendritic cell progenitors (MDPs). MDPs further differentiate into common dendritic cell progenitors (CDPs), which can to generate major dendritic cell (DC) subsets: plasmacytoid DCs (pDCs), conventional DC1 (cDC1), and conventional DC2 (cDC2). pDCs undergo terminal differentiation in the bone marrow, while pre-cDCs migrate through the bloodstream to lymphoid and non-lymphoid tissues, where they generate cDC1 and cDC2 subsets. On the other hand, MDPs differentiate into monocytes, and upon exposure to inflammatory cues, some monocytes differentiate into inflammatory dendritic cells under the influence of cytokines or chemokines
The subsets of DCs
cDCs
The pre-cDCs, or precursors of cDCs, first enter the bloodstream from the bone marrow and then spread to different tissues. Within these tissues, pre-cDCs undergo differentiation into two distinct subtypes, namely type I (cDC1) and type II (cDC2). cDC1s are characterized by their exceptional antigen cross-presentation capabilities, effectively presenting extracellular antigens to cytotoxic CD8+ T cells via MHC-I. They play a major role in boosting anticancer immune responses and are excellent at fighting intracellular infections [25–28]. Although a few reports suggest that other cell types, such as macrophages, human pDCs, or mouse CD8α+CD11b+DCs, may also possess antigen cross-presentation capabilities [29, 30], the unique adaptation of this cross-presentation function primarily relies on the cDC1 subset, owing to its distinctive subcellular molecular mechanisms and vesicular transport [31]. On the other hand, cDC2s mainly use MHC-II to deliver antigens to distinct helper CD4+ T cell subsets, controlling how the body reacts to external infections.
While mouse and human DCs share high functional homology, they exhibit disparities in their molecular phenotypes [32]. Mouse cDC1s demonstrate elevated levels of CD11c, MHC-II, CD8α, and CD24 expression and typically express X-C motif chemokine receptor 1 (XCR1), CD141, C-type lectin receptor 9A (Clec9a), DEC-205, and CD103. Their development relies on a cascade of transcription factors, such as interferon regulatory factor 8 (IRF8), inhibitor of DNA binding 2 (Id2), and basic leucine zipper ATF-like transcription factor 3 (Batf3). In contrast, human cDCs development is governed by distinct transcription factors, including IRF8 and Batf3, in conjunction with the expression of CD141, Clec9a, and XCR1 [33–38]. Notably, human IRF8 can concurrently regulate the development of both cDC1s and cDC2s. Furthermore, both mouse and human cDC2s promote CD4+ T cell responses, albeit they possess relatively diminished capacities for antigen presentation to CD8+ T cells. A number of similar markers, such as CD11c, MHC-II, CD11b, and CD172a, are shared by cDC2s in both species.
pDCs
pDCs exhibit a distinctive secretory morphology reminiscent of plasma cells. Similar to cDCs, pDCs express the cytokine receptor Flt3 (CD135) and rely exclusively on its ligand, Flt3L, for their development. pDCs possess a unique capacity for robust IFN-I production, rendering them pivotal in antiviral and antitumor immunity. pDCs are predominantly located within lymphoid organs, where they circulate and typically constitute 0.1% to 0.5% of the total nucleated cells. Key markers expressed by human pDCs include CD303 (BDCA2) and CD123 (IL-3RA) [39–41].
Tumor-infiltrating DCs
Following tumorigenesis, DCs infiltrate into solid tumors, which are referred to as tumor-infiltrating DCs (TIDCs) within the TME. TIDCs encompass a diverse array of cell types, including cDCs and pDCs [42]. TIDCs are capable of presenting tumor antigens to T cells, thereby facilitating the tumor-immunity cycle. Reports indicate the clinical significance of TIDCs in various solid tumors, showing a strong correlation between TIDCs and clinical prognosis [43, 44]. However, TIDCs frequently show reduced antigen cross-presentation capacities [45]. A characteristic feature of immunologically dysfunctional TIDCs is an increased intracellular lipid content and augmented mitochondrial respiration. Within the TME, numerous immunosuppressive factors impede the maturation and antigen-presenting function of DCs function of DCs. For example, the expression of MHC class I and II molecules and their regulatory factors (such as CIITA) is reduced on TIDCs, along with downregulation of genes encoding ER-resident aminopeptidases (ERAP) and antigen processing-related transporters (TAP) [46]. The density and activation status of TIDCs can be determined by various molecules, with S-100/CD1a and CD83/DC-LAMP being the most widely used. Bell et al. confirmed that immature DCs expressing CD1a and langerin are distributed throughout tumors in breast cancer, while CD83+ and DC-LAMP+ (mature DCs) are localized in the peritumoral region [47]. Additionally, emerging studies have established lipid accumulation in dendritic cells (DCs) as a critical mechanism driving the dysfunction observed in TIDCs [48, 49]. The studies mentioned above indicate that to facilitate the completion of the tumor-immunity cycle, it is imperative not only to harness cytokines to attract an increased infiltration of DCs into the neoplastic tissue but also to promote antigen presentation and maturation of DCs effectively.
Innate activation of DCs
The transmission of innate immunological signals is essential for triggering T and B cell responses. Central to this mechanism are DCs, whose activation by innate signals can induce their differentiation into immunogenic APCs and facilitate the transmission of information to lymphocytes. In this context, our main goals are to clarify the processes by which DCs are activated by innate immunological signals and investigate the impact of these processes on the immune response.
TLRs are well-known pattern recognition receptors (PRR) that can effectively activate DCs. A wide variety of TLR ligands function as adjuvants to enhance antigen cross-presentation [50, 51]. The actions of TLRs on DCs result in increased expression of MHC-peptide complexes, co-stimulatory molecules, and immune-regulatory cytokines, thereby fostering the initiation and activation of T cells. Except for TLR3, all TLRs activate TGF-β-activated kinase 1 via the adapter molecule MyD88 on DCs, subsequently activating the MAPK and NF-κB signaling pathways. This activation leads to the production of tumor necrosis factor-alpha (TNF-α), IL-12, and IL-6. cDC1s typically express most TLRs, except TLR5 and TLR7, with particularly high expression of TLR3. Human cDC1s express TLRs and secrete proinflammatory cytokines, including IL-12p70 and IFN-α, in response to infection-induced Th1 responses. Comparable to other steady-state DC subsets, cDC2s express a variety of TLRs but show higher levels of NOD-like receptor thermal protein domain associated proteins (NLRPs) and other inflammation-related signaling molecules, suggesting their functional specificity in identifying distinct danger signals. They usually secrete elevated levels of inflammatory cytokines, such as IL-6 and IL-8, and typically possess a mature phenotype in the lung-draining lymph nodes. They are distributed in the B cell zones of lymph nodes and are generally associated with antigen presentation to CD4+ T cells. While TLR1, 2, 4, 6, 8, and 9 are expressed in all examined DC subsets, the expression of TLR3, 5, and 7 varies significantly among DC subpopulations [52]. pDCs express high levels of intracellular nucleic acid-sensing TLRs, such as TLR7 and TLR9, which respectively recognize single-stranded RNA and unmethylated CpG-containing DNA sequences. pDCs respond to these nucleic acids by secreting a substantial amount of IFN-I and activating ISGs in other target cells [53]. Human pDCs do not express TLR4 and are unresponsive to lipopolysaccharide (LPS). On the other hand, while having low TLR4 expression, CD11c + DCs and monocyte-derived DCs (Mo-DCs) show considerable sensitivity to LPS activation [54, 55].
Despite TLR's potent ability to activate DCs, the specific activation mechanism remains unclear. Currently, it is believed that TLRs act on DCs by activating various members of the IRF family, thereby inducing IFN-I responses [56]. Notably, Hoshino et al. showed a significant decrease in the activation of mouse DCs when treated with TLR4 or TLR9 agonists in the absence of STAT-1 or IFNAR [57]. Another study revealed that IFN-I is a critical mediator induced by TLR7 agonist for cross-presentation [58]. Additionally, DCs from IFN-αβR−/− mice exhibit a significant deficiency in stimulating T cells compared to DCs from wild-type (WT) mice. Therefore, the complete activation of DCs in response to TLR signals largely depends on the production of IFN-I.
Bacteria and viruses that invade the body can activate the RIG-I/MDA5-MAVS, cGAS-STING, and TLR-MyD88 pathways, which are the canonical pathways of IFN-I activation [59]. Seng-Ryong Woo et al. demonstrated that host cell STING and IRF3 play essential roles in initiating spontaneous CD8+ T cell responses against immunogenic tumors. This was observed in mice lacking MyD88, TLR4, TLR9, TRIF, P2X7R, MAVS, STING, and IRF3 [60]. Furthermore, the group illustrated that DNA derived from tumor cells activates IFN-β production and DCs via the cGAS, STING, and IRF3 pathways. Additionally, other studies have shown that the adaptor protein STING, rather than MyD88, is essential for the IFN-I-dependent antitumor effects mediated by radiation [61]. Based on these data, it appears that the DCs' cGAS-STING pathway is essential for the innate immune system to recognize DNA originating from tumors.
DCs in cancer
Function-wise, cDC1s support CD8 + T cell responses and demonstrate strong antigen cross-presentation capacities. Additionally, cDC1s play a pivotal role in promoting tumor rejection responses. Studies have shown that mice lacking cDC1 expression in the tumor microenvironment (TME), either entirely or partially, exhibit a failure to generate antitumor responses [62]. Mice with a deficit in Batf3, for example, have impaired cDC1 formation and maturation, are unable to activate tumor-specific cytotoxic T cells, and are unable to generate effective antiviral responses [19, 63, 64]. In addition, recent findings underscore the importance of cDC1s in CD4+ T cell activation, with a few studies reporting that deficiency of cDC1s leads to a failure of CD4+ T cell activation [65, 66]. Although cDC1s are not very abundant in the TME, there is a positive association between their abundance and patient survival rates, which can be used as a measure of how well ICI therapy is working [18, 67].
cDC2s possess the capacity to elicit antitumor immunity [68]. They produce various cytokines, such as IL-10 and IL-23, and present TAAs to CD4+ T cells or transfer them to resident DCs within lymphoid tissues. cCD2s activate effector T cells, primarily Tregs, encompassing Th2 and Th17 cells responsible for immune tolerance. However, in both human and murine models, cDC2s are associated with a positive prognosis. For this reason, both cDC1s and cDC2s are beneficial for tumor immunity.
pDCs play an indispensable role in initiating antiviral immune responses. In mice, pDCs respond to CpG ODN and virus genomes with similar structures via TLR9 activation [69]. Given that pDCs are recognized as key players in the defense against viral infections [70], TLR9's primary physiological function likely involves the recognition of viral DNA. However, the role of pDCs in cancer is multifaceted. Conversely, the presence of pDCs within tumors has been linked to unfavorable outcomes in a number of malignancies, such as melanoma, ovarian cancer, breast cancer, and head and neck cancer [71–75]. Potential mechanisms may include pDCs inducing the co-stimulatory ligand inducible T cell co-stimulator, thereby promoting the expansion of Tregs [76]. Additionally, tumor-associated pDCs can activate Foxp3+ Tregs through indoleamine 2,3-dioxygenase (IDO) [77]. In patients with melanoma, pDCs express IDO, resulting in tryptophan depletion, impaired T cell function, and immune tolerance suggesting that pDCs can also elicit immunosuppressive responses. Furthermore, the infiltration of pDCs into tumors leads to reduced levels of IFN-α, sustaining the expansion of intratumoral Foxp3+ Tregs, thereby promoting immune tolerance and tumor progression.
Positively, pDCs have the ability tocan trigger immune-stimulatory reactions [78]. For example, a higher infiltration of pDCs has been significantly associated with non-progression and prolonged overall survival in patients with colon cancer. Functional deficits in pDCs have been observed across various cancer subtypes, characterized by attenuated responses to TLR7 or TLR9 activation and/or impaired IFN-I responses [74]. Furthermore, activation of pDCs has been demonstrated to induce antitumor immune responses, with several clinical trials employing pDCs demonstrating significant clinical benefits in human cancer treatment, underscoring the potential of pDCs in fostering antitumor immunity. Within the peritumoral region of primary melanoma, CD123+ pDCs closely interact with CD8+ T cells. Functional analyses have revealed that both human and murine pDCs can activate CD8+ T cells, leading to their differentiation into cytolytic and IFN-γ-producing effector T cells, thereby promoting tumor regression in vivo. In the TME, human CD2high pDCs exhibit elevated levels of granzyme B, TRAIL, and lysozyme, which curtail tumor cell proliferation and mediate contact-dependent killing of tumor cells. Additionally, CD2high pDCs efficiently secrete IL-12p70, stimulating naive T cells and resulting in T cell expansion and immune responses. Therefore, pDCs serve a dual role in tumor development, both promoting and inhibiting tumor growth. However, further investigation is required to elucidate the antigen presentation capabilities of pDCs.
In summary, DCs play a crucial role in maintaining immune homeostasis. However, the precise functions and significant contributions of individual DC subsets in maintaining immune equilibrium remain unclear. In addition to the aforementioned subtypes, DCs also exhibit other variations, including Langerhans cells (LCs) and Mo-DCs. Cross-presentation is a pivotal mechanism by which CD8+ T cells respond to exogenous antigens, especially those originating from deceased cells. Current research predominantly attributes the cross-presentation of tumor antigens to CD8α+ DCs. Nonetheless, intrinsically generated Mo-DCs can also efficiently participate in cross-presentation through a vesicular-dependent pathway [30]. Furthermore, unlike their murine counterparts, human pDCs have been reported to proficiently cross-present soluble cell-associated antigens [79]. Notably, a study showed that LCs adeptly cross-present antigens and promote the expansion of CD8+ T cells both in vitro and in vivo [58]. While most DC subtypes can engage in cross-presentation under specific experimental conditions, the optimal delivery of tumor cell-related antigens seems to be primarily limited to a subset of cDC1s [29]. Thus, further research is warranted to elucidate the specific roles of distinct DC subtypes in the TME during various stages of tumor development, which should establish a foundation for the advancement of cancer therapeutics.
cGAS-STING-IFN-I axis mediates anti-tumor immune response
Numerous studies conducted since the discovery of cGAS have revealed a tight relationship between cancer immunotherapy and the cGAS-STING pathway [80–83]. Classical theories postulate that the cGAS-STING pathway triggers the IRF3-mediated IFN-I pathway and the NF-κB-mediated inflammatory pathway [84]. Recent studies have uncovered additional roles of the cGAS-STING pathway, including its involvement in autophagy, endoplasmic reticulum stress, and the regulation of cellular metabolism [85–88]. In this section, we provide an overview of both IFN-dependent and IFN-independent STING-mediated antitumor responses. We also investigate the known and possible mechanisms of DCs' response to STING.
IFN-I-dependent cGAS-STING signaling-mediated tumor immune response
In recent years, mounting evidence suggests that, the integrity of the IFN-I signaling pathway is essential for radiotherapy, chemotherapy, targeted therapy, and immunotherapy [61, 89–91]. One significant reason for this is that normal intracellular expression of IFN-I serves as a form of immune surveillance, helping to prevent the development of tumors [92]. For instance, human breast cancer bone metastasis often occurs in conjunction with the presence of IFN-I-deficient cancer cells, attributed to reduced levels of IRF7 expression [93]. Reintroduction of IRF7 to IRF7-deficient tumor cells or supplementation with IFN-α effectively inhibits bone metastasis in a mouse model of breast cancer [93]. Furthermore, deficiency of either IFNAR1 or IFNAR2 increases the incidence of methylcholanthrene-induced fibrosarcoma in mice [94]. Ifnar1−/− mice exhibit more severe chemically induced skin papilloma compared to their WT counterparts [95]. Therefore, the body's normal IFN-I signaling is frequently necessary for cancer immune surveillance.
As a critical component of intracellular DNA recognition, the cGAS-STING pathway primarily modulates tumor immune functions by eliciting the expression of IFN-I. For instance, in several mouse tumor models, tumors exhibit accelerated growth in mice deficient in cGAS or STING, concomitant with a diminished functionality of DCs [60, 61, 96]. Notably, the impairment in DC function was ameliorated by exogenous IFN-β treatment, thereby rescuing the cross-priming capability of cGAS or STING-deficient DCs [60].
Cellular DNA normally resides in the cell nucleus or mitochondria in a homeostatic state to avert spontaneous inflammatory reactions [97]. However, due to uncontrolled cell division and defective DNA repair, tumor cells often exhibit extensive DNA mutations and genomic instability. Damaged DNA or chromatin fragments are typically located within micronuclei (MN), and the membranes of these MN are highly prone to breakdown [98], increasing the likelihood of DNA leakage into the cytoplasm [99]. Studies have demonstrated that chemotherapy drugs can induce the production of immunogenic MN to initiate antitumor immune responses [100]. A significant portion of these MN co-localizes with cGAS, leading to the activation of the IFN-I response in these cells [101]. Therefore, the generation of mitotically dependent immunogenic MN can effectively activate the cGAS-STING pathway, thereby eliciting a systemic antitumor immune response.
However, many tumor cells employ immune evasion mechanisms by inhibiting this pathway. Therefore, effectively activating the cGAS-STING pathway within tumor cells emerges as a critical strategy to enhance the effectiveness of cancer treatment. Our prior research showed that the utilization of the demethylating drug zebularine induces the accumulation of DNA in the cytoplasm of tumor cells, enhances the expression of the STING gene, triggers the generation of IFN-I and the expression of ISGs, thereby increasing the sensitivity of tumor cells to the cGAS-STING pathway [102]. Subsequently, another study revealed that the reversal of STING methylation in mouse melanoma cell lines using a clinically available DNA methylation inhibitor, decitabine, ameliorates STING agonist-induced activation and IFN-I induction, thereby enhancing the CD8+ T-cell-dependent immune response that promotes tumor regression [103]. Several studies have reported that demethylating drugs effectively stimulate the activation of endogenous retrovirus (ERV) within cells, subsequently activating the cGAS-STING and RIG-I-MAVS pathways and enhancing the expression of IFN-I to bolster antitumor immune activity [104, 105].
Furthermore, the cGAS-STING pathway is widely expressed in immune cells such as DCs, macrophages, and T cells. The activation of this pathway effectively promotes the maturation of these immune cells. Notably, recent studies have revealed that a substantial number of tumor cells undergo cell death as a result of chemotherapy or radiotherapy, leading to the release of double-stranded DNA. DCs engulf this released DNA, which subsequently accumulates in their cytoplasm, thereby triggering the activation of the cGAS-STING signaling pathway. This activation leads to the robust production of IFN-I and inflammatory cytokines, such as TNF-α and IL-6. Thus, the intact cGAS-STING signaling pathway serves as a pivotal determinant not only for tumor cells but also for regulating the antitumor immune response in immune cells.
IFN-I-independent STING-mediated tumor therapy
The antitumor response facilitated by STING extends beyond its conventional reliance on the cGAS-STING pathway and IFN-I. Recent studies have revealed that STING can influence tumor dynamics through various mechanisms such as IFN-independent mechanisms (Fig. 3) [80].
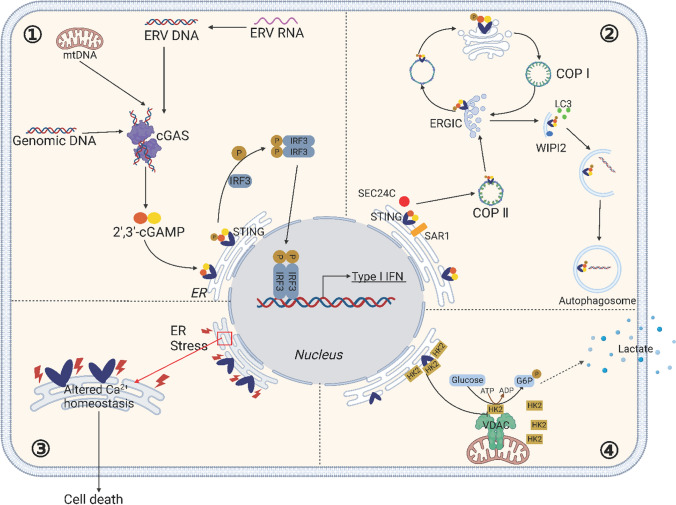
Fig. 3.
The Versatile Mechanisms of STING. ①cGAS-STING signaling pathway. The cGAS recognizes endogenous viral reverse-transcribed DNA or its DNA (from mitochondria or tumor cells). cGAS recognizes the DNA and then synthesizes the second messenger, 2′3'-cGAMP, which activates STING. The translocation of STING from the endoplasmic reticulum to the Golgi apparatus is a prerequisite for its involvement in downstream signaling and the regulation of IFN-I transcription. Activated STING recruits and activates TBK1, which phosphorylates IRF3. Subsequently, phosphorylated IRF3 forms a dimer and translocate to the nucleus, where it initiates the expression of immunomodulatory factors such as IFN-I. ② STING and cellular autophagy. DNA from pathogens or damaged cells activates cGAS to synthesize cGAMP. cGAMP binds to STING and triggers the translocation of STING from the ER to the ERGIC and the Golgi apparatus, a process that is dependent on SAR1, SEC24C. The ERGIC, which contains STING, serves as a membrane source for LC3 recruit and lipolysis through a WIPI2-dependent mechanism. The LC3-positive membranes target DNA and pathogens to autophagosomes, which subsequently fuse with lysosomes. Accumulation of STING in lysosomes leads to lysosomal membrane permeabilization, which in turn leads to lysosome-dependent cell death. ③: STING and endoplasmic reticulum stress Certain mutations in STING disrupt calcium homeostasis in T cells, which induces ER stress, leading to cell death. ④: STING and cellular metabolism. STING binds to hexokinase 2 (HK2), the rate-limiting enzyme of glycolysis, and restricts the mitochondrial localization of HK2, thereby inhibiting its activity and, consequently, the process of glycolysis. ERV, Endogenous Retrovirus; ER, Endoplasmic reticulum; ERGIC, The ER-Golgi intermediate compart; COP I, coat protein I; COP II, coat protein II; VDAC, voltage-dependent anion channel
In 2009, Saitoh et al. reported the colocalization of STING and LC3 [106], implying an association between STING activation and cellular autophagy. In 2019, Xiang et al. systematically elucidated STING's role in autophagy [85]. They demonstrated that STING can activate autophagy through mechanisms independent of TBK1 activation and IFN-I induction, elucidating an ancient and highly conserved function of the cGAS-STING pathway in autophagy induction that predates the emergence of the IFN-I pathway in vertebrates. Subsequent studies revealed that STING activation not only induces classical autophagy but also triggers non-canonical autophagy pathways [107]. Additionally, STING can act as a proton channel mediating Golgi proton efflux, resulting in pH elevation and facilitating non-canonical autophagy and NLRP3 inflammasome activation [108]. Recent investigations have revealed that activated STING not only facilitates direct tumor cell death through the induction of autophagy but also mediates intercellular transfer to bolster anti-tumor immunity [109]. Mechanistically, this research has identified that RAB22A mediates STING-induced non-canonical autophagy. RAB22A-mediated atypical autophagosomes encapsulate activated STING, merging with RAB22A-positive early endosomes to form a novel structure, termed the Rafesome [109]. The Rafesome can then be secreted by the cell to extracellular space, forming RAB22A-induced extracellular vesicles (R-EVs). These STING-bearing R-EVs are competent to transfer between tumor cells, eliciting the secretion of IFNβ and thereby propelling anti-tumor immunity [109]. These results provide opportunities to investigate the possible involvement of STING in different downstream pathways related to different physiological processes. Such studies may significantly impact the development of STING-based treatment approaches.
The NF-κB-mediated inflammatory response constitutes a significant downstream consequence of STING activation. A recent investigation highlighted the pivotal role of the NF-κB-mediated inflammatory pathway in the antitumor effects of STING agonists [110]. In mice harboring the STING-∆CTT mutant, characterized by a C-terminal deletion of STING that interferes with its interaction with the downstream TBK1 protein, the activation of both IRF3 and NF-κB is compromised, while STING-mediated autophagy remains unaffected. Conversely, in STING-S365A mutant mice, a mutation that disrupts STING's ability to bind with IRF3, resulting in decreased IFN-I production, the activation of NF-κB and autophagic responses still occur. Notably, both WT and STING-S365A mutant mice exhibit robust antitumor immune responses when exposed to cGAMP in the LL2 lung cancer model, whereas such responses are conspicuously absent in ∆CTT mutant mice. These findings suggest that the antitumor responses induced by STING activation are contingent upon NF-κB-induced inflammation rather than autophagy.
STING also functions as an intracellular metabolic checkpoint, limiting aerobic glycolysis to promote antitumor immune responses [111]. Recently, Zhang et al. elucidated STING's role in curbing aerobic glycolysis, demonstrating that this function operates independently of its innate immune functions [112]. In this mechanical process, STING binds to the rate-limiting enzyme of glycolysis, hexokinase II (HK2), restraining HK2's mitochondrial localization, thereby inhibiting its activity and consequently suppressing glycolysis [112]. Thus, STING exerts inhibitory control over HK2 to curtail tumor aerobic glycolysis, ultimately fostering in vivo antitumor immunity. This discovery provides novel insights into the development of STING-based anticancer drugs and sheds light on why STING remains quiescent or is expressed at subdued levels in various cancer cells.
cGAS-STING-IFN-I axis and DC activation
IFN-I exerts diverse effects on the activation and function of DCs. IFN-I can facilitate DC maturation by upregulating the expression of surface molecules such as CD80, CD83, CD86, and MHC class molecules, thereby enhancing their antigen presentation capacity. Additionally, IFN-I can induce DCs to express a spectrum of ISGs, further promoting the activation and proliferation of T cells. It is noteworthy that distinct DC subtypes may exhibit varying sensitivity to IFN-I, potentially resulting in heterogeneous responses.
IFN-I has been shown to facilitate cross-priming of CD8+ T cells to soluble antigens and plays a crucial role in activating DCs [113]. IFN-I treatment enhances antigen retention within DCs and decreases the acidification rate of intracellular endo-lysosomes [114]. A study validated that IFN-I promotes sustained antigen presence in CD8α+ DCs by regulating intracellular pH, consequently bolstering antigen cross-presentation by CD8α+ DCs [114]. Similar investigations have also demonstrated that IFN-secreting DCs significantly prolong the intracellular presence of antigen and decelerate intracellular acidification following OVA antigen uptake, consequently increasing MHC-I cross-presentation of the antigen [115]. Moreover, IFN-I promotes the transcription and translation of immunoproteasome subunits β1i (LMP2), β2i (MECL-1), and β5i (LMP7), thus enhancing peptide binding and presentation on MHC-I [116, 117]. Additionally, IFN-α not only promotes the localization of MHC-I to antigen storage compartments within DCs but also elevates the levels of MHC-I and MHC-II on the cell membrane [115]. Furthermore, IFN-α enhances the activation of CD8+ T cells by upregulating the surface expression of the stimulatory molecules CD40, CD80, and CD86 on DCs, as well as intracellular signaling in T cells [118].
As is well known, IFN-I primarily exerts its effects through downstream ISGs. IFN-I regulates the expression of numerous ISGs by activating the JAK-STAT pathway, thereby modulating the immune response [119]. To investigate the impact of ISGs on DC activity, a specific subset of DCs, characterized by a high expression of ISGs, has been identified as ISG+ DCs [113]. These ISG+ DCs lose their ability to induce effective antitumor T cell responses in Ifnar1−/− mice. AXL, an IFN-induced engulfment receptor, has also been validated as a phenotypic marker for ISG+ DCs [120]. In colorectal cancer and melanoma, a small population of unactivated ISG+ DCs has been observed, which promote CD8+ T cells to attack tumor cells upon IFN-I treatment. While the specific ISGs responsible for mediating DC activation still need further identification, the expression analysis of various ISGs, including STAT1, MX dynamin-like GTPase 1 (MX1), C-X-C motif chemokine ligand (CXCL) 10, CXCL9, C–C motif chemokine ligand (CCL) 4, CCL5, HLA class I genes, absent in melanoma 2 (AIM2), and guanylate binding protein 1 (GBP1), in breast carcinoma has demonstrated predictive value for a favorable long-term outcome [121].
As previously mentioned, IFN-I can induce the maturation of DCs and supply tumor-specific antigens to CD4+ and protective CD8+ T cells [115]. Burnette et al. conducted experiments employing MyD88-deficient mice, TRIF-deficient mice, cGAS-deficient mice, and STING-deficient mice, and validated that IFN-I induced by radiotherapy can be attributed to the cGAS-STING pathway [61]. However, the robust antitumor effects observed in STING-induced IFN-I-independent functions suggest that other mechanisms, such as metabolic regulation and NF-κB-induced inflammatory functions, may also play significant roles in DC activation. Further research is required to fully elucidate the comprehensive regulation of STING-mediated antitumor effects.
cGAS-STING-IFN-I signaling axis as a master regulator in the cancer immunity cycle
As a multifunctional signaling axis, cGAS-STING-IFN-I participates in nearly all stages of the tumor immune cycle, playing a pivotal role in the tumorigenesis process. Here, we will elucidate the roles and distinctions of IFN-I treatment and cGAS-STING pathway activation in the tumor immune cycle (Fig. 4).
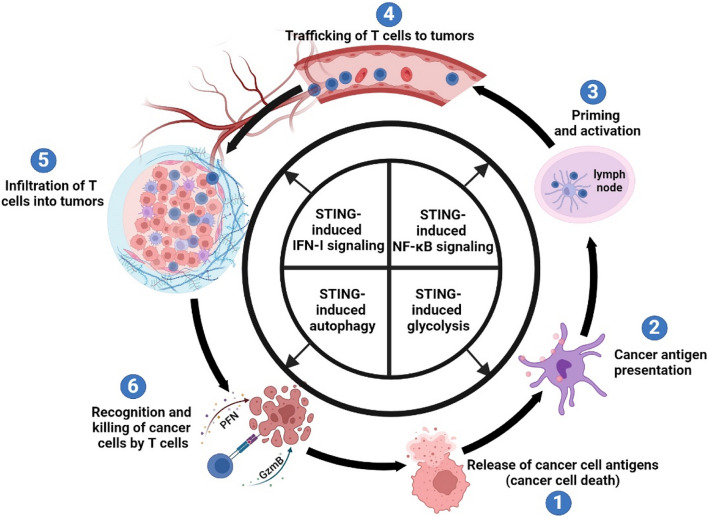
Fig. 4.
Activation of the cGAS-STING-IFN-I signaling axis positively regulates each step of the cancer-immunity cycle. The activation of the cGAS-STING pathway influences cancer immune responses through both IFN-I-dependent and IFN-I-independent mechanisms, involving autophagy, inflammation, and glycolysis
One primary source of tumor antigens is tumor cell death. IFN-I directly inhibits tumor growth by inducing cell apoptosis, impeding cell cycle progression, and regulating tumor cell proliferation and differentiation [122, 123]. While the capacity of IFN-I to induce cell death in malignant cells has been recognized for over three decades, the underlying mechanisms of IFN-I action appear to be rather complex [124]. Nevertheless, IFN-I signaling has frequently been associated with apoptosis, involving widespread caspase activation [125]. Furthermore, IFN-I can inhibit tumor growth by suppressing angiogenesis. The inhibition of IFN-I on angiogenesis is primarily attributed to the upregulation of interferon-stimulated genes (ISGs) that suppress angiogenesis. ISGs such as STAT1, PML, IRF7, GBP1, ISG20, miR-106, and miR-16 exert a direct anti-angiogenic effect [121]. IFN-I also plays a crucial role in DC activation and antigen presentation. Recent studies indicate that IFN-I promotes the terminal differentiation and maturation of DCs, leading to increased surface expression of MHC-I, thereby enhancing immune recognition capabilities [118, 126–128]. This cytokine is essential for activating tumor-specific T cells and facilitating tumor elimination [129]. In the TME, CXCL9, CCL5, and CXCL10 are associated with CD8+ T cell infiltration [130]. IFN-I can induce the expression of CXCL9 and CXCL10, thereby regulating the trafficking and infiltration of CD8+ T cells [131].
Indeed, activation of the cGAS-STING pathway not only induces IFN-I for participation in the cancer immune cycle but also exhibits unique characteristics. For instance, while IFN-I induces cell apoptosis through various pathways, the cGAS-STING pathway can trigger IFN-independent autophagy in tumors. Moreover, the activation of the NF-κB pathway and glycolysis by the cGAS-STING pathway effectively promotes DC activation and immune cell infiltration in the TME.
In addition, a recent study [86] revealed that chromosomal instability (CIN) in tumor cells leads to sustained activation of the STING pathway. Notably, despite the persistent activation of the cGAS-STING pathway within tumor cells, it not only fails to promote the immune system's ability to eliminate these cells but also facilitates tumor migration. Specifically, this chronic activation of STING does not trigger the IFN-I response associated with tumor immune clearance. Instead, it initiates endoplasmic reticulum stress, leading to a pro-migratory TME and ultimately facilitating tumor immune evasion. The utilization of STING inhibitors has been shown to reduce metastasis in melanoma, breast cancer, and colorectal cancer driven by CIN [132]. This discovery establishes a theoretical basis for therapeutic strategies targeting CIN in tumor cells. Additionally, it underscores the intricate relationship between the activation status of STING in tumor cells and cancer treatment, connecting its role in immune and inflammatory functions with autophagy, metabolism, and the endoplasmic reticulum stress response.
STING agonists not only stimulate the production of IFN-I and the expression of ISGs but also activate cellular stress and death pathways [133]. In fact, within T cells, the activation of STING promotes T cell apoptosis [134]. Research has revealed the elevated production of the Noxa protein (a protein associated with cell death) following STING activation, resulting in cell apoptosis. STING induces T cell apoptosis by modulating endoplasmic reticulum stress and the unfolded protein response, leading to hyperactivation and cell death [135]. Through experiments involving STING S365A mutant mice, Wu et al. demonstrated that STING agonists robustly induce apoptosis in CD8+ T cells. Importantly, they showed that this effect occurs independently of the STING-induced IFN signaling pathway [136]. These findings indicate that tumor cells can trigger STING-mediated T cell apoptosis, facilitating immune evasion. Consequently, optimizing the dosage of STING agonists is crucial in tumor immunotherapy, striking a delicate balance between maximizing therapeutic benefits and preventing T cell apoptosis to enhance cancer treatment outcomes.
Advances in the development of clinical drugs targeting the cGAS-STING-IFN I signaling axis
IFN-I has served as a potent immunomodulatory agent in clinical cancer therapy for over 30 years. However, its utility has been hampered by a short half-life and the onset of severe flu-like symptoms associated with its systemic administration. Recent years have witnessed a resurgence in the clinical application of IFN-I, owing to innovations such as antibody-conjugated IFN-I and the emergence of gene therapy. Additionally, the discovery of the cGAS-STING pathway has revealed novel avenues in cancer treatment, with STING agonists displaying remarkable potentials. STING agonists act as upstream signaling molecules for IFN-I, orchestrating a range of downstream responses, including IFN-I-independent autophagy, inflammation, endoplasmic reticulum stress, and metabolic regulation. Therefore, the development of STING agonists presents a broader scope within the realm of cancer therapy compared to IFN-I. Currently, drugs targeting the cGAS-STING-IFN-I signaling axis encompass diverse formats, such as protein drugs, small-molecule drugs, and gene therapy drugs (Table 1). We summarize the difficulties related to the cGAS-STING-IFN-I signaling axis and give an overview of the clinical advances made with these medications in the section that follows.
Table 1.
STING agonists in partial clinical trials
| STING agonists | Drug name | Condition | Species | Method of administration | Study Phase |
|---|---|---|---|---|---|
| DMXAA | Vadimezan | Mouse lung cancer, mesothelioma, human lung cancer and prostate cancer | Human, mouse | Intraperitoneal Injections | III |
| CDN | ADU-S100 [148] | Metastatic solid tumor or lymphoma | Human, mouse | Intertumoral Injections | I |
| IMSA101 | Breast cancer, malignant solid tumors | Human, mouse | Intertumoral Injections | I / IIa | |
| MK1454 [171] | Advanced/Metastatic Solid Tumors, Lymphoma, metastatic or unresectable recurrent squamous cell carcinoma of the head and neck | Human, mouse | Intertumoral Injections | II | |
| MK2118 | Advanced/metastatic solid tumor or lymphoma | Human, mouse | Intertumoral Administration | I | |
| BI-1387446 [172] | Advanced, unresectable and or metastatic solid tumors | Human, mouse | Intertumoral Administration | I | |
| TAK-676 [173] | Advanced non-small cell lung cancer, triple-negative breast cancer, or squamous cell carcinoma of the head and neck | Human, mouse | Intravenous injection | I | |
| E-7766 [174] | High-risk non-muscle invasive bladder cancer | Human, mouse | Intertumoral Administration | I | |
| BMS-986301 | Squamous cell carcinoma of the head and neck | Human, mouse | Intramuscular, intravenous and intra-tumoral administration | I | |
| SB-11285 [175] | Squamous cell carcinoma of the head and neck | Human, mouse | Intravenous injection | I / II | |
| DN-015089 | advanced solid tumor | Human, mouse | Subcutaneous or Intertumoral Injections | Ia / Ib | |
| VB-85247 [176] | Non-muscle invasive bladder cancer | Mouse | Intravesical drip | Preclinical studies | |
| ONM-501 | Advanced malignant solid tumors, lymphomas | Human、mouse | Subcutaneous or Intertumoral Injections | I | |
| STING agonists—ADC | JAB-BX400 | solid tumor | Human | None | Preclinical studies |
| JAB-X1800 | solid tumor | Human | None | Preclinical studies | |
| XMT-2056 | gastric and breast cancer | Human, mouse | Systemic administration | Preclinical studies | |
| IMSA201 | solid tumor | Human, mouse | none | Preclinical studies | |
| engineering bacteria | SYNB1891 [177] | Melanoma, advanced solid tumors or lymphomas | Human, mouse | Intertumoral Administration | I |
| Others | diABZI STING agonist [178] | colorectal cancer | Mouse | Intravenous injection | Preclinical studies |
| MSA-2 [179] | colorectal cancer | Mouse | Oral | Preclinical studies | |
| SR-717 [180] | Melanoma, colorectal cancer | Mouse | Intraperitoneal Injections | Preclinical studies | |
| SNX-281 | Solid tumors, lymphomas | Human, mouse | Intravenous injection | I | |
| XMT-2056 | HER2-positive breast cancer | Huma | Intravenous injection | I |
Progress in the clinical development of IFN-I drugs
IFN-I was initially approved for the high-dose treatment of various hematologic malignancies, including chronic lymphocytic leukemia [137]. It was believed that IFN-α's anti-angiogenic properties play a pivotal role in its antitumor efficacy by influencing tumor vasculature [138]. Subsequently, IFN-α received approval for the treatment of follicular non-Hodgkin's lymphoma, melanoma, and AIDS-related Kaposi's sarcoma [139]. However, the administration of high-dose IFN-α via intravenous route leads to acute symptoms, including flu-like reactions, thrombocytopenia, leukopenia, neutropenia, and depression. Therefore, the development of novel IFN-I agents and localized treatment approaches has emerged as a primary focus in the field of IFN drug development.
Polyethylene glycol IFN (PEG-IFN), designed to enhance IFN stability and extend its half-life, is commonly employed in the treatment of specific tumor types. In the context of chronic myeloid leukemia, combining PEG-IFN with targeted therapeutic drugs, such as imatinib, has proven effective in improving treatment outcomes [140]. Furthermore, conjugating IFN-I with antibodies represents another effective strategy for improving the efficacy of IFN-I therapy. For example, heterodimers of anti-CD20 antibodies and IFN-α fusion proteins (IGN002), which incorporate rituximab's antigen-binding sequences and full-length human IgG1/κ constant regions, are in development for the treatment of B cell lymphoma [141]. Yang et al. demonstrated the efficacy of antibody-IFN-β fusion proteins against various solid tumors in mice, targeting the epidermal growth factor receptor (EGFR) and Her2/neu [142]. Given the robust immune-activating properties of PD-L1 antibodies and IFN-α, the outlook for PD-L1 antibody-IFN-α fusion proteins in cancer treatment appears promising.
An illustrative case involved the incorporation of IFN-β into the VSV virus for the treatment of liver cancer in mice, rats, and rhesus monkeys [143]. Furthermore, in a phase IB clinical trial utilizing an oncolytic virus, the G207 vector derived from HSV-1, featuring a γ34.5 deletion, demonstrated the ability to induce heightened inflammatory responses and increased ISG expression [144]. Therefore, enhancing the expression of IFN-β during oncolytic virus therapy may more effectively reinforce the antitumor immune processes mediated by the virus. Nevertheless, given that IFN-I can effectively activate antiviral immune responses, the integration of IFN-I into viral particles for gene therapy still presents daunting challenges.
Progress in the clinical development of STING-agonist drugs
The flavone acetic acid derivative DMXAA, known for its vascular-disrupting capabilities on established tumor vasculature, induces tumor cell death without affecting normal tissues [145]. Subsequently, DMXAA was identified as an agonist for STING. In murine tumor experiments, DMXAA demonstrated promise, reducing mouse fibrosarcoma size and increasing specific T cell counts [146]. In a phase II clinical trial, a combination therapy of DMXAA with carboplatin (CBP) and paclitaxel exhibited superior efficacy in treating advanced non-small cell lung cancer compared to CBP and paclitaxel alone [147]. However, these favorable outcomes were not replicated in a phase III clinical trial, leading to its discontinuation. This discrepancy arises from the species-specific nature of DMXAA, as it is not sensitive to human STING activation. Therefore, the primary focus in developing STING agonists has shifted towards human STING activation.
ADU-S100/MIW815 is a synthetic cyclic dinucleotide that mimics the natural ligand required for the activation of human STING [148]. Preclinical studies have shown the induction of tumor-specific CD8+ T cells through intratumoral administration of ADU-S100 [148]. However, clinical research has indicated that ADU-S100 as a monotherapy exhibits limited efficacy. Nonetheless, when combined with PD-1 antibody drugs, it demonstrates significant antitumor effects [149]. In a phase Ib clinical trial (NCT03172936) assessing the effectiveness and safety of spartalizumab in combination with ADU-S100 for patients with advanced solid tumors or lymphomas, only five patients exhibited significant responses, comprising two complete responses and three partial responses, resulting in a 9.4% response rate. Additionally, only 6 out of 30 patients achieved stable disease (SD) in the monthly injection group [149]. Another STING agonist, MK-1454, did not lead to any remissions when used as monotherapy, as indicated by preliminary data presented at the 2018 ESMO conference (Abstract 5475). However, when used in combination with the PD-1 inhibitor pembrolizumab, a partial response rate of 24% was observed. Preclinical results for STING agonists, such as SB11285 and MSA-2, have demonstrated significant antitumor effects. However, more clinical studies are necessary.
These clinical findings suggest that the therapeutic efficacy of STING agonists as monotherapy in cancer treatment is limited for several reasons. Firstly, a complete tumor immune cycle necessitates the release of antigens, a process in which STING agonists alone exhibit restricted effectiveness. Secondly, the key step in activating antitumor immunity involves alleviating immune checkpoint inhibition on T cells. However, STING agonists not only fail to downregulate the expression of PD-1/PD-L1 but also significantly enhance the expression of multiple immune checkpoints [150]. Thirdly, in clinical trials, achieving optimal therapeutic outcomes with low doses of STING agonists poses challenges, while high doses may lead to severe adverse reactions. In addition to common side effects, such as injection site pain, fever, fatigue, and itching, high-dose STING agonists induce apoptosis in CD8+ T cells, compromising the immune response. These challenges complicate safety assessments during regulatory scrutiny. Lastly, prolonged exposure to high doses of STING agonists can induce tolerance in target cells, thereby promoting tumor formation. Therefore, it is imperative to investigate the optimal dosage and duration of STING agonist administration.
In addition, the issues of drug delivery and off-target effects present significant challenges in the development of small-molecule STING agonists. A recent study involved the genetic engineering of bacteria for the purpose of delivering STING agonists [151]. Escherichia coli Nissle 1917 carrying the diadenosine cyclase gene, when injected into melanoma mouse tumors, was observed within the tumor mass but not in the subcutaneous space surrounding the tumor. This localization contributed to a reduction in tumor size [151]. Additionally, in the context of the increasing utilization of ADC drugs, STING agonists are now being employed as conjugate drugs for ADC. The STING agonist ADC employs antibodies to facilitate the targeted delivery of small-molecule immunostimulants to the TME and their subsequent localized release. This approach addresses the challenge of achieving a narrow safety window for immunostimulant system delivery while simultaneously enhancing the effectiveness of antitumor therapy [152]. An ADC has been synthesized that combines a STING agonist with an antibody targeting the EGFR. This resulting ADC effectively activated immune cells in vitro and demonstrated potent therapeutic effects with minimal toxicity in a syngeneic mouse tumor model [152].
Furthermore, the application of STING agonists within cancer vaccines may represent a novel approach to circumvent the systemic administration of these agents. STINGVAX, a combination cancer vaccine targeting the STING pathway, combines cyclic dinucleotides (CDNs) with tumor antigens and GM-CSF [153]. Relevant experiments have shown that it can activate dendritic cells (DCs) in the draining lymph nodes (DLNs) in vivo, and its ability to activate DCs is stronger than lipopolysaccharide (LPS). Experimental results from mice with STING gene mutations and IFNαR − / − mice demonstrate that the anti-tumor effect of STINGVAX requires functional STING and type I interferon involvement [153]. Tumor-derived microvesicles (T-MPs) represent another type of tumor vaccine containing specific proteins and nucleic acids from parental cells [154]. Research has indicated that T-MPs influence the immunogenic phenotype of DCs and produce potent anti-tumor immunity in processes of prevention and therapy by mediating antigen transfer from macrophages to DCs and inducing type I interferon production via the cGAS/STING pathway. Additionally, evidence suggests that microvesicles from allogeneic tumor cell lines appear to harbor shared tumor antigens that can be cross-presented by host DCs, providing potential simplification for clinical application of T-MP-based vaccines [154]. DNA@CaCO3, a cGAS-STING agonist synthesized by simple biomineralization growth of dsDNA-calcium carbonate (CaCO3) microparticles, activate DCs through intracellular cGAS-STING pathways by promoting dsDNA escape from endosomes, thereby inducing their maturation and activation to initiate an immune response [155].
Another challenge pertains to the presence of single-nucleotide polymorphisms within the STING gene, i.e., there exist five primary STING variant types in the human population: R232 (57.9%), HAQ (20.4%), R232H (13.7%), AQ (5.2%), and R293Q (1.5%). Research indicates that these human STING variants exhibit varying responses to STING agonists. Furthermore, the prevalence of these variants displays significant disparities among distinct population groups. For instance, the HAQ variant is prevalent among East Asians but rare among Africans [156]. Therefore, it becomes imperative to account for the distribution of STING variants within the population when selecting patients for clinical trials.
In summary, these findings underscore the crucial role played by the STING-mediated IFN-I-dependent signaling pathway in antitumor immune responses. As our understanding of these strategies advances, they offer promising avenues for further research and application in the field of cancer immunotherapy.
Discussion
In recent years, significant progress has been achieved in the field of cancer treatment, with a particular emphasis on T cells, including PD-1 antibodies, CAR-T, and TILs. The essential role played by T cells in recognizing and eradicating tumors is crucial. However, the activation of DCs, a critical step in T cell-mediated immunity, is frequently impeded by the highly immunosuppressive TME in most solid tumors [157, 158]. Tumor cells often disrupt the normal process of antigen presentation by inhibiting the maturation of DCs, resulting in the failure of T cell activation and, ultimately, the development of immune tolerance [159, 160]. Hence, the effective presentation of antigens through the activation of DCs is of paramount importance for the establishment of T cell-specific immunity.
Several in vivo studies have indicated that cDCs are indispensable in antigen cross-presentation, whereas pDCs and LCs exhibit lowered effectiveness compared to cDCs [29, 63]. When danger signals are detected, cDCs become activated by recognizing PAMPs or DAMPs [161]. Activated cDCs capture antigens and migrate to lymph nodes, where they present these antigens to both CD8+ and CD4+ T cells, initiating the immune response [37, 162]. Current research on DCs extends beyond cDCs alone, with animal experiments typically involving DCs derived from bone marrow. The activation of DCs often relies on innate immune signals, primarily mediated by cytokines. In preclinical experiments, cytokines such as IFN-α, GM-CSF, IL-2, IL-12, IL-15, and IL-21 have demonstrated effective treatment of mouse tumors. When investigating factors contributing to DC maturation, Thomas Luft et al. discovered that only IFN-α or IFN-β accelerated maturation, with most cells acquiring mature DC characteristics within three days [118]. However, the precise mechanism of IFN-I-mediated activation of DCs is not fully understood.
In recent years, research into the cGAS-STING signaling pathway has unveiled a close association between IFN-I and cGAS-STING activation in DC activation. While they share multiple functions, they exhibit distinct mechanisms. Both IFN-I and cGAS-STING agonists induce cellular apoptosis, but IFNs primarily affect tumor cells, whereas STING agonists impact various immune cell types, including T and B cells. Additionally, IFN-I activation in DCs leads to the expression of ISGs, which activate immune cells. In contrast, STING agonists not only induce IFN-I but also prompt DCs to release cytokines such as TNF-α and IL-6, thereby comprehensively activating the immune response. As an upstream signal for IFN-Is, STING agonists also mediate IFN-I-independent responses, including autophagy, inflammation, endoplasmic reticulum stress, and metabolic regulation. Consequently, the development of STING agonists holds broader potential in cancer therapy compared to IFN-I.
IFN-I encompasses a group of proteins, including IFN-α, IFN-β, and less explored variants such as IFN-ε, IFN-κ, and IFN-ω. For drug development targeting this pathway, IFN-α2 has been utilized for nearly 30 years, while IFN-β and other IFN-α types are still in the investigational phase. Studies have revealed that various IFN-α subtypes, particularly IFN-α14, exhibit diverse abilities to stimulate downstream pathways. IFN-α14 has demonstrated significant advantages over IFN-α2 in combating HBV infection, implying potential divergent antitumor effects among various IFN-α variants. While IFN-α and IFN-β share similar mechanisms in the treatment of tumors, IFN-β exhibits a higher affinity for IFNAR1/IFNAR2 receptors [163]. Consequently, compared to various IFN-α isoforms, IFN-β can modulate cellular functions at lower concentrations. The findings suggest that the development of targeted therapies for specific IFN-α subtypes or the formulation of enhanced IFN-beta preparations may represent novel strategies for leveraging Type I interferons in the treatment of cancer.
Relative to IFN-I, STING agonists possess the capacity to modulate an extensive array of downstream pathways. Presently, various STING agonists have demonstrated promising results in preclinical animal models, showing significant tumor burden reduction and immune activation. Moreover, as a potent activator of the cGAS-STING pathway, manganese ions can significantly enhance the host's anti-tumor immune function, effectively reducing the cost of tumor immune therapy [164]. This presents a novel avenue for drug development in STING agonists. Nonetheless, the advancement of STING agonists is met with considerable challenges, particularly concerning the potential damage to T cells, which is of paramount importance. This underscores the necessity for precision in the development of STING agonists, emphasizing their specificity to certain cell types.
Furthermore, recent studies have demonstrated that STING agonists may promote the development of tumors in some cancer types [165, 166]. On one hand, STING activation can promote chronic inflammation, thereby driving cutaneous carcinogenesis. Compared to wild-type mice, STING-deficient mice exhibit resistance to mutagen-induced skin tumorigenesis [165]. On the other hand, activation of STING supports tumor development by facilitating the infiltration of Tregs into the tumor microenvironment, upregulation of immune checkpoint molecules, and secretion of IL-10, which collectively serve to suppress T cell activity [166–168]. Overall, STING facilitates the growth of tumors by promoting chronic inflammation, which lays the groundwork for an immunosuppressive tumor microenvironment. It is noteworthy that chronic inflammation is a prolonged process in tumorigenesis, suggesting that sustained activation of STING at sites of inflammation can lead to tumor development. However, in the context of cancer therapy, the application of STING agonists constitutes a short-term, high-dose treatment strategy capable of effectively initiating the tumor-immunity cycle and promoting tumor regression highlighting the necessity for further comprehensive research and consideration in the development of small-molecule STING agonists. Fortunately, preclinical research combining ADC medications with gene-based strategies and STING agonists has produced encouraging outcomes, suggesting a new path for STING agonist development [152]. The development of cancer vaccines targeting the cGAS-STING-IFN I signaling pathway represents another direction in drug development. However, the high incidence of adverse events such as nausea, fatigue, and flu-like symptoms during DC vaccine use still requires further improvement [169, 170].
In summary, both IFN-I and STING agonists play essential roles in activating DCs and promoting immune responses. However, they exhibit distinct mechanisms and functions. A comprehensive understanding of these commonalities and disparities is vital for comprehending immune regulation and disease treatment mechanisms. As our comprehension advances, these insights offer potential for further research and applications in the field of cancer immunotherapy.
Acknowledgements
We sincerely thank colleagues in the Qi Chen lab for their discussion and support.
Author contributions
Manuscript conceptualization QC, YKS and GHL; Literature search and articles acquisition GHL, XQZ, HCZ, ZDZ and YDW; Funding acquisition, QC, YKS; Writing original manuscript draft YKS, GHL; Figures drawing, GHL; Final manuscript review and editing, QC, YKS, GHL, XQZ, HCZ, ZDZ and YDW.
Funding
This work was supported by grants from the Natural Science Foundation of Fujian Province (Grant No. 2022J01652), National Natural Science Foundation of China (Grant No. U22A20326), Natural Science Foundation of Fujian Province (Grant No. 2023J011275), Natural Science Foundation of Fujian Province (Grant No. 2023J011568).
Data availability
This review article did not look at any new data. Only results published in previous studies and identified in the reference list below were used.
Declarations
Conflict of interest
The authors declare they have no conflict of interest. Figures were created with biorender.com.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved the manuscript for publication at Cellular and Molecular Life Sciences.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yangkun Shen, Email: shenyk@fjnu.edu.cn.
Qi Chen, Email: chenqi@fjnu.edu.cn.
References
- 1.Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30(6):507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim TK, Vandsemb EN, Herbst RS, et al. Adaptive immune resistance at the tumour site: mechanisms and therapeutic opportunities. Nat Rev Drug Discov. 2022;21(7):529–540. doi: 10.1038/s41573-022-00493-5. [DOI] [PubMed] [Google Scholar]
- 3.Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. The Lancet. 2018;391(10135):2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 4.Shum B, Larkin J, Turajlic S. Predictive biomarkers for response to immune checkpoint inhibition. Semin Cancer Biol. 2022;79:4–17. doi: 10.1016/j.semcancer.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L, Buqué A, Kepp O, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 7.Linsley PS, Speake C, Whalen E, et al. Copy number loss of the interferon gene cluster in melanomas is linked to reduced T cell infiltrate and poor patient prognosis. PLoS ONE. 2014;9(10):e109760. doi: 10.1371/journal.pone.0109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L, Wu J, Du F, et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 2020;10(1):26–39. doi: 10.1158/2159-8290.CD-19-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Zhao H, Shen Y, et al. A Variety of nucleic acid species are sensed by cGAS, implications for its diverse functions. Front Immunol. 2022;13:826880. doi: 10.3389/fimmu.2022.826880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang KS, Xu CQ, Lv J. Identification and validation of the prognostic value of cyclic GMP-AMP synthase-stimulator of interferon (cGAS-STING) related genes in gastric cancer. Bioengineered. 2021;12(1):1238–1250. doi: 10.1080/21655979.2021.1911557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luksch H, Stinson WA, Platt DJ, et al. STING-associated lung disease in mice relies on T cells but not type I interferon. J Allergy Clin Immunol. 2019;144(1):254–266.e258. doi: 10.1016/j.jaci.2019.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilliams M, Ginhoux F, Jakubzick C, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balan S, Saxena M, Bhardwaj N. Dendritic cell subsets and locations. Int Rev Cell Mol Biol. 2019;348:1–68. doi: 10.1016/bs.ircmb.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Maraskovsky E, Daro E, Roux E, et al. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96(3):878–884. doi: 10.1182/blood.V96.3.878. [DOI] [PubMed] [Google Scholar]
- 17.Laoui D, Keirsse J, Morias Y, et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun. 2016;7:13720. doi: 10.1038/ncomms13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michea P, Noel F, Zakine E, et al. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat Immunol. 2018;19(8):885–897. doi: 10.1038/s41590-018-0145-8. [DOI] [PubMed] [Google Scholar]
- 19.Salmon H, Idoyaga J, Rahman A, et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44(4):924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Zhang X, Cheng Y, et al. Dendritic cell migration in inflammation and immunity. Cell Mol Immunol. 2021;18(11):2461–2471. doi: 10.1038/s41423-021-00726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruedl C, Kopf M, Bachmann MF. CD8(+) T cells mediate CD40-independent maturation of dendritic cells in vivo. J Exp Med. 1999;189(12):1875–1884. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manickasingham S, Reis e Sousa C. Microbial and T cell-derived stimuli regulate antigen presentation by dendritic cells in vivo. J Immunol. 2000;165(9):5027–5034. doi: 10.4049/jimmunol.165.9.5027. [DOI] [PubMed] [Google Scholar]
- 23.Muraille E, De Trez C, Pajak B, et al. T cell-dependent maturation of dendritic cells in response to bacterial superantigens. J Immunol. 2002;168(9):4352–4360. doi: 10.4049/jimmunol.168.9.4352. [DOI] [PubMed] [Google Scholar]
- 24.Mailliard RB, Egawa S, Cai Q, et al. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195(4):473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancel JC, Crozat K, Dalod M, et al. Are conventional type 1 dendritic cells critical for protective antitumor immunity and how? Front Immunol. 2019;10:9. doi: 10.3389/fimmu.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachem A, Guttler S, Hartung E, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207(6):1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crozat K, Guiton R, Contreras V, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207(6):1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flinsenberg TW, Compeer EB, Koning D, et al. Fcgamma receptor antigen targeting potentiates cross-presentation by human blood and lymphoid tissue BDCA-3+ dendritic cells. Blood. 2012;120(26):5163–5172. doi: 10.1182/blood-2012-06-434498. [DOI] [PubMed] [Google Scholar]
- 29.Nierkens S, Tel J, Janssen E, et al. Antigen cross-presentation by dendritic cell subsets: one general or all sergeants? Trends Immunol. 2013;34(8):361–370. doi: 10.1016/j.it.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang-Huau TL, Gueguen P, Goudot C, et al. Human in vivo-generated monocyte-derived dendritic cells and macrophages cross-present antigens through a vacuolar pathway. Nat Commun. 2018;9(1):2570. doi: 10.1038/s41467-018-04985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz FM, Colbert JD, Merino E, et al. The biology and underlying mechanisms of cross-presentation of exogenous antigens on MHC-I molecules. Annu Rev Immunol. 2017;35:149–176. doi: 10.1146/annurev-immunol-041015-055254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins SH, Walzer T, Dembele D, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9(1):R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durai V, Murphy KM. Functions of murine dendritic cells. Immunity. 2016;45(4):719–736. doi: 10.1016/j.immuni.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachem A, Hartung E, Guttler S, et al. Expression of XCR1 characterizes the Batf3-dependent lineage of dendritic cells capable of antigen cross-presentation. Front Immunol. 2012;3:214. doi: 10.3389/fimmu.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown CC, Gudjonson H, Pritykin Y, et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell. 2019;179(4):846–863. doi: 10.1016/j.cell.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154(1):3–20. doi: 10.1111/imm.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merad M, Sathe P, Helft J, et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller JC, Brown BD, Shay T, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13(9):888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furie RA, van Vollenhoven RF, Kalunian K, et al. Trial of Anti-BDCA2 antibody litifilimab for systemic lupus erythematosus. N Engl J Med. 2022;387(10):894–904. doi: 10.1056/NEJMoa2118025. [DOI] [PubMed] [Google Scholar]
- 40.Furie R, Werth VP, Merola JF, et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J Clin Invest. 2019;129(3):1359–1371. doi: 10.1172/JCI124466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JW, Budzak J, Liu Y, et al. Identification of serum glycoprotein ligands for the immunomodulatory receptor blood dendritic cell antigen 2. Glycobiology. 2018;28(8):592–600. doi: 10.1093/glycob/cwy050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng S, Li Z, Gao R, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021;184(3):792–809.e723. doi: 10.1016/j.cell.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Herber DL, Cao W, Nefedova Y, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16(8):880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramakrishnan R, Tyurin VA, Veglia F, et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol. 2014;192(6):2920–2931. doi: 10.4049/jimmunol.1302801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bandola-Simon J, Roche PA. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol Immunol. 2019;113:31–37. doi: 10.1016/j.molimm.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4(12):941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 47.Bell D, Chomarat P, Broyles D, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190(10):1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161(7):1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin X, Zeng W, Wu B, et al. PPARα inhibition overcomes tumor-derived exosomal lipid-induced dendritic cell dysfunction. Cell Rep. 2020;33(3):108278. doi: 10.1016/j.celrep.2020.108278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kool M, Geurtsvankessel C, Muskens F, et al. Facilitated antigen uptake and timed exposure to TLR ligands dictate the antigen-presenting potential of plasmacytoid DCs. J Leukoc Biol. 2011;90(6):1177–1190. doi: 10.1189/jlb.0610342. [DOI] [PubMed] [Google Scholar]
- 51.Faure F, Mantegazza A, Sadaka C, et al. Long-lasting cross-presentation of tumor antigen in human DC. Eur J Immunol. 2009;39(2):380–390. doi: 10.1002/eji.200838669. [DOI] [PubMed] [Google Scholar]
- 52.Edwards AD, Diebold SS, Slack EM, et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33(4):827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 53.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168(9):4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 54.Jarrossay D, Napolitani G, Colonna M, et al. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31(11):3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 55.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194(6):863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colonna M. TLR pathways and IFN-regulatory factors: to each its own. Eur J Immunol. 2007;37(2):306–309. doi: 10.1002/eji.200637009. [DOI] [PubMed] [Google Scholar]
- 57.Hoshino K, Kaisho T, Iwabe T, et al. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int Immunol. 2002;14(10):1225–1231. doi: 10.1093/intimm/dxf089. [DOI] [PubMed] [Google Scholar]
- 58.Oh JZ, Kurche JS, Burchill MA, et al. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2011;118(11):3028–3038. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16(2):141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- 60.Woo SR, Fuertes MB, Corrales L, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type i interferon-dependent antitumor immunity in immunogenic Tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21(5):298–312. doi: 10.1038/s41568-021-00339-z. [DOI] [PubMed] [Google Scholar]
- 63.Hildner K, Edelson BT, Purtha WE, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sánchez-Paulete AR, Cueto FJ, Martínez-López M, et al. Cancer immunotherapy with immunomodulatory Anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. 2016;6(1):71–79. doi: 10.1158/2159-8290.Cd-15-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferris ST, Durai V, Wu R, et al. cDC1 prime and are licensed by CD4(+) T cells to induce anti-tumour immunity. Nature. 2020;584(7822):624–629. doi: 10.1038/s41586-020-2611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferris ST, Carrero JA, Mohan JF, et al. A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity. 2014;41(4):657–669. doi: 10.1016/j.immuni.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26(5):638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saito Y, Komori S, Kotani T, et al. The role of type-2 conventional dendritic cells in the regulation of tumor immunity. Cancers (Basel) 2022 doi: 10.3390/cancers14081976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lund J, Sato A, Akira S, et al. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198(3):513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colonna M, Krug A, Cella M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr Opin Immunol. 2002;14(3):373–379. doi: 10.1016/s0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 71.Aspord C, Leccia MT, Charles J, et al. Melanoma hijacks plasmacytoid dendritic cells to promote its own progression. Oncoimmunology. 2014;3(1):e27402. doi: 10.4161/onci.27402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demoulin S, Herfs M, Delvenne P, et al. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol. 2013;93(3):343–352. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- 73.Li S, Wu J, Zhu S, et al. Disease-associated plasmacytoid dendritic cells. Front Immunol. 2017;8:1268. doi: 10.3389/fimmu.2017.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitchell D, Chintala S, Dey M. Plasmacytoid dendritic cell in immunity and cancer. J Neuroimmunol. 2018;322:63–73. doi: 10.1016/j.jneuroim.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 75.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15(8):471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aspord C, Leccia MT, Charles J, et al. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol Res. 2013;1(6):402–415. doi: 10.1158/2326-6066.CIR-13-0114-T. [DOI] [PubMed] [Google Scholar]
- 77.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117(9):2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Eck van der Sluijs J, van Ens D, Brummelman J, et al (2023) Human CD34(+)-derived complete plasmacytoid and conventional dendritic cell vaccine effectively induces antigen-specific CD8(+) T cell and NK cell responses in vitro and in vivo. Cell Mol Life Sci 80(10):298. 10.1007/s00018-023-04923-4 [DOI] [PMC free article] [PubMed]
- 79.Tel J, Schreibelt G, Sittig SP, et al. Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T cells despite lower Ag uptake than myeloid dendritic cell subsets. Blood. 2013;121(3):459–467. doi: 10.1182/blood-2012-06-435644. [DOI] [PubMed] [Google Scholar]
- 80.Jiang M, Chen P, Wang L, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13(1):81. doi: 10.1186/s13045-020-00916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng J, Mo J, Zhu T, et al. Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer. 2020;19(1):133. doi: 10.1186/s12943-020-01250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Long ZJ, Wang JD, Xu JQ, et al. cGAS/STING cross-talks with cell cycle and potentiates cancer immunotherapy. Mol Ther. 2022;30(3):1006–1017. doi: 10.1016/j.ymthe.2022.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Samson N, Ablasser A. The cGAS-STING pathway and cancer. Nat Cancer. 2022;3(12):1452–1463. doi: 10.1038/s43018-022-00468-w. [DOI] [PubMed] [Google Scholar]
- 84.Zierhut C, Yamaguchi N, Paredes M, et al. The cytoplasmic DNA sensor cGAS promotes mitotic cell death. Cell. 2019;178(2):302–315.e323. doi: 10.1016/j.cell.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gui X, Yang H, Li T, et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567(7747):262–266. doi: 10.1038/s41586-019-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J, Hubisz MJ, Earlie EM, et al. Non-cell-autonomous cancer progression from chromosomal instability. Nature. 2023;620(7976):1080–1088. doi: 10.1038/s41586-023-06464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oduro PK, Zheng X, Wei J, et al. The cGAS-STING signaling in cardiovascular and metabolic diseases: future novel target option for pharmacotherapy. Acta Pharm Sin B. 2022;12(1):50–75. doi: 10.1016/j.apsb.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu H, Zhang R, Yi L, et al. UNC93B1 attenuates the cGAS-STING signaling pathway by targeting STING for autophagy-lysosome degradation. J Med Virol. 2022;94(9):4490–4501. doi: 10.1002/jmv.27860. [DOI] [PubMed] [Google Scholar]
- 89.Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 90.Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–2496. doi: 10.1158/0008-5472.Can-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stagg J, Loi S, Divisekera U, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108(17):7142–7147. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang W, Wang G, Xu ZG, et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell. 2019;178(1):176–189.e115. doi: 10.1016/j.cell.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bidwell BN, Slaney CY, Withana NP, et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med. 2012;18(8):1224–1231. doi: 10.1038/nm.2830. [DOI] [PubMed] [Google Scholar]
- 94.Dunn GP, Bruce AT, Sheehan KC, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6(7):722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 95.Chen HM, Tanaka N, Mitani Y, et al. Critical role for constitutive type I interferon signaling in the prevention of cellular transformation. Cancer Sci. 2009;100(3):449–456. doi: 10.1111/j.1349-7006.2008.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang H, Hu S, Chen X, et al. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc Natl Acad Sci U S A. 2017;114(7):1637–1642. doi: 10.1073/pnas.1621363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roers A, Hiller B, Hornung V. Recognition of endogenous nucleic acids by the innate immune system. Immunity. 2016;44(4):739–754. doi: 10.1016/j.immuni.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 98.Zhang CZ, Spektor A, Cornils H, et al. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522(7555):179–184. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yum S, Li M, Chen ZJ. Old dogs, new trick: classic cancer therapies activate cGAS. Cell Res. 2020;30(8):639–648. doi: 10.1038/s41422-020-0346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chao HH, Karagounis IV, Thomas C, et al. Combination of CHEK1/2 inhibition and ionizing radiation results in abscopal tumor response through increased micronuclei formation. Oncogene. 2020;39(22):4344–4357. doi: 10.1038/s41388-020-1300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lai J, Fu Y, Tian S, et al. Zebularine elevates STING expression and enhances cGAMP cancer immunotherapy in mice. Mol Ther. 2021;29(5):1758–1771. doi: 10.1016/j.ymthe.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Falahat R, Berglund A, Perez-Villarroel P, et al. Epigenetic state determines the in vivo efficacy of STING agonist therapy. Nat Commun. 2023;14(1):1573. doi: 10.1038/s41467-023-37217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ku Y, Park JH, Cho R, et al. Noncanonical immune response to the inhibition of DNA methylation by Staufen1 via stabilization of endogenous retrovirus RNAs. Proc Natl Acad Sci U S A. 2021 doi: 10.1073/pnas.2016289118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fang L, Hao Y, Yu H, et al. Methionine restriction promotes cGAS activation and chromatin untethering through demethylation to enhance antitumor immunity. Cancer Cell. 2023;41(6):1118–1133.e1112. doi: 10.1016/j.ccell.2023.05.005. [DOI] [PubMed] [Google Scholar]
- 106.Saitoh T, Fujita N, Hayashi T, et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci U S A. 2009;106(49):20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng Z, Zhao M, Shan H, et al. Noncanonical autophagy is a new strategy to inhibit HSV-1 through STING1 activation. Autophagy. 2023 doi: 10.1080/15548627.2023.2237794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu B, Carlson RJ, Pires IS, et al. Human STING is a proton channel. Science. 2023;381(6657):508–514. doi: 10.1126/science.adf8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao Y, Zheng X, Chang B, et al. Intercellular transfer of activated STING triggered by RAB22A-mediated non-canonical autophagy promotes antitumor immunity. Cell Res. 2022;32(12):1086–1104. doi: 10.1038/s41422-022-00731-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yum S, Li M, Fang Y, et al. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc Natl Acad Sci U S A. 2021 doi: 10.1073/pnas.2100225118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu Z, Yu X, Ding R, et al. Glycolysis drives STING signaling to facilitate dendritic cell antitumor function. J Clin Invest. 2023 doi: 10.1172/jci166031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang L, Jiang C, Zhong Y, et al. STING is a cell-intrinsic metabolic checkpoint restricting aerobic glycolysis by targeting HK2. Nat Cell Biol. 2023;25(8):1208–1222. doi: 10.1038/s41556-023-01185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duong E, Fessenden TB, Lutz E, et al. Type I interferon activates MHC class I-dressed CD11b(+) conventional dendritic cells to promote protective anti-tumor CD8(+) T cell immunity. Immunity. 2022;55(2):308–323.e309. doi: 10.1016/j.immuni.2021.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lorenzi S, Mattei F, Sistigu A, et al. Type I IFNs control antigen retention and survival of CD8α(+) dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J Immunol. 2011;186(9):5142–5150. doi: 10.4049/jimmunol.1004163. [DOI] [PubMed] [Google Scholar]
- 115.Spadaro F, Lapenta C, Donati S, et al. IFN-α enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119(6):1407–1417. doi: 10.1182/blood-2011-06-363564. [DOI] [PubMed] [Google Scholar]
- 116.Shin EC, Seifert U, Kato T, et al. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006;116(11):3006–3014. doi: 10.1172/jci29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shin EC, Seifert U, Urban S, et al. Proteasome activator and antigen-processing aminopeptidases are regulated by virus-induced type I interferon in the hepatitis C virus-infected liver. J Interferon Cytokine Res. 2007;27(12):985–990. doi: 10.1089/jir.2007.0039. [DOI] [PubMed] [Google Scholar]
- 118.Luft T, Pang KC, Thomas E, et al. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161(4):1947–1953. doi: 10.4049/jimmunol.161.4.1947. [DOI] [PubMed] [Google Scholar]
- 119.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schmid ET, Pang IK, Carrera Silva EA, et al. AXL receptor tyrosine kinase is required for T cell priming and antiviral immunity. Elife. 2016 doi: 10.7554/eLife.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Borden EC. Interferons α and β in cancer: therapeutic opportunities from new insights. Nat Rev Drug Discov. 2019;18(3):219–234. doi: 10.1038/s41573-018-0011-2. [DOI] [PubMed] [Google Scholar]
- 122.Pokrovskaja K, Panaretakis T, Grandér D. Alternative signaling pathways regulating type I interferon-induced apoptosis. J Interferon Cytokine Res. 2005;25(12):799–810. doi: 10.1089/jir.2005.25.799. [DOI] [PubMed] [Google Scholar]
- 123.Katayama T, Nakanishi K, Nishihara H, et al. Type I interferon prolongs cell cycle progression via p21WAF1/CIP1 induction in human colon cancer cells. Int J Oncol. 2007;31(3):613–620. doi: 10.3892/ijo.31.3.613. [DOI] [PubMed] [Google Scholar]
- 124.Manabe A, Yi T, Kumagai M, et al. Use of stroma-supported cultures of leukemic cells to assess antileukemic drugs. I Cytotoxicity of interferon alpha in acute lymphoblastic leukemia. Leukemia. 1993;7(12):1990–1995. [PubMed] [Google Scholar]
- 125.Holicek P, Guilbaud E, Klapp V, et al. Type I interferon and cancer. Immunol Rev. 2023 doi: 10.1111/imr.13272. [DOI] [PubMed] [Google Scholar]
- 126.Paquette RL, Hsu NC, Kiertscher SM, et al. Interferon-alpha and granulocyte-macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J Leukoc Biol. 1998;64(3):358–367. doi: 10.1002/jlb.64.3.358. [DOI] [PubMed] [Google Scholar]
- 127.Ignarro RS, Bombeiro AL, Chiarotto GB, et al. Interferon-beta induces major histocompatibility complex of class I (MHC-I) expression and a proinflammatory phenotype in cultivated human astrocytes. Differentiation. 2022;128:43–56. doi: 10.1016/j.diff.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 128.Radvanyi LG, Banerjee A, Weir M, et al. Low levels of interferon-alpha induce CD86 (B7.2) expression and accelerates dendritic cell maturation from human peripheral blood mononuclear cells. Scand J Immunol. 1999;50(5):499–509. doi: 10.1046/j.1365-3083.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 129.Fuertes MB, Kacha AK, Kline J, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7):3077–3085. doi: 10.1158/0008-5472.Can-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Padovan E, Spagnoli GC, Ferrantini M, et al. IFN-alpha2a induces IP-10/CXCL10 and MIG/CXCL9 production in monocyte-derived dendritic cells and enhances their capacity to attract and stimulate CD8+ effector T cells. J Leukoc Biol. 2002;71(4):669–676. doi: 10.1189/jlb.71.4.669. [DOI] [PubMed] [Google Scholar]
- 132.Bakhoum SF, Ngo B, Laughney AM, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553(7689):467–472. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Larkin B, Ilyukha V, Sorokin M, et al. Cutting edge: activation of STING in T cells induces Type I IFN responses and cell death. J Immunol. 2017;199(2):397–402. doi: 10.4049/jimmunol.1601999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gulen MF, Koch U, Haag SM, et al. Signalling strength determines proapoptotic functions of STING. Nat Commun. 2017;8(1):427. doi: 10.1038/s41467-017-00573-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu J, Chen YJ, Dobbs N, et al. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med. 2019;216(4):867–883. doi: 10.1084/jem.20182192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu J, Dobbs N, Yang K, et al. Interferon-independent activities of mammalian STING mediate antiviral response and tumor immune evasion. Immunity. 2020;53(1):115–126.e115. doi: 10.1016/j.immuni.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pestka S. The human interferon-alpha species and hybrid proteins. Semin Oncol. 1997;24(3 Suppl 9):S9-4-s9-17. [PubMed] [Google Scholar]
- 138.Persano L, Crescenzi M, Indraccolo S. Anti-angiogenic gene therapy of cancer: current status and future prospects. Mol Aspects Med. 2007;28(1):87–114. doi: 10.1016/j.mam.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 139.Berraondo P, Sanmamed MF, Ochoa MC, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120(1):6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Geelen IGP, Gullaksen SE, Ilander MM, et al. Switching from imatinib to nilotinib plus pegylated interferon-α2b in chronic phase CML failing to achieve deep molecular response: clinical and immunological effects. Ann Hematol. 2023;102(6):1395–1408. doi: 10.1007/s00277-023-05199-1. [DOI] [PubMed] [Google Scholar]
- 141.Yamada R, Steward K. Antibody-CD20-interferon-alpha fusion protein has superior in vivo activity against human B cell lymphomas compared to Rituximab, and enhanced complement-dependent cytotoxicity in vitro. J ImmunoTher Cancer. 2013;1(263):P263–P263. doi: 10.1186/2051-1426-1-S1-P263. [DOI] [Google Scholar]
- 142.Yang X, Zhang X, Fu ML, et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25(1):37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jenks N, Myers R, Greiner SM, et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Hum Gene Ther. 2010;21(4):451–462. doi: 10.1089/hum.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Waters AM, Johnston JM, Reddy AT, et al. Rationale and design of a phase 1 clinical trial to evaluate HSV G207 alone or with a single radiation dose in children with progressive or recurrent malignant supratentorial brain tumors. Hum Gene Ther Clin Dev. 2017;28(1):7–16. doi: 10.1089/humc.2017.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baguley BC. Antivascular therapy of cancer: DMXAA. Lancet Oncol. 2003;4(3):141–148. doi: 10.1016/s1470-2045(03)01018-0. [DOI] [PubMed] [Google Scholar]
- 146.Graham PT, Nowak AK, Cornwall SMJ, et al. The STING agonist, DMXAA, reduces tumor vessels and enhances mesothelioma tumor antigen presentation yet blunts cytotoxic T cell function in a murine model. Front Immunol. 2022;13:969678. doi: 10.3389/fimmu.2022.969678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McKeage MJ, Von Pawel J, Reck M, et al. Randomised phase II study of ASA404 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Br J Cancer. 2008;99(12):2006–2012. doi: 10.1038/sj.bjc.6604808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meric-Bernstam F, Sweis RF, Hodi FS, et al. Phase I dose-escalation trial of MIW815 (ADU-S100), an intratumoral STING agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin Cancer Res. 2022;28(4):677–688. doi: 10.1158/1078-0432.Ccr-21-1963. [DOI] [PubMed] [Google Scholar]
- 149.Meric-Bernstam F, Sweis RF, Kasper S, et al. Combination of the STING agonist MIW815 (ADU-S100) and PD-1 inhibitor spartalizumab in advanced/metastatic solid tumors or lymphomas: an open-label, multicenter Phase Ib Study. Clin Cancer Res. 2023;29(1):110–121. doi: 10.1158/1078-0432.Ccr-22-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Grabosch S, Bulatovic M, Zeng F, et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene. 2019;38(13):2380–2393. doi: 10.1038/s41388-018-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leventhal DS, Sokolovska A, Li N, et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat Commun. 2020;11(1):2739. doi: 10.1038/s41467-020-16602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu YT, Fang Y, Wei Q, et al. Tumor-targeted delivery of a STING agonist improvescancer immunotherapy. Proc Natl Acad Sci U S A. 2022;119(49):e2214278119. doi: 10.1073/pnas.2214278119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fu J, Kanne DB, Leong M, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7(283):283ra252. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang H, Tang K, Zhang Y, et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol Res. 2015;3(2):196–205. doi: 10.1158/2326-6066.CIR-14-0177. [DOI] [PubMed] [Google Scholar]
- 155.Li Q, Dong Z, Cao Z, et al. A general biomineralization strategy to synthesize autologous cancer vaccines with cGAS-STING activating capacity for postsurgical immunotherapy. ACS Nano. 2023;17(11):10496–10510. doi: 10.1021/acsnano.3c01404. [DOI] [PubMed] [Google Scholar]
- 156.Wang Z, Zhao C, Wang C, et al. Synthesis and biological evaluation of all possible inosine-mixed cyclic dinucleotides that activate different hSTING variants. Bioorg Med Chem. 2021;29:115899. doi: 10.1016/j.bmc.2020.115899. [DOI] [PubMed] [Google Scholar]
- 157.Restifo NP, Smyth MJ, Snyder A. Acquired resistance to immunotherapy and future challenges. Nat Rev Cancer. 2016;16(2):121–126. doi: 10.1038/nrc.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Phuengkham H, Ren L, Shin IW, et al. Nanoengineered immune niches for reprogramming the immunosuppressive tumor microenvironment and enhancing cancer immunotherapy. Adv Mater. 2019;31(34):e1803322. doi: 10.1002/adma.201803322. [DOI] [PubMed] [Google Scholar]
- 159.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kranz LM, Diken M, Haas H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 161.Whiteside TL, Odoux C. Dendritic cell biology and cancer therapy. Cancer Immunol Immunother. 2004;53(3):240–248. doi: 10.1007/s00262-003-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhu S, Yang N, Wu J, et al. Tumor microenvironment-related dendritic cell deficiency: a target to enhance tumor immunotherapy. Pharmacol Res. 2020;159:104980. doi: 10.1016/j.phrs.2020.104980. [DOI] [PubMed] [Google Scholar]
- 163.Zitvogel L, Galluzzi L, Kepp O, et al. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 164.Lv M, Chen M, Zhang R, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30(11):966–979. doi: 10.1038/s41422-020-00395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ahn J, Xia T, Konno H, et al. Inflammation-driven carcinogenesis is mediated through STING. Nat Commun. 2014;5:5166. doi: 10.1038/ncomms6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liang D, Xiao-Feng H, Guan-Jun D, et al. (2015) Activated STING enhances Tregs infiltration in the HPV-related carcinogenesis of tongue squamous cells via the c-jun/CCL22 signal. Biochim Biophys Acta. 1852;11:2494–2503. doi: 10.1016/j.bbadis.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 167.An X, Zhu Y, Zheng T, et al. An analysis of the expression and association with immune cell infiltration of the cGAS/STING pathway in pan-cancer. Mol Ther Nucleic Acids. 2019;14:80–89. doi: 10.1016/j.omtn.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moore E, Clavijo PE, Davis R, et al. Established T cell-inflamed tumors rejected after adaptive resistance was reversed by combination STING activation and PD-1 pathway blockade. Cancer Immunol Res. 2016;4(12):1061–1071. doi: 10.1158/2326-6066.CIR-16-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boudewijns S, Bloemendal M, de Haas N, et al. Autologous monocyte-derived DC vaccination combined with cisplatin in stage III and IV melanoma patients: a prospective, randomized phase 2 trial. Cancer Immunol Immunother. 2020;69(3):477–488. doi: 10.1007/s00262-019-02466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wilgenhof S, Corthals J, Heirman C, et al. Phase II study of autologous monocyte-derived mRNA Electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol. 2016;34(12):1330–1338. doi: 10.1200/jco.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 171.Chang W, Altman MD, Lesburg CA, et al. Discovery of MK-1454: a potent cyclic dinucleotide stimulator of interferon genes agonist for the treatment of cancer. J Med Chem. 2022;65(7):5675–5689. doi: 10.1021/acs.jmedchem.1c02197. [DOI] [PubMed] [Google Scholar]
- 172.Harrington K, Parkes E, Weiss J, et al. Abstract CT217: Phase I, first-in-human trial evaluating BI 1387446 (STING agonist) alone and in combination with ezabenlimab (BI 754091; anti-PD-1) in solid tumors. Can Res. 2021;81(13_Supplement):CT217–CT217. doi: 10.1158/1538-7445.Am2021-ct217. [DOI] [Google Scholar]
- 173.Cooper BT, Chmura SJ, Luke JJ, et al. TAK-676 in combination with pembrolizumab after radiation therapy in patients (pts) with advanced non–small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), or squamous-cell carcinoma of the head and neck (SCCHN): phase 1 study design. J Clin Oncol. 2022;40(16):2698–2698. doi: 10.1200/JCO.2022.40.16_suppl.TPS2698. [DOI] [Google Scholar]
- 174.Huang KC, Chanda D, McGrath S, et al. Pharmacologic activation of STING in the bladder induces potent antitumor immunity in non-muscle invasive murine bladder cancer. Mol Cancer Ther. 2022;21(6):914–924. doi: 10.1158/1535-7163.Mct-21-0780. [DOI] [PubMed] [Google Scholar]
- 175.Hayman TJ, Baro M, MacNeil T, et al. STING enhances cell death through regulation of reactive oxygen species and DNA damage. Nat Commun. 2021;12(1):2327. doi: 10.1038/s41467-021-22572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prabagar M, Bommireddy V, Siegel R, et al. Abstract 524: The novel STING agonist VB-85247 induces robust durable antitumor immune responses by intravesical administration in a non-muscle invasive bladder cancer model. Cancer Res. 2021;81(13):524–524. doi: 10.1158/1538-7445.Am2021-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luke JJ, Piha-Paul SA, Medina T, et al. Phase I Study of SYNB1891, an engineered E. coli nissle strain expressing STING agonist, with and without atezolizumab in advanced malignancies. Clin Cancer Res. 2023;29(13):2435–2444. doi: 10.1158/1078-0432.Ccr-23-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ramanjulu JM, Pesiridis GS, Yang J, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564(7736):439–443. doi: 10.1038/s41586-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 179.Pan BS, Perera SA, Piesvaux JA, et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 2020 doi: 10.1126/science.aba6098. [DOI] [PubMed] [Google Scholar]
- 180.Chin EN, Yu C, Vartabedian VF, et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science. 2020;369(6506):993–999. doi: 10.1126/science.abb4255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review article did not look at any new data. Only results published in previous studies and identified in the reference list below were used.